Abstract
Background
In women with large and ptotic breasts who require a mastectomy and immediate, implant-based reconstruction, long flaps pose a high risk for flap ischemia and necrosis. A new trans-vertical incision for skin-reducing mastectomy is described, which reduces the skin envelope and lifts the breast.
Objectives
The authors sought to describe the new mastectomy access incision and assess its efficacy and safety when followed by immediate implant-based reconstruction.
Methods
This retrospective analysis included 70 consecutive patients (101 breasts) with large and ptotic breasts who underwent a unilateral (n = 39; 55.7%) or bilateral (n = 31; 44.3%), skin-reducing mastectomy utilizing the trans-vertical approach for either breast cancer or risk reduction. All received immediate one- (n = 86; 85.5%) or two-stage (n = 15; 14.5%), implant-based reconstruction utilizing acellular dermal matrix.
Results
Mean age was 50.1 years and mean body mass index was 25.6 kg/m2. After a median follow-up of 4.9 years, the number of breasts with minor and major complications was 21 (20.8%) and 26 (25.7%), respectively. The most common major complications were skin-flap necrosis (n = 12; 11.9%) and infection (n = 8; 7.9%). All occurred within 3 months postsurgically. There were 7 cases of capsular contracture (6.9%) and 5 reconstruction failures (5.0%). Higher body mass index (P < 0.01) and breast weight (P < 0.05) were associated with increased complication rates. According to BREAST-Q, 55/64 patients (85.9%) were somewhat or very satisfied with the aesthetic outcome.
Conclusions
The trans-vertical approach is an effective, reproducible, and safe alternative to conventional skin-reducing mastectomy, with favorable aesthetic outcomes, in patients with large and ptotic breasts.
Level of Evidence: 4

Breast reconstruction can moderate the negative aesthetic and quality-of-life effects of mastectomy.1 In the United States, more than two-thirds of breast reconstructions in women who underwent therapeutic or risk-reducing mastectomy are immediate procedures;2 around 80% of all of breast reconstructions are implant based, and more than half include an acellular dermal matrix (ADM).2
Whenever possible, a nipple- or skin-sparing mastectomy is preferred that utilizes the patient’s entire breast skin envelope to cover the reconstructed scaffold made of implant / tissue expander, an ADM, and sometimes the pectoralis major muscle. In large and ptotic breasts, preservation of the entire skin envelope results in long skin flaps, which may develop ischemia and flap necrosis.3 Furthermore, the skin envelope of the original large and ptotic breast may not conform to the shape of the reconstructed breast.
A 2-stage procedure has been described that includes a preliminary reduction mammaplasty followed after several months by a nipple-sparing mastectomy.4 This approach may not be applicable for breast cancer patients, in whom delaying the definitive mastectomy or adjuvant therapies for 2 to 3 months is not feasible. Alternatively, an autologous vascularized flap can be transferred to reconstruct the breast while tailoring the skin envelope to the desired size.5 Obvious drawbacks of these approaches are the need for at least 2 operations in the former and donor site morbidity in the latter.
The trans-vertical approach is well suited for these cases, because it incorporates excision of the skin in the centro-lateral portion of the breast, including the nipple-areola complex (NAC). Thus, it leaves a skin envelope that can be easily adjusted in 2 dimensions to the shape of the reconstructed scaffold (implant, muscle, and ADM), forming a natural, smaller, and uplifted breast mound. In this report, we describe our experience with this procedure.
METHODS
Study Design
This was a retrospective cohort study of consecutive patients who underwent a trans-vertical mastectomy and immediate implant- and ADM-based breast reconstruction between January 2009 and June 2017. Data were collected from the registry of a single hospital (Assuta Medical Center), 5 breast surgeons, and one plastic surgeon. The study protocol was approved by the Assuta-Maccabie Institutional Review Board (IRB). All patients provided written informed consent.
Patients
All patients were females aged ≥18 years, with large and ptotic breasts, who underwent mastectomy with immediate reconstruction either for breast cancer or risk reduction due to a high-risk mutation and/or strong family history of breast cancer. We defined large breasts as those that would benefit functionally from a breast reduction and ptotic breasts as those that would benefit aesthetically from a breast lift. All patients required both.
The procedure was performed in 1 stage (direct to implant) or 2 stages (tissue expander, later changed to a permanent implant), depending on risk factors and clinical assessment of perfusion of the mastectomy skin flaps. Self-reported smokers were instructed to avoid smoking for at least 2 weeks before surgery (and for 2 months afterwards) and were required to have a negative urine nicotine test 3 days prior to surgery. Patients who did not cease smoking were advised to undergo delayed reconstruction.
Morbidly obese individuals (>100 lb over ideal weight, body mass index [BMI] ≥ 40 kg/m2, or BMI ≥ 35 kg/m2 with obesity-related health conditions) underwent a 2-stage (tissue expander) reconstruction.
Surgical Techniques
All patients were marked preoperatively for trans-vertical mastectomy, in the standing position, with 2 spindle-shaped ellipses that encompassed the NAC: a horizontal ellipse around the NAC, extending transversely or obliquely to the lateral breast pole (not reaching the lateral breast fold), and a vertical ellipse overlapping around the NAC and extending downward along the mid-axis of the breast on the lower pole (not reaching the inframammary fold [IMF]). The width of each ellipse was determined by tailor tucking the skin while the patient was in a standing position (Video). The horizontal skin ellipse was excised full thickness with the breast, and the vertical skin ellipse was either completely excised or de-epithelialized (Stefano Pompei, personal communication, October 2012) to preserve the blood supply coming from the subdermal plexus, mechanically reinforcing the lower pole.
Video 1.
Watch now at https://academic.oup.com/asj/article-lookup/doi/10.1093/asj/sjy181
Patients received a dose of intravenous antibiotics 20 minutes prior to surgical incision. For those who did not receive neoadjuvant chemotherapy, antimicrobial treatment consisted only of 1 preoperative dose; others received intravenous antibiotics over the first 24 hours following surgery.
Sentinel lymph-node biopsy or axillary lymph-node dissection were conducted according to the oncological status of each patient, either through a separate axillary incision or through the mastectomy incision.
At least 10 minutes before mastectomy incision, breasts were infiltrated subcutaneously with a blunt cannula with 60 to 180 mL of tumescent solution (500 mL lactated Ringer’s solution, 0.5 mg adrenaline, and 40 mL 1% lidocaine) per breast.
Skin incisions were full thickness around the NAC and transverse ellipse and either full or partial thickness along the vertical ellipse.
Atraumatic mastectomy and reconstructive techniques were employed in all cases. Dissection with blunt-tip scissors was used to separate the skin and subcutaneous tissues from the breast, and electrocautery was employed to separate the breast from the chest wall muscles. Injury to the skin flaps and subdermal plexus was prevented by avoiding use of sharp or crushing instruments. No retractors, clamps, or hooks were used on the skin envelope during mastectomy or reconstruction. Skin-flap retraction was carried out using fingers only (preferably those of the surgeon), thus minimizing the possibly of crush injury to the subdermal plexus.
Fenestrated or 2:1 meshed ADM (SurgiMend, Integra LifeSciences, Plainsboro, NJ) was used to bridge the defect between the cut edge of the pectoral muscle and the IMF, to cover and support the lower and lateral aspects of the implant, and to offload the weight of the implant when upright. The serratus muscle was not elevated.
In most patients, the decision whether to employ a 1-stage (direct to implant) or 2-stage (tissue expander) approach was made preoperatively based on risk factors. However, patients initially assigned to a 1-stage reconstruction underwent a 2-stage procedure if intraoperative clinical evaluation of the flaps suggested suboptimal perfusion.
Various steps were taken to reduce the likelihood of bacterial contamination. ADMs were rehydrated in normal saline solution and then soaked in 500 mL of normal saline containing 1 g cefazolin, 80 mg gentamicin, and 500 mL betadine solution (“triple antibiotic solution”) for at least 10 minutes. Implant pockets were irrigated with normal saline solution and then triple antibiotic solution. In addition, the skin surrounding the incision was painted with betadine solution, and gloves were changed prior to handling the ADM and the implant. Vicryl PLUS sutures (Ethicon Inc., Somerville, NJ) were used to suture the ADM to the muscle and IMF and to close the subcutaneous layers.
To reduce postoperative pain, an interpectoral block was performed using 10 mL per breast of bupivacaine hydrochloride with adrenaline, either by the surgeon following the mastectomy or by the anesthesiologists under ultrasound guidance prior to the procedure.
Two 10-mm Jackson-Pratt drains were inserted through a 5-cm subcutaneous tunnel, one in the lateral breast gutter and the other in the IMF (in the subcutaneous space). The first drain was removed prior to discharge of the patient from hospital, typically by day 3, and the second when drainage decreased to <30 mL in 24 hours.
Tension-free skin closure was performed to redrape the mastectomy flaps over the scaffold of the reconstructed breast.
Assessments
The following data were retrieved from a prospectively maintained database and the medical records of all patients: baseline characteristics and demographic variables (age, patient weight, BMI, and breast weight); medical history and risk factors (comorbidities, obesity, smoking, prior radiation therapy, neoadjuvant chemotherapy, and prior breast surgery); variables relating to the procedure (reason for surgery [breast cancer vs risk reduction], breasts operated [unilateral or bilateral], quality of skin flaps [based on clinical evaluation only], size and type of implant, volume fill for tissue expanders, contralateral procedure, and fat grafting); and postoperative course and complications (time to drain removal, postoperative bleeding, skin-flap necrosis, infection, seroma, capsular contracture, explantation, and need for reoperation for any reason). Capsular contracture was graded according to the Baker classification.
Minor complications included hematomas or seromas that resolved spontaneously or were drained in the office and cases of localized surgical-site infection or skin-flap necrosis that resolved spontaneously or were treated with oral antibiotics or debrided in the office. Major complications included hematomas or seromas that were drained in the operating room and cases involving skin necrosis or infection that led to implant exchange or explantation.
Breast volume was estimated preoperatively using Vectra 3D photography (Canfield Scientific, Parsippany, NJ) and volumetric plastic bowls (Plastikmott, Gothenburg, Sweden). All breast specimens were weighed in the operating room.
Patients assessed aesthetic outcome at least 6 months following surgery by means of a translated and validated BREAST-Q questionnaire6 sent to patients by fax or e-mail and returned anonymously. Patients were given 16 questions on aesthetic outcome and asked to rate their satisfaction with each aspect on a scale of 1 to 4 representing “very dissatisfied,” “somewhat dissatisfied,” “somewhat satisfied,” and “very satisfied”. Individual scores were summed (range, 16-64), and patients who scored ≤32 were classified as “somewhat dissatisfied” or “very dissatisfied” overall, and those who scored >32 were considered to be “somewhat satisfied” or “very satisfied” overall.
Data Analysis
For all continuous variables, mean, standard deviation (SD), and range were calculated. For all categorical variables, absolute frequencies and percentages were calculated. Then t tests were performed to compare risk factors in those who experienced complication vs those who did not and in those who had skin-flap necrosis vs those who did not. Chi-square for independence was performed to test associations between risk factors, capsular contracture, and complications.
Statistical analyses were conducted using the SPSS statistical software (Version 20; IBM Inc., Chicago, IL). Statistical significance was defined as Alpha (α) = 0.05 (1-sided).
RESULTS
Baseline Characteristics
A total 70 consecutive patients (101 breasts) with large and ptotic breasts who underwent trans-vertical mastectomy and immediate breast reconstruction were included in this analysis. The mean patient age was 50.1 years (SD, 10.9; range, 27.0-78.0 years) and mean BMI was 25.6 kg/m2 (SD, 4.2; range, 17.9-34.9 kg/m2) (Table 1). The mean follow-up was 4.9 years (SD, 2.1; range, 0.55-8.5 years).
Table 1.
Baseline Demographics and Concurrent Treatments
| Variable | Patients (N = 70) / breasts (N = 101) |
|---|---|
| Age,a mean (SD; range) | 50.1 (10.9; 27.0-78.0) |
| BMIa (kg/m2), mean (SD; range) | 25.6 (4.2; 17.9-34.9) |
| Obesity,a n (%) | 10 (14.3) |
| Smoking,a n (%) | 10 (14.3) |
| Breast weight (g), mean (SD; range) | 675.5 (277.8; 117.0-1750.0) |
| Chemotherapy,a N = 54, n (%) | |
| None received | 22 (40.7) |
| Neoadjuvant | 17 (31.5) |
| Adjuvant | 14 (25.9) |
| Neoadjuvant and adjuvant | 1 (1.9) |
| Radiotherapy, N = 85, n (%) | |
| None received | 52 (61.2) |
| Prior | 10 (11.8) |
| Postoperative | 23 (27.1) |
aPatients (N = 70). Obesity was defined as BMI > 30 kg/m2. BMI, body mass index; SD, standard deviation.
A total of 39 patients (55.7%) underwent unilateral and 31 (44.3%) bilateral mastectomy followed by immediate implant- and ADM-based breast reconstruction (Table 2). Eighty-six breasts (85.1%) underwent a 1-stage (direct-to-implant) approach, and 15 (14.9%) had a 2-stage (tissue expander) reconstruction. Mean breast weight was 675.5 g (SD, 277.8; range, 117.0-1750.0 g).
Table 2.
Reconstructive Procedure
| Variable | Patients (N = 70) / breasts (N = 101) |
|---|---|
| Mastectomy / reconstruction,a n (%) | |
| Unilateral | 39 (55.7) |
| Bilateral | 31 (44.3) |
| Procedure, n (%) | |
| One stage (direct to implant) | 86 (85.1) |
| Two stages (TE) | 15 (14.9) |
| Implant size (g), mean (SD) | 478.8 (99.6) |
| Total TE expansion (g), mean (SD) | 557.0 (154.1) |
| Implant surface, N = 85, n (%) | |
| Macrotextured | 76 (89.4) |
| Microtextured | 6 (7.1) |
| Nanotextured | 3 (3.5) |
| Contralateral procedure, n (%) | |
| Mastectomy and reconstruction | 62 (61.4) |
| Breast reduction | 5 (5.0) |
| Mastopexy | 3 (3.0) |
| Mastopexy augmentation | 3 (3.0) |
| None | 28 (27.6) |
| Fat injection, n (%) | 26 (25.7) |
| Fat injection (mL), mean (SD) | 157.8 (94.6) |
| Fat injection (number of injections), mean (SD) | 1.2 (0.5) |
| Follow up (years),a mean (SD; range) | 4.9 (2.1; 0.55-8.5) |
a Patients (N = 70). SD, standard deviation; TE, tissue expander.
Among 54 patients for whom data are available, 17 (31.5%) received neoadjuvant chemotherapy, 14 (25.9%) received adjuvant chemotherapy, and 1 patient (1.9%) had both (Table 1). Among 85 operated breasts for which data are available, 10 (11.8%) had prior radiation therapy and 23 (27.1%) had postoperative radiation.
Two drains were used in all cases; the last drain was removed after a mean of 12.3 days (SD, 6.2; range, 3-25 days).
Aesthetic Outcomes
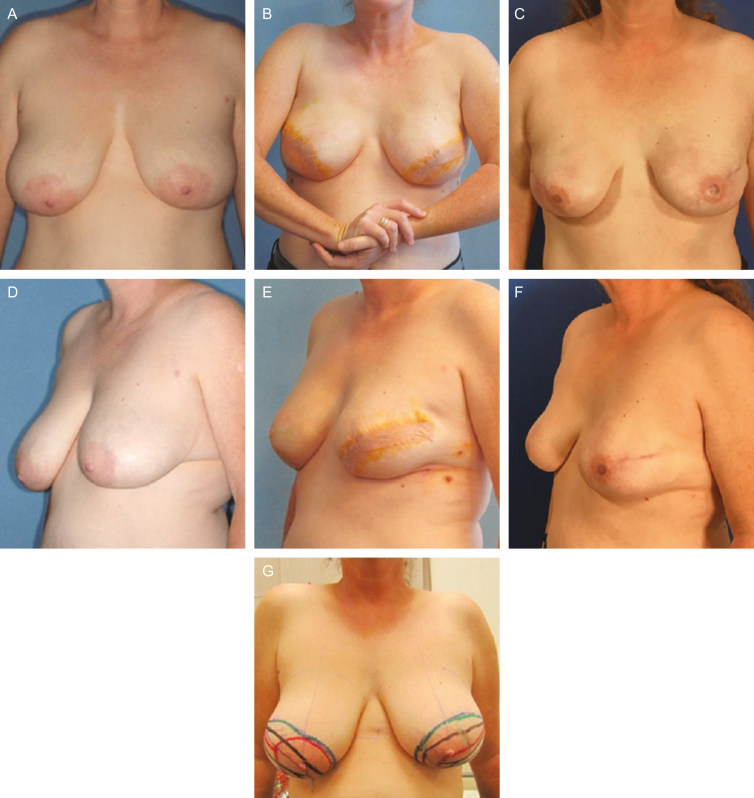
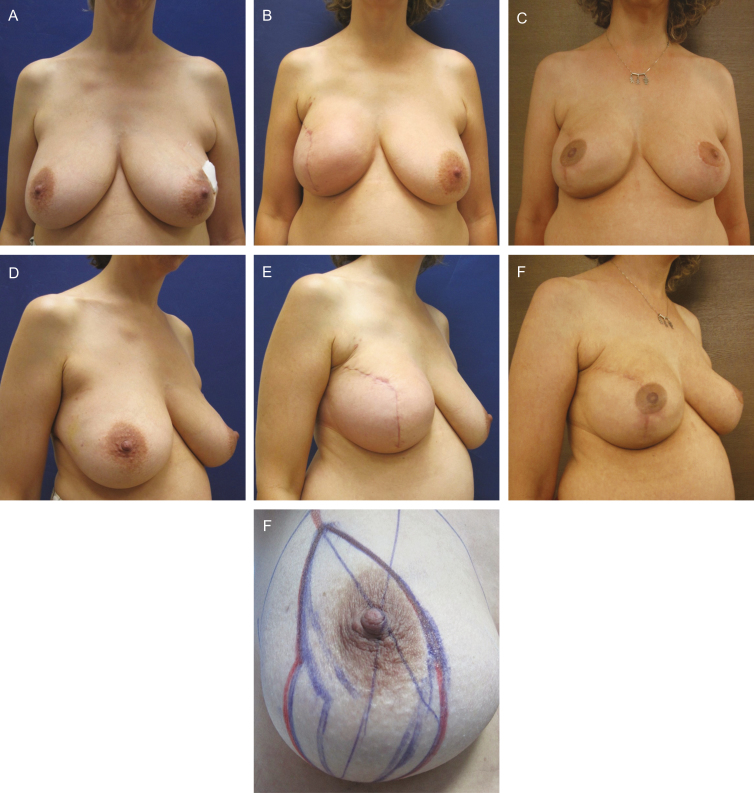
Example before-and-after photographs are provided in Figures 1 and 2.
Figure 1.
This 47-year-old woman underwent bilateral, trans-vertical, risk-reducing mastectomy and immediate 1-stage implant- and acellular dermal matrix-based reconstruction. Before surgery (A, C) and with preoperative markings (G); at 3 weeks postoperatively (B, E, Natrelle FX 495 g), with minimal animation due to maintained attachment of pectoralis major muscles low on the sternum (B); and at 4 years postoperatively (D, F).
Figure 2.
Unilateral (right), trans-vertical, skin-reducing mastectomy and immediate 1-stage implant- and acellular dermal matrix-based reconstruction in this 45-year-old woman. Before surgery (A, C) and with preoperative markings (G); at 6 weeks postoperatively (B, E, right side Natrelle FX 615 g); and at 18 months post-right-side radiation therapy and 12 months post-left-side contralateral breast reduction (D, F).
In a BREAST-Q analysis (64/70 responders), 55 patients (85.9%) said that they were somewhat or very satisfied with the aesthetic outcome and 9 patients (14.1%) said that they were somewhat or very dissatisfied.
Patient dissatisfaction was related to radiation treatment (P = 0.006). Patient satisfaction was found to be unrelated to complications, unilateral or bilateral reconstruction, or whether fat grafting was conducted or not.
Complications
Minor complications resolving spontaneously or with local treatment occurred in 21 of 101 breasts (20.8%), and major complications occurred in 26 breasts (25.7%) (Table 3). The most frequent major complications were skin-flap necrosis (n = 12; 11.9%) and infection (n = 8; 7.9%). All occurred within 3 months post-surgery. Explantation was required in 5 cases (5.0%).
Table 3.
Complications
| Variable | Breasts (N = 101) |
|---|---|
| Total, n (%) | |
| Minor | 21 (20.8) |
| Major | 26 (25.7) |
| Hematoma, n (%) | |
| Minor | 0 (0) |
| Major | 5 (5.0) |
| Seroma, n (%) | |
| Minor | 11 (10.9) |
| Major | 1 (1.0) |
| Infection, n (%) | |
| Minor | 5 (5.0) |
| Major | 8 (7.9) |
| Skin-flap necrosis, n (%) | |
| Minor | 5 (5.0) |
| Major | 12 (11.9) |
| Capsular contracture (grade 3-4), n (%) | 7 (6.9) |
| Explantation, n (%) | 5 (5.0) |
There were 7 cases of grade 3-4 capsular contracture (6.9%), of which 6 had either been irradiated (n = 4) or experienced a complication (n = 2 nonirradiated patients with skin-flap necrosis). All capsule contractures appeared within the first year of follow-up.
No cases of red breast syndrome were observed.
Associations Between Risk Factors and Complications
Potential links between risk factors and the likelihood of experiencing a complication were assessed in this group of patients with large and ptotic breasts. The overall risk of complications (both minor and major) increased in patients with greater breast weight (mean breast weight of patients experiencing and not experiencing a complication: 736.8 vs 622.6 g; P < 0.05) (Table 4). Higher breast weight was also associated with an increased rate of skin-flap necrosis (mean breast weight of patients experiencing and not experiencing skin-flap necrosis: 821.7 vs 643.6 g; P = 0.008). The overall risk of complications was also associated with increasing BMI: mean BMIs were 26.6 and 24.6 kg/m2, respectively, in patients experiencing and not experiencing a complication (P < 0.01). Large implant size, radiation therapy, and smoking were not associated with significantly increased rates of complications in this series. However, irradiated patients tended towards a higher rate of reconstructive failure (explantation) (P = 0.064). No difference in minor or major complications was found between 1- and 2-stage procedures.
Table 4.
Associations Between Risk Factors, Capsular Contracture, and Complications
| Variable | No complication (N = 54) | Complication (N = 47) | P |
|---|---|---|---|
| Mean breast weight | 622.6 (264.0) | 736.8 (283.7) | <0.05 |
| Mean BMI (kg/m2) | 24.6 (3.6) | 26.6 (4.4) | <0.01 |
| Radiotherapya | 15 (33) | 18 (46) | 0.202 |
| Smoking | 9 (17) | 6 (13) | 0.529 |
| Capsular contracture | 4 (7) | 3 (6) | 1.000 |
| Variable | No capsular contracture (N = 94) | Capsular contracture (N = 7) | P |
| Radiotherapya | 29 (37) | 4 (67) | 0.201 |
Data are mean (SD) or n (%). aPre- or postoperative or both. BMI, body mass index; SD, standard deviation.
DISCUSSION
Women with large and ptotic breasts present a particular challenge for surgeons performing mastectomy with immediate reconstruction.3,7 Large and ptotic breasts, often with stretched and thin skin envelopes, more commonly experience ischemia, skin-flap necrosis, and other complications following mastectomy.3,8,9 Patients with large or ptotic breasts who undergo immediate, implant-based reconstruction are more likely to require revision surgery than small-breasted women.10
In patients with large breasts, nipple-sparing mastectomy may not be possible due to inadequate perfusion. Indeed, a recent analysis of 809 nipple-sparing mastectomies found that increasing mastectomy weight was a significant predictor of complications (including necrosis) and explantation.11 Close proximity of the tumor to the NAC was also an indication for removing the NAC in some patients in the present study. Regarding the surgical technique used, the Wise pattern mastectomy incision, with or without nipple-sparing and immediate reconstruction, has largely been abandoned due to the unpredictable blood supply of the skin flaps on both sides of the vertical scar and a high incidence of skin necrosis. The use of a vertical incision is possible,12-14 but this pattern is not suitable for large breasts that require skin excess reduction both in the vertical and horizontal planes. A 2-stage procedure, based on breast reduction first followed by delayed nipple-sparing mastectomy, has been described by Spear and colleagues.4 However, this approach requires 2 to 3 procedures to complete and may not be a practical option for many patients and surgeons. Techniques based on mastopexy at the time of nipple-sparing mastectomy have also been described15,16 but may be associated with ischemia of the NAC and skin envelope. Another option is to immediately fill the space with vascularized autologous tissue,5 an approach suitable for some patients and some surgeons, but at the price of a significantly longer procedure and recovery as well as donor-site morbidity.
Here, we have described outcomes from a retrospective analysis of 70 women (101 breasts) with large and ptotic breasts who underwent skin-reducing mastectomy employing the trans-vertical approach, with immediate breast reconstruction. This method reduces the overall size and length of the skin flaps and allows reconstruction of a smaller and uplifted breast, based on a technique that has not been previously described. The use of ADM allows for larger, more controlled implant pockets and improved implant positioning, and may also offload the weight of the implant from the lower pole skin envelope.17,18
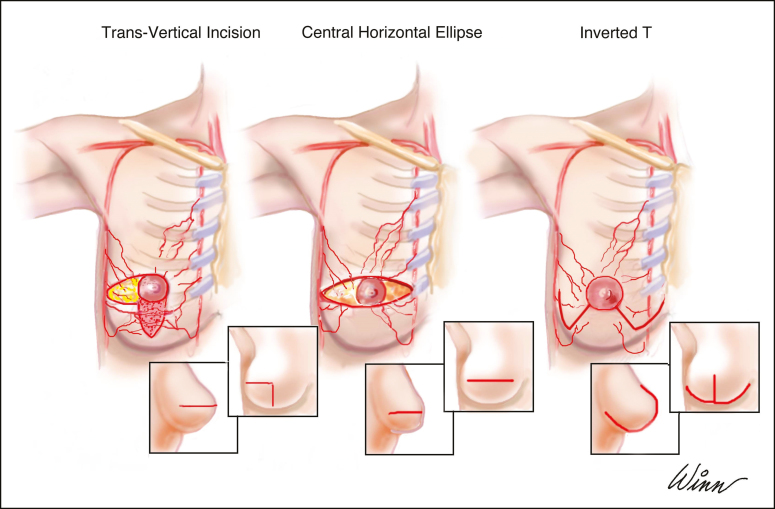
The most commonly used mastectomy incision/access with large and ptotic breasts is a large horizontal ellipse centered on the NAC (Figure 3). This amputates the tip of the cone of the breast, thereby producing a flat and boxy breast with the medial end of the scar often violating the cleavage area. The trans-vertical approach yields a more conical and uplifted shape. It also facilitates exposure and breast removal while leaving regular and nontraumatized skin flaps, because the need for skin retraction is limited. The transverse scar that results could be a limitation, but it is typically obscured by the bra and may therefore be an acceptable trade-off given that the procedure is performed to treat cancer or for risk reduction, postoperative breast shape is improved due to the lift given by the vertical component, and the complication rate may be lower than with the Wise pattern.
Figure 3.
Some of the different incisions that can be used for mastectomy in women with large and ptotic breasts.
In the present series, rates of minor and major complications were each around 20% to 25%. This is higher than the complication rates typically observed in “normal”-breasted women who underwent implant- and ADM-based reconstructions,19-25 including those performed by the same surgeon.9 This is expected given the additional complexity and greater risk of complications during breast reconstruction in women with large and ptotic breasts, and hence the reported complication rate is acceptable and comparable to other reported series. Furthermore, the explantation (reconstruction failure) rate was only 5.0%, which is similar or lower than that observed in previous series with non-high-risk patients.19-25
Breast weight and BMI were associated with increased complication rates in accordance with previous studies of implant-based reconstruction.9,26-30 However, although breast weight was linked with complications in this group of patients, implant size was not. This may have been because the implants used were typically smaller than the original breast and were placed in a smaller skin envelope, utilizing an ADM to help offload the weight of the implant from the lower pole skin.
Smoking and radiation therapy were not associated with increased complication rates, which is at variance with previous data.9,26-28 This is most likely due to 2 limitations of this study: its relatively small sample and the fact that active smokers were excluded or underwent a 2-stage procedure. Other limitations are that this is a retrospective, nonrandomized, single-center study involving 1 plastic surgeon and 5 general surgeons. Hence a large, prospective, multicenter trial may be needed to confirm these results. A larger series might also reveal additional associations between patient characteristics, risk factors, and postoperative complications. One further limitation was not preserving the NAC in cases in which there was no oncological indication for removing it due to high risk of flap necrosis. Further studies are needed to prove the safety of that approach in patients with large and ptotic breasts given that the aesthetic advantages of NAC preservation are well established.
CONCLUSIONS
Overall, in mastectomy / reconstruction candidates with large and ptotic breasts, the trans-vertical approach appears to be an effective, reproducible, and safe option for skin-reducing mastectomy. Aesthetic outcomes appear to be favorable compared to the standard horizontal ellipse.
Supplementary Material
Acknowledgments
The authors would like to thank Dr Timothy Ryder from Biological Communications Limited for medical writing and editorial assistance in developing this manuscript.
Disclosures
Dr Scheflan is a consultant, investigator, and speaker for Integra LifeSciences (Plainsboro, NJ). Drs Lotan and Allweis declared no potential conflicts of interest with respect to the research, authorship, and publication of this article.
Funding
Integra LifeSciences provided a grant to support data collection and statistical analysis.
REFERENCES
- 1. Serletti JM, Fosnot J, Nelson JA, Disa JJ, Bucky LP. Breast reconstruction after breast cancer. Plast Reconstr Surg. 2011;127(6):124e-135e. [DOI] [PubMed] [Google Scholar]
- 2. American Society of Plastic Surgeons. Plastic surgery statistics report 2016. www.plasticsurgery.org/documents/News/Statistics/2016/plastic-surgery-statistics-full-report-2016.pdf. Accessed April 27, 2018. [Google Scholar]
- 3. Spear SL, Carter ME, Schwarz K. Prophylactic mastectomy: indications, options, and reconstructive alternatives. Plast Reconstr Surg. 2005;115(3):891-909. [DOI] [PubMed] [Google Scholar]
- 4. Spear SL, Rottman SJ, Seiboth LA, Hannan CM. Breast reconstruction using a staged nipple-sparing mastectomy following mastopexy or reduction. Plast Reconstr Surg. 2012;129(3):572-581. [DOI] [PubMed] [Google Scholar]
- 5. DellaCroce FJ, Blum CA, Sullivan SK, et al. Nipple-sparing mastectomy and ptosis: perforator flap breast reconstruction allows full secondary mastopexy with complete nipple areolar repositioning. Plast Reconstr Surg. 2015;136(1):1e-9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pusic AL, Klassen AF, Scott AM, Klok JA, Cordeiro PG, Cano SJ. Development of a new patient-reported outcome measure for breast surgery: the BREAST-Q. Plast Reconstr Surg. 2009;124(2):345-353. [DOI] [PubMed] [Google Scholar]
- 7. Nava MB, Cortinovis U, Ottolenghi J, et al. Skin-reducing mastectomy. Plast Reconstr Surg. 2006;118:603-610. [DOI] [PubMed] [Google Scholar]
- 8. Ang Z, Gaunt E, Agrawal A, et al. Short-term outcomes of immediate breast reconstruction in a university teaching hospital. Breast Cancer Res Treat. 2018;167:356. [Google Scholar]
- 9. Scheflan M, Grinberg-Rashi H, Hod K. Bovine acellular dermal matrix in immediate breast reconstruction: a retrospective, observational study with SurgiMend. Plast Reconstr Surg. 2018;141(1):1e-10e. [DOI] [PubMed] [Google Scholar]
- 10. Roostaeian J, Pavone L, Da Lio A, Lipa J, Festekjian J, Crisera C. Immediate placement of implants in breast reconstruction: patient selection and outcomes. Plast Reconstr Surg. 2011;127(4):1407-1416. [DOI] [PubMed] [Google Scholar]
- 11. Frey JD, Salibian AA, Karp NS, Choi M. The impact of mastectomy weight on reconstructive trends and outcomes in nipple-sparing mastectomy: progressively greater complications with larger breast size. Plast Reconstr Surg. 2018;141(6):795e-804e. [DOI] [PubMed] [Google Scholar]
- 12. Chapman-Jackson ED, Griner D, Brzezienski MA. Circumvertical mastectomy incision: refinement in the surgical scar of implant-based breast reconstruction. Ann Plast Surg. 2014;72:S97-S102. [DOI] [PubMed] [Google Scholar]
- 13. Bourne DA, Ahuja N, Gimbel ML. Analysis of the vertical mammaplasty design in skin-sparing mastectomy and immediate autologous reconstruction. J Plast Reconstr Aesthet Surg. 2016;69(1):23-29. [DOI] [PubMed] [Google Scholar]
- 14. Dayicioglu D, Tugertimur B, Zemina K, et al. Vertical mastectomy incision in implant breast reconstruction after skin sparing mastectomy: advantages and outcomes. Ann Plast Surg. 2016;76(Suppl 4):S290-S294. [DOI] [PubMed] [Google Scholar]
- 15. Rivolin A, Kubatzki F, Marocco F, et al. Nipple-areola complex sparing mastectomy with periareolar pexy for breast cancer patients with moderately ptotic breasts. J Plast Reconstr Aesthet Surg. 2012;65(3):296-303. [DOI] [PubMed] [Google Scholar]
- 16. Al-Mufarrej FM, Woods JE, Jacobson SR. Simultaneous mastopexy in patients undergoing prophylactic nipple-sparing mastectomies and immediate reconstruction. J Plast Reconstr Aesthet Surg. 2013;66(6):747-755. [DOI] [PubMed] [Google Scholar]
- 17. Scheflan M, Brown I. Immediate implant-based breast reconstruction using variable lower pole support. In: Urban C, Rietjens M, eds. Oncoplastic and Reconstructive Breast Surgery. 1st ed. Milan: Springer-Verlag Mailand; 2013:235-252. [Google Scholar]
- 18. Scheflan M, Colwell AS. Tissue reinforcement in implant-based breast reconstruction. Plast Reconstr Surg Glob Open. 2014;2(8):e192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Colwell AS, Damjanovic B, Zahedi B, et al. Retrospective review of 331 consecutive immediate single-stage implant reconstructions with acellular dermal matrix: Indications, complications, trends, and costs. Plast Reconstr Surg. 2011;128:1170-1178. [DOI] [PubMed] [Google Scholar]
- 20. Salzberg CA, Ashikari AY, Koch RM, et al. An 8-year experience of direct-to-implant immediate breast reconstruction using human acellular dermal matrix (AlloDerm). Plast Reconstr Surg. 2011;127:514-524. [DOI] [PubMed] [Google Scholar]
- 21. Butterfield JL. 440 Consecutive immediate, implant-based, single-surgeon breast reconstructions in 281 patients: a comparison of early outcomes and costs between SurgiMend fetal bovine and AlloDerm human cadaveric acellular dermal matrices. Plast Reconstr Surg. 2013;131(5):940-951. [DOI] [PubMed] [Google Scholar]
- 22. Palaia DA, Arthur KS, Cahan AC, Rosenberg MH. Incidence of seromas and infections using fenestrated versus nonfenestrated acellular dermal matrix in breast reconstructions. Plast Reconstr Surg Glob Open. 2015;3(11):e569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Headon H, Kasem A, Manson A, Choy C, Carmichael AR, Mokbel K. Clinical outcome and patient satisfaction with the use of bovine-derived acellular dermal matrix (SurgiMend™) in implant based immediate reconstruction following skin sparing mastectomy: a prospective observational study in a single centre. Surg Oncol. 2016;25(2):104-110. [DOI] [PubMed] [Google Scholar]
- 24. Zhao X, Wu X, Dong J, Liu Y, Zheng L, Zhang L. A meta-analysis of postoperative complications of tissue expander/implant breast reconstruction using acellular dermal matrix. Aesthetic Plast Surg. 2015;39(6):892-901. [DOI] [PubMed] [Google Scholar]
- 25. Lee KT, Mun GH. Updated evidence of acellular dermal matrix use for implant-based breast reconstruction: a meta-analysis. Ann Surg Oncol. 2016;23(2):600-610. [DOI] [PubMed] [Google Scholar]
- 26. McCarthy CM, Mehrara BJ, Riedel E, et al. Predicting complications following expander/implant breast reconstruction: an outcomes analysis based on preoperative clinical risk. Plast Reconstr Surg. 2008;121(6):1886-1892. [DOI] [PubMed] [Google Scholar]
- 27. Davila AA, Seth AK, Wang E, et al. Human acellular dermis versus submuscular tissue expander breast reconstruction: a multivariate analysis of short-term complications. Arch Plast Surg. 2013;40(1):19-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pannucci CJ, Antony AK, Wilkins EG. The impact of acellular dermal matrix on tissue expander/implant loss in breast reconstruction: an analysis of the tracking outcomes and operations in plastic surgery database. Plast Reconstr Surg. 2013;132(1):1-10. [DOI] [PubMed] [Google Scholar]
- 29. Lardi AM, Ho-Asjoe M, Mohanna PN, Farhadi J. Immediate breast reconstruction with acellular dermal matrix: factors affecting outcome. J Plast Reconstr Aesthet Surg. 2014;67(8):1098-1105. [DOI] [PubMed] [Google Scholar]
- 30. Woo KJ, Paik JM, Mun GH, Pyon JK, Bang SI. Risk factors for complications in immediate expander-implant breast reconstruction for non-obese patients: impact of breast size on complications. Aesthetic Plast Surg. 2016;40(1):71-78. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.