Abstract
Estrogen receptor-alpha (ERα) and -beta (ERβ) occur in key elements of the brain gluco-homeostatic network in both sexes, including the hindbrain dorsal vagal complex (DVC), but the influence of distinct receptor populations on this critical function is unclear. The ventromedial hypothalamic nucleus (VMN) maintains glucose balance by integrating nutrient, endocrine, and neurochemical cues, including metabolic sensory information supplied by DVC A2 noradrenergic neurons. Current research utilized the selective ERα and ERβ antagonists MPP and PHTPP to characterize effects of DVC ERs on VMN norepinephrine (NE) activity and metabolic neurotransmitter signaling in insulin-induced hypoglycemic (IIH) male rats. Data show that ERβ inhibits VMN glycogen synthase and stimulates phosphorylase protein expression, while attenuating hypoglycemic augmentation of glycogen content. Furthermore, both ERs attenuate VMN glucose concentrations during IIH. Hypoglycemic up-regulation of nitric oxide (NO) and brain-derived neurotrophic factor (BDNF) signaling was correspondingly driven by ERα or -β, whereas GABA and steroidogenic factor-1 were respectively suppressed independently of ER input or by ERβ. IIH intensified VMN NE accumulation by ERβ-dependent mechanisms, but did not alter NE levels in other gluco-regulatory loci. ERβ amplified the magnitude of insulin-induced decline in blood glucose. Both ER regulate corticosterone, but not glucagon secretion during IIH and oppose hypoglycemic diminution of circulating free fatty acids. These findings identify distinguishing versus common VMN functions targeted by DVC ERα and -β. Sex differences in hypoglycemic VMN NE accumulation, glycogen metabolism, and transmitter signaling may involve, in part, discrepant regulatory involvement or differential magnitude of impact of these hindbrain ERs.
Keywords: MPP, PHTPP, norepinephrine, nitric oxide synthase, glutamate decarboxylase, glycogen
Introduction
Neurons require a continuous supply of glucose to support vital high energy-consuming functions. Insulin-induced hypoglycemia (IIH) is a recurring complication of pharmacotherapeutic management of insulin-dependent diabetes mellitus that causes metabolic fuel deprivation of the brain. The ventromedial hypothalamic nucleus (VMN) responds to neuroglucopenia by shaping counter-balancing autonomic, neuroendocrine, and behavioral functions that restore glucostasis. The hindbrain dorsal vagal complex (DVC) is a vital source of metabolic-sensory input to the brain gluco-regulatory network as deficits of the oxidizable glycolytic end-product L-lactate in that site activate neural mechanisms that elevate blood glucose [Patil and Briski, 2005]. Administration of exogenous L-lactate to the DVC normalizes hypothalamic metabolic transmitter expression, indicating the critical influence of local energy status on downstream hypothalamic elements of that circuitry [Gujar et al., 2014]. A2 noradrenergic neurons likely communicate hypoglycemia-associated DVC substrate fuel imbalance, as these cells exhibit lactate-reversible activation of the ultra-sensitive energy sensor adenosine 5’-monophosphate-activated protein kinase (AMPK), coincident with lactate-dependent augmentation of hypothalamic norepinephrine (NE) activity [Shrestha et al., 2014].
DVC NE is implicated in regulation of VMN reactivity to IIH, as neurotoxic destruction of DVC catecholamine neurons modifies hypoglycemic patterns of VMN AMPK activity and neurotransmitter protein expression in male rats [Alhamami et al., 2018]. In both sexes, estrogen receptor-alpha (ERα) and -beta (ERβ) are expressed in key elements of the brain gluco-homeostatic network, including the DVC [Simerly et al., 1990], but distinct roles of central receptor subtype populations in glucose balance have not been investigated in male or female. Evidence that A2 neurons in the female rat DVC express ERα and -β proteins [Ibrahim et al., 2013] supports the possibility that estradiol may govern noradrenergic input to the male VMN. Present research examined the hypothesis that in the male, hindbrain regulation of characterized VMN gluco-inhibitory (γ-aminobutyric acid (GABA) [Chan et al., 2006]), and gluco-stimulatory (nitric oxide (NO) and steroidogenic factor-1 (SF-1) [Fioramonti et al., 2011; Routh et al., 2014] transmitters during IIH is subject to DVC ERα and/or -β control.
Brain astrocytes contribute to neuro-metabolic stability by maintaining an energy fuel reserve in the form of the complex carbohydrate polymer glycogen [Stobart and Anderson, 2013]. Glucose, the primary energy source to the brain, is acquired from the circulation by these glia and either incorporated into glycogen or converted to the oxidizable fuel L-lactate for trafficking to neurons [Laming et al., 2000]. In the brain and periphery, glycogen metabolism is controlled by opposing actions of glycogen synthase (GS) and glycogen phosphorylase (GP), which respectively catalyze glycogen synthesis or breakdown. NE has well-documented stimulatory effects on astrocyte glycogenolysis in vitro [Magistretti, 1988; Quach et al., 1988; Subbarao and Hertz, 1990]. Recent studies show that VMN nitrergic transmission in males is up-regulated in response to diminished substrate fuel supply from glycogen [Alhamami et al., 2018]. Thus, we examined whether DVC ERs regulate the VMN glycogen metabolic enzymes glycogen synthase (GS) and glycogen phosphorylase (GP) protein expression and tissue glycogen accumulation. VMN tissue was attained by micropunch-dissection from brains of insulin-injected male rats pretreated by intra-caudal fourth ventricular (CV4) administration of the selective ERα antagonist 1,3-Bis(4-hydroxyphenyl)-4-methyl-5-[4-(2-piperidinylethoxy)phenol]-1H-pyrazole dihydro-chloride (MPP), the ERβ antagonist 4-[2-phenyl-5,7-bis(trifluoromethyl)pyrazolo[1,5-a]pyrimidin-3-yl]phenol (PHTPP), or vehicle alone for 1) Western blot analysis of metabolic transmitter, GS, and GP protein expression, 2) ELISA measurement of norepinephrine (NE) content of the VMN and hypothalamic metabolic structures that innervate the VMN [Bouret, 2017], and 3) HPLC/mass spectrometric determination of VMN tissue glycogen and glucose levels. Discussion below of the significance of current research involving male rats focuses on sex-specific effects of DVC ERs on forebrain VMN function in hypoglycemic male versus female rats [Napit and Briski, personal communication].
Materials and Methods
Experimental Design
Adult male Sprague-Dawley rats (2–3 months of age) were housed in groups of 2–3 per cage, under a 14 hr light/10 hr dark lighting schedule (lights on at 05:00 hr). Animals were fed standard laboratory rat chow (Harlan Teklad LM-485; Harlan Industries, Madison, WI) and watered ad-libitum. Rats were accustomed to daily handling commencement of the study. Animal use protocol was compliant with NIH guidelines, under approval by ULM Institutional Animal Care and Use Committee. On study day 1, rats were anesthetized by intraperitoneal injection of ketamine/xylazine anesthesia (0.1 mL/100 g bw, 90 mg ketamine:10 mg xylazine/mL; Henry Schein Inc., Melville, NY), then stereotactically implanted with a PE-20 cannula aimed at the CV4 at the following coordinates: 0.0 mm from midline; −13.3 mm posterior to bregma, 6.60 mm ventral to skull surface. After surgery, animals were injected with enrofloxacin (10mg/kg im; Norbrook Laboratories Limited, Newry, Northern Ireland) and ketoprofen (3mg/kg sc; Zoetis Inc., Kalamazoo MI), and transferred to individual cages for recovery. At 8:45 hr on Day 10, vehicle [dimethyl sulfoxide (DMSO, 200nL; VWR, Solon, OH); groups 1 (n=5) and 2 (n=5)], the ERα antagonist MPP [10 uM/200 nL; Tocris/Bio-Techne Corp., Minneapolis, MN; group 3 (n=5)], or ERβ antagonist PHTPP [10 uM/200 nL; Tocris; group 4 (n=5)] was delivered to the CV4. At 9:00 hr on Day 10, rats in group 1 were injected sc with vehicle (V; sterile diluent; Eli Lilly & Co., Indianapolis, IN); other groups were injected with neutral protamine Hagedorn insulin (INS; 10 U/kg bw; Eli Lilly). At 10:00 hr, unanesthetized restrained animals were euthanized by 1.4 sec exposure to focused-beam microwave irradiation within an In Vivo Microwave Fixation System (5kW; Stoelting Co., Wood Dale, IL) prior to brain tissue and trunk blood collection.
Micropunch Dissection of the VMN, Arcuate Hypothalamic Nucleus (ARH), Dorsomedial Hypothalamic Nucleus (DMN), and Lateral Hypothalamic Area (LHA)
Each hypothalamus was cut into serial 100 – 150 micron-thick frozen sections through the VMN, ARH, DMN, and LHA, using calibrated Brain Punch set punch tools (prod. no. 57401; Stoelting), for micropunch dissection. Distinct sets of sections were collected over specific rostro-caudal lengths for different analytical objectives: 1) between −2.00 and −2.20 mm: two 100 μm-thick sections; Western blot analysis; 2) between −2.20 and −2.40 mm: two 100 μm sections, NE ELISA analysis; 3) between −2.40 and −2.70 mm: two 150 μm sections, HPLC/mass spectrometric analysis; 4) beginning at −2.70 mm: six (VMN, ARH, LHA) or four (DMN) 100 μm-thick sections, Western blotting.
Micropunches used for immunoblotting were collected into lysis buffer consisting of 2.0% sodium dodecyl sulfate, 0.05 M dithiothreitol, 10.0% glycerol, 1.0 mM EDTA, 60.0 mM Tris-HCl, pH 7.2; tissues utilized for ELISA were saved in lysis buffer made of 0.01 N HCl supplemented with 1.0 mM EDTA, 4.0 mM sodium metabisulfite; punches for HPLC/mass spectrometric measurements were collected into 0.02 M Tris buffer, pH 7.2.
Western Blot Analysis
For each rat, VMN tissue punches were pooled and heat-denatured at 95°C. In each treatment group, tissue aliquots from individual subjects were combined to create separate triplicate pools for analysis of each protein of interest, prior to separation in BioRad TGX 10–12% Stain-Free gels [Shakya et al., 2018]. Gels were activated by UV light (1 min) in a BioRad ChemiDoc TM Touch Imaging System before overnight transblotting (4–5°C) to 0.45-μm PVDF-Plus membranes (prod. no. PV4HY00010; ThermoFisherScientific, Waltham, MA). After blocking (2 hr) with Tris-buffered saline, pH 7.4, containing 0.1 % Tween-20 and 2% bovine serum albumin, membranes were incubated (24–48 hrs; 4–5° C) with rabbit primary polyclonal antisera against glycogen synthase (GS; 1:2,000; prod. no. 3893S; Cell Signaling Technology, Danvers, MA), glycogen phosphorylase (GP; 1:2,000; prod. no. NBP1–32799; Novus Biologicals, Littleton, CO), glutamate decarboxylase65/67 (GAD65/67; 1:10,000; prod. no. ABN904; MilliporeSigma (Burlington, MA), neuronal nitric oxide synthase (nNOS; 1:2,000; prod. no. NBP1–39681; Novus Biol.), brain-derived neurotrophic factor (BDNF; 1:2,000; prod. no. NBP1–46750; Novus Biol.), or steroidogenic factor-1 (SF1; 1:2,000; prod. no. PA5–41967; ThermoFisherSci.). Membranes were next incubated (1 hr) with goat anti-rabbit secondary antibodies (1:5,000; prod. no. NEF812001EA; PerkinElmer, Waltham, MA) prior to exposure to Supersignal West Femto maximum sensitivity chemiluminescent substrate (prod. no. 34096; ThermoFisherSci.). Membrane buffer washes and antibody incubations were carried out by Freedom Rocker™ Blotlbot® automation (Next Advance, Inc., Troy NY). Chemiluminescence band optical density (O.D.) values obtained in the ChemiDoc MP system were normalized to total in-lane protein with Imagelab software (Image Lab™ 6.0.0; Bio-Rad). Precision plus protein molecular weight dual color standards (prod. no. 161–0374; Bio-Rad) were included in each Western blot analysis.
ELISA Measurement of VMN, ARH, DMN, and LHA NE Content
For each animal, micropunched tissue from each structure was analyzed for NE content using Noradrenaline Research ELISA™ kit reagents (Labor Diagnostika Nord GmbH & Co KG, Nordhorn, Germany), as reported [Shrestha et al., 2014].
Glycogen HPLC Analyses
VMN tissue was heat-denatured and homogenized by ultra-sonification. Supernatant aliquots (20 μL) were hydrolyzed by incubation with 0.5 mg/ml amyloglucosidase (10 μL) and 0.1M sodium acetate (10 μL) for 2 hr prior to heating to 100ºC (5 min), followed by cooling to room temperature. Supernatant glycogen concentrations were determined by reverse-phase HPLC in a Hitachi LaChrom Elite® System (Hitachi America, Ltd., Tarrytown, NY), by modification of published methods [Honda et al., 1989; Fuller et al., 2012; Bai et al., 2015]. Hydrolyzed and non-hydrolyzed sample aliquots were derivatized with 100 μL 0.5 M 1-phenyl-3-methyl-5-pyrazolone (PMP) reagent supplemented with 0.3 M NaOH. After acidification with 400 μL 0.75% formic acid, derivatized samples were extracted with chloroform, vacuum concentrated to remove chloroform and formic acid, transferred to fresh tubes, frozen at −80°C, and lyophilized. Lyophilized samples were diluted to 1.0 mL with acetonitrile/0.01 M triethylamine (65:35) v/v; 20 μL aliquots were then separated on a Zorbax ODS (4.6 cm × 250 mm, 5 μm), with an acetonitrile/0.005 M triethylamine (65:35) v/v mobile phase, at a flow rate of 1 ml/min. Wavelength of detection was 245 nm. Tissue glycogen concentrations, as calculated by subtracting from hydrolysis-derived total glucose concentrations, were determined using a calibration curve. Glycogen and D-glucose concentrations were expressed as ng/mL and ug/mL respectively. Prepared D-glucose-PMP and HPLC eluent containing D-glucose-PMP were analyzed in a Q®TRAP LC-MS/MS 3200 System (AB Sciex LLC, Framingham, MA). 1 μg/mL D-glucose-PMP was diluted with HPLC-grade methanol prior to injection, and eluted with acetonitrile/0.005 M triethylamine (65:35) v/v over consecutive 1–3 min intervals. HPLC eluent was evaporated at 45°C for 60 min, frozen to −80°C, lyophilized at −55°C, and reconstituted with methanol prior to injection.
Glucose and Counter-Regulatory Hormone Measurements
Blood glucose levels were determined using an ACCU-CHECK Aviva plus glucometer (Roche Diagnostic Corporation, Indianapolis, IN) [Kale et al., 2006]. Plasma free fatty acid concentrations were assessed using Free Fatty Acid Quantitation Kit reagents (MAK044; Sigma Aldrich, St. Louis, Mo) [Briski et al., 2017]. Plasma corticosterone (ADI-900–097; Enzo Life Sciences, Inc., Farmingdale, NY) and glucagon (EZGLU-30K, EMD Millipore, Billerica, MA) concentrations were determined using commercial ELISA kit reagents, as described [Alhamami et al., 2018].
Statistical analyses
Mean normalized Western blot protein O.D., micropunch tissue NE, VMN glycogen, glucose, glucagon, corticosterone, and free fatty acid values were evaluated between treatment groups by one-way analysis of variance and Student Newman Keuls post-hoc test. Differences of p<0.05 were considered significant. In each Figure, statistical differences between specific pairs of treatment groups are denoted with the following symbols: *p<0.05; **p<0.01; ***p<0.001.
Results
Figure 1 depicts the effects of CV4 pretreatment with MPP or PHTPP on hypoglycemia-associated patterns of VMN metabolic neurotransmitter marker protein expression in the male rat. Data presented in Panel A show that VMN GAD65/67 protein profiles were significantly decreased in insulin- versus to vehicle-injected controls [V/INS versus V/V] (F(3,8) = 22.64; p < 0.0001), and that this response was refractory to antagonism of either hindbrain ER. As illustrated in Panel B, IIH-associated augmentation of VMN nNOS content was attenuated by DVC ERα or -β blockade (F(3,8) = 8.11; p < 0.008). Hypoglycemic male rats exhibited a significant reduction in VMN SF-1 protein (Panel C; F(3,8) = 28.31; p = 0.0001. This decline was intensified by MPP or PHTPP pretreatment [MPP/INS and PHTPP/INS versus V/INS]. Panel D shows that IIH augmented BDNF profiles (F(3,8) = 8.69; p = 0.007), and that this response was reversed by DVC ERβ antagonism.
Figure 1.
Effects of Caudal Fourth Ventricular (CV4) Administration of the ERα Antagonist 1,3-Bis(4-hydroxyphenyl)-4-methyl-5-[4-(2-piperidinylethoxy)phenol]-1H-pyrazole dihydrochloride (MPP) or ERβ Antagonist 4-[2-phenyl-5,7-bis(trifluoromethyl)pyrazolo[1,5-a]pyrimidin-3-yl]phenol (PHTPP) on Ventromedial Hypothalamic Nucleus (VMN) Metabolic Neurotransmitter Marker Protein Responses to Insulin-Induced Hypoglycemia (IIH) in the Male Rat. Micropunch-dissected VMN tissue from animals pretreated by CV4 delivery of MPP, PHTPP, or vehicle prior to sc insulin (INS) injection was pooled within treatment groups for Western blot analysis of glutamate decarboxylase65/67 (GAD65/67; Panel A), neuronal nitric oxide synthase (nNOS; Panel B), steroidogenic factor-1 (SF-1; Panel C), or brain-derived neurotrophic factor (BDNF; Panel D). Data depict mean normalized protein optical density (O.D.) values ± S.E.M. for rats given vehicle both as CV4 pretreatment and sc injection (V/V; solid white bars; n=5) or animals injected sc with INS after pretreatment with V (V/INS; solid gray bars; n=5), MPP (MPP/INS; diagonal-striped gray bars; n=5) or PHTPP (PHTPP/INS; cross-hatched gray bars; n=5. *p<0.05; **p<0.01; ***p<0.001.
Data presented in Figure 2 illustrate the roles of DVC ERα and -β in hypoglycemic patterns of VMN GS and GP protein expression and VMN glycogen and glucose content. Panel A shows that GS profiles did not differ between V/INS versus V/V groups, but that ERβ signaling is inhibitory to this protein profiles during IIH (F(3,8) = 8.93; p = 0.002). VMN GP content was significantly elevated in V/INS animals (F(3,8) = 8.15; p = 0.0032); this response was prevented by PHTPP pretreatment [PHTPP/INS versus V/INS]. VMN glycogen levels were significantly higher in the INS- versus V-injected group (Panel C), and further augmented by ERβ blockade [PHTPP/INS versus V/INS] (F(3,16) = 10.94; p = 0.002). Data in Panel D indicate that VMN glucose content was unaffected by IIH (39.02; p < 0.0001), but that both hindbrain ERα and -β impose an inhibitory tone on these tissue levels.
Figure 2.
Impact of DVC ERα versus ERβ Antagonism on Hypoglycemic Patterns of VMN Glycogen Synthase (GS) and Glycogen Phosphorylase (GP) Protein Expression and Adjustments in VMN Glycogen and Glucose Content in the Male Rat. Panels A and B depict mean normalized GS and GP O.D. values ± S.E.M., respectively, from triplicate Western blot analyses of pooled VMN tissue from V/V (solid white bars), V/INS (solid gray bars), MPP/INS (diagonal-striped gray bars), and PHTPP/INS (cross-hatched gray bars) treatment groups. Panels C and D illustrate effects of INS in the presence or absence of pharmacological blockade of DVC ER on mean HPLC measures of VMN tissue glycogen and glucose content ± S.E.M., respectively, for n=5 animals per treatment group. *p<0.05; **p<0.01; ***p<0.001.
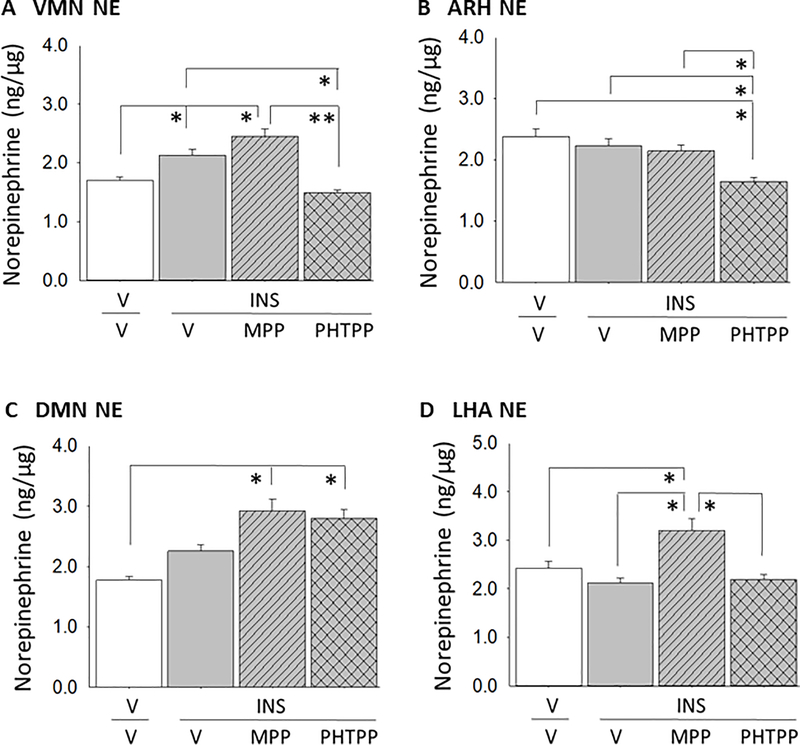
Figure 3 shows the effects of MPP or PHTPP pretreatment on NE accumulation in the VMN (Panel A), ARH (Panel B), DMN (Panel C), and LHA (Panel D). Data reveal that IIH increased NE activity in the VMN (F(3,16 ) = 28.31; p = 0.0001), but not other structures evaluated. Hypoglycemic augmentation of VMN NE was reversed by ERβ antagonism. DVC ERα input was stimulatory or inhibitory to ARH (F(3,16) = 9.93; p = 0.025) or LHA (F(3,16) = 9.29; p = 0.03) NE profiles in hypoglycemic animals. DMN NE content (F(3,16) = 14.95; p = 0.01) did not differ between MPP- or PHTPP- versus V-pretreated INS-injected groups.
Figure 3.
Effects of DVC ERα or ERβ Antagonism on Hypoglycemic Site-Specific Norepinephrine (NE) Accumulation in Hypoglycemic Male Rats. Micropunches of the VMN (Panel A), arcuate hypothalamic nucleus (Panel B), dorsomedial hypothalamic nucleus (DMN), and lateral hypothalamic area (LHA) were analyzed by ELISA methods for measurement of tissue NE content. Data depict mean NE levels ± S.E.M. for V/V (solid white bars; n=5), V/INS (solid gray bars; n=5), MPP/INS (diagonal-striped gray bars; n=5), and PHTPP/INS (cross-hatched gray bars; n=5) treatment groups. *p<0.05; **p<0.01; ***p<0.001.
Data in Figure 4 depict glycemic, counter-regulatory hormone, and FFA responses to INS injection. Results indicate that blood glucose concentrations were significantly decreased to an equivalent extent in all INS treatment groups (Panel A; F(3,16) = 31.74; p < 0.0001), and that DVC ERβ signaling is inhibitory to circulating glucose during IIH [PHTPP/INS versus V/INS]. Plasma glucagon levels (Panel B) were elevated in response to INS injection, a response that was refractory to MPP or PHTPP pretreatment (F(3,16) = 6.02; p = 0.01). Corticosterone secretion was reduced at the time examined here by DVC ERα- and ERβ-dependent mechanisms (Panel C; F(3,16) = 22.51; p = 0.0003). Circulating FFAs were significantly diminished in V/INS versus V/V groups (Panel D; F(3,16) = 23.63; p = 0.0002); this decline was exacerbated in MPP- and PHTPP-pretreated hypoglycemic rats.
Figure 4.
CV4 MPP versus PHTPP Pretreatment on Glucose, Glucagon, Corticosterone, and Free Fatty Acid (FFA) Responses to Insulin Injection of Male Rats. Data show circulating glucose (Panel A), glucagon (Panel B), corticosterone (Panel C), and FFA (Panel D) levels ± S.E.M. levels in V/V (solid white bars; n=5), V/INS (solid gray bars; n=5), MPP/INS (diagonal-striped gray bars; n=5), and PHTPP/INS (cross-hatched gray bars; n=5) treatment groups. *p<0.05; **p<0.01; ***p<0.001.
Discussion
The VMN is a key component of the brain gluco-homeostatic network, utilizing sensory input about cellular energy stability and peripheral fuel stores to shape counter-regulatory responses to glucose imbalance. The DVC is a major location of visceral, including metabolic sensory information that is highly receptive to estradiol in both sexes [Simerly et al., 1990]. Present research investigated the premise that DVC ERα and ERβ control VMN NE activity, glycogen metabolism, and gluco-regulatory neurotransmitter signaling in hypoglycemic male rats. Data show that ERβ controls VMN GP and GP expression, glycogen content, and NE activity, whereas input from both ERs governs VMN tissue glucose levels. Hypoglycemic up-regulation of the gluco-stimulatory neurotransmitter NO was mediated by DVC ERα, whereas concurrent suppression of the counter-regulatory inhibitor GABA occurred independently of either receptor. Results reveal that both hindbrain ER exert control over circulating glucose, corticosterone, and FFA levels over the post-INS injection time frame evaluated here. Outcomes provide novel proof of estradiol-dependent hindbrain control of critical VMN functions during IIH in the male rat.
Current data show that VMN GS protein expression was unaffected by IIH, but that hypoglycemia up-regulated GP profiles in this site. This positive GP response was reversed by PHTPP, indicating a stimulatory role for DVC ERβ signaling. Paradoxically, VMN glycogen content was simultaneously increased, despite inhibitory effects of ERβ on glycogen accumulation. These findings imply that effects of IIH on GP enzyme activity may not parallel changes in total GP protein expression, or on the other hand, that total GP expression is biphasic over the interval between INS injection and sacrifice, with augmented profiles occurring secondary to an initial decline in protein expression. Interestingly, results also unexpectedly showed that VMN tissue glycogen was elevated after only one hour of hypoglycemia, which is in contrast to the conventional view that brain glycogen is rapidly depleted during this metabolic stress. The functional implications of this local amplification of substrate fuel reserve remain unclear. Interestingly, in the VMN of female rats, GP and glycogen content are both diminished at an equivalent time point after induction of IIH, with ERα acting to oppose the former response and ERβ driving the latter [Napit and Briski, personal communication]. Moreover, VMN GS protein levels were refractory to IIH in both sexes, but these profiles reflect an inhibitory tonus imposed by hindbrain ERα versus ERβ in females versus males, respectively. Taken together, these results indicate that VMN glycogen metabolic enzymes are controlled by different ERs in male versus female rats, whereas glycogen content is governed by ERβ in both sexes. It is possible that hindbrain regulation of GS and GP may involve different neurotransmitter(s) in each sex, or alternatively, a common neuron population in which the ratio of ERα and ERβ expression is sex-specific.
VMN GAD65/67 expression was inhibited in hypoglycemic male rats, an outcome consistent with reports that GABA neurotransmission suppresses hypoglycemic up-regulation counter-regulatory hormone secretion in males [Chan et al., 2006]; however, current findings show that DVC ER are unrelated to this response. GAD65/67 protein was similarly repressed in INS-injected females, but ERβ signaling is critical for that negative response in that sex. Further research is required to determine if GAD65/67 profiles in the male are governed exclusively by local intra-VMN stimuli and/or ER-independent cues of DVC origin. Hypoglycemic male rats exhibited DVC ERα-driven augmentation of VMN nNOS protein content, whereas both hindbrain ER mediate this positive response to INS treatment in females. Further studies are needed to determine if the same ERα-driven hindbrain neurotransmitter(s) up-regulates nNOS in male and female, and if in the latter sex, ERβ is additive/synergistic to ERα and/or stimulates additional neurochemicals that augment nNOS. VMN SF-1 and BDNF proteins each exhibited sex-specific responses to IIH. Interestingly, SF-1 was decreased or unchanged in hypoglycemic male versus female rats, respectively. Both ER actively mediate this decline in males and impose a negative tone on SF-1 expression in females, findings that imply that the magnitude of this negative input may differ between sexes. It is also possible that opposing stimulatory cues may be stronger in the female. Further studies are required to determine which hypothesis is correct. BDNF profiles were elevated in males, but reduced in females. As DVC ERβ drives this augmentation in males and imparts a positive impact on this profile in females, this receptor may promote higher intensity stimulatory signaling in the male as compared to the female. Alternatively, counter-balancing inhibitory signals may be stronger in the female.
Experimental evidence demonstrates that DVC ERβ plays a role in IIH augmentation of VMN NE accumulation in the male. This site-specific augmentation coincided with a lack of change in ARH, DMN, and LHA NE activity. These findings support the suggestion that regulatory effects of this receptor on VMN substrates are mediated, at least in part, by noradrenergic signaling. At the same time, mechanisms by which DVC ERα influence male VMN targets remain unclear. At one hour after INS injection, female rats exhibited a significant hindbrain ER-independent decline in VMN NE content. Females were also characterized by diminished ARH, DMN, and LHA NE accumulation. DVC ERβ imposed a stimulatory influence on ARH NE activity in males, whereas ERα actively suppressed accumulation of this catecholamine in females. LHA NE buildup was regulated by a positive ERα-mediated tone in males, but was not subject to DVC ER control in females. DMN NE responses to IIH in both sexes did not involve hindbrain ER input. Within each sex, discrepant involvement of ERα versus ERβ in site-specific NE accumulation implies that distinct subsets of DVC noradrenergic neurons projecting to specific hypothalamic metabolic loci may express varying levels and/or ratios of ERα and -β proteins, or that other ER-sensitive transmitter signals derived from the DVC may differentially regulate local ratios of NE synthesis, metabolism, and release.
Current results show that in male rats, PHTPP attenuated the insulin-induced decline in circulating glucose, signifying that DVC ERβ signaling elicits this decrement. Hindbrain ERβ induction of hypoglycemia likely precludes glucagon involvement, as ERβ antagonism did not modify hypoglycemic hyperglucagonemia. In contrast, hindbrain ERβ is likely involved in hypoglycemic reductions in corticosterone secretion, as PHTPP normalized this hormone profile in hypoglycemic rats. Thus, the possibility exists that ERβ may contribute, in part, to a reduction in circulating glucose levels through a suppression in corticosterone secretion. VMN responses to IIH that require input from DVC ERβ include up-regulated GP and BDNF protein expression, as PHTPP prevented effects of hypoglycemia on these profiles. Further effort is needed to determine if one or both proteins may mediate ERβ regulation of glucose and corticosterone profiles during hypoglycemia. PHTPP effects on hypoglycemic patterns of VMN GP expression and corticosterone secretion may be causally related as pharmacological suppression of GP by 1,4-dideoxy-1,4-imino-D-arabinitol is reported to normalize corticosterone release during hypoglycemia Alhamami et al., 2018]. Collective findings from present work and companion studies on females show that unlike males, hypoglycemic hyperglucagonemia in females is opposed by DVC ERα and ERβ [Napit and Briski, personal communication], whereas corticosterone secretion is regulated by ERβ in both sexes as well as by ERα in males. Current data show that MPP pretreatment normalized VMN nNOS expression during hypoglycemia, results that implicate hindbrain ERα signaling in this protein response. However, evidenced that MPP did not modify glucagon and corticosterone secretory responses to hypoglycemia suggests that NO signaling from the VMN locations investigated here does not regulate these counter-regulatory hormones in this sex.
As the current experimental design did not evaluate effects of either antagonist on parameters of interest in the absence of hypoglycemia, ongoing work aims to determine if the extent of hindbrain ER influence on those functions is equivalent or different during the distinctive energy states of eu- versus hypoglycemia. At present, the possibility that data described here may reflect, to some extent, additive effects of IIH and ER antagonist cannot be overlooked.
An important issue that remains unresolved concerns the source(s) of ligand(s) that activates DVC ER signaling in the male. It is highly probable that these ER likely interact with estradiol metabolized from testosterone by aromatase enzyme action outside the brain as well as within the DVC. An additional possibility is that these receptors may also be subject to control by local de novo neurosteroid synthesis.
In summary, the present research demonstrates that DVC ERα and -β regulate unique (ERα: nNOS; ERβ: GS, BDNF), as well as common (SF-1) VMN target protein expression in hypoglycemic male rats, and that VMN glycogen and glucose content is correspondingly controlled by ERβ versus both ERs. ERβ-dependent up-regulation of VMN NE activity during IIH supports the likelihood that noradrenergic signaling may mediate effects of this receptor on VMN substrates. Results also imply that discrepancies between sexes in patterns of change in VMN NE activity, glycogen metabolism, and transmitter signaling in males versus females may involve, at least in part, differences in regulatory involvement or differential magnitude of control imposed by DVC ERs.
Acknowledgements
This research was funded by NIH DK 109382.
Abbreviations
- GABA
γ-aminobutyric acid
- GAD65/67
glutamate decarboxylase65/67
- GP
glycogen phosphorylase
- GS
glycogen synthase
- IIH
insulin-induced hypoglycemia
- LHA
lateral hypothalamic area
- MPP
1,3-Bis(4-hydroxyphenyl)-4-methyl-5-[4-(2-piperidinylethoxy)phenol]-1H-pyrazole dihydrochloride
- NE
norepinephrine
- NO
nitric oxide
- nNOS
neuronal nitric oxide synthase
- PHTPP
4-[2-phenyl-5,7-bis(trifluoromethyl)pyrazolo[1,5-a]pyrimidin-3-yl]phenol
- SF-1
steroidogenic factor-1
- VMN
ventromedial hypothalamic nucleus
References
- Alhamami HN, Alshamrani A, Briski. Effects of the glycogen phosphorylase inhibitor 1,4-dideoxy-1,4-imino-D-arabinitol on ventromedial hypothalamic nucleus 5’-adenosine monophosphate-activated protein kinase activity and metabolic neurotransmitter biosynthetic enzyme protein expression in eu- versus hypoglycemic male rats. Physiological Reports 2018; 103: 236–249 [Google Scholar]
- Bai W, Fang X, Zhao W, Huang S, Zhang H, Qian M. Determination of oligosaccharides and monosaccharides in Hakka rice wine by precolumn high-performance liquid chromatography. J. Food Drug Anal 2015; 23: 645–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouret SG. Development of Hypothalamic Circuits That Control Food Intake and Energy Balance In: Harris RBS, editor. Appetite and Food Intake: Central Control. 2nd edition. Boca Raton (FL): CRC Press/Taylor & Francis; 2017. Chapter 7. [PubMed] [Google Scholar]
- Chan O, Zhu W, Ding Y, McCrimmon RJ, Sherwin RS. Blockade of GABA(A) receptors in the ventromedial hypothalamus further stimulates glucagon and sympathoadrenal but not the hypothalamo-pituitary-adrenal response to hypoglycemia. Diabetes 2006; 55: 1080–1087. [DOI] [PubMed] [Google Scholar]
- Fioramonti X, Marsollier N, Song Z, Fakira KA, Patel RM, Brown S, Duparc T, Pica-Mendez A, Sanders NM, Knauf C, Valet P, McCrimmon RJ, Beuve A, Magnan C, Routh VH. Ventromedial hypothalamic nitric oxide production is necessary for hypoglycemia detection and counterregulation. Diabetes 2010; 59: 519–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller M, Duplock S, Turner C, Davey P, Brooks DA, Hopwood JJ, Meikle PJ. Mass spectrometric quantification of glycogen to assess primary substrate accumulation in the Pompe mouse. Anal. Biochem 2012: 421: 759–763. [DOI] [PubMed] [Google Scholar]
- Gujar AD, Ibrahim BA, Tamrakar P, Koshy Cherian A, Briski KP Hindbrain lactostasis regulates hypothalamic AMPK activity and hypothalamic metabolic neurotransmitter mRNA and protein responses to hypoglycemia. Amer. J. Physiol 2014; 306: R457–R69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda S, Akao E, Suzuki S, Okuda M, Kakehi K, Nakamura J. High-performance liquid chromatography of reducing carbohydrates as strongly ultraviolet-absorbing and electrochemically-sensitive 1-phenyl-3-methyl-5-pyrazolone derivatives. Anal. Biochem 1989; 180: 351–357. [DOI] [PubMed] [Google Scholar]
- Ibrahim BA, Tamrakar P, Gujar AD, Koshy Cherian A, Briski KP. Caudal fourth ventricular administration of the AMPK activator 5-aminoimiazole-4-carboxamide-riboside regulates glucose and counterregulatory hormone profiles, dorsal vagal complex metabolosensory neuron function, and hypothalamic Fos expression. J. Neurosci. Res 2013; 91: 1226–1238. [DOI] [PubMed] [Google Scholar]
- Kale AY, Paranjape SA, Briski KP. I.c.v. administration of the nonsteroidal glucocorticoid receptor antagonist, CP4–72555, prevents exacerbated hypoglycemia during repeated insulin administration. Neuroscience 2006; 140: 555–565. [DOI] [PubMed] [Google Scholar]
- Laming PR, Kimelberg H, Robinson S, Salm A, Hawrylak N, Muller C, Roots B, Ng K, 2000. Neuronal-glial interactions and behavior. Neurosci. Biobehav. Rev 24, 295–340. [DOI] [PubMed] [Google Scholar]
- Magistretti PJ, 1988. Regulation of glycogenolysis by neurotransmitters in the central nervous system. Diabetes Metab. 14, 237–246. [PubMed] [Google Scholar]
- Napit PR, Ali MH, Shakya M, Mandal SK, Bheemanapally K, Mahmood ASMH, Ibrahim MMH, Briski KP. Hindbrain estrogen receptor regulation of ventromedial hypothalamic nucleus glucoregulatory transmitter signaling and counter-regulatory hormone secretion in hypoglycemic female rats. [Google Scholar]
- Patil GD, Briski KP Lactate is a critical ‘sensed’ variable in caudal hindbrain monitoring of CNS metabolic stasis. Amer. J. Physiol 2005a; 289: R1777–R11786. [DOI] [PubMed] [Google Scholar]
- Routh VH, Hao L, Santiago AM, Sheng Z, Zhou C. Hypothalamic glucose sensing: making ends meet. Front. Syst. Neurosci 2014; 8:236. doi: 10.3389/fnsys.2014.00236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakya M, Shrestha PK, Briski KP. Hindbrain 5’-monophosphate-activated protein kinase mediates short-term food deprivation inhibition of the gonadotropin-releasing hormone-luteinizing hormone axis: role of nitric oxide. Neuroscience 2018; 383: 46–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrestha PK, Tamrakar P, Ibrahim BA, Briski KP. Hindbrain medulla catecholamine cell group involvement in lactate-sensitive hypoglycemia-associated patterns of hypothalamic norepinephrine and epinephrine activity. Neuroscience 2014; 278: 20–30. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Chang C, Muramatsu M, Swanson LW. Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: an in situ hybridization study. J Comp Neurol. 1990; 294: 76–95. [DOI] [PubMed] [Google Scholar]
- Stobart JL, Anderson CM, 2013. Multifunctional role of astrocytes as gatekeepers of neuronal energy supply. Cell. Neurosci 7, 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]