Abstract
Lyme disease is a common multisystem disease caused by infection with a tick‐transmitted spirochete, Borrelia burgdorferi and related Borrelia species. The monoglycosylated diacylglycerol known as B. burgdorferi glycolipid II (BbGL‐II) is a major target of antibodies in sera from infected individuals. Here, we show that CD1b presents BbGL‐II to human T cells and that the TCR mediates the recognition. However, we did not detect increased frequency of CD1b‐BbGL‐II binding T cells in the peripheral blood of Lyme disease patients compared to controls. Unexpectedly, mapping the T cell specificity for BbGL‐II‐like molecules using tetramers and activation assays revealed a concomitant response to CD1b‐expressing APCs in absence of BbGL‐II. Further, among all major classes of self‐lipid tested, BbGL‐II responsive TCRs show strong cross‐reactivity to diacylglycerol, a self‐lipid antigen with structural similarities to BbGL‐II. Extending prior work on MHC and CD1b, CD1c, and CD1d proteins, this study provides evidence for cross‐reactive CD1b‐restricted T cell responses to bacterial and self‐antigens, and identifies chemically defined targets for future discovery of self and foreign antigen cross‐reactive T cells.
Keywords: antigen specificity, CD1b, lipid antigen, Lyme disease, T cells
Introduction
MHC molecules present peptides from self or foreign origin to T cells. Outside the MHC, the human genome encodes additional antigen presenting molecules that produce proteins that are structurally related to MHC class I proteins, including CD1a, CD1b, CD1c, and CD1d 1. CD1‐β2microglobulin complexes are expressed on key APCs such as B cells, macrophages, and dendritic cells 2, where they bind many types of lipid antigens for presentation to TCRs. An important difference between MHC and CD1 genes is that CD1 genes show extremely low rates of polymorphism. Although single nucleotide polymorphisms in CD1 are known, they have not been shown to affect lipid antigen display or TCR recognition. Thus, unlike peptide antigens, where the pattern of response varies substantially among individuals, CD1 allows development of lipid antigens as immunogens for vaccines or targets of diagnostic studies that could work in any genetic background 3, 4.
Among human CD1 molecules, CD1b was the first for which lipid antigen presentation was shown 5, 6. High levels of CD1b are present on DC's or induced on monocytes differentiating into DCs 7, 8, where it presents self and foreign lipids to T cells. Many foreign lipids that are known to be presented by CD1b are derived from mycobacteria or closely related actinobacteria, such as glucose monomycolate, glycerol monomycolate 9, 10, 11, mycolic acid 5, 12, 13, and diacylated sulfoglycolipids 14, 15. Also, some self‐lipids from mammalian cells are presented by CD1b, including phosphatidylglycerol, phosphatidic acid (PA), phosphatidylethanolamine, phosphatidylinositol, and gangliosides 16, 17, 18, 19. Interestingly, for some of these self‐lipids, such as phosphatidylglycerol and PA, the T cells that recognize them cross‐react with bacterial versions of the lipids, such as lipids found in Salmonella and Staphylococcus species 18.
Here, we tested the possibility that CD1b might play a role in Lyme disease, which is a tick‐borne bacterial infection 20. The initial site of infection is the skin, where the spread of bacteria and immune response often begins as an expanding skin lesion, erythema migrans. In untreated patients, this skin lesion may be followed weeks later by neuroborreliosis or carditis, and months later, by arthritis. In North America Borrelia burgdorferi is the causative agent of Lyme disease, while in Europe and Asia B. afzelii and B. garinii are the predominant species 21. Infections caused by B. burgdorferi in North America tend to be more arthritogenic, whereas B. garinii causes a more neurotropic disease. B. afzelii predominantly causes skin manifestations 22. Most patients respond to sterilizing regimens of antibiotics. However, some patients have persistent or worsening, long lasting synovitis, called post‐infectious or antibiotic‐refractory Lyme arthritis where live Borrelia cannot be detected 23. The synovial lesion in these patients is associated with chronic inflammation, fibrosis, and autoimmune phenomena 24, 25, 26, 27.
B. burgdorferi has two major glycolipids, Borrelia burgdorferi glycolipid I (BbGL‐I) and Borrelia burgdorferi glycolipid II (BbGL‐II), which together comprise 36% of the total lipid mass of the bacteria. These molecules belong to different lipid classes: BbGL‐I is cholesterol based, and BbGL‐II is diacylglycerol based. However, both lipids are components of both the inner and outer membrane 28, 29, and both elicit antibody responses in infected people 30, 31. In one study of North American patients, 52% of patients with neuroborreliosis had weak IgG antibody responses to BbGL‐II, and almost all patients with Lyme arthritis, had strong IgG reactivity with both BbGL‐1 and BbGL‐II 32. Although BbGL‐I and BbGL‐II were identified in B. burgdorferi, both lipids were also detected in B. afzelii and B. garinii 33. The gene named monogalactosyl‐1,2‐diacylglycerol synthase, mgs, which is thought to catalyze the synthesis of BbGL‐II, is present in all three pathogenic Borrelia species 34. Thus, BbGL‐II would be a potential glycolipid antigen for use in vaccines and diagnostics.
Several prior studies suggested the hypothesis that CD1b might present Borrelia lipids to human T cells. First, while modeling suggested that BbGL‐I fits into the antigen binding cleft of human CD1c 35, we predicted that BbGL‐II would fit in the antigen binding cleft of CD1b based on its two‐tailed glycolipid structure and size 36, 37. Second, NKT cells are activated by CD1d and BbGL‐II 38, and depletion of NKT cells or CD1d render mice more sensitive to infection 39, 40. Third, human CD1b and CD1c transcripts and proteins are upregulated by TLR ligands or live B. burgdorferi. Studies of ex vivo B. burgdorferi infected human skin explants and erythema migrans biopsies show CD1b protein upregulation to high levels at the site of infection 41. Because CD1b is rarely expressed in human tissues in the periphery, these findings suggested a model in which localized infection rapidly upregulates CD1b proteins at the site of infection, where bacterial lipids are being shed 42. However, direct evidence for Borrelia lipid antigens for CD1b is lacking. In this study, we investigated whether BbGL‐II can be presented by CD1b to human T cells.
Results
Characterization of BbGL‐II
To characterize in‐house produced synthetic BbGL‐II (1, 2 di‐O‐oleoyl‐3‐O‐α‐d‐galactopyranosyl‐sn‐glycerol) 31, we carried out positive mode, multistage collision‐induced dissociation (CID)–electrospray ionization (ESI)–mass spectrometry (MS) (Fig. 1). The MS2 collision pattern of BbGL‐II (C45H82O10Na+, calculated m/z 805.6, detected m/z 805.9) shows loss of 282 u, representing the mass of any oleic acyl (C18:1) moiety. The MS3 collision pattern shows subsequent loss of the hexose (162 u), another oleic acid (282 u), or acylglycerol (338 u). These data confirm that the BbGL‐II used in this study is intact and identifies key components, including two oleic acyl units.
Figure 1.
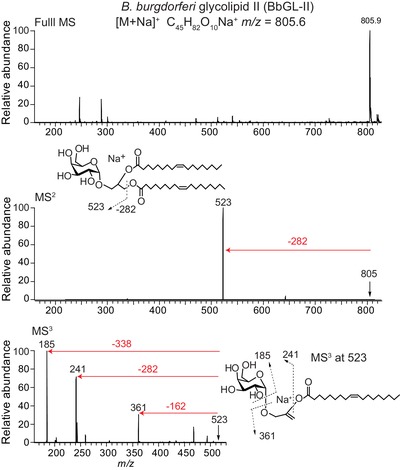
Mass spectrometry on synthetic BbGL‐II lipid. Positive ion mode ESI–MS of synthetic BbGL‐II gave an expected major ion at m/z 805.9 (top panel), which was subjected to multistage CID‐MS to yield fragment ions as indicated (middle and bottom panels).
BbGL‐II specific T cells
To identify CD1b‐BbGL‐II specific T cells, we used peripheral blood mononuclear cells (PBMC) from a buffy coat from donor 24 (BC24). This donor was considered immune based on the presence of an erythema migrans lesion but did not have clinical signs of Lyme disease. After 14 days of expansion of T cells with anti‐CD3, ∼0.3% of cells stained positively with CD1b‐BbGL‐II tetramers (Fig. 2A). These cells were sorted and expanded to yield CD1b‐BbGL‐II tetramer positivity in 1.4% of cells. After a second round of sorting and expansion with CD1b‐BbGL‐II tetramers, a clear CD1b‐BbGL‐II tetramer+ CD4– population was identified. This large, homogenously staining population represented 14% of cells, which we named T cell line BC24A. The CD4+ cells contained CD1b‐specific populations with a very broad reactivity pattern, including CD1b‐phosphatidylglycerol 19, and were not analyzed here because we were interested in discovering BbGL‐II‐specific T cells. Further analysis showed that BC24A T cells do not detectably bind to mock‐treated CD1b tetramers (Fig. 2B), which are called CD1b‐endo tetramers because they contain endogenous cellular lipids from the mammalian expression system for CD1b 18. In contrast to nearly all previously reported CD1b reactive T cells lines, which are CD4+ or CD4–CD8– 11, 18, 43, BC24A cells uniformly express high levels of CD8.
Figure 2.
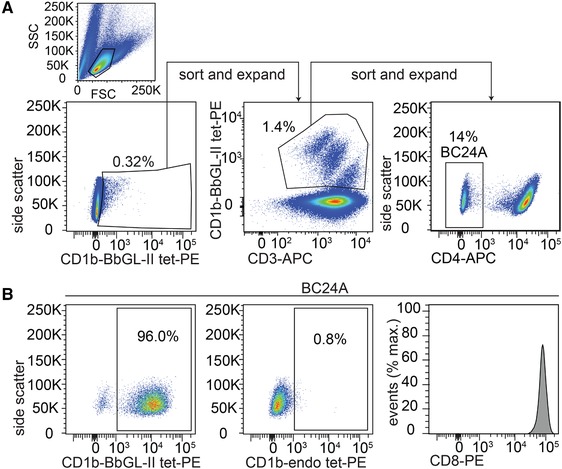
Isolation of CD1b‐BbGL‐II binding T cells. (A) PBMC from blood donor BC24 (Table 1) with erythema migrans were sorted based on CD1b‐BbGL‐II tetramer binding. After three rounds of sorting and expansion, the 96% of CD4‒ T cells that stain with CD1b‐BbGL‐II tetramers were further tested for staining with CD1b‐endo tetramers carrying endogenous (endo) lipids and an antibody against CD8α. Positive CD1b‐BbGL‐II tetramer, negative CD1b‐endo, and negative CD4 staining have been observed in five independent experiments. CD8 staining was performed once.
BC24A T cells were subjected to a single‐cell TCR sequencing method 44, which yielded six separate interpretable TCR‐α and ‐β chain pairs that were all identical. The BC24A TCR consists of an α‐chain containing the gene segments TRAV36/DV7*04 and TRAJ22*01 and a β chain containing the gene segments TRBV4‐1*01 and TRBJ1‐4*01 (Fig. 3A). Unlike the conserved α chain sequences of invariant T cells, such as NKT cells, the BC24A TCR contains seven non‐template‐encoded nucleotides. Invariant TCRs such as NKT cells and germline‐encoded mycolyl reactive (GEM) T cells use predominantly or solely germline encoded sequences. So this finding suggests that BC24A is a representative of the private T cell repertoire unique to this individual. Whereas CD1 tetramers typically bind TCRs, there is some prior evidence for staining of non‐T cells or binding to non‐TCR proteins like ILT4 45, 46. Therefore, to assess whether CD1b‐BbGL‐II tetramers bind this αβ TCR, we performed a TCR transfer experiment. Jurkat 76 (J76) cells, which are deficient for both TCR‐α and ‐β chains 47, were transduced with the BC24A TCR and CD3, both using GFP as a transduction marker. CD1b‐BbGL‐II tetramers selectively stained TCR‐transduced, GFP+ cells but not untransduced cells, demonstrating that tetramer binding is TCR dependent (Fig. 3B). Like the primary T cell population BC24A, staining of TCR transduced J76 cells with CD1b‐endo tetramers did not show detectable binding, demonstrating that binding between CD1b and the TCR is dependent on the BbGL‐II glycolipid (Fig. 3B). Thus, BbGL‐II is a CD1b‐presented antigen for T cells that undergoes cognate recognition by an αβ TCR.
Figure 3.
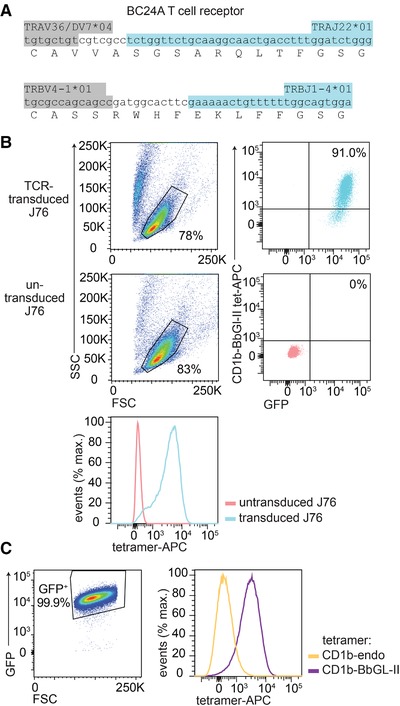
TCR dependent tetramer binding. (A) The sequence of the CD1b‐BbGL‐II binding TCR was determined by single cell TCR sequencing, where six independently obtained single cells showed the same CDR3 sequence. The nucleotides of the variable region (light grey) N‐region additions (white) and joining segment (dark grey) are color‐coded. (B) J76 cells transduced with the BC24A TCR and untransduced J76 cells were stained with CD1b‐BbGL‐II tetramer. (C) J76 cells transduced with the CD1b‐BbGL‐II specific TCR were stained with CD1b‐BbGL‐II and CD1b‐endo tetramers. Panel (C) was pregated based on forward scatter (FSC) and side scatter (SSC) as shown in (B). Four independent experiments were performed with comparable results. One representative experiment is shown.
T cell populations in random donors and Borrelia‐infected people
Next, we sought to measure CD1b‐BbGL‐II and CD1b‐endo tetramer staining in polyclonal T cell populations (Fig. 4, Table 1, Supporting Information Figs. 1–5). We used anti‐CD3‐expanded T cells from PBMC from four Lyme arthritis, eight Lyme neuroborreliosis patients, and six random blood bank donors from the Netherlands (Fig. 4A). In contrast to the brightly staining cells seen after CD1b‐BbGL‐II tetramer sorting and expansion (Fig. 2), ex vivo staining did not yield cell populations that were well separated from the tetramer negative cells and did not result in significant differences between CD1b‐endo and CD1b‐BbGL‐II tetramer staining in any of the groups. Prior studies 18, 46 suggest that T cell precursor frequencies of 10−5 to 10−4 above background are needed for detection of antigen‐specific T cells. Thus, CD1b‐BbGL‐II tetramer staining was insufficiently sensitive to detect T cells in these expanded polyclonal T cell populations. Because phospholipids are abundantly present in CD1b‐endo 19, another way of formulating this conclusion is to say that BbGL‐II‐specific T cells are not more abundant than phospholipid‐specific T cells. T cell expansion was necessary from PBMC from Lyme disease patients because cell numbers directly ex vivo were insufficient for these experiments. However, we were able to perform experiments on PBMC from blood bank donors directly ex vivo (Fig. 4B). Also directly ex vivo, no significant differences between CD1b‐endo and CD1b‐BbGL‐II tetramer staining were detected.
Figure 4.
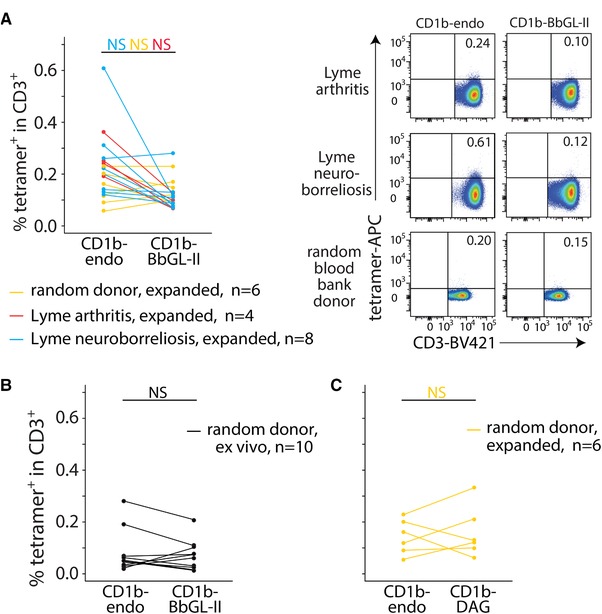
Tetramer staining of T cells from Lyme disease patients. T cells that were expanded for 2 weeks (A and C) or PBMC that were used directly ex vivo (B) were stained with an αCD3 antibody and the indicated tetramers (CD1b‐BbGL‐II, CD1b‐endo, or CD1b‐DAG). Facs data of three representative subjects (one Lyme arthritis, one Lyme neuroborreliosis, and one random blood bank donor) are shown in (A). The complete set of flowcytometric data and pregating strategy is shown in Supporting Information Figures 1–5. The percentages CD3+ tetramer+ cells are indicated. NS: not significant (p > 0.05) as calculated by paired Wilcoxon ranked sums tests and Benjamini and Hochberg correction for multiple testing.
Table 1.
Overview of the blood donors used in this study. All patients and blood bank donors are from the Netherlands
| Donor | Donor status | Time since diagnosis (months) |
|---|---|---|
| 1 | Lyme neuroborreliosis | 6 |
| 2 | Lyme arthritis | 6 |
| 3 | Lyme neuroborreliosis | 0 |
| 4 | Lyme neuroborreliosis | 24 |
| 5 | Lyme arthritis | 12 |
| 6 | Lyme arthritis | 0 |
| 7 | Lyme neuroborreliosis | 12 |
| 8 | Lyme arthritis | 1 |
| 9 | Lyme neuroborreliosis | 0 |
| 11 | Lyme neuroborreliosis | 1 |
| 12 | Lyme neuroborreliosis | 3 |
| 14 | Lyme neuroborreliosis | 24 |
| BC1 | Random blood bank donor | NA |
| BC2 | Random blood bank donor | NA |
| BC8 | Random blood bank donor | NA |
| BC9 | Random blood bank donor | NA |
| BC13 | Random blood bank donor | NA |
| BC14 | Random blood bank donor | NA |
| BC17 | Random blood bank donor | NA |
| BC18 | Random blood bank donor | NA |
| BC19 | Random blood bank donor | NA |
| BC20 | Random blood bank donor | NA |
| BC24 | Erythema migrans | Unknown |
NA, not applicable; BC, buffy coat.
T cell activation assays demonstrate CD1b and self‐antigen reactivity
To determine whether BC24A T cells can be functionally activated by cellular CD1b‐BbGL‐II complexes, IFN‐γ production was measured with an ELISPOT assay using CD1b‐expressing C1R cells as APCs (Fig. 5A). No response was seen upon stimulation with C1R.CD1a cells or C1R.CD1b treated with anti‐CD1b monoclonal antibodies (BCD1b3.1), demonstrating an absolute requirement for CD1b expression. However, we observed similarly high levels of IFN‐γ production in response to C1R.CD1b with no added antigen, C1R.CD1b cells treated with BbGL‐II lipid, or C1R.CD1b cells treated with sonicated B. burgdorferi bacteria. In this system, CD1b‐dependent reactivity to human APCs in the absence of exogenously added antigen is interpreted as CD1b autoreactivity. Together, the data suggested the existence of a self‐antigen in C1R cells, which we sought to discover.
Figure 5.
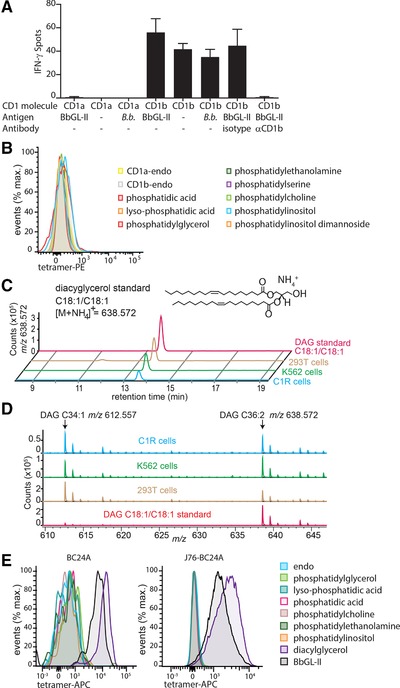
CD1b‐BbGL‐II‐binding T cells are autoreactive and recognize diacylglycerol. (A) IFN‐γ production was measured by co‐culture of BC24A T cells and C1R cells transduced to express human CD1a or CD1b. The cells were incubated in the presence of BbGL‐II lipid, sonicated Borrelia burgdorferi (B.b.), or without antigen (–). A blocking antibody or isotype control was added. Data is mean value of triplicates with error bars showing SD. Data shown is representative of three independent experiments. (B) Staining of BC24A with the indicated tetramers. (C) Detection of C36:2 DAG standard or lipid extracts of human cell lines C1R, K562, and 293T cells by high mass resolution mass HPLC time of flight MS. (D) Mass spectrum between 13.5 and 13.7 min shows the presence of C34:1 DAG and C36:2 DAG in cells. (E) Cultured primary T cells or J76 cells transduced with the BC24A TCR were stained with CD1b tetramers loaded with the indicated antigens. Pregating strategy is shown in Figures 2A and 3C, respectively. BC24A staining was performed twice independently, J76‐BC24A staining was performed three times independently. One representative staining is shown.
Diacylglycerol is a self‐antigen
Recent studies have identified CD1b‐presented lipid autoantigens, including PA, phosphatidylglycerol, phosphatidylcholine, and related molecules 16, 18. Structural studies of CD1b‐phosphatidylglycerol‐TCR show that CD1b binds the diacylglycerol (DAG) moiety and presents the phosphoglycerol anion to cationic cup in the TCR. Therefore, we tested staining with a panel of CD1b tetramers loaded with phospholipids, including PA, phosphatidylglycerol, phosphatidylethanolamine, phosphatidylserine, phosphatidylcholine, as well as one smaller (lysophosphatidic acid) and larger (phosphatidylinositol mannoside) lipid with a similar core structure (Fig. 5B). Although some of these alternately loaded tetramers have been previously shown to bind TCRs 18, BC24A did not stain, ruling out the many of the most abundant self‐phospholipids as antigens in this case.
Next, we considered self‐lipids that more closely resemble the structure of BbGL‐II, which has a simple DAG backbone coupled to a hexose sugar (Fig. 1). Hexose‐DAGs are not known to exist in human cells. However, free DAG has been reported in low amounts in human cells as a second messenger and as an intermediate of triglyceride synthesis. Therefore, we tested whether the C1R cells, which were used as APCs in our functional assay, as well as two other commonly used APCs, K562 and 293T, expressed detectable amounts of DAG. To detect DAG, we used reverse phase HPLC‐quadrupole time‐of‐flight–MS, which provides high mass accuracy and retention time information for the detected ions (Fig. 5C). In all cell lines we detected ions with a mass and retention time matching synthetic dioleoyl‐DAG standard. A mass spectrum at that retention time (13.5‐14 min) showed ammoniated ions of the chain length analogs C34:1 (m/z 612.5567) and C36:2 (m/z 638.5723) DAGs. (Fig. 5D).
Next, we found that dioleoyl DAG‐treated CD1b tetramers brightly stained BC24A cells. This result was confirmed in BC24A TCR‐transduced J76 cells (Fig. 5E). Polyclonal T cells from six random blood bank donors did not show significant differences between CD1b‐endo and CD1b‐DAG tetramer staining (Fig. 4C). So, even though BC24A staining provides proof of principle that this reactivity pattern exists, detection of such T cells is not possible in peripheral blood at precursor frequencies of >10−4, which is needed for detection above background staining levels. Whereas prior work has identified sphingolipid 17 and phospholipid autoantigens for CD1b 16, 18, these data identify a neutral lipid self‐antigen: simple diacylglycerols mediate CD1b autoreactivity.
Discussion
Whereas most studies of human αβ T cell responses focus on MHC and peptide antigens, new information on the CD1 system increasingly points to a parallel system of antigen response involving recognition of foreign lipids. Direct evidence for T cell responses to B. burgdorferi lipids started with Kinjo's seminal work, demonstrating that CD1d‐BbGL‐II complexes activate invariant NKT cells 38. NKT cells form an interdonor‐conserved innate T cell population. Based on the non‐template encoded nucleotide additions in its TCR α chain, the BC24A TCR most likely represents one example of the private, diverse repertoire. A further difference between the CD1d and CD1b systems is that CD1d is constitutively expressed on most cell types, whereas CD1a, CD1b and CD1c protein expression is normally undetectable on myeloid cells in the periphery until Toll‐like receptors, cytokines or other stimuli induce CD1b transcription 8. Because B. burgdorferi infection stimulates CD1b expression in human skin, the existence of Borrelia lipid‐specific T cells was predicted 41. Here we provide proof of principle for CD1b‐mediated T cell recognition of a major glycolipid that is expressed in the three major disease‐causing species of Borrelia.
Whether or not Borrelia lipid recognition plays a role in Lyme disease outcomes will require further study, given the lack of increased ex vivo CD1b‐BbGL‐II tetramer staining seen in our pilot study. However, BbGL‐II and DAG provide specific molecular targets for future clinical studies in Lyme disease patients. Given the importance of intradermal T cell responses in the erythema migrans lesion, prior evidence for local upregulation of CD1b in skin and the expectation that lipids would be shed locally, the next phase of studies will take advantage of skin to focus on tissue‐specific T cells in human responses to Borrelia lipids. Further, the pattern of recognition observed here matches a broader theme of immune response in Lyme disease. At the molecular level, B cell and MHC‐restricted T cell responses in Lyme disease show demonstrable responses to self‐antigens 25, 26, 48 and cross‐reactivities among bacterial and self‐antigens 27. Thus Borrelia‐initiated, self‐propagating immune responses may contribute to post‐infectious Lyme arthritis.
From a molecular perspective, these studies identify DAG as a previously unknown autoantigen in the CD1b system. Although recognition of a moderately abundant self‐lipid was unexpected, the data demonstrate antigen recognition using several types of assays. A cloned TCR binds DAG lipids loaded onto CD1b complexes, and a CD1b‐mediated autoreactive response to antigen presenting cells that produce DAG was found.
This study reports a T cell line with combined self and foreign lipid antigen specificity. Given the low staining of BC24A T cells with CD1b‐endo tetramers, and the high staining with CD1b‐BbGL‐II tetramers, the strong response of this T cell line to CD1b expressed on C1R cells was unexpected, but can be readily explained by the different valency of TCR ligands on tetramers and cells. DAG constitutes a low percentage of cellular lipids compared to phospholipids. Tetramers typically require that two or more arms be loaded with a TCR binding ligand to achieve adequate avidity to bind T cells. In contrast, the surface of a CD1b‐expressing cell has a much larger number of CD1b proteins from which to generate binding partners for TCRs, so rare lipids can generate multivalent TCR ligands. Our data directly document DAG presence in C1R cells, competence of CD1b‐DAG tetramers to stain BC24A brightly, and maximal cytokine response to CD1b‐expressing C1R cells. These results highlight the ability of cells to present relatively rare antigens to T cells, as contrasted with tetramers, which require higher absolute occupancy.
While the precise molecular mechanism for the observed cross‐reactivity between BbGL‐II and DAG remains to be established through structural studies, recent work on CD1b‐phosphatidylglycerol‐TCR complexes suggest a likely model. DAG, BbGL‐II, and phosphatidylglycerol have essentially the same diacylglycerol anchor, and this shared chemical moiety also matches the lipid‐anchoring moiety seen in the CD1b‐phosphatidylglycerol‐TCR structure 16. Here the DAG anchor sits atop a lipid spacer and positions the glycerol unit just at the opening of the F' portal of CD1b. If the shared chemical moieties of DAG and BbGL‐II are positioned similarly inside CD1b, the hexose sugar unique to BbGL‐II would protrude through the F' portal to rest on the outer surface of CD1b in some way. Our data indicate that the glucose is not required for TCR binding, and it does not inhibit TCR response. There are several known mechanisms by which the distal moiety on lipid antigen can be ‘ignored’ by an approaching TCR. The simplest is that the BbGL‐II molecule sits completely inside CD1b, but this is less favored given the lack of clear precedent for carbohydrates to be seated inside the hydrophobic cleft. A second, more favored possibility is that the hexose sugar protrudes to the surface of CD1 through the F’ portal, whereas the TCR binds near the center of CD1. This mechanism is known as left‐right mismatch 49. Last, CD1b‐reactive TCRs can have an escape channel whereby larger head groups escape sideways between the TCR α and β chains 19. Overall, these findings support and extend key emerging concepts in CD1b biology: TCR cross‐reactivity for foreign and self‐antigens.
Materials and methods
Mass spectrometry
For nanospray analysis, a 1 μM solution of lipid in methanol was loaded onto a nanospray tip and analyzed by ESI–MS and CID–MS on the LXQ Ion Trap Mass Spectrometer (Thermo Fisher Scientific) in positive ion mode. Collision energy was 20 to 30 % of the maximum, and product ions were trapped with a q value of 0.25. Chloroform/methanol extracted total lipids from cell lines were prepared at 0.5 mg/ml and 10 μl was injected for HPLC‐ESI‐MS analysis (Agilent 6520 Accurate‐Mass Q‐TOF and 1200 series HPLC system using a reverse phase Eclipse Plus‐C18 column (3.5 μM, 2.1 mm × 30 mm, Agilent Technologies) according to the published method 46.
Tetramers and flow cytometry
Biotinylated CD1b monomers were obtained from the National Institute Health (NIH) and loaded with 32 μg phosphatidylglycerol (#841188P, Avanti polar lipids), PA (#840857, Avanti polar lipids), lyso‐PA (#857130, Avanti polar lipids), phosphatidylethanolamine (#850757, Avanti polar lipids), phosphatidylserine (#840032, Avanti polar lipids), phosphatidylcholine (#850475, Avanti polar lipids), phosphatidylinositol (#840042, Avanti polar lipids), phosphatidylinositol dimannoside (Bill and Melinda Gates Foundation lipid bank), 1,2 dioleoyl‐sn‐glycerol (#D0138, Sigma), or in‐house produced synthetic BbGL‐II 31. Lipids were dissolved in citrate buffer at pH 4.5 with 0.5% CHAPS (Sigma) by sonication for 10 min at 37°C, followed by incubation for 2 h at 65°C and repeat sonication for 10 min at 37°C, after which the CD1b monomers were added. This mixture was incubated overnight at 37°C. After incubation, the pH was neutralized by adding TRIS pH 8.5. As control, CD1b monomers were treated in the same way without adding lipid (CD1b‐endo). Tetramers were generated by linking monomer together with streptavidin‐allophycocyanin (Life Technologies) or streptavidin‐PE (Life Technologies). Experimental setup of flow cytometric experiments adhered to published guidelines 50.Cells were stained with tetramers and αCD3 monoclonal antibody (clone SK7, BD biosciences). Cells were first incubated for 10 min with tetramers at room temperature, then the αCD3 antibody was added and incubated again for 10 min at room temperature, followed by an incubation of 20 min at 4°C. The cells were either sorted on the FACSAria (BD biosciences) or analyzed on the FACSCanto II (BD biosciences).
Human subjects
PBMC of random blood donors were derived from the blood bank (Sanquin, Amsterdam). Lyme disease patient‐derived blood was obtained as part of an ongoing Lyme disease study, which is approved by the regional Medical Research Ethics Committees United (Nieuwegein, the Netherlands; MEC‐U: NL36407.100.11).
T cell culture
PBMC were isolated from buffy coats or whole blood by density gradient centrifugation. PBMC or sorted T cells were cultured in T cell medium 51. Cell were cultured in either 25 mL (1 × 105–1 × 106 T cells) with 25 × 106 irradiated PBMC and 5 × 106 irradiated EBV‐transformed B cells as feeder cells or in 200 μL (800‐8,000 T cells) with 2 × 105 irradiated PBMC and 4×104 irradiated EBV‐transformed B cells as feeder cells. Cells were stimulated with 30 ng/mL αCD3 antibody (OKT3) in the absence of IL‐2 followed by adding 1 ng/mL IL‐2 after 24 h and thereafter. After 14–21 days, cells were resorted and cultured.
Single cell TCR sequencing
Ninety‐six‐well Eppendorf plates were coated with Vapor‐Lock (Qiagen). A total of 0.5 μL 5× Iscript buffer, 0.5 μL reverse transcriptase (Iscript, Bio‐Rad), 0.1% triton X‐100, and 1.25 μL H2O were added per well for cDNA synthesis. Single cells were sorted in these 96‐well plates on the FACSAria followed by centrifugation at 3000 RPM at 4°C for 10 min. CDNA synthesis and nested PCRs were performed as described by Wang et al. 44 with small modifications. For the first PCR reaction, the following conditions were used: 95°C for 2 min, 35 cycles of 95°C for 20 s, 50°C for 20 s, 72°C for 45 s, followed by one cycle of 72°C for 7 min. For the second PCR the following conditions were used: 95°C for 2 min, 35 cycles of 95°C for 20 s, 56°C for 20 s, 72°C for 45 s, followed by one cycle of 72°C for 7 min. Samples were loaded on 1.5% (α‐chain) or 2% (β‐chain) agarose gel for electrophoresis. Multiple wells that showed a result for both α‐chain and β‐chain on gel electrophoresis underwent Sanger sequencing.
ELISPOT
Ninety‐six‐well 0.45 μm Hydrophobic Multiscreen plates (Milipore) were coated with αIFN‐γ mAb (clone 1‐D1K, Mabtech) overnight at 4°C. The plate was blocked for 2 h after which 20 000 C1R cells transfected with CD1a or CD1b were co‐cultured with 200 T cells in the present or absence of a blocking antibody (clone BCD1b.3) and different antigens. The antigens used in this assay are BbGL‐II lipid (5 μg/mL), sonicated B. burgdorferi (200 000 bacteria per well) and media as control. After incubation overnight at 37°C cells were lysed and washed away with PBS‐Tween, and the plates were incubated for 2 h with a biotinylated αIFN‐γ antibody (clone 7‐B6‐1, Mabtech). The wells were washed with PBS‐Tween and incubated with Extravidin‐ALP (Sigma–Aldrich) for 1 h. After washing with PBS‐Tween followed by washing with PBS, 5‐Bromo‐4‐chloro‐3‐indolyl phosphate/Nitro blue tetrazolium (SigmaFAST tablets, Sigma) was added to visualize the spots. Spots were counted by the Immunospot reader (C.T.L Technologies).
Conflict of interest
The authors declare no commercial or financial conflict of interest.
Abbreviations
- BbGL‐II
Borrelia burgdorferi glycolipid II
- CID
collision‐induced dissociation
- DAG
diacylglycerol
- ESI
electrospray ionization
- MS
mass spectrometry
- PBMC
peripheral blood mononuclear cells
Supporting information
Supplemental _gure 1. Tetramer staining on Lyme arthritis patients
Raw Facs plots of data summarized in Fig. 4A. PBMCs of four Lyme arthritis patients in di_erent disease states (Table 1) were expanded via αCD3 stimulation for two weeks and stained with αCD3 antibody and CD1b‐BbGL‐II or CD1b‐endo tetramers. Cells are pre‐gated as shown.
Supplemental _gure 2. Tetramer staining on Lyme neuroborreliosis patients
Raw Facs plots of data summarized in Fig. 4A. PBMCs of eight Lyme neuroborreliosis patients in di_erent disease states (Table 1) were expanded via αCD3 stimulation for two weeks and stained with an αCD3 antibody and CD1b‐BbGL‐II or CD1b‐endo tetramers. Cells are pre‐gated as shown in Supplemental Fig.1.
Supplemental _gure 3. Tetramer staining on random blood donors
Raw Facs plots of data summarized in Fig. 4A. PBMCs of six random blood bank donors were expanded via αCD3 stimulation for two weeks and stained with an αCD3 antibody and CD1b‐BbGL‐II or CD1b‐endo tetramers. Cells are pre‐gated as shown in Supplemental Fig.1.
Supplemental _gure 4. Tetramer staining on random donors.
Raw Facs plots of data summarized in Fig. 4B. Ex vivo PBMCs of ten random blood bank donors were stained with tetramers. Gates are set based on staining with _CD3 antibody only. Cells are as shown in Supplemental Fig.1.
Supplemental _gure 5. Tetramer staining on random blood donors
Raw Facs plots of data summarized in Fig. 4C. PBMCs of six random blood bank donors were expanded via αCD3 stimulation for two weeks and stained with an αCD3 antibody and CD1b‐DAG or CD1b‐endo tetramers. Cells are pre‐gated as shown in Supplemental Fig.1.
Acknowledgements
This work was supported by funding from Nederlands Wetenschappelijk Onderzoek grant 824.02.002 and the NIH (AI116604, AI04393). P.R. was supported by a Boehringer Ingelheim Fonds travel grant. M.N.T.S. was supported by an Australian Postgraduate Award. M.J.S., D.I.G., and D.G.P. are supported by the National Health and Medical Research Council (NHMRC, 1063587) and the Australian Research Council (ARC; CE140100011). D.I.G. is also supported by NHMRC Senior Principal Research Fellowship (1020770) and D.G.P. is supported by an NHMRC Career Development Fellowship (APP1144308).
The peer review history for this article is available at https://publons.com/publon/10.1002/eji.201847949
References
- 1. Calabi, F. , Jarvis, J. M. , Martin, L. and Milstein, C. , Two classes of CD1 genes. Eur. J. Immunol. 1989. 19: 285–292. [DOI] [PubMed] [Google Scholar]
- 2. Dougan, S. K. , Kaser, A. and Blumberg, R. S. , CD1 expression on antigen‐presenting cells. Curr. Top Microbiol. Immunol. 2007. 314: 113–141. [DOI] [PubMed] [Google Scholar]
- 3. Rossjohn, J. , Gras, S. , Miles, J. J. , Turner, S. J. , Godfrey, D. I. and McCluskey, J. , T cell antigen receptor recognition of antigen‐presenting molecules. Annu. Rev. Immunol. 2015. 33: 169–200. [DOI] [PubMed] [Google Scholar]
- 4. Van Rhijn, I. and Moody, D. B. , Donor unrestricted T cells: A shared human t cell response. J. Immunol. 2015. 195: 1927–1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Beckman, E. M. , Porcelli, S. A. , Morita, C. T. , Behar, S. M. , Furlong, S. T. and Brenner, M. B. , Recognition of a lipid antigen by CD1‐restricted alpha beta+ T cells. Nature 1994. 372: 691–694. [DOI] [PubMed] [Google Scholar]
- 6. Porcelli, S. , Brenner, M. B. , Greenstein, J. L. , Balk, S. P. , Terhorst, C. and Bleicher, P. A. , Recognition of cluster of differentiation 1 antigens by human CD4‐CD8‐cytolytic T lymphocytes. Nature 1989. 341: 447–450. [DOI] [PubMed] [Google Scholar]
- 7. Krutzik, S. R. , Tan, B. , Li, H. , Ochoa, M. T. , Liu, P. T. , Sharfstein, S. E. , Graeber, T. G. et al., TLR activation triggers the rapid differentiation of monocytes into macrophages and dendritic cells. Nat. Med. 2005. 11: 653–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Roura‐Mir, C. , Wang, L. , Cheng, T. Y. , Matsunaga, I. , Dascher, C. C. , Peng, S. L. , Fenton, M. J. et al., Mycobacterium tuberculosis regulates CD1 antigen presentation pathways through TLR‐2. J. Immunol. 2005. 175: 1758–1766. [DOI] [PubMed] [Google Scholar]
- 9. Layre, E. , Collmann, A. , Bastian, M. , Mariotti, S. , Czaplicki, J. , Prandi, J. , Mori, L. et al., Mycolic acids constitute a scaffold for mycobacterial lipid antigens stimulating CD1‐restricted T cells. Chem. Biol. 2009. 16: 82–92. [DOI] [PubMed] [Google Scholar]
- 10. Moody, D. B. , Reinhold, B. B. , Guy, M. R. , Beckman, E. M. , Frederique, D. E. , Furlong, S. T. , Ye, S. et al., Structural requirements for glycolipid antigen recognition by CD1b‐restricted T cells. Science 1997. 278: 283–286. [DOI] [PubMed] [Google Scholar]
- 11. Van Rhijn, I. , Kasmar, A. , de Jong, A. , Gras, S. , Bhati, M. , Doorenspleet, M. E. , de Vries, N. et al., A conserved human T cell population targets mycobacterial antigens presented by CD1b. Nat. Immunol. 2013. 14: 706–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chancellor, A. , Tocheva, A. S. , Cave‐Ayland, C. , Tezera, L. , White, A. , Al Dulayymi, J. R. , Bridgeman, J. S. et al., CD1b‐restricted GEM T cell responses are modulated by Mycobacterium tuberculosis mycolic acid meromycolate chains. Proc. Natl. Acad. Sci. USA 2017. 114: E10956–E10964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Van Rhijn, I. , Iwany, S. K. , Fodran, P. , Cheng, T. Y. , Gapin, L. , Minnaard, A. J. and Moody, D. B. , CD1b‐mycolic acid tetramers demonstrate T‐cell fine specificity for mycobacterial lipid tails. Eur. J. Immunol. 2017. 47: 1525–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gilleron, M. , Stenger, S. , Mazorra, Z. , Wittke, F. , Mariotti, S. , Bohmer, G. , Prandi, J. et al., Diacylated sulfoglycolipids are novel mycobacterial antigens stimulating CD1‐restricted T cells during infection with mycobacterium tuberculosis. J. Exp. Med. 2004. 199: 649–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. James, C. A. , Yu, K. K. Q. , Gilleron, M. , Prandi, J. , Yedulla, V. R. , Moleda, Z. Z. , Diamanti, E. et al., CD1b tetramers identify T cells that recognize natural and synthetic diacylated sulfoglycolipids from Mycobacterium tuberculosis . Cell Chem Biol 2018. 25: 392–402 e314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shahine, A. , Van Rhijn, I. , Cheng, T. Y. , Iwany, S. , Gras, S. , Moody, D. B. and Rossjohn, J. , A molecular basis of human T cell receptor autoreactivity toward self‐phospholipids. Sci Immunol 2017. 2: eaao1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shamshiev, A. , Donda, A. , Prigozy, T. I. , Mori, L. , Chigorno, V. , Benedict, C. A. , Kappos, L. et al., The alphabeta T cell response to self‐glycolipids shows a novel mechanism of CD1b loading and a requirement for complex oligosaccharides. Immunity. 2000. 13: 255–264. [DOI] [PubMed] [Google Scholar]
- 18. Van Rhijn, I. , van Berlo, T. , Hilmenyuk, T. , Cheng, T. Y. , Wolf, B. J. , Tatituri, R. V. , Uldrich, A. P. et al., Human autoreactive T cells recognize CD1b and phospholipids. Proc. Natl. Acad. Sci. USA 2016. 113: 380–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shahine, A. , Reinink, P. , Reijneveld, J. F. , Gras, S. , Holzheimer, M. , Cheng, T. Y. , Minnaard, A. J. et al., A T‐cell receptor escape channel allows broad T‐cell response to CD1b and membrane phospholipids. Nat. Commun. 2019. 10: 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pfister, H. W. , Wilske, B. and Weber, K. , Lyme borreliosis: basic science and clinical aspects. Lancet 1994. 343: 1013–1016. [DOI] [PubMed] [Google Scholar]
- 21. Steere, A. C. , Strle, F. , Wormser, G. P. , Hu, L. T. , Branda, J. A. , Hovius, J. W. , Li, X et al., Lyme borreliosis. Nat. Rev. Dis. Primers 2016. 2: 16090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Stanek, G. , Wormser, G. P. , Gray, J. and Strle, F. , Lyme borreliosis. Lancet 2012. 379: 461–473. [DOI] [PubMed] [Google Scholar]
- 23. Arvikar, S. L. and Steere, A. C. , Diagnosis and treatment of Lyme arthritis. Infect. Dis. Clin. North Am. 2015. 29: 269–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Collins, C. , Shi, C. , Russell, J. Q. , Fortner, K. A. and Budd, R. C. , Activation of gamma delta T cells by Borrelia burgdorferi is indirect via a TLR‐ and caspase‐dependent pathway. J. Immunol. 2008. 181: 2392–2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Crowley, J. T. , Drouin, E. E. , Pianta, A. , Strle, K. , Wang, Q. , Costello, C. E. and Steere, A. C. , A highly expressed human protein, apolipoprotein B‐100, serves as an autoantigen in a subgroup of patients with Lyme disease. J. Infect. Dis. 2015. 212: 1841–1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Crowley, J. T. , Strle, K. , Drouin, E. E. , Pianta, A. , Arvikar, S. L. , Wang, Q. , Costello, C. E. et al., Matrix metalloproteinase‐10 is a target of T and B cell responses that correlate with synovial pathology in patients with antibiotic‐refractory Lyme arthritis. J. Autoimmun. 2016. 69: 24–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Drouin, E. E. , Seward, R. J. , Strle, K. , McHugh, G. , Katchar, K. , Londono, D. , Yao, C. et al., A novel human autoantigen, endothelial cell growth factor, is a target of T and B cell responses in patients with Lyme disease. Arthritis Rheum. 2013. 65: 186–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ben‐Menachem, G. , Kubler‐Kielb, J. , Coxon, B. , Yergey, A. and Schneerson, R. , A newly discovered cholesteryl galactoside from Borrelia burgdorferi. Proc. Natl. Acad. Sci. USA 2003. 100: 7913–7918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Toledo, A. , Huang, Z. , Coleman, J. L. , London, E. and Benach, J. L. , Lipid rafts can form in the inner and outer membranes of Borrelia burgdorferi and have different properties and associated proteins. Mol. Microbiol. 2018. 108: 63–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hossain, H. , Wellensiek, H. J. , Geyer, R. and Lochnit, G. , Structural analysis of glycolipids from Borrelia burgdorferi . Biochimie 2001. 83: 683–692. [DOI] [PubMed] [Google Scholar]
- 31. Pozsgay, V. , Kubler‐Kielb, J. , Coxon, B. , Marques, A. , Robbins, J. B. and Schneerson, R. , Synthesis and antigenicity of BBGL‐2 glycolipids of Borrelia burgdorferi, the causative agent of Lyme disease. Carbohydr. Res. 2011. 346: 1551–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jones, K. L. , Seward, R. J. , Ben‐Menachem, G. , Glickstein, L. J. , Costello, C. E. and Steere, A. C. , Strong IgG antibody responses to Borrelia burgdorferi glycolipids in patients with Lyme arthritis, a late manifestation of the infection. Clin. Immunol. 2009. 132: 93–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stubs, G. , Fingerle, V. , Wilske, B. , Gobel, U. B. , Zahringer, U. , Schumann, R. R. and Schroder, N. W. , Acylated cholesteryl galactosides are specific antigens of borrelia causing lyme disease and frequently induce antibodies in late stages of disease. J. Biol. Chem. 2009. 284: 13326–13334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ostberg, Y. , Berg, S. , Comstedt, P. , Wieslander, A. and Bergstrom, S. , Functional analysis of a lipid galactosyltransferase synthesizing the major envelope lipid in the Lyme disease spirochete Borrelia burgdorferi . FEMS Microbiol. Lett. 2007. 272: 22–29. [DOI] [PubMed] [Google Scholar]
- 35. Mansour, S. , Tocheva, A. S. , Cave‐Ayland, C. , Machelett, M. M. , Sander, B. , Lissin, N. M. , Molloy, P. E. et al., Cholesteryl esters stabilize human CD1c conformations for recognition by self‐reactive T cells. Proc. Natl. Acad. Sci. USA 2016. 113: E1266–E1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gadola, S. D. , Zaccai, N. R. , Harlos, K. , Shepherd, D. , Castro‐Palomino, J. C. , Ritter, G. , Schmidt, R. R. et al., Structure of human CD1b with bound ligands at 2.3 A, a maze for alkyl chains. Nat. Immunol. 2002. 3: 721–726. [DOI] [PubMed] [Google Scholar]
- 37. Huang, S. , Cheng, T. Y. , Young, D. C. , Layre, E. , Madigan, C. A. , Shires, J. , Cerundolo, V. et al., Discovery of deoxyceramides and diacylglycerols as CD1b scaffold lipids among diverse groove‐blocking lipids of the human CD1 system. Proc. Natl. Acad. Sci. USA 2011. 108: 19335–19340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kinjo, Y. , Tupin, E. , Wu, D. , Fujio, M. , Garcia‐Navarro, R. , Benhnia, M. R. , Zajonc, D. M. et al., Natural killer T cells recognize diacylglycerol antigens from pathogenic bacteria. Nat. Immunol. 2006. 7: 978–986. [DOI] [PubMed] [Google Scholar]
- 39. Kumar, H. , Belperron, A. , Barthold, S. W. and Bockenstedt, L. K. , Cutting edge: CD1d deficiency impairs murine host defense against the spirochete, Borrelia burgdorferi . J. Immunol. 2000. 165: 4797–4801. [DOI] [PubMed] [Google Scholar]
- 40. Tupin, E. , Benhnia, M. R. , Kinjo, Y. , Patsey, R. , Lena, C. J. , Haller, M. C. , Caimano, M. J. et al., NKT cells prevent chronic joint inflammation after infection with Borrelia burgdorferi . Proc. Natl. Acad. Sci. USA 2008. 105: 19863–19868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yakimchuk, K. , Roura‐Mir, C. , Magalhaes, K. G. , de Jong, A. , Kasmar, A. G. , Granter, S. R. , Budd, R. et al., Borrelia burgdorferi infection regulates CD1 expression in human cells and tissues via IL1‐beta. Eur. J. Immunol. 2011. 41: 694–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Moody, D. B. , TLR gateways to CD1 function. Nat. Immunol. 2006. 7: 811–817. [DOI] [PubMed] [Google Scholar]
- 43. Van Rhijn, I. , Gherardin, N. A. , Kasmar, A. , de Jager, W. , Pellicci, D. G. , Kostenko, L. , Tan, L. L. et al., TCR bias and affinity define two compartments of the CD1b‐glycolipid‐specific T cell repertoire. J. Immunol. 2014. 193: 5338–5344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wang, G. C. , Dash, P. , McCullers, J. A. , Doherty, P. C. and Thomas, P. G. , T cell receptor alphabeta diversity inversely correlates with pathogen‐specific antibody levels in human cytomegalovirus infection. Sci. Transl. Med. 2012. 4: 128ra142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Li, D. , Hong, A. , Lu, Q. , Gao, G. F. , Jin, B. , Screaton, G. R. and Xu, X. N. , A novel role of CD1c in regulating CD1d‐mediated NKT cell recognition by competitive binding to Ig‐like transcript 4. Int. Immunol. 2012. [DOI] [PubMed] [Google Scholar]
- 46. Wun, K. S. , Reijneveld, J. F. , Cheng, T. Y. , Ladell, K. , Uldrich, A. P. , Le Nours, J. , Miners, K. L. et al., T cell autoreactivity directed toward CD1c itself rather than toward carried self lipids. Nat. Immunol. 2018. 19: 397–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Heemskerk, M. H. , Hoogeboom, M. , de Paus, R. A. , Kester, M. G. , van der Hoorn, M. A. , Goulmy, E. , Willemze, R. et al., Redirection of antileukemic reactivity of peripheral T lymphocytes using gene transfer of minor histocompatibility antigen HA‐2‐specific T‐cell receptor complexes expressing a conserved alpha joining region. Blood 2003. 102: 3530–3540. [DOI] [PubMed] [Google Scholar]
- 48. Pianta, A. , Drouin, E. E. , Crowley, J. T. , Arvikar, S. , Strle, K. , Costello, C. E. and Steere, A. C. , Annexin A2 is a target of autoimmune T and B cell responses associated with synovial fibroblast proliferation in patients with antibiotic‐refractory Lyme arthritis. Clin. Immunol. 2015. 160: 336–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cotton, R. N. , Shahine, A. , Rossjohn, J. and Moody, D. B. , Lipids hide or step aside for CD1‐autoreactive T cell receptors. Curr. Opin. Immunol. 2018. 52: 93–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Cossarizza, A. , Chang, H. D. , Radbruch, A. , Akdis, M. , Andra, I. , Annunziato, F. , Bacher, P. et al., Guidelines for the use of flow cytometry and cell sorting in immunological studies. Eur. J. Immunol. 2017. 47: 1584–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ly, D. , Kasmar, A. G. , Cheng, T. Y. , de Jong, A. , Huang, S. , Roy, S. , Bhatt, A. et al., CD1c tetramers detect ex vivo T cell responses to processed phosphomycoketide antigens. J. Exp. Med. 2013. 210: 729–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental _gure 1. Tetramer staining on Lyme arthritis patients
Raw Facs plots of data summarized in Fig. 4A. PBMCs of four Lyme arthritis patients in di_erent disease states (Table 1) were expanded via αCD3 stimulation for two weeks and stained with αCD3 antibody and CD1b‐BbGL‐II or CD1b‐endo tetramers. Cells are pre‐gated as shown.
Supplemental _gure 2. Tetramer staining on Lyme neuroborreliosis patients
Raw Facs plots of data summarized in Fig. 4A. PBMCs of eight Lyme neuroborreliosis patients in di_erent disease states (Table 1) were expanded via αCD3 stimulation for two weeks and stained with an αCD3 antibody and CD1b‐BbGL‐II or CD1b‐endo tetramers. Cells are pre‐gated as shown in Supplemental Fig.1.
Supplemental _gure 3. Tetramer staining on random blood donors
Raw Facs plots of data summarized in Fig. 4A. PBMCs of six random blood bank donors were expanded via αCD3 stimulation for two weeks and stained with an αCD3 antibody and CD1b‐BbGL‐II or CD1b‐endo tetramers. Cells are pre‐gated as shown in Supplemental Fig.1.
Supplemental _gure 4. Tetramer staining on random donors.
Raw Facs plots of data summarized in Fig. 4B. Ex vivo PBMCs of ten random blood bank donors were stained with tetramers. Gates are set based on staining with _CD3 antibody only. Cells are as shown in Supplemental Fig.1.
Supplemental _gure 5. Tetramer staining on random blood donors
Raw Facs plots of data summarized in Fig. 4C. PBMCs of six random blood bank donors were expanded via αCD3 stimulation for two weeks and stained with an αCD3 antibody and CD1b‐DAG or CD1b‐endo tetramers. Cells are pre‐gated as shown in Supplemental Fig.1.


