Abstract
Moderate to severe atopic dermatitis (AD) has a high disease burden and a significant effect on quality of life. Observational studies are necessary to determine the patient disease burden and long‐term disease control in the Japanese population. ADDRESS‐J is a non‐interventional, observational registry of adult Japanese patients with moderate to severe AD. Herein, we report baseline data from the ADDRESS‐J study describing disease characteristics and current treatment practices. At baseline, 300 adult AD patients with Investigator's Global Assessment (IGA) scores (range, 0–4) of 3 (moderate) or 4 (severe) whose treatments for AD were intensified, were assessed for clinical and patient‐reported outcomes and current AD treatments. The registry patients’ median age was 34.0 years; 60.7% were male and 71.7% had had AD for more than 20 years. At baseline, 220 study patients had an IGA score of 3 and 80 had an IGA score of 4. The median Eczema Area and Severity Index score was 21.7 (range, 0–72), the median body surface area involvement was 46.25%, and the median pruritus numerical rating scale score was 7.0 (range, 0–10); for each of these measures, higher scores represent greater severity. Most registry patients (86.7%) had recently used topical corticosteroids or topical calcineurin inhibitors as treatment for AD. This registry cohort represents a population of Japanese patients with moderate to severe AD and provides an important resource for characterizing the disease burden and evaluating the safety and effectiveness of various AD treatments.
Keywords: adult, atopic dermatitis, burden of disease, Japan, registry
Introduction
Atopic dermatitis (AD) is a chronic inflammatory skin disease characterized by fluctuating symptoms, including pruritus and eczematous lesions, and occurs most commonly in patients with atopic diathesis.1, 2, 3 Allergic/atopic (type 2) comorbidities are prevalent in patients with AD; these include asthma, allergic rhinitis, allergic conjunctivitis and food allergy.3 AD is one of the most common dermatological conditions in Japan,4, 5 with a prevalence of 6.5% in adults and 11.2% in elementary school children.6, 7, 8
Patients with AD often experience a high disease burden, with symptoms frequently affecting sleep, quality of life and psychosocial factors.9, 10 The severity of disease symptoms, particularly pruritus, correlates with poor quality of life and depression, and results in a substantially negative impact on work productivity and day‐to‐day activities.11, 12 Recent studies have highlighted the significant disease burden of AD in Japanese patients.12, 13
According to the guidelines for AD management by the Japanese Dermatological Association, the current standard pharmacotherapies for AD include topical corticosteroids (TCS) and tacrolimus ointments to control inflammation, and oral antihistamines and anti‐allergic drugs as adjunctive treatments for pruritus.2 The oral immunosuppressant cyclosporin is recommended for patients over 16 years of age with resistance to other therapies.3 Adjunctive therapies include ultraviolet (UV) light therapy and herbal medicines.2
While TCS are a common treatment for AD, many patients have AD that is not adequately controlled with TCS. In a prospective study of patients with moderate to severe AD in Japan, AD symptoms were not controlled by TCS in 7% of infants, 10% of children and 19% of adolescent and adult patients.14 Moreover, a recent study showed that adherence levels for oral and topical AD treatments are low in Japan.15 Indeed, many patients with AD have fears of using TCS due to concerns of skin thinning and risks of systemic absorption.16 While oral corticosteroids are also used as rescue therapy, these agents do not provide complete control of the disease, and they are not advised for long‐term use due to safety concerns.17
To understand the status of current AD treatments, patient disease burden and long‐term outcomes of available AD therapies in Japan, large‐scale prospective multicenter studies are needed. ADDRESS‐J is a non‐interventional, observational registry of adult Japanese patients with moderate to severe AD. This paper reports the baseline demographics and disease characteristics of the patients enrolled in ADDRESS‐J, and describes current treatment practices for moderate to severe AD in Japan.
Methods
Registry study design
ADDRESS‐J is an ongoing, prospective, non‐interventional, observational, longitudinal study to evaluate the real‐world effectiveness and safety of existing AD treatments in the long‐term management (2 years) of adult patients with moderate to severe AD in Japan, with a particular interest in frequency of flare occurrence. The study period is from July 2016 to July 2019, including a 12‐month enrollment period. Patients were enrolled at their baseline visit. Post‐baseline assessments are planned for approximately every 3 months over a 24‐month observation period (Fig. 1). Patients are receiving treatment per routine clinical practice and undergo pre‐specified clinical observation and assessments at the sites at visit intervals of 3 months.
Figure 1.
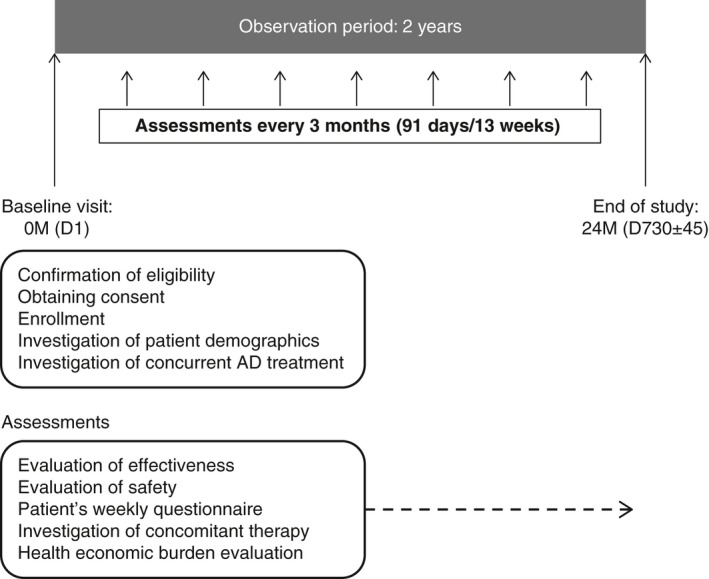
ADDRESS‐J registry study design. AD, atopic dermatitis; D, day; M, month.
Study sites
Study sites were categorized as hospitals or clinics. Hospital is defined as a medical office with 20 or more beds for admission, whereas a clinic is defined as one with 19 or fewer beds for admission. In this study, there are 10 hospitals and 20 clinics; all the clinics do not have beds for admission.
Enrollment criteria
The inclusion criteria for study were: Japanese patients aged 20 to less than 60 years who had a diagnosis of AD at least 6 months before the baseline visit and an Investigator's Global Assessment (IGA) score of at least three at the baseline visit. Patients also must have had any of the following treatment changes at the baseline visit: introduction of new AD medications such as topical/oral corticosteroids or topical/oral immunosuppressants (UV irradiation or antihistamine/anti‐allergic drugs excluded); change to a higher‐potency class of TCS or an increase in the dose of an AD medication (topical/oral corticosteroids or topical/oral immunosuppressants) currently being used; implementation of structured patient education regarding standard care for AD medication (brief instructions on treatment that are usually provided on a routine outpatient basis were not considered patient education); and willing and able to comply with specified study‐related procedures (including understanding and answering various questionnaires) for a 2‐year observation period. Patients were required to provide signed informed consent. Exclusion criteria included treatment with an investigational drug within 8 weeks before the baseline visit and presence of skin comorbidities that may interfere with study assessments.
Data collection and assessments
Clinical observation and assessments were implemented at baseline (day 1) and every 3 months starting from baseline. The assessments conducted at baseline are presented herein. Enrollment data were collected at the baseline visit, and included confirmation of eligibility, written informed consent and current treatment for AD.
In addition to enrollment information, the following data were collected at baseline: demographics including sex, age, height, weight, education and employment classification; disease characteristics including AD duration and number of clinic or hospital visits for AD treatments in the past year; and medical history of other allergic diseases including history of parents and siblings.
The following clinical and patient‐reported measures of AD signs and symptoms were also recorded at baseline: IGA, Eczema Area and Severity Index (EASI), percent body surface area (BSA) affected (%), Dermatology Life Quality Index (DLQI), EuroQOL group health questionnaire with five dimensions (EQ‐5D) utility score, EQ‐5D visual analog scale (VAS) score, Patient‐Oriented Eczema Measure (POEM), pruritus numerical rating scale (NRS) maximum itch intensity in the past week, and condition of nodular prurigo. The IGA is a tool for evaluating AD severity and is scored by an investigator or a physician using a 5‐point scale: 0 = clear; 1 = almost clear; 2 = mild; 3 = moderate; and 4 = severe.18 Unless otherwise specified, “moderate” refers to IGA = 3 and “severe” refers to IGA = 4. EASI is scored on a scale ranging 0–72 and includes measures of regional BSA with key signs of inflammation including erythema, induration/papulation/edema, excoriations and lichenification.19 The DLQI is a practical 10‐item questionnaire for clinical use in assessing the impacts of a disease and its treatment on patient quality of life. The DLQI score is calculated by summing up the score of each question, with 30 as the maximum total score.20 The EQ‐5D is a standardized instrument for measuring generic health status, with patient self‐assessment of mobility, self‐care, usual activities, pain or discomfort and anxiety/depression.21 The EQ‐5D VAS is a patient‐reported score of their health on a scale of 0 (worst health) to 100 (best health).22 POEM is a patient‐oriented assessment of disease severity in patients with AD. The questionnaire evaluates seven symptoms of AD (itching, sleep disturbance, bleeding, weeping/oozing of skin, cracking of skin, flaking of skin and dryness/roughness of skin) on a 5‐point scale of frequency during the previous week. The maximum total score is 28.23 For pruritus NRS, patients report average pruritus intensity (over the last 7 days) using an 11‐point scale (0–10, 0 = no itch and 10 = worst itch).24 Prurigo nodules are hyperkeratotic, excoriated, pruritic nodules commonly associated with AD and chronic pruritus.25, 26, 27 As there are no standardized prurigo measures, we collected the following exploratory data on prurigo nodules: Presence/absence (0 = absent, 1 = present); number (<10, ≥10 to <100, ≥100); and size of most significant prurigo (diameter) (<2 mm, ≥2 to <4 mm, ≥4 to <8 mm, ≥8 mm).
The most recent AD treatments prior to the baseline visit were also recorded at baseline, including medications and procedures. The following information was reported by prescription‐based dose adjustment: pre‐specified therapeutic class, total dose prescribed, duration of treatment, dosing frequency and reason for prescription. For procedures intended for AD treatment, pre‐specified therapeutic class, the date of implementation and the reason for procedure was reported. For patient education intended as standard care for AD, the date of implementation and its reason was reported.
Statistical analysis
A target number of 300 patients was planned for this study. This number allows the average incidence rate of flare to be estimated with a 95% confidence interval of 0.2 times/patient‐years, assuming a flare rate of 1.5 times/year within the 2‐year follow‐up period. The incidence rate of flare is the primary end‐point of the study; however, post‐baseline data including flares are not reported in this manuscript.
Baseline data were analyzed using descriptive statistics values. Continuous data were summarized using the number of patients observed (n), mean, standard deviation, minimum, median and maximum. Categorical data were summarized using counts and percentages. Missing data were not included. To examine the relationship among continuous variables, Spearman's rank correlation coefficients were used. The statistical package SAS® (version 9.2 or later; SAS Institute, Cary, NC, USA) was used for all analyses.
Ethical and regulatory standards
This study is being conducted in accordance with the principles defined by the 18th World Medical Association General Assembly Declaration of Helsinki and all subsequent amendments. This study is also being conducted in accordance with ‘Ethical guidelines for clinical trials in medical research involving human subjects’ (established 22 December 2014).28 The study protocol was reviewed and approved by institutional review boards before the first patient recruitment. All patients provided written informed consent before study procedures were initiated. The study was registered in the University Hospital Medical Information Network Clinical Trials Registry (UMIN‐CTR no. UMIN000022623).
Results
Baseline demographics and characteristics
A total of 300 patients were enrolled in the registry between 28 July 2016 and 4 July 2017. The demographics and disease characteristics of the registry patients are listed in Table 1. At baseline, median age was 34.0 years, the mean baseline body mass index (BMI) was 22.64 kg/m2 and 60.7% of the patients were male.
Table 1.
Baseline demographics and disease characteristics
| Characteristic | n | All | n | Moderate: IGA = 3 | n | Severe: IGA = 4 |
|---|---|---|---|---|---|---|
| Male, n (%) | 300 | 182 (60.7) | 220 | 135 (61.4) | 80 | 47 (58.8) |
| Female, n (%) | 300 | 118 (39.3) | 220 | 85 (38.6) | 80 | 33 (41.3) |
| Age (years; median [range]) | 300 | 34.0 (20–58) | 220 | 35.0 (20–58) | 80 | 33.0 (20–57) |
| Height (cm; median [range]) | 299 | 165.30 (145.0–185.3) | 219 | 165.50 (145.0–185.3) | 80 | 165.15 (145.0–180.0) |
| Weight (kg; median [range]) | 299 | 60.50 (36.0–111.3) | 219 | 62.00 (36.0–111.3) | 80 | 59.50 (43.0–102.8) |
| BMI (kg/m2; median [range]) | 299 | 21.91 (15.7–41.4) | 219 | 22.03 (15.9–41.4) | 80 | 21.75 (15.7–39.3) |
| Age of onset (years; median [range]) | 300 | 3.0 (0–52) | 220 | 4.0 (0–52) | 80 | 3.0 (0–48) |
| AD disease duration (years; median [range]) | 300 | 25.53 (0.6–55.5) | 220 | 25.56 (0.6–55.5) | 80 | 25.32 (1.1–52.3) |
| No. of visits for AD treatments in the past 1 year (times; median [range]) | 298 | 8.0 (0–120) | 220 | 8.0 (0–50) | 78 | 6.5 (0–120) |
| IGA score (median [range]) | 300 | 3.0 (3–4) | 220 | 3.0 (3–3) | 80 | 4.0 (4–4) |
| EASI total score (median [range]) | 300 | 21.700 (3.40–72.00) | 220 | 18.000 (3.40–53.40) | 80 | 40.750 (17.00–72.00) |
| BSA (%; median [range]) | 300 | 46.25 (10.0–100.0) | 220 | 38.70 (10.0–99.0) | 80 | 78.50 (24.0–100.0) |
| DLQI total score (median [range]) | 300 | 6.0 (0–30) | 220 | 6.0 (0–30) | 80 | 12.5 (2–29) |
| EQ‐5D utility score (median [range]) | 299 | 0.8228 (0.102–1.000) | 219 | 0.8709 (0.121–1.000) | 80 | 0.7184 (0.102–1.000) |
| EQ‐5D VAS (median [range]) | 297 | 70.0 (0–100) | 217 | 70.0 (0–100) | 80 | 60.0 (0–100) |
| POEM total score (median [range]) | 300 | 16.0 (2–28) | 220 | 14.5 (2–28) | 80 | 21.5 (7–28) |
| Pruritus score by NRS (median [range]) | 297 | 7.0 (0–10) | 217 | 6.0 (0–10) | 80 | 8.0 (3–10) |
AD, atopic dermatitis; BMI, body mass index; BSA, body surface area; DLQI, Dermatology Life Quality Index; EASI, Eczema Area and Severity Index; EQ‐5D, EuroQOL group health questionnaire with five dimensions; IGA, Investigator's Global Assessment; NRS, numerical rating scale; POEM, Patient‐Oriented Eczema Measure.
Among the patients in the registry, the median age of onset of AD was 3 years and the median disease duration of AD was 25.5 years. At baseline, most (73.3%) of the patients had moderate AD: 220 (73.3%) patients had an IGA of 3, 80 (26.7%) had an IGA of 4, and the median total EASI score was 21.7, which is considered severe on the 0–72‐point scale. On average, approximately half (46.25%) of the patients’ BSA was affected by AD. Patients had a median pruritus NRS for maximum itch intensity of 7.0 (on a scale of 0–10) and a median DLQI score of 6.0 (on a scale of 0–30). The median EQ‐5D utility score was 0.8228 and the median EQ‐5D VAS score was 70.0. The median POEM total score was 16.0 (on a scale of 0–28). The baseline demographics were similar between the patients visiting the hospital sites and the clinic sites, whereas the patients visiting the hospital sites exhibited consistently more severe AD symptoms than those visiting the clinic sites. Among patients with an IGA of 3, 64% visited a clinic and 36% visited a hospital, while among those with an IGA of 4, 29% visited a clinic and 71% visited a hospital (Table S2).
Allergic/atopic comorbidities
The allergic/atopic comorbidities of the registry patients are listed in Table 2. Of the registry patients, 74.3% had allergic/atopic diseases in addition to AD. The most prevalent current allergic/atopic comorbidities were allergic rhinitis (55.7%), allergic conjunctivitis (23.7%), food allergy (22.0%) and asthma (18.3%). Asthma was more prevalent among patients with moderate AD (IGA = 3) compared with patients having severe AD (IGA = 4), at 20.5% and 12.5%, respectively. In addition, hives (urticaria) were more prevalent among patients with moderate AD compared with patients having severe AD, at 12.3% and 6.3%, respectively, and were more prevalent in females than males (Table S3). There was a higher prevalence of food allergy among registry patients with childhood AD onset, compared with those who had adult AD onset (Fig. S1).
Table 2.
Allergic/atopic comorbidities
| Comorbidity | n (%) | ||
|---|---|---|---|
| All | Moderate: IGA = 3 | Severe: IGA = 4 | |
| Total | 300 (100) | 220 (100) | 80 (100) |
| Allergic diseases (except for atopic dermatitis) | 223 (74.3) | 166 (75.5) | 57 (71.3) |
| Allergic rhinitis | 167 (55.7) | 135 (61.4) | 32 (40.0) |
| Allergic conjunctivitis | 71 (23.7) | 54 (24.5) | 17 (21.3) |
| Food allergy | 66 (22.0) | 45 (20.5) | 21 (26.3) |
| Asthma | 55 (18.3) | 45 (20.5) | 10 (12.5) |
| Hives | 32 (10.7) | 27 (12.3) | 5 (6.3) |
| Allergic contact dermatitis | 29 (9.7) | 20 (9.1) | 9 (11.3) |
| Drug allergy | 14 (4.7) | 8 (3.6) | 6 (7.5) |
| Chronic sinusitis | 13 (4.3) | 10 (4.5) | 3 (3.8) |
| Atopic keratoconjunctivitis | 8 (2.7) | 5 (2.3) | 3 (3.8) |
IGA, Investigator's Global Assessment.
Prurigo nodules
The prevalence of prurigo nodules is higher with higher disease severity: 30.9% of patients with an IGA of 3 (moderate) had prurigo nodules and 56.3% of patients with an IGA of 4 (severe) had prurigo nodules (Table 3). The prevalence of prurigo was different between males and females (46.2% and 24.6%, respectively). Among patients with prurigo, 23.0% had less than 10 prurigo nodules, 60.2% had 10 or more to less than 100, and 16.8% had 100 or more (Table 4). The size of the prurigo was related to disease severity: Among patients with prurigo nodules whose most significant nodule was less than 2 mm in size, 10 out of 11 patients had moderate disease severity (IGA = 3) and only one patient had severe (IGA = 4), while among patients whose most significant prurigo nodule was 8 mm or more, seven out of 23 patients had moderate and 16 out of 23 patients had severe AD. While the presence of prurigo nodules did not clearly affect the degree of pruritus NRS scores in this study, we observed a tendency of higher mean pruritus NRS score as the number and maximal size of prurigo nodules increased (Fig. 2).
Table 3.
Presence of prurigo nodules
| n (%) | |||||
|---|---|---|---|---|---|
| All | Male | Female | Moderate: IGA = 3 | Severe: IGA = 4 | |
| 300 (100) | 182 (100) | 118 (100) | 220 (100) | 80 (100) | |
| Prurigo nodules | |||||
| Yes | 113 (37.7) | 84 (46.2) | 29 (24.6) | 68 (30.9) | 45 (56.3) |
| No | 184 (61.3) | 98 (53.8) | 86 (72.9) | 150 (68.2) | 34 (42.5) |
| Missing | 3 (1.0) | 0 | 3 (2.5) | 2 (0.9) | 1 (1.3) |
IGA, Investigator's Global Assessment.
Table 4.
Number and size of prurigo nodules
| n (%) | |||||
|---|---|---|---|---|---|
| Prurigo: yes | Male | Female | Moderate: IGA = 3 | Severe: IGA = 4 | |
| 113 (100) | 84 (100) | 29 (100) | 68 (100) | 45 (100) | |
| No. of prurigo nodules | |||||
| <10 | 26 (23.0) | 18 (21.4) | 8 (27.6) | 20 (29.4) | 6 (13.3) |
| ≥10 to <100 | 68 (60.2) | 50 (59.5) | 18 (62.1) | 42 (61.8) | 26 (57.8) |
| ≥100 | 19 (16.8) | 16 (19.0) | 3 (10.3) | 6 (8.8) | 13 (28.9) |
| Size of the most significant nodule (mm) | |||||
| <2 | 11 (9.7) | 8 (9.5) | 3 (10.3) | 10 (14.7) | 1 (2.2) |
| ≥2 to <4 | 51 (45.1) | 36 (42.9) | 15 (51.7) | 36 (52.9) | 15 (33.3) |
| ≥4 to <8 | 28 (24.8) | 23 (27.4) | 5 (17.2) | 15 (22.1) | 13 (28.9) |
| ≥8 | 23 (20.4) | 17 (20.2) | 6 (20.7) | 7 (10.3) | 16 (35.6) |
IGA, Investigator's Global Assessment.
Figure 2.
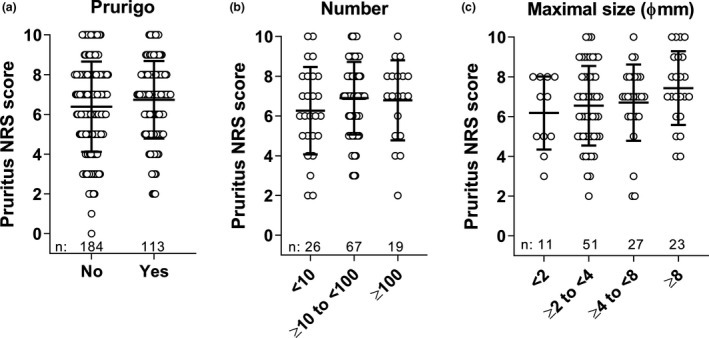
Association of prurigo and pruritus NRS. (a) Pruritus NRS scores plotted for patients with and without prurigo nodules at baseline. (b) Pruritus NRS scores plotted for patients with prurigo nodules by number category and (c) by maximal size category. Number of patients in each category are shown as n. Bars depict mean ± standard deviation. NRS, numerical rating scale.
Number of physician visits for AD treatments in the past year
Among patients with moderate AD (IGA = 3), 17.3% had 13 or more physician visits within the past year, while 57.7% had five to 12 visits, and 25.0% had four or less visits (Table 5). Among patients with severe AD (IGA = 4), 15.0% had 13 or more physician visits for AD in the past year, while 51.3% had five to 12 visits, and 31.3% had four or less visits. There was no substantial difference in the number of physician visits for AD treatments in the past year between patients in hospitals and clinics (Table 5).
Table 5.
Number of physician visits for AD treatment in the past year
| n (%) | |||||
|---|---|---|---|---|---|
| All | IGA | Medical institution | |||
| Moderate: IGA = 3 | Severe: IGA = 4 | Hospital | Clinic | ||
| No. of visits in the past 1 year | 300 (100) | 220 (100) | 80 (100) | 137 (100) | 162 (100) |
| ≥0 to <4 | 80 (26.7) | 55 (25.0) | 25 (31.3) | 37 (27.0) | 43 (26.4) |
| ≥5 to <12 | 168 (56.0) | 127 (57.7) | 41 (51.3) | 78 (56.9) | 90 (55.2) |
| ≥13 | 50 (16.7) | 38 (17.3) | 12 (15.0) | 22 (16.1) | 28 (17.2) |
| Missing | 2 (0.7) | 0 | 2 (2.5) | 0 | 2 (1.2) |
IGA, Investigator's Global Assessment.
Most recent medication or treatment before baseline visit
Prior to baseline, 86.7% of patients used either TCS or a topical calcineurin inhibitor (TCI) to treat AD (Table 6). Among patients using TCS to treat AD, the majority used “very strong” TCS (63.3%), with 21.0% using the “strongest” TCS, 22.7% using a “strong” TCS, 40.7% using a “medium” TCS, and only 2.3% using a “weak” TCS. Overall, 30.7% of patients used TCI. TCS/TCI use was similar between patients with moderate (IGA = 3) and severe (IGA = 4) disease. Among other treatments, 63.3% of patients used a skin moisturizer, 10.7% used a (for consistency with paragraph below) skin protectant, 6.0% used an oral immunosuppressant, 3.0% used an oral corticosteroid, 70.0% used antihistamine/anti‐allergic drugs, 4.7% of patients had UV phototherapy treatment and no patients received psychotherapy immediately prior to baseline.
Table 6.
Most recent medication/treatment before baseline visits
| n (%) | |||||
|---|---|---|---|---|---|
| All | IGA | Medical institution | |||
| Moderate: IGA = 3 | Severe: IGA = 4 | Hospital | Clinic | ||
| Total | 300 | 220 (100) | 80 (100) | 137 | 162 |
| Any TCS and/or TCI | 260 (86.7) | 194 (88.2) | 66 (82.5) | 117 (85.4) | 143 (87.7) |
| TCS: strongest | 63 (21.0) | 45 (20.5) | 18 (22.5) | 26 (19.0) | 37 (22.7) |
| TCS: very strong | 190 (63.3) | 139 (63.2) | 51 (63.8) | 86 (62.8) | 104 (63.8) |
| TCS: strong | 68 (22.7) | 51 (23.2) | 17 (21.3) | 25 (18.2) | 43 (26.4) |
| TCS: medium | 122 (40.7) | 91 (41.4) | 31 (38.8) | 44 (32.1) | 78 (47.9) |
| TCS: weak | 7 (2.3) | 5 (2.3) | 2 (2.5) | 3 (2.2) | 4 (2.5) |
| TCI | 92 (30.7) | 69 (31.4) | 23 (28.8) | 44 (32.1) | 48 (29.4) |
| Antihistamine drug/anti‐allergic drug | 210 (70.0) | 161 (73.2) | 49 (61.3) | 86 (62.8) | 124 (76.1) |
| Skin moisturizer | 190 (63.3) | 148 (67.3) | 42 (52.5) | 79 (57.7) | 111 (68.1) |
| Skin protectant | 32 (10.7) | 18 (8.2) | 14 (17.5) | 18 (13.1) | 14 (8.6) |
| Oral immunosuppressant | 18 (6.0) | 12 (5.5) | 6 (7.5) | 13 (9.5) | 5 (3.1) |
| Kampo medicines (herbal drug) | 17 (5.7) | 10 (4.5) | 7 (8.8) | 12 (8.8) | 5 (3.1) |
| UV phototherapy | 14 (4.7) | 14 (6.4) | 0 | 4 (2.9) | 10 (6.1) |
| Compound drug (antihistamine + corticosteroid) | 10 (3.3) | 8 (3.6) | 2 (2.5) | 4 (2.9) | 6 (3.7) |
| NSAID | 8 (2.7) | 5 (2.3) | 3 (3.8) | 4 (2.9) | 4 (2.5) |
| Oral corticosteroid | 9 (3.0) | 5 (2.3) | 4 (5.0) | 5 (3.6) | 4 (2.5) |
| Psychotherapy | 0 | 0 | 0 | 0 | 0 |
IGA, Investigator's Global Assessment; NSAID, non‐steroidal anti‐inflammatory drug; TCI, topical calcineurin inhibitor; TCS, topical corticosteroid; UV, ultraviolet.
A slightly larger proportion of patients with severe disease than with moderate disease used a skin protectant (17.5% vs 8.2%), oral immunosuppressant (7.5% vs 5.5%), oral corticosteroid (5.0% vs 2.3%) and herbal drugs (8.8% vs 4.5%). A slightly greater proportion of patients with moderate disease than with severe disease used antihistamines or anti‐allergic drugs (73.2% vs 61.3%, respectively) and UV phototherapy (6.4% vs 0%).
Interrelationships among multiple disease measures from both physicians’ and patients’ perspectives
A correlation analysis provided insight into the interrelationships between clinical and patient‐reported disease measures (Figs 2, 3, 4). Physician's assessments of AD severity by EASI and BSA showed associations with AD severity measured by IGA. For patients with an IGA of 3, the median EASI score was 18.00 and the median BSA was 38.70; among patients with an IGA of 4, the median EASI score was 40.75 and the median BSA score was 78.50, whereas patients’ assessments of disease symptoms and quality of life by DLQI, POEM, EQ‐5D and pruritus NRS did not clearly associate with disease severity by IGA. Some patients with an IGA of 3 had poor POEM, EQ‐5D and pruritus NRS scores, whereas some patients with an IGA of 4 had better results in these self‐assessment scores (Fig. 3).
Figure 3.
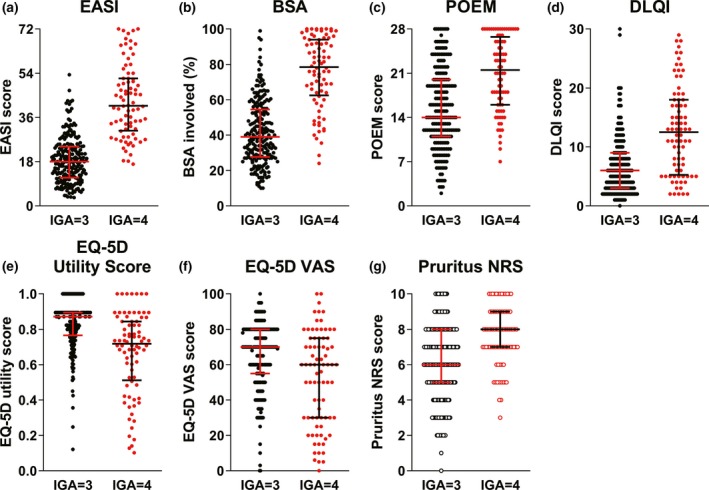
Association of IGA score and other disease measures, including patient‐reported outcomes. (a–g) Bars depict median with interquartile values (Q1, Q3), and black and red dots depict the patients with an IGA of 3 and 4, respectively. BSA, body surface area; DLQI, Dermatology Life Quality Index; EASI, Eczema Area and Severity Index; EQ‐5D, EuroQOL group health questionnaire with five dimensions; IGA, Investigator's Global Assessment; NRS, numerical rating scale; POEM, Patient‐Oriented Eczema Measure; VAS, visual analog scale.
Figure 4.
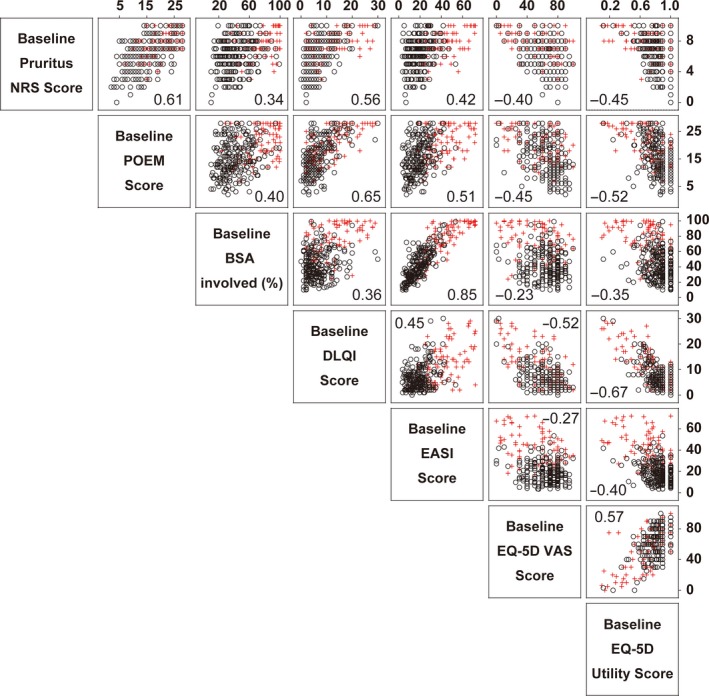
Relationship between various disease measures and disease severity. Correlations between disease severity measures are shown in a matrix. The values in the graphs are Spearman's rank correlation coefficient (r). Black and red symbols depict the patients with an IGA of 3 and 4, respectively. BSA, body surface area; DLQI, Dermatology Life Quality Index; EASI, Eczema Area and Severity Index; EQ‐5D, EuroQOL group health questionnaire with five dimensions; IGA, Investigator's Global Assessment; NRS, numerical rating scale; POEM, Patient‐Oriented Eczema Measure; VAS, visual analog scale.
Eczema Area and Severity Index score and BSA percentage involved were markedly correlated, confirming that they are mutually dependent variables. There were positive correlations between POEM and DLQI (r = 0.65), pruritus NRS (r = 0.61), EASI (r = 0.51) and BSA (r = 0.40). There were also positive correlations between pruritus NRS and DLQI (r = 0.56), EASI (r = 0.42) and BSA (r = 0.34). These correlation profiles show that patients with worse POEM and pruritus NRS scores had worse quality of life as assessed by DLQI, and tended to have greater severity of disease, as assessed by EASI or BSA; but some of them had milder severity of disease, concordant with the previous observations (Fig. 4).
Biomarkers
Biomarker results were collected if the data were available in the medical records. In general, the levels of AD biomarkers thymus and activation regulated chemokine (TARC), eosinophils, immunoglobulin (Ig)E and lactate dehydrogenase (LDH) related with disease severity, measured by IGA (Table S4; Fig. S2). The median TARC level was 1565.0 pg/mL in patients with moderate AD and 3943.0 pg/mL in patients with severe AD. The median eosinophil count was 575/μL in patients with moderate AD and 1062/μL in patients with severe AD. The median IgE level was 1908 IU/mL in patients with moderate AD and 8772 IU/mL in patients with severe AD, and the median LDH level was 275.0 and 325.5 IU/mL, respectively.
Discussion
The baseline data from the ADDRESS‐J registry provide important information on the current demographics and disease characteristics of Japanese adults with moderate to severe AD, while also reporting recent treatments and allergic comorbidities. The registry includes patients undergoing treatment intensification and IGA scores equal to or higher than 3, in order to measure long‐term control of symptoms. Such long‐term observational studies are essential for capturing long‐term data on treatment practices and their real‐world effectiveness. This observational study uses multiple standard outcome measures for AD, including assessments of clinical signs by IGA, EASI and BSA, and patient‐reported outcomes including pruritus NRS, POEM and DLQI, as well as exploratory outcome measures for prurigo nodules.
Subgroup analyses of AD characteristics by age of onset and presence of allergic comorbidities provide valuable information about the different presentations of AD and the relationships between different disease measures. A recent study found no significant difference in sex, family history, BMI or EASI between patients with childhood‐onset AD and patients with adult‐onset AD; however, there were differences in the rates of food allergy and age of AD onset.29, 30 Similar findings of higher prevalence of food allergy among patients with childhood‐onset compared with adult‐onset AD were observed in the ADDRESS‐J cohort, suggesting that it is representative of the general AD patient population.
Among ADDRESS‐J patients, a history of asthma was more prevalent in patients with moderate AD compared with those having severe AD, suggesting that severe AD may not involve airway comorbidities as much as moderate AD does. Hives were also more prevalent among patients with moderate AD compared with patients having severe AD. There are conflicting reports as to whether the severity of AD is associated with increased asthma and other allergic comorbidities.13, 31, 32 This could be partially attributed to the differential definitions of severity employed in each analysis. Further investigations are required to clarify the relationship among the extent of skin sensitization, AD severity and other allergic comorbidities.
We and others recently observed a similar tendency in which the prevalence of atopic comorbidities such as asthma and rhinitis in AD patients does not significantly increase with the severity of AD in population‐based surveys in Japan13 and the USA.9 In children with AD, there is a population‐based report that AD severity influences the prevalence of asthma, allergic rhinitis and food allergy, as well as severity of those comorbidities.33 The present study did not collect severity information for such comorbidities, which is a limitation. However, even in the aforementioned pediatric study, overall allergic rhinitis within the past 12 months was not associated with the severity of AD.33 The prevalence of comorbidities may vary between children and adults, and among the severity stratum of comorbid diseases. Furthermore, severe AD patients may be treated with some systemic therapy, resulting in the alleviation of other atopic comorbidities. More studies are obviously needed to determine the relationship between the severity of AD and prevalence and severity of atopic comorbidities.
Unlike what we observed with atopic comorbidities, a larger proportion of patients with severe AD tended to have prurigo – compared with patients with moderate AD – as both the size and prevalence of prurigo increased with disease severity. Among ADDRESS‐J patients, prurigo was more prevalent in males than in females. According to a study of dermatological disorders in Japan,4 among Japanese patients, males have a higher prevalence of prurigo than females. This difference we observed in prevalence of prurigo nodules between males and females was in the proportion of patients with more than 100 prurigo nodules (higher in males), and in the proportion of patients with prurigo nodules having a diameter larger than 4 mm (also higher in males). Mechanistically, one may postulate that patients with a larger prurigo burden exhibit higher pruritus NRS scores, which induces more scratching behavior and results in more prurigo. Existence of prurigo per se is not related to baseline pruritus NRS score.
According to Japanese AD treatment guidelines,2, 3 the current standard medicinal therapies for AD include TCS and TCI as the main treatment for inflammation, with topical application of emollients for treatment of skin barrier dysfunction as basic care, and systemic antihistamines and anti‐allergic drugs as adjunctive treatments for pruritus. Herbal treatments (Kampo medicine) are advised as last‐resort treatment for patients whose symptoms are uncontrolled with more conventional therapies. The prevalence of treatments among registry patients well reflects these treatment guidelines.
Generally, at baseline, there was no obvious difference in most recent drug treatments between the patient populations with moderate versus severe AD. There were, however, minor differences in the use of oral immunosuppressants and oral corticosteroids, but it is unclear whether this was ongoing treatment for chronic disease or whether these were treatments for recent flares. The percentage of patients prescribed oral corticosteroids for AD was low (3%), as these are not recommended for long‐term use due to severe side‐effects.2, 3 It is possible that patients with a high number of visits for treatment per year are undergoing phototherapy treatment as this requires frequent visits,34 which is another example of higher burden on these patients. The number of patient visits for AD treatment in the past year was similar between hospitals and clinics, suggesting that patients utilize both types of medical institutions at an approximately equal rate for AD treatment.
Information about correlations between different disease measures may help in monitoring long‐term control of AD; for example, identifying consistent positive correlations between two disease measures may help determine effectiveness of treatments long term. The correlation analysis of the baseline data potentially highlights a gap in perception between physicians and patients, as there is some apparent inconsistency between objective disease severity and patient‐reported outcomes.35 Physician's assessments of AD disease severity such as EASI and BSA showed a clear association with AD severity (determined by IGA), while DLQI, POEM, EQ‐5D and pruritus NRS did not show such an association, reflecting that some patients with moderate AD have impaired quality of life, while others with severe AD have better quality of life. This could be explained by patient‐to‐patient variation of objective, patient‐reported observations of disease symptoms, or it could indicate that patient‐reported disease severity is independent of physician‐assessed measures. For example, some patients may assess their quality of life independently from their symptoms.36 These discrepancies highlight the importance of the Harmonising Outcome Measures for Eczema (HOME) initiative to include multi‐modal evaluation aspects in long‐term disease control. The HOME initiative is a global initiative of patients and health‐care professionals that aims to standardize the outcome measures in patients with AD in clinical trials and in clinical practice, including clinical signs, symptoms, quality of life measures and long‐term control of symptoms.36, 37, 38, 39
This study may be limited by recall bias in patient‐reported data. Outcome differences between sexes may be confounded due to recall and reporting bias between males and females,40, 41 and in general recall bias may also affect comorbidity and onset analyses. For example, the higher prevalence of adult‐onset AD versus childhood‐onset AD in this study could be due to transient remission after infancy in some cases that were self‐reported as “adult‐onset” as up to 70% of children diagnosed with AD under the age of 2 years undergo spontaneous remission of their disease.42
There is also potential bias with the biomarker data (TARC, IgE, eosinophils and LDH) because only patients with more severe AD might be examined for these indices. Subgroup analysis is required for the population who has baseline biomarker data. Also, not all of the data for eosinophils were available at baseline.
In conclusion, the ADDRESS‐J Japanese registry cohort represents a population of patients with a long duration of AD undergoing a variety of treatments, providing an important resource for characterizing the disease burden of AD in Japan. The baseline data from this study indicate that among Japanese adults with moderate to severe AD, most have had AD since childhood, and the burden presented by their disease symptoms affects their quality of life independently of disease severity. This population has severe AD symptoms according to patient‐reported measures such as POEM score and pruritus NRS for maximum itch intensity, and clinical measures such as EASI total score. The results of the long‐term analysis from ADDRESS‐J will provide further insight into the safety and effectiveness of various treatments for adult patients with moderate to severe AD.
Supporting information
Table S1. ADDRESS‐J study investigators
Table S2. Atopic dermatitis (AD) severity (Investigator's Global Assessment [IGA] score) by type of study site
Table S3. Allergic comorbidities presented by atopic dermatitis (AD) onset and sex
Table S4. Baseline biomarkers for atopic dermatitis (AD)
Figure S1. Allergic comorbidities presented by atopic dermatitis (AD) onset.
Figure S2. Relationship between biomarkers and Investigator's Global Assessment (IGA; limited data).
Acknowledgments
Research was sponsored by Sanofi and Regeneron Pharmaceuticals, Inc. UMIN‐CTR: UMIN000022623. Medical writing/editorial assistance was provided by Lola MacRae, Ph.D., of Excerpta Medica, funded by Sanofi Genzyme and Regeneron Pharmaceuticals, Inc.
Conflict of Interest
N. K. received honoraria for lectures from Mitsubishi Tanabe Pharma and Maruho; and research grants from Maruho, Kyowa Hakko Kirin, Novartis Pharma, Janssen Pharmaceutical, AbbVie and Sanofi. H. S. received honoraria for lectures from Mitsubishi Tanabe Pharma, Maruho, Taiho Pharmaceutical, Kyowa Hakko Kirin, Kyorin Pharmaceutical, and Sanofi; and research grants from Eisai, Tokiwa Pharmaceutical and Torii Pharmaceutical. Y. K. received contract research grants from Sanofi. T. E. received honoraria for lectures from Maruho and Kyowa Hakko Kirin. S. T. received consultant fees from Daiichi Sankyo, Takeda Pharmaceutical, Sanofi, Solasia Pharma, Sysmex, and Gunze. H. T., Y. T., E. R. and K. A. are employees of Sanofi and may be shareholders. M. A. is an employee and shareholder of Regeneron Pharmaceuticals, Inc.
References
- 1. Gittler JK, Shemer A, Suárez‐Fariñas M et al Progressive activation of TH2/TH22 cytokines and selective epidermal proteins characterizes acute and chronic atopic dermatitis. J Allergy Clin Immunol 2012; 130(6): 1344–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Saeki H, Nakahara T, Tanaka A et al Clinical practice guidelines for the management of atopic dermatitis 2016. J Dermatol 2016; 43(10): 1117–1145. [DOI] [PubMed] [Google Scholar]
- 3. Katayama I, Aihara M, Ohya Y et al Japanese guidelines for atopic dermatitis 2017. Allergol Int 2017; 66(2): 230–247. [DOI] [PubMed] [Google Scholar]
- 4. Furue M, Yamazaki S, Jimbow K et al Prevalence of dermatological disorders in Japan: a nationwide, cross‐sectional, seasonal, multicenter, hospital‐based study. J Dermatol 2011; 38(4): 310–320. [DOI] [PubMed] [Google Scholar]
- 5. Furue M, Chiba T, Takeuchi S. Current status of atopic dermatitis in Japan. Asia Pac Allergy 2011; 1(2): 64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Saeki H, Iizuka H, Mori Y et al Prevalence of atopic dermatitis in Japanese elementary schoolchildren. Br J Dermatol 2005; 152(1): 110–114. [DOI] [PubMed] [Google Scholar]
- 7. Saeki H, Tsunemi Y, Fujita H et al Prevalence of atopic dermatitis determined by clinical examination in Japanese adults. J Dermatol 2006; 33(11): 817–819. [DOI] [PubMed] [Google Scholar]
- 8. Saeki H, Furue M, Furukawa F et al Guidelines for management of atopic dermatitis. J Dermatol 2009; 36(10): 563–577. [DOI] [PubMed] [Google Scholar]
- 9. Eckert L, Gupta S, Amand C, Gadkari A, Mahajan P, Gelfand JM. Impact of atopic dermatitis on health‐related quality of life and productivity in adults in the United States: an analysis using the National Health and Wellness Survey. J Am Acad Dermatol 2017; 77(2): 274–279. [DOI] [PubMed] [Google Scholar]
- 10. Simpson EL, Bieber T, Eckert L et al Patient burden of moderate to severe atopic dermatitis (AD): insights from a phase 2b clinical trial of dupilumab in adults. J Am Acad Dermatol 2016; 74(3): 491–498. [DOI] [PubMed] [Google Scholar]
- 11. Chrostowska‐Plak D, Reich A, Szepietowski JC. Relationship between itch and psychological status of patients with atopic dermatitis. J Eur Acad Dermatol Venereol 2013; 27(2): e239–e242. [DOI] [PubMed] [Google Scholar]
- 12. Yano C, Saeki H, Ishiji T et al Impact of disease severity on work productivity and activity impairment in Japanese patients with atopic dermatitis. J Dermatol 2013; 40(9): 736–739. [DOI] [PubMed] [Google Scholar]
- 13. Arima K, Gupta S, Gadkari A et al Burden of atopic dermatitis in Japanese adults: analysis of data from the 2013 National Health and Wellness Survey. J Dermatol 2018; 45(4): 390–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Furue M, Terao H, Rikihisa W et al Clinical dose and adverse effects of topical steroids in daily management of atopic dermatitis. Br J Dermatol 2003; 148(1): 128–133. [DOI] [PubMed] [Google Scholar]
- 15. Murota H, Takeuchi S, Sugaya M et al Characterization of socioeconomic status of Japanese patients with atopic dermatitis showing poor medical adherence and reasons for drug discontinuation. J Dermatol Sci 2015; 79(3): 279–287. [DOI] [PubMed] [Google Scholar]
- 16. Charman C, Williams H. The use of corticosteroids and corticosteroid phobia in atopic dermatitis. Clin Dermatol 2003; 21(3): 193–200. [DOI] [PubMed] [Google Scholar]
- 17. Yu S, Drucker AM, Lebwohl M, Silverberg JI. A systematic review of safety and efficacy of systemic corticosteroids in atopic dermatitis. J Am Acad Dermatol 2017; 78(4): 733–740. [DOI] [PubMed] [Google Scholar]
- 18. Futamura M, Leshem YA, Thomas KS, Nankervis H, Williams HC, Simpson EL. A systematic review of Investigator Global Assessment (IGA) in atopic dermatitis (AD) trials: many options, no standards. J Am Acad Dermatol 2016; 74(2): 288–294. [DOI] [PubMed] [Google Scholar]
- 19. Hanifin JM, Thurson M, Otomo M, Cherill R, Tofte SJ, Graeber M. The eczema area and severity index (EASI): assessment of reliability in atopic dermatitis. EASI Evaluator Group. Exp Dermatol 2001; 10(1): 11–18. [DOI] [PubMed] [Google Scholar]
- 20. Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI) – a simple practical measure for routine clinical use. Clin Exp Dermatol 1994; 19(3): 210–216. [DOI] [PubMed] [Google Scholar]
- 21. van Reenan M, Janssen B. EQ‐5D‐5L User Guide: Basic Information on How to Use the EQ‐5D Instrument. Rotterdam, the Netherlands: EuroQol Research Foundation, 2015. [Google Scholar]
- 22. Whynes D, the TOMBOLA Group . Correspondence between EQ‐5D health state classifications and EQ VAS scores. Health Qual Life Outcomes 2008; 6: 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Charman CR, Venn AJ, Williams HC. The patient‐oriented eczema measure: development and initial validation of a new tool for measuring atopic dermatitis severity from the patients’ perspective. Arch Dermatol 2004; 140(12): 1513–1519. [DOI] [PubMed] [Google Scholar]
- 24. Yosipovitch G, Reaney M, Mastey V et al Validation of peak pruritus numerical rating scale: results from clinical studies of dupilumab in adult patients with moderate‐to‐severe atopic dermatitis. Presented at American Academy of Dermatology Annual Meeting, March 3–7, 2017; Orlando, FL, USA: Abstract 5063.
- 25. Pereira MP, Steinke S, Zeidler C et al European academy of dermatology and venereology European prurigo project: expert consensus on the definition, classification and terminology of chronic prurigo. J Eur Acad Dermatol Venereol 2018; 32(7): 1059–1065. [DOI] [PubMed] [Google Scholar]
- 26. Fostini AC, Girolomoni G, Tessari G. Prurigo nodularis: an update on etiopathogenesis and therapy. J Dermatolog Treat 2013; 24(6): 458–462. [DOI] [PubMed] [Google Scholar]
- 27. Mullins TB, Bhimji SS. Prurigo Nodularis. Treasure Island, FL: StatPearls Publishing, 2017. [PubMed] [Google Scholar]
- 28. Sone S. Ethical guidelines for clinical trials in medical research involving human subjects. Gan To Kagaku Ryoho 2015; 42(8): 893–902. [PubMed] [Google Scholar]
- 29. Bantz S, Zhu Z, Zheng T. The atopic march: progression from atopic dermatitis to allergic rhinitis and asthma. J Clin Cell Immunol 2014; 5(2): 202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Flohr C, Perkin M, Logan K et al Atopic dermatitis and disease severity are the main risk factors for food sensitization in exclusively breastfed infants. J Invest Dermatol 2014; 134(2): 345–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Brunner PM, Silverberg JI, Guttman‐Yassky E et al Increasing comorbidities suggest that atopic dermatitis is a systemic disorder. J Invest Dermatol 2017; 137(1): 18–25. [DOI] [PubMed] [Google Scholar]
- 32. Celakovská J, Bukač J. The severity of atopic dermatitis evaluated with the SCORAD index and the occurrence of bronchial asthma and rhinitis, and the duration of atopic dermatitis. Allergy Rhinol (Providence) 2016; 7(1): e8–e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Silverberg JI, Simpson EL. Association between severe eczema in children and multiple comorbid conditions and increased healthcare utilization. Pediatr Allergy Immunol 2013; 24(5): 476–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Patrizi A, Raone B, Ravaioli GM. Management of atopic dermatitis: safety and efficacy of phototherapy. Clin Cosmet Investig Dermatol 2015; 8: 511–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chopra R, Vakharia PP, Sacotte R et al Relationship between EASI and SCORAD severity assessments for atopic dermatitis. J Allergy Clin Immunol 2017; 140(6): 1708–1710.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Heinl D, Prinsen CA, Deckert S. Measurement properties of adult quality‐of‐life measurement instruments for eczema: a systematic review. Allergy 2016; 71(3): 358–370. [DOI] [PubMed] [Google Scholar]
- 37. Schmitt J, Spuls P, Boers M et al Towards global consensus on outcome measures for atopic eczema research: results of the HOME II meeting. Allergy 2012; 67(9): 1111–1117. [DOI] [PubMed] [Google Scholar]
- 38. Schmitt J, Spuls PI, Thomas KS et al The Harmonising Outcome Measures for Eczema (HOME) statement to assess clinical signs of atopic eczema in trials. J Allergy Clin Immunol 2014; 134(4): 800–807. [DOI] [PubMed] [Google Scholar]
- 39. Spuls PI, Gerbens LAA, Simpson E et al Patient‐Oriented Eczema Measure (POEM), a core instrument to measure symptoms in clinical trials: a Harmonising Outcome Measures for Eczema (HOME) statement. Br J Dermatol 2017; 176(4): 979–984. [DOI] [PubMed] [Google Scholar]
- 40. Barsky AJ, Peekna HM, Borus JF. Somatic symptom reporting in women and men. J Gen Intern Med 2001; 16(4): 266–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. van Wijk CM, Kolk AM. Sex differences in physical symptoms: the contribution of symptom perception theory. Soc Sci Med 1997; 45(2): 231–246. [DOI] [PubMed] [Google Scholar]
- 42. Bieber T. Atopic Dermatitis. N Engl J Med 2008; 358(14): 1483–1494. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. ADDRESS‐J study investigators
Table S2. Atopic dermatitis (AD) severity (Investigator's Global Assessment [IGA] score) by type of study site
Table S3. Allergic comorbidities presented by atopic dermatitis (AD) onset and sex
Table S4. Baseline biomarkers for atopic dermatitis (AD)
Figure S1. Allergic comorbidities presented by atopic dermatitis (AD) onset.
Figure S2. Relationship between biomarkers and Investigator's Global Assessment (IGA; limited data).


