Abstract
Dysgeusia (abnormal taste) is common in those with chronic kidney disease and contributes to poor nutritional intake. Previous sensory work has shown that taste improves after dialysis sessions. The goal of this pilot study was to characterize altered taste perceptions in patients on dialysis compared with healthy adults, and to evaluate relationships between serum parameters with taste perceptions. We hypothesized that patients undergoing dialysis would experience blunted taste intensities compared with controls, and that serum levels of potential tastants would be inversely related to taste perception of compounds. Using a cross-sectional design, we carried out suprathreshold sensory assessments (flavor intensity and liking) of tastants/flavors potentially influenced by kidney disease and/or the dialysis procedure. These included sodium chloride, potassium chloride, calcium chloride, sodium phosphate, phosphoric acid, urea, ferrous sulfate, and monosodium glutamate. Individuals on maintenance hemodialysis (n= 17, 10 males, range 23–87 years) were compared with controls with normal gustatory function (n=29, 13 males, range 21–61 years). Unadjusted values for intensity and liking for the solutions showed minimal differences. However, when values were adjusted for participants’ perceptions of water (as a control for taste abnormalities), intensity of monosodium glutamate, sodium chloride, and sodium phosphate solutions were more intense for patients on dialysis compared with controls. Some significant correlations were also observed between serum parameters, particularly potassium, for dialysis patients and sensory ratings. These results suggest altered taste perception in patients during dialysis warrants further study.
Keywords: chronic kidney disease, dysgeusia, hemodialysis, taste
Introduction
Chronic kidney disease (CKD) affects approximately 11–13% of the worldwide population (Hill et al. 2016). Progression of the disease can often warrant the commencement of dialysis, with hemodialysis being the most common modality of renal replacement therapy. Patients receiving dialysis are subject to prescriptive diets (Kalantar-Zadeh et al. 2015), which can help increase dialysis effectiveness by improving parameters such as serum electrolytes, acid–base balance, and blood pressure (Mc Causland et al. 2012; Beerendrakumar and Haridasan 2018). Despite the multitude of benefits attributed to these prescribed diets, poor dietary adherence is still a major issue, as recent systematic review (Oquendo et al. 2017) noted that 25–86% of hemodialysis patients do not adhere to these diets. This can predispose patients to a higher risk of malnutrition and hence, poorer survival outcomes and quality of life (Boltong and Campbell 2013; Lynch et al. 2013).
One explanation for this poor adherence is dysgeusia, abnormal taste sensation, which affects ~35% of end-stage renal disease patients (Lynch et al. 2013). Some commonly noted taste disturbances include reduced taste acuity, impaired detection of salty taste and reporting that certain foods taste“metallic-like” (Boltong and Campbell 2013; McMahon et al. 2014). Abnormalities in taste sensation may adversely affect the palatability of food and thus decrease adherence to renal diets.
Fluid imbalances, uremic toxin accumulation, metabolic derangements, and zinc deficiency are some hypothesized mechanisms linked with the onset of dysgeusia (Carrero 2011; Boltong and Campbell 2013; Lynch et al. 2013; Neto et al. 2016). Specific to CKD patients, imbalances in ions, uremic toxins, or other small compounds in blood could be contributing to altered vascular and salivary concentrations of solutes (Manley et al. 2012). This may alter the baseline at which oral chemoreceptor cells are responding to stimuli in foods. Vascular taste is when taste cells respond to tastants in the blood from the basolateral side of the receptor cell; as CKD patients have altered dynamics and levels of various taste active stimuli in blood (e.g., sodium, potassium, urea, etc.), vascular taste could be altered in these individuals. Further, oral chemosensation could also be altered through salivary changes, as prior research has shown that CKD patients have altered salivary composition of several compounds that are active chemosensory stimuli in foods, including calcium, potassium, and urea (Manley et al. 2012; Seethalakshmi et al. 2014; Rodrigues et al 2016). This may be escalated by specific taste genetics that are sensitive to the increased salivary urea often found in this particular patient group (Manley 2015). Additionally, previous studies have implied that salivary and serum concentrations of these compounds are correlated and that taste sensations improve following dialysis sessions (Burge et al. 1979; Shepherd et al. 1986; Farleigh et al. 1987; Seethalakshmi et al. 2014; Rodrigues et al 2016). Hence, alterations in saliva or vascular taste due to serum abnormalities may play a mechanistic role in these altered taste perceptions.
Previous studies have examined this hypothesis for five primary tastes: sweet, salty, bitter, sour, and umami (Burge et al. 1979; Shepherd et al. 1986; Farleigh et al. 1987; Manley et al. 2012; McMahon et al. 2014). However, other salts and small molecules are also chemosensory stimuli, and the differences among these less prototypical “tastants” has not been evaluated. Thus, this pilot study aimed to test how hemodialysis patients perceive a wider range of chemosensory stimuli, specifically focusing on ions and other small molecules that are likely to be altered in serum for CKD patients compared with healthy controls.
Materials and methods
Study design
This pilot study used a cross-sectional design to compare perception of taste-active compounds in dialysis patients (n = 17) versus a control group (n = 29). A sensory assessment was conducted in which participants provided feedback on flavor intensity and liking/disliking for a variety of stimuli that may be present at abnormal concentrations in the blood and/or saliva of patients undergoing dialysis.
Participants
The target population for this study was adult patients with end-stage renal disease attending a local dialysis clinic in Lafayette, IN, for thrice weekly maintenance hemodialysis (n = 17). All participants were invited to take part in the study during their normal scheduled dialysis treatment session. Control subjects (n = 29) were recruited through the Purdue University Saliva, Perception, Ingestion, and Tongues (SPIT) Laboratory participant pool. Inclusion criteria for the control subjects included the following: self-reported normal gustatory function, no issues with salivation, or dry mouth; ≥18 years of age; and no tongue, lip, or cheek piercings. All participants gave written informed consent prior to participating in this study. The protocol was approved by the Human Subjects Institutional Review Board of Purdue University and registered at www.clinicaltrials.gov (NCT03495271).
Tasting solutions
Solutions are listed in Table 1. All chemicals were food grade, and all were purchased from Sigma-Aldrich with the exception of calcium chloride (Modernist Pantry); and monosodium glutamate (Ajinomoto). The solutions were presented to subjects in 15-mL aliquots at room temperature. All solutions were prepared on the day before each testing.
Table 1.
Concentration of solutions used in the taste assessment
| Compounds | Molarity (M) | %(w/w) | Sensory quality |
| Sodium chloride | 0.2 | 1.16 | Salty |
| Potassium chloride | 0.01 | 0.74 | Salty, bitter |
| Calcium chloride | 0.15 | 1.62 | Calcium tastea, metallic |
| Sodium phosphate | 0.0063 | 0.09 | Salty, phosphorous tastea |
| Phosphoric acid | 0.007 | 0.37 | Sour |
| urea | 0.5 | 2.91 | Bitter |
| Ferrous sulfate | 0.025 | 0.69 | Metallic |
| Monosodium glutamate | 0.01 | 0.17 | Umami |
| Deionised water | — | — | Control (solvent) |
aThese “tastes” are under debate as potential gustatory sensations; we will refer to them as tastes for simplicity in this report, but readers should consult other articles to understand the state of the science regarding these compounds as taste stimuli (Tordoff et al. 2012; Tordoff 2017).
Tasting protocol
Each solution was presented at room temperature to participants in a blinded fashion and in counterbalanced order. We aimed to carry out the dialysis taste assessments at the beginning of the patient’s dialysis session, but this was not always consistent due to the clinical setup.
As these stimuli are generally unpleasant, all participants tasted a mixture of urea and potassium chloride sample first to control for bias during the initial exposure to the unpleasant sensation (termed “first sample effect” in the sensory field, or “initial elevation bias” in psychology; Shrout et al. 2018). Participants tasted 15-mL aliquots of each sample and expectorated after 10 s. After tasting each solution, participants reported perceived flavor intensity and liking/disliking of the solution. Participants rinsed their mouths with spring water (Ice Mountain brand bottled water) between each sample.
Sensory questionnaire
Sensory questions were asked verbally by experimenters and data were recorded using RedJade sensory software. For each sample, the experimenter asked the participant to rate the overall flavor intensity of the solution on a scale from 0 to 100, with 0 being no sensation and 100 being the strongest sensation ever experienced. Participants were familiarized with this intensity scale using a warm-up questionnaire, which asked about the brightness of this room, the brightness of the sun, the loudness of a shout, the loudness of a whisper, the bitterness of black coffee, and the sweetness of pure sugar (adapted from Hayes et al. 2013). For the samples, participants also reported their liking for the sensation, with 0 being the “worst thing ever” and 100 being the “best thing ever”.
Blood sample collections
Nonfasting serum blood samples (8 mL) were drawn from dialysis access following taste assessments and analyzed by Mid America Clinical Laboratories. Samples were targeted to be collected within 30 min of the taste assessment, but this varied considerably from subject to subject due to the active clinical environment.
Statistical analysis
Data were analyzed using SAS for Windows, version 9.4. Significant differences between the variables were assessed using mixed models controlling for year of birth, sex, order effects, and subjects (as a repeated measure); the Kenward Roger method was applied for calculation of degrees of freedom. The dependent variables were flavor intensity or liking/disliking rating, and the variables of interest were the sample type, group (control or dialysis), and the interaction of group and sample type. Statistical code is available in supplemental files. Sensory ratings were analyzed both as unadjusted as well as adjusted for each participant’s perception of water (Water adjusted rating = Original rating – water rating). This approach not only controlled for between-subject variability in how they used the scale but also controlled for baseline abnormalities in perception of water. Water is not a neutral stimulus, and different sources of water can lead to changes in perception of flavor intensity and/or sensitivity to tastes (Dalton et al. 2000; Hoehl et al. 2010). Deionized water, which was the solvent in this study, is often described as bitter or metallic, perhaps because the pH is actually below neutral (Whelton et al. 2007). Subtracting the rating of the water from the rating of the tastant solutions, thus, gives a better idea of how individual participants perceived the solutes in contrast to a standard (deionized water) with minimal solutes. Thus, the water-adjusted ratings were calculated for each individuals’ intensity and liking ratings for every test solution. Alpha was set at 0.05 across all tests. Spearman correlations were used to identify possible relationships between serum parameters and taste perceptions in the dialysis patients.
Results
Baseline characteristics of the study population
Participant characteristics are reported in Table 2. The control group was significantly younger than the dialysis group (P < 0.001). Baseline taste abnormalities were reported by 43.8% of the dialysis cohort. Abnormal sensations reported included that “everything tastes bitter/sour,” “some fruits don’t taste as sweet,” “higher salt threshold,” and “metallic tastes.”
Table 2.
Participant characteristics
| Control | Dialysis | |
| N | 29 | 17 |
| Gender Male, n (%)Female, n (%) | 13 (48.1)16 (51.9) | 10 (62.5)7 (37.5) |
| Age (years) | 32 (range 21–61) | 61 (range 23–87)* |
| Taste abnormalities, N (%) | - | 7 (43.8) |
*P < 0.05, dialysis vs. control.
Flavor intensity
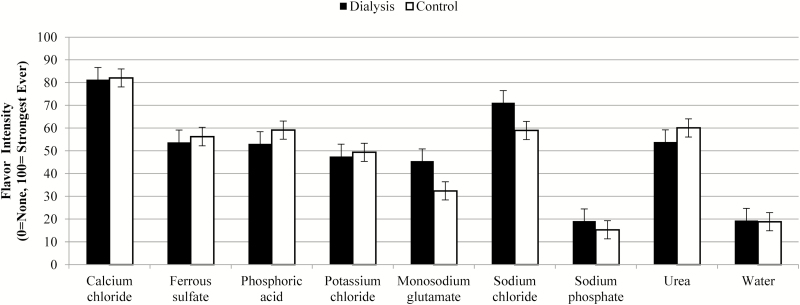
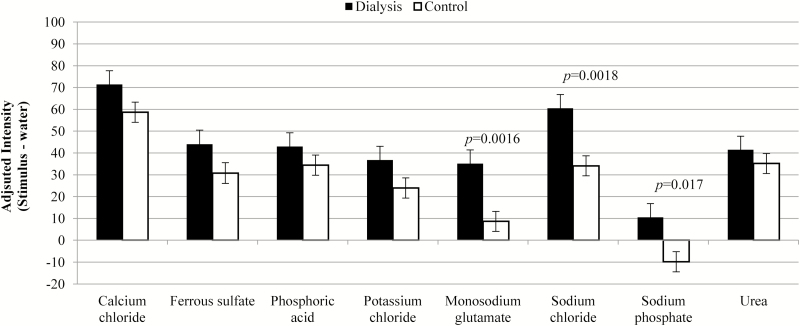
Unadjusted flavor intensity values are presented in Figure 1 and showed no differences (P = 0.73) between groups overall, only trends in effects for interactions within sample types. After adjustment for deionized water taste, significant differences emerged (Figure 2, P = 0.044 between groups). Specifically, water-corrected ratings for monosodium glutamate (P = 0.0016), sodium chloride (P = 0.0018), and sodium phosphate (P = 0.017) were higher for dialysis patients compared with control participants.
Figure 1.
Mean and standard error for flavor intensity, unadjusted.
Figure 2.
Mean and standard error for flavor intensity after adjustment for the perception of water (original rating—water rating; positive values indicate the sample was rated as more intense than water).
Hedonic ratings
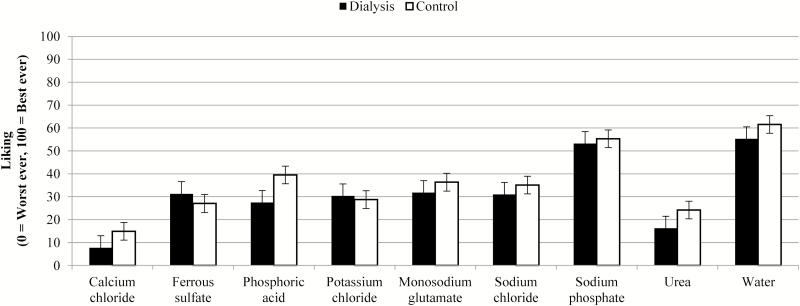
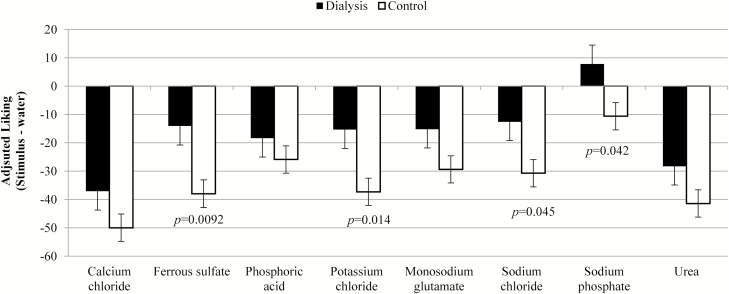
Liking/disliking values are presented in Figures 3 and 4. Unadjusted liking scores (Figure 3) highlights general, and similar (P = 0.37 between groups, no significant interactions) disliking for the solutions across both groups, which is signified by a mean score of <50 (i.e., values were closer to “worst ever” side of the scale). Adjusted liking data are shown in Figure 4 and are more negative due to more dislike for the flavors versus water. The dialysis group’s adjusted liking ratings were less negative than the control group’s, indicating the patients on dialysis rated the samples closer to water for liking than controls (P = 0.023), which could indicate the dialysis group actually found the solutions closer to hedonically neutral than the control group. Specific samples driving this difference between the groups were ferrous sulfate (P = 0.0092), potassium chloride (P = 0.014), sodium chloride (P = 0.045), and sodium phosphate (P = 0.042).
Figure 3.
Mean and standard error for liking of compounds, unadjusted.
Figure 4.
Mean and standard error for liking of compounds after adjustment for the perception of water (Original rating—water rating; negative numbers indicate water was liked more than the sample, and numbers to closer to zero mean the sample was rated more similarly to water).
Serum parameters and taste
Serum results for the patients on dialysis are reported in Table 3, and significant correlations are shown in Table 4. One sample was excluded due to hemolysis. Spearman correlations were conducted between the sensory ratings and serum levels of compounds of interest. In unadjusted ratings, a negative correlation was observed between serum glucose and urea flavor intensity (P = 0.035); negative correlations for unadjusted liking ratings were also observed between flavor intensity of monosodium glutamate and creatinine (P = 0.033). In water-adjusted ratings, a positive correlation was observed between serum potassium and taste intensity of monosodium glutamate (P = 0.019); in adjusted liking ratings, positive correlations were observed between serum potassium and phosphoric acid (P = 0.0008), potassium chloride (P = 0.027), urea (P = 0.028), and calcium chloride (P = 0.028). Negative correlations were observed between adjusted liking ratings for urea and serum carbon dioxide (P = 0.038) and between ferrous sulfate and serum sodium (P = 0.045).
Table 3.
Serum parameters for dialysis patients
| Blood parameters | Ref. range a | Mean |
| Magnesium (mg/dL) | 1.6–2.6 | 2.04 ± 0.17 |
| Sodium (mmol/L) | 136–145 | 137.60 ± 2.06 |
| Potassium (mmol/L) | 3.5–5.1 | 4.31 ± 0.56 |
| Calcium (mg/dL) | 8.4–10.5 | 8.91 ± 0.63 |
| Phosphorous (mg/dL) | 2.5–4.7 | 3.37 ± 1.69 |
| Chloride (mmol/L) | 98–110 | 98.93 ± 2.25 |
| Carbon dioxide (mmol/L) | 20–29 | 24.27 ± 3.90 |
| Glucose (mg/dL) | 65–99 | 128.07 ± 58.39 |
| Urea nitrogen (mg/dL) | 10–20 | 33.40 ± 17.14 |
| Creatinine (mg/dL) | 0.70–1.20 | 5.24± 3.14 |
| Albumin (mg/dL) | 3.5–5.0 | 3.57 ± .35 |
aReference range provided by Mid-America Clinical Laboratories.
Table 4.
Spearman correlations between sensory ratings and serum parameters
| Rating type | Sensory stimulus | Serum parameter | Spearman Rho | P-value |
| Unadjusted flavor | Urea | Glucose | −0.55 | 0.035 |
| Water adjusted flavor | Monosodium glutamate | Potassium | 0.60 | 0.019 |
| Unadjusted liking | Monosodium glutamate | Creatinine | −0.55 | 0.033 |
| Water adjusted liking | Phosphoric acid | Potassium | 0.77 | 0.0008 |
| Potassium chloride | Potassium | 0.57 | 0.027 | |
| Urea | Potassium | 0.57 | 0.028 | |
| Calcium chloride | Potassium | 0.56 | 0.028 | |
| Urea | Carbon dioxide | −0.54 | 0.038 | |
| Ferrous sulfate | Sodium | −0.52 | 0.045 |
Discussion
In the present pilot study, we found water-adjusted flavor and liking intensity scores were different between control and dialysis patients. Specifically, dialysis patients reported a more intense sensation for three sodium containing salts (monosodium glutamate, sodium chloride, and sodium phosphate). Differences in adjusted liking ratings appear to be primarily due to ferrous sulfate, potassium chloride, sodium chloride, and sodium phosphate being rated closer to water ratings (near neutral on the hedonic scale) for the dialysis group compared to control. The differences found in the water-adjusted data, but not unadjusted data, suggest that baseline taste perception may be an important factor for dysgeusia in dialysis patients and should be better characterized in future studies.
Prior studies have generally shown that patients with CKD often experience lower taste intensity and/or sensitivity for sodium containing compounds, along with other tastants. One study (Manley et al. 2012) conducted suggested that CKD patients have an impaired ability to identify sour, bitter, and glutamate tastes. Another study (McMahon et al. 2014) also reported significantly lower intensity scores for monosodium glutamate and sodium chloride. In that particular study, higher salivary and serum sodium levels correlated with lower sensitivity to tasting sodium (McMahon et al. 2014). A possible explanation for differences between these reports and our current work is that our taste assessments were not performed in the dialysis patients until they had undergone some of their dialysis treatment. Although we aimed to complete the assessment at the beginning of treatment, this was not feasible due to the busy clinical setting, and on occasion was not conducted until >30 min after dialysis commencement. It is possible that excess salivary and serum sodium was filtered through the dialysate, reducing their sodium taste-threshold and improving sensitivity. Indeed, previous research has shown that dialysis treatment removes excess salivary metabolites in a mirror-like fashion to serum filtration (Seethalakshmi et al. 2014; Khanum, Mysore-Shivalingu et al. 2017). In addition, this has been linked to improved taste function postdialysis (Burge et al. 1979). Older studies have indicated increased sensitivity and decreased preference for sodium chloride post dialysis which may further explain the higher ratings noted in our dialysis group by comparison to healthy controls (Farleigh et al. 1987; Shepherd et al. 1987; Leshem and Rudoy 1997). Furthermore, given the difference in our findings between water-adjusted and unadjusted assessments, and the lack of major correlations with serum levels for sodium, it is possible that baseline abnormalities in taste are more important than acute changes during dialysis.
In our study, unadjusted liking scores were generally rated <50 on the scales in both patients and controls which indicated overall negative hedonic reaction to the solutions. These lower ratings were expected given that the solutions were characteristically unpalatable, with some leaving lingering tastes (e.g., ferrous sulfate and monosodium glutamate, in particular). However, food ingredients lead to very different affective responses when presented in foods versus in solution. Monosodium glutamate, for example, can make a variety of foods more palatable but is generally unpleasant when tasted in isolation. Patients undergoing dialysis indicated that sodium phosphate, sodium chloride, potassium chloride, and ferrous sulfate solutions tasted closer to a “neutral” water their control counterparts. However, distractions from the dialysis procedure itself may have influenced these ratings. In general, we would expect the busy clinical environment of a dialysis unit to confound liking ratings. However, we would have expected the negative feelings of the environment (due to having to go through the process of dialysis) could leech into negative affect for the stimuli presented. This was not the case. Future studies should be conducted in a better controlled environment, or with controls in a similar clinical environment to the patients attending dialysis.
We detected few associations between serum parameters and hemodialysis patient’s flavor ratings in the present study. We did however observe that serum potassium, in particular, correlated with water-adjusted hedonic ratings for a number of compounds. This may imply a role for potassium in the hedonic perception of other flavors. As several potassium channels are proposed to influence different types of taste (particularly sour and fatty tastes; Gilbertson et al. 1997; Challis and Ma 2016), imbalances in potassium may alter taste cell signaling, resulting in abnormalities in the quality of sensations and changes in effect. This should be pursued in further work, both in patients on dialysis as well as healthy controls.
Prior research indicates that taste thresholds of renal patients increase with age and this finding is also in agreement with results of studies on healthy subjects (Ciechanover et al. 1980; Vreman et al. 1980; Ng et al. 2004; Ogawa et al. 2017). Therefore, it is important to consider the fact that our dialysis and control groups were not demographically well matched, especially in terms of age. Age was included as a covariate in our statistical model and indeed indicated that younger subjects had higher ratings, even when adjusted for water. This is consistent with other work. However, our patients on dialysis actually gave higher ratings than the younger controls, which is directly the opposite of what we would expect for an age effect, and indeed is also opposite from what we saw in our own model’s age effect. Certainly, matching the groups for age could improve our understanding of these potential differences between groups, but a multitude of other confounding variables may also impact on our ability to conduct taste tests in renal patients. Medications, diet, and other chronic diseases can play an influential role on taste perception, each of which are difficult to control for, especially in older subjects who have many health issues (Boltong and Campbell 2013).
There are several other limitations to this study which must also be considered. As a pilot study, the sample size was small and thus results should be considered preliminary. Second, the control group did not have serum parameters measured for comparison. Furthermore, our ability to assess the serum–taste perception relationship was restrained considering our serum samples were drawn late into the dialysis session. Future larger studies should be pursued using controlled, or at least comparable, environments and protocols to minimize confounding factors in our clinical setting.
Finally, our findings of greater differences when controlling for water perception should be further investigated. Deionized water itself stimulates sensation in the mouth, often of greater intensity than tap or spring waters (Hoehl et al. 2010). We did not find a difference in taste intensity of deionized water between our groups in the current study, but this concept should be further investigated to determine if individual differences in serum and salivary solutes contribute to differences in perception of water, or some sort of partitioning of solutes within the deionized water, which could then alter perception of other dissolved solids. Our findings indicate that it may be important to correct for this baseline sensation of the solvent in future work to investigate dysgeusia in patients undergoing hemodialysis.
Conclusion
The findings of this study add to the body of evidence suggesting that taste changes occur with CKD. Our work emphasizes the need to investigate taste and flavor active compounds beyond the prototypical taste stimuli for sweet, sour, salty, bitter, and umami tastes. As many known tastants are found in human serum and saliva, and are dysregulated with CKD, these nontypical stimuli are prime candidates for contributing to dysgeusia accompanying CKD. We identified CKD patients experienced altered taste intensity for compounds that include a sodium ion (greater intensity for monosodium glutamate, sodium chloride, and sodium phosphate) and lesser dislike for ferrous sulfate, potassium chloride, sodium chloride, and sodium phosphate compared with healthy controls, when correcting for the subjects’ perceptions of deionized water. More research is required to fully evaluate how dysgeusia is experienced by CKD patients.
Funding
This work was supported by an Indiana CTSI Project Development Team [grant number NIH UL1TR00118] to K.M.H.G.; K.M.H.G. receives support through K01 DK102864 and R.N.M. receives support through K23 DK102824.
Conflict of interest
The authors have no conflicts of interest to declare.
Acknowledgements
This work was presented in part at the American Society of Nephrology Kidney Week 2017 Meeting in New Orleans, LA (Fitzgerald C, Moorthi RN, Moe SM, Hill Gallant KM, Running CA. A pilot study characterizing dysgeusia in hemodialysis patients. J Am Soc Nephrol 28, 2017: 723)
References
- Beerendrakumar N, Ramamoorthy L, Haridasan S. 2018. Dietary and fluid regime adherence in chronic kidney disease patients. J Caring Sci. 7:17–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boltong A., Campbell K. 2013. ‘Taste’ changes: a problem for patients and their dietitians. Nutr Diet. 70(4):262–269. [Google Scholar]
- Burge JC, Park HS, Whitlock CP, Schemmel RA. 1979. Taste acuity in patients undergoing long-term hemodialysis. Kidney Int. 15:49–53. [DOI] [PubMed] [Google Scholar]
- Carrero JJ. 2011. Mechanisms of altered regulation of food intake in chronic kidney disease. J Ren Nutr. 21:7–11. [DOI] [PubMed] [Google Scholar]
- Challis RC, Ma M. 2016. Sour taste finds closure in a potassium channel. Proc Natl Acad Sci U S A. 113:246–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciechanover M, Peresecenschi G, Aviram A, Steiner JE. 1980. Malrecognition of taste in uremia. Nephron. 26:20–22. [DOI] [PubMed] [Google Scholar]
- Dalton P, Doolittle N, Nagata H, Breslin PA. 2000. The merging of the senses: integration of subthreshold taste and smell. Nat Neurosci. 3:431–432. [DOI] [PubMed] [Google Scholar]
- Farleigh CA, Shepherd R, Jevons S, Pryor JS. 1987. Effects of haemodialysis on taste for salt in relation to changes in blood constituents. Hum Nutr Clin Nutr. 41:441–451. [PubMed] [Google Scholar]
- Gilbertson TA, Fontenot DT, Liu L, Zhang H, Monroe WT. 1997. Fatty acid modulation of K+ channels in taste receptor cells: gustatory cues for dietary fat. Am J Physiol. 272:C1203–C1210. [DOI] [PubMed] [Google Scholar]
- Hayes JE, Allen AL, Bennett SM. 2013. Direct comparison of the generalized Visual Analog Scale (gVAS) and general Labeled Magnitude Scale (gLMS). Food Qual Prefer. 28:36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill NR, Fatoba ST, Oke JL, Hirst JA, O’Callaghan CA, Lasserson DS, Hobbs FD. 2016. Global prevalence of chronic kidney disease—a systematic review and meta-analysis. PLoS One. 11:e0158765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoehl K., Schoenberger G., U. and e. al. 2010. Water quality and taste sensitivity for basic tastes and metallic sensation. Food Qual Pref. 21(2):243–249. [Google Scholar]
- Kalantar-Zadeh K, Tortorici AR, Chen JL, Kamgar M, Lau WL, Moradi H, Rhee CM, Streja E, Kovesdy CP. 2015. Dietary restrictions in dialysis patients: is there anything left to eat? Semin Dial. 28:159–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanum N, Mysore-Shivalingu M, Basappa S, Patil A, Kanwar S. 2017. Evaluation of changes in salivary composition in renal failure patients before and after hemodialysis. J Clin Exp Dent. 9:e1340–e1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leshem M, Rudoy J. 1997. Hemodialysis increases the preference for salt in soup. Physiol Behav. 61:65–69. [DOI] [PubMed] [Google Scholar]
- Lynch KE, Lynch R, Curhan GC, Brunelli SM. 2013. Altered taste perception and nutritional status among hemodialysis patients. J Ren Nutr. 23:288–295.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manley KJ. 2015. Taste genetics and gastrointestinal symptoms experienced in chronic kidney disease. Eur J Clin Nutr. 69:781–785. [DOI] [PubMed] [Google Scholar]
- Manley KJ, Haryono RY, Keast RSJ. 2012. Taste changes and saliva composition in chronic kidney disease. Ren Soc Austr J. 8(2):56–60. [Google Scholar]
- Mc Causland FR, Waikar SS, Brunelli SM. 2012. Increased dietary sodium is independently associated with greater mortality among prevalent hemodialysis patients. Kidney Int. 82:204–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon EJ, Campbell KL, Bauer JD. 2014. Taste perception in kidney disease and relationship to dietary sodium intake. Appetite. 83:236–241. [DOI] [PubMed] [Google Scholar]
- Neto LC, Bacci MR., Sverzutt LC, Costa MG, Alves BCA, Fonseca FL. 2016. The role of zinc in chronic kidney disease patients on hemodialysis: a systematic review. Health 8:344–352. [Google Scholar]
- Ng K, Woo J, Kwan M, Sea M, Wang A, Lo R, Chan A, Henry CJ. 2004. Effect of age and disease on taste perception. J Pain Symptom Manage. 28:28–34. [DOI] [PubMed] [Google Scholar]
- Ogawa T, Annear MJ, Ikebe K, Maeda Y. 2017. Taste-related sensations in old age. J Oral Rehabil. 44:626–635. [DOI] [PubMed] [Google Scholar]
- Oquendo LG, Asencio JMM, de Las Nieves CB. 2017. Contributing factors for therapeutic diet adherence in patients receiving haemodialysis treatment: an integrative review. J Clin Nurs. 26:3893–3905. [DOI] [PubMed] [Google Scholar]
- Rodrigues VP, Franco MM, Marques CP, de Carvalho RC, Leite SA, Pereira AL, Benatti BB. 2016. Salivary levels of calcium, phosphorus, potassium, albumin and correlation with serum biomarkers in hemodialysis patients. Arch Oral Biol. 62:58–63. [DOI] [PubMed] [Google Scholar]
- Seethalakshmi C, Koteeswaran D, Chiranjeevi V. 2014. Correlation of Serum and Salivary biochemical parameters in end stage renal disease patients undergoing hemodialysis in pre and post-dialysis state. J Clin Diagn Res. 8:CC12–CC14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd R, Farleigh CA, Atkinson C, Pryor JS. 1987. Effects of haemodialysis on taste and thirst. Appetite. 9:79–88. [DOI] [PubMed] [Google Scholar]
- Shepherd R, Farleigh CA, Pryor JS. 1986. Changes in salt taste in dialysis and their relationship to blood constituents. Percept Mot Skills. 62:343–347. [DOI] [PubMed] [Google Scholar]
- Shrout PE, Stadler G, Lane SP, McClure MJ, Jackson GL, Clavél FD, Iida M, Gleason MEJ, Xu JH, Bolger N. 2018. Initial elevation bias in subjective reports. Proc Natl Acad Sci U S A. 115:E15–E23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tordoff MG. 2017. Phosphorus taste involves T1R2 and T1R3. Chem Senses. 42:425–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tordoff MG, Alarcón LK, Valmeki S, Jiang P. 2012. T1R3: a human calcium taste receptor. Sci Rep. 2:496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vreman HJ, Venter C, Leegwater J, Oliver C, Weiner MW. 1980. Taste, smell and zinc metabolism in patients with chronic renal failure. Nephron. 26:163–170. [DOI] [PubMed] [Google Scholar]
- Whelton AJ, Dietrich AM, Burlingame GA, Schechs M, Duncan SE. 2007. Minerals in drinking water: impacts on taste and importance to consumer health. Water Sci Technol. 55:283–291. [DOI] [PubMed] [Google Scholar]