Abstract
Background:
Data from a US multicenter longitudinal study of bariatric surgery was used to compare weight change (primary outcome) and comorbidities (secondary outcome) in patients who underwent SG versus RYGB.
METHODS:
This study includes participants who underwent SG and matched participants who underwent RYGB from the Longitudinal Assessment of Bariatric Surgery-2 (LABS-2) study. Adults undergoing initial bariatric surgical procedures between 2006–2009 were enrolled. Participants who underwent SG were high-risk or superobese and intended to have a second stage procedure. Mixed models were used to evaluate percent weight change from baseline through 7 years, and diabetes, dyslipidemia, and hypertension prevalence through 5 years.
RESULTS:
Fifty-seven of 59 participants who underwent SG were matched one-to-one. Most were female (68%), white (81%), had a median age of 49 (37, 56) years and median BMI of 56.4 (35.5–76.8) kg/m2 pre-surgery. Weight loss was significantly less 1–7 years following SG versus matched RYGB (e.g., year-7 mean weight loss was 23.6% versus 30.4%, respectively; p=.001). For both surgical groups, prevalence of diabetes, low HDL and hypertension were significantly (p<.05) lower five years post-surgery vs baseline.
CONCLUSION:
Higher risk or super obese participants following SG lost less weight than did matched RYGB counterparts throughout 7 years. Both groups exhibited improvements in comorbidities from pre-surgery through 5 years.
BACKGROUND
Sleeve Gastrectomy (SG) has grown in popularity over the last two decades, progressing from investigational to mainstream (1). Initially, SG was not performed as a primary, definitive bariatric surgical procedure rather was intended to be the initial stage of a 2-stage procedure on higher risk bariatric surgical candidates due to super obesity or complex medical/surgical conditions (2, 3). The second stage was to be either a Roux-en-Y gastric bypass (RYGB) or biliopancreatic diversion and duodenal switch (BPD-DS) after initial weight loss and improved medical status and a decreased operative risk. Early studies demonstrated good preliminary outcomes with this approach (4).
Prior to 2012, SG was not formally accepted as a primary procedure by the American Society of Metabolic and Bariatric Surgery (ASMBS) (5) and many insurers did not cover the procedure. In 2007, the ASMBS expressed support for SG, especially in the 2-stage procedure approach, but cited a lack of greater than 3-year follow-up data as a concern (6). In 2009, the ASMBS stated there were limited 3–5-year data available and conditionally accepted SG, primarily because of its established value as a first-stage operation for high risk patients (3). As recently as 2011 the SG accounted for less than one-fifth of bariatric surgical procedures performed in the United States (7). That same year, a report utilizing national data from the American College of Surgeons-Bariatric Surgery Center Network accreditation program positioned SG between laparoscopic adjustable gastric banding (LAGB) and RYGB in terms of safety, weight loss, and co-morbidity resolution in the first postoperative year (8), which led to formal support from ASMBS (5). In 2012, a study of 1000 patients underwent stand-alone SG with a follow up of 3 years demonstrated safety, weight and comorbidities outcomes close to RYGB (9). Just four years later (2016), SG accounted for over half of US bariatric surgical procedures (7). However, there are few prospective studies of SG with long-term follow-up. Additionally, there are few comparisons of long-term outcomes following SG vs. RYGB, especially among higher risk super obese or medically complex patients.
The Longitudinal Assessment of Bariatric Surgery-2 (LABS-2), a large multi-center cohort study, was designed to evaluate the effectiveness of surgery, durability of effect, and long-term outcomes. Participants underwent surgery between 2006–2009 and were followed for 6–7 years. The main findings following the two most common procedures during that timeframe, RYGB and LAGB, have been reported (10). This report addresses an important knowledge gap in the literature by examining the durability and variability of weight loss (primary outcomes) and the comorbidity response (secondary outcomes) following SG, which was performed among super obese or high-risk patients and originally intended to be the initial stage of a 2-stage process, and compares the response to a matched RYGB group
METHODS:
The LABS-2 is a multi-center observational cohort study at 10 US hospitals in 6 geographically diverse clinical centers. Adults undergoing first-time bariatric surgical procedures as part of routine clinical care by participating surgeons were recruited between 2006 and 2009 and followed through January 31, 2015. Research assessments were conducted within 30 days prior to surgery, and approximately 6 months, 1 year, and then annually following surgery for at least 6 years and up to 7 years through the study end date (January 31, 2015). The Institutional Review Boards at each center approved the protocol and all participants gave informed consent to participate in the study. The LABS study is registered at ClinicalTrials.gov (NCT00465829).
During the time of LABS recruitment (2006–2009), SG was recommended to be utilized as a staged procedure for high-risk or super obese patients and was not reimbursed as a primary procedure by insurance. All SG submitted into the LABS cohort were designated by their surgeons as “high-risk or superobese” (i.e. either high-risk from a medical or surgical perspective) who would significantly benefit from the 2-stage approach.
This report includes 57 participants who underwent a SG and their matched RYGB counterparts. Participants were matched on sex, race, age (within 5 years), and baseline BMI (within 5kg/m2). Furthermore, when possible, participants were also matched on additional criteria using the following hierarchy ethnicity, smoking, diabetes, hyperlipidemia, high triglycerides, low HDL and hypertension status.
Research assessments, conducted by LABS-certified personnel, were primarily conducted in-person, with the exception of the 6-month and 6-year assessments, which were brief, and largely completed by telephone or mail. Sociodemographic characteristics were self-reported. Weight measurements and calculation of weight change in the LABS cohort have been described (10). Weight change was calculated as the percent change from baseline (primary outcome) and in kilograms (kg) (secondary outcome). The lowest weight among participants whose weight was measured at five or more assessments, at least one of which occurred during or after the five-year assessment, was classified as weight nadir if weight was not missing at the assessments due immediately prior to and immediately following it. Weight regain from nadir was calculated as percentage of maximum weight lost, i.e., [100*(post-nadir weight – nadir weight)]/ (baseline weight – nadir weight) (11) and percentage of baseline weight.
Comorbid conditions were not assessed at the brief assessments conducted at 6 months and year 6 but were at other time points. Given that data collection ended prior to many year 7 assessments and the relatively rare comorbidity outcomes (prevalence, remission, incidence), comorbidities are reported only through year 5. The LABS definitions of diabetes, high low-density lipoprotein cholesterol (LDL), low high-density lipoprotein cholesterol (HDL), high triglycerides, and hypertension have been reported (12). In addition to prevalence, remission and incidence of comorbidities at follow-up were determined in reference to baseline status. Remission was defined as having had the comorbidity at baseline with absence of the comorbidity at follow-up. Incidence was the absence of the comorbidity at baseline and having the comorbidity at follow-up.
Vital status was determined through annual study follow-up. In addition, a query of the National Death Index, a centralized database of death record information on file in state vital statistics offices, was performed through the year 2015 (13). Subsequent bariatric procedures within 7 years of initial bariatric surgery were identified by LABS surgeons who performed the procedures, medical record review or participant self-report, using a standardized protocol.
Statistical analyses were conducted using SAS version 9.4 (SAS Institute, Cary NC. USA). All reported P values are two-sided: P values less than 0.05 were considered to be statistically significant. The data for each participant and his or her match were censored following a second stage bariatric procedure. Descriptive statistics summarize baseline characteristics, subsequent bariatric procedures, and outcomes by time point in the two procedure groups. Frequencies and percentages are reported for categorical data. Medians, 25th and 75th percentiles, are reported for continuous data. Statistical significance of pre-surgery group differences in distributions was tested using Wilcoxon’s test for continuous variables and Pearson’s chi-square test or exact tests for categorical variables, as appropriate.
Difference in weight change between SG vs. RYGB was tested by fitting a linear mixed effect model via maximum likelihood with weight change over time as the outcome, time since initial bariatric surgery (assessment), procedure (SG vs. RYGB) and a procedure by time interaction as discrete fixed effects, and the matched pair as random effects. The model controlled for baseline age, smoking and site, which were related to missing follow-up data (14), as fixed effects. Statistical significance of the difference in distributions of weight change across the 7 years was tested with a likelihood ratio test. Because the procedure by time interaction was significant, the equality of the distributions of weight change at each time point for those undergoing SG vs. RYGB were tested. The mean weight change by procedure, and the mean difference in weight change between procedures, with corresponding 95% confidence intervals, are presented by post-surgery assessment.
A linear mixed effect model was also used to test for a difference in weight regain from post-surgery weight nadir between SG vs. RYGB. Procedure, linear and quadratic terms for time since weight nadir (time), and procedure by time interactions were entered as fixed effects, and matched pair as random effects. This model also controlled for baseline age, smoking and site as fixed effects. Because the procedure by time interactions were not statistically significant, they were not included in final models.
Not all participants undergoing SG could be matched with someone who underwent RYGB by baseline comorbidity status. Due to the small sample size and sparseness of comorbidity data, there were few matched pairs that had data available at the same follow-up time points. Thus the analyses for comorbidities were assessed within each surgical group, using unmatched Poisson mixed models with robust error variance to estimate prevalence of each comorbidity with time (assessment) as a discrete fixed effect (15). Modeled proportions and 95% CI are reported by assessment. Pairwise comparison were made between baseline and year 5 prevalence. There was insufficient statistical power to model remission and incidence. However, remission and incidence were calculated with observed data.
RESULTS
Study Participants and Retention
Of 59 participants who underwent a SG procedure, 57 were matched to a participant who underwent RYGB as his or her first bariatric operation; 46 (80.7%) of the RYGB were performed laparoscopically. The two participants who could not be matched on sex, age and BMI were both males: one age 35 years with a BMI of 80.4 kg/m2, one 43 years old with a BMI of 94.3 kg/m2. Both participants lost at least 20% of baseline weight by the six-month assessment and 30% by the year 1 assessment. Neither underwent a subsequent bariatric procedure or died.
Not all participants were due for their 7-year follow-up assessment before data collection ended. In the analysis sample of 57 SG and 57 RYGB, excluding weights after a second stage bariatric procedure or measured during pregnancy, weights were attained in 98.2% (55/56) of SG participants at 6 months, 100.0% (54/54) at year 1, 89.8% (44/49) at year 2, 87.2% (4¼7) at year 3, 89.6% (4¾8) at year 4, 80.4% (37/46) at year 5, 93.5% (4¾6) at year 6, and 90.0% (27/30) at year 7. Applying the same criteria, among the matched RYGB participants weights were attained 96.5% (55/57) at 6 months, 88.9% (48/54) at year 1, 75.0% (36/48) at year 2, 68.1% (32/47) at year 3, 68.8% (3¾8) at year 4, 64.6% (3¼8) at year 5, 65.9% (29/44) at year 6, and 57.6% (19/33) at year 7.
Baseline characteristics
Approximately two-thirds of participants were female and 81% were white. The median (25th-75th percentile) age was 49 (37–56) years and median (25th-75th percentile) BMI was 56.4 (46.8–63.2) kg/m2. Baseline characteristics and locations where procedures were performed by surgical procedure are reported in Table 1. Among SG, 82.5% were done with a 40 Fr bougie size.
Table 1.
Baseline characteristics of LABS-2 participants who underwent sleeve gastrectomy to matcheda participants who underwent Roux-en-Y gastric bypass.
| Sleeve Gastrectomy (N=57b) |
Roux-en-Y Gastric Bypass (N=57b) |
p valuec | |
|---|---|---|---|
| Age, years | 0.90 | ||
| Median (25th,75th %-ile) | 50.0 (36.0, 55.0) | 48.0 (38.0, 57.0) | |
| Range | 21.0–73.0 | 20.0–69.0 | |
| Body mass index, kg/m2 | 0.89 | ||
| Median (25th,75th %-ile) | 57.7 (46.8, 63.1) | 56.0 (47.2, 63.2) | |
| Range | 35.5–75.2 | 35.7–76.8 | |
| Sex, n (%) | 1.00 | ||
| Male | 18 (31.6) | 18 (31.6) | |
| Female | 39 (68.4) | 39 (68.4) | |
| Race, n (%) | (n=55) | 0.92 | |
| White | 45 (81.8) | 46 (80.7) | |
| Black | 8 (14.5) | 8 (14.0) | |
| Other | 2 (3.6) | 3 (5.3) | |
| Ethnicity, n (%) | 0.51 | ||
| Hispanic | 6 (10.5) | 4 (7.0) | |
| Non-Hispanic | 51 (89.5) | 53 (93.0) | |
| Current/recent smoker, n (%) | 0.43 | ||
| No | 47 (82.5) | 50 (87.7) | |
| Yes | 10 (17.5) | 7 (12.3) | |
| Diabetes, n (%) | (n=53) | 0.89 | |
| No | 36 (67.9) | 38 (66.7) | |
| Yes | 17 (32.1) | 19 (33.3) | |
| High LDL, n (%) | (n=52) | (n=54) | 0.57 |
| No | 27 (51.9) | 31 (57.4) | |
| Yes | 25 (48.1) | 23 (42.6) | |
| High triglycerides, n (%) | (n=53) | (n=55) | 0.18 |
| No | 41 (77.4) | 48 (87.3) | |
| Yes | 12 (22.6) | 7 (12.7) | |
| Low HDL, n (%) | (n=54) | (n=56) | 0.96 |
| No | 34 (63.0) | 35 (62.5) | |
| Yes | 20 (37.0) | 21 (37.5) | |
| Hypertension, n (%) | (n=54) | 0.52 | |
| No | 10 (18.5) | 8 (14.0) | |
| Yes | 44 (81.5) | 49 (86.0) | |
| Location surgery preformed | |||
| Oregon Health & Science University | 0 (0.0) | 4 (7.0) | |
| East Carolina Medical Center | 1 (1.8) | 13 (22.8) | |
| Cornell University Medical Center | 49 (86.0) | 4 (7.0) | |
| Neuropsychiatric Research Institute | 1 (1.8) | 6 (10.5) | |
| University of Pittsburgh Medical Center | 1 (1.8) | 9 (15.8) | |
| University of Washington | 1 (1.8) | 7 (12.3) | |
| Columbia University Medical Center | 4 (7.0) | 1 (1.8) | |
| Virginia Mason Medical Center | 0 (0.0) | 8 (14.0) | |
| Legacy Good Samaritan Hospital | 0 (0.0) | 5 (8.8) | |
Abbreviations: HDL=High-density lipoprotein, LDL=Low-density lipoprotein.
Deno
minators differ because of missing data. Only n’s less than 57 are shown.
Wilcoxon’s test for continuous variables; Pearson’s chi-square test or exact tests, as appropriate, for categorical variables.
Weight Change
Figure 1 shows both modeled and observed percent of baseline weight change by time point and surgical procedure, and the mean difference (i.e., SG-RYGB) in weight change between procedures by time point. The difference in weight change between procedures differed over time (p<.001 for procedure x time interaction). At 6-months there was not a significant difference between SG versus RYGB (estimated mean percent of baseline weight change of 24.1% vs. 26.3%; p=.19). However, by year 1 weight change was significantly less following SG compared with RYGB (29.4% vs. 34.4%; P<.01) and remained less through year 7 (23.6% vs. 30.4%; p=.001). Supporting data, including weight change in kg, are reported in Table 2.
Figure 1. Observed and Modeled Percent Weight Change Following Sleeve Gastrectomy and Roux-en-Y Gastric Bypass.
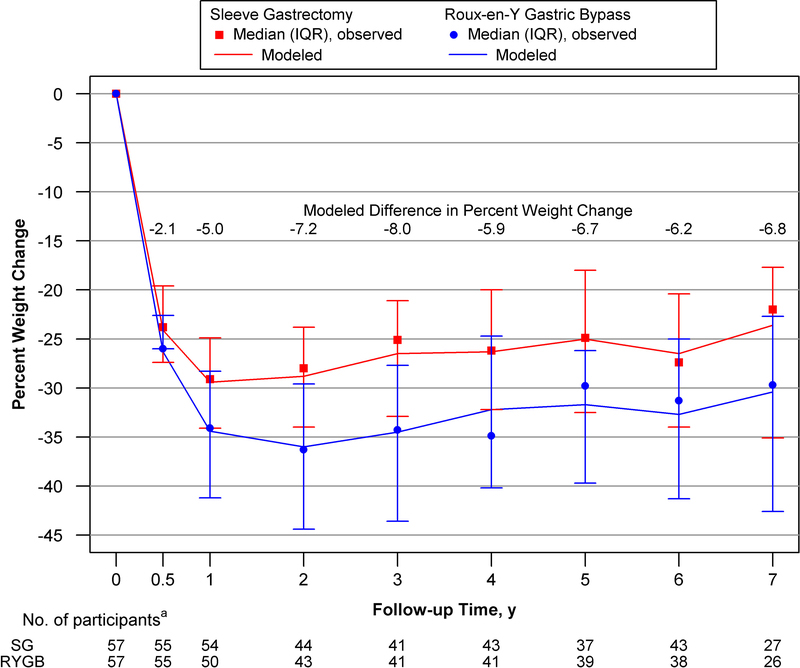
Abbreviations: IQR, interquartile range.
Lines indicate modeled weight change based on mixed models with the matched pair as random effects. Data markers, median values; bars, interquartile range=25th-75th percentile of observed data. Negative value indicates weight loss from baseline. The difference between procedures was calculated as SG minus RYGB.
aData was censored during pregnancies and following a participants’ second stage bariatric procedure or the second state procedure of a participant’s match. Data collection ended before the 7-year assessment of 18 SG and 12 RYGB participants.
Table 2.
Observed and modeled weight changea by time point in relation to initial bariatric procedure
| 6 month | Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | Year 6 | Year 7 | |
|---|---|---|---|---|---|---|---|---|
| Observed Median (25th, 75thpercentile) | ||||||||
| Percent Weight Change from Baseline | ||||||||
| SG | n=55 | n=54 | n=44 | n=41 | n=43 | n=37 | n=43 | n=27 |
| −23.8 | −29.1 | −28.0 | −25.1 | −26.2 | −24.9 | −27.4 | −22.0 | |
| (−27.4, −19.6) | (−34.1, −24.9) | (−34.0, −23.8) | (−32.9, −21.1) | (−32.2, −20.0) | (−32.5, −18.0) | (−34.0, −20.4) | (−35.1, −17.7) | |
| RYGB | n=55 | n=50 | n=43 | n=41 | n=41 | n=39 | n=38 | n=26 |
| −26.0 | −34.1 | −36.3 | −34.3 | −34.9 | −29.8 | −31.3 | −29.7 | |
| (−31.0, −22.6) | (−41.2, −28.3) | (−44.4, −29.6) | (−43.6, −27.7) | (−40.2, −24.7) | (−39.7, −26.2) | (−41.3, −25.0) | (−42.6, −22.7) | |
| Differenceb | 2.2 | 4.8 | 7.6 | 7.6 | 6.7 | 5.8 | 4.4 | 5.8 |
| (−3.4, 7.9) | (−1.7, 12.8) | (2.0, 15.1) | (0.9, 15.7) | (−2.6, 15.0) | (0.4, 14.2) | (−4.1, 17.1) | (−4.4, 20.6) | |
| Weight Change, kg | ||||||||
| SG | n=55 | n=54 | n=44 | n=41 | n=43 | n=37 | n=43 | n=27 |
| −35.0 | −41.4 | −38.0 | −34.5 | −31.8 | −34.1 | −34.1 | −32.3 | |
| (−43.2, −30.5) | (−54.1, −35.0) | (−50.1, −29.1) | (−46.4, −26.4) | (−50.9, −25.9) | (−54.1, −24.5) | (−48.6, −26.8) | (−48.2, −23.6) | |
| RYGB | n=55 | n=50 | n=43 | n=41 | n=41 | n=39 | n=38 | n=26 |
| −38.6 | −50.7 | −54.1 | −52.3 | −47.3 | −41.4 | −45.0 | −40.2 | |
| (−45.5, −30.9) | (−67.7, −38.2) | (−70.5, −37.7) | (−61.4, −36.8) | (−56.8, −32.7) | (−55.9, −33.6) | (−58.2, −36.4) | (−55.9, −31.4) | |
| 3.2 | 7.5 | 10.9 | 8.9 | 11.1 | 8.6 | 6.6 | 6.1 | |
| Differenceb | (−5.5, 11.1) | (−2.7, 21.8) | (1.8, 24.8) | (−1.4, 26.3) | (−1.8, 24.9) | (0.2, 28.9) | (−5.6, 25.1) | (−8.4, 30.5) |
| Model-based Estimates, Mean (95% Confidence Interval)c | ||||||||
| Percent Weight Change from Baseline | ||||||||
| SG | −24.1 | −29.4 | −28.8 | −26.5 | −26.3 | −25.0 | −26.5 | −23.6 |
| (−26.4, −21.9) | (−31.7, −27.2) | (−31.2, −26.4) | (−29.0, −24.0) | (−28.7, −23.9) | (−27.5, −22.4) | (−29.0, −24.1) | (−26.4, −20.7) | |
| RYGB | −26.3 | −34.4 | −36.0 | −34.5 | −32.2 | −31.7 | −32.7 | −30.4 |
| (−28.5, −24.0) | (−36.8, −32.1) | (−38.4, −33.5) | (−37.0, −32.0) | (−34.7, −29.7) | (−34.2, −29.2) | (−35.3, −30.2) | (−33.3, −27.5) | |
| Differenceb | 2.1 | 5.0 | 7.2 | 8.0 | 5.9 | 6.7 | 6.2 | 6.8 |
| (−1.1, 5.3) | (1.7, 8.2) | (3.7, 10.6) | (4.5, 11.5) | (2.4, 9.4) | (3.1, 10.3) | (2.6, 9.7) | (2.8, 10.9) | |
| P value for differenced | .19 | <.01 | <.001 | <.001 | <.01 | <.001 | <.001 | .001 |
| Weight Change, kg | ||||||||
| SG | −36.7 | −45.0 | −43.0 | −40.2 | −39.8 | −37.7 | −40.2 | −35.7 |
| (−40.7, −32.6) | (−49.1, −40.9) | (−47.4, −38.6) | (−44.7, −35.7) | (−44.2, −35.4) | (−42.4, −33.1) | (−44.6, −35.7) | (−40.8, −30.5) | |
| RYGB | −40.2 | −53.7 | −55.3 | −53.2 | −49.5 | −49.0 | −50.4 | −47.1 |
| (−44.2, −36.1) | (−57.9, −49.5) | (−59.7, −50.9) | (−57.7, −48.7) | (−54.0, −45.0) | (−53.6, −44.4) | (−55.0, −45.8) | (−52.4, −41.9) | |
| Differenceb | 3.7 | 8.9 | 12.5 | 13.2 | 9.9 | 11.5 | 10.5 | 11.7 |
| (−2.0, 9.5) | (3.1, 14.8) | (6.3, 18.7) | (6.9, 19.6) | (3.6, 16.2) | (5.0, 18.0) | (4.1, 16.8) | (4.3, 19.0) | |
| P value for differenced | .21 | <.01 | <.001 | <.001 | <.01 | <.001 | <.01 | <.01 |
Abbreviations: RYGB, Roux-en-Y gastric bypass, SG, sleeve gastrectomy.
Negative values indicate weight loss. Data were censored during pregnancies and following a subsequent bariatric procedure or the subsequent bariatric procedure of a match. Data collection ended before the 7 year assessment for 18 SG and 12 RYGB participants
The difference is the weight change in SG minus the weight change in RYGB. Thus, a positive number indicates less weight loss following SG vs. RYGB.
Estimates are based on a mixed model that control for baseline age, smoking and site, which were related to missing follow-up data.
p values for model: procedure: <.001, time: <.001, procedure x time: <.001. Because the procedure by time interaction was statistically significant, the equality of mean change at each time point was tested.
Among pairs of participants whose weight nadir could be determined and who gained weight prior to the last study assessment (n=31; Table 33), there was not a significant difference in weight regain from post-surgery weight nadir between surgical groups (i.e., SG-RYGB) whether weight regain was measured as the percentage of baseline weight (Beta=0.53 (95% CI, −2.6–3.6); p=.74) or the percentage of maximum weight lost (Beta=−1.37 (95% CI, −11.7–9.0); p=.79).
Second Stage procedures
Ten participants who underwent SG had a second bariatric procedure during follow-up; all were planned and occurred within 2 years of the initial SG. None of the matched RYGB participants underwent a reversal or second bariatric procedure during follow-up. Information on the participants who underwent a second stage procedure following SG, including their weight loss prior to the second stage procedure, are provided in Table 4.
Table 4.
Information on participants who had a second stage procedure following a sleeve gastrectomy
| Pre-SG | At time of new procedure | After new procedure | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Participant | Year of SG |
Weight, kg |
BMI, kg/m2 |
Years since SG |
% baseline weight change since SG |
New procedure |
Years since new procedure |
% weight change since new procedure |
% baseline weight change since SG |
| 1 | 2007 | 135.2 | 70.4 | 0.4 | −22.6 | BPDS | 6.4 | −18.8 | −37.0 |
| 2 | 2006 | 138.8 | 52.7 | 0.5 | −21.3 | BPDS | 6.5 | −32.0 | −46.4 |
| 3 | 2007 | 193.2 | 69.4 | 0.8 | −23.3 | RYGB | 5.0 | 17.4 | −9.7 |
| 4 | 2008 | 140.6 | 69.6 | 1.1 | −36.1 | BPDS | 5.8 | −14.8 | −45.5 |
| 5 | 2006 | 138.8 | 68.8 | 1.1 | −23.7 | BPDS | 6.1 | −36.6 | −51.5 |
| 6 | 2006 | 111.6 | 51.5 | 1.3 | −25.2 | BPDS | 5.6 | −29.7 | −47.3 |
| 7 | 2007 | 142.4 | 53.1 | 1.4 | −15.1 | RYGB | 5.8 | −32.8 | −42.8 |
| 8 | 2008 | 153.3 | 66.6 | 1.4 | −31.2 | BPDSa | 4.5 | −10.9 | −38.6 |
| 9 | 2008 | 121.6 | 57.7 | 1.6 | −29.4 | RYGB | 0.1 | −9.0 | −35.6 |
| 10 | 2007 | 122.0 | 59.7 | 1.9 | −25.0 | BPDS | 5.0 | −30.5 | −47.8 |
Abbreviations: BMI= Body mass index; BPDS= biliopancreatic diversion with duodenal switch, RYGB= Roux-en-Y Gastric Bypass, SG=sleeve gastrectomy.
BPDS was revised 2.1 years after it was performed.
Comorbid Conditions
Figure 2, panels A and B, shows the modeled prevalence of comorbidities by time point in SG and RYGB, respectively. For both SG and RYGB procedure groups prevalence of diabetes, low HDL and hypertension were significantly lower five years after surgery vs baseline; high LDL was also significantly lower for RYGB (Table 5), but not SG (44.5 (95% CI, 30.5–58.6) to 24.2 (95%CI, 7.6–40.7); p=0.10). However, statistical power was limited. Likewise, there was not a significant difference in prevalence of high triglycerides between baseline and year 5 for either procedure (23.3 (95% CI, 11.9–34.8) to 7.8 (95% CI, −2.8–18.4); p=0.16, for SG; 13.1 (95% CI, 3.9–22.4) to 3.5 (95% CI, −2.4–9.3); p=0.17, for RYGB). The observed comorbidity prevalence, remission and incidence by time point and by surgical procedure are presented in Table 5.
Figure 2. Modeled Prevalence of Comorbid Conditions by Time Point in Relation to Sleeve Gastrectomy and Roux-en-Y Gastric Bypass, Respectively.
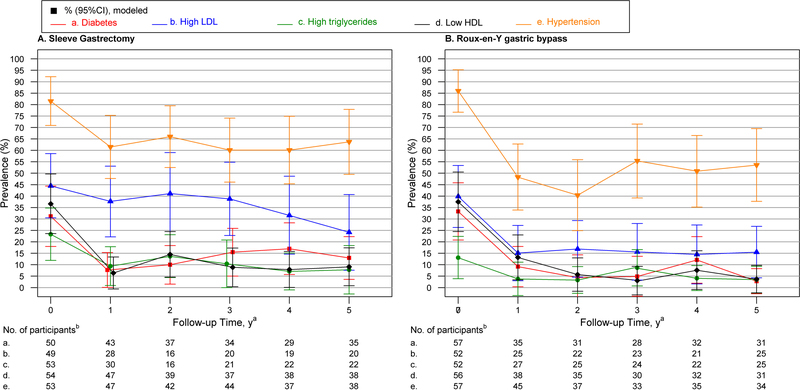
Abbreviations: HDL, high-density lipoprotein cholesterol, LDL=Low-density lipoprotein. Lines indicate modeled prevalence, bars, 95% CI, based on mixed models.
Table 5.
Observed Prevalence, Remission and Incidence of Comorbiditiesa and Modeled Prevalence Estimates by Time Point in Relation to Initial Bariatric Procedure, by Procedure.
| Observed Prevalence, Remission and Incidence of Comorbidities | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| %, No./Totala |
||||||||||||
| Baseline | Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | |||||||
| Sleeve Gastrectomy | ||||||||||||
| Diabetes | ||||||||||||
| Prevalence | 30.0 | 15/50 | 7.0 | 3/43 | 8.1 | 3/37 | 14.7 | 5/34 | 13.8 | 4/29 | 8.6 | 3/35 |
| Remission | NA | NA | 77.8 | 7/9 | 62.5 | 5/8 | 62.5 | 5/8 | 66.7 | 4/6 | 66.7 | 4/6 |
| Incidence | NA | NA | 0.0 | 0/30 | 0.0 | 0/27 | 4.4 | 1/23 | 9.1 | 2/22 | 3.7 | 1/27 |
| High LDL | ||||||||||||
| Prevalence | 44.9 | 22/49 | 35.7 | 10/28 | 37.5 | 6/16 | 45.0 | 9/20 | 31.6 | 6/19 | 25.0 | 5/20 |
| Remission | NA | NA | 22.2 | 2/9 | 33.3 | 2/6 | 30.0 | 3/10 | 33.3 | 3/9 | 44.4 | 4/9 |
| Incidence | NA | NA | 11.8 | 2/17 | 20.0 | 2/10 | 12.5 | 1/8 | 0.0 | 0/10 | 0.0 | 0/10 |
| High triglycerides | ||||||||||||
| Prevalence | 22.6 | 12/53 | 6.7 | 2/30 | 12.5 | 2/16 | 9.5 | 2/21 | 4.6 | 1/22 | 9.1 | 2/22 |
| Remission | NA | NA | 33.3 | 1/3 | 0.0 | 0/2 | 75.0 | 3/4 | 50.0 | 1/2 | 60.0 | 3/5 |
| Incidence | NA | NA | 0.0 | 0/25 | 0.0 | 0/14 | 0.0 | 0/16 | 0.0 | 0/20 | 0.0 | 0/17 |
| Low HDL | ||||||||||||
| Prevalence | 37.0 | 20/54 | 6.4 | 3/47 | 15.4 | 6/39 | 10.8 | 4/37 | 7.9 | 3/38 | 7.9 | 3/38 |
| Remission | NA | NA | 80.0 | 12/15 | 61.5 | 8/13 | 66.7 | 8/12 | 75.0 | 9/12 | 76.9 | 10/13 |
| Incidence | NA | NA | 0.0 | 0/30 | 3.9 | 1/26 | 0.0 | 0/24 | 0.0 | 0/25 | 0.0 | 0/25 |
| Hypertension | ||||||||||||
| Prevalence | 81.1 | 43/53 | 61.7 | 29/47 | 69.1 | 29/42 | 59.1 | 26/44 | 51.4 | 19/37 | 55.3 | 21/38 |
| Remission | NA | NA | 30.6 | 11/36 | 24.2 | 8/33 | 31.4 | 11/35 | 37.9 | 11/29 | 31.0 | 9/29 |
| Incidence | NA | NA | 14.3 | 1/7 | 42.9 | 3/7 | 0.0 | 0/6 | 16.7 | 1/6 | 14.3 | 1/7 |
| Roux-en-Y Gastric Bypass | ||||||||||||
| Diabetes | ||||||||||||
| Prevalence | 33.3 | 19/57 | 8.6 | 3/35 | 6.5 | 2/31 | 7.1 | 2/28 | 9.4 | 3/32 | 3.2 | 1/31 |
| Remission | NA | NA | 75.0 | 9/12 | 87.5 | 7/8 | 100.0 | 8/8 | 77.8 | 7/9 | 88.9 | 8/9 |
| Incidence | NA | NA | 0.0 | 0/23 | 4.4 | 1/23 | 10.0 | 2/20 | 4.4 | 1/23 | 0.0 | 0/22 |
| High LDL | ||||||||||||
| Prevalence | 40.4 | 21/52 | 16.0 | 4/25 | 13.6 | 3/22 | 13.0 | 3/23 | 14.3 | 3/21 | 16.0 | 4/25 |
| Remission | NA | NA | 69.2 | 9/13 | 66.7 | 6/9 | 80.0 | 8/10 | 75.0 | 6/8 | 63.6 | 7/11 |
| Incidence | NA | NA | 0.0 | 0/11 | 0.0 | 0/12 | 9.1 | 1/11 | 8.3 | 1/12 | 0.0 | 0/13 |
| High triglycerides | ||||||||||||
| Prevalence | 13.5 | 7/52 | 3.7 | 1/27 | 4.0 | 1/25 | 8.3 | 2/24 | 4.6 | 1/22 | 4.0 | 1/25 |
| Remission | NA | NA | 100.0 | 2/2 | 50.0 | 1/2 | 0.0 | 0/2 | 0.0 | 0/1 | 66.7 | 2/3 |
| Incidence | NA | NA | 4.2 | 1/24 | 0.0 | 0/22 | 0.0 | 0/20 | 0.0 | 0/20 | 0.0 | 0/21 |
| Low HDL | ||||||||||||
| Prevalence | 37.5 | 21/56 | 13.2 | 5/38 | 5.7 | 2/35 | 3.3 | 1/30 | 9.4 | 3/32 | 3.2 | 1/31 |
| Remission | NA | NA | 61.5 | 8/13 | 75.0 | 6/8 | 100.0 | 7/7 | 77.8 | 7/9 | 85.7 | 6/7 |
| Incidence | NA | NA | 0.0 | 0/25 | 0.0 | 0/27 | 4.4 | 1/23 | 4.4 | 1/23 | 0.0 | 0/24 |
| Hypertension | ||||||||||||
| Prevalence | 86.0 | 49/57 | 48.9 | 22/45 | 46.0 | 17/37 | 57.6 | 19/33 | 51.4 | 18/35 | 50.0 | 17/34 |
| Remission | NA | NA | 44.7 | 17/38 | 50.0 | 16/32 | 37.9 | 11/29 | 41.9 | 13/31 | 44.8 | 13/29 |
| Incidence | NA | NA | 14.3 | 1/7 | 20.0 | 1/5 | 25.0 | 1/4 | 0.0 | 0/4 | 20.0 | 1/5 |
| Model-Based Estimates, % (95% Confidence Interval) b | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P |
|||||||||||||
| Baseline | Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | Year 5 vs. BL |
|||||||
| Sleeve Gastrectomy | |||||||||||||
| Diabetes (n=54) | 31.2 | (18.0–44.4) | 7.7 | (0.1–15.3) | 10.0 | (1.5–18.4) | 15.5 | (5.0–25.9) | 17.0 | (5.7–28.3) | 13.0 | (3.6–22.3) | 0.02 |
| High LDL (n=53) | 44.5 | (30.5–58.6) | 37.7 | (22.2–53.1) | 41.1 | (23.1–59.0) | 38.8 | (22.8–54.8) | 31.6 | (14.6–48.7) | 24.2 | (7.6–40.7) | 0.10 |
| High trig. (n=55) | 23.3 | (11.9–34.8) | 9.4 | (0.9–17.9) | 13.7 | (4.4–23.1) | 10.4 | (0.0–20.8) | 7.1 | (−1.0–15.1) | 7.8 | (−2.8–18.4) | 0.16 |
| Low HDL (n=56) | 36.6 | (23.6–49.7) | 6.4 | (−0.6–13.4) | 14.5 | (4.6–24.5) | 8.9 | (0.4–17.3) | 7.9 | (0.1–15.8) | 9.1 | (0.8–17.4) | <0.01 |
| Hypertension (n=57) | 81.5 | (70.9–92.2) | 61.5 | (47.7–75.3) | 66.0 | (52.5–79.5) | 60.1 | (46.1–74.1) | 60.1 | (45.4–74.9) | 63.7 | (49.5–77.9) | 0.048 |
| Roux-en-Y Gastric Bypass | |||||||||||||
| Diabetes (n=57) | 33.3 | (20.8–45.8) | 9.2 | (0.4–18.0) | 4.4 | (−5.5–14.3) | 4.9 | (−3.8–13.7) | 12.1 | (1.9–22.3) | 2.8 | (−2.7–8.3) | 0.03 |
| High LDL (n=55) | 39.9 | (26.3–53.4) | 15.1 | (3.0–27.2) | 16.9 | (4.6–29.3) | 15.6 | (3.3–28.0) | 14.5 | (1.6–27.4) | 15.5 | (4.3–26.8) | 0.02 |
| High trig. (n=55) | 13.1 | (3.9–22.4) | 3.8 | (−3.5–11.1) | 3.3 | (−2.5–9.2) | 8.7 | (0.7–16.6) | 4.1 | (−1.3–9.6) | 3.5 | (−2.4–9.3) | 0.17 |
| Low HDL (n=56) | 37.5 | (24.5–50.5) | 13.2 | (3.4–23.0) | 5.7 | (−1.6–13.0) | 3.1 | (−3.2–9.3) | 7.6 | (−0.8–16.1) | 3.7 | (−2.3–9.7) | 0.01 |
| Hypertension (n=57) | 86.0 | (76.7–95.2) | 48.3 | (33.9–62.8) | 40.4 | (24.9–55.9) | 55.4 | (39.2–71.5) | 50.9 | (35.2–66.5) | 53.6 | (37.7–69.5) | <0.01 |
Abbreviations: HDL, high-density lipoprotein; LDL, low-density lipoprotein; NA, not applicable.
There are 57 SG and 57 RYGB participants. Missing data vary for each comorbid condition due to varying data requirements.
Estimates are based on mixed models that controlled for baseline age, smoking and site, which were significantly associated with missing follow-up data.
Mortality and Death Rates After Bariatric Surgery
There were 2 deaths within 7 years of SG and 3 deaths within 7 years of RYGB. The SG deaths occurred 8 days and 4.9 years after SG. The RYGB deaths occurred 0.8, 5.2 and 5.7 years after RYGB.
DISCUSSION
This longitudinal study employs standardized data collection and compares weight change through 7 years and comorbidity prevalence through 5 years in a group of super-obese or high-risk patients who underwent laparoscopic SG with a matched group of laparoscopic RYGB. By year 1, weight change was significantly less following SG compared with RYGB and remained less through year 7. There was not a significant difference in weight regained from post-surgery weight nadir by surgical procedure group. For both SG and RYGB, the prevalence of diabetes, low HDL and hypertension, were significantly lower five years after surgery vs baseline (e.g., diabetes prevalence decreased from 31% to 13% among SG vs. 33% to 3% among RYGB).
There are a limited number of studies with up to 5-year outcomes that have focused on the outcomes of higher risk and/or super obese patients who underwent SG, many with the plan of second stage definitive procedure to follow. These studies were either retrospective (16), without comparison group (16), unmatched (17–19) or had lacked long-term follow up (17–19). Even so, the reported weight change, weight change comparison and obesity-related comorbidity improvement outcomes are similar, for the most part, to this LABS-2 report. For example, Eid et al. studied 74 super obese patients, whose mean preoperative BMI was 66±7 kg/m2, who underwent SG but did not proceed to the second weight loss procedure (16). This retrospective study, which evaluated status 6 to 8 years (mean: 73 months) post-surgery, reported EWL of 48%, roughly equivalent to the 7 year weight loss of 24% of baseline weight in our sample (20). Additionally, 70% of the patients with diabetes showed improvement or remission of the disease across follow up. While this study had long-term data with 93% data completeness, there was no comparison group, 43% of the outcomes were self-reported, and improvement and remission of diabetes were not reported separately.
Three studies have included RYGB comparison groups. Zerwick et al. compared 32 RYGB and 45 SG patients’ short-term outcomes (mean preoperative BMIs of 53.9 kg/m2 and 52.7 kg/m2 SG and RYGB, respectively (18). They demonstrated greater weight loss in the RYGB group at 6, 9 and 12 months. Thereaux et al. in a single site study identified 74 SG (mean BMI 57.2 kg/m2) and 285 RYGB (mean BMI 56.7 kg/m2) patients and compared weight change and DM improvement at one year; RYGB demonstrated better weight loss and resolution of DM (17). Most recently, Hong et al. in a retrospective study identified 106 SG with 501 RYGB patients with a preoperative BMI greater than 50 kg/m2 with follow up to 3 years (19). There was not a statistically significant difference between procedures in the weight loss or rate of type 2 DM remission at any point in the 3-year follow up period. This study, however, had a very high attrition rate with only 6% of SG group and 11% of RYGB group remaining to complete the 3 years follow up time.
Mehaffey et al. described outcomes of the 2009 patients undergoing laparoscopic RYGB over 20 years; 328 of them were super super obese (SSO), who had BMI > 60 kg/m2 (21). There was no significant difference in postoperative outcomes or complications compared to non-SSO population. Weight loss was similar to our matched comparator RYGB cohort. However, their follow up in Mehaffey’s SSO cohort decreased to 15% at 4 years.
Recently, two randomized clinical trials have compared SG as a stand-a-lone procedure versus RYGB. Salminen et al. conducted the Sleeve vs Bypass (SLEEVEPASS) multicenter, randomized clinical equivalence trial in Finland (22). The trial enrolled 240 morbidly obese patients with mean BMI of 45.9, who were randomly assigned to SG or RYGB with a 5-year follow-up period. The difference in weight loss between the two groups was not statistically significant. A similar study from Switzerland (The SM-BOSS Randomized Clinical Trial) also found no significant difference in weight loss in the short-term, but weight loss in RYGB participants surpassed SG 5 year follow up (23). These studies, however, were not restricted to super-obese or high-risk patients. Additionally, they did not show significant statistical difference between SG and RYGB for DM remission (22, 23).
Li et al. performed a meta-analysis of 62 studies that included 10,498 RYGB and 7951 SG patients (preoperative BMI not reported) with follow up between 0.5 and 5 years (24). They did not find a significant difference in DM improvement, but concluded that RYGB resulted in greater weight loss and better resolution of hypertension, dyslipidemia, GERD, and arthritis. In another meta-analysis, Shoar & Saber focused on outcomes of 5264 laparoscopic SG and laparoscopic RYGB patients with 36 to 75.8 months of follow-up (25). Despite the insignificant difference between RYGB and SG in mid-term term (3–5 years) weight loss, RYGB produced better weight loss in the long-term (greater than 5 years). There was no significant difference between the two procedures for co-morbidity resolution (Type 2 diabetes, hypertension, hyperlipidemia and obstructive sleep apnea).
There are few comparative data on weight regain between bariatric procedures. De Hollanda et al. compared mid-term (3–5 years) weight loss trajectories between SG and RYGB and their data suggested that weight regain was more common following SG (11).
The findings reported here confirm and extend the literature by showing that procedure-specific weight loss diverged 6 months post-surgery, such that the difference between procedures increased until approximately 2 years and then remained relatively consistent through 7 years. Furthermore, with the higher risk or super obese cohort in LABS reported here, there was not a significant difference in weight regain from nadir by procedure whether measured in reference to baseline weight or in reference to post-surgery weight nadir. Rather, these data suggest it is the weight loss following surgery, as opposed to the weight regain following nadir, that accounts for the difference in long-term weight loss between procedures.
The sample size, inability to match participants on baseline comorbidity status and discordance in available data throughout follow-up precluded a paired analysis for change in comorbidity prevalence following surgery. However, long-term change in comorbidity status was examined within surgical procedures which had similar sex, race, age and BMI distributions. This study shows that SG and RYGB were both effective in decreasing prevalence of DM through five years of follow-up. The same was true for low HDL and hypertension, although more than half of participants were hypertensive at year 5 (i.e., 64%, down from 82% at baseline, among SG, vs. 54%, down from 86% at baseline, among RYGB).
Overall, the literature indicates that high quality studies, including randomized trials with bigger samples are needed to compare both procedures in high-risk super-obese patients regarding weight change, obesity-related comorbidity improvement, remission and occurrence and weight regain outcomes.
This study, while rigorously designed and executed, does have some limitations. Because SG was an uncommon procedure at the time of LABS-2 recruitment, the sample size is small, and most cases were done at one site. In addition, because this was an observational (i.e., non-randomized) study, even after matching on key characteristics there may be differences between treatment groups related to change in weight or comorbidities. Data completeness over 7 years was excellent for the SG group (80% or higher) for weight across six to seven years of follow-up . However, it was lower for the matched RYGB group (57%−89%), for unknown reasons. Data completeness for comorbidities was also lower, reflecting the multiple data requirements (i.e., fasting blood draw or non-fasting blood draw or blood pressure measurement plus prescription medication assessment). Despite these limitations, this study improves upon previous studies of SG with standardized data collection, a matched RYGB comparator group and longer-term follow-up.
CONCLUSION:
Higher risk or super obese participants following SG lost less weight than matched RYGB counterparts from 1 to 7 years following surgery. Both groups exhibited improvements in comorbidities through 5 years.
Table 3.
Observed percentage of maximum weight lost that was regaineda by years since weight nadir.
| Years since weight nadir |
|||||
|---|---|---|---|---|---|
| 1 year | 2 years | 3 years | 4 years | 5 years | |
| Observed Median (25th, 75thpercentile) | |||||
| % maximum weight loss regained | |||||
| SG (n=39) | n=38 | n=31 | n=31 | n=27 | n=23 |
| 8.8 (4.0–15.9) | 16.7 (6.8–25.0) | 24.1 (14.8–35.4) | 21.1 (11.6–41.3) | 30.2 (16.5–41.6) | |
| RYGB (n=38) | n=38 | n=31 | n=32 | n=27 | n=20 |
| 10.5 (5.4–16.3) | 17.5 (8.9–26.4) | 18.4 (10.1–37.0) | 22.2 (12.4–46.1) | 31.8 (19.3–46.1) | |
| Difference (n=31)b | n=28 | n=20 | n=23 | n=14 | n=10 |
| −1.6 (−7.1–4.2) | −0.4 (−13.4,8.1) | 4.4 (−7.5–13.2) | −0.7 (−9.0–10.9) | −8.7 (−25.6–18.7) | |
Abbreviations: RYGB, Roux-en-Y gastric bypass, SG, sleeve gastrectomy.
Data were censored during pregnancies and following a subsequent bariatric procedure or the subsequent bariatric procedure of a match.
The difference between pairs is calculated as the weight regain in SG minus the weight regain in their RYGB matched pair. Of the 57 pairs in the sample, this difference could be calculated among 31 pairs at one or more post-nadir assessments. Sixteen pairs were ineligible because at least one of the pairs did not regain weight prior to death (2 SG, 1 RYGB) having another procedure (10 SG) or the final LABS-2 assessment (2 SG, 1 RYGB). Ten pairs were excluded due to insufficient weight measurements (2 SG, 6 RYGB, and 2 both SG and RYGB).
ACKNOWLEDGEMENTS OF:
Dr. Ahmed reports grants from Allurion Technologies Inc. outside the submitted work. Dr. Courcoulas reports grants from Covidien/Ethicon J&J, and Allurion Technologies Inc. outside the submitted work. Dr. Gourash reports grants from Covidien/Ethicon outside of the submitted work.
FUNDING:
This clinical study was a cooperative agreement funded by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). Grant numbers: DCC -U01 DK066557; Columbia - U01-DK66667 (in collaboration with Cornell University Medical Center CTRC, Grant UL1-RR024996); University of Washington - U01-DK66568 (in collaboration with CTRC, Grant M01RR-00037); Neuropsychiatric Research Institute - U01-DK66471; East Carolina University – U01-DK66526; University of Pittsburgh Medical Center – U01-DK66585 (in collaboration with CTRC, Grant UL1-RR024153); Oregon Health & Science University – U01-DK66555.
Acknowledgement of the LABS personnel contributing to the study include:
Columbia University Medical Center, New York, NY: Paul D. Berk, MD, Marc Bessler, MD, Amna Daud, Harrison Lobdell IV, Jemela Mwelu, Beth Schrope, MD, PhD, Akuezunkpa Ude, MD Cornell University Medical Center, New York, NY: Jamie Honohan BA, Michelle Capasso, BA, Ricardo Costa, BS, Greg Dakin, MD, Faith Ebel RD, MPH, Michel Gagner, MD, Jane Hsieh BS, Alfons Pomp, MD, Gladys Strain, PhD East Carolina Medical Center, Greenville, NC: Rita Bowden, RN, William Chapman, MD, FACS, Blair Cundiff, BS, Mallory Ball, BS, Emily Cunningham, BA, Lynis Dohm, PhD, John Pender MD, Walter Pories, MD, FACS Neuropsychiatric Research Institute, Fargo, ND: Jennifer Barker, MBA, Michael Howell, MD, Luis Garcia, MD, FACS, MBA, Kathy Lancaster, BA, Erika Lovaas, BS, James E. Mitchell, MD, Tim Monson, MD, Oregon Health & Science University: Chelsea Cassady, BS, Emily Coburn, MPH, Emily Moher, MPH, Clifford Deveney, MD, Katherine Elder, PhD, Stefanie Greene, Jonathan Purnell, MD, Robert O’Rourke, MD, Chad Sorenson, Bruce M. Wolfe, MD, Legacy Good Samaritan Hospital, Portland, OR: Emma Patterson, MD, William Raum, MD, Lisa VanDerWerff, PAC, Jason Kwiatkowski, PAC, University of Pittsburgh Medical Center, Pittsburgh, PA: Anita P. Courcoulas, MD, MPH, FACS, William Gourash, RN, CRNP, PhD, Ramesh Ramanathan, MD, Melissa Kalarchian PhD, Marsha Marcus PhD, Eleanor Shirley, MA, BS, University of Washington, Seattle, WA: David R. Flum, MD, MPH, E. Patchen Dellinger, MD, Saurabh Khandelwal, MD, Skye D. Stewart, MS, CCRC, Morgan M. Cooley, Rebecca Blissell, Megan J. Miller, MEd Virginia Mason Medical Center, Seattle, WA: Richard Thirlby, MD Lily Chang, MD, Jeffrey Hunter, MD, Ravi Moonka, MD, Debbie Ng, MPH, MA Data Coordinating Center, Graduate School of Public Health at the University of Pittsburgh, Pittsburgh, PA: Steven H. Belle, PhD, MScHyg, Wendy C. King, PhD, Debbie Martin, BA, Rocco Mercurio, MBA, Abdus Wahed, PhD, Frani Averbach, MPH, RDN National Institute of Diabetes and Digestive and Kidney Diseases: Mary Horlick, MD, Carolyn W. Miles, PhD, Myrlene A. Staten, MD, Susan Z. Yanovski, MD National Cancer Institute: David E. Kleiner, MD, PhD
Footnotes
CONFLICT OF INTEREST
Dr. Pomp receives speaker honoraria form WL Gore & associates, Ethicon and Medtronics., Drs. King, Belle and Dakin and Ms. Hinerman have nothing to disclose.
Contributor Information
Bestoun Ahmed, Department of Surgery, Division of Minimally Invasive Bariatric and General Surgery, University of Pittsburgh Medical Center.
Wendy C. King, Graduate School of Public Health, Epidemiology, University of Pittsburgh.
William Gourash, Department of Surgery, Division of Minimally Invasive Bariatric and General Surgery, University of Pittsburgh Medical Center.
Steven H. Belle, Graduate School of Public Health, Epidemiology & Biostatistics, University of Pittsburgh.
Amanda Hinerman, Graduate School of Public Health, Epidemiology, University of Pittsburgh.
Alfons Pomp, Department of Surgery, Division of GI, Metabolic, & Bariatric Surgery, Weill Cornell Medicine.
Greg Dakin, Department of Surgery, Division of GI, Metabolic, & Bariatric Surgery, Weill Cornell Medicine.
Anita P Courcoulas, Department of Surgery, Division of Minimally Invasive Bariatric and General Surgery, University of Pittsburgh Medical Center.
REFERENCES:
- 1.Mechanick JI, Youdim A, Jones DB, Garvey WT, Hurley DL, McMahon MM, et al. Clinical practice guidelines for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient−−2013 update: cosponsored by American Association of Clinical Endocrinologists, The Obesity Society, and American Society for Metabolic & Bariatric Surgery. Obesity. 2013;21 Suppl 1:S1–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brethauer SA, Kothari S, Sudan R, Williams B, English WJ, Bregman M, et al. Systematic review on reoperative bariatric surgery American Society for Metabolic and Bariatric Surgery Revision Task Force. SOARD. 2014. [DOI] [PubMed] [Google Scholar]
- 3.Clinical Issues Committee of the American Society for M, Bariatric S. Updated position statement on sleeve gastrectomy as a bariatric procedure. Surgery for obesity and related diseases : official journal of the American Society for Bariatric Surgery. 2010;6(1):1–5. [DOI] [PubMed] [Google Scholar]
- 4.Cottam D, Qureshi FG, Mattar SG, Sharma S, Holover S, Bonanomi G, et al. Laparoscopic sleeve gastrectomy as an initial weight-loss procedure for high-risk patients with morbid obesity. Surg Endosc. 2006;20(6):859–63. [DOI] [PubMed] [Google Scholar]
- 5.Committee ACI. Updated position statement on sleeve gastrectomy as a bariatric procedure. Surgery for obesity and related diseases : official journal of the American Society for Bariatric Surgery. 2012;8(3):e21–6. [DOI] [PubMed] [Google Scholar]
- 6.Clinical Issues Committee of American Society for M, Bariatric S. Sleeve gastrectomy as a bariatric procedure. Surgery for obesity and related diseases : official journal of the American Society for Bariatric Surgery. 2007;3(6):573–6. [DOI] [PubMed] [Google Scholar]
- 7.English WJ, DeMaria EJ, Brethauer SA, Mattar SG, Rosenthal RJ, Morton JM. American Society for Metabolic and Bariatric Surgery estimation of metabolic and bariatric procedures performed in the United States in 2016. Surgery for obesity and related diseases : official journal of the American Society for Bariatric Surgery. 2017. [DOI] [PubMed] [Google Scholar]
- 8.Hutter MM, Schirmer BD, Jones DB, Ko CY, Cohen ME, Merkow RP, et al. First report from the American College of Surgeons Bariatric Surgery Center Network: laparoscopic sleeve gastrectomy has morbidity and effectiveness positioned between the band and the bypass. Annals of surgery. 2011;254(3):410–20; discussion 20–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boza C, Salinas J, Perez G, Raddatz A, Runke R, Prmentel F, et al. Laparoscopic sleeve gastrectomy as a stand-alone procedure for morbid obesity: report of 1,000 cases and 3-year follow-up. Obesity surgery. 2012;22(6):866–71. [DOI] [PubMed] [Google Scholar]
- 10.Courcoulas AP, King WC, Belle SH, Berk P, Flum DR, Garcia L, et al. Seven-Year Weight Trajectories and Health Outcomes in the Longitudinal Assessment of Bariatric Surgery (LABS) Study. JAMA surgery. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Hollanda A, Ruiz T, Jimenez A, Flores L, Lacy A, Vidal J. Patterns of Weight Loss Response Following Gastric Bypass and Sleeve Gastrectomy. Obesity surgery. 2015;25(7):1177–83. [DOI] [PubMed] [Google Scholar]
- 12.Belle SH, Berk PD, Chapman WH, Christian NJ, Courcoulas AP, Dakin GF, et al. Baseline characteristics of participants in the Longitudinal Assessment of Bariatric Surgery-2 (LABS-2) study. Surgery for obesity and related diseases : official journal of the American Society for Bariatric Surgery. 2013;9(6):926–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention. National Death Index. Centers for Disease Control and Prevention Website.2017. [
- 14.King WC, Chen JY, Belle SH, Courcoulas AP, Dakin GF, Elder KA, et al. Change in Pain and Physical Function Following Bariatric Surgery for Severe Obesity. Jama. 2016;315(13):1362–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zou GA. A Modified Poisson Regression Approach to Prospective Studies with Binary Data. American journal of epidemiology. 2004;159(7):5. [DOI] [PubMed] [Google Scholar]
- 16.Eid GM, Brethauer S, Mattar SG, Titchner RL, Gourash W, Schauer PR. Laparoscopic sleeve gastrectomy for super obese patients: forty-eight percent excess weight loss after 6 to 8 years with 93% follow-up. Annals of surgery. 2012;256(2):262–5. [DOI] [PubMed] [Google Scholar]
- 17.Thereaux J, Corigliano N, Poitou C, Oppert JM, Czernichow S, Bouillot JL. Comparison of results after one year between sleeve gastrectomy and gastric bypass in patients with BMI >/= 50 kg/m(2). Surgery for obesity and related diseases : official journal of the American Society for Bariatric Surgery. 2015;11(4):785–90. [DOI] [PubMed] [Google Scholar]
- 18.Zerrweck C, Sepulveda EM, Maydon HG, Campos F, Spaventa AG, Pratti V, et al. Laparoscopic gastric bypass vs. sleeve gastrectomy in the super obese patient: early outcomes of an observational study. Obesity surgery. 2014;24(5):712–7. [DOI] [PubMed] [Google Scholar]
- 19.Hong J, Park S, Menzo EL, Rosenthal R. Midterm outcomes of laproscopic sleeve gastrectomy as a stand-alone prodedure in super-obese patients. SOARD. 2017:7. [DOI] [PubMed] [Google Scholar]
- 20.Belle SH, Berk PD, Courcoulas AP, Engel S, Flum DR, Gourash W, et al. Reporting weight change: standardized reporting accounting for baseline weight. Surgery for obesity and related diseases : official journal of the American Society for Bariatric Surgery. 2013;9(5):782–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mahaffey JH, LaPar DJ, Turrentine FE, Miller MS, Hallowell PT, Schirmer BD. Ourcomes of laparoscopic Roux-en-Y gastric bypass in super-super obese patients. SOARD. 2015;11:814–20. [DOI] [PubMed] [Google Scholar]
- 22.Salminen P, Helmio M, Ovaska J, Juuti A, Leivonen M, Peromaa-Haavisto P, et al. Effect of Laparoscopic Sleeve Gastrectomy vs Laparoscopic Roux-en-Y Gastric Bypass on Weight Loss at 5 Years Among Patients With Morbid Obesity: The SLEEVEPASS Randomized Clinical Trial. Jama. 2018;319(3):241–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peterli R, Wolnerhanssen BK, Peters T, Vetter D, Kroll D, Borbely Y, et al. Effect of Laparoscopic Sleeve Gastrectomy vs Laparoscopic Roux-en-Y Gastric Bypass on Weight Loss in Patients With Morbid Obesity: The SM-BOSS Randomized Clinical Trial. Jama. 2018;319(3):255–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li J, Lai D, Wu D. Laparoscopic Roux-en-Y Gastric Bypass Versus Laparoscopic Sleeve Gastrectomy to Treat Morbid Obesity-Related Comorbidities: a Systematic Review and Meta-analysis. Obesity surgery. 2016;26(2):429–42. [DOI] [PubMed] [Google Scholar]
- 25.Shoar S, Saber AA. Long-term and midterm outcomes of laparoscopic sleeve gastrectomy versus Roux-en-Y gastric bypass: a systematic review and meta-analysis of comparative studies. Surgery for obesity and related diseases : official journal of the American Society for Bariatric Surgery. 2017;13(2):170–80. [DOI] [PubMed] [Google Scholar]


