Abstract
Background:
The EMPA-REG OUTCOME trial showed that empagliflozin, a sodium-glucose co-transporter-2 inhibitor (SGLT2i), reduces the risk of hospitalization for heart failure (HHF) by 35%, on top of standard of care in patients with type 2 diabetes (T2D) and established CV disease (CVD). The EMPagliflozin compaRative effectIveness and SafEty (EMPRISE) study aims to assess empagliflozin’s effectiveness, safety, and healthcare utilization in routine care from 08/2014 through 09/2019. In this first interim analysis, we investigated the risk of HHF among T2D patients initiating empagliflozin vs. sitagliptin, a dipeptidyl peptidase-4 inhibitor (DPP-4i).
Methods:
Within two commercial and one federal (Medicare) claims data sources in the U.S., we identified a 1:1 propensity-score (PS) matched cohort of T2D patients ≥18 years initiating empagliflozin or sitagliptin from 08/2014 through 09/2016. The HHF outcome was defined as a HF discharge diagnosis in the primary position (HHF-specific); a broader definition was based on a HF discharge diagnosis in any position (HHF-broad). Hazard ratios (HR) and 95% confidence intervals (CI) were estimated controlling for over 140 baseline characteristics in each data source and pooled by fixed-effects meta-analysis.
Results:
After PS-matching, we identified 16,443 patient pairs who initiated empagliflozin or sitagliptin. Average age was approximately 59 years, almost 54% of the participants were males, and approximately 25% had records of existing cardiovascular disease. Compared to sitagliptin, the initiation of empagliflozin decreased the risk of HHF-specific by 50% (HR = 0.50; 95% CI = 0.28-0.91), and the risk of HHF-broad by 49% (HR: 0.51;95% CI: 0.39–0.68), over a mean follow-up of 5.3 months. Results were consistent in patients with and without baseline cardiovascular disease, and for both empagliflozin 10 mg or 25mg daily dose; analyses comparing empagliflozin vs. the DPP-4i class, and comparing SGLT2i vs. DPP-4i classes also produced consistent findings.
Conclusions:
The first interim analysis from EMPRISE showed that compared with sitagliptin, the initiation of empagliflozin was associated with a decreased risk of HHF among patients with T2D as treated in routine care, with and without a history of cardiovascular disease.
Clinical Trial Registration:
https://clinicaltrials.gov/ct2/show/NCT03363464 ( NCT03363464)
Keywords: Empagliflozin, sodium-glucose co-transporter-2 inhibitor, dipeptidyl peptidase-4 inhibitor, type 2 diabetes, heart failure, comparative effectiveness
Background
The cardiovascular outcome trial(1) EMPA-REG OUTCOME showed that empagliflozin, a sodium-glucose co-transporter-2 (SGLT2) inhibitor, reduces the relative risk of cardiovascular death by 38% (HR 0.62; 95% CI 0.49 – 0.77), all-cause mortality by 32% (HR 0.68; 95% CI 0.57 – 0.82) , and hospitalization for heart failure by 35% (HR 0.65; 95% CI 0.50 – 0.85) when added onto standard of care in patients with type 2 diabetes (T2D) and established cardiovascular disease. However, these beneficial effects are yet to be evaluated in routine clinical care, which includes patients across a broader spectrum of cardiovascular risk, including patients without clinical evidence of cardiovascular disease.
The EMPagliflozin compaRative effectIveness and SafEty (EMPRISE) study program aims to assess the comparative effectiveness, safety, and impact on healthcare utilization of empagliflozin, using real-world data from three databases in the U.S. EMPRISE is a sequentially built new-user active-comparator cohort study of 1:1 propensity-score-matched patients initiating empagliflozin or a comparator, which will collect accumulating data for a period of five years following the date of empagliflozin’s approval in the U.S., i.e., August 1, 2014 through September 30, 2019; it is comprised of four planned interim analyses and a final analysis, each performed based on twelve-month-data updates. EMPRISE is expected to include over 200,000 1:1 propensity-score matched patients by its completion.(2)
In this interim analysis from EMPRISE, based on data from August 2014 through September 2016, we evaluated the risk of HHF associated with the initiation of empagliflozin compared with the initiation of sitagliptin, the most frequently prescribed dipeptidyl peptidase 4 inhibitors (DPP-4i) in the U.S, which has demonstrated a neutral effect on the risk of HHF (HR 1.00; 95% CI 0.83–1.20).(3)
Methods
The authors declare that all supporting data are available within the article (and its online supplementary files).
Data source and study design
Within two commercial (Optum Clinformatics and IBM MarketScan) and one federal (Medicare fee-for-service) data sources in the U.S., we identified a 1:1 propensity-score (PS) matched cohort of T2D patients ≥18 years initiating empagliflozin or sitagliptin. Cohort entry date was the day of the first filled prescription of empagliflozin or sitagliptin, with no SGLT2i or DPP-4i use in the preceding year among patients with at least one year of continuous enrollment prior to cohort entry. The follow-up began on the day after cohort entry and continued in an “as-treated” approach until the first occurrence of treatment discontinuation or switch to a drug in the comparator class, the occurrence of an outcome, a nursing home admission, death, plan disenrollment, or September 30, 2016. In case of treatment interruption or discontinuation, we extended the exposure effect window until 30 days after the end of the last prescription’s supply.
In secondary analyses, we re-defined the comparator group as initiation of the overall DPP-4i class (sitagliptin, linagliptin, saxagliptin, or alogliptin) and the exposure as initiation of the overall SGLT2i class (canagliflozin, empagliflozin, or dapagliflozin).
Outcomes and patient characteristics
The HHF outcome was defined as a heart failure discharge diagnosis in the primary position (HHF-specific; positive predictive value [PPV] = 84–100%)(4); we also assessed a broader definition of HHF, defined as a heart failure discharge diagnosis in any position (HHF-broad; PPV = 79–96%).(4) Patient baseline characteristics were measured on the basis of enrollment information and claims during the 12 months prior to cohort entry, and included demographics, calendar time (in quarters and days), comorbidities, diabetes-specific complications, use of diabetes drugs, use of other medications, indicators of health care utilization as proxy for overall disease state, care intensity and surveillance, and laboratory test results, which were available for a subset of 45–50% of patients in Optum and 5–10% in MarketScan. Particular emphasis was placed on the identification of claims-measured indicators of diabetes severity, including number of glucose-lowering medications at index date and specific past or concurrent diabetes therapy, diabetic nephropathy, neuropathy, retinopathy, diabetic foot and lower-limb amputations, number of hemoglobin A1c (HbA1c) or glucose tests ordered (Supplemental Table 1). We assessed the potential for residual confounding by unmeasured factors not included in the claims-based propensity score model by evaluating balance in laboratory test results in the subset of the population with this information available. An equivalent study design on second-line oral antidiabetic medications had shown successful balance in unmeasured patient characteristics like duration of diabetes, body mass index, HbA1c, creatinine, or lipid levels.(5)
Statistical analysis
Within each data source, propensity scores (PS) were estimated using a multivariable logistic regression predicting the initiation of empagliflozin vs. sitagliptin, conditional upon over 140 pre-defined baseline characteristics (Supplemental Table 1).(6) Patients were 1:1 PS-matched using the nearest neighbor methodology with a maximum caliper of 0.01 of the PS.(7, 8) Post-matching covariate balance between treatments was assessed for each covariate by the calculation of standardized differences, i.e., the difference in means or proportions divided by the pooled standard deviation, with meaningful imbalances set at values greater than 0.1.(9, 10) Hazard ratios (HRs) and 95% confidence intervals (CI) were estimated in each data source and pooled across the data sources using a fixed-effects meta-analysis,7 since random effects pooling can be biased in the context of few databases.8 In order to address potential unmeasured confounding, we conducted the following sensitivity analyses – (1) we performed 1:1 high-dimensional propensity score (hdPS) matching, which enriched the original PS with 100 additional empirically identified covariates;(11) and (2) we assessed the association with a control outcome with an expected null finding, i.e., the occurrence of flu vaccination during follow-up. We also conducted subgroup analyses stratified by (1) presence of cardiovascular disease at baseline, defined as a diagnosis or procedure for myocardial infarction, unstable angina, coronary atherosclerosis or other forms of chronic ischemic heart disease, coronary procedure, congestive heart failure, ischemic or hemorrhagic stroke, transient ischemic attack, peripheral arterial disease or surgery, or lower extremity amputation, recorded in the 12 months before cohort entry; (2) presence of heart failure at baseline, defined as a diagnosis of heart failure or use of loop diuretics during the 12 months before cohort entry; (3) gender; and (4) empagliflozin dose initiated (10 or 25 mg/day). Within each subgroup, PS was re-estimated and PS-matching and analyses were re-performed. Analyses re-defining the comparator group as initiation of the overall DPP-4i class (sitagliptin, linagliptin, saxagliptin, or alogliptin) and the exposure as initiation of the overall SGLT2i class (canagliflozin, empagliflozin, or dapagliflozin), were also conducted.
All analyses were performed using Aetion platform version 3.2 with R version 3.2, which has previously been scientifically validated by accurately repeating a range of previously-published studies(12) and by replicating clinical trial findings.(13),,(14)All individual data were de-identified, the study was approved by the Brigham and Women’s Hospital institutional review board, signed data license agreements were in place for all data sources. The study was registered at EnCEPP (EUPAS20677) and on ClinicalTrials.gov ( NCT03363464).
Results
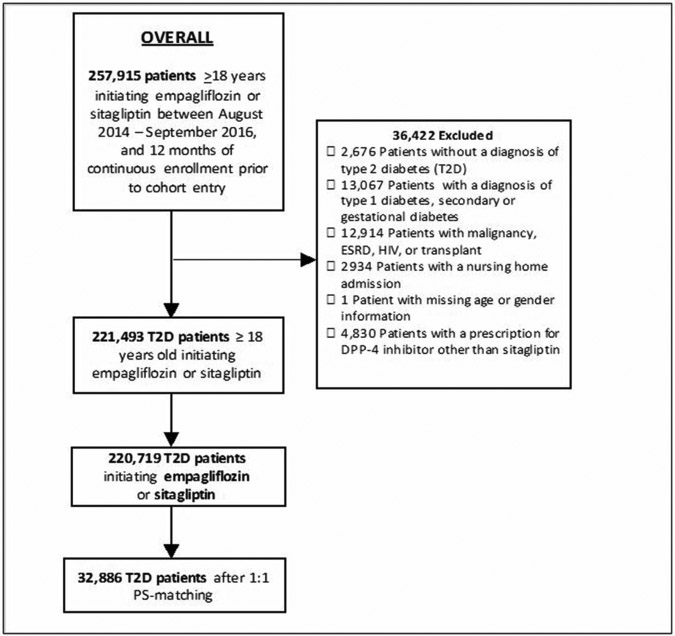
We identified a total of 18,880 empagliflozin and 201,839 sitagliptin initiators. Empagliflozin initiators were younger, more frequently male, less frail as measured by the Claims-Based Frailty Index (CFI),(15) and had a lower general burden of comorbidities as measured by the Combined Comorbidity Score(16) compared to sitagliptin. Conversely, they had higher prevalence of obesity, higher baseline use of insulin or glucagon-like peptide (GLP)-1 receptor agonists, and higher number of antidiabetic medications at cohort entry (Table 1). 87% of empagliflozin initiators were successfully matched to sitagliptin initiators resulting in 16,443 patient pairs (Figure 1, Table 1). PS-matched patients showed similar distribution of characteristics at baseline. In the patient subset with laboratory test results those values were equally balanced including HbA1c and creatinine, despite not having been included in the PS model (Table 1, Supplemental Table 1). The average age was 59 years, and almost 54% of the participants were males. Individuals with history of cardiovascular disease, including recent acute cardiovascular events, represented about 25% of study participants and approximately 5% of the population had history of heart failure. The additional comparisons of empagliflozin vs. the overall DPP-4i class (N=17,551 PS-matched pairs) and the overall SGLT2i class vs. the DPP-4i class (N=112,264 PS-matched pairs) showed comparable characteristics and balance achievement after PS-matching (Supplemental Figure 1, Supplemental Table 2).
Table 1.
Selected baseline characteristics of patients initiating empagliflozin vs. sitagliptin before and after 1:1 propensity-score matching
| UNMATCHED | PROPENSITY-SCORE MATCHED | |||||
|---|---|---|---|---|---|---|
| Baseline characteristics | Sitagliptin (N = 201,839) |
Empagliflozin (N = 18,880) |
St. Diff. | Sitagliptin (N = 16,443) |
Empagliflozin (N = 16,443) |
St. Diff. |
| Demographics | ||||||
| Age; mean (sd) | 67.54 (9.46) | 58.41 (8.93) | 0.99 | 59.11 (9.11) | 59.09 (8.94) | 0.00 |
| Male; n (%) | 96,641 (47.9%) | 10,168 (53.9%) | −0.12 | 8,777 (53.4%) | 8,816 (53.6%) | 0.00 |
| Burden of comorbidities | ||||||
| Combined comorbidity score; mean (sd) | 2.89 (2.22) | 2.20 (1.59) | 0.36 | 2.22 (1.66) | 2.19 (1.63) | 0.02 |
| Frailty score, mean (sd) | 0.15 (0.05) | 0.13 (0.04) | 0.44 | 0.14 (0.04) | 0.14 (0.04) | 0.00 |
| Diabetes-related complications | ||||||
| Diabetic nephropathy; n (%) | 20,082 (9.9%) | 1,490 (7.9%) | 0.07 | 1,257 (7.6%) | 1,247 (7.6%) | 0.00 |
| Diabetic retinopathy; n (%) | 14,153 (7.0%) | 1,122 (5.9%) | 0.04 | 966 (5.9%) | 957 (5.8%) | 0.00 |
| Diabetic neuropathy; n (%) | 36,387 (18.0%) | 3,216 (17.0%) | 0.03 | 2,698 (16.4%) | 2,694 (16.4%) | 0.00 |
| Diabetes with peripheral circulatory disorders; n (%) | 12,811 (6.3%) | 762 (4.0%) | 0.10 | 701 (4.3%) | 674 (4.1%) | 0.01 |
| Diabetic Foot; n (%) | 4,986 (2.5%) | 357 (1.9%) | 0.04 | 329 (2.0%) | 298 (1.8%) | 0.01 |
| Hypoglycemia; n (%) | 14,631 (7.2%) | 1,160 (6.1%) | 0.04 | 1,060 (6.4%) | 1,057 (6.4%) | 0.00 |
| Features of diabetes medication initiation and baseline diabetes therapy | ||||||
| No. antidiabetic drugs at cohort entry; mean (sd) | 2.16 (0.77) | 2.29 (0.95) | −0.15 | 2.21 (0.85) | 2.22 (0.90) | −0.01 |
| Naive new user*; n (%) | 27,139 (13.4%) | 1,380 (7.3%) | 0.20 | 1,294 (7.9%) | 1,363 (8.3%) | −0.01 |
| Monotherapy; n (%) | 20,203 (10.0%) | 1,130 (6.0%) | 0.15 | 1,061 (6.5%) | 1,127 (6.9%) | −0.02 |
| Concomitant initiation or current use of metformin; n (%) | 131,791 (65.3%) | 11,305 (59.9%) | 0.11 | 10,010 (60.9%) | 10,092 (61.4%) | −0.01 |
| Concomitant initiation or current use of sulfonylureas; n (%) | 67,409 (33.4%) | 4,849 (25.7%) | 0.17 | 4,385 (26.7%) | 4,378 (26.6%) | 0.00 |
| Concomitant initiation or current use of insulin; n (%) | 19,559 (9.7%) | 3,922 (20.8%) | −0.31 | 3,010 (18.3%) | 2,954 (18.0%) | 0.01 |
| Other comorbidities at baseline | ||||||
| History of CV disease; n (%) | 74,342 (36.8%) | 4,608 (24.4%) | 0.27 | 4,115 (25.0%) | 4,094 (24.9%) | 0.00 |
| Ischemic heart disease; n (%) | 51,715 (25.6%) | 3,382 (17.9%) | 0.19 | 3,013 (18.3%) | 2,980 (18.1%) | 0.01 |
| Previous coronary revascularization; n (%) | 15,386 (7.6%) | 864 (4.6%) | 0.13 | 754 (4.6%) | 772 (4.7%) | 0.00 |
| Ischemic or hemorrhagic stroke; n (%) | 19,753 (9.8%) | 997 (5.3%) | 0.17 | 948 (5.8%) | 913 (5.6%) | 0.01 |
| Heart failure; n (%) | 21,514 (10.7%) | 920 (4.9%) | 0.22 | 884 (5.4%) | 834 (5.1%) | 0.01 |
| Peripheral arterial disease or surgery; n (%) | 20,610 (10.2%) | 959 (5.1%) | 0.19 | 863 (5.2%) | 872 (5.3%) | 0.00 |
| Hypertension; n (%) | 166,283 (82.4%) | 14,422 (76.4%) | 0.15 | 12,565 (76.4%) | 12,513 (76.1%) | 0.01 |
| Chronic kidney disease; n (%) | 31,924 (15.8%) | 1,268 (6.7%) | 0.29 | 1,203 (7.3%) | 1,164 (7.1%) | 0.01 |
| Laboratory test results† | ||||||
| HbA1c (%); mean (sd) | 8.33 (1.78) | 8.50 (1.77) | −0.10 | 8.60 (1.86) | 8.46 (1.77) | 0.08 |
| Patients with HbA1c results available; n (%) | 17,214 (19.3%) | 2,649 (18.4%) | 0.02 | 2,395 (19.7%) | 2,091 (17.2%) | 0.06 |
| Creatinine (mg/dl); mean (sd) | 0.97 (0.33) | 0.88 (0.22) | 0.32 | 0.90 (0.26) | 0.89 (0.22) | 0.04 |
| Patients with creatinine results available; n (%) | 17,436 (19.6%) | 2,812 (19.5%) | 0.00 | 2,441 (20.1%) | 2,197 (18.1%) | 0.05 |
| Total cholesterol (mg/dl); mean (sd) | 176.96 (45.82) | 176.69 (45.22) | 0.01 | 179.36 (47.83) | 177.42 (45.80) | 0.04 |
| Patients with total cholesterol results available; n (%) | 15,478 (17.4%) | 2,556 (17.8%) | −0.01 | 2,195 (18.1%) | 2,012 (16.5%) | 0.04 |
| LDL level (mg/dl); mean (sd) | 89.89 (47.55) | 87.41 (39.68) | 0.06 | 91.14 (40.49) | 88.07 (39.53) | 0.08 |
| Patients with LDL results available; n (%) | 16,147 (18.1%) | 2,543 (17.7%) | 0.01 | 2,243 (18.4%) | 2,003 (16.5%) | 0.05 |
| HDL level (mg/dl); mean (sd) | 46.32 (44.30) | 44.07 (13.07) | 0.07 | 43.98 (12.61) | 44.24 (13.16) | −0.02 |
| Patients with HDL results available; n (%) | 15,345 (17.2%) | 2,516 (17.5%) | −0.01 | 2,179 (17.9%) | 1,978 (16.3%) | 0.04 |
DPP-4: dipeptidyl peptidase-4; St. Diff: standardized differences, i.e., the difference in means or proportions divided by the pooled standard deviation;9 sd: standard deviation; Q: quarter; GLP-1 RA: glucagon-like peptide-1 receptor agonists; COPD: Chronic obstructive pulmonary disease; BUN: blood urea nitrogen; HbA1c: hemoglobin A1c; LDL: low-density lipoprotein; HDL: high-density lipoproteins
Defined as patients without any use of glucose-lowering medications during the 12 months prior to cohort entry
Only available in Optum Clinformatics and Truven MarketScan
Figure 1. Flowchart of overall study population of empagliflozin vs. sitagliptin initiators.
ESRD: end stage renal disease; HIV: human immunodeficiency virus; DPP-4: dipeptidyl peptidase-4; PS: propensity score
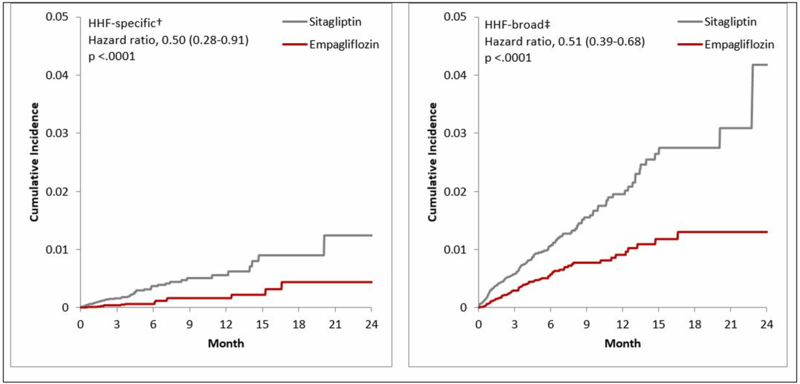
The incidence rates/1,000 person-years in empagliflozin vs. sitagliptin PS-matched initiators were 2.1 vs. 6.7 for HHF-specific and 10.5 vs. 22.2 for HHF-broad outcomes. Compared to sitagliptin, the initiation of empagliflozin decreased the risk of HHF-specific by 50% (HR=0.50; 95% CI 0.28–0.91), and the risk of HHF-broad by 49% (HR=0.51; 95% CI 0.39–0.68), over a mean follow-up of 5.3 months (Table 2). Database-specific estimates suggested concordant direction of the effect (Supplemental Table 3). Cumulative incidence plots were consistent with these findings and tended to separate within six months after treatment initiation (Figure 2). Further adjustment by hdPS-matching produced consistent results (HR=0.54; 95% CI 0.29–0.98 for HHF-specific; HR=0.54; 95% CI 0.41–0.71 for HHF-broad), as well as stratified analyses by duration of follow-up (Supplemental Tables 4-5). There was no association between empagliflozin and a control outcome with an expected null finding, i.e., occurrence of flu vaccination during follow-up (HR=0.96; 95% CI 0.90–1.02) (Supplemental Table 6). Subgroup analyses by presence of baseline cardiovascular disease, history of heart failure, gender, and empagliflozin daily dose initiated, produced consistent results (Table 2), as did analyses comparing empagliflozin vs. the overall class of DPP-4i and comparing the overall SGLT2i vs. the DPP-4i class (Table 3, Supplemental Figure 2).
Table 2.
Incidence rates and hazard ratios for hospitalization for heart failure in 1:1 PS-matched initiators of empagliflozin vs. sitagliptin.
| Empagliflozin | Sitagliptin | ||
|---|---|---|---|
| Patient population | N events (IR/1000 PY) |
N events (IR/1000 PY) |
HR (95% CI) |
| All patients | 16,443 | 16,443 | |
| HHF-Specific* | 16 (2.1) | 48 (6.7) | 0.50 (0.28-0.91) |
| HHF-Broad† | 78 (10.5) | 158 (22.2) | 0.51 (0.39-0.68) |
| Patients with CV history‡ | 4,034 | 4,034 | |
| HHF-Specific* | 12 (7.0) | 30 (16.9) | 0.55 (0.27-1.10) |
| HHF-Broad† | 60 (35.1) | 106 (60.7) | 0.60 (0.44-0.83) |
| Patients without CV history‡ | 12,342 | 12,342 | |
| HHF-Specific* | <11§ (0.5) | 14 (2.6) | 0.41 (0.10-1.68) |
| HHF-Broad† | 16 (2.8) | 40 (7.4) | 0.40 (0.22-0.73) |
| Patients with HF history∥ | 1,934 | 1,934 | |
| HHF-Specific* | 12 (14.5) | 25 (29.4) | 0.54 (0.27-1.09) |
| HHF-Broad† | 51 (62.7) | 87 (104.7) | 0.61 (0.43-0.86) |
| Patients without HF history∥ | 14,405 | 14,405 | |
| HHF-Specific* | <11§ (0.6) | 11 (1.7) | 0.60 (0.18-2.07) |
| HHF-Broad† | 25 (3.8) | 50 (7.9) | 0.52 (0.32-0.85) |
| Gender-Male | 8,690 | 8,690 | |
| HHF-Specific* | <11§ (2.5) | 26 (6.7) | 0.48 (0.23-1.02) |
| HHF-Broad† | 48 (11.8) | 89 (23.0) | 0.56 (0.40-0.80) |
| Gender-Female | 7,637 | 7,637 | |
| HHF-Specific* | <11§ (1.8) | 17 (5.2) | 0.67 (0.25-1.83) |
| HHF-Broad† | 31 (9.4) | 58 (17.7) | 0.57 (0.37-0.89) |
| Empagliflozin 10mg | 10,204 | 10,204 | |
| HHF-Specific* | 11 (2.6) | 28 (6.2) | 0.66 (0.32-1.39) |
| HHF-Broad† | 45 (10.8) | 99 (22.0) | 0.53 (0.37-0.75) |
| Empagliflozin 25mg | 7,396 | 7,396 | |
| HHF-Specific* | <11§ (1.8) | 23 (7.2) | 0.48 (0.18-1.26) |
| HHF-Broad† | 34 (10.3) | 73 (22.8) | 0.49 (0.32-0.74) |
HHF: hospitalization for heart failure; PS: propensity-score; IR: incidence rate; PY: person-years; HR: hazard ratio; CI: confidence intervals; CV: cardiovascular.
Discharge diagnosis of HF in the primary position
Discharge diagnosis of HF in any position
Defined as history of myocardial infarction, unstable angina, coronary atherosclerosis and other forms of chronic ischemic heart disease, coronary procedure, heart failure, ischemic or hemorrhagic stroke, transient ischemic attack, peripheral arterial disease or surgery, lower extremity amputation
In accordance with the data use agreement, we do not report information for frequency cells with less than 11 cases. These are noted as <11
Defined as history of heart failure or use of loop diuretics
Figure 2. Cumulative incidence of hospitalization for heart failure comparing empagliflozin vs. sitagliptin initiators *.
HHF: hospitalization for heart failure; HHF-broad: broad definition of HHF; HHF-specific: narrow definition of HHF.
* Analyses were 1:1 propensity score- matched among new users of the study agents.
†Discharge diagnosis of HF in the primary position
‡Discharge diagnosis of HF in any position
Table 3.
Incidence rates and hazard ratios for hospitalization for heart failure in 1:1 PS-matched initiators of empagliflozin vs. DPP-4i and SGLT2i vs. DPP-4i.
| Empagliflozin | DPP-4i | SGLT2i | DPP-4i | |||
|---|---|---|---|---|---|---|
| Patient population | N events (IR/1000 PY) |
N events (IR/1000 PY) |
HR (95% CI) |
N events (IR/1000 PY) |
N events (IR/1000 PY) |
HR (95% CI) |
| All patients | 17,551 | 17,551 | 112,264 | 112,264 | ||
| HHF-Specific* | 16 (2.0) | 42 (5.6) | 0.49 (0.27-0.89) | 175 (2.7) | 414 (6.9) | 0.42 (0.35-0.50) |
| HHF-Broad† | 82 (10.3) | 146 (19.6) | 0.56 (0.43-0.73) | 1,055 (16.2) | 1,486 (24.9) | 0.70 (0.65-0.75) |
| Patients with CV history‡ | 4,245 | 4,245 | 29,941 | 29,941 | ||
| HHF-Specific* | 13 (7.1) | 42 (23.5) | 0.34 (0.18-0.63) | 149 (8.9) | 319 (19.6) | 0.48 (0.39-0.58) |
| HHF-Broad† | 64 (35.4) | 115 (65.0) | 0.56 (0.41-0.76) | 841 (51.2) | 1,117 (69.6) | 0.75 (0.68-0.82) |
| Patients without CV history‡ | 13,238 | 13,238 | 82,089 | 82,089 | ||
| HHF-Specific* | <11§ (0.5) | <11§ (1.7) | 0.49 (0.12-2.03) | 36 (0.7) | 90 (2.1) | 0.37 (0.25-0.54) |
| HHF-Broad† | 17 (2.8) | 36 (6.3) | 0.46 (0.26-0.83) | 217 (4.5) | 358 (8.3) | 0.57 (0.48-0.68) |
| Patients with HF history∥ | 2,051 | 2,051 | 15,105 | 15,105 | ||
| HHF-Specific* | 13 (14.7) | 22 (24.7) | 0.78 (0.37-1.63) | 136 (16.4) | 304 (36.3) | 0.46 (0.38-0.57) |
| HHF-Broad† | 54 (62.1) | 96 (110.2) | 0.56 (0.41-0.78) | 755 (93.1) | 1,012 (124.5) | 0.77 (0.70-0.84) |
| Patients without HF history∥ | 15,421 | 15,421 | 96,734 | 96,734 | ||
| HHF-Specific* | <11§ (0.6) | 13 (2.0) | 0.35 (0.11-1.12) | 46 (0.8) | 96 (1.9) | 0.46 (0.32-0.66) |
| HHF-Broad† | 27 (3.8) | 41 (6.2) | 0.65 (0.40-1.06) | 315 (5.6) | 472 (9.2) | 0.64 (0.55-0.73) |
| Gender-Male | 9,347 | 9,347 | 59,788 | 59,788 | ||
| HHF-Specific* | <11* (2.3) | 26 (6.3) | 0.43 (0.20-0.92) | 100 (2.7) | 207 (6.4) | 0.47 (0.36-0.60) |
| HHF-Broad† | 49 (11.1) | 91 (22.0) | 0.53 (0.37-0.74) | 571 (15.7) | 789 (24.4) | 0.69 (0.62-0.77) |
| Gender-Female | 8,131 | 8,131 | 52,023 | 52,023 | ||
| HHF-Specific* | <11§ (1.7) | 14 (4.1) | 0.52 (0.20-1.39) | 80 (2.8) | 195 (7.2) | 0.43 (0.33-0.55) |
| HHF-Broad† | 33 (9.4) | 56 (16.6) | 0.58 (0.37-0.91) | 480 (16.9) | 744 (27.6) | 0.67 (0.60-0.75) |
| Empagliflozin 10mg | 10,620 | 10,620 | Not applicable | |||
| HHF-Specific* | 11 (2.5) | 19 (4.1) | 0.83 (0.38-1.80) | |||
| HHF-Broad† | 46 (10.5) | 95 (20.6) | 0.55 (0.39-0.78) | |||
| Empagliflozin 25mg | 7,744 | 7,744 | ||||
| HHF-Specific* | <11§ (1.7) | 19 (5.8) | 0.40 (0.16-1.02) | |||
| HHF-Broad† | 35 (10.1) | 56 (17.3) | 0.62 (0.41-0.95) | |||
HHF: hospitalization for heart failure; PS: propensity-score; DPP-4i: dipeptidyl peptidase-4 inhibitors (alogliptin=3.1%,linagliptin=19.1%, sitagliptin=66.5%, saxagliptin=11.3%); SGLT2i: sodium-glucose co-transporter-2 inhibitors (canagliflozin=68.6%, dapagliflozin=18.6%, empagliflozin=12.8%); IR: incidence rate; PY: person-years; HR: hazard ratio; CI: confidence intervals; CV: cardiovascular.
Discharge diagnosis of HF in the primary position
Discharge diagnosis of HF in any position
Defined as history of myocardial infarction, unstable angina, coronary atherosclerosis and other forms of chronic ischemic heart disease, coronary procedure, heart failure, ischemic or hemorrhagic stroke, transient ischemic attack, peripheral arterial disease or surgery, lower extremity amputation
In accordance with the data use agreement, we do not report information for frequency cells with less than 11 cases. These are noted as <11
Defined as history of heart failure or use of loop diuretics
Discussion
A first assessment from EMPRISE showed that compared with sitagliptin, the initiation of empagliflozin was associated with a decreased risk of HHF in routine care comparable in timing and magnitude to the EMPA-REG OUTCOME trial results.(1) Results remained consistent among patients with and without history of cardiovascular disease at baseline, although the number of events was still small in this interim analysis.
These findings complement the EMPA-REG OUTCOME trial results and support the notion that empagliflozin prevents HHF in routine care patients with a possible benefit across the spectrum of T2D people with and without history of cardiovascular disease. It has been proposed that one of the main mechanisms that may explain the cardioprotective benefits of empagliflozin and other SGLT2 inhibitors(17, 18) is via improvement in ventricular loading conditions through a reduction in preload (secondary to natriuresis and osmotic diuresis) and afterload (through a reduction in blood pressure and improvement in vascular function). Other postulated mechanisms include the improvement in cardiac metabolism and bioenergetics leading to enhanced cardiac efficiency and cardiac output; the inhibition of the myocardial Na+/H+ exchange which would restore whole-body sodium homeostasis and ultimately reduce cardiac failure; the reduction of necrosis and cardiac fibrosis, a common pathway through which heart failure develops; and an alteration in adipokines, cytokine production and epicardial adipose tissue mass, a common mechanism through which cardiovascular disease and insulin resistance develops.(19)
The EMPRISE study was designed to enhance clinical equipoise across treatment groups and minimize chances of confounding and time-related biases.(20, 21),(22) Specifically, (1) EMPRISE did not implement a hierarchical exposure definition allowing patients who started sitagliptin and then switched to empagliflozin to be included as empagliflozin initiators resulting in possible immortal time bias,(20, 21) but instead it included new users of either empagliflozin or sitagliptin, without any use of either SGLT2 inhibitors or DPP-4 inhibitors during the year prior to cohort entry;(23) (2) EMPRISE did not compare empagliflozin to diabetes agents used at the extremes of the treatment pathway for T2D, e.g., metformin or insulin, but it used comparators (i.e., sitagliptin or overall DPP-4 inhibitors) that represented comparable therapeutic alternatives for patients with T2D at the time,(24) thus enhancing clinical equipoise for diabetes severity and duration between exposure groups and reducing chances of time-lag bias;16 and (3) EMPRISE implemented an extensive propensity-score adjustment on many proxies of diabetes severity and duration, including baseline use of insulin and other specific diabetes agents, diabetes-related complications, and healthcare utilization, which have demonstrated success in confounding control in studies of patients with T2D(5) and which can also reduce time-lag bias.16 Furthermore, the inclusion of patients as treated in routine care without restrictions enabled assessment of the effects of empagliflozin across T2D patients with and without history of cardiovascular disease, and head-to-head comparisons of specific alternative diabetes treatment options allowed answering the clinically relevant question of which medication to choose for optimal diabetes care. Finally, observed absolute rates of HHF among EMPRISE patients were comparable to those previously reported among real-world T2D patients as captured in healthcare utilization data sources.(25),(26)
Residual confounding by some unmeasured characteristics cannot be entirely ruled out, although it is unlikely to be consequential. A hdPS-matched analysis, which enriched the original PS with 100 additional empirically identified covariates, produced results consistent with the main analysis, and we were able to reproduce a null finding in an analysis evaluating the association between empagliflozin and a control neutral outcome. In addition, selected laboratory test results, including HbA1c, were balanced after propensity-score adjustment, despite not having been included in the propensity-score model suggesting that we were able to successfully balance key unmeasured factors. Even though heart failure outcomes were defined using previously-validated claims-based algorithms with high positive predictive value,(4) some extent of outcome misclassification remains a possibility. At this stage of EMPRISE, the short duration of follow-up, mainly driven by the availability for analysis of only 2 years of empagliflozin use, limits the assessment of the long-term effects of empagliflozin. However, the decreased risk of HHF observed in RCTs appeared equally early,(1, 17, 18) thus, the short follow-up observed in the current study is not expected to affect the assessment of HHF. The subgroup of patients without cardiovascular disease at baseline is of specific interest although the number of events is still small. We excluded all patients from this subgroup analysis who had a cardiovascular diagnosis or procedure coded during an encounter with the professional healthcare system in the 12 months before cohort entry. We cannot fully rule out that some patients have undiagnosed or low severity cardiovascular disease, that was not recorded. As more data from EMPRISE become available over the study period, analyses will be conducted to test the robustness of such a definition.
In conclusion, this first interim analysis of the EMPRISE study showed that compared with sitagliptin, the initiation of empagliflozin was associated with a decreased risk of HHF among patients with T2D as treated in routine care, with and without a history of cardiovascular disease. Future analyses will include increasing numbers of patients to study additional outcomes and more patient subgroups.
Supplementary Material
Clinical perspective.
What is new?
A first assessment from The EMPagliflozin compaRative effectIveness and SafEty (EMPRISE) study, which aims to assess the comparative effectiveness, safety, and impact on healthcare utilization of empagliflozin using real-world data, showed that compared with sitagliptin, the initiation of empagliflozin was associated with a decreased risk of hospitalization for heart failure (HHF) in routine care comparable in timing and magnitude to the EMPA-REG OUTCOME trial results.
The decrease in risk of HHF remained consistent among patients with and without history of cardiovascular disease at baseline, although the number of events was still small in this interim analysis.
What are the clinical implications?
These findings complement the EMPA-REG OUTCOME trial results and consolidate the notion that empagliflozin prevents HHF in routine care patients with an observed benefit across the spectrum of T2D people with and without history of cardiovascular disease.
Clinicians need to weigh in the cardiovascular benefits of empagliflozin when prescribing glucose-lowering therapies in routine patients with type 2 diabetes.
Acknowledgments
Funding Sources
This study was supported by a research grant to the Brigham and Women’s Hospital from Boehringer-Ingelheim. The study was conducted by the authors independent of the sponsor. The authors retained the right of publication and determined the final wording of the manuscript.
Conflict of Interest Disclosures
Elisabetta Patorno: Supported by a career development grant K08AG055670 from the National Institute on Aging. Elisabetta Patorno is investigator of investigator-initiated grants to the Brigham and Women’s Hospital from GSK, not directly related to the topic of the submitted work.
Ajinkya Pawar: None
Jessica M Franklin: None
Mehdi Najafzadeh: None
Anouk Déruaz-Luyet: Employment (Boehringer-Ingelheim)
Kimberly G. Brodovicz: Employment (Boehringer-Ingelheim)
Steven Sambevski: Employment (Boehringer-Ingelheim)
Lily G. Bessette: None
Adrian J. Santiago Ortiz: None
Martin Kulldorff: Supported by the National Institute of General Medical Sciences, grant RO1GM108999.
Sebastian Schneeweiss: Principal investigator of investigator-initiated grants to the Brigham and Women’s Hospital from Bayer and Vertex unrelated to the topic of this study. He is a consultant to WHISCON and to Aetion, a software manufacturer of which he owns equity. His interests were declared, reviewed, and approved by the Brigham and Women’s Hospital and Partners HealthCare System in accordance with their institutional compliance policies.
References
- 1.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE; EMPA-REG OUTCOME Investigators. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med. 2015;373:2117–2128. [DOI] [PubMed] [Google Scholar]
- 2.Patorno EPA, Franklin JM, Déruaz-Luyet A, Brodovicz KG, Bartels DB, Kulldorff M, Schneeweiss S. Baseline information from a post-marketing monitoring program on empagliflozin: Implications for study validity and exposure accrual. Pharmacoepidemiol Drug Saf. 2018;27(Supplement 2):52–53.29152808 [Google Scholar]
- 3.Green JB, Bethel MA, Armstrong PW, Buse JB, Engel SS, Garg J, Josse R, Kaufman KD, Koglin J, Korn S, Lachin JM, McGuire DK, Pencina MJ, Standl E, Stein PP, Suryawanshi S, Van de Werf F, Peterson ED, Holman RR; TECOS Study Group. Effect of Sitagliptin on Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2015;373:232–242. [DOI] [PubMed] [Google Scholar]
- 4.Saczynski JS, Andrade SE, Harrold LR, Tjia J, Cutrona SL, Dodd KS, Goldberg RJ, Gurwitz JH. A systematic review of validated methods for identifying heart failure using administrative data. Pharmacoepidemiol Drug Saf. 2012;21 Suppl 1:129–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patorno E, Gopalakrishnan C, Franklin JM, Brodovicz KG, Masso-Gonzalez E, Bartels DB, Liu J, Schneeweiss SI. Claims-based studies of oral glucose-lowering medications can achieve balance in critical clinical variables only observed in electronic health records. Diabetes Obes Metab. 2018;20:974–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rubin DB. Estimating causal effects from large data sets using propensity scores. Ann Intern Med. 1997;127:757–763. [DOI] [PubMed] [Google Scholar]
- 7.Ripollone JE, Huybrechts KF, Rothman KJ, Ferguson RE, Franklin JM. Implications of the Propensity Score Matching Paradox in Pharmacoepidemiology. Am J Epidemiol. 2018;187:1951–1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rassen JA, Shelat AA, Myers J, Glynn RJ, Rothman KJ, Schneeweiss S. One-to-many propensity score matching in cohort studies. Pharmacoepidemiol Drug Saf. 2012;21 Suppl 2:69–80. [DOI] [PubMed] [Google Scholar]
- 9.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28:3083–3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Franklin JM, Rassen JA, Ackermann D, Bartels DB, Schneeweiss S. Metrics for covariate balance in cohort studies of causal effects. Stat Med. 2014;33:1685–1699. [DOI] [PubMed] [Google Scholar]
- 11.Schneeweiss S, Rassen JA, Glynn RJ, Avorn J, Mogun H, Brookhart MA. High-dimensional propensity score adjustment in studies of treatment effects using health care claims data. Epidemiology. 2009;20:512–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang SV, Verpillat P, Rassen JA, Patrick A, Garry EM, Bartels DB. Transparency and Reproducibility of Observational Cohort Studies Using Large Healthcare Databases. Clin Pharmacol Ther. 2016;99:325–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fralick M, Kesselheim AS, Avorn J, Schneeweiss S. Use of Health Care Databases to Support Supplemental Indications of Approved Medications. JAMA Intern Med. 2018;178:55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim SC, Solomon DH, Rogers JR, Gale S, Klearman M, Sarsour K, Schneeweiss S. Cardiovascular Safety of Tocilizumab Versus Tumor Necrosis Factor Inhibitors in Patients With Rheumatoid Arthritis: A Multi-Database Cohort Study. Arthritis Rheumatol. 2017;69:1154–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim DH, Glynn RJ, Avorn J, Lipsitz LA, Rockwood K, Pawar A, Schneeweiss S. Validation of a Claims-Based Frailty Index Against Physical Performance and Adverse Health Outcomes in the Health and Retirement Study. J Gerontol A Biol Sci Med Sci. 2018. doi: 10.1093/gerona/gly197. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gagne JJ, Glynn RJ, Avorn J, Levin R, Schneeweiss S. A combined comorbidity score predicted mortality in elderly patients better than existing scores. J Clin Epidemiol. 2011;64:749–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR; CANVAS Program Collaborative Group. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N Engl J Med. 2017;377:644–657. [DOI] [PubMed] [Google Scholar]
- 18.Wiviott SD RI, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Silverman MG, Zelniker TA, Kuder JF, Murphy SA, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Ruff CT, Gause-Nilsson IAM, Fredriksson M, Johansson PA, Langkilde AM, Sabatine MS; DECLARE–TIMI 58 Investigators. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2019;380:347–357. [DOI] [PubMed] [Google Scholar]
- 19.Verma S, McMurray JJV. SGLT2 inhibitors and mechanisms of cardiovascular benefit: a state-of-the-art review. Diabetologia. 2018;61:2108–2117. [DOI] [PubMed] [Google Scholar]
- 20.Suissa S Lower Risk of Death With SGLT2 Inhibitors in Observational Studies: Real or Bias? Diabetes Care. 2018;41:6–10. [DOI] [PubMed] [Google Scholar]
- 21.Suissa S Reduced Mortality With Sodium-Glucose Cotransporter-2 Inhibitors in Observational Studies: Avoiding Immortal Time Bias. Circulation. 2018;137:1432–1434. [DOI] [PubMed] [Google Scholar]
- 22.Patorno E, Patrick AR, Garry EM, Schneeweiss S, Gillet VG, Bartels DB, Masso-Gonzalez E, Seeger JD. Observational studies of the association between glucose-lowering medications and cardiovascular outcomes: addressing methodological limitations. Diabetologia. 2014;57:2237–2250. [DOI] [PubMed] [Google Scholar]
- 23.Schneeweiss S A basic study design for expedited safety signal evaluation based on electronic healthcare data. Pharmacoepidemiol Drug Saf. 2010;19:858–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, Peters AL, Tsapas A, Wender R, Matthews DR. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38:140–149. [DOI] [PubMed] [Google Scholar]
- 25.Kosiborod M, Cavender MA, Fu AZ, Wilding JP, Khunti K, Holl RW, Norhammar A, Birkeland KI, Jørgensen ME, Thuresson M, Arya N, Bodegård J, Hammar N, Fenici P; CVD-REAL Investigators and Study Group. Lower Risk of Heart Failure and Death in Patients Initiated on Sodium-Glucose Cotransporter-2 Inhibitors Versus Other Glucose-Lowering Drugs: The CVD-REAL Study (Comparative Effectiveness of Cardiovascular Outcomes in New Users of Sodium-Glucose Cotransporter-2 Inhibitors). Circulation. 2017;136:249–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patorno E, Goldfine AB, Schneeweiss S, Everett BM, Glynn RJ, Liu J, Kim SC. Cardiovascular outcomes associated with canagliflozin versus other non-gliflozin antidiabetic drugs: population based cohort study. BMJ. 2018;360:k119. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.