Abstract
Background
While asbestos has long been known to cause mesothelioma, quantitative exposure‐response data on the relation of mesothelioma risk and exposure to chrysotile asbestos are sparse.
Methods
Quantitative relationships of mortality from mesothelioma and pleural cancer were investigated in an established cohort of 5397 asbestos textile manufacturing workers in North Carolina, USA. Eligible workers were those employed between 1950 and 1973 with mortality follow‐up through 2003. Individual exposure to chrysotile fibres was estimated on the basis of 3420 air samples covering the entire study period linked to work history records. Exposure coefficients adjusted for age, race, and time‐related covariates were estimated by Poisson regression.
Results
Positive, statistically significant associations were observed between mortality from all pleural cancer (including mesothelioma) and time since first exposure (TSFE) to asbestos (rate ratio [RR], 1.19; 95% confidence interval [CI], 1.06‐1.34 per year), duration of exposure, and cumulative asbestos fibre exposure (RR, 1.15; 95% CI, 1.04‐1.28 per 100 f‐years/mL; 10‐year lag). Analyses of the shape of exposure‐response functions suggested a linear relationship with TSFE and a less‐than‐linear relationship with cumulative exposure. Restricting the analysis to years when mesothelioma was coded as a unique cause of death yielded stronger but less precise associations.
Conclusions
These observations support with quantitative data the conclusion that chrysotile causes mesothelioma and encourage exposure‐response analyses of mesothelioma in other cohorts exposed to chrysotile.
Keywords: asbestos, cancer, epidemiology, mesothelioma, risk assessment
1. INTRODUCTION
Asbestos has long been known to cause mesothelioma.1, 2, 3 Nevertheless, investigation of asbestos exposure‐response relationships for mesothelioma is hampered by the rarity of the disease, which constrains statistical power, and the long induction and latency period characteristic of the relationship, which requires a study design in which the asbestos exposure and ascertainment of mesothelioma cases are highly separated in time. Concerns about the quality of ascertainment of the outcome further hamper investigations of this association, due largely to the lack of specific codes for mesothelioma in the International Classification of Diseases (ICD) before the 10th Revision (ICD‐10) was introduced in 1999.
A further challenge to investigation of asbestos exposure‐mesothelioma relationships concerns differences between studies in characterization of the exposure. While all forms of asbestos have been determined to cause mesothelioma,2 there is evidence that the quantitative risk of mesothelioma varies with asbestos fibre type, with higher unit risks usually observed for exposure to commercial amphibole asbestos minerals (mainly amosite and crocidolite) than for exposure to chrysotile asbestos.2, 4 The magnitude of the risk of mesothelioma from exposure to chrysotile is particularly relevant because chrysotile comprises the vast majority of the asbestos produced worldwide and is currently the only form of asbestos that is legal for use in many countries. However, the small number of study populations exposed only to chrysotile that have both enough mesothelioma cases for analysis and individual quantitative exposure data further limits the ability to investigate exposure‐response relationships for mesothelioma and chrysotile.
The largest occupational cohort that has been studied for the effects of exposure to chrysotile includes asbestos miners and millers in Québec, Canada. The last follow‐up of this cohort though 1992 included over 10 000 workers and observed 38 deaths from mesothelioma5; a standardized mortality ratio (SMR) for mesothelioma was not reported due to lack of suitable reference rates, however. More recent studies of other occupational cohorts exposed to chrysotile have reported elevated SMRs based on smaller numbers of mesothelioma deaths: seven deaths from pleural cancer were observed among 1056 men used at a chrysotile mine in Balangero, Italy, with an SMR of 5.5 (95% CI, 2.2‐11.4),6 and three mesothelioma deaths among 865 asbestos textile workers in Chongqing, China gave an SMR of 45.5 (95% CI, 9.4‐132.8).7 Three mesothelioma deaths were also observed among 3072 asbestos textile workers in South Carolina, USA, but no SMR or exposure‐response analysis was reported for mesothelioma in that cohort.8
To investigate quantitative relationships between mesothelioma risk and exposure to chrysotile asbestos, we carried out further analyses of mortality and exposure data from an established cohort of asbestos textile workers in North Carolina; we previously reported SMRs of 10.9 (95% CI, 3.0‐28.0) and 12.4 (95% CI, 3.4‐31.8) for mesothelioma and cancer of the pleura, respectively, each based on four deaths, in that cohort.9 Here, we investigate quantitative relationships with several metrics of exposure, including time since first exposure, duration of exposure, and cumulative exposure and fit an absolute‐risk model used for risk assessment by US occupational and environmental health agencies.
2. METHODS
2.1. Population and vital status ascertainment
The study population and facilities have been described previously.9 Briefly, men and women employed for at least 1 day between 1950 and 1973 in three North Carolina mills that produced asbestos yarns and woven goods from raw fibres were enumerated from company records and files held by state and national health agencies. A fourth, smaller plant that did not process raw fibres was also included in the original study, but did not have adequate exposure data and is excluded from this analysis.
Vital status of the cohort was ascertained through 2003 by searches of the National Death Index (NDI) for deaths in 1979 and later and of the North Carolina Death Index, Social Security Administration Master Benefit File, and other sources for deaths in earlier years. Cause of death information was obtained from NDI‐Plus for deaths identified through NDI and was coded to the 9th or 10th revision of the ICD. For deaths before 1979, death certificates were obtained from state vital records offices and causes of death were coded manually by a nosologist to the revision of the ICD in force at the time of death. Contributing causes of death and other significant conditions were coded, in addition to the underlying cause. For years before the ICD‐10 was adopted, we reviewed original death certificates for deaths with ICD codes that were often assigned historically for mesothelioma10 and searched for mentions of mesothelioma in any field of the death certificate.
2.2. Exposure assessment
Quantitative individual exposures to asbestos fibres were estimated from 3420 air samples taken from the 1930s to the 1980s. Air sampling in the early years used midget impinger samplers to measure total dust concentrations. Measurement of fibre concentrations by membrane filter sampling and phase‐contrast microscopy (PCM) was introduced in 1964 and both methods were used until 1971, after which time PCM was used exclusively. We used data from approximately 1000 paired and concurrent samples obtained by both methods to estimate plant‐ and period‐specific factors to convert measurements of dust concentration to estimated PCM‐equivalent fibre concentrations. We then analyzed the PCM fibre concentration data using multivariable mixed models to estimate average fibre concentrations by plant, department, job, and time period. These data were linked to individual work‐history records to estimate cumulative exposure to asbestos fibres for each worker. Further details of the exposure assessment methods and results have been reported previously.11
2.3. Data analysis
The analysis included 5387 workers who were employed for at least 1 day in any of the three plants. Exposure‐response relations were estimated by maximum likelihood, fitting a Poisson regression model of the form log(rate) = , where rate is the rate of the outcome of interest, Z is a vector of covariates, and E is the exposure metric of interest. We fit models for the outcome of pleural cancer combined with mesothelioma (including deaths coded as either mesothelioma or cancer of the pleura), as well as for the outcome of mesothelioma (including only deaths with ICD‐10 codes for mesothelioma) in the subcohort of workers who survived until at least 1999.
We examined associations of mesothelioma and pleural cancer with exposure duration, time since first exposure (TSFE) and cumulative fibre exposure. In analyses of cumulative exposure, we evaluated exposure lags of 10 to 50 years in 10‐year increments. We also considered models in which TSFE was entered simultaneously with cumulative exposure. Because of the small numbers of deaths, variables for exposure and quantitative covariates were entered in continuous form. We examined the shape of exposure‐response functions by fitting smoothed penalized‐spline curves to the data. The results were compared to those obtained by conventional log‐linear Poisson regression by examining the degrees of freedom and improvement in model fit indicated by the Akaike Information Criterion (AIC).
All models were adjusted for age at risk (continuous) and race (white or nonwhite). Adjustment for gender had little impact on any model and was omitted from our final models. The time‐related covariates calendar year and length of follow‐up were evaluated with respect to their contributions to goodness of model fit and the change in the estimated coefficient for the exposure indicator. After examining these criteria, calendar year was retained in models for exposure duration, while length of follow‐up was retained in models for cumulative exposure. In models for TSFE, calendar year did not notably affect either the point estimate or model fit and length of follow‐up was not assessed because of its close correlation with TSFE (note that TSFE and length of follow‐up are equal in value if first exposure is coincident with entry into observation; however the first exposure can also occur before or after entry).
We also evaluated a mesothelioma risk model used by US Environmental Protection Agency (EPA) and Occupational Safety and Health Administration (OSHA). The EPA/OSHA model was developed in the 1980s12, 13 from proposals by Newhouse and Berry14 and Peto et al15 that mortality from mesothelioma is independent of age at exposure and increases as approximately the third power of TSFE . The mortality rate (R) is assumed to be a function of TSFE, average exposure concentration (C) and exposure duration (D) discounted by 10 years to account for latency, such that
To estimate the coefficient K, we fit the preceding equations to individual‐level data as a generalized linear model with an identity link function and Poisson error structure similar to that described by Berman and Crump.4
Statistical analyses were carried out using the glm and mgcv packages in the R system.16, 17
3. RESULTS
Descriptive data for the 5397 workers included in the study population are shown in Table 1. In observation from 1950 to 2003, the cohort registered 172 860 person‐years at risk, four deaths with mesothelioma (ICD‐10 C45) and four deaths with cancer of the pleura (ICD‐9 163 or 163.9) as the underlying cause. All of these deaths occurred more than 20 years after beginning employment.
Table 1.
Descriptive data by follow‐up period, North Carolina asbestos textile cohort
| Follow‐up 1950 to 2003 | Follow‐up 1999 to 2003 | |||
|---|---|---|---|---|
| Mean or n | Range | Mean or n | Range | |
| Workers, n | 5397 | – | 2803 | – |
| Person‐years, n | 172 860 | – | 13 022 | – |
| Mesothelioma deaths a , n | 4 | – | 4 | – |
| Pleural cancer deaths b , n | 4 | – | 0 | – |
| Year of hire | 1960 | 1917‐1973 | 1963 | 1932‐1973 |
| Length of follow‐up, y | 31.4 | 0.1‐54.0 | 39.6 | 25.2‐54.0 |
| Exposure duration, y | 3.5 | <0.1‐47.5 | 2.8 | <0.1‐42.4 |
| Mean exposure, f/mL | 17.8 | 0.1‐248.7 | 13.4 | 0.1‐170.9 |
| Cumulative exposure, f‐y/mL | 79.1 | <0.1‐5677.9 | 35.9 | <0.1‐1271.1 |
| Time since first exposure, y | 31.9 | 0‐72.0 | 39.4 | 26.0‐69.0 |
ICD‐10 C45.
ICD‐9 163 or 163.9.
Mortality from all pleural cancer, including deaths coded as mesothelioma or cancer of the pleura, was positively associated with exposure duration (rate ratio [RR], 1.10; 95% CI, 1.04‐1.16 per year) and with TSFE (RR, 1.19; 95% CI, 1.06‐1.34 per year) (Table 2). Adding a term for the third power of TSFE did not improve model fit, and neither TSFE or its third power was significant. When cumulative exposure was added to the model for TSFE, the effect of TSFE remained essentially the same and there was no significant association with cumulative exposure (Table 2). A term for the interaction of TSFE and cumulative exposure was also nonsignificant and did not improve model fit (data not shown). Fitting a smooth curve to the data for TSFE suggested a linear relationship with the log‐RR of pleural cancer mortality with RRs below unity for TSFE < 20 years (Figure 1).
Table 2.
Association of pleural cancer mortality with duration of exposure to asbestos and time since first exposure (TSFE); North Carolina asbestos textile workers, 1950 to 2003
| Exposure metric | RR a | 95% CIb | AICc |
|---|---|---|---|
| Duration, y | 1.10 | 1.04‐1.16 | 149.95 |
| TSFE, y | 1.19 | 1.06‐1.34 | 149.98 |
| TSFE3 | 1.00002 | 1.00001‐1.00003 | 156.31 |
| TSFE | 1.42 | 0.99‐2.05 | 150.31 |
| TSFE3 | 0.99997 | 0.99991‐1.00003 | – |
| TSFE | 1.18 | 1.05‐1.33 | 151.57 |
| Cumulative exposure (100 f‐years/mL), 10 y lag | 1.05 | 0.92‐1.20 | – |
Abbreviations: AIC, Akaike Information Criterion; CI, confidence interval; RR, rate ratio.
Rate ratio, per year for duration and time since first exposure; per 100 f‐years/mL for cumulative exposure. Models for duration adjusted for age, race, and year of follow‐up; models for time since first exposure adjusted for age and race.
Figure 1.
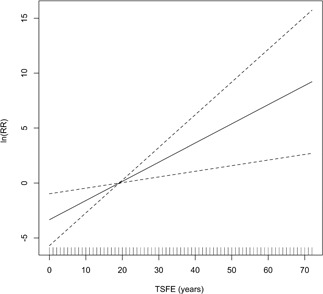
Penalized spline smooth (1 degree of freedom) of log relative rate of mesothelioma and time since first exposure (TSFE) in years. Vertical lines on horizontal axis indicate distribution of TSFE
In a model for cumulative exposure, lagged 10 years, the RR for pleural cancer mortality per 100 f‐years/mL was 1.15 (95% CI, 1.04‐1.28), and for exposure lags 20 and 30 years the RRs were 1.16 (95% CI, 1.04‐1.29) and 1.17 (95% CI, 1.03‐1.34) (Table 3). The RR per 100 f‐years/mL under a 40‐year lag assumption was slightly larger in magnitude but less precise (RR, 1.19; 95% CI, 0.89‐1.59), and the estimated RR per 100 f‐years/mL under a 50‐year lag had the widest confidence interval (RR, 1.14; 95% CI, 0.64‐2.01).
Table 3.
Association of pleural cancer mortality with cumulative exposure to asbestos by lag period; North Carolina asbestos textile workers, 1950 to 2003
| Lag period, y | RR a | 95% CIb | AICc |
|---|---|---|---|
| 10 | 1.15 | 1.04‐1.28 | 152.26 |
| 20 | 1.16 | 1.04‐1.29 | 152.39 |
| 30 | 1.17 | 1.03‐1.34 | 152.87 |
| 40 | 1.19 | 0.89‐1.59 | 154.67 |
| 50 | 1.14 | 0.64‐2.01 | 155.42 |
Abbreviations: AIC, Akaike Information Criterion; CI, confidence interval; RR, rate ratio.
Rate ratio perper 100 f‐years/mL, adjusted for race and length of follow‐up.
Smoothing data for cumulative exposure indicated a curvilinear association between exposure and the log‐RR of pleural cancer mortality, suggesting less than an exponential association, with the RR per unit exposure increasing less above about 2000 f‐years/mL, as illustrated for a 10‐year exposure lag in Figure 2. Exposure‐response functions were similar for other lag periods (data not shown).
Figure 2.
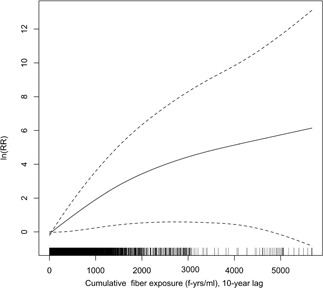
Penalized spline smooth (1.37 degrees of freedom) of log relative rate of mesothelioma and cumulative fibre exposure (f‐years/ml), lagged 10 years. Vertical lines on horizontal axis indicate distribution of cumulative exposure
Fitting the EPA/OSHA absolute risk model to data for the cohort gave a coefficient of 0.088 × 10−8 (95% CI, 0.027 × 10−8 to 0.149 × 10−8) per f‐year/ml (AIC 150.12).
Analysis of mesothelioma mortality in the subcohort still at risk as of 1999 showed patterns qualitatively similar to the full cohort: positive associations were observed with exposure duration, TSFE and cumulative exposure (Table 4). RRs for TSFE and cumulative exposure were larger in magnitude but less precise relative to those for the full cohort. Lagging cumulative exposure by 40 to 50 years gave increased RRs, but did not improve model fit. The coefficient for the EPA/OSHA risk model was 0.296 × 10−8 (95% CI, 0.0059 × 10−8 to 0.587 × 10−8) per f‐year/mL in the subcohort.
Table 4.
Association of mesothelioma mortality with time since first exposure and cumulative exposure to asbestos, North Carolina asbestos textile workers, 1999 to 2003
| Exposure metric | RR a | 95% CI | AIC |
|---|---|---|---|
| Duration, y | 1.12 | 1.04‐1.21 | 69.3 |
| TSFE, y | 1.28 | 1.02‐1.60 | 69.0 |
| Cumulative exposure (100 f‐years/mL) | |||
| 10‐y lag | 1.39 | 0.96‐2.04 | 67.5 |
| 20‐y lag | 1.39 | 0.95‐2.04 | 67.5 |
| 30‐y lag | 1.40 | 0.95‐2.05 | 67.5 |
| 40‐y lag | 1.52 | 0.96‐2.43 | 67.4 |
| 50‐y lag | 1.60 | 0.57‐4.48 | 69.3 |
Abbreviations: AIC, Akaike Information Criterion; CI, Confidence interval; RR, rate ratio.
Rate ratio per 100 f‐years/mL, adjusted for race and length of follow‐up.
4. DISCUSSION
Among North Carolina asbestos textile workers exposed to chrysotile, we observed positive, statistically significant associations between mortality from pleural cancer (including mesothelioma) and time since first exposure to asbestos, duration of exposure, and cumulative asbestos fibre exposure. These associations were greater in magnitude in analyses restricted to years on study after 1999 when mesothelioma was coded as a specific cause of death, but the precision of the estimated associations was reduced. The relationship of pleural cancer mortality rates and cumulative chrysotile exposure appeared to be curvilinear (ie, less than exponential), rising less steeply above about 2000 f‐years/mL.
These findings provide evidence that mortality from mesothelioma and pleural cancer is quantitatively associated with cumulative exposure to chrysotile fibers as well as with time since first exposure. The magnitude of these associations is not directly comparable, however, and the independent effects of each metric are difficult to estimate because of limited power and their mutual dependence on time.
Comparable exposure‐response data for mesothelioma are not available for any other group of chrysotile textile workers. Among chrysotile miners and millers, Pira et al6 reported increasing SMRs with longer duration of exposure and time since first exposure, but no similar pattern for cumulative exposure and no statistically significant trend for any exposure metric. Liddell et al5 reported generally increasing rates of mesothelioma mortality with increasing cumulative dust exposure, but did not provide data for other exposure indicators. No quantitative risk coefficients or formal investigations of the shape of exposure‐response functions were reported for either study.
Although no such data are available for cohorts exposed only to chrysotile, several authors have investigated the shape of the temporal relationship between mesothelioma risk and time since exposure among workers exposed to amphibole asbestos or mixed chrysotile and amphibole fibres. Our observation of an essentially linear increase in log‐relative risk with time since exposure is consistent with findings reported by Peto et al15 that mesothelioma mortality in several cohorts of asbestos workers continued to increase up to 60 years after exposure. However, Barone‐Adesi et al,18 presented contrasting data suggesting that mortality from mesothelioma may begin to plateau about 40 years after exposure.
The EPA/OSHA absolute risk model did not fit the data notably better than conventional relative risk models for TSFE or cumulative exposure. The model results are nevertheless useful for comparison. Estimates of mesothelioma risk from fitting equivalent models are available for South Carolina asbestos textile workers and Québec chrysotile miners and millers.4 Our estimate of the mesothelioma risk coefficient (0.088 × 10−8) falls between the estimates of 0.012 × 10−8 and 0.15 × 10−8 for Québec miners and South Carolina textile workers, respectively. The production operations in the North Carolina and South Carolina textile mills were similar, so similar unit risks would be expected. However, while exposures were assessed in the same manner for both studies, relatively fewer exposure measurements and less detailed work history and process information were available for North Carolina.19 Attenuation due to a greater degree of nondifferential measurement error may therefore be a potential explanation for relatively weaker exposure‐disease associations in North Carolina, compared to South Carolina.20
The principal strength of this study relative to other investigations of mesothelioma risk among workers exposed to chrysotile is the availability of extensive individual exposure estimates, which facilitate a range of exposure‐response analyses. The main limitations are reduced precision due to the small number of informative deaths and concerns about the quality of mesothelioma ascertainment, particularly before specific ICD codes for mesothelioma were available.
The magnitude and direction of misclassification of mesothelioma occurrence are not completely known and are likely to vary by time and place. Underestimation of the true number of cases is nevertheless the primary concern. Studies conducted in the United States before the ICD‐10 compared numbers of mesotheliomas recognized by cancer registries11 or in histopathological case series21, 22 to data from matched death certificates. The results suggest substantial under‐ascertainment during that time period: roughly 60% to 80% of mesotheliomas were mentioned on death certificates, while as few as 12% were coded as the underlying cause of death.10 These studies also found that 20% to 30% of deaths coded on death certificates as cancer of the pleura and about 50% of those coded as cancer of the peritoneum had no mention of mesothelioma in cancer registry records Davis et al.10 and that about 8% of mesotheliomas mentioned on death certificates were misdiagnoses of other cancers, most often lung cancer.22 Thus, it is possible that death certificate data from before the ICD‐10 include some false‐positive mesotheliomas while underestimating mesothelioma occurrence overall. The addition of a unique code for mesothelioma in the ICD‐10 appears to have led to substantially improved, but still incomplete, ascertainment, with about 80% correspondence between incident mesothelioma in the SEER registry and certified mesothelioma deaths in the years 1999 to 2000.23
We sought to reduce undercounting of mesothelioma in the cohort by examining death certificate data for mesothelioma mentioned in any field and for codes often applied to mesothelioma in the pre‐ICD‐10 period. Nevertheless, the strengthening of associations with all exposure indicators when the analysis was restricted to years after adoption of the ICD‐10 is consistent with nondifferential under‐ascertainment of mesothelioma deaths in earlier years. These findings should be viewed with caution, however, as the subcohort that survived until 1999 included only 8% of total person‐years and favoured workers hired later, followed longer, and with lower cumulative exposures. Further follow‐up would improve the ascertainment mesothelioma in the full cohort.
In conclusion, our observations of positive, statistically significant associations of pleural cancer and mesothelioma mortality with cumulative exposure to chrysotile asbestos fibres, as well as with the duration of exposure and time since exposure, support the conclusion that chrysotile causes mesothelioma and provide quantitative data for risk assessment. Similar exposure‐response analyses of mesothelioma in other cohorts exposed to chrysotile are encouraged.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
DL conceived the study, directed the original data collection, performed statistical analyses, and drafted the manuscript. DBR and LE contributed to the parent study and to drafting and critically revising the manuscript. All authors approve the final version and agree to take responsibility for the work.
Supporting information
Supplementary information
DISCLOSURE BY AJIM EDITOR OF RECORD
John Meyer declares that he has no conflict of interest in the review and publication decision regarding this article.
ETHICS APPROVAL AND INFORMED CONSENT
This study was determined to be exempt from IRB review by the Institutional Review Board of the University of Nevada, Reno. The research was based entirely on existing data for a historical occupational cohort, therefore subject consent was not, and could not be, obtained.
Loomis D, Richardson DB, Elliott L. Quantitative relationships of exposure to chrysotile asbestos and mesothelioma mortality. Am J Ind Med. 2019;62:471‐477. 10.1002/ajim.22985
References
REFERENCES
- 1. International Agency for Research on Cancer (IARC) . Chemicals and industrial processes associated with cancer in humans: an updating of IARC monographs Volumes 1 to 20. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. 1979; suppl 1. [PubMed]
- 2. International Agency for Research on Cancer (IARC) . A review of human carcinogens, Part C: arsenic, metals, fibres and dusts. IARC monographs on the evaluation of carcinogenic risks to humans. 2012;100C. [PMC free article] [PubMed]
- 3. National Toxicology Program (NTP) . 2016. Report on carcinogens, 14th ed. Research Triangle Park, NC: US Department of Health and Human Services, Public Health Service. https://ntp.niehs.nih.gov/go/roc14/. Accessed January 7, 2019.
- 4. Berman DW, Crump KS. Update of potency factors for asbestos‐related lung cancer and mesothelioma. Crit Rev Toxicol. 2008;38(suppl 1):1‐47. [DOI] [PubMed] [Google Scholar]
- 5. Liddell FD, McDonald AD, McDonald JC. The 1891‐1920 birth cohort of Quebec chrysotile miners and millers: development from 1904 and mortality to 1992. Ann Occup Hyg. 1997;41:13‐36. [DOI] [PubMed] [Google Scholar]
- 6. Pira E, Romano C, Donato F, Pelucchi C, Vecchia C, Boffetta P. Mortality from cancer and other causes among Italian chrysotile asbestos miners. Occup Environ Med. 2017;74:558‐563. [DOI] [PubMed] [Google Scholar]
- 7. Wang X, Lin S, Yu I, Qiu H, Lan Y, Yano E. Cause‐specific mortality in a Chinese chrysotile textile worker cohort. Cancer Sci. 2013;104:245‐249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hein MJ, Stayner LT, Lehman E, Dement JM. Follow‐up study of chrysotile textile workers: cohort mortality and exposure‐response. Occup Environ Med. 2007;64:616‐625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Loomis D, Dement JM, Wolf SH, Richardson DB. Lung cancer mortality and fibre exposures among North Carolina asbestos textile workers. Occup Environ Med. 2009;66:535‐542. [DOI] [PubMed] [Google Scholar]
- 10. Davis LK, Martin TR, Kligler B. Use of death certificates for mesothelioma surveillance. Public Health Rep. 1992;107:481‐483. [PMC free article] [PubMed] [Google Scholar]
- 11. Dement JM, Meyers D, Loomis D, Richardson D. Estimates of historical exposures by phase contrast and transmission electron microscopy in North Carolina USA asbestos textile plants. Occup Environ Med. 2009;66:574‐583. [DOI] [PubMed] [Google Scholar]
- 12. Occupational Safety and Health Administration (OSHA) . Occupational exposure to asbestos. 29 CFR 1910. Fed Reg. 1983;48(215):51086‐51140. [Google Scholar]
- 13. Environmental Protection Agency (EPA) . Airborne asbestos health assessment update. EPA/600/8‐84/003F Washington, DC: US EPA; 1986. [Google Scholar]
- 14. Newhouse ML, Berry G. Predictions of mortality from mesothelioma tumours in asbestos factory workers. Br J Ind Med. 1976;33:147‐151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Peto J, Seidman H, Selikoff IJ. Mesothelioma mortality in asbestos workers: implications for models of carcinogenesis and risk assessment. Br J Cancer. 1982;45:124‐135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. R Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2018. [Google Scholar]
- 17. Wood SN. Generalized additive models: an introduction with R. Boca Raton, FL: Chapman and Hall/CRC; 2006. [Google Scholar]
- 18. Barone‐Adesi F, Ferrante D, Bertolotti M, et al. Long‐term mortality from pleural and peritoneal cancer after exposure to asbestos: possible role of asbestos clearance. Int J Cancer. 2008;123:912‐916. [DOI] [PubMed] [Google Scholar]
- 19. Dement JM, Loomis D, Richardson D, Wolf SH, Kuempel ED. Estimates of historical exposures by phase contrast and transmission electron miscroscopy of pooled exposure‐response analyses of North Carolina and South Carolina, USA asbestos textile cohorts. Occup Environ Med. 2011;68:593‐598. [DOI] [PubMed] [Google Scholar]
- 20. Elliott L, Loomis D, Dement J, Hein MJ, Richardson D, Stayner L. Lung cancer mortality in North Carolina and South Carolina chrysotile asbestos textile workers. Occup Environ Med. 2012;69:385‐390. [DOI] [PubMed] [Google Scholar]
- 21. Ribak J, Lilis R, Suzuki Y, Penner L, Selikoff IJ. Death certificate categorization of malignant pleural and peritoneal mesothelioma in a cohort of asbestos insulation workers. J Soc Occup Med. 1991;41:137‐139. [DOI] [PubMed] [Google Scholar]
- 22. Selikoff IJ, Seidman H. Use of death certificates in epidemiological studies, including occupational hazards: variations in discordance of different asbestos‐associated diseases on best evidence ascertainment. Am J Ind Med. 1992;22:481‐492. [DOI] [PubMed] [Google Scholar]
- 23. Pinheiro GA, Antao VCS, Bang KM, Attfield MD. Malignant mesothelioma surveillance: a comparison of ICD 10 mortality data with SEER incidence data in nine areas of the United States. Int J Occup Environ Health. 2004;10:251‐255. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information


