Summary
Chemotherapy plus rituximab has been the mainstay of treatment for follicular lymphoma (FL) for two decades but is associated with immunosuppression and relapse. In phase 2 studies, lenalidomide combined with rituximab (R2) has shown clinical synergy in front‐line and relapsed/refractory FL. Here, we show that lenalidomide reactivated dysfunctional T and Natural Killer (NK) cells ex vivo from FL patients by enhancing proliferative capacity and T‐helper cell type 1 (Th1) cytokine release. In combination with rituximab, lenalidomide improved antibody‐dependent cellular cytotoxicity in sensitive and chemo‐resistant FL cells, via a cereblon‐dependent mechanism. While single‐agent lenalidomide and rituximab increased formation of lytic NK cell immunological synapses with primary FL tumour cells, the combination was superior and correlated with enhanced cytotoxicity. Immunophenotyping of FL patient samples from a phase 3 trial revealed that R2 treatment increased circulating T‐ and NK‐cell counts, while R‐chemotherapy was associated with reduced cell numbers. Finally, using an in vitro model of myeloid differentiation, we demonstrated that lenalidomide caused a reversible arrest in neutrophil maturation that was distinct from a cytotoxic chemotherapeutic agent, which may help explain the lower rates of neutropenia observed with R2 versus R‐chemotherapy. Taken together, we believe these data support a paradigm shift in the treatment of FL – moving from combination immunochemotherapy to chemotherapy‐free immunotherapy.
Keywords: lenalidomide, follicular lymphoma, non‐Hodgkin lymphoma, immunomodulation, antibody‐dependent cell‐mediated cytotoxicity
Follicular lymphoma (FL) is the most common form of indolent non‐Hodgkin lymphoma (NHL) in the United States and Europe and accounts for roughly 20% of NHL cases globally (Perry et al, 2016; Teras et al, 2016). FL presents a variable clinical course but is ultimately incurable. Treatment approaches vary, from a watch‐and‐wait strategy to monotherapy with anti‐CD20 antibody to immunochemotherapy. The latter combines rituximab with standard chemotherapeutic regimens, such as cyclophosphamide, vincristine, and prednisone, with or without doxorubicin (CVP or CHOP, respectively) or bendamustine, with remission rates up to 90% and 5‐year overall survival rates approaching 90% (Rummel et al, 2013; Flinn et al, 2014; Luminari et al, 2016). However, relapse is common and the median progression‐free survival (PFS) after induction therapy is 6–7 years, with PFS declining after each additional relapse (Rivas‐Delgado et al, 2017; Salles et al, 2017). Moreover, chemotherapeutic agents have been associated with immune suppression and impaired lymphocyte recovery that can persist for up to 2 years after treatment (Saito et al, 2015; Ito et al, 2016; Martin et al, 2017a; Olszewski et al, 2018). Thus, the clinical challenge in FL is to deliver more tolerable and effective treatment regimens that minimize immunosuppressive effects and long‐term toxicity.
Scientific advances are providing insights into mechanisms that drive pathogenesis in FL disease. Mutations that can influence disease progression have been identified in FL cells (Pastore et al, 2015; Weigert & Weinstock, 2017), and cellular and molecular features of the tumour microenvironment have been associated with disease severity and clinical outcome (Dave et al, 2004; Yang & Ansell, 2012). Natural killer (NK) and cytotoxic T cells are rendered dysfunctional through diverse mechanisms, including selective loss of activating ligands, upregulation of inhibitory checkpoint molecules, and induction of anergy in the absence of inflammatory cytokines (Yang & Ansell, 2012; Gravelle et al, 2016). In addition, tumour‐infiltrating T cells from patients with FL have been shown to form defective immune synapses with malignant B cells, which inhibits their ability to recognize and lyse target cells (Ramsay et al, 2009).
Rituximab is an anti‐CD20 antibody; its mechanisms of action are to augment NK cell‐mediated killing of malignant B cells via antibody‐dependent cellular cytotoxicity (ADCC), to enhance antibody‐dependent cellular phagocytosis (ADCP) and to induce complement‐mediated killing. However, rituximab activity may be adversely affected by the underlying immune dysfunction observed in FL patients. For example, low absolute lymphocyte counts and reduced numbers of circulating NK cells in FL patients prior to treatment are predictive of an inferior response to rituximab (Decaudin et al, 2003; Shafer et al, 2013). Moreover, combining rituximab with chemotherapy, such as bendamustine or CHOP, does not mitigate the sustained immunosuppression seen in patients with B‐cell lymphoma (Saito et al, 2015; Ito et al, 2016).
Lenalidomide binds to cereblon in the Cullin‐4 RING E3 ubiquitin ligase and promotes degradation of the haematopoietic transcription factors IKAROS and AIOLOS (Lopez‐Girona et al, 2012; Chamberlain et al, 2014). In neoplastic B cells, degradation of these substrates causes apoptosis, whereas in T cells the result is enhanced co‐stimulation signalling and increased interleukin 2 (IL2) production (Gandhi et al, 2014). As a single agent, lenalidomide restores the ability of tumour‐infiltrating T cells from patients with B‐cell lymphoma to form functional immune synapses with autologous malignant B cells (Ramsay et al, 2008, 2009; Hagner et al, 2017). Lenalidomide in combination with rituximab (R2) has been shown to enhance activation of healthy donor NK cells and increase ADCC of various NHL cell lines (Wu et al, 2008; Zhang et al, 2009; Lagrue et al, 2015). In the clinic, R2 has demonstrated synergy in FL in both front‐line and relapsed/refractory settings in phase 2 trials (Fowler et al, 2014; Tuscano et al, 2014; Leonard et al, 2015; Martin et al, 2017b). Results of the recently reported phase 3 RELEVANCE trial, evaluating R2 versus R‐chemotherapy in previously untreated advanced FL patients, demonstrated similar efficacy in the two treatment arms, but found a greater frequency of grade 3/4 neutropenia was associated with R‐chemotherapy (Morschhauser et al, 2018). R2 immunotherapy has also shown activity in marginal zone lymphoma (MZL), where the combination achieved an overall response rate (ORR) of up to 89% in phase 2 studies (Fowler et al, 2014; Sacchi et al, 2016) and is currently being evaluated in the phase 3 MAGNIFY study (Andorsky et al, 2018).
Despite demonstrated clinical benefits of R2 in FL and MZL, the mechanism of action of combination treatment in the indolent NHL setting has not been well studied. Here, using primary FL patient samples, we demonstrate that the combination of lenalidomide and rituximab reactivates dysfunctional NK and T cells, leading to increased cytokine production and immune synapse signalling, with enhancement of NK‐mediated ADCC and CD8+ T cell anti‐FL activity. We show that R2 treatment of FL patients enrolled in the RELEVANCE trial led to increased circulating T‐ and NK‐cells compared to R‐chemotherapy, which was associated with a decline in immune cell numbers. Additionally, we investigated treatment‐associated neutropenia and provide in vitro evidence that lenalidomide induced a block in neutrophil maturation that was reversible and distinct from the cytotoxic effects of a chemotherapeutic agent. Taken together, our laboratory studies and correlative results provide a mechanistic basis for the R2 synergy observed in the clinic that supports the rational use of combination chemotherapy‐free immunotherapy for the treatment of FL.
Materials and methods
Patient samples and primary human cells
All patient samples were obtained after written informed consent, in accordance with the Declaration of Helsinki and International Council on Harmonization Good Clinical Practice guidelines, and with approval from the research ethics committees of all participating institutions. Peripheral blood samples were collected from patients enrolled in the RELEVANCE study at screening and at the end of induction therapy with R‐CHOP or R2. For immune synapse bioassays, cryopreserved lymph node (LN) single‐cell suspension samples were obtained from six treatment‐naïve patients with FL (clinical grades 1–3A) who were undergoing diagnostic biopsies. In addition, peripheral blood samples were obtained from six treatment‐naïve patients with leukaemic‐phase FL (grade IV; lymphocyte counts >20 × 109/l). CD4+ and CD8+ T cells were isolated from patient samples by positive magnetic selection, and malignant B cells were isolated by negative magnetic selection (to ~95% purity by flow cytometry), using MagniSort Cell Separation kits (Thermo Fisher Scientific, Waltham, MA, USA). CD56+ NK cells were isolated (to ~85% purity by flow cytometry) by magnetic selection (MagniSort NK Cell Enrichment Kit) from peripheral blood mononuclear cells (PBMC) that were harvested by density‐gradient centrifugation (Histopaque, Sigma‐Aldrich, St Louis, MO, USA).
For other ex vivo experiments, PBMC were isolated from buffy coats of healthy donors (New York Blood Center, New York, NY, USA), as previously described (Hagner et al, 2015). Viably‐frozen PBMC from naïve and relapsed/refractory FL and MZL patients were purchased from ConversantBio (Huntsville, AL, USA), ProteoGenex, (Inglewood, CA, USA) and BioreclammationIVT (Hicksville, NY, USA).
Cell lines and cell culture
The FL cell lines, DOHH2 and RL, were obtained from American Type Culture Collection (ATCC; Manassas, VA, USA) and Leibniz Institute‐DSMZ (Braunschweig, Germany), respectively. The splenic MZL cell line SLVL was from RIKEN BioResource Center (Ibaraki, Japan), and the NK‐92 cell line was from ATCC. All cell lines were maintained in RPMI medium [RPMI‐1640 with 10% fetal bovine serum (FBS), supplemented with 2 mmol/L L‐glutamine, 1% penicillin/streptomycin and 1 mmol/L sodium pyruvate]. DOHH2 and RL cells were made resistant to bendamustine, 4 hydroperoxycyclophosphamide (4‐HC), and doxorubicin by stepwise exposure to increasing drug concentrations up to 100, 5 and 250 μmol/l, respectively.
Enumeration of T and NK cells in peripheral blood of patients
Enumeration of CD4 and CD8 T and NK cells in peripheral blood of patients enrolled in the RELEVANCE trial was performed as previously described (Plonquet et al, 2007). Briefly, absolute cell counts were derived directly from the flow cytometry data by using fluorescent beads of calibrated concentration (‘single platform’ technology). Lymphocytes were gated according to high‐CD45 fluorescence intensity and low side scatter intensity; CD4 T cells were defined as CD3+CD4+ lymphocytes, CD8 T cells were defined as CD3+CD8+ lymphocytes, and NK cells as CD3−CD16+ and/or CD56+ lymphocytes.
See the Data S1 for additional methods.
Results
Lenalidomide demonstrated ex vivo immune stimulatory effects on T cells and NK cells from FL patient and healthy donor PBMC
The effect of lenalidomide on CD3‐stimulated PBMC from healthy donors and FL patients, both treatment‐naïve and relapsed/refractory, was examined (Fig 1). Lenalidomide treatment of PBMC from FL patients led to a significant increase (P < 0·05) of 1·6‐ to 2·5‐fold in surface expression of CD56, OX40, IL2Rα, and NKp30 on NK cells compared to dimethyl sulfoxide (DMSO)‐treated controls (Fig 1A). Notably, lenalidomide significantly increased intracellular granzyme B levels in FL NK cells by 1·6‐fold over DMSO. Lenalidomide increased expression of CD56 and OX40 1·7‐ and 3·0‐fold in healthy donor NK cells (P < 0·05) compared to DMSO. In lenalidomide‐treated FL PBMC, CD8+ T cells showed up to 1·9‐fold higher expression of CD40L, OX40, HLA‐DR, and ICOS (P < 0·05), while CD40L and ICOS were significantly increased up to 2·5‐fold by lenalidomide treatment of healthy donor CD8+ T cells compared to DMSO (Fig 1B).
Figure 1.
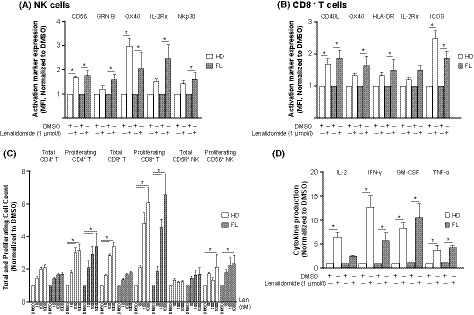
Lenalidomide enhances activation of T and NK cells in PBMC from healthy donors and FL patients. CD3‐stimulated peripheral blood mononuclear cells (PBMC) from healthy donors (HD) (n = 3–4) and follicular lymphoma (FL) patients (n = 3–4) were treated with dimethyl sulfoxide (DMSO) or lenalidomide (1 μmol/l) for 5 days and analysed by flow cytometry. (A‐B) Expression of activation markers as mean fluorescent intensity (MFI) on (A) Natural Killer (NK) cells and (B) CD8+ T cells. (C) Total and proliferating [carboxyfluorescein succinimidyl ester (CFSE)dim] CD4+ T, CD8+ T and CD56+ NK cell counts. (D) Supernatants from stimulated/treated PBMC were collected after 72 h and cytokine production was analysed by Luminex. All data are normalized to DMSO and presented as mean ± standard error of the mean (SEM). *P < 0·05 by two‐way anova.
Lenalidomide treatment led to 2·3‐ to 6·6‐fold increases in absolute counts of proliferating (CellTracedim) CD4+ T cells, CD8+ T cells and NK cells in PBMC from FL patients and 2·1‐ to 6·1‐fold increases in these populations in healthy donor PBMC (P < 0·05) compared to their respective DMSO‐treated controls (Fig 1C). Lenalidomide treatment of healthy donor PBMC resulted in a 3·4‐fold increase in total CD8+ T cell counts but had no effect on total CD8+ T cell counts in PBMC from FL patients (Fig 1C). Total CD4+ T and NK cell counts in both FL and healthy donor PBMC were unaffected by lenalidomide. Notably, a comparison of absolute cell counts in DMSO‐treated negative controls revealed differences between PBMC from FL patients and those from healthy donors. In control FL PBMC, absolute counts of proliferating CD4+ T cells and CD8+ T cells (0·12 ± 0·06 × 109/l and 0·03 ± 0·01 × 109/l, respectively) were lower compared with absolute counts of proliferating CD4+ T cells and CD8+ T cells (0·24 ± 0·05 × 109/l and 0·11 ± 0·03 × 109/l, respectively) in DMSO‐treated healthy donor PBMC (data not shown). This observation is consistent with a deficit in T cell proliferative capacity in PBMC from FL patients. Although lenalidomide treatment did not bring the absolute cell counts in FL PBMC to levels detected in healthy donor PBMC, the magnitude (fold) of the lenalidomide‐mediated stimulation, relative to DMSO controls, was comparable in both sample groups. Thus, lenalidomide was able to restore proliferative capacity despite the FL immune deficit.
We next examined the effect of lenalidomide on ex vivo cytokine release by CD3‐stimulated PBMC from FL patients and healthy donors at 72 h. Lenalidomide significantly increased interferon‐γ (IFN‐γ), granulocyte‐macrophage colony‐stimulating factor (GM‐CSF) and tumour necrosis factor‐α (TNF‐α) production up to 11‐fold in FL patient cells, relative to DMSO‐treated controls. In healthy donor PBMC, IL2, IFN‐γ, GM‐CSF and TNF‐α production were increased up to 13‐fold by lenalidomide (Fig 1D). Absolute levels of released cytokines were 3 to 5 times lower in DMSO‐treated FL PBMC (IFN‐γ, 33 ± 18; GM‐CSF, 25 ± 17; and TNF‐α, 97 ± 52 pg/ml) compared to DMSO‐treated healthy donor PBMC (IFN‐γ, 98 ± 35; GM‐CSF, 75 ± 19; and TNF‐α, 480 ± 29 pg/ml) (data not shown). These data provide additional evidence that, in the absence of lenalidomide, immune cells from FL patients had suppressed effector responses upon cross‐linking with CD3 compared to healthy donor cells. Taken together, these results show that ex vivo lenalidomide treatment can augment the expression of critical co‐stimulatory receptors on T and NK cells, increase their proliferative capacity and enhance the secretion of T‐helper cell type 1 (Th1) cytokines. Importantly, the immunostimulatory activity of lenalidomide was able to overcome defective effector responses in immune cells from FL patients.
R2 immunotherapy enhanced numbers of circulating T and NK cells in FL patients in comparison with R‐CHOP therapy
As part of correlative biomarker studies associated with the RELEVANCE trial (Morschhauser et al, 2018), changes in T and NK cells in the peripheral blood of 193 FL patients receiving treatment with either R2 (n = 101) or R‐CHOP (n = 92) were examined. Blood samples were collected at screening and at the end of induction therapy (week 24). T cell (CD3/CD4/CD8) and NK cell (CD56/CD16) populations were enumerated by flow cytometry (Figs 2 and 3). In patients receiving R‐CHOP, median CD4+ T cell counts declined from baseline to week 24 (from 0·48 × 109/l to 0·43 × 109/l, respectively), whereas CD4+ T cell counts were significantly increased (P < 0·05) in the R2 arm (from 0·51 × 109/l to 0·79 × 109/l) (Fig 2A, B). CD8+ T cell counts showed a slight increase from baseline to week 24 with R2, although the difference was not significant (Fig 2C). CD8+ T cell counts were unaltered following R‐CHOP (Fig 2C, D). In contrast, NK cell counts in the R‐CHOP group decreased significantly (P < 0·05) following therapy (from 0·18 × 109/l to 0·12 × 109/l), whereas no significant change was observed with R2 treatment (Fig 3A, B).
Figure 2.
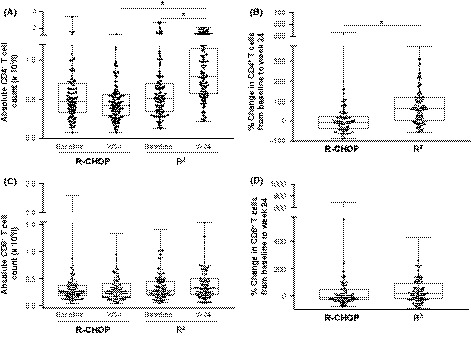
Elevation of CD4+ T cell counts in peripheral blood of FL patients receiving R2 compared to R‐CHOP. CD4+ T and CD8+ T cells were enumerated in peripheral blood mononuclear cells (PBMC) from 193 patients with follicular lymphoma (FL) at baseline and after 24 weeks of rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone (R‐CHOP; n = 92) or rituximab and lenalidomide (R2; n = 101) therapy, using flow cytometry. (A) Absolute CD4+ T cell counts at baseline and 24 weeks. (B) Percentage change in CD4+ T cell counts from baseline to week 24 (W24) of R‐CHOP or R2 therapy. (C) Absolute CD8+ T cell counts at baseline and 24 weeks. (D) Percentage change in CD8+ T cell counts from baseline to week 24 of R‐CHOP or R2 therapy. Data are presented as median with interquartile range. *P < 0·05 by one‐way anova (for A and C); *P < 0·05 by Unpaired t‐test (for B and D).
Figure 3.
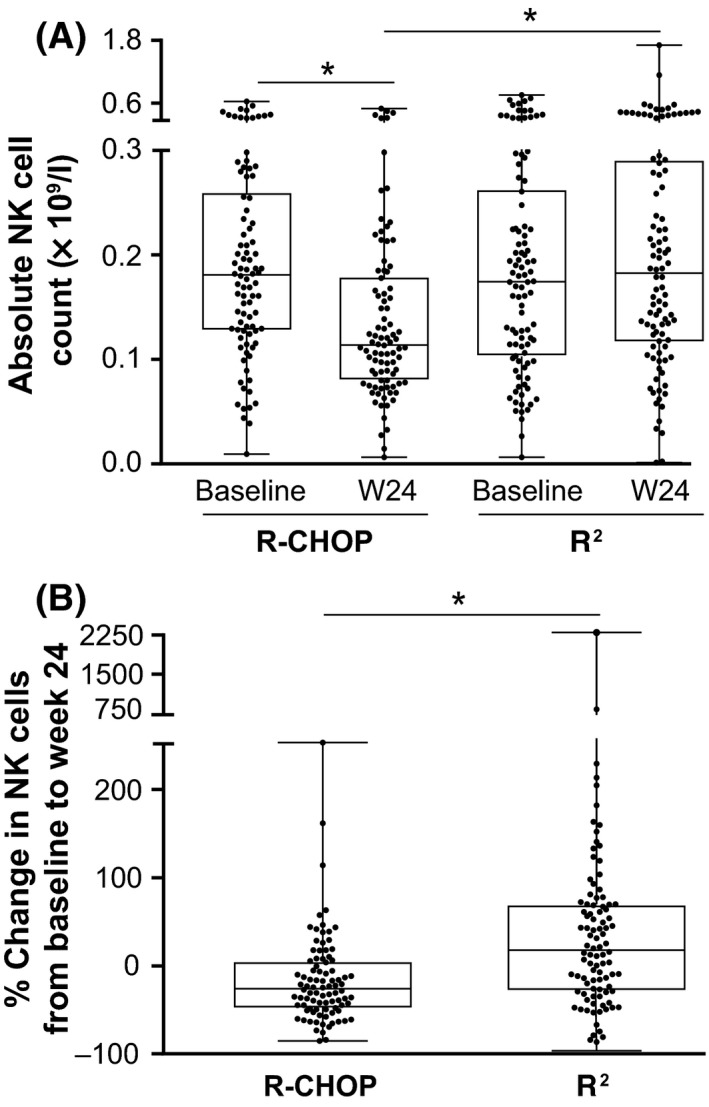
Reduction of NK cell counts in peripheral blood of FL patients receiving R‐CHOP compared to R2. CD56+ Natural Killer (NK) cells were enumerated in peripheral blood mononuclear cells from 188 patients with follicular lymphoma (FL) at baseline and after 24 weeks of rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone (R‐CHOP; n = 90) or rituximab and lenalidomide (R2; n = 98) therapy, using flow cytometry. (A) Absolute NK cell counts at baseline and 24 weeks (W24). Data are presented as median with interquartile range. *P < 0·05 by one‐way anova. (B) Percentage change in NK cell counts from baseline to week 24 of R‐CHOP or R2 therapy. Data are presented as median with interquartile range. *P < 0·05 by Unpaired t‐test.
Lenalidomide plus rituximab restored the ability of NK and T cells from FL patients to form functional immune synapses with autologous FL B cells
A key mechanism of immune dysfunction identified in tumour‐infiltrating CD4+ or CD8+ T lymphocytes from lymph node biopsies of FL patients is their inability to mobilize F‐actin to the immune synapse with autologous FL B cells (Ramsay et al, 2009). Here, we investigated formation of the F‐actin lytic NK cell immune synapse using peripheral blood‐derived NK cells and autologous circulating tumour B cells from patients presenting with leukaemic‐phase FL. Using confocal microscopy with image analysis, we showed that treatment with rituximab, lenalidomide or their combination resulted in significant increases in F‐actin polymerization (Fig 4A) and increased expression of granzyme B at NK cell:tumour cell immune synapses (Fig 4B). The combination was superior in enhancing NK cell immunological synapse formation against FL B cells compared with single agents (Fig 4A, B). We also asked whether enhanced immune synapse formation correlated with NK cell killing function. Cytotoxicity assays revealed that treating FL B cells with rituximab alone (ADCC) or treating both NK and FL B cells with lenalidomide induced comparable significant increases in NK cell‐mediated tumour cell killing compared to DMSO (P < 0·05). Rituximab‐treated cells significantly induced B cell death by 10% compared to DMSO‐treated cells at 4% (P < 0·05). Single‐agent lenalidomide significantly increased tumour cell death to 11% (P < 0·05). However, the combination demonstrated superior induction of NK lytic killing activity compared to either drug alone and increased tumour cell death to 18% (Fig 4C).
Figure 4.
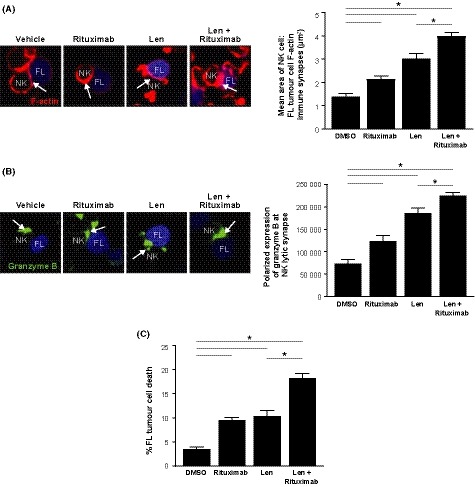
Combination lenalidomide‐rituximab repairs NK cell immune synapse dysfunction and enhances NK cytolytic activity in autologous tumour cells from FL patients. (A–B) Treated Natural Killer (NK) cells (interleukin 2 treatment with or without lenalidomide (Len, 1 μmol/l), were conjugated with autologous labelled follicular lymphoma (FL)B cells (blue) treated with rituximab (1 μg/ml), Len (1 μmol/l), or the combination. Confocal microscopy images and quantitative analysis of (A) NK cell F‐actin immune synapse formation (F‐actin, red) interactions with autologous tumour FL cells (blue) and (B) expression of granzyme B (green) from representative patient and drug treatment groups. Quantification of the total area (μm2) of F‐actin polymerization or the sum intensity of granzyme B at NK cell contact sites and synapses with FL B cells (arrows) is shown. (C) NK cell‐mediated killing of FL B cells in Len, rituximab or Len + rituximab treated cells. Data are presented as mean ± standard error of the mean (n = 6). *P < 0·05 by one‐way anova.
Immune synapse formation between lenalidomide treated CD4+ or CD8+ T cells from lymph node biopsies and autologous tumour cells showed significantly enhanced F‐actin immune synapse formation with FL tumour cells compared to DMSO‐treated cells (Fig 5A, B), consistent with previous findings (Ramsay et al, 2009). However, treatment of FL tumour cells with rituximab did not improve the ability of untreated FL T cells to form immune synapses, and the combination had no impact on lenalidomide's effect on F‐actin polymerization. Enhanced immune synapse formation was associated with increased recruitment of tyrosine‐phosphorylated proteins (P‐Tyr) in CD4+ T cells and an increased polarized expression of granzyme B to CD8+ T cell:FL B cell conjugates (Fig 5A, B lower images). Cytotoxicity assays demonstrated that lenalidomide treatment alone or in combination with rituximab enhanced cytotoxic T cell activity against primary tumour B cells compared to rituximab alone or DMSO controls (Fig 5C).
Figure 5.
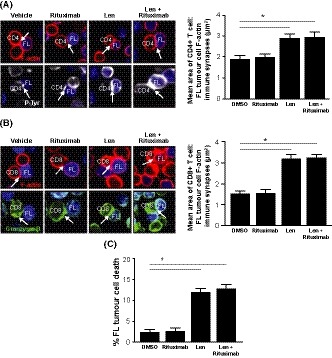
Combination lenalidomide‐rituximab repairs T cell immune synapse dysfunction and enhances CD8+ T cytolytic activity in autologous tumour cells from FL patients. (A–B) CD4+ and CD8+ T cells treated with dimethyl sulfoxide (DMSO; vehicle) or lenalidomide (Len, 1 μmol/l) were conjugated with autologous labelled follicular lymphoma (FL)B cells (blue) pulsed with superantigen (sAg) cocktail and treated with rituximab (1 μg/ml), lenalidomide (1 μmol/l), or the combination. Confocal images and quantitative analysis of (A) CD4+ T cell F‐actin immune synapse formation (F‐actin, red or tyrosine‐phosphorylated proteins, P‐Tyr, white) interactions, and (B) CD8+ T cell F‐actin immune synapse formation (F‐actin, red) or granzyme B (green) interactions from representative patient and drug treatment groups. Quantitative image analysis measured the total area (μm2) of F‐actin polymerization at CD4+ or CD8+ T cell contact sites and synapses with FL B cells. (C) FL patient CD8+ T‐cell killing against autologous tumour B cells (+sAg) as target cells following the drug treatments. Data are presented as mean ± SEM (n = 6). *P < 0·05 by one‐way anova.
Lenalidomide in combination with rituximab led to enhanced ADCC against parental and chemo‐resistant FL cell lines
Previous publications have shown that lenalidomide activates healthy donor NK cells, leading to greater ADCC against chronic lymphocytic leukaemia and mantle cell lymphoma (MCL) cells (Wu et al, 2008; Zhang et al, 2009). We investigated whether treatment of NK cells from FL patients with lenalidomide would improve ADCC of rituximab‐treated or untreated FL cell lines, DOHH2 and RL (Fig 6). In these experiments, we also determined whether treatment of FL NK cells with lenalidomide would potentiate immune‐mediated killing of chemo‐resistant DOHH2 and RL cells. Both lenalidomide and rituximab as single agents enhanced ADCC, reducing counts of viable parental DOHH2 cells by 58% and 44%, respectively, and the combination further enhanced ADCC to 69% compared to DMSO‐treated controls (Fig 6A). The effects of lenalidomide and rituximab as single agents on bendamustine‐, doxorubicin‐ or 4‐HC‐resistant DOHH2 cells showed significantly enhanced ADCC over DMSO (P < 0·05), which was greatest with combination immunotherapy (Fig 6A–C). The effect of treatment on ADCC was similar when RL cells were used as target FL cells. Lenalidomide treatment of NK cells from FL patients significantly (P < 0·05) enhanced the ADCC of parental RL cells to 31%, while rituximab treatment of the target cells led to 25% decline in viable RL cells; the combination increased ADCC to 57% significantly (P < 0·05) compared to DMSO (Fig 6A). Lenalidomide and rituximab as single agents significantly induced immune‐mediated cytotoxicity in all chemo‐resistant RL cells (P < 0·05), and the combination of lenalidomide and rituximab further enhanced ADCC against all resistant RL cells (Fig 6A–C). Taken together, these data demonstrated comparable ADCC activity against both parental and chemo‐resistant target lymphoma B cells, thus providing supporting evidence for the clinical activity of lenalidomide plus rituximab in the setting of chemo‐resistant FL.
Figure 6.

Lenalidomide enhances the effect of rituximab on NK‐mediated ADCC in parental and chemotherapeutic drug‐resistant FL cell lines via a cereblon‐dependent manner. Antibody‐dependent cellular cytotoxicity (ADCC) was mediated by Natural Killer (NK) cells from healthy donors that were cultured in conditioned medium from CD3‐stimulated peripheral blood mononuclear cells treated with dimethyl sulfoxide (DMSO) or lenalidomide, prior to co‐culture with parental and drug resistant follicular lymphoma cell lines (DOHH2 and RL) treated with DMSO or rituximab. Apoptosis was analysed by Annexin V and TO‐PRO‐3 staining. Data are shown as percent viable cells (normalized to DMSO) in (A) parental and bendamustine‐resistant; (B) parental and doxorubicin‐resistant; and (C) parental and 4 hydroperoxycyclophosphamide (HC)‐resistant target cells. Data from 8 to 9 independent experiments are presented as mean ± SEM *P < 0·05 by Student's t‐test. (D) Parental NK‐92 and NK‐92 CRISPR cereblon (CRBN)−/− cells were treated with lenalidomide (100 and 1000 nmol/l) for 12 h and co‐cultured with DMSO‐ or rituximab‐treated DOHH2 cells for 4 h. Percent viable DOHH2 cells (normalized to DMSO) are shown. Data are presented as mean ± SEM (n = 3–6) *P < 0·05 by one‐way anova.
We tested the functional requirement for cereblon in the ability of lenalidomide to enhance ADCC against lymphoma cells. As ADCC is predominantly mediated by NK cells, we generated NK‐92 CRISPR CRBN −/− cells lacking cereblon expression. As expected, lenalidomide treatment of parental NK‐92 cells led to increased ADCC killing of rituximab‐treated DOHH2 cells. However, this activity was significantly diminished when NK‐92 CRISPR CRBN −/− cells were used as effector cells (Fig 6D). These results demonstrate for the first time that lenalidomide acts directly on NK cells via a cereblon‐dependent mechanism to enhance rituximab‐mediated ADCC.
Lenalidomide is differentiated from chemotherapeutic agents
Current treatment options for FL encompass a range of therapeutic regimens, including bendamustine in combination with obinutuzumab or rituximab (first‐line therapy or second‐line and subsequent therapy) (Freedman, 2018). PBMC from healthy donors were treated with bendamustine, ibrutinib or lenalidomide, using drug concentrations comparable to doses used in the clinic, and then co‐cultured with rituximab‐treated DOHH2 and RL cells (Figure S1). Treatment of PBMC with bendamustine or ibrutinib prior to co‐culture caused significant reductions in viability (46% and 41%, respectively) compared to DMSO‐treated controls (Figure S1A). In contrast, lenalidomide had no impact on PBMC viability. In co‐culture experiments, lenalidomide in combination with rituximab significantly enhanced ADCC of DOHH2 and RL cells by 44% and 45%, respectively, compared to DMSO (Figure S1B, C). In contrast, the combination of rituximab with bendamustine or ibrutinib did not increase ADCC. Rather, these combinations showed an inverse dose response in which ADCC was decreased at the highest drug concentrations tested. These observations were probably partly due to the cytotoxic effects of bendamustine and ibrutinib on PBMC. In addition, ibrutinib has been shown to interfere with in vitro ADCC mediated by therapeutic anti‐CD20 antibodies, that is linked to BTK and ITK inhibition in NK cells (Da Roit et al, 2015; Jerkeman et al, 2017).
The combination of lenalidomide and rituximab showed enhanced activity in marginal zone lymphoma (MZL)
We evaluated lenalidomide and rituximab in the context of MZL, using the splenic B cell lymphoma with circulating villous lymphocytes (SLVL) cell line as a model. Single agent lenalidomide and rituximab stimulated PBMC‐mediated ADCC of SLVL cells by up to 18% and 31%, respectively, while the combination enhanced ADCC by up to 37% (Figure S2). Thus, lenalidomide, alone or in combination with rituximab, displayed anti‐tumour immunostimulatory activity in a model of MZL.
Lenalidomide caused a reversible block in neutrophil maturation, unlike the cytotoxic effects of a chemotherapeutic agent on neutrophil precursor cells
The effects of lenalidomide and bendamustine were compared on myeloid maturation using clonogenic assays, as well as an in vitro differentiation assay using bone marrow (BM) myeloid progenitor (CD34+) cells. Treatment of myeloid progenitor cells with bendamustine at a range of drug concentrations, dosed on 2 consecutive days for short durations (3 h) followed by washout, caused significant reductions (up to 98%) in granulocyte/macrophage colony‐forming units (CFU‐GM) (Fig 7A). In contrast, BM progenitor cells exposed to lenalidomide under the same conditions showed a reduction of only 27% in CFU‐GM at the highest dose. In vitro differentiation of haematopoietic stem cells was followed by flow cytometry with automatic gating according to CD33 and CD11b expression to identify 10 myeloid stages of neutrophil differentiation (Fig 7B). Mature neutrophils begin developing between myeloid stages 7 and 8. To determine the effects on neutrophil maturation, CD34+ cells were treated with bendamustine or lenalidomide for 14 days, followed by 7 days with no drug. Exposure to bendamustine caused a significant reduction in cell viability, such that cultures were terminated after 7 days of treatment and differentiation could not be measured. Exposure to lenalidomide blocked in vitro neutrophil differentiation; however, the block was reversible, and differentiation was restored after the 7‐day off‐drug period (Fig 7C). Taken together, our data showed that lenalidomide induced a block in neutrophil maturation, which was reversible upon drug washout, while having no cytotoxic effect on CFU‐GMs or mature neutrophils. In contrast, bendamustine was cytotoxic towards neutrophil progenitor cells.
Figure 7.
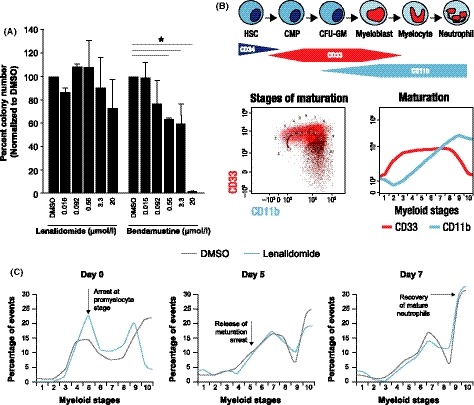
Lenalidomide induces a reversible myeloid maturation arrest while bendamustine induces an irreversible neutrophil cytotoxicity. (A) Bone marrow CD34+ cells from healthy donors were treated for 3 h exposure with dimethyl sulfoxide (DMSO), lenalidomide or bendamustine on 2 consecutive days, followed by washout. (B) Schematic diagram and representative flow cytometric gating for in vitro differentiation of haematopoietic stem cells to mature neutrophils. Myeloid stages (1–10) of neutrophil differentiation were automatically gated according to CD33 and CD11b expression. (C) Bone marrow CD34+ cells from healthy donors were cultured with stem cell factor, fms‐related tyrosine kinase 3, and granulocyte colony‐stimulating factor to promote in vitro myeloid maturation. Cultures were treated with DMSO (vehicle) or lenalidomide (20 μM) for 14 days, followed by a 7‐day washout. Data represent cells in myeloid stages 1–10 at 0, 5, and 7 days post‐drug washout. Cell differentiation and apoptosis were measured by flow cytometry. CFU‐GM, granulocyte/macrophage colony‐forming unit; CMP, common myeloid progenitor; HSC, haematopoietic stem cell.
Discussion
R2 has demonstrated clinical activity in FL in both front‐line and relapsed/refractory settings, achieving ORRs of up to 98% and ~77%, respectively (Fowler et al, 2014; Tuscano et al, 2014; Leonard et al, 2015; Martin et al, 2017b; Morschhauser et al, 2018). However, the molecular mechanisms underlying R2 clinical synergy in indolent NHL have not been fully characterized. In this study, we used primary patient samples to demonstrate that R2 immunotherapy enhances immune effector cell functions via complementary mechanisms of action, summarized in Fig 8.
Figure 8.
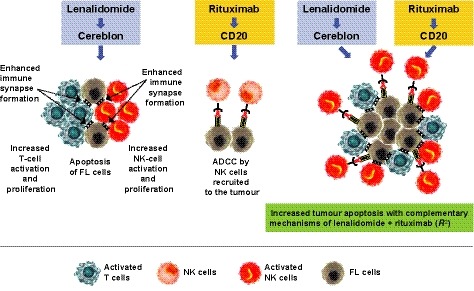
Complementary mechanisms of lenalidomide and rituximab induce immune‐mediated cytotoxicity in FL tumour cells. Lenalidomide single‐agent resulted in increased Natural Killer (NK)cell (orange) and T cell (blue) activation and proliferation and enhanced immune synapse formation, leading to apoptosis of follicular lymphoma (FL) cells (grey). Rituximab treatment of target FL cells led to NK cell–mediated antibody‐dependent cellular cytotoxicity (ADCC) of FL cells. Combining lenalidomide with rituximab resulted in enhancement of tumour apoptosis through complementary mechanisms.
FL is known to be a disease of immune dysfunction. Here, we show that lenalidomide single agent reactivates dysfunctional T and NK cells from FL patients, as indicated by elevated expression of co‐stimulatory receptors, enhanced proliferative capacity, and increased Th1 cytokine release. These results support recent findings from two clinical studies. One study noted early T‐cell activation in patients with relapsed FL or DLBCL in response to lenalidomide monotherapy (Menard et al, 2017), and another revealed lenalidomide‐mediated enhancement of NK cell proliferation in patients with MCL (Hagner et al, 2017). Results of our analysis of patient samples from the phase 3 RELEVANCE trial (Morschhauser et al, 2018) are consistent with those from a phase 2 trial of R2 in FL, where increased numbers of memory T cells, NK cells, and other immune cell populations were detected in peripheral blood of patients, starting from cycle 2 day 1 of therapy (Fowler et al, 2014; Morschhauser et al, 2018). In contrast, we found that R‐CHOP was associated with declines in both NK and CD4+ T cell counts in patients in the RELEVANCE study. We also demonstrated enhancement of immune‐mediated cytotoxicity against an MZL cell line by lenalidomide in combination with rituximab. These preclinical findings provide important insight for the ongoing phase 3 MAGNIFY trial of R2 in patients with relapsed/refractory indolent NHL, in which MZL patients have achieved ORR and confirmed/unconfirmed complete response (CR/CRu) rates of 66% and 44%, respectively (Coleman et al, 2017).
Using genetically knocked‐out cereblon NK cells, we show for the first time that lenalidomide directly activates NK cells via a cereblon‐dependent mechanism, leading to enhanced ADCC. Notably, lenalidomide demonstrated immunostimulatory effects on PBMC from both healthy donors and FL patients, despite evidence of profound functional defects in immune cells from FL patients. Our autologous immunological assays revealed that combination lenalidomide‐rituximab enhanced formation of NK cell lytic immune synapses with target FL cells compared to either drug alone. In contrast, the restoration of tumour‐infiltrating CD4+ and CD8+ T cell immune synapses with FL tumour B cells was predominantly mediated by lenalidomide treatment. Critically, our ex vivo functional data using FL‐derived primary cells demonstrated that the ability of R2 immunotherapy to reactivate NK cell and T cell immune synapses correlated with increased cytotoxic killing of autologous FL tumour cells. Notably, the combination was active against both parental and chemo‐resistant lymphoma B cell lines, suggesting that activation of immune effector cells is a major contributing mechanism of action for lenalidomide that is distinct from chemotherapy. These findings support early results of a phase 3 trial of R2 in relapsed/refractory FL, in which patients with double‐refractory FL have achieved 1‐year PFS rates of 66% and CR/CRu rates of 21% (Andorsky et al, 2017). Lenalidomide has also demonstrated immune stimulatory activity in combination with the type II anti‐CD20 antibody obinutuzumab, as recently reported by Morschhauser et al from their phase 1b (GALEN) study, where they are assessing the combination in patients with relapsed/refractory FL (Morschhauser et al, 2018; Vo et al, 2018). Their analysis showed that lenalidomide monotherapy prior to the first infusion of obinutuzumab resulted in reactivation of peripheral blood T cells (Menard et al, 2017). Moreover, lenalidomide in combination with obinutuzumab reversed an immature NK phenotype and induced activation of circulating NK cells (Vo et al, 2018). These findings are similar to the effects we observed when NK cells from FL patients were treated with combination lenalidomide‐rituximab ex vivo.
For FL patients, whose course of disease spans many years, the goal is to offer safer treatment options with a more tolerable safety profile compared to standard chemotherapy, which is often associated with immune suppression and lymphopenia (Garcia Munoz et al, 2014; Sarkozy et al, 2017; Olszewski et al, 2018). In addition to the effect lenalidomide has on lymphocytes, there are also effects on the myeloid lineage of cells. Using an in vitro model of myeloid differentiation, we show that lenalidomide induced a block in neutrophil maturation, which was reversible by drug wash‐out, with no loss of cell viability. Restoration of bone marrow precursor cell populations after treatment with a cytotoxic agent would require considerable time, consistent with clinical observations of impaired lymphocyte recovery in patients treated with R‐bendamustine or R‐CHOP (Garcia Munoz et al, 2014; Ito et al, 2016). Thus, while neutropenia remains one of the major toxicities associated with both chemotherapy and lenalidomide‐rituximab, our studies suggest the duration of neutrophil recovery is likely to be longer in chemotherapy‐treated patients in comparison with lenalidomide‐treated patients. Our preclinical findings are consistent with the safety analysis from the RELEVANCE study, which reported that a higher percentage of patients in the R‐chemotherapy group had grade 3 or 4 neutropenia, compared with the R2 group (50% vs. 32%, respectively) (Morschhauser et al, 2018).
In conclusion, we provide preclinical evidence that lenalidomide and rituximab have complementary mechanisms of action and this unique combination is characterized by immune enhancement, not immunosuppression, as has been observed with immunochemotherapy. This work, along with recent phase 3 clinical data, challenges the paradigm of conventional immunochemotherapy and supports the rationale for combination chemotherapy‐free immunotherapy for FL patients.
Author contributions
HC, CB, SC, EGT, DP, and ABF performed the research. HC, PT, CB, SC, EGT, CG, AGR, PRH, ABF, KT, MHDL, FM, and AKG designed research and interpreted results. PT, SC, EGT, AGR, and AKG assisted with manuscript preparation.
Conflict of Interest
HC, PT, CB, SC, EGT, CG, PRH, and AKG are employed by and have equity ownership in Celgene Corporation. AGR and KT receive research funding from Celgene. FM receives advisory board and lecture fees from Celgene. DP, ABF, and MHDL report no conflict of interest.
Sources of support
Research funding was provided by Celgene Corporation, Summit, NJ and San Francisco, CA.
Supporting information
Data S1. Materials and Methods.
Figure S1. Chemotherapeutic and novel agents reduce PBMC viability and do not enhance rituximab‐mediated antibody‐dependent cellular cytotoxicity.
Figure S2. Combination lenalidomide‐rituximab enhances antibody‐dependent cellular cytotoxicity in the MZL cell line SLVL.
Acknowledgements
The authors thank the Nikon Imaging Facility at King’s College London for use of the Point Scanning Confocal A1R microscope, and all facility staff (Daniel Matthews, Isma Ali, Benjamin Robinson and Daniel Metcalf) who provided support. Research funding was provided by Celgene Corporation, Summit, NJ and San Francisco, CA. Medical writing assistance was provided by Dorothy Fallows and Bio Connections, LLC, and funded by Celgene Corporation.
References
- Andorsky, D.J. , Yacoub, A. , Melear, J.M. , Coleman, M. , Kolibaba, K. , Brooks, H.D. , Bitran, J.D. , Fanning, S.R. , Lansigan, F. , Ricker, J.L. , Foon, K.A. , Liu, D. , Llorente, M. , Li, J. & Sharman, J.P. (2017) Phase IIIb randomized study of lenalidomide plus rituximab (R2) followed by maintenance in relapsed/refractory NHL: analysis of patients with double‐refractory or early relapsed follicular lymphoma (FL). Journal of Clinical Oncology, 35(Suppl 15), 7502 (abstract 7502). [Google Scholar]
- Andorsky, D.J. , Coleman, M. , Yacoub, A. , Melear, J.M. , Brooks, H.D. , Fanning, S.R. , Kolibaba, K. , Lansigan, F. , Reynolds, C. , Li, J. , Liu, D. , Llorente, M. , Ricker, J.L. & Sharman, J.P. (2018) Response rate to lenalidomide plus rituximab (R2) as independent of number of prior lines of therapy: interim analysis of initial phase of MAGNIFY phase IIIb study of R2 followed by maintenance in relapsed/refractory indolent NHL. Journal of Clinical Oncology, 36(Suppl 15), 715–716 (abstract 7516). [Google Scholar]
- Chamberlain, P.P. , Lopez‐Girona, A. , Miller, K. , Carmel, G. , Pagarigan, B. , Chie‐Leon, B. , Rychak, E. , Corral, L.G. , Ren, Y.J. , Wang, M. , Riley, M. , Delker, S.L. , Ito, T. , Ando, H. , Mori, T. , Hirano, Y. , Handa, H. , Hakoshima, T. , Daniel, T.O. & Cathers, B.E. (2014) Structure of the human Cereblon‐DDB1‐lenalidomide complex reveals basis for responsiveness to thalidomide analogs. Nature Structural & Molecular Biology, 21, 803–809. [DOI] [PubMed] [Google Scholar]
- Coleman, M. , Andorsky, D.J. , Yacoub, A. , Melear, J.M. , Kolibaba, K. , Brooks, H.D. , Bitran, J.D. , Fanning, S.R. , Lansigan, F. , Ricker, J.L. , Foon, K.A. , Llorente, M. , Li, J. & Sharman, J.P. (2017) Phase IIIB study of lenalidomide plus rituximab followed by maintenance in relapsed or refractory NHL: analysis of marginal zone lymphoma. Hematological Oncology, 35(Suppl S2), 148 (abstract 139). [Google Scholar]
- Da Roit, F. , Engelberts, P.J. , Taylor, R.P. , Breij, E.C. , Gritti, G. , Rambaldi, A. , Introna, M. , Parren, P.W. , Beurskens, F.J. & Golay, J. (2015) Ibrutinib interferes with the cell‐mediated anti‐tumor activities of therapeutic CD20 antibodies: implications for combination therapy. Haematologica, 100, 77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dave, S.S. , Wright, G. , Tan, B. , Rosenwald, A. , Gascoyne, R.D. , Chan, W.C. , Fisher, R.I. , Braziel, R.M. , Rimsza, L.M. , Grogan, T.M. , Miller, T.P. , LeBlanc, M. , Greiner, T.C. , Weisenburger, D.D. , Lynch, J.C. , Vose, J. , Armitage, J.O. , Smeland, E.B. , Kvaloy, S. , Holte, H. , Delabie, J. , Connors, J.M. , Lansdorp, P.M. , Ouyang, Q. , Lister, T.A. , Davies, A.J. , Norton, A.J. , Muller‐Hermelink, H.K. , Ott, G. , Campo, E. , Montserrat, E. , Wilson, W.H. , Jaffe, E.S. , Simon, R. , Yang, L. , Powell, J. , Zhao, H. , Goldschmidt, N. , Chiorazzi, M. & Staudt, L.M. (2004) Prediction of survival in follicular lymphoma based on molecular features of tumor‐infiltrating immune cells. New England Journal of Medicine, 351, 2159–2169. [DOI] [PubMed] [Google Scholar]
- Decaudin, D. , Des Guetz, G. , Mathiot, C. , Dumont, J. , Hubert, P. , Vincent‐Salomon, A. & Pouillart, P. (2003) Absolute lymphocyte count as a predictive factor for response to monoclonal anti‐CD20 antibody therapy. Annals of Oncology, 14, 171–172. [DOI] [PubMed] [Google Scholar]
- Flinn, I.W. , van der Jagt, R. , Kahl, B.S. , Wood, P. , Hawkins, T.E. , Macdonald, D. , Hertzberg, M. , Kwan, Y.L. , Simpson, D. , Craig, M. , Kolibaba, K. , Issa, S. , Clementi, R. , Hallman, D.M. , Munteanu, M. , Chen, L. & Burke, J.M. (2014) Randomized trial of bendamustine‐rituximab or R‐CHOP/R‐CVP in first‐line treatment of indolent NHL or MCL: the BRIGHT study. Blood, 123, 2944–2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler, N.H. , Davis, R.E. , Rawal, S. , Nastoupil, L. , Hagemeister, F.B. , McLaughlin, P. , Kwak, L.W. , Romaguera, J.E. , Fanale, M.A. , Fayad, L.E. , Westin, J.R. , Shah, J. , Orlowski, R.Z. , Wang, M. , Turturro, F. , Oki, Y. , Claret, L.C. , Feng, L. , Baladandayuthapani, V. , Muzzafar, T. , Tsai, K.Y. , Samaniego, F. & Neelapu, S.S. (2014) Safety and activity of lenalidomide and rituximab in untreated indolent lymphoma: an open‐label, phase 2 trial. The Lancet. Oncology, 15, 1311–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman, A. (2018) Follicular lymphoma: 2018 update on diagnosis and management. American Journal of Hematology, 93, 296–305. [DOI] [PubMed] [Google Scholar]
- Gandhi, A.K. , Kang, J. , Havens, C.G. , Conklin, T. , Ning, Y. , Wu, L. , Ito, T. , Ando, H. , Waldman, M.F. , Thakurta, A. , Klippel, A. , Handa, H. , Daniel, T.O. , Schafer, P.H. & Chopra, R. (2014) Immunomodulatory agents lenalidomide and pomalidomide co‐stimulate T cells by inducing degradation of T cell repressors Ikaros and Aiolos via modulation of the E3 ubiquitin ligase complex CRL4(CRBN.). British Journal of Haematology, 164, 811–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia Munoz, R. , Izquierdo‐Gil, A. , Munoz, A. , Roldan‐Galiacho, V. , Rabasa, P. & Panizo, C. (2014) Lymphocyte recovery is impaired in patients with chronic lymphocytic leukemia and indolent non‐Hodgkin lymphomas treated with bendamustine plus rituximab. Annals of Hematology, 93, 1879–1887. [DOI] [PubMed] [Google Scholar]
- Gravelle, P. , Do, C. , Franchet, C. , Mueller, S. , Oberic, L. , Ysebaert, L. , Larocca, L.M. , Hohaus, S. , Calmels, M.N. , Frenois, F.X. , Kridel, R. , Gascoyne, R.D. , Laurent, G. , Brousset, P. , Valitutti, S. & Laurent, C. (2016) Impaired functional responses in follicular lymphoma CD8+TIM‐3+ T lymphocytes following TCR engagement. Oncoimmunology, 5, e1224044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagner, P.R. , Man, H.W. , Fontanillo, C. , Wang, M. , Couto, S. , Breider, M. , Bjorklund, C. , Havens, C.G. , Lu, G. , Rychak, E. , Raymon, H. , Narla, R.K. , Barnes, L. , Khambatta, G. , Chiu, H. , Kosek, J. , Kang, J. , Amantangelo, M.D. , Waldman, M. , Lopez‐Girona, A. , Cai, T. , Pourdehnad, M. , Trotter, M. , Daniel, T.O. , Schafer, P.H. , Klippel, A. , Thakurta, A. , Chopra, R. & Gandhi, A.K. (2015) CC‐122, a pleiotropic pathway modifier, mimics an interferon response and has antitumor activity in DLBCL. Blood, 126, 779–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagner, P.R. , Chiu, H. , Ortiz, M. , Apollonio, B. , Wang, M. , Couto, S. , Waldman, M.F. , Flynt, E. , Ramsay, A.G. , Trotter, M. , Gandhi, A.K. , Chopra, R. & Thakurta, A. (2017) Activity of lenalidomide in mantle cell lymphoma can be explained by NK cell‐mediated cytotoxicity. British Journal of Haematology, 179, 399–409. [DOI] [PubMed] [Google Scholar]
- Ito, K. , Okamoto, M. , Inaguma, Y. , Okamoto, A. , Ando, M. , Ando, Y. , Tsuge, M. , Tomono, A. , Kakumae, Y. , Hayashi, T. , Yamada, S. & Emi, N. (2016) Influence of R‐CHOP therapy on immune system restoration in patients with B‐cell lymphoma. Oncology, 91, 302–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerkeman, M. , Ek, S. , Freiburghaus, C. & Lindblad, A.A. (2017) Ibrutinib in combination with anti‐CD20‐antibody negatively affects antibody dependent cellular cytotoxic (ADCC) on mantle cell lymphoma cell lines, not reversed by the addition of lenalidomide. Blood, 130(Suppl 1), 1266. [Google Scholar]
- Lagrue, K. , Carisey, A. , Morgan, D.J. , Chopra, R. & Davis, D.M. (2015) Lenalidomide augments actin remodeling and lowers NK‐cell activation thresholds. Blood, 126, 50–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard, J.P. , Jung, S.H. , Johnson, J. , Pitcher, B.N. , Bartlett, N.L. , Blum, K.A. , Czuczman, M. , Giguere, J.K. & Cheson, B.D. (2015) Randomized trial of lenalidomide alone versus lenalidomide plus rituximab in patients with recurrent follicular lymphoma: CALGB 50401 (Alliance). Journal of Clinical Oncology, 33, 3635–3640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez‐Girona, A. , Mendy, D. , Ito, T. , Miller, K. , Gandhi, A.K. , Kang, J. , Karasawa, S. , Carmel, G. , Jackson, P. , Abbasian, M. , Mahmoudi, A. , Cathers, B. , Rychak, E. , Gaidarova, S. , Chen, R. , Schafer, P.H. , Handa, H. , Daniel, T.O. , Evans, J.F. & Chopra, R. (2012) Cereblon is a direct protein target for immunomodulatory and antiproliferative activities of lenalidomide and pomalidomide. Leukemia, 26, 2326–2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luminari, S. , Goldaniga, M. , Cesaretti, M. , Orsucci, L. , Tucci, A. , Pulsoni, A. , Salvi, F. , Arcaini, L. , Carella, A.M. , Tedeschi, A. , Pinto, A. , Stelitano, C. & Baldini, L. (2016) A phase II study of bendamustine in combination with rituximab as initial treatment for patients with indolent non‐follicular non‐Hodgkin lymphoma. Leukaemia & Lymphoma, 57, 880–887. [DOI] [PubMed] [Google Scholar]
- Martin, P. , Chen, Z. , Cheson, B.D. , Robinson, K.S. , Williams, M. , Rajguru, S.A. , Friedberg, J.W. , van der Jagt, R.H. , LaCasce, A.S. , Joyce, R. , Ganjoo, K.N. , Bartlett, N.L. , Lemieux, B. , VanderWalde, A. , Herst, J. , Szer, J. , Bar, M.H. , Cabanillas, F. , Dodds, A.J. , Montgomery, P.G. , Pressnail, B. , Ellis, T. , Smith, M.R. & Leonard, J.P. (2017a) Long‐term outcomes, secondary malignancies and stem cell collection following bendamustine in patients with previously treated non‐Hodgkin lymphoma. British Journal of Haematology, 178, 250–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin, P. , Jung, S.H. , Pitcher, B. , Bartlett, N.L. , Blum, K.A. , Shea, T. , Hsi, E.D. , Ruan, J. , Smith, S.E. , Leonard, J.P. & Cheson, B.D. (2017b) A phase II trial of lenalidomide plus rituximab in previously untreated follicular non‐Hodgkin's lymphoma (NHL): CALGB 50803 (Alliance). Annals of Oncology, 28, 2806–2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menard, C. , Dulong, J. , Nguyen, T.T. , Bescher, N. , Latour, M. , Bézier, I. , Loisel, S. , Houot, R. , Lamy, T. , Morschhauser, F. & Tarte, K. (2017) Lenalidomide treatment restores in vivo T cell activity in relapsed/refractory FL and DLBCL. Blood, 130, 729. [Google Scholar]
- Morschhauser, F. , Fowler, N.H. , Feugier, P. , Bouabdallah, R. , Tilly, H. , Palomba, M.L. , Fruchart, C. , Libby, E.N. , Casasnovas, R.O. , Flinn, I.W. , Haioun, C. , Maisonneuve, H. , Ysebaert, L. , Bartlett, N.L. , Bouabdallah, K. , Brice, P. , Ribrag, V. , Daguindau, N. , Le Gouill, S. , Pica, G.M. , Martin Garcia‐Sancho, A. , Lopez‐Guillermo, A. , Larouche, J.F. , Ando, K. , Gomes da Silva, M. , Andre, M. , Zachee, P. , Sehn, L.H. , Tobinai, K. , Cartron, G. , Liu, D. , Wang, J. , Xerri, L. , Salles, G.A. & Investigators, R.T. (2018) Rituximab plus lenalidomide in advanced untreated follicular lymphoma. New England Journal of Medicine, 379, 934–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olszewski, A.J. , Reagan, J.L. & Castillo, J.J. (2018) Late infections and secondary malignancies after bendamustine/rituximab or RCHOP/RCVP chemotherapy for B‐cell lymphomas. American Journal of Hematology, 93, E1–E3. [DOI] [PubMed] [Google Scholar]
- Pastore, A. , Jurinovic, V. , Kridel, R. , Hoster, E. , Staiger, A.M. , Szczepanowski, M. , Pott, C. , Kopp, N. , Murakami, M. , Horn, H. , Leich, E. , Moccia, A.A. , Mottok, A. , Sunkavalli, A. , Van Hummelen, P. , Ducar, M. , Ennishi, D. , Shulha, H.P. , Hother, C. , Connors, J.M. , Sehn, L.H. , Dreyling, M. , Neuberg, D. , Moller, P. , Feller, A.C. , Hansmann, M.L. , Stein, H. , Rosenwald, A. , Ott, G. , Klapper, W. , Unterhalt, M. , Hiddemann, W. , Gascoyne, R.D. , Weinstock, D.M. & Weigert, O. (2015) Integration of gene mutations in risk prognostication for patients receiving first‐line immunochemotherapy for follicular lymphoma: a retrospective analysis of a prospective clinical trial and validation in a population‐based registry. The Lancet. Oncology, 16, 1111–1122. [DOI] [PubMed] [Google Scholar]
- Perry, A.M. , Diebold, J. , Nathwani, B.N. , MacLennan, K.A. , Muller‐Hermelink, H.K. , Bast, M. , Boilesen, E. , Armitage, J.O. & Weisenburger, D.D. (2016) Non‐Hodgkin lymphoma in the developing world: review of 4539 cases from the International Non‐Hodgkin Lymphoma Classification Project. Haematologica, 101, 1244–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plonquet, A. , Haioun, C. , Jais, J.P. , Debard, A.L. , Salles, G. , Bene, M.C. , Feugier, P. , Rabian, C. , Casasnovas, O. , Labalette, M. , Kuhlein, E. , Farcet, J.P. , Emile, J.F. , Gisselbrecht, C. & Delfau‐Larue, M.H. ; Groupe d’étude des lymphomes de l'adulte . (2007) Peripheral blood natural killer cell count is associated with clinical outcome in patients with aaIPI 2‐3 diffuse large B‐cell lymphoma. Annals of Oncology, 18, 1209–1215. [DOI] [PubMed] [Google Scholar]
- Ramsay, A.G. , Johnson, A.J. , Lee, A.M. , Gorgun, G. , Le Dieu, R. , Blum, W. , Byrd, J.C. & Gribben, J.G. (2008) Chronic lymphocytic leukemia T cells show impaired immunological synapse formation that can be reversed with an immunomodulating drug. The Journal of Clinical Investigation, 118, 2427–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsay, A.G. , Clear, A.J. , Kelly, G. , Fatah, R. , Matthews, J. , Macdougall, F. , Lister, T.A. , Lee, A.M. , Calaminici, M. & Gribben, J.G. (2009) Follicular lymphoma cells induce T‐cell immunologic synapse dysfunction that can be repaired with lenalidomide: implications for the tumor microenvironment and immunotherapy. Blood, 114, 4713–4720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivas‐Delgado, A. , Magnano, L. , Moreno‐Velazquez, M. , Garcia, O. , Mozas, P. , Dlouhy, I. , Baumann, T. , Rovira, J. , Gonzalez, B. , Martinez, A. , Balague, O. , Delgado, J. , Villamor, N. , Campo, E. , Gine, E. , Sancho, J.M. & Lopez‐Guillermo, A. (2017) Progression‐free survival shortens after each relapse in patients with follicular lymphoma treated in the rituximab era. Hematological Oncology, 35(Suppl S2), 360–361 (abstract 405). [Google Scholar]
- Rummel, M.J. , Niederle, N. , Maschmeyer, G. , Banat, G.A. , von Grunhagen, U. , Losem, C. , Kofahl‐Krause, D. , Heil, G. , Welslau, M. , Balser, C. , Kaiser, U. , Weidmann, E. , Durk, H. , Ballo, H. , Stauch, M. , Roller, F. , Barth, J. , Hoelzer, D. , Hinke, A. & Brugger, W. (2013) Bendamustine plus rituximab versus CHOP plus rituximab as first‐line treatment for patients with indolent and mantle‐cell lymphomas: an open‐label, multicentre, randomised, phase 3 non‐inferiority trial. Lancet, 381, 1203–1210. [DOI] [PubMed] [Google Scholar]
- Sacchi, S. , Marcheselli, R. , Bari, A. , Buda, G. , Molinari, A.L. , Baldini, L. , Vallisa, D. , Cesaretti, M. , Musto, P. , Ronconi, S. , Specchia, G. , Silvestris, F. , Guardigni, L. , Ferrari, A. , Chiapella, A. , Carella, A.M. , Santoro, A. , Di Raimondo, F. , Marcheselli, L. & Pozzi, S. (2016) Safety and efficacy of lenalidomide in combination with rituximab in recurrent indolent non‐follicular lymphoma: final results of a phase II study conducted by the Fondazione Italiana Linfomi. Haematologica, 101, e196–e199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito, H. , Maruyama, D. , Maeshima, A.M. , Makita, S. , Kitahara, H. , Miyamoto, K. , Fukuhara, S. , Munakata, W. , Suzuki, T. , Kobayashi, Y. , Taniguchi, H. & Tobinai, K. (2015) Prolonged lymphocytopenia after bendamustine therapy in patients with relapsed or refractory indolent B‐cell and mantle cell lymphoma. Blood Cancer Journal, 5, e362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salles, G.A. , Seymour, J.F. , Feugier, P. , Offner, F. , Lopez‐Guillermo, A. , Belada, D. , Xerri, L. , Bouabdallah, R. , Catalano, J. , Brice, P. , Haioun, C. , Martín, A. , Pedersen, L.M. , Delmer, A.J. , Simpson, D. , Leppa, S. , Soubeyran, P. , Casasnovas, R. , Intragumtornchai, T. , Ribrag, V. , Silva, M.G. , Nicolas‐Virelizier, E. , Lister, T. , Estell, J. , Milone, G. , Sonet, A. , Assemat, J. , Zeuner, H. , Coiffier, B. & Tilly, H. (2017) Long term follow‐up of the PRIMA Study: half of patients receiving rituximab maintenance remain progression free at 10 years. Blood, 130, 486. [Google Scholar]
- Sarkozy, C. , Link, B.K. , Ghesquieres, H. , Maurer, M. , Nicolas‐Virelizier, E. , Thompson, C. , Traverse‐Glehen, A. , Feldman, A. , Allmer, C. , Slager, S. , Ansell, S. , Habermann, T. , Bachy, E. , Cerhan, J. & Salles, G. (2017) Cause of death in follicular lymphoma in the rituximab era: a pooled analysis of French and US cohorts. Hematological Oncology, 35(Suppl S2), 34–35 (abstract 16). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafer, D. , Smith, M.R. , Borghaei, H. , Millenson, M.M. , Li, T. , Litwin, S. , Anad, R. & Al‐Saleem, T. (2013) Low NK cell counts in peripheral blood are associated with inferior overall survival in patients with follicular lymphoma. Leukemia Research, 37, 1213–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teras, L.R. , DeSantis, C.E. , Cerhan, J.R. , Morton, L.M. , Jemal, A. & Flowers, C.R. (2016) 2016 US lymphoid malignancy statistics by World Health Organization subtypes. CA: A Cancer Journal for Clinicians, 66, 443–459. [DOI] [PubMed] [Google Scholar]
- Tuscano, J.M. , Dutia, M. , Chee, K. , Brunson, A. , Reed‐Pease, C. , Abedi, M. , Welborn, J. & O'Donnell, R.T. (2014) Lenalidomide plus rituximab can produce durable clinical responses in patients with relapsed or refractory, indolent non‐Hodgkin lymphoma. British Journal of Haematology, 165, 375–381. [DOI] [PubMed] [Google Scholar]
- Vo, D.N. , Alexia, C. , Allende‐Vega, N. , Morschhauser, F. , Houot, R. , Menard, C. , Tarte, K. , Cartron, G. & Villalba, M. (2018) NK cell activation and recovery of NK cell subsets in lymphoma patients after obinutuzumab and lenalidomide treatment. Oncoimmunology, 7, e1409322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigert, O. & Weinstock, D.M. (2017) The promises and challenges of using gene mutations for patient stratification in follicular lymphoma. Blood, 130, 1491–1498. [DOI] [PubMed] [Google Scholar]
- Wu, L. , Adams, M. , Carter, T. , Chen, R. , Muller, G. , Stirling, D. , Schafer, P. & Bartlett, J.B. (2008) lenalidomide enhances natural killer cell and monocyte‐mediated antibody‐dependent cellular cytotoxicity of rituximab‐treated CD20+ tumor cells. Clinical Cancer Research, 14, 4650–4657. [DOI] [PubMed] [Google Scholar]
- Yang, Z.Z. & Ansell, S.M. (2012) The tumor microenvironment in follicular lymphoma. Clinical Advances in Hematology & Oncology: H&O, 10, 810–818. [PubMed] [Google Scholar]
- Zhang, L. , Qian, Z. , Cai, Z. , Sun, L. , Wang, H. , Bartlett, J.B. , Yi, Q. & Wang, M. (2009) Synergistic antitumor effects of lenalidomide and rituximab on mantle cell lymphoma in vitro and in vivo. American Journal of Hematology, 84, 553–559. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Materials and Methods.
Figure S1. Chemotherapeutic and novel agents reduce PBMC viability and do not enhance rituximab‐mediated antibody‐dependent cellular cytotoxicity.
Figure S2. Combination lenalidomide‐rituximab enhances antibody‐dependent cellular cytotoxicity in the MZL cell line SLVL.


