Abstract
Background:
Albuminuria predicts adverse events in heart failure with preserved ejection fraction (HFpEF). No therapies to date have reduced albuminuria in HFpEF.
Methods and Results:
We analyzed 1175 participants from the Americas from the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist (TOPCAT) study with urinary albumin-to-creatinine ratio (UACR) measurements at baseline. We examined the association of UACR with the primary outcome (cardiovascular death, aborted cardiac arrest, or HF hospitalization) its individual components, all-cause mortality, and several safety endpoints using multivariable-adjusted Cox regression. We evaluated whether spironolactone reduced albuminuria at the 1-year visit in a subpopulation (N=744). 35% had microalbuminuria, 13% had macroalbuminuria, and 80% were receiving angiotensin-converting enzyme-inhibitors or angiotensin receptor blockers. Increasing UACR was associated with male gender, higher systolic blood pressure (SBP), diabetes mellitus, and renal dysfunction. Macroalbuminuria (hazard ratio [HR] 1.67 [95%CI 1.22, 2.28]) and microalbuminuria (HR 1.47 [95%CI 1.15, 1.86]) were independently associated with the TOPCAT primary endpoint (compared to normoalbuminuria). Adjusting for placebo response, spironolactone reduced albuminuria by 39% in all participants at the 1-year visit compared to baseline (geometric mean ratio 0.61, 95%CI 0.49–0.77), and by 76% (geometric mean ratio 0.24, 95%CI 0.10, 0.56) among those with macroalbuminuria. Reducing UACR by 50% was independently associated with a reduction in HF hospitalization (HR 0.90, p=0.017) and all-cause mortality (HR 0.91, p=0.019). The change in UACR was significantly associated with change in SBP (p=0.001).
Conclusions:
In TOCPAT, albuminuria was independently associated with worse cardiovascular outcomes. Spironolactone significantly reduced albuminuria compared to placebo. Reducing albuminuria was independently associated with improved outcomes.
Clinical Trials Registration:
ClinicalTrials.gov; Identifier: NCT00094302
Keywords: heart failure with preserved ejection fraction, kidney, spironolactone, albuminuria
INTRODUCTION
Albuminuria is present in nearly half of patients with heart failure with preserved ejection fraction (HFpEF) and portends a worse prognosis.1–3 An analysis from the Candesartan in Heart failure: Assessment of Reduction in Mortality and Morbidity (CHARM) Programme demonstrated that the presence of albuminuria was associated with nearly a two-fold increase in the risk of cardiovascular death or HF admission in HFpEF.1 Whereas clinical practice guidelines recommend screening for and treatment of albuminuria in the management of patients with diabetes mellitus, hypertension, and chronic kidney disease, similar guidelines are not available for management of HFpEF.4, 5
Albuminuria is a biomarker of multiple pathophysiological processes including systemic inflammation and endothelial and microvascular dysfunction, which have been postulated to play a role in HFpEF.6, 7 Therefore, albuminuria has been considered as a target to reduce cardiovascular events in HFpEF.8 Unfortunately, no therapies to date from randomized trials in HFpEF, including angiotensin receptor blockers (ARB) or angiotensin receptor-neprilysin inhibitors, have demonstrated a reduction in albuminuria.1, 9
Spironolactone has been shown to reduce albuminuria in patients with diabetic nephropathy and chronic kidney disease, even on top of angiotensin converting enzyme inhibitor (ACE-I) or ARB therapy.10–15 However, whether spironolactone reduces albumin excretion in a broad array of HFpEF patients has not been studied. In this analysis, we first assessed the prognostic role of albuminuria among patients with HFpEF enrolled in the Americas in the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist (TOPCAT) trial and evaluated the effect of spironolactone in reducing albuminuria in the entire cohort and in several subgroups.
METHODS
TOPCAT study design and objectives
The design of the TOPCAT study has been described in detail previously.16 The data, analytic methods, and study materials have been made available to other researchers for purposes of reproducing the results or replicating the procedure located on the National Institutes of Health website. Briefly, TOPCAT was a multi-center, international, randomized, double blind, placebo-controlled trial of spironolactone in adults with HFpEF recruited from over 270 clinical sites. The trial was funded by the National Heart, Lung, and Blood Institute as a contract with the Brigham and Women’s Hospital (Clinical Coordinating Center) and the New England Research Institute (Data Coordinating Center). Enrollment began in August 2006 and ended in January 2012, and the primary results of the trial were published in April 201417 (mean follow-up was 3.5 years). The primary aim of the TOPCAT study was to determine whether treatment with spironolactone, compared to placebo, can produce a clinically meaningful reduction in the composite outcome of cardiovascular mortality, aborted cardiac arrest, or HF hospitalization in adults with symptomatic HF and documented LVEF ≥ 45%. All study participants provided written informed consent.
Inclusion criteria for TOPCAT were as follows: age ≥ 50 years; diagnosis of HF based on at least one HF symptom at the time of study screening and at least one HF sign within the 12 months prior to screening; LVEF ≥ 45% (per local reading); at least 1 HF hospitalization in the 12 months prior to study screening or B-type natriuretic peptide (BNP) > 100 pg/ml or N-terminal pro-BNP > 360 pg/ml (in the absence of an alternative explanation for elevated natriuretic peptide level) within the 60 days prior to screening; serum potassium < 5.0 mmol/L prior to randomization.16 There were multiple exclusion criteria for TOPCAT, as detailed previously.16 Examples of exclusion criteria include severe chronic kidney disease (defined as estimated glomerular filtration rate [eGFR] <30 mL/min per 1.73 m2 or serum creatinine ≥2.5 mg/dL), severe systemic illness with a life expectancy of less than 3 years, a history of significant hyperkalemia, known intolerance to aldosterone antagonists, and recent myocardial infarction, coronary artery bypass grafting, or percutaneous coronary intervention. By trial design, all enrolled patients were asked to provide a spot urine specimen to measure UACR at baseline and yearly thereafter. Laboratory measurements for urine chemistries (including UACR) were performed locally at the enrolling site.
For the present study, we first excluded participants from Russia and Georgia (N=1678), given the significant regional differences previously described,18 and those with missing urinary albumin to creatinine ratio (UACR) at baseline (N=590) or implausible values (N=2), yielding 1175 participants for the present analyses. Subsequent analyses investigating the change in albuminuria at 1-year further excluded participants with missing 1-year UACR values. Of the initial 1175 participants, 1033 were present at the 1-year visit, 289 of which did not have an available UACR measurement, leaving 744 participants for the subanalysis of change from baseline to 1-year UACR. All HF hospitalizations were adjudicated by a clinical end-point committee at Brigham and Women’s Hospital, blinded to study-drug assignments, and according to pre-specified criteria.16 The primary endpoint of the study was the time to death from cardiovascular causes, aborted cardiac arrest, or HF hospitalization. Secondary endpoints included cardiovascular mortality, all cause mortality, and HF hospitalization. The safety outcomes of our study included doubling of serum creatinine, hyperkalemia (potassium > 5.5 mEq/L), and discontinuation of study drug.
Statistical analysis
Baseline characteristics are summarized by clinical groups of albuminuria (normoalbuminuria, microalbuminuria, and macroalbuminuria) using mean and standard deviation for normally distributed variables and geometric mean and/or median [25th-75th percentile] if non-normally distributed. Categorical variables are presented as percentages of observations. Microalbuminuria was defined by UACR ≥ 30 mg/g and macroalbuminuria by UACR ≥ 300 mg/g.19 The values of UACR are right-skewed and therefore required log transformation to approximate a normal distribution for analysis as a continuous variable.9 ANOVA and chi-squared tests were performed as appropriate, with p-values shown for trend.
The association between albuminuria groups and the efficacy and safety outcomes were assessed using crude and multivariable-adjusted Cox regression. UACR was evaluated both as a categorical as well as a continuous variable, given the graded relationship between UACR and adverse events.1 Linearity was demonstrated between the association of log UACR and all outcomes. Covariates were chosen based upon a combination of clinical relevance and previous prognostic implication in TOPCAT.20, 21 Multivariable models were adjusted for New York Heart Association class, diabetes status, serum creatinine, heart rate, age, sex, race, smoking status, atrial fibrillation, peripheral artery disease, ejection fraction, systolic blood pressure (SBP), and assignment to spironolactone vs. placebo.
We next determined the placebo-adjusted change in UACR from baseline to the 1-year visit. Post-randomization changes from baseline were compared using linear regression, controlling for treatment allocation and baseline UACR as independent variables. To assess whether the treatment effect was independent of several subgroups, an interaction term between treatment and the subgroup was tested. Further, we assessed clinical and laboratory covariates that were independently associated with change in UACR using multivariable regression. Then, we performed Cox regression between the change in UACR and efficacy outcomes, expressing hazard ratios per 50% reduction in UACR. We adjusted for all covariates associated with the change in UACR at a significance level of p<0.10. We tested for an interaction between UACR change and treatment allocation.
We also performed linear regression between the change in SBP and the change in albuminuria (from the 1-year and baseline visits), adjusting for baseline UACR and SBP, to determine whether albuminuria reduction was influenced by SBP reduction. Analyses were performed using STATA version 12, and a two-sided p-value < 0.05 was considered statistically significant.
RESULTS
Baseline characteristics
Of the initial study population of 1767 participants in the Americas, UACR was available in 1175 participants. Supplementary Table 1 displays characteristics of participants with and without baseline UACR measurements, demonstrating few clinical differences between the groups. Table 1 lists the baseline characteristics of the study population, stratified by groups of albuminuria: 52% had a normal UACR, 35% had microalbuminuria, and 13% had macroalbuminuria. The median (25th-75th percentile) UACR was 27 (9–117) mg/g. The average age was 72±10 years, 48% were women, and 80% were white. Hypertension (89%), renal dysfunction (defined by eGFR ≤ 60 mL/min/m2, 48%), and diabetes mellitus (44%) were common. Nearly 80% were on ACE-I or ARB therapy. Patients with increased UACR were more often male, enrolled through the HF hospitalization stratum, had higher SBP, more frequently had diabetes mellitus and renal dysfunction, and had lower hemoglobin levels (p<0.05 for all comparisons). While they were more likely to take beta-blockers or calcium channel blockers, there was no difference in ACE-I/ARB use (p=0.37).
TABLE 1.
Baseline Clinical Characteristics by Urinary Albumin to Creatinine Ratio
| Normoalbuminuria N=605 |
Microalbuminuria N=414 |
Macroalbuminuria N=156 |
P-value* | |
|---|---|---|---|---|
| Albumin to creatinine ratio, mg/g** | 9 (5 – 18) | 79 (45 – 142) | 704 (451 – 1393) | |
| Randomization to spironolactone, n (%) | 318 (52.6%) | 206 (49.8%) | 73 (46.8%) | 0.16 |
| Age, years | 72 ± 10 | 73 ± 9 | 70 ± 10 | 0.07 |
| Female, n (%) | 307 (50.7%) | 188 (45.4%) | 67 (42.9%) | 0.037 |
| White race, n (%) | 491 (81.2%) | 327 (79.0%) | 118 (75.6%) | 0.12 |
| NYHA Class III or IV, n (%) | 189 (31.3%) | 159 (38.5%) | 50 (32.1%) | 0.26 |
| Enrollment through HF hospitalization stratum, n (%) | 322 (53.2%) | 244 (58.9%) | 102 (65.4%) | 0.003 |
| Physical Characteristics | ||||
| Systolic blood pressure, mmHg | 126 ± 15 | 128 ± 17 | 133 ± 16 | <0.001 |
| Diastolic blood pressure, mmHg | 71 ± 11 | 72 ± 12 | 73 ± 12 | 0.16 |
| Heart rate (beats/min) | 69 ± 11 | 69 ± 11 | 69 ± 10 | 0.91 |
| Body mass index (kg/m2) | 33.9 ± 7.8 | 33.2 ± 8.0 | 34.5 ± 9.3 | 0.94 |
| Comorbidities, n (%) | ||||
| Hypertension | 537 (88.8%) | 369 (89.1%) | 141 (90.4%) | 0.59 |
| Atrial fibrillation | 285 (47.1%) | 200 (48.3%) | 59 (37.8%) | 0.13 |
| Diabetes mellitus | 232 (38.3%) | 190 (45.9%) | 93 (59.6%) | <0.001 |
| Myocardial Infarction | 120 (19.8%) | 89 (21.5%) | 26 (16.7%) | 0.67 |
| COPD | 102 (16.9%) | 66 (15.9%) | 22 (14.1%) | 0.41 |
| Stroke | 62 (10.2%) | 37 (8.9%) | 11 (7.1%) | 0.21 |
| Peripheral artery disease | 59 (9.8%) | 52 (12.6%) | 17 (10.9%) | 0.36 |
| Chronic kidney disease | 259 (42.8%) | 209 (50.5%) | 90 (57.7%) | <0.001 |
| Current smoker | 38 (6.3%) | 22 (5.3%) | 13 (8.3%) | 0.62 |
| Medication Use, n (%) | ||||
| ACE-I and/or ARB | 491 (81.3%) | 322 (77.8%) | 123 (78.8%) | 0.27 |
| Beta-blocker | 451 (74.7%) | 324 (78.3%) | 132 (84.6%) | 0.008 |
| Calcium channel blocker | 205 (33.9%) | 169 (40.8%) | 87 (55.8%) | <0.001 |
| Diuretic | 531 (87.9%) | 378 (91.3%) | 142 (91.0%) | 0.10 |
| Other anti-hypertensive medication | 99 (16.4%) | 65 (15.7%) | 34 (21.8%) | 0.25 |
| Statin | 373 (61.8%) | 275 (66.4%) | 109 (69.9%) | 0.032 |
| Laboratory Testing | ||||
| Estimated glomerular filtration rate (mL/min/1.78 m22) | 67 ± 21 | 64 ± 22 | 60 ± 20 | <0.001 |
| Hemoglobin (mg/dL) | 13.1 ± 1.6 | 12.8 ± 1.7 | 12.7 ± 1.7 | <0.001 |
| Electrocardiographic and Imaging Data | ||||
| Ejection fraction (%) | 58 ± 8 | 58 ± 7 | 57 ± 7 | 0.46 |
| ECG Left ventricular hypertrophy (%) | 47 (10.3%) | 53 (15.9%) | 14 (12.6%) | 0.12 |
NYHA, New York Heart Association; COPD, chronic obstructive pulmonary disease; ACE-I, angiotensin-converting-enzyme inhibitor; ARB, angiotensin II receptor blocker; HF, heart failure.
P-value shown for trend.
Presented as median (25th – 75th percentile) since the variable is right-skewed.
Table 2 shows event rates and crude and multivariable-adjusted hazard ratios for efficacy and safety outcomes, stratified by albuminuria group (with normoalbuminuria designated as the referent group). There was a significant relationship between albuminuria group and several outcomes after multivariable adjustment. For example, the risk for the primary outcome was 1.47 (95% CI 1.15, 1.86) for microalbuminuria and 1.67 (95% CI 1.22, 2.28) for macroalbuminuria even after adjustment for several potentially confounding variables. This was influenced predominantly by an increased risk for HF hospitalization (1.56 [95% CI 1.18, 2.06] for microalbuminuria and 2.09 [95% CI 1.48, 2.97] for macroalbuminuria). Similar associations were found for cardiovascular death (for the microalbuminuria group), worsening renal function (for the macroalbuminuria group), and hyperkalemia (for both groups).
TABLE 2.
Event Rates and Crude and Adjusted Hazard Ratios for Efficacy and Safety Outcomes by Urinary Albumin to Creatinine Ratio
| Outcomes, n (%) | Normoalbuminuria N=605 |
Microalbuminuria N=414 |
Macroalbuminuria N=156 |
|---|---|---|---|
| Composite endpoint | |||
| • Event rate (per 100 person-years, 95% CI) | 8.4 (7.1, 10.0) | 14.3 (12.2, 16.8) | 16.8 (13.1, 21.6) |
| • Crude model HR (95% CI) | ref | 1.68 (1.33, 2.12) | 1.96 (1.45, 2.65) |
| • Multivariable adjusted model HR (95% CI) | ref | 1.47 (1.15, 1.86) | 1.67 (1.22, 2.28) |
| Cardiovascular mortality | |||
| • Event rate (per 100 person-years, 95% CI) | 3.5 (2.7, 4.4) | 5.8 (4.6, 7.3) | 4.3 (2.7, 6.7) |
| • Crude model HR (95% CI) | ref | 1.65 (1.17, 2.32) | 1.24 (0.74, 2.07) |
| • Multivariable adjusted model HR (95% CI) | ref | 1.53 (1.08, 2.17) | 1.12 (0.66, 1.92) |
| HF hospitalization | |||
| • Event rate (per 100 person-years, 95% CI) | 5.9 (4.8, 7.1) | 10.9 (9.1, 13.1) | 14.8 (11.4, 19.4) |
| • Crude model HR (95% CI) | ref | 1.84 (1.40, 2.42) | 2.47 (1.77, 3.45) |
| • Multivariable adjusted model HR (95% CI) | ref | 1.56 (1.18, 2.06) | 2.09 (1.48, 2.97) |
| All cause mortality | |||
| • Event rate (per 100 person-years, 95% CI) | 6.0 (5.0, 7.3) | 8.2 (6.8, 10.0) | 7.5 (5.4, 10.5) |
| • Crude model HR (95% CI) | ref | 1.35 (1.03, 1.76) | 1.25 (0.85, 1.83) |
| • Multivariable adjusted model HR (95% CI) | ref | 1.29 (0.99, 1.70) | 1.20 (0.80, 1.79) |
| Doubling of creatinine | |||
| • Event rate (per 100 person-years, 95% CI) | 4.6 (3.7, 5.8) | 4.9 (3.7, 6.4) | 7.8 (5.5, 11.2) |
| • Crude model HR (95% CI) | ref | 1.05 (0.74, 1.50) | 1.69 (1.11, 2.56) |
| • Multivariable adjusted model HR (95% CI) | ref | 0.97 (0.68, 1.38) | 1.56 (1.01, 2.42) |
| Hyperkalemia | |||
| • Event rate (per 100 person-years, 95% CI) | 4.6 (3.7, 5.8) | 7.4 (5.9, 9.2) | 9.6 (6.9, 13.3) |
| • Crude model HR (95% CI) | ref | 1.57 (1.14, 2.16) | 2.02 (1.36, 3.01) |
| • Multivariable adjusted model HR (95% CI) | ref | 1.51 (1.09, 2.10) | 1.80 (1.18, 2.76) |
| Discontinuation of study drug | |||
| • Event rate (per 100 person-years, 95% CI) | 18.1 (16.0, 20.6) | 21.7 (18.8, 25.0) | 23.7 (18.7, 29.9) |
| • Crude model HR (95% CI) | ref | 1.18 (0.97, 1.43) | 1.27 (0.97, 1.66) |
| • Multivariable adjusted model HR (95% CI) | ref | 1.10 (0.91, 1.35) | 1.16 (0.88, 1.53) |
HF, heart failure; CI, confidence interval.
Multivariable models adjusted for New York Heart Association class, diabetes status, serum creatinine, heart rate, age, sex, race, smoking status, atrial fibrillation, peripheral artery disease, ejection fraction, systolic blood pressure, and assignment to spironolactone vs. placebo.
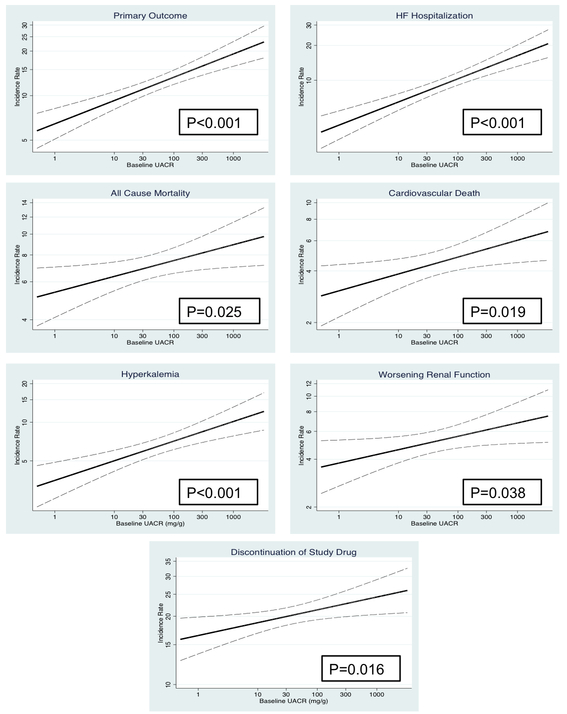
Figure 1 and Supplementary Table 2 show the graded relationship between UACR and adverse outcomes modeling UACR as a continuous variable. Hazard ratios in Supplementary Table 1 are shown per doubling of UACR. There was a consistent increased hazard ratio for the primary endpoint [1.08 (1.04, 1.13), p<0.001], HF hospitalization [1.11 (1.06, 1.16), p<0.001], and all-cause mortality [1.05 (1.00, 1.10), p=0.041] per doubling of UACR. There was no interaction between baseline UACR and treatment response for the primary endpoint (Supplementary Figure 1) or any other outcome (Supplementary Table 2), indicating that the effect of spironolactone was similar in those with low vs. high UACR values.
Figure 1: Event Rates by Baseline Urinary Albumin to Creatinine Ratio.
Event rates and 95% confidence intervals (dashed lines) are displayed for safety and efficacy outcomes by baseline urinary albumin to creatinine ratio (UACR). P-value shown for linear trend.
We performed a sub-analysis of participants attending the 1-year visit with available UACR measurements (N=744). Adjusting for placebo response, spironolactone significantly reduced albuminuria at the 1-year visit compared to baseline by 39% (geometric mean ratio 0.61, 95% CI 0.49, 0.77, p<0.001) (Table 3). Analyzed per trial arm, treatment with spironolactone reduced albuminuria by 29% (geometric mean ratio 0.71 [95% CI 0.58, 0.88], p=0.001] at the 1-year visit, while there was no significant difference in the placebo group (geometric mean ratio 0.97 [95% CI 0.83, 1.18], p=0.88] (Supplementary Table 3). We performed subgroup analyses by baseline albuminuria group, presence of diabetes mellitus, use of ACE-I/ARB, eGFR ≤60 ml/min/m2versus >60 mL/min/m2, and baseline SBP ≥ 130 mmHg. There was a significant interaction only by baseline albuminuria group, such that there was a 76% reduction in UACR among those with macroalbuminuria (geometric mean ratio 0.24, 95% CI 0.10, 0.56). We also assessed the association of clinical and laboratory covariates with change in UACR. After multivariable adjustment, baseline UACR, eGFR and diabetes were all independently associated with change in UACR (Supplementary Table 4).
TABLE 3.
Placebo-adjusted Ratio in Urinary Albumin to Creatinine Ratio at the 1-Year Visit vs. Baseline by Treatment Arm
| Treatment Effect of Spironolactone vs. Placebo | ||
|---|---|---|
| Placebo-adjusted Geometric Mean Ratio of 1- Year to Baseline UACR (95% CI), P-value |
Interaction P-value | |
| All Patients | 0.61 (0.49, 0.77), p<0.001 | NA |
| Albuminuria Status | 0.006 | |
| Normalbuminuria | 0.65 (0.48, 0.89), p=0.006 | |
| Microalbuminuria | 0.78 (0.55, 1.11), p=0.17 | |
| Macroalbuminuria | 0.24 (0.10, 0.56), p=0.001 | |
| Diabetes Mellitus | 0.27 | |
| Present | 0.53 (0.37, 0.76), p=0.001 | |
| Absent | 0.68 (0.50, 0.91), p=0.011 | |
| eGFR<60 ml/min/1.73m22 | 0.10 | |
| Present | 0.51 (0.37, 0.70), p<0.001 | |
| Absent | 0.72 (0.52, 1.54), p=0.05 | |
| Use of ACE-I/ARB | 0.18 | |
| Yes | 0.57 (0.44, 0.74), p<0.001 | |
| No | 0.81 (0.52, 1.32), p=0.43 | |
| Systolic Blood Pressure ≥ 130 mmHg | 0.17 | |
| Present | 0.53 (0.38, 0.73), p<0.001 | |
| Absent | 0.71 (0.51, 0.98), p=0.034 | |
UACR, urinary albumin to creatinine ratio; eGFR, estimated glomerular filtration rate; ACE-I, angiotensin-converting-enzyme inhibitor; ARB, angiotensin II receptor blocker
Table 4 shows the effect of halving UACR at the 1-year visit compared to baseline on efficacy outcomes. On crude analysis, reducing UACR by 50% was associated with a nearly consistent 10% decrease in the risk for the primary endpoint, cardiovascular death, HF hospitalization, and all-cause mortality (p<0.05 for all comparisons). After adjusting for baseline UACR, diabetes status, eGFR, age, and assignment to spironolactone vs. placebo, reducing albuminuria was independently associated with a reduction in HF hospitalization (HR 0.90, 95% CI 0.82, 0.98) and all cause mortality (HR 0.91, 95% CI 0.84, 0.98). In the multivariable analyses, there was no interaction by assignment to spironolactone.
TABLE 4.
Crude and Adjusted Hazard Ratios for Efficacy Outcomes per Halving of Urinary Albumin to Creatinine Ratio at the 1-Year Visit Compared to Baseline
| Outcomes | Crude Model HR (95% CI)* |
P-value | Adjusted Model HR (95% CI)*^ |
P-value | p for treatment interaction ^ |
|---|---|---|---|---|---|
| Composite endpoint | 0.91 (0.85, 0.98) | 0.01 | 0.93 (0.86, 1.00) | 0.054 | 0.91 |
| Cardiovascular mortality | 0.89 (0.80, 0.98) | 0.015 | 0.91 (0.82, 1.01) | 0.064 | 0.83 |
| HF hospitalization | 0.89 (0.82, 0.97) | 0.007 | 0.90 (0.82, 0.98) | 0.017 | 0.80 |
| All cause mortality | 0.90 (0.83, 0.97) | 0.008 | 0.91 (0.84, 0.98) | 0.019 | 0.71 |
HF, heart failure; CI, confidence interval; UACR, urinary albumin to creatinine ratio.
Hazard ratios are expressed per 50% reduction in UACR at the 1-year visit compared to baseline.
Models adjusted baseline UACR, diabetes status, estimated glomerular filtration rate, age, and assignment to spironolactone vs. placebo.
Analyses were landmarked at the 1-year visit.
We performed further analyses to understand mechanisms by which spironolactone reduces albuminuria. There was a significant association between change in SBP and change in albuminuria (p=0.001) (Supplementary Figure 2), which was not influenced by randomization to spironolactone (p=0.54). However, spironolactone remained associated with a reduction in UACR even after adjusting for change in SBP (p<0.001). There was no association between change in eGFR and change in albuminuria (p=0.07).
DISCUSSION
In this analysis of the TOPCAT trial, we demonstrated several important findings regarding the prognostic relevance of albuminuria and the influence of spironolactone in reducing urinary albumin excretion in HFpEF. First, increasing baseline albuminuria conferred a significantly increased, and graded, risk for several major adverse cardiovascular events, even after multivariable adjustment. In addition, spironolactone reduced albuminuria by 39% compared to placebo. While there was consistent albuminuria reduction among those with diabetes mellitus, hypertension, and chronic kidney disease, there was significant effect modification by baseline albuminuria, such that patients with baseline macroalbuminuria observed the greatest reduction in albuminuria (76% reduction). Next, we found reducing albuminuria was independently associated with a reduction in HF hospitalization and all-cause mortality. Finally, there was a significant association between SBP and albuminuria reduction, though SBP reduction did not account for the entire mechanism by which spironolactone reduced albuminuria. Despite significant interest in reducing albuminuria to improve cardiovascular outcomes in HFpEF, previous studies have failed to demonstrate such a reduction.1, 9
Albuminuria is very common in HFpEF, and nearly half the participants in TOPCAT had evidence of albuminuria, similar to previous studies.1,2 This may reflect the high prevalence of relevant comorbidities including hypertension, chronic kidney disease, and diabetes mellitus, and/or the presence of a systemic endothelial dysfunction process that results in albuminuria due to endothelial dysfunction in the kidney. The prevalence of albuminuria is particularly striking given that 80% of all participants were already on ACE-I/ARBs, therapies known to reduce albuminuria in individuals with these comorbidities. Notably, given the exclusion of participants with severe renal dysfunction in TOPCAT, our study likely underestimates the true prevalence of albuminuria in HFpEF. Albuminuria also conferred an increased, and graded, risk for the primary outcome (hazard ratios 1.47 and 1.67 for microalbuminuria and macroalbuminuria, respectively, or 8% increased risk per doubling of UACR), driven predominantly by an increased risk for HF hospitalization. Our estimates are similar to those found in a CHARM sub-analysis of HFpEF patients.1
Importantly, we found that spironolactone had a significant effect in reducing albuminuria, which was observed regardless of background ACE-I/ARB therapy. The reduction in albuminuria was greatest in those with baseline macroalbuminuria (76% reduction) and normoalbuminuria (35% reduction), even after adjusting for placebo-response (which accounts for regression to the mean among those with high UACR). There was also a 22% reduction in those with baseline microalbuminuria, but this was not statistically significant, likely due to lack of power to detect a more subtle association. Although spironolactone can lead to a reduction in renal function, treatment was still associated with overall improved cardiovascular events in the Americas.18 Notably, there was a significant association between the SBP lowering effect of spironolactone and its reduction in albuminuria. However, other therapies that lower SBP in HFpEF have not shown a reduction in albuminuria.1, 9 In addition, the association between spironolactone and albuminuria reduction remained significant even after adjusting for the change in SBP. Hence, the mechanism of action by which spironolactone reduces albuminuria may be multifactorial and goes beyond BP reduction alone. Spironolactone, for instance, also improves endothelial function and vascular compliance and even reduces oxidative stress, which are important derangements in HFpEF that may be associated with albuminuria.16
There have been mixed results in previous trials of both HFrEF and HFpEF groups in the anti-proteinuric effect of various inhibitors of the renin-angiotensin-aldosterone system. Trials of aliskiren, sacubitril-valsartan, and candesartan have not demonstrated such a benefit.1, 9, 22 A substudy from Studies of Left Ventricular Dysfunction (SOLVD) demonstrated an anti-proteinuric effect of enalapril among HFrEF patients, but this was only seen in diabetic patients, a group expected to benefit.23 A phase II trial of BAY 94–8862 (now known as finerenone, a next generation non-steroidal mineralocorticoid antagonist) demonstrated that both finerenone and spironolactone significantly reduced albuminuria compared to placebo.24 However, this study likewise only evaluated HFrEF patients. Notably, no other trial in HFpEF has demonstrated a reduction in albumin excretion.1, 9 While spironolactone did not reduce the primary endpoint in the overall population, there was significant regional variation with a benefit demonstrated in the Americas, and concerns have been raised regarding study conduct in Russia.18, 25, 26 Our results demonstrate additional physiological rationale for spironolactone and support its re-appraisal in HFpEF in an outcomes-based trial, which is currently underway (ClinicalTrials.gov Identifier NCT02901184).27
Notably, reducing albuminuria was associated with a reduction in all studied adverse cardiovascular events on unadjusted analysis in our analysis. These relationships persisted for HF hospitalization and all-cause mortality even after multivariable adjustment. Interestingly, there was no interaction by treatment arm, indicating that primary reduction in albuminuria may be an important intervention to reduce adverse events in HFpEF. How albuminuria relates to adverse events in HFpEF may be multifactorial. Albuminuria is a marker of endothelial dysfunction, microvascular disease, global vascular disease, and systemic inflammation, which are all processes that may play a role in the pathogenesis of symptoms in HFpEF.2, 28–30 Comprehensive echocardiographic studies of HFpEF have likewise demonstrated links between albuminuria and adverse cardiovascular remodeling and impaired biventricular function.2, 31 Specifically, albuminuria is associated with subclinical cardiac dysfunction, as demonstrated by worsening longitudinal strain.2, 32 Interestingly, albuminuria may be more specific to the pathogenesis of HFpEF, and not HFrEF, as a previous study showed that albuminuria predicted progression to HFpEF only.33 In addition, albuminuria may reflect a cardiorenal phenotype, and in particular renal venous congestion, as seen in animal models,34 or reflect reduced renal blood flow as demonstrated in HF patients.35 Finally, albuminuria itself may provoke diuretic resistance, since albumin-bound diuretic filtered in the renal tubules impairs interaction with luminal cotransporting proteins.36 It should be noted, however, that while albuminuria reduction may be helpful particularly with regards to reducing renal injury, the relationship between albuminuria and renal function is not always straightforward. For instance, sucubitril-valsartan actually increased albuminuria compared to enalapril in HFpEF patients, but still demonstrated greater preservation of eGFR.9
There are some potential limitations of the study. We used spot measurements to estimate albuminuria, while 24-hour urine collection is the gold standard. However, the latter is impractical in large trials or epidemiologic surveys of albuminuria and often fraught with inaccurate timing of collections. In addition, spot estimates of UACR correlate with 24-hour collection measurements at the population level.37 Next, severe renal dysfunction was an exclusion criterion in TOPCAT, which limits its generalizability to this subset of patients. However, spironolactone is relatively contraindicated in patients with severe renal dysfunction. In addition, since urine specimens were not available at baseline or the 1-year visit for all participants, a challenge of similar magnitude demonstrated in other trials,1, 13, 22 this may alter the estimates of risk by albuminuria and albuminuria reduction. However, as we’ve demonstrated, there is little difference in baseline characteristics between those with and without UACR measurements, and therefore unlikely to significantly bias these results. In addition, out of the 1175 participants, only 142 participants did not come to the 1-year visit (57 of whom had died). Finally, since only 744 participants have baseline and 1-year UACR values, we were underpowered to perform a mediation analysis to determine whether the beneficial effects of spironolactone were attributable to reduction in albuminuria.
In summary, in patients with HFpEF, increasing UACR confers a significantly increased risk for major adverse cardiovascular events and worsening renal function. Spironolactone reduced albuminuria compared to placebo by 39%, an effect augmented in those with macroalbuminuria (76%). Reducing albuminuria was associated with a reduction in several adverse cardiovascular events. While there was a significant association between SBP and albuminuria reduction, SBP reduction did not account for the entire mechanism by which spironolactone reduced albuminuria. Our results provide further physiological rationale for the ongoing efforts to reassess the benefits of spironolactone in HpEF.27
Supplementary Material
WHAT IS NEW?
This study reports the relationship between albuminuria and adverse cardiovascular events in patients with heart failure with preserved ejection fraction (HFpEF).
Spironolactone reduced albuminuria significantly compared to placebo, regardless of underlying comorbidities or medication use.
We also demonstrated that reducing albuminuria was associated with significant clinical benefit, including a reduction in mortality.
The reduction in albuminuria was related to blood pressure control, but the mechanism by which spironolactone reduced albuminuria was not entirely explained by blood pressure reduction.
WHAT ARE THE CLINICAL IMPLICATIONS?
We demonstrate another potential benefit for the use of spironolactone in HFpEF.
Spironolactone could be considered part of the therapeutic armamentarium in treating albuminuria in treating patients with HFpEF.
Our results provide further physiological rationale for the ongoing efforts to reassess the benefits of spironolactone in HpEF.
Acknowledgments
FUNDING SOURCE
This work was funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, contract HHSN268200425207C. The content of this article does not necessarily represent the views of the National Heart, Lung, and Blood Institute or of the Department of Health and Human Services.
Footnotes
DISCLOSURES
Dr Rouleau is a consultant for Novartis, Bayer and Astra-Zeneca. Dr Pfeffer has received consulting fees from Aastrom, Abbott Vascular, Amgen, Cerenis, Concert, Daiichi Sankyo, Fibrogen, Genzyme, GlaxoSmithKline, Hamilton Health Sciences, Medtronic, Merck, Novo Nordisk, Roche, Salix, Sanderling, Sanofi Aventis, Serono, Servier, and Teva, as well as research grants from New England Research Institute via subcontract from the National Institutes of Health, Amgen, Celladon, Novartis, and Sanofi-Aventis. Dr. O’Meara reports research support/clinical trial participation disclosures with Novartis, Bayer, Astra Zeneca, Merck, Amgen, Servier; as well as speaker or consultant services for Novartis, Pfizer, Servier, Astra Zeneca, Amgen. The Brigham and Women’s Hospital has patents for the use of inhibitors of the renin-angiotensin system in selected survivors of myocardial infarction with Novartis Pharmaceuticals on which Dr Pfeffer is a coinventor. His share of the licensing agreement is irrevocably transferred to charity. Dr Desai has received consulting fees from Novartis, Abbott, AstraZeneca, DalCor Pharma, Boston Scientific, and Relypsa, as well as research grants from Novartis and from AtCor Medical (to support the Vascular Stiffness Ancillary Study to the TOPCAT trial). Dr Lewis has received research grants from the National Heart, Lung, and Blood Institute, Novartis, and Sanofi Aventis. Dr S. Shah has received research grants from the American Heart Association, National Institutes of Health, Actelion, AstraZeneca, Corvia, and Novartis, and consulting fees from Actelion, Amgen, AstraZeneca, Bayer, Boehringer-Ingelheim, Cardiora, Eisai, Gilead, Ironwood, Merck, Novartis, Pfizer, Sanofi, Tenax, and United Therapeutics. Dr Sweitzer has received research grants from the National Institutes of Health. Dr Pitt reports receiving consulting fees from Amorcyte, AstraZeneca, Aurasense, Bayer, BG Medicine, Gambro, Johnson & Johnson, Mesoblast, Novartis, Pfizer, Relypsa, and Takeda; receiving research grant support from Forest Laboratories; and holding stock in Aurasense, Relypsa, BG Medicine, and Aurasense. Dr Pitt also reports a pending patent related to site-specific delivery of eplerenone to the myocardium. Dr Solomon has received consulting fees from Novartis and Bayer and research grants from the National Heart, Lung, and Blood Institute. The other authors report no conflicts.
Contributor Information
Senthil Selvaraj, Division of Cardiology, Department of Medicine, Hospital of the University of Pennsylvania, Philadelphia, PA.
Brian Claggett, Division of Cardiology, Department of Medicine, Brigham and Women’s Hospital, Boston, MA.
Sanjiv J. Shah, Division of Cardiology, Department of Medicine, Northwestern University Feinberg School of Medicine, Chicago, IL.
Inder Anand, VA Medical Center and University of Minnesota, Minneapolis, MN.
Jean L. Rouleau, Department of Medicine, Montreal Heart Institute, University of Montreal, Quebec, Canada.
Eileen O’Meara, Department of Medicine, Montreal Heart Institute, University of Montreal, Quebec, Canada.
Akshay S. Desai, Division of Cardiology, Department of Medicine, Brigham and Women’s Hospital, Boston, MA.
Eldrin F. Lewis, Division of Cardiology, Department of Medicine, Brigham and Women’s Hospital, Boston, MA.
Bertram Pitt, University of Michigan, Ann Arbor, MI.
Nancy K. Sweitzer, Sarver Heart Center, University of Arizona College of Medicine, Tucson.
James C. Fang, University of Utah, Salt Lake City, UT, USA.
Marc A. Pfeffer, Division of Cardiology, Department of Medicine, Brigham and Women’s Hospital, Boston, MA.
Scott D. Solomon, Division of Cardiology, Department of Medicine, Brigham and Women’s Hospital, Boston, MA.
REFERENCES
- 1.Jackson CE, Solomon SD, Gerstein HC, Zetterstrand S, Olofsson B, Michelson EL, Granger CB, Swedberg K, Pfeffer MA, Yusuf S, McMurray JJ, Investigators C, Committees. Albuminuria in chronic heart failure: prevalence and prognostic importance. Lancet 2009;374:543–50. [DOI] [PubMed] [Google Scholar]
- 2.Katz DH, Burns JA, Aguilar FG, Beussink L, Shah SJ. Albuminuria is independently associated with cardiac remodeling, abnormal right and left ventricular function, and worse outcomes in heart failure with preserved ejection fraction. JACC Heart Fail 2014;2:586–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miura M, Shiba N, Nochioka K, Takada T, Takahashi J, Kohno H, Shimokawa H, Investigators C-. Urinary albumin excretion in heart failure with preserved ejection fraction: an interim analysis of the CHART 2 study. Eur J Heart Fail 2012;14:367–76. [DOI] [PubMed] [Google Scholar]
- 4.Stevens PE, Levin A, Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group M. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med 2013;158:825–30. [DOI] [PubMed] [Google Scholar]
- 5.Standards of Medical Care in Diabetes-2017: Summary of Revisions. Diabetes Care 2017;40(Suppl 1):S4–S5. [DOI] [PubMed] [Google Scholar]
- 6.Weir MR. Microalbuminuria and cardiovascular disease. Clin J Am Soc Nephrol 2007;2:581–90. [DOI] [PubMed] [Google Scholar]
- 7.Paulus WJ, Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol 2013;62(4):263–71. [DOI] [PubMed] [Google Scholar]
- 8.Ghali JK. A new direction for albuminuria: an enigmatic multibiomarker. JACC Heart Fail 2014;2:597–9. [DOI] [PubMed] [Google Scholar]
- 9.Voors AA, Gori M, Liu LC, Claggett B, Zile MR, Pieske B, McMurray JJ, Packer M, Shi V, Lefkowitz MP, Solomon SD, Investigators P. Renal effects of the angiotensin receptor neprilysin inhibitor LCZ696 in patients with heart failure and preserved ejection fraction. Eur J Heart Fail 2015;17:510–7. [DOI] [PubMed] [Google Scholar]
- 10.Rachmani R, Slavachevsky I, Amit M, Levi Z, Kedar Y, Berla M, Ravid M. The effect of spironolactone, cilazapril and their combination on albuminuria in patients with hypertension and diabetic nephropathy is independent of blood pressure reduction: a randomized controlled study. Diabet Med 2004;21:471–5. [DOI] [PubMed] [Google Scholar]
- 11.Mehdi UF, Adams-Huet B, Raskin P, Vega GL, Toto RD. Addition of angiotensin receptor blockade or mineralocorticoid antagonism to maximal angiotensin-converting enzyme inhibition in diabetic nephropathy. J Am Soc Nephrol 2009;20:2641–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sato A, Hayashi K, Naruse M, Saruta T. Effectiveness of aldosterone blockade in patients with diabetic nephropathy. Hypertension 2003;41:64–8. [DOI] [PubMed] [Google Scholar]
- 13.Bakris GL, Agarwal R, Chan JC, Cooper ME, Gansevoort RT, Haller H, Remuzzi G, Rossing P, Schmieder RE, Nowack C, Kolkhof P, Joseph A, Pieper A, Kimmeskamp-Kirschbaum N, Ruilope LM, Mineralocorticoid Receptor Antagonist Tolerability Study-Diabetic Nephropathy Study G. Effect of Finerenone on Albuminuria in Patients With Diabetic Nephropathy: A Randomized Clinical Trial. JAMA 2015;314:884–94. [DOI] [PubMed] [Google Scholar]
- 14.Bomback AS, Kshirsagar AV, Amamoo MA, Klemmer PJ. Change in proteinuria after adding aldosterone blockers to ACE inhibitors or angiotensin receptor blockers in CKD: a systematic review. Am J Kidney Dis 2008;51:199–211. [DOI] [PubMed] [Google Scholar]
- 15.Bakris GL, Molitch M. Microalbuminuria as a risk predictor in diabetes: the continuing saga. Diabetes Care 2014;37:867–75. [DOI] [PubMed] [Google Scholar]
- 16.Desai AS, Lewis EF, Li R, Solomon SD, Assmann SF, Boineau R, Clausell N, Diaz R, Fleg JL, Gordeev I, McKinlay S, O’Meara E, Shaburishvili T, Pitt B, Pfeffer MA. Rationale and design of the treatment of preserved cardiac function heart failure with an aldosterone antagonist trial: a randomized, controlled study of spironolactone in patients with symptomatic heart failure and preserved ejection fraction. Am Heart J 2011;162:966–972 e10. [DOI] [PubMed] [Google Scholar]
- 17.Pfeffer MA, Pitt B, McKinlay SM. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med 2014;371:181–2. [DOI] [PubMed] [Google Scholar]
- 18.Pfeffer MA, Claggett B, Assmann SF, Boineau R, Anand IS, Clausell N, Desai AS, Diaz R, Fleg JL, Gordeev I, Heitner JF, Lewis EF, O’Meara E, Rouleau JL, Probstfield JL, Shaburishvili T, Shah SJ, Solomon SD, Sweitzer NK, McKinlay SM, Pitt B. Regional variation in patients and outcomes in the Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist (TOPCAT) trial. Circulation 2015;131:34–42. [DOI] [PubMed] [Google Scholar]
- 19.Eknoyan G, Hostetter T, Bakris GL, Hebert L, Levey AS, Parving HH, Steffes MW, Toto R. Proteinuria and other markers of chronic kidney disease: a position statement of the national kidney foundation (NKF) and the national institute of diabetes and digestive and kidney diseases (NIDDK). Am J Kidney Dis 2003;42:617–22. [DOI] [PubMed] [Google Scholar]
- 20.Solomon SD, Claggett B, Lewis EF, Desai A, Anand I, Sweitzer NK, O’Meara E, Shah SJ, McKinlay S, Fleg JL, Sopko G, Pitt B, Pfeffer MA, Investigators T. Influence of ejection fraction on outcomes and efficacy of spironolactone in patients with heart failure with preserved ejection fraction. Eur Heart J 2016;37:455–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Selvaraj S, Claggett B, Shah SJ, Anand I, Rouleau JL, Desai AS, Lewis EF, Pitt B, Sweitzer NK, Pfeffer MA, Solomon SD. Systolic blood pressure and cardiovascular outcomes in heart failure with preserved ejection fraction: an analysis of the TOPCAT trial. Eur J Heart Fail 2018;20:483–490. [DOI] [PubMed] [Google Scholar]
- 22.Jackson CE, MacDonald MR, Petrie MC, Solomon SD, Pitt B, Latini R, Maggioni AP, Smith BA, Prescott MF, Lewsey J, McMurray JJ, investigators ALOohFT. Associations of albuminuria in patients with chronic heart failure: findings in the ALiskiren Observation of heart Failure Treatment study. Eur J Heart Fail 2011;13:746–54. [DOI] [PubMed] [Google Scholar]
- 23.Capes SE, Gerstein HC, Negassa A, Yusuf S. Enalapril prevents clinical proteinuria in diabetic patients with low ejection fraction. Diabetes Care 2000;23:377–80. [DOI] [PubMed] [Google Scholar]
- 24.Pitt B, Kober L, Ponikowski P, Gheorghiade M, Filippatos G, Krum H, Nowack C, Kolkhof P, Kim SY, Zannad F. Safety and tolerability of the novel non-steroidal mineralocorticoid receptor antagonist BAY 94–8862 in patients with chronic heart failure and mild or moderate chronic kidney disease: a randomized, double-blind trial. Eur Heart J 2013;34:2453–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Denus S, O’Meara E, Desai AS, Claggett B, Lewis EF, Leclair G, Jutras M, Lavoie J, Solomon SD, Pitt B, Pfeffer MA, Rouleau JL. Spironolactone Metabolites in TOPCAT - New Insights into Regional Variation. N Engl J Med 2017;376:1690–1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, Clausell N, Desai AS, Diaz R, Fleg JL, Gordeev I, Harty B, Heitner JF, Kenwood CT, Lewis EF, O’Meara E, Probstfield JL, Shaburishvili T, Shah SJ, Solomon SD, Sweitzer NK, Yang S, McKinlay SM, Investigators T. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med 2014;370:1383–92. [DOI] [PubMed] [Google Scholar]
- 27.Lund LH, Oldgren J, James S. Registry-Based Pragmatic Trials in Heart Failure: Current Experience and Future Directions. Curr Heart Fail Rep 2017;14:59–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taqueti VR, Solomon SD, Shah AM, Desai AS, Groarke JD, Osborne MT, Hainer J, Bibbo CF, Dorbala S, Blankstein R, Di Carli MF. Coronary microvascular dysfunction and future risk of heart failure with preserved ejection fraction. Eur Heart J 2018;39:840–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dryer K, Gajjar M, Narang N, Lee M, Paul J, Shah AP, Nathan S, Butler J, Davidson CJ, Fearon WF, Shah SJ, Blair JEA. Coronary microvascular dysfunction in patients with heart failure with preserved ejection fraction. Am J Physiol Heart Circ Physiol 2018;314:H1033–H1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Akiyama E, Sugiyama S, Matsuzawa Y, Konishi M, Suzuki H, Nozaki T, Ohba K, Matsubara J, Maeda H, Horibata Y, Sakamoto K, Sugamura K, Yamamuro M, Sumida H, Kaikita K, Iwashita S, Matsui K, Kimura K, Umemura S, Ogawa H. Incremental prognostic significance of peripheral endothelial dysfunction in patients with heart failure with normal left ventricular ejection fraction. J Am Coll Cardiol 2012;60:1778–86. [DOI] [PubMed] [Google Scholar]
- 31.Gori M, Senni M, Gupta DK, Charytan DM, Kraigher-Krainer E, Pieske B, Claggett B, Shah AM, Santos AB, Zile MR, Voors AA, McMurray JJ, Packer M, Bransford T, Lefkowitz M, Solomon SD, Investigators P. Association between renal function and cardiovascular structure and function in heart failure with preserved ejection fraction. Eur Heart J 2014;35:3442–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Katz DH, Selvaraj S, Aguilar FG, Martinez EE, Beussink L, Kim KY, Peng J, Sha J, Irvin MR, Eckfeldt JH, Turner ST, Freedman BI, Arnett DK, Shah SJ. Association of low-grade albuminuria with adverse cardiac mechanics: findings from the hypertension genetic epidemiology network (HyperGEN) study. Circulation 2014;129:42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brouwers FP, de Boer RA, van der Harst P, Voors AA, Gansevoort RT, Bakker SJ, Hillege HL, van Veldhuisen DJ, van Gilst WH. Incidence and epidemiology of new onset heart failure with preserved vs. reduced ejection fraction in a community-based cohort: 11-year follow-up of PREVEND. Eur Heart J 2013;34:1424–31. [DOI] [PubMed] [Google Scholar]
- 34.Wegria R, Capeci NE, Blumenthal MR, Kornfeld P, Hays DR, Elias RA, Hilton JG. The pathogenesis of proteinuria in the acutely congested kidney. J Clin Invest 1955;34:737–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smilde TD, Damman K, van der Harst P, Navis G, Westenbrink BD, Voors AA, Boomsma F, van Veldhuisen DJ, Hillege HL. Differential associations between renal function and “modifiable” risk factors in patients with chronic heart failure. Clin Res Cardiol 2009;98:121–9. [DOI] [PubMed] [Google Scholar]
- 36.Wilcox CS. New insights into diuretic use in patients with chronic renal disease. J Am Soc Nephrol 2002;13:798–805. [DOI] [PubMed] [Google Scholar]
- 37.Nathan DM, Rosenbaum C, Protasowicki VD. Single-void urine samples can be used to estimate quantitative microalbuminuria. Diabetes Care 1987;10:414–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.