Abstract
Nutraceuticals present in food are molecules able to exert biological activity for the prevention and treatment of various diseases, in form of pharmaceutical preparations, such as capsules, cream, or pills. Myrtus communis L. is a spontaneous Mediterranean evergreen shrub, widely known for the liqueur obtained from its berries rich in phytochemicals such as tannins and flavonoids. In the present study, we aimed to evaluate the properties of myrtle byproducts, residual of the industrial liqueur processing, in Adipose-derived stem cells (ADSCs) induced at oxidative stress by in vitro H2O2 treatment. Cells were exposed for 12-24 and 48h at treatment with extracts and then senescence-induced. ROS production was then determined. The real-time PCR was performed to evaluate the expression of inflammatory cytokines and sirtuin-dependent epigenetic changes, as well the modifications in terms of stem cell pluripotency. The β-galactosidase assay was conducted to analyze stem cell senescence after treatment. Our results show that industrial myrtle byproducts retain a high antioxidant and antisenescence activity, protecting cells from oxidative stress damages. The results obtained suggest that residues from myrtle liqueur production could be used as resource in formulation of food supplements or pharmaceutical preparations with antioxidant, antiaging, and anti-inflammatory activity.
1. Introduction
Nutraceuticals can be defined as a branch of biomedical science based on the biological action of specific molecules, which are part of food [1]. The officinal plants, described also as medicinal, have long been studied for their antiseptic, antibacterial, and anti-inflammatory properties [2]. They are also useful in the presence of allergies or diseases of the respiratory system and for cutaneous local treatment, for example, in case of psoriasis, eczema, and sunburn, thus performing protective action against various diseases [3, 4].
In recent years, the attention of researchers has been addressed to the study and the use of medicinal plants in clinical practice [5], thanks to their special antioxidant properties, cell cycle inhibition, promotion of tissue regeneration, and inhibition of acute inflammation [6, 7]. The role of free radical reactions, in fact, is well known for some pathologies.
Deregulation of reactive oxygen species (ROS) balancing is a key event during the onset and progression of pathological conditions related to aging, as cancer, diabetes, atherosclerosis, neurodegeneration, and inflammatory diseases [8–10]. Currently, it is also believed that even prooxidant agents or processes may exert a role in cell homeostasis, by stimulating the antioxidant defense system in cells and tissues, the so-called “oxidative stress preconditioning” or hormesis [11]. The term hormesis indicates an agent exerting a biphasic effect: at low doses representing a stimulus, while being toxic at high doses [12].
Recently, the development of nutraceuticals has become a great issue also applied to regenerative medicine. Regenerative medicine is based on the capability of stem cells to repair tissue damage and restore cellular homeostasis, by substituting damaged elements [13]. Although bone marrow has been used as the main source of hMSCs (hBMSCs), bone marrow collection remains a relatively invasive and painful procedure. Furthermore, the use of hBMSCs is potentially associated with a high degree of viral infection and a significant decline in cell viability and differentiation with donor age [14]. An ideal hMSCs source should allow the isolation of large amount of stem cells, collected with a minimally invasive procedure, and provide a hMSCs population maintaining good vitality and differentiating potential with donor's age [15, 16]. Adipose tissue represents a great source of stem cells, called Adipose-derived stem cells (ADSCs) [17], usually considered wasting material. hADSCs exhibit phenotypic and gene expression profiles similar to hMSCs obtained from bone marrow [18–20] and, under chemical and physical stimuli, are able to acquire different phenotypes, thus participating in tissue regenerative process [21–23]. Within this context, we have previously demonstrated that vitamin D together with melatonin exerts an important role in orchestrating stem cell fate [24] through activation of HDAC and sirtuins [25]. Moreover, other different natural molecules have been previously used by our group to achieve a specific cellular phenotype from human stem cells obtained from different sources [26, 27] or to control cell proliferation in hepatocarcinoma cells [28]. Another important key point in regenerative medicine is the loss of regenerative potential of stem cells during aging [29], which is deeply related to cellular senescence and ROS production [30]. Within these contexts, finding a natural product able to counteract senescence in stem cells and restore their regenerative potential could have a great impact in the interventions for age-related diseases. Berries of Myrtus communis L. (Myrtaceae), an evergreen spontaneous shrub, typical of the Mediterranean areas, contain a large amount of bioactive polyphenols [31, 32], mainly anthocyanins [33–35], and have long been known for anti-inflammatory, antiseptic, and antimicrobial properties [36]. In Sardinia, Myrtus communis is commonly used in the food industry, for the production of sweet liqueur obtained by hydroalcoholic extraction of the berries [37], to which follows the production of large quantities of residues (about 30.000 Kg/year) and waste material. The use of these biomasses is still limited being underexploited as compost for agricultural purposes. However, they represent large potential resources for use as food supplements, food additives, or as a raw material for the preparation of extracts with antimicrobial activity [38].
The aim of the present study was to analyze the effect of myrtle extracts on the molecular pathway controlling ADSCs senescence, obtained by H2O2 exposure in vitro. The use of these residues in medicine could represent large alternative resources in the cosmetic and pharmacological industry, to be used for the treatment of various diseases [39].
2. Materials and Methods
2.1. Biomass Collection
The biomasses of myrtle (byproducts), residual of the preparation of the homonymous liqueur, were used in this study. The industrial byproducts, kindly provided by a distillery located in Sardinia, were obtained after a process of infusion of the myrtle berries in hydroalcoholic solution for about 30 days. At the end of the infusion period, the berries were pressed and left to dry at room temperature to remove residual alcohol. In order to mimic the industrial process, a small-scale infusion was carried out in laboratory. With this purpose, berries belonging to 8 myrtle cultivars were harvested at maturity at the experimental orchard located in the “Antonio Milella” station of the University of Sassari (central western Sardinia, Italy) and mixed together with the aim of providing a uniform sample like that of the industrial supply. According to the industrial process, 200 g of berries was mixed with 750 mL of a hydroalcoholic solution (70% v/v) and stored for 30 days at room temperature (20°C) in dark bottles. At the end of the infusion period, the byproducts were separated from the hydroalcoholic extract, pressed, and freeze-dried. Once lyophilized, pulp and seeds were separated. The pulp of the industrial byproducts and that of the byproducts obtained in laboratory were used to prepare the extracts.
2.1.1. Chemicals
All reagents and solvents used in this process were of analytical grade, unless otherwise specified and used without further purification. 2,2-Diphenyl-1-picryhydrazyl radical (DPPH) was purchased from Alfa Aesar (London, UK). 5,5-Dimethyl-1-pyrroline-N-oxide (DMPO) was purchased from Enzo Life Sciences.
2.2. Preparation and Characterization of Myrtus Extracts
2.2.1. Preparation of Plant Extracts
Two grams of freeze-dried myrtle pulp byproducts were extracted twice with 40 mL of a methanol/water solution (70% MeOH) and sonicated in an ultrasonic cleaner (VWR International, Leuven, Belgium) for 1 hour at 25°C. The mixtures were centrifuged at 3000x g for 10 minutes. The organic extracts were filtered with Whatman 4 filter paper, evaporated to dryness under a nitrogen flow to remove methanol, and then freeze-dried to remove water. The freeze-dried extracts were used to assess the hydroxyl radical scavenging activity and the subsequent tests in vitro on stem cells.
2.3. Radical Scavenging Activity
2.3.1. DPPH Radical Scavenging Activity
The DPPH radical scavenging activity was determined spectrophotometrically according to Fadda et al., 2014 [40]. The water extract properly diluted was mixed to 100μL of DPPH (1 mM in absolute ethanol). The mixture was stored in the dark at room temperature for 1 h and UV-Vis-VIS readings were carried out with a spectrophotometer Agilent 8453 at 517 nm. The antiradical activity was expressed as TEAC units (mmols trolox/g of DW) using a Trolox calibration curve (5-20 μM, R2 = 0.99).
2.3.2. Hydroxyl Radical Scavenging Activity
The hydroxyl radical scavenging activity was determined with the spin trapping method coupled with Electron Paramagnetic Resonance (EPR) spectroscopy. The hydroxyl radicals were generated by Fenton reaction and trapped with a nitrone spin trap DMPO obtaining a DMPO-OH adduct [41]. In the Fenton reaction, iron(II) is oxidized by hydrogen peroxide to iron(III) generating a hydroxyl radical and a hydroxide ion. Before the analysis, the freeze-dried extracts were mixed with water in order to get the final concentration of 15 mg mL−1. The water extract, properly diluted, was mixed with 0.1 mM iron(II) sulfate, DMPO 26 mM, and hydrogen peroxide 0.03% (w/w) to a final volume of 1 mL with water. The DMPO adduct was detected with a Bruker EMX spectrometer operating at the X-band (9.4 GHz) using a Bruker Aqua-X capillary cell. EPR instrument was set under the following conditions: modulation frequency, 100 kHz; modulation amplitude, 1 G; receiver gain, 1 x 105; and microwave power, 20 mW. EPR spectra were recorded at room temperature immediately after the preparation of the reaction mixture. The concentration of the DMPO-OH adduct was estimated from the double integration of spectra. The percentage of inhibition was calculated against a blank with no extract applying the following formula:
| (1) |
where A0 is the concentration of the spin adduct without extract and As is the concentration of the adduct after the reaction with the extract. Results were expressed as EC50. Three replications were performed for each extract.
2.4. Cell Culturing and Treatment
ADSCs of human adult subcutaneous adipose tissue were obtained from male and female patients, during surgery processes (n=12, age=45± 15 years, BMI: 22 ± 3 kg/m2), after signing a written informed consent. Ethics Committee Review Boards for Human Studies in Sassari approved the study (n_ETIC 240I/CE 26 July 2016, Ethical committee, ASL Sassari). The samples collected were processed and the cells were isolated and characterized as previously described [24]. Cells at passage 5 were cultured in a basic growing medium composed of Dulbecco's modified Eagle's Medium (DMEM, Life Technologies, USA), 20% fetal bovine serum (FBS, Life Technologies, USA), 200 mM L-glutamine (Euroclone, Italy), and 200 U/mL penicillin−0.1 mg/mL streptomycin (Euroclone, Milano, Italy). To perform the tests in vitro, the freeze-dried Myrtus extracts was suspended in cultured medium at a final concentration of 0,5 mg/ml, derived from previously tests (data not shown), and then used directly in cell culture, for 12-24 and 48h. Cells used as control are cultured in the growing medium only. To induce senescence, after treatment with the extracts, cells were incubated for 1h with 100 μM H2O2 in basic growing medium. For positive control of antioxidant activity and stressful conditions, ADSCs were cultured in the same conditions with 100 μg/ml ascorbic acid (Sigma-Aldrich, Germany), known for its properties in regulation of immune system and chronic inflammation [42].
2.5. MTT Viability Assay
Cellular metabolic activity was evaluated by the Thiazolyl Blue Tetrazolium Bromide (MTT) assay (Sigma-Aldrich, Germany). Cells, cultured in the presence or absence of Myrtus extracts, in which senescence was induced as previously described, were seeded at a concentration of 10,000 cells/well in 96-well plates. After the attachment, cells were incubated with 200 μl of different extracts for 12, 24, and 48 hours and then induced to senescence with H2O2. The medium was removed and 100 μl MTT at final concentration of 0.65 mg/ml was added in each well and incubated for 2h. After incubation, formazan was dissolved in DMSO and absorbance detected at 570 nm using Varian50 MPR, Microplate reader. The viability of H2O2-senescenct cells precultured with Myrtus extracts (treated cells) was calculated as % cell viability referred to untreated control cells = (OD570 treated cells) × 100/(OD570 control).
2.6. SA-β-Gal Staining
To identify senescent cells in culture, “The Senescence Cells Histochemical Staining Kit” (Sigma-Aldrich, Germany) was used. ADSCs in 6-well plate are cultured for 12-24-48h in the presence or absence of Myrtus extracts and then induced to senescence with H2O2. At the end of the incubation time, the medium containing H2O2 was removed and the cells were fixed and processed according to the manufacturer's instructions. For evaluation of SA-β-Gal activity, cells were then observed by light microscopy. The number of positively blue-stained cells was calculated as the percentage of total number of cells.
2.7. Measuring of Nitric Oxide Production
To test the variation in nitric oxide (NO) production by the cells at different time points, Griess Reagent Kit for Nitrite Determination (Thermo Fisher Scientific, USA) was used. According to the manufacturer's instructions, 150 μl of nitrite standard solution was added to each well of 96-well plate and incubated for 30 minutes. The nitrite concentrations were read as the absorbance at 548 nm wavelength of the nitrite-containing samples in a spectrophotometric microplate reader.
2.8. Gene Expression Analysis of Real-Time PCR
Total mRNA was isolated at times 0, 12, 24, and 48 hours from cells treated in previously described conditions, at passage 5, and used for quantitative polymerase chain reaction. RNA extraction was performed using the ChargeSwitch total RNA Cell Kits (Life Technologies, Grand Island, NY, USA) and approximately 1μg of total RNA was reverse-transcribed into cDNA using the Superscript Vilo cDNA synthesis kit (Life Technologies, USA), according to the manufacturer's protocol. Quantitative polymerase chain reaction was performed in triplicate under standard qRT-PCR conditions (50°C for 2 min, 95°C for 2 min, and then cycled at 95°C for 15 s, 55–59°C for 30 s, and 60°C for 1 min, for 40 cycles), according to the qRT-PCR protocol specified in the Platinum® Quantitative PCR SuperMix-UDG Kit, using a CFX Thermal Cycler (Bio-Rad) (Applied Biosystems). The total volume of each reaction was 25 μL, composed by 2X SuperMix with SYBR Green I, 0.1 μM of each primer, and 3 μL cDNA generated from 1μg of the total RNA template. Target Ct values were normalized on hGAPDH, considered as a reference gene, while the mRNA levels of ADSCs treated in different conditions were expressed as fold of change (2−∆∆Ct) relative to the mRNA levels observed in ADSCs at time 0, before starting treatment. Each experiment included a distilled water control.
The qRT-PCR analysis was performed for the following genes: octamer-binding transcription factor 4 (Oct-4); Sex determining region Y-box 2 (Sox2); Homeobox protein Nanog (NANOG); NAD-dependent deacetylase sirtuin-1 (SIRT1); Interleukin 6 (IL-6); Tumor necrosis factor alpha (TNF-α); and Heat Shock Protein 90b (Hsp90b). All primers were designed with Primer3, spanning all exons and highly specific. They are from Invitrogen and are described in Table 1.
Table 1.
Primers sequences.
| Primers | Forward | Reverse |
|---|---|---|
| hGAPDH | GAGTCAACGGAATTTGGTCGT | GACAAGCTTCCCGTTCTCAG |
| Oct-4 | GAGGAGTCCCAGGCAATCAA | CATCGGCCTGTGTATATCCC |
| Sox2 | CCGTTCATGTAGGTCTCGGAGCTG | CAACGGCAGCTACAGCTAGATGC |
| NANOG | CATGAGTGTGGATCCAGCT | CCTGAATAAGCAGATCCAT |
| SIRT1 | CATTTTCCATGGCGCTGAGG | TGCTGGTGGAACAATTCCTGT |
| IL-6 | TCTCAACCCCCAATAA | GCCGTCGAGGATGTA |
| TNF-α | CCTCAGACGCCACAT | GAGGGCTGATTAGAGAGA |
| Hsp90b | AGTTGGAATTCAGGGCATTG | TTTCTCGGGAGATGTTCAGG |
2.9. Statistical Analysis
Statistical analysis was performed using Statistical Package for the Social Sciences version 13 Software (SPSS Inc., Chicago, IL, USA). The experiments were performed two times with three technical replicates for each treatment. The distributions of each group variance were evaluated with Kruskal-Wallis rank sum and Wilcoxon signed-rank test, assuming a p value <0.05 as statistically significant.
3. Results
3.1. Radical Scavenging Activity
Table 2 shows the hydroxyl and the DPPH radicals scavenging activities of myrtle byproducts. The DPPH radical scavenging activity of myrtle residues obtained from the industrial process was consistent with results of antiradical activity measured on fresh myrtle berries [43].
Table 2.
DPPH and hydroxyl radicals scavenging activities of myrtle byproducts obtained from the production of myrtle liqueur at industrial and laboratory level.
| Antiradical activity | DPPH | Hydroxyl radical |
|---|---|---|
| mmols trolox/g d.w. | EC50 (mg/mL) | |
| Industrial byproducts | 1,45 ± 0,01 | 0,48 ± 0,03 |
| Laboratory byproducts | ||
| Pulp | 1,06 ± 0,2 | 0,64 ± 0,01 |
Both in industrial products and in fresh berries, the pulp, rich in anthocyanins and flavonoids, showed a high DPPH radical scavenging activity as compared to other plant species [44]. The hydroxyl radical scavenging activity, measured in the same extracts, confirmed the highest antiradical activity of the evaluated products. The hydroxyl radical (·OH) is one of the major causes of oxidative stress in living cells; its low selectivity makes this radical extremely reactive towards lipids, proteins, and nucleic acids [44, 45].
3.2. Myrtus Extract Regulates Cytokine Secretion in Inflammatory Response
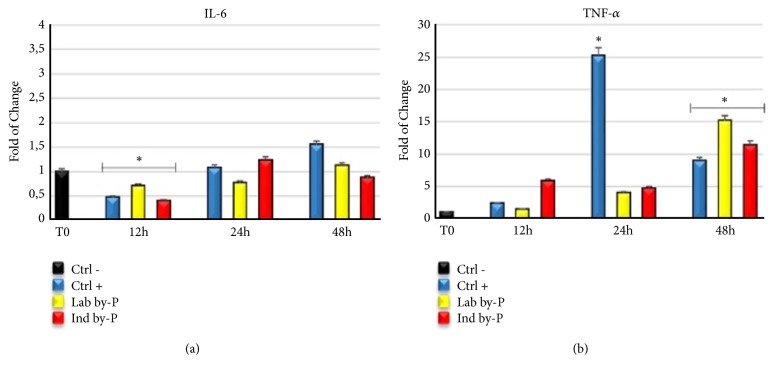
Figure 1 shows the expression of proinflammatory cytokines Interleukin 6 (IL-6) (Figure 1(a)) and Tumor necrosis factor alpha (TNF-α) (Figure 1(b)) in cells exposed to Myrtus extracts for 12, 24, or 48h. IL-6 significantly decreased at 12h of treatment, compared to untreated cells, for all cultured conditions, including industrial byproduct, a sign that after industrial liqueur production the berries retain some of their properties. On the other hand, TNF-α is upregulated after 48h of extracts exposure, suggesting that Myrtus can counteract the inflammation induced by oxidative stress, but at the same time, it may promote tissue regeneration by cytokine secretion and stem cell recruitment.
Figure 1.
Expression of proinflammatory cytokines Il-6 and TNF-α. The expression of Interleukin 6 (IL-6) (a) and Tumor necrosis factor alpha (TNF-α) (b) was evaluated in H2O2-senescent ADSCs exposed for 12, 24, or 48h to ascorbic acid (CTRL+, blue bar) or to Lab by-P (yellow bar) or Ind by-P (red bar). The mRNA levels for each gene were expressed as fold of change (2−∆∆Ct) of mRNA levels observed in untreated ADSCs (CTRL-, black bar) defined as 1 (mean ±SD; n=6) and normalized to Glyceraldehyde-3-Phosphate-Dehidrogenase (GAPDH). Data are represented as mean± SD referring to the control (∗ p ≤ 0.05).
3.3. Modulation of Nitric Oxide Production by Myrtus Treatment
Myrtus byproducts, both laboratory and industrial, have shown a potent antioxidant activity, decreasing significantly the nitric oxide (NO) production after induction of oxidative stress. This reduction was higher at 12 and 24h of treatment for both of Myrtus extracts, compared to untreated cells (Figure 2). The berries residual of liquor production have maintained their properties, exerting an important antioxidant response at stressor event.
Figure 2.
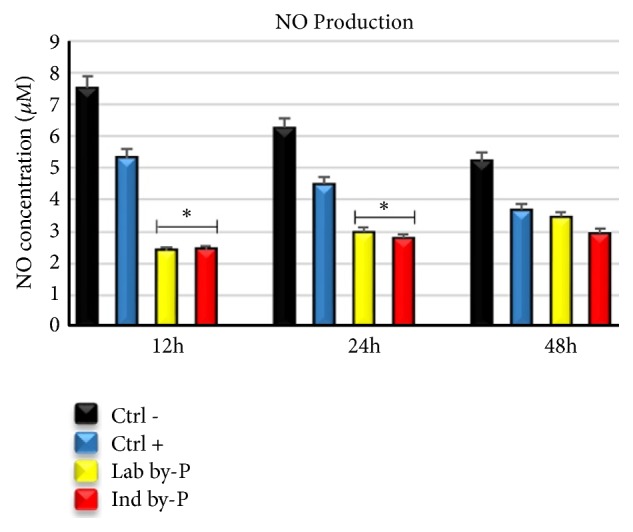
Measuring nitric oxide production after oxidative stress induction. The NO concentration was evaluated in ADSCs exposed for 12, 24, or 48h to ascorbic acid (CTRL+, blue bar), at Lab by-P (yellow bar), or at Ind by-P (red bar) and then induced to oxidative stress, compared to untreated H2O2-senescent cells (CTRL-, black bar). The nitrite concentrations were read as the absorbance at 548 nm for each sample and were expressed as mean± SD referring to the control (∗ p ≤ 0.05).
3.4. Gene Expression Analysis of Pluripotency Related Genes
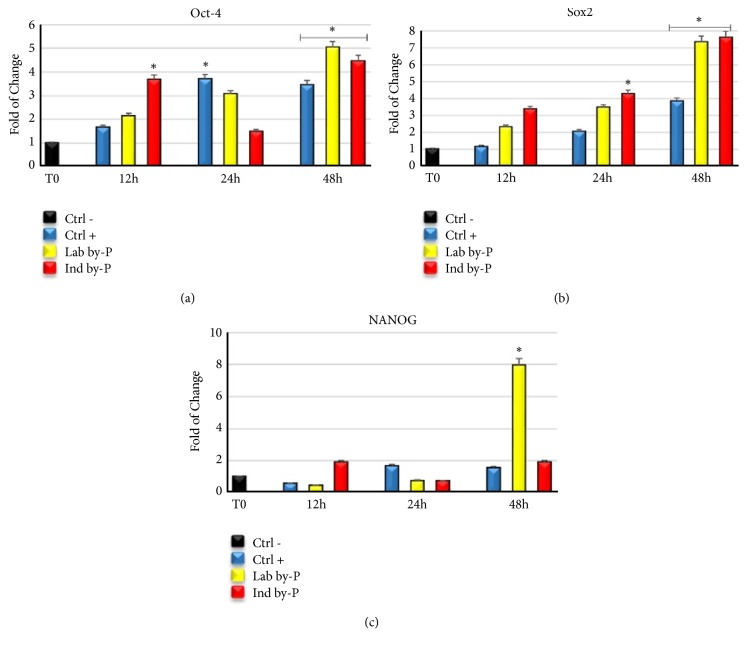
Exposure of ADSCs to laboratory and industrial byproducts revealed a significative upregulation of pluripotency related genes, Oct-4 (Figure 3(a)), Sox2 (Figure 3(b)), and NANOG (Figure 3(c)) compared to untreated cells, suggesting a promotion of regenerative potential of stem cells after stressful conditions. This overexpression of mRNA levels was already evident since the first hours of treatment, but reached its maximum after 48h.
Figure 3.
Expression of pluripotency related genes. The expression of octamer-binding transcription factor 4 (Oct-4) (a). Sex determining region Y-box 2 (Sox2) (b) and Homeobox protein Nanog (NANOG) (c) were evaluated in H2O2-senescent ADSCs exposed for 12, 24, or 48h to ascorbic acid (CTRL+, blue bar), at Lab by-P (yellow bar), or at Ind by-P (red bar). The mRNA levels for each gene were expressed as fold of change (2−∆∆Ct) of mRNA levels observed in untreated ADSCs (CTRL-, black bar) defined as 1 (mean ±SD; n=6) and normalized to Glyceraldehyde-3-Phosphate-Dehidrogenase (GAPDH). Data are represented as mean± SD referring to the control (∗ p ≤ 0.05).
3.5. The Antisenescent Effect of Myrtus Involves Sirtuin-Dependent Epigenetic Changes and Regulates the Expression of HSP
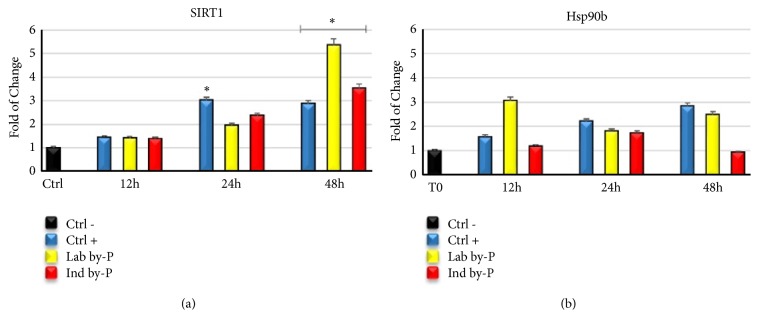
Figure 4 shows the capability of Myrtus extracts to induce SIRT1 activity with a significant increase in mRNA levels at 48h of treatment (panel (a)). Furthermore, treatment with Myrtus extracts has increased the levels of HSP90b (panel (b)), suggesting a role of this compound to protect cells from oxidative stress damage.
Figure 4.
Expression of Sirtuins and Heat Shock Proteins in ADSCs induced to oxidative stress. The expression of NAD-dependent deacetylase sirtuin-1 (SIRT1) (a) and Heat Shock Protein 90b (Hsp90B) (b) was evaluated in H2O2-senescent ADSCs exposed for 12, 24, or 48h to ascorbic acid (CTRL+, blue bar), to Lab by-P (yellow bar), or to Ind by-P (red bar). The mRNA levels for each gene were expressed as fold of change (2−∆∆Ct) of mRNA levels observed in untreated ADSCs (CTRL-, black bar) defined as 1 (mean ±SD; n=6) and normalized to Glyceraldehyde-3-Phosphate-Dehidrogenase (GAPDH). Data are represented as mean± SD referring to the control (∗ p ≤ 0.05).
3.6. Effects of Myrtus on ADSCs Senescence-Induced by H2O2 Treatment
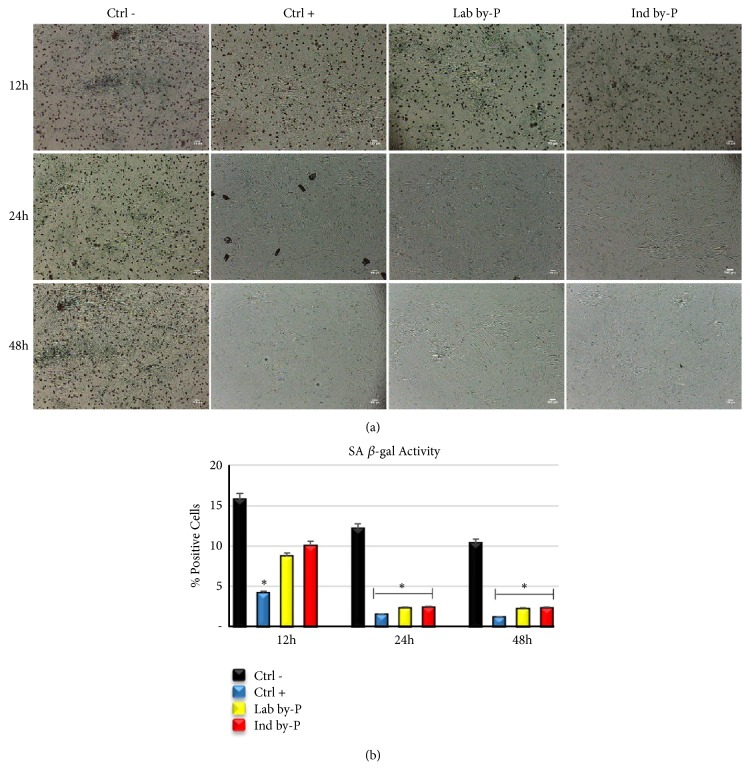
Consistent with previously described real-time PCR analysis, of protection from oxidative stress damages, Figure 5 shows the results from β-galactosidase staining assay, used to evaluate whether ADSCs treatment with Myrtus byproducts may oppose the premature senescence elicited by H2O2 treatment. Results have revealed that the extracts are able to significantly counteract the senescence process (panel (b)) and protect cells by oxidative stress damages. β-gal analysis at light microscope has revealed that both Lab and Ind by-P exert an antisenescence activity, that was higher for 24 and 48h of treatment, compared to untreated cells (panel (a)).
Figure 5.
Senescence-associated β-galactosidase activity. (a) β-galactosidase was evaluated in H2O2-senescent ADSCs treated with ascorbic acid (CTRL+), with Lab by-P, or with Ind by-P for 12, 24, or 48h, compared to untreated ADSCs (CTRL-). Scale bar=100 μm. (b) The numbers of positive (blue) and negative cells were counted under the light microscope and the percentage of SA-β-Gal-positive cells for each treatment was calculated as the number of positive cells divided by the total number of cells counted using an image software analysis (ImageJ). Data are expressed as mean± SD referring to the control (∗ p ≤ 0.05).
3.7. Myrtus Maintains Mitochondrial Activity in H2O2-Senescent ADSCs
The yellow tetrazolium salt is enzymatically converted into purple formazan precipitate in viable cells by mitochondrial succinate dehydrogenase. Our results showed that Myrtus extracts are able to preserve the mitochondrial activity and the viability of treated cells even after H2O2 exposure, for all different time-points, compared to the control untreated-senescent cells (Figure 6). Moreover, the waste industrial residues of Myrtus compounds are not cytotoxic for the cells, whose vitality is maintained, if not even increased, as compared to untreated controls not exposed to oxidative stress.
Figure 6.
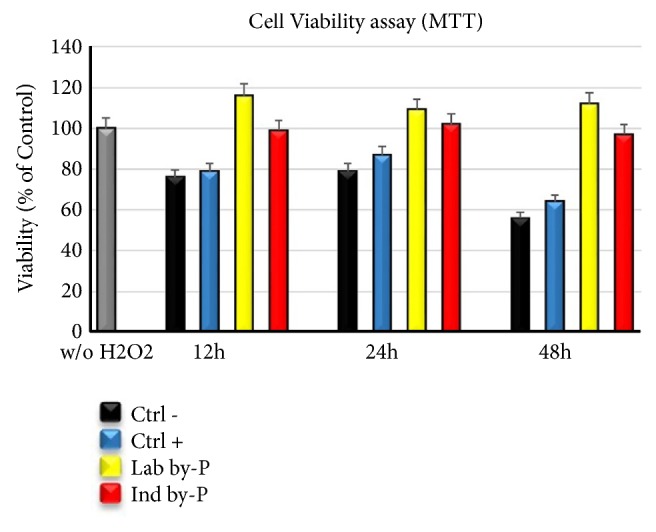
Mtt assay of the ADSCs treated with Myrtus extracts related to the untreated cells (grey bar). Cell Viability = {OD570 of treated cells} × 100%/{OD570 of control cells, considered as 100}. The data were entered using SPSS Version 2.0 (IBM SPSS, 2013). Data are expressed as mean± SD referring to the control (∗ p ≤ 0.05).
4. Discussion
The human body has the capability to cope with various environmental stresses, introducing adaptation mechanisms to restore the physiological balance. Altered functioning of biological processes is due to some pathological conditions such as aging, diabetes, atherosclerosis, and cancer [46]. In recent years, numerous studies have reported that natural molecules, like flavonoids and anthocyanins contained in food, show anticancer properties and protect against various diseases [47]. Some authors describe a significant cytotoxic effect of polyphenols on tumor cells with the inhibition of proliferation and consequent induction of apoptosis after treatment [48, 49].
Also flavonoids content in P. macrocarpa fruits have cytotoxic activities on different carcinoma cell lines, like human cervical, colon, and breast [50].
Myrtus is a spontaneous Mediterranean evergreen shrub used in the industrial field for the formulation of the liqueur [51], but it is also known for containing classes of biomolecules used as phytochemicals, including phenolic compounds such as tannins, quercetin, and gallic and ellagic acids in the seeds of its berry pulp [42, 50, 51]. As shown in Table 2, the laboratory and industrial byproducts used in this study have high radical scavenging activity, which depends on the percentage of anthocyanins, flavonoids, and tannins able to protect living cells from oxidative stress.
The molecules contained in myrtle berry are able to exert antimicrobic, anti-inflammatory, and antiaging activities and in particular are known for their free radical-scavenging activities, so they are used as a potent antioxidant [52]. Several in vitro studies have demonstrated that quercetin protects against oxidative damage and has a protective effect in stress-induced cells [53]. Extensive ROS production correlates with the loss of tissue homeostasis, leading to dysfunctional patterns, associated with inflammatory response [52, 53].
Inflammation is a frequently occurring response to several pathological conditions to protect tissue integrity against injuries, being also dangerous according to the dose and time of exposure [54, 55]. Oxidative stress induction with H2O2 treatment causes premature cellular senescence [56] and can activate an inflammatory response, associated with cytokine production [57]. Chemical components of Mediterranean plants, such as polyphenols, gallic acid derivatives, and flavonols, exert antioxidant and anti-inflammatory activity by inhibition of enzymes involved in ROS production [57, 58]. Polyphenols are capable of reducing ROS secretion and modulate the nitric oxide (NO) production and enzymatic activity involved in inflammatory response and cell activation, by downregulating TNF-α release and the levels of serological markers like IL-6 cytokine [56, 57]. Other authors described that treatment with S. grandiflora extracts decreased the level of IL-6 and TNF-α and colon inflammation in mice, related to ROS scavenging activity, and inhibitory action on inflammatory response [59]. Consistent with these studies, Myrtus extracts have shown a significative decrease in IL-6 expression at 12h of treatment, in cells cultured in the presence of Lab by-P, but also with Ind by-P, compared to H2O2-senescent ADSCs cultured in growing medium alone, demonstrating a potential anti-inflammatory effect of industrial waste materials (Figure 1(a)). The upregulation of IL6 and TNF-α (Figure 1(b)) suggests that Myrtus extracts may effectively counteract the inflammation induced by oxidative stress and at the same time promote tissue regeneration by stem cells recruitment. Inflammation in fact is an important response of the organisms after damage and plays an important role in tissue regeneration, through the secretion of TNF-α and IL-6 and in the presence of low ROS production [60]. Organisms possess a redox balance system able to modulate the cellular and tissue stress responses to maximize physiological defense processes [61]. High ROS production contributes to the development of many pathological conditions, proteins alteration, and activation of inflammatory acute response and premature cell senescence [62]. Phytochemicals and natural compounds can directly interact with nuclear receptors and enzymes of cell signaling, modulating natural antioxidant responses [59, 60]. Myrtle is described as able to exert antioxidant and anti-inflammatory activity in the treatment of different respiratory disorders, related to a fibrosis of the tissue and progressive loss of function [63]. The pretreatment for 12h-24h of H2O2-senescent ADSCs with different Myrtus byproducts has proved able to significantly decrease the NO production induced by H2O2-oxidative stress stimulation, compared to untreated cells (Figure 2). Myrtus extracts can, therefore, be considered involved in the activation of cell redox system, scavenging high amounts of ROS induced by oxidative stress and modulating the activity of enzymes recruited in antioxidant response and balancing.
A limited ROS production together with the modulation of TNF-α release plays a crucial role in stem cell recruitment in the sites of injuries, promoting cell migration and tissue regeneration [64]. Adipose-derived stem cells have self-renewal potential and can differentiate into different cell lineages, becoming adipocytes, chondrocytes, and osteocytes, representing an important resource in regenerative medicine [65]. In the present paper, ADSCs exposed to extracts, in particular Lab by-P, but also Ind by-P, showed a significant increase in mRNA levels of the principal markers of pluripotency, Oct-4, Sox2, and NANOG, as compared with untreated cells (Figure 3), suggesting that Myrtus berries have a high antioxidant activity and promote tissue regeneration after damage. This effect has also been previously shown by other authors, who describe an interesting circuit between SIRT1 and Nanog gene expression, modulated by ROS and p53 [66]. In the present paper, we show a similar gene expression trend between SIRT1 and Nanog after Myrtus extracts stimulation, reaching a maximum of their expression after 48 hours in culture (Figure 3).
Oct-4 is the main actor in stem cell pluripotency by suppressing molecular pathways of differentiation and by directly activating SIRT1 deacetylase. In actual fact, Oct-4 is correlated to the downregulation of SIRT1 and cell differentiation [67].
The NAD-dependent class III histone deacetylase sirtuin-1 (SIRT1) regulates various physiological processes and is involved in metabolism, stress response, and aging [68]. Some studies in vitro have demonstrated that inhibition of SIRT1 promotes the secretion of inflammatory cytokine, while, on the contrary, its overexpression prevents premature senescence [69]. Gene expression analysis in ADSCs treated with different Myrtus extracts (Lab by-P and Ind by-P) demonstrated that SIRT1 expression is upregulated starting from 12h of treatment, reaching a maximum after 24h-48h of treatment (Figure 4). This overexpression is strictly related to the prevention of premature senescence (Figure 5) and to a higher resistance to oxidative stress. β-gal analysis has revealed that both Lab and Ind by-P exert an antisenescence activity, in a time-dependent manner (higher for 24h and 48h of treatment), counteracting the premature ADSCs senescence, induced by H2O2 treatment, (Figure 5).
Our results demonstrate that Myrtus extracts have the capability to induce SIRT1 activity and prevent cell senescence in vitro in cells exposed to oxidative stress. Furthermore, phytochemicals are involved in Heat Shock-induced response as a mechanism for Self-Defense [70]. Heat Shock Proteins (HSPs) are mainly responsible for maintaining protein homeostasis and play an important role in aging [71].
Hsp90b is constitutively expressed in human cells, and its levels increased when cells are exposed to different kinds of stressors to maintain viability [72]. HSP inhibition associated with altered H2O2 balance leads to the generation of oxidative stress [73]. ADSCs treatment with Myrtus extracts has determined an increase in mRNA levels of Hsp90b (Figure 4(b)), which may suggest a role for this compound in stimulating the secretion of Hsp90b and protecting cells from oxidative stress damage. Myrtus industrial waste is able to protect the cells increasing their viability and maintaining their mitochondrial activity under stressing conditions (Figure 6).
5. Conclusions
Taken together, these results suggest that Myrtus have important antioxidant and protective activities to defend cells from stressful and harmful conditions, by epigenetically modulating HSP90b gene expression via SIRT1.
In addition, our findings demonstrate that Myrtus extracts can also have a regenerative potential by modulating stem cell pluripotency and inflammatory response. An intriguing observation suggests that the Ind by-P, residual of industrial production, maintains a large part of Myrtus properties and could be used as alternative resource in formulation of food supplements or cosmetics, as well as pharmaceutical preparations to be used for the treatment of various diseases.
Acknowledgments
The authors are grateful for the financial support from Eldor Lab SRL, Milan, Italy, Fondazione di Sardegna, Project “Myrtus 2.0: from waste to resource (Bioactivity of myrtle by-products)” and P.O.R. FSE 2014/2020 Regione Autonoma della Sardegna.
Data Availability
The data used to support the findings of this study are included within the article.
Conflicts of Interest
The authors declare that they have no conflicts of interest regarding the publication of this paper.
Authors' Contributions
Sara Cruciani and Sara Santaniello contributed equally to this work. Sara Cruciani, Sara Santaniello, and Margherita Maioli designed the experimental plan and conceived the idea of the article. Sara Cruciani, Angela Fadda, Margherita Maioli, and Carlo Ventura wrote the paper and reviewed scientific literature. Sara Cruciani performed data analysis and figures preparation. Sara Santaniello performed the experiments. Angela Fadda and Maurizio Mulas collected and characterized the plants. Luana Sale prepared all the berry extracts. Giorgia Sarais and Daniele Sanna characterized the extracts. Giorgio Carlo Ginesu, Maria Laura Cossu, and Pier Andrea Serra participated in sample collection. All the authors gave the final approval of the version to be submitted.
References
- 1.Kalra E. K. Nutraceutical - definition and introduction. AAPS PharmSciTech. 2003 doi: 10.1208/ps050325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bahiense J. B., Marques F. M., Figueira M. M., et al. Potential anti-inflammatory, antioxidant and antimicrobial activities of Sambucus australis. Pharmaceutical Biology. 2017;55(1):991–997. doi: 10.1080/13880209.2017.1285324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schönknecht K., Krauss H., Jambor J., Fal A. M. Treatment of cough in respiratory tract infections - the effect of combining the natural active compounds with thymol. Wiadomosci lekarskie (Warsaw, Poland : 1960) 2016;69(6):791–798. [PubMed] [Google Scholar]
- 4.Soory M. Relevance of nutritional antioxidants in metabolic syndrome, ageing and cancer: Potential for therapeutic targeting. Infectious Disorders - Drug Targets. 2009;9(4):400–414. doi: 10.2174/187152609788922537. [DOI] [PubMed] [Google Scholar]
- 5.Kaur K. Functional Nutraceuticals: Past, Present, and Future. 2016. [Google Scholar]
- 6.Gupta C., Prakash D. Phytonutrients as therapeutic agents. Journal of Complementary and Integrative Medicine. 2014;11(3):151–169. doi: 10.1515/jcim-2013-0021. [DOI] [PubMed] [Google Scholar]
- 7.Di Lorenzo C., Dell'agli M., Badea M., et al. Plant food supplements with anti-inflammatory properties: a systematic review (II) Critical Reviews in Food Science and Nutrition. 2013;53(5):507–516. doi: 10.1080/10408398.2012.691916. [DOI] [PubMed] [Google Scholar]
- 8.Le Lay S., Simard G., Martinez M. C., Andriantsitohaina R. Oxidative stress and metabolic pathologies: from an adipocentric point of view. Oxidative Medicine and Cellular Longevity. 2014;2014:18. doi: 10.1155/2014/908539.908539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blokhina O., Virolainen E., Fagerstedt K. V. Antioxidants, oxidative damage and oxygen deprivation stress: a review. Annals of Botany. 2003;91:179–194. doi: 10.1093/aob/mcf118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosa A., Tuberoso C. I. G., Atzeri A., Melis M. P., Bifulco E., Dessì M. A. Antioxidant profile of strawberry tree honey and its marker homogentisic acid in several models of oxidative stress. Food Chemistry. 2011;129(3):1045–1053. doi: 10.1016/j.foodchem.2011.05.072. [DOI] [PubMed] [Google Scholar]
- 11.Son T. G., Camandola S., Mattson M. P. Hormetic dietary phytochemicals. NeuroMolecular Medicine. 2008;10(4):236–246. doi: 10.1007/s12017-008-8037-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Calabrese E. J., Bachmann K. A., Bailer A. J., et al. Biological stress response terminology: Integrating the concepts of adaptive response and preconditioning stress within a hormetic dose-response framework. Toxicology and Applied Pharmacology. 2007 doi: 10.1016/j.taap.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 13.Patel D. M., Shah J., Srivastava A. S. Therapeutic potential of mesenchymal stem cells in regenerative medicine. Stem Cells International. 2013;2013:15. doi: 10.1155/2013/496218.496218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stolzing A., Jones E., McGonagle D., Scutt A. Age-related changes in human bone marrow-derived mesenchymal stem cells: consequences for cell therapies. Mechanisms of Ageing and Development. 2008;129(3):163–173. doi: 10.1016/j.mad.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 15.Tremolada C., Palmieri G., Ricordi C. Adipocyte transplantation and stem cells: plastic surgery meets regenerative medicine. Cell Transplantation. 2010;19(10):1217–1223. doi: 10.3727/096368910x507187. [DOI] [PubMed] [Google Scholar]
- 16.Bianchi F., Maioli M., Leonardi E., et al. A new non-enzymatic method and device to obtain a fat tissue derivative highly enriched in pericyte-like elements by mild mechanical forces from human lipoaspirates. Cell Transplantation. 2013;22(11):2063–2077. doi: 10.3727/096368912x657855. [DOI] [PubMed] [Google Scholar]
- 17.Gimble J. M., Guilak F. Differentiation potential of adipose derived adult stem (ADAS) cells. Current Topics in Developmental Biology. 2003;58:137–160. doi: 10.1016/S0070-2153(03)58005-X. [DOI] [PubMed] [Google Scholar]
- 18.de Ugarte D. A., Alfonso Z., Zuk P. A., et al. Differential expression of stem cell mobilization-associated molecules on multi-lineage cells from adipose tissue and bone marrow. Immunology Letters. 2003;89(2-3):267–270. doi: 10.1016/s0165-2478(03)00108-1. [DOI] [PubMed] [Google Scholar]
- 19.de Ugarte D. A., Morizono K., Elbarbary A., et al. Comparison of multi-lineage cells from human adipose tissue and bone marrow. Cells Tissues Organs. 2003;174(3):101–109. doi: 10.1159/000071150. [DOI] [PubMed] [Google Scholar]
- 20.Zuk P. A., Zhu M., Ashjian P., et al. Human adipose tissue is a source of multipotent stem cells. Molecular Biology of the Cell. 2002 doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maioli M., Rinaldi S., Santaniello S., et al. Radioelectric asymmetric conveyed fields and human adipose-derived stem cells obtained with a nonenzymatic method and device: A novel approach to multipotency. Cell Transplantation. 2014;23(12):1489–1500. doi: 10.3727/096368913X672037. [DOI] [PubMed] [Google Scholar]
- 22.Sterodimas A., de Faria J., Nicaretta B., Pitanguy I. Tissue engineering with adipose-derived stem cells (ADSCs): current and future applications. Journal of Plastic, Reconstructive & Aesthetic Surgery. 2010;63(11):1886–1892. doi: 10.1016/j.bjps.2009.10.028. [DOI] [PubMed] [Google Scholar]
- 23.Tallone T., Realini C., Böhmler A., et al. Adult human adipose tissue contains several types of multipotent cells. Journal of Cardiovascular Translational Research. 2011;4(2):200–210. doi: 10.1007/s12265-011-9257-3. [DOI] [PubMed] [Google Scholar]
- 24.Basoli V., Santaniello S., Cruciani S., et al. Melatonin and vitamin D interfere with the adipogenic fate of adipose-derived stem cells. International Journal of Molecular Sciences. 2017;18(5):p. 981. doi: 10.3390/ijms18050981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Santaniello S., Cruciani S., Basoli V., et al. Melatonin and vitamin D orchestrate adipose derived stem cell fate by modulating epigenetic regulatory genes. International Journal of Medical Sciences. 2018;15(14):1631–1639. doi: 10.7150/ijms.27669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maioli M., Contini G., Santaniello S., et al. Amniotic fluid stem cells morph into a cardiovascular lineage: analysis of a chemically induced cardiac and vascular commitment. Drug Design, Development and Therapy. 2013;7:1063–1073. doi: 10.2147/dddt.s44706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maioli M., Basoli V., Santaniello S., et al. Osteogenesis from dental pulp derived stem cells: a novel conditioned medium including melatonin within a mixture of hyaluronic, butyric, and retinoic acids. Stem Cells International. 2016;2016:8. doi: 10.1155/2016/2056416.2056416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maioli M., Basoli V., Carta P., et al. Synthesis of magnolol and honokiol derivatives and their effect against hepatocarcinoma cells. Plos One. 2018;13(2) doi: 10.1371/journal.pone.0192178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rinaldi S., Maioli M., Santaniello S., et al. Regenerative treatment using a radioelectric asymmetric conveyor as a novel tool in antiaging medicine: An in vitro beta-galactosidase study. Clinical Interventions in Aging. 2012;7:191–194. doi: 10.2147/CIA.S33312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rinaldi S., Maioli M., Pigliaru G., et al. Stem cell senescence. Effects of REAC technology on telomerase-independent and telomerase-dependent pathways. Scientific Reports. 2015;4(1) doi: 10.1038/srep06373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nassar M. I., Aboutabl E.-S. A., Ahmed R. F., El-Khrisy E.-D. A., Ibrahim K. M., Sleem A. A. Secondary metabolites and bioactivities of Myrtus communis. Pharmacognosy Research. 2010;2(6):325–329. doi: 10.4103/0974-8490.75449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alipour G., Dashti S., Hosseinzadeh H. Review of pharmacological effects of Myrtus communis L. and its active constituents. Phytotherapy Research. 2014;28(8):1125–1136. doi: 10.1002/ptr.5122. [DOI] [PubMed] [Google Scholar]
- 33.Sumbul S., Ahmad M. A., Asif M., Akhtar M., Saud I. Physicochemical and phytochemical standardization of berries of Myrtus communis Linn. Journal of Pharmacy and Bioallied Sciences. 2012;4(4):322–326. doi: 10.4103/0975-7406.103266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maldini M., Chessa M., Petretto G. L., et al. Profiling and simultaneous quantitative determination of anthocyanins in wild Myrtus communis L. berries from different geographical areas in sardinia and their comparative evaluation. Phytochemical Analysis. 2016:249–256. doi: 10.1002/pca.2623. [DOI] [PubMed] [Google Scholar]
- 35.Montoro P., Tuberoso C. I. G., Piacente S., et al. Stability and antioxidant activity of polyphenols in extracts of Myrtus communis L. berries used for the preparation of myrtle liqueur. Journal of Pharmaceutical and Biomedical Analysis. 2006;41(5):1614–1619. doi: 10.1016/j.jpba.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 36.Deriu A., Branca G., Molicotti P., et al. In vitro activity of essential oil of Myrtus communis L. against Helicobacter pylori. International Journal of Antimicrobial Agents. 2007;30(6):562–563. doi: 10.1016/j.ijantimicag.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 37.Serreli G., Jerković I., Gil K. A., Marijanović Z., Pacini V., Tuberoso C. I. G. Phenolic compounds, volatiles and antioxidant capacity of white myrtle berry liqueurs. Plant Foods for Human Nutrition. 2017;72(2):205–210. doi: 10.1007/s11130-017-0611-8. [DOI] [PubMed] [Google Scholar]
- 38.Gündüz G. T., Gönül Ş. A., Karapinar M. Efficacy of myrtle oil against Salmonella Typhimurium on fresh produce. International Journal of Food Microbiology. 2009;130(2):147–150. doi: 10.1016/j.ijfoodmicro.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 39.Tretiakova I., Blaesius D., Maxia L., et al. Myrtucommulone from Myrtus communis induces apoptosis in cancer cells via the mitochondrial pathway involving caspase-9. Apoptosis. 2008;13(1):119–131. doi: 10.1007/s10495-007-0150-0. [DOI] [PubMed] [Google Scholar]
- 40.Fadda A., Serra M., Molinu M. G., Azara E., Barberis A., Sanna D. Reaction time and DPPH concentration influence antioxidant activity and kinetic parameters of bioactive molecules and plant extracts in the reaction with the DPPH radical. Journal of Food Composition and Analysis. 2014;35(2):112–119. doi: 10.1016/j.jfca.2014.06.006. [DOI] [Google Scholar]
- 41.Fadda A., Barberis A., Sanna D. Influence of pH, buffers and role of quinolinic acid, a novel iron chelating agent, in the determination of hydroxyl radical scavenging activity of plant extracts by Electron Paramagnetic Resonance (EPR) Food Chemistry. 2018;240:174–182. doi: 10.1016/j.foodchem.2017.07.076. [DOI] [PubMed] [Google Scholar]
- 42.Sorice A., Guerriero E., Capone F., Colonna G., Castello G., Costantini S. Ascorbic acid: Its role in immune system and chronic inflammation diseases. Mini-Reviews in Medicinal Chemistry. 2014;14(5):444–452. doi: 10.2174/1389557514666140428112602. [DOI] [PubMed] [Google Scholar]
- 43.Tuberoso C. I. G., Rosa A., Bifulco E., et al. Chemical composition and antioxidant activities of Myrtus communis L. berries extracts. Food Chemistry. 2010;123(4):1242–1251. doi: 10.1016/j.foodchem.2010.05.094. [DOI] [Google Scholar]
- 44.Fernandes R. P. P., Trindade M. A., Tonin F. G., et al. Evaluation of antioxidant capacity of 13 plant extracts by three different methods: cluster analyses applied for selection of the natural extracts with higher antioxidant capacity to replace synthetic antioxidant in lamb burgers. Journal of Food Science and Technology. 2016;53(1):451–460. doi: 10.1007/s13197-015-1994-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Salgado P., Melin V., Contreras D., Moreno Y., Mansilla H. D. Fenton reaction driven by iron ligands. Journal of the Chilean Chemical Society. 2013;58(4):2096–2101. doi: 10.4067/S0717-97072013000400043. [DOI] [Google Scholar]
- 46.Davies K. J. A. Adaptive homeostasis. Molecular Aspects of Medicine. 2016;49:1–7. doi: 10.1016/j.mam.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Khoo H. E., Azlan A., Tang S. T., Lim S. M. Anthocyanidins and anthocyanins: colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food & Nutrition Research. 2017 doi: 10.1080/16546628.2017.1361779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Azadmehr A., Hajiaghaee R., Mazandarani M. Induction of apoptosis and G2/M cell cycle arrest by Scrophularia striata in a human leukaemia cell line. Cell Proliferation. 2013;46(6):637–643. doi: 10.1111/cpr.12074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ferhi S., Santaniello S., Zerizer S., et al. Total phenols from grape leaves counteract cell proliferation and modulate apoptosis-related gene expression in MCF-7 and HepG2 human cancer cell lines. Molecules. 2019;24(3):p. 612. doi: 10.3390/molecules24030612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lay M. M., Karsani S. A., Mohajer S., Abd Malek S. N. Phytochemical constituents, nutritional values, phenolics, flavonols, flavonoids, antioxidant and cytotoxicity studies on Phaleria macrocarpa (Scheff.) Boerl fruits. BMC Complementary and Alternative Medicine. 2014;14 doi: 10.1186/1472-6882-14-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sumbul S., Aftab Ahmad M., Asif M., Akhtar M. Myrtus communis Linn. - A review. Indian Journal of Natural Products and Resources (IJNPR) 2011;2(4):395–402. [Google Scholar]
- 52.Kanoun K., Belyagoubi-Benhammou N., Ghembaza N., Atik Bekkara F. Comparative studies on antioxidant activities of extracts from the leaf, stem and berry of Myrtus communis L. International Food Research Journal. 2014;21(5):1957–1962. [Google Scholar]
- 53.Kook D., Wolf A. H., Yu A. L., et al. The protective effect of quercetin against oxidative stress in the human RPE in vitro. Investigative Ophthalmology & Visual Science. 2008;49(4):1712–1720. doi: 10.1167/iovs.07-0477. [DOI] [PubMed] [Google Scholar]
- 54.Chatterjee S. Oxidative stress, inflammation, and disease. Oxidative Stress and Biomaterials. 2016:35–58. [Google Scholar]
- 55.Maioli M., Rinaldi S., Santaniello S., et al. Radio electric conveyed fields directly reprogram human dermal skin fibroblasts toward cardiac, neuronal, and skeletal muscle-like lineages. Cell Transplantation. 2013;22(7):1227–1235. doi: 10.3727/096368912X657297. [DOI] [PubMed] [Google Scholar]
- 56.Facchin F., Bianconi E., Romano M., et al. Comparison of oxidative stress effects on senescence patterning of human adult and perinatal tissue-derived stem cells in short and long-term cultures. International Journal of Medical Sciences. 2018;15(13):1486–1501. doi: 10.7150/ijms.27181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kiyoshima T., Enoki N., Kobayashi I., et al. Oxidative stress caused by a low concentration of hydrogen peroxide induces senescence-like changes in mouse gingival fibroblasts. International Journal of Molecular Medicine. 2012;30(5):1007–1012. doi: 10.3892/ijmm.2012.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hennia A., Miguel M., Nemmiche S., Miguel M. G. Antioxidant activity of myrtus communis l. and myrtus nivellei batt. & trab. extracts: a brief review. Medicines. 2018;5(3):p. 89. doi: 10.3390/medicines5030089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gupta R. A., Motiwala M. N., Mahajan U. N., Sabre S. G. Protective effect of Sesbania grandiflora on acetic acid induced ulcerative colitis in mice by inhibition of TNF-α and IL-6. Journal of Ethnopharmacology. 2018;219:222–232. doi: 10.1016/j.jep.2018.02.043. [DOI] [PubMed] [Google Scholar]
- 60.Karin M., Clevers H. Reparative inflammation takes charge of tissue regeneration. Nature. 2016;529(7586):307–315. doi: 10.1038/nature17039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Foyer C. H., Noctor G. Redox homeostasis and antioxidant signaling: a metabolic interface between stress perception and physiological responses. The Plant Cell. 2005;17(7):1866–1875. doi: 10.1105/tpc.105.033589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Poljsak B., Šuput D., Milisav I. Achieving the balance between ROS and antioxidants: when to use the synthetic antioxidants. Oxidative Medicine and Cellular Longevity. 2013;2013:11. doi: 10.1155/2013/956792.956792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Samareh Fekri M., Mandegary A., Sharififar F., et al. Protective effect of standardized extract of Myrtus communis L. (myrtle) on experimentally bleomycin-induced pulmonary fibrosis: biochemical and histopathological study. Drug and Chemical Toxicology. 2018;41(4):408–414. doi: 10.1080/01480545.2018.1459670. [DOI] [PubMed] [Google Scholar]
- 64.Shi L., Fu S., Fahim S., et al. TNF-alpha stimulation increases dental pulp stem cell migration in vitro through integrin alpha-6 subunit upregulation. Archives of Oral Biolog. 2017;75:48–54. doi: 10.1016/j.archoralbio.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 65.Miana V. V., Prieto González E. A. Adipose tissue stem cells in regenerative medicine. Ecancermedicalscience. 2018;12 doi: 10.3332/ecancer.2018.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Han M.-K., Song E.-K., Guo Y., Ou X., Mantel C., Broxmeyer H. E. SIRT1 regulates apoptosis and Nanog expression in mouse embryonic stem cells by controlling p53 subcellular localization. Cell Stem Cell. 2008;2(3):241–251. doi: 10.1016/j.stem.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang Z.-N., Chung S.-K., Xu Z., Xu Y. Oct4 maintains the pluripotency of human embryonic stem cells by inactivating p53 through sirt1-mediated deacetylation. Stem Cells. 2014;32(1):157–165. doi: 10.1002/stem.1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ota H., Akishita M., Eto M., Iijima K., Kaneki M., Ouchi Y. Sirt1 modulates premature senescence-like phenotype in human endothelial cells. Journal of Molecular and Cellular Cardiology. 2007;43(5):571–579. doi: 10.1016/j.yjmcc.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 69.Volonte D., Zou H., Bartholomew J. N., Liu Z., Morel P. A., Galbiati F. Oxidative stress-induced inhibition of Sirt1 by caveolin-1 promotes p53-dependent premature senescence and stimulates the secretion of interleukin 6 (IL-6) The Journal of Biological Chemistry. 2015;290(7):4202–4214. doi: 10.1074/jbc.m114.598268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ohnishi K., Ohkura S., Nakahata E., et al. Non-specific protein modifications by a phytochemical induce heat shock response for self-defense. Plos One. 2013;8(3) doi: 10.1371/journal.pone.0058641.e58641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mahat D. B., Salamanca H. H., Duarte F. M., Danko C. G., Lis J. T. Mammalian heat shock response and mechanisms underlying its genome-wide transcriptional regulation. Molecular Cell. 2016;62(1):63–78. doi: 10.1016/j.molcel.2016.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Prodromou C. Mechanisms of Hsp90 regulation. Biochemical Journal. 2016;473(16):2439–2452. doi: 10.1042/BCJ20160005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sable A., Rai K. M., Choudhary A., Yadav V. K., Agarwal S. K., Sawant S. V. Inhibition of Heat Shock proteins HSP90 and HSP70 induce oxidative stress, suppressing cotton fiber development. Scientific Reports. 2018;8(1) doi: 10.1038/s41598-018-21866-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are included within the article.