Abstract
Background:
The aims were to compare the cardiovascular disease (CVD) risk among children with chronic kidney disease (CKD) secondary to focal segmental glomerulosclerosis (FSGS) with the CVD risk of children with CKD due to other diagnoses.
Methods:
Casual blood pressure (BP), ambulatory blood pressure monitoring (APBM), echocardiogram, lipids, carotid intima medial thickness (cIMT) and uric acid obtained from participants in the Chronic Kidney Disease in Children (CKiD) cohort were analyzed longitudinally. Seventy-nine children with FSGS (FSGS-CKD) were compared to 196 children with non-FSGS glomerular disease (GDO-CKD) and 616 children with non-glomerular disease (NG-CKD).
Results:
At baseline, FSGS-CKD (median 14 years) had ambulatory hypertension (24.6%), masked hypertension (46.2%), left ventricular hypertrophy (LVH) (26.3%) and dyslipidemia (60.0%). In adjusted models, FSGS-CKD had higher systolic BP z-score (0.52 vs 0.11 and 0.23, p=0.002 and 0.02), triglycerides (133 vs 109 and 102 mg/dl, p=0.007 and <0.001) and non-high density lipoprotein (144 vs 132 and 119 mg/dl, p=0.07 and <0.001) at baseline when compared to GDO-CKD and NG-CKD, respectively. Left ventricular mass index (LVMI) (36.0 vs 31.7 g/m2.7, p <0.001) and the odds of LVH (OR: 3.38, 95%CI 1.42,8.08) at baseline were greater in FSGS-CKD compared to NG-CKD. Adjusted longitudinal analysis showed that FSGS-CKD had a faster decline in LVMI than NGCKD and FSGS-CKD had a faster increase in uric acid compared to both groups.
Conclusions:
Children with CKD due to FSGS had a relatively high prevalence of CVD risk factors. FSGS was associated with greater CVD risk when compared to other CKD diagnoses.
Keywords: glomerular disease, pediatrics, carotid intima medial thickness, left ventricular hypertrophy, hypertension, dyslipidemia
Introduction
Focal segmental glomerulosclerosis (FSGS), the second leading cause of nephrotic syndrome in children, has increased in incidence in recent years and accounts for 11.7% percent of pediatric transplants in North America [1–3]. Beyond the risk of kidney morbidity, children with FSGS often have hypertension, prolonged exposure to medications and a pronounced alteration of lipoprotein metabolism that may place them at increased risk for adverse cardiovascular outcomes.
Hypertension is frequently a presenting feature of FSGS due to glomerular scarring and subsequent activation of the renin angiotensin aldosterone system, sodium/water retention and sympathetic nervous system activation [4]. In addition, medications used for FSGS treatment (e.g. steroids, calcineurin inhibitors) can cause hypertension. Unfortunately, some children do not achieve remission and continue to be nephrotic and/or hyperlipidemic. In addition to the risk of hyperlipidemia, genetic studies of lipoproteins have indicated that variants in APOL1 increase both the risk for FSGS and atherosclerotic cardiovascular disease risk in African-American adults and children [5–7].
Recent studies from the Chronic Kidney Disease in Children (CKiD) cohort have shown that children with chronic kidney disease (CKD) often have increased arterial wall thickness, uncontrolled hypertension, masked hypertension, left ventricular hypertrophy (LVH) and dyslipidemia [8–11]. A population of children with FSGS is included in this cohort; however, little is known about the extent of cardiovascular disease (CVD) risk burden specifically in children with FSGS. Thus, the purpose of the current study was to characterize the cardiovascular health of children with FSGS enrolled in CKiD. In addition, we aimed to compare the CVD risk and longitudinal changes in CVD indicators among children with CKD secondary to FSGS with the CVD risk of children with CKD due to other glomerular disease and non-glomerular causes of CKD.
Methods
Study Design and Population
The CKiD study is a multi-center, prospective cohort of children with mild-moderate CKD. A total of 891 participants, aged 1–16 years with a diagnosis of CKD and an estimated glomerular filtration rate (eGFR) <90 ml/min|1.73m2 were enrolled at 54 sites in North America from 2005–2014 in two recruitment waves [12]. Study visits consisted of data and biosample collection annually. Cardiovascular testing was performed every two years, starting with the year 2 visit, which included ambulatory blood pressure monitoring (ABPM), lipid studies, echocardiography and carotid intima medial thickness (cIMT, measured at selected sites). A full description of the CKiD study has been previously published [13]. The study protocol followed the Declaration of Helsinki and was approved by the Institutional Review Board at each participating site. Informed consent/assent was obtained from each participant.
Our study population was divided into three diagnosis categories for comparison: FSGS (FSGS-CKD), glomerular diagnosis other than FSGS (GDO-CKD) and nonglomerular disease (NG-CKD). Diagnoses were based on clinical chart review by CKiD site principal investigators. Primary versus secondary etiologies of FSGS were not recorded by CKiD. In addition, genetic data were not collected by CKiD, therefore, genetic vs. non-genetic forms of FSGS were not able to be distinguished in the cohort.
Measures of Cardiovascular Health
Clinic blood pressure (BP) was measured using an aneroid sphygmomanometer (Mabis MedicKit 5, Mabis Healthcare, Waukegan, IL). The average of three readings was obtained from a sitting position after five minutes of rest. Systolic (SBP) and diastolic (DBP) BPs were transformed to BP z-scores and corresponding percentiles [14]. Hypertensive BP was defined as SBP or DBP ≥95% for age, sex and height or self-reported history of hypertension on anti-hypertensive medications. Uncontrolled hypertension was defined as hypertensive BP for those with a prior diagnosis of hypertension.
ABPM was measured and centrally read according to recommended guidelines (Spacelab 90217 monitors, Spacelabs Medical, Redmond, CA) [15]. Ambulatory hypertension was defined as clinic BP >95th percentile, 24-hour SBP or DBP >95th percentile and SBP or DBP load >25% [16]. Masked hypertension was defined as clinic SBP and DBP <95th percentile, 24-hour SBP or DBP >95th percentile and SBP or DBP load >25%. Children with mean systolic or diastolic nocturnal dip <10% were classified as non-dippers [16, 17].
2D/M-mode echocardiography was performed for left ventricular (LV) measurements [18]. LV mass was calculated using the formula by Devereux et al [19]. LV mass index (LVMI) was derived by dividing LV mass (g) by the participant’s height (m2.7) [20]. Values ≥ 95 percentile for age and sex indicated the presence of LVH [21]. Additionally, systolic and diastolic dysfunction was assessed by shortening fraction (SF), velocity of early (E-wave) and late (A-wave) diastolic trans-mitral flow, and E/A ratio.
B-mode ultrasonography cIMT was used to examine the common carotid arteries. The combined width of the intima plus the media was used as a measure of arterial wall thickness. Certified readings were obtained from the Cardiovascular Core Imaging Research Laboratory (Cincinnati, OH). cIMT z-scores were calculated for children ≥ 6 years old using normative reference data [22].
Fasting lipid studies (triglycerides, total cholesterol, high density lipoprotein [HDL] –cholesterol) were measured using a Bayer Advia 2400 analyzer. Non-HDL cholesterol was calculated as the difference between total cholesterol and HDL-cholesterol. Abnormally high triglycerides was defined as >110 mg/dl for children <10 years old, and >130 mg/dl for children ≥10 years old. Abnormal HDL and non-HDL cholesterol were defined as <40 mg/dl and >160 mg/dl, respectively [23]. Dyslipidemia was defined as the presence of any of these three abnormalities.
Demographic, clinical and kidney health variables
Covariates of interest were age at diagnosis, age at study entry, race/ethnicity, maternal education, low birth weight (LBW, <2500g), and gestational age. Height, weight and body mass index (BMI) z-scores and percentiles were created [24]. BMI ≥85th percentile was categorized as overweight and BMI ≥95th percentile was categorized as obese. Blood chemistry included serum calcium (mg/dL), phosphorus (mg/dl) and uric acid (mg/dl). Renal health was assessed by eGFR (ml/min|1.73m2) using the 2012 Schwartz formula [12] and urine protein:creatinine ratio (UP/Cr) from the first morning urine. Nephrotic range proteinuria was defined as UP/Cr >2 mg/mg. Use of medications was self-reported.
Statistical Analysis
For variables which were measured at every visit, baseline was defined as visit 1 (study entry), and for variables which were measured at only even visits, baseline was defined as visit 2 (1 year after study entry). Baseline characteristics of the three diagnosis groups were described and pairwise comparisons were based on the rank sum test and Fisher’s exact test, as appropriate.
We fitted separate logistic regression models comparing CKD groups for outcomes of uncontrolled hypertension, LVH, dyslipidemia, and non-dipper status. Two models were fit for each outcome: the first was unadjusted, the second was adjusted for age, sex, race, overweight/obese status, cohort, and log transformed eGFR (to achieve normality) at the baseline visit.
To compare baseline and longitudinal changes of indicators of cardiovascular health, linear mixed effects models with random intercepts and slopes were used. Models were adjusted for baseline age, sex, race, overweight/obese status, recruitment wave, and log transformed eGFR at baseline. For LVMI, time-updated height was included instead of age since LVM is more strongly dependent on height [21]. Triglycerides, HDL-cholesterol and non-HDL cholesterol were log-transformed to achieve normality. Group-specific estimates and 95% confidence intervals (CI) for the initial level and slopes were derived from linear combinations of the model parameters. Pair-wise comparisons assessed how the GDO-CKD and NG-CKD groups were different from FSGS-CKD (as the reference group). Statistical significance was assessed at the p<0.05 level and all analyses were conducted in SAS 9.3 (SAS Institute, Carey, NC).
Results
Demographics and Therapy Use
A total of 891 children were enrolled in CKiD during the study period. Of these, 8.9% (79) were children with FSGS-CKD, 22.0% (196) had GDO-CKD and 69.1% (616) had NG-CKD. Table 1 presents participant characteristics at baseline. Those with FSGS-CKD tended to be of black race and with lower maternal education compared to non-FSGS groups. FSGS-CKD was more likely to be of LBW and premature compared to the GDOCKD group. The FSGS-CKD group was more likely to have a biological family member with FSGS compared to the other etiologic groups. When compared to NG-CKD, children with FSGS-CKD were older, less likely male and heavier. The median age of CKD onset was 9 years for those with FSGS-CKD and 7.5 years for GDO-CKD, compared to birth for those with NG-CKD. The median eGFR among children with FSGS was lower compared to GDO-CKD, and higher compared to children with NGCKD. The proportion with nephrotic range proteinuria was substantially higher among those with FSGS-CKD. Children with FSGS-CKD and GDO-CKD had similar proportions receiving antihypertensive therapy, corticosteroids and any immunosuppressive therapy. The prevalence of these therapies was higher among these two groups compared to those with NG-CKD. The use of diuretic and lipid lowering medication was higher in the FSGS-CKD group than in the other two groups.
Table 1.
Baseline demographic, growth, chronic kidney disease and therapy characteristics of participants in the Chronic Kidney Disease in Children (CKiD) cohort, by underlying CKD diagnosis.
| Variable | FSGS-CKD N = 79 | GDO-CKD N = 196 | NG-CKD N = 616 | P value comparing FSGS-CKD to GDO-CKD | P value comparing FSGS-CKD to NG-CKD |
|---|---|---|---|---|---|
| Demographic and birth history | |||||
| Age at study entry, years | 14.5 [10.3, 15.8] | 13.9 [10.8, 15.9] | 10.1 [6.5, 13.6] | 0.82 | <0.001 |
| Male sex | 54.4% (43) | 53.1% (104) | 65.7% (405) | 0.84 | 0.048 |
| Black race | 54.4% (43) | 20.9% (41) | 18.7% (115) | <0.001 | <0.001 |
| Maternal education college level or higher | 44.2% (34) | 59.8% (113) | 62.3% (376) | 0.02 | 0.002 |
| Low birth weight (< 2500g) | 23.6% (17) | 12.2% (22) | 19.6% (115) | 0.02 | 0.42 |
| Premature birth (< 36 weeks) | 15.5% (11) | 6.8% (13) | 12.6% (75) | 0.03 | 0.49 |
| CKiD II recruitment | 45.6% (36) | 56.6% (111) | 25.6% (158) | 0.09 | <0.001 |
| Duration of follow up (years) | 3.0 [1.1, 4.4] | 2.9 [1.8, 4.0] | 4.3 [3.0, 7.0] | 0.88 | <0.001 |
| Any relative with FSGS | 9.7% (7) | 1.1% (2) | 0.7% (4) | <0.001 | <0.001 |
| Growth | |||||
| Height z-score | −0.28 [−1.31, 0.24] | −0.18 [−0.94, 0.70] | −0.61 [−1.45, 0.16] | 0.06 | 0.21 |
| Weight z-score | 0.56 [−0.32, 1.98] | 0.64 [−0.23, 1.57] | −0.13 [−0.97, 0.74] | 0.99 | <0.001 |
| BMI z-score | 0.92 [0.16, 2.01] | 0.93 [−0.02, 1.60] | 0.32 [−0.43, 1.21] | 0.35 | <0.001 |
| BMI categories | 0.50 | <0.001 | |||
| Normal (< 85th percentile) | 51.9% (41) | 55.9% (109) | 70.7% (419) | ||
| Overweight (85th to < 95th percentile) | 17.7% (14) | 20.5% (40) | 14.7% (87) | ||
| Obese (≥ 95th percentile) | 30.4% (24) | 23.6% (46) | 14.7% (87) | ||
| Chronic Kidney Disease | |||||
| Age at CKD onset, years | 9.0 [4.5, 12.5] | 7.5 [2.5, 12.5] | 0.0 [0.0, 0.0] | 0.3 | <0.001 |
| Proportion of life with CKD | 32.5 [15.5, 61.5] | 29.5 [9.7, 68.9] | 100.0 [100.0, 100.0] | 0.89 | <0.001 |
| eGFR (ml/min|1.73m2) | 56.0 [39.0, 67.2] | 62.5 [45.2, 78.5] | 49.4 [36.7, 61.9] | 0.02 | 0.03 |
| Urine protein:creatinine, mg/mg | 1.11 [0.30, 4.17] | 0.61 [0.19, 1.80] | 0.28 [0.11, 0.80] | 0.02 | <0.001 |
| Nephrotic proteinuria (>2.0 mg/mg) | 32.0% (24) | 22.0% (42) | 8.3% (49) | 0.089 | <0.001 |
| Therapy use | |||||
| Any anti-hypertensive medication | 93.7% (74) | 90.8% (178) | 53.4% (329) | 0.44 | <0.001 |
| ACE inhibitor | 75.9% (60) | 70.9% (139) | 39.9% (246) | 0.4 | <0.001 |
| ARB | 31.6% (25) | 21.9% (43) | 6.0% (37) | 0.09 | <0.001 |
| Diuretic | 20.3% (16) | 10.2% (20) | 4.2% (26) | 0.025 | <0.001 |
| Corticosteroid | 24.1% (19) | 32.7% (64) | 1.1% (7) | 0.16 | <0.001 |
| Any immunosuppressive medication | 51.9% (41) | 42.9% (84) | 1.3% (8) | 0.17 | <0.001 |
| Lipid lowering medication | 24.1% (19) | 4.1% (8) | 1.0% (6) | <0.001 | <0.001 |
CKD - chronic kidney disease; FSGS-CKD - focal segmental glomerulosclerosis group; GDO-CKD - glomerular disease other group; NG-CKD - non-glomerular group; eGFR - estimated glomerular filtration rate; BMI - body mass index; ACE - angiotensin converting enzyme; ARB - angiotensin II receptor blocker
Cardiovascular Risk Profile for FSGS
Table 2 presents the characteristics at baseline for cardiovascular variables. A substantial proportion of children with FSGS-CKD had abnormal BP parameters including 24.4% with uncontrolled clinic BP, 24.6% with confirmed ABPM hypertension, and 46.2% with masked HTN. Nocturnal non-dipper status was present in 51%. LVH was present in 26.3% of FSGS-CKD with eccentric hypertrophy in 17.5% and concentric hypertrophy in 8.8%. The prevalence of dyslipidemia was 60.0% in those with FSGS-CKD, with 29.3% having ≥2 lipid abnormalities. The median cIMT z-score was 0.68 (IQR −0.21, 1.57) (excluding 50 observations from children <6 years due to lack of normative reference data) [22].
Table 2.
Baseline characteristics of cardiovascular health indicators among participants, by underlying CKD diagnosis.
| Variable | FSGS-CKD N = 79 | GDO-CKD N = 196 | NG-CKD N = 616 | P value comparing FSGS-CKD to GDO-CKD | P value comparing FSGS-CKD to NG-CKD |
|---|---|---|---|---|---|
| Blood Pressure | |||||
| SBP z-score | 0.60 [−0.02, 1.38] | 0.03 [−0.76, 0.97] | 0.42 [−0.30, 1.11] | <0.001 | 0.04 |
| DBP z-score | 0.47 [−0.10, 1.17] | 0.22 [−0.42, 0.86] | 0.56 [−0.04, 1.18] | 0.05 | 0.58 |
| Measured BP status | 0.163 | 0.639 | |||
| Normal (<90th percentile) | 66.2% (51) | 76.2% (147) | 68.4% (405) | ||
| Prehypertension (90th to < 95th percentile) | 9.1% (7) | 8.8% (17) | 11.1% (66) | ||
| Hypertension (> 95th percentile) | 24.7% (19) | 15.0% (29) | 20.4% (121) | ||
| Uncontrolled hypertensive clinic BP | 24.4% (19) | 14.9% (29) | 19.9% (121) | 0.06 | 0.36 |
| Ambulatory and clinic BP categories | 0.01 | 0.17 | |||
| White coat hypertension | 3.1% (2) | 1.8% (3) | 2.1% (11) | ||
| Masked hypertension | 46.2% (30) | 55.8% (91) | 54.0% (285) | ||
| Confirmed hypertension | 24.6% (16) | 8.6% (14) | 14.4% (76) | ||
| SBP dipping < 10% | 47.1% (24) | 38.3% (49) | 39.5% (175) | 0.28 | 0.3 |
| DBP dipping < 10% | 31.4% (16) | 18.8% (24) | 15.6% (69) | 0.07 | 0.005 |
| Non dipper status | 51.0% (26) | 39.8% (51) | 40.4% (179) | 0.17 | 0.15 |
| Cardiovascular Tests | |||||
| Left ventricular mass index, g/m2.7 | 31.4 [27.4, 39.6] | 28.7 [24.9, 34.8] | 31.5 [25.6, 37.6] | 0.02 | 0.27 |
| LVH | 26.3% (15) | 11.0% (16) | 10.9% (52) | 0.007 | <0.001 |
| Left ventricular geometry | 0.06 | 0.007 | |||
| Normal | 68.4% (39) | 80.7% (117) | 78.9% (378) | ||
| Eccentric Hypertrophy | 17.5% (10) | 7.6% (11) | 6.5% (31) | ||
| Concentric Remodeling | 5.3% (3) | 8.3% (12) | 10.2% (49) | ||
| Concentric Hypertrophy | 8.8% (5) | 3.4% (5) | 4.4% (21) | ||
| Shortening Fraction | 0.37 [0.34, 0.41] | 0.36 [0.33, 0.39] | 0.38 [0.34, 0.42] | 0.15 | 0.27 |
| E/A ratio | 1.7 [1.6, 2.3] | 1.9 [1.5, 2.3] | 1.8 [1.5, 2.3] | 0.81 | 0.68 |
| Carotid Artery Intima-Medial Thickness, mm | 0.43 [0.32, 0.47] | 0.41 [0.35, 0.46] | 0.42 [0.37, 0.48] | 0.85 | 0.67 |
| Biomarkers | |||||
| Uric acid, mg/dL | 6.5 [5.7, 7.3] | 5.8 [4.8, 6.8] | 5.8 [4.7, 7.0] | 0.005 | 0.005 |
| Calcium, mg/dL | 9.4 [8.9, 9.7] | 9.3 [8.8, 9.6] | 9.6 [9.3, 9.9] | 0.84 | <0.001 |
| Phosphorus, mg/dL | 4.4 [3.8, 4.9] | 4.4 [3.7, 4.8] | 4.5 [4.1, 5.0] | 0.83 | 0.13 |
| Ca × P product | 40.0 [35.5, 45.5] | 39.8 [35.1, 45.1] | 43.2 [38.8, 47.5] | 0.95 | <0.001 |
| Lipid levels | |||||
| Diabetes Mellitus at baseline | 0.0% (0) | 3.6% (7) | 0.3% (2) | 0.2 | 1.00 |
| Ever diagnosed with Diabetes Mellitus during study follow-up | 3.8% (3) | 6.2% (12) | 2.1% (13) | 0.57 | 0.41 |
| Triglycerides, mg/dL | 121 [86, 191] | 93 [70, 149] | 97 [72, 140] | 0.01 | 0.01 |
| High triglycerides | 49.2% (32) | 31.5% (53) | 42.4% (231) | 0.01 | 0.29 |
| HDL cholesterol, mg/dL | 53 [41, 61] | 50 [40, 60] | 49 [42, 58] | 0.38 | 0.24 |
| Low HDL cholesterol | 6.2% (4) | 9.5% (16) | 9.0% (49) | 0.41 | 0.44 |
| Non-HDL cholesterol, mg/dL | 121 [108, 162] | 118 [95, 145] | 118 [97, 138] | 0.06 | 0.02 |
| High non-HDL cholesterol | 27.7% (18) | 14.9% (25) | 10.3% (56) | 0.02 | <0.001 |
| Counts of abnormal lipids values (out of 3) | 0.07 | 0.02 | |||
| 0 | 40.0% (26) | 58.9% (99) | 56.8% (310) | ||
| 1 | 30.8% (20) | 22.6% (38) | 26.0% (142) | ||
| 2 | 23.1% (15) | 14.3% (24) | 15.2% (83) | ||
| 3 | 6.2% (4) | 4.2% (7) | 2.0% (11) | ||
| Dyslipidemia | 60.0% (39) | 41.1% (69) | 43.2% (236) | 0.009 | 0.01 |
CKD - chronic kidney disease; FSGS-CKD - focal segmental glomerulosclerosis group; GDO-CKD - glomerular disease other group; NG-CKD - non-glomerular group; SBP - systolic blood pressure; DBP - diastolic blood pressure; BP - blood pressure; LVH - left ventricular hypertrophy; E - early wave diastolic trans-mitral flow; A - late wave diastolic trans-mitral flow; Ca x P calcium x phosphorus; HDL - high density lipoprotein.
Comparison of Cardiovascular Risk among CKD Groups
At baseline, there were significant differences in cardiovascular markers between FSGS-CKD and non-FSGS CKD groups. Children with FSGS-CKD had significantly higher median SBP z-score, uric acid and triglycerides, as well as a greater prevalence with LVH, high non-HDL cholesterol and dyslipidemia compared to the other CKD groups (Table 2). Children with FSGS-CKD also had higher ambulatory BP and LVMI compared to children with GDO-CKD. Children with FSGS-CKD had more diastolic non-dipping and abnormal LV geometry, but lower Ca × P product values compared to children with NG-CKD. No significant differences in E/A ratio or cIMT values were found among the groups.
Table 3 shows the association between cardiovascular outcomes and CKD diagnosis. In unadjusted analyses, FSGS-CKD was a risk factor for all cardiovascular outcomes examined (i.e., the odds ratios > 1), however, only LVH, dyslipidemia and diastolic dipping were significant. In models adjusting for age, gender, race, BMI category, cohort wave and eGFR, restricting to those with NG-CKD and FSGS-CKD, only LVH and dyslipidemia were significantly associated with FSGS. It should be noted that the overall odds ratio estimates for each outcome remained > 1 (i.e., increased risk), but those with FSGS were not significantly different than GDO-CKD.
Table 3.
Association between cardiovascular and metabolic outcomes and CKD diagnosis at analytical baseline
| Outcome | Comparison | Unadjusted | Adjusteda |
|---|---|---|---|
| Uncontrolled hypertension | FSGS-CKD vs. GDO-CKD | 1.84 (0.96, 3.53) | 1.30 (0.60, 2.83) |
| FSGS-CKD vs. NG-CKD | 1.30 (0.74, 2.26) | 1.63 (0.87, 3.05) | |
| Left ventricular hypertrophy | FSGS-CKD vs. GDO-CKD | 2.88 (1.31, 6.32) | 1.30 (0.49, 3.47) |
| FSGS-CKD vs. NG-CKD | 2.93 (1.52, 5.65) | 3.38 (1.42, 8.08) | |
| Dyslipidemia | FSGS-CKD vs. GDO-CKD | 2.15 (1.20, 3.86) | 1.75 (0.87, 3.50) |
| FSGS-CKD vs. NG-CKD | 1.96 (1.16, 3.32) | 2.31 (1.21, 4.44) | |
| Diastolic dipping < 10% | FSGS-CKD vs. GDO-CKD | 1.98 (0.95, 4.15) | 1.46 (0.62, 3.43) |
| FSGS-CKD vs. NG-CKD | 2.48 (1.30, 4.72) | 1.96 (0.92, 4.18) | |
| Systolic dipping < 10% | FSGS-CKD vs. GDO-CKD | 1.43 (0.74, 2.76) | 1.30 (0.63, 2.69) |
| FSGS-CKD vs. NG-CKD | 1.36 (0.76, 2.44) | 1.13 (0.59, 2.18) | |
| Non dipper status | FSGS-CKD vs. GDO-CKD | 1.57 (0.82, 3.02) | 1.36 (0.66, 2.80) |
| FSGS-CKD vs. NG-CKD | 1.53 (0.86, 2.74) | 1.30 (0.68, 2.50) |
Adjusted for age, gender, race, overweight/obese status, cohort wave and eGFR (log transformed)
Longitudinal Changes
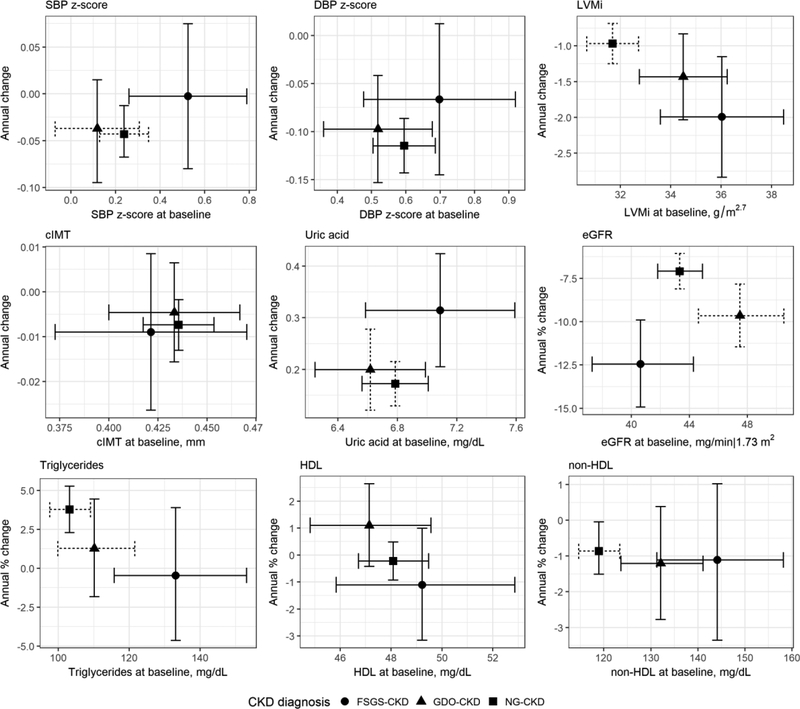
Figure 1 shows the adjusted estimates of baseline levels and longitudinal changes in cardiovascular markers across the diagnosis groups. The number of visits per participant for each cardiovascular variable is presented in Supplementary Table 1. FSGS-CKD had higher SBP z-scores at baseline than in the other two groups, but the change over time was not significantly different across diagnosis groups. DBP z-scores were not significantly different at baseline and change over time was not different by CKD group. LVMI was higher among those with FSGS-CKD compared to those with NG-CKD. LVMI decreased over time among all the three groups, and children with FSGS had a faster decline in LVMI than children with NG-CKD. No significant differences in either baseline values or changes over time were detected for cIMT. We also investigated age- and gender-specific cIMT z-scores based on previously published reference values and found no evidence of differences across diagnostic groups (data not shown). Those with FSGS-CKD had increasing uric acid over time and this was a significantly faster increase compared to those with GDO-CKD and NG-CKD. Children with FSGS-CKD had a higher concentration of non-HDL cholesterol and triglycerides at baseline than children with the other two CKD diagnoses. For FSGS-CKD, average triglycerides decreased 0.50% per year, which was borderline significantly (p = 0.05) different from the NGCKD group, who had a faster increase of 3.71% per year. There were no significant differences in baseline HDL among the three groups. Those with FSGS-CKD entered the study with a lower eGFR and had a significantly faster decline than the other etiologic groups. The average change was −12.5% per year for those with FSGS-CKD; those with GDO-CKD declined nearly 10% per year, while those with NG-CKD declined approximately 7% per year.
Figure 1.
Longitudinal changes in cardiovascular indicators based adjusted3 linear mixed effects models with random intercepts and random slopes comparing CKD diagnosis groups.
Dashed horizontal bar: Intercept statistically different (p < 0.05) from FSGS-CKD group.
Dashed vertical bar: Slope (or annual % change) statistically different from FSGS-CKD group.a Models were adjusted for age (all outcomes except for LVMi), sex, race, overweight/obese status, cohort wave and eGFR (log transformed). For interpretation of intercept, the reference characteristic was for a non-black, 10 year old normal weight boy from cohort wave 1 whose eGFR at baseline was 45 ml/min|1.73m2; for LVMI model: time-updated height (centered at 150 cm) was included instead of age as a covariate
CKD - chronic kidney disease; FSGS - focal segmental glomerulosclerosis; G - glomerular; NG - non-glomerular; SBP - systolic blood pressure; DBP - diastolic blood pressure; BMI - body mass index;
Discussion
This analysis of a multi-center, national cohort of children with mild-moderate CKD demonstrated that the CVD risk factors of ambulatory hypertension, masked hypertension, non-dipper status, LVH and dyslipidemia are prevalent in children with FSGS. Additionally, we found that FSGS was associated with a worse cardiovascular risk profile when compared with other CKD diagnoses. Children with FSGS had greater SBP z-scores and triglycerides at baseline compared to non-FSGS CKD. Non-HDL cholesterol, LVMI and LVH at baseline were also greater in children with FSGS than in the NG-CKD cohort. Longitudinal analysis showed children with FSGS had a faster decline in LVMI compared to NG-CKD and FSGS had a faster increase in uric acid compared to both CKD groups.
FSGS, the most common primary glomerular disorder in children causing ESRD, is a complex disease that remains only partially understood [2]. Our finding that the FSGS cohort had increased cardiovascular risk compared to non-FSGS CKD is likely multi-factorial, being related to the severity of ongoing proteinuria and exposure to multiple immunosuppressive medications with cardiovascular side-effects. In addition, genetic variants in APOL1 have been shown to confer increased risk of both FSGS and CVD in African-American adults and children [5–7]. Furthermore, obesity, lower socioeconomic status with reduced opportunity to exercise and decreased access to healthy food, altered Vitamin D metabolism, and disease specific related factors may contribute to the cardiovascular risk in this population [23, 25–27].
Children with FSGS have a more rapid onset of CKD at an older age with subsequent greater exposure to immune modulating agents which are likely to have an increased effect on obesity and the progression of disease than in non-FSGS cohorts, especially when compared to non-glomerular CKD. In this study, children with NGCKD whose renal disorders were commonly due to congenital abnormalities, had a longer time since disease onset and a lower eGFR compared to FSGS-CKD, and yet FSGS-CKD had a worse cardiovascular profile, suggesting that FSGS is associated with a more aggressive cardiovascular burden. Interestingly, the FSGS-CKD cardiovascular profile was also worse when compared to GDO-CKD, even though disease duration, age of onset, weight status and medication exposure were similar in the latter cohort.
As mentioned previously, children with FSGS are at risk for hypertension for a variety of reasons [28]. Despite the fact that 94% of children with FSGS in our study were on anti-hypertensive medication, 25% had ambulatory hypertension, 46% had masked hypertension and their clinical SBP z-score did not change over time, further documenting that hypertension is not adequately being treated in this population. Previous studies that describe the high burden of hypertension and its suboptimal control among children with FSGS are consistent with these results [29, 30].
The literature on LVH and other target organ effects in children with FSGS is limited. A small single center study reported LVH in 56% (10/18) of nephrotic children with a podocin mutation, a mutation known to cause FSGS [31]. In another CKiD study, Woroniecki et al. found that African-American children with FSGS and the high risk APOL1 genotype had increased odds of LVH and obesity compared to children with the APOL1 low risk genotype [6]. There is mounting evidence that obesity is relevant to CVD as demonstrated by the strong association between obesity and LVH in children in the general population, which is independent of BP [32]. Hemodynamic, metabolic and inflammatory factors are thought to contribute to the development of LVH in obese children [33]. In this study, however, although the children with FSGS were significantly more obese than those with NG-CKD, obesity alone was likely not responsible for differences in LVMI and LVH as children with FSGS still had greater LVMI and LVH compared to NG-CKD children after adjusting for weight status.
Given that lipoprotein metabolism is known to be altered in nephrotic syndrome, the finding of greater dyslipidemia in FSGS children compared to the non-FSGS cohort was not unexpected. In nephrotic syndrome, lipid abnormalities typically include elevated total cholesterol, LDL, VLDL, triglycerides and apolipoprotein B with varied HDL levels [34]. These lipid changes are due to decreased lipoprotein lipase activity in the liver and peripheral tissues, increased biosynthesis in the liver, and compositional changes of the lipoproteins [34]. However, the role of hyperlipidemia on the long-term risk of atherosclerosis and CVD in children with FSGS is not fully understood. There were no differences in cIMT, a surrogate for atherosclerosis, between FSGS and non-FSGS children in our study, which may be explained in part by the small sample size that completed cIMT testing in the FSGS group. A study by Brady et al. demonstrated that cIMT in children in CKiD was elevated compared with healthy children [35].
These findings in children with FSGS highlight a critical need to improve identification and treatment of these CVD comorbidities in this high-risk population. Clinicians should focus on rigorous BP control in children with FSGS [36] and greater attention should be paid to metabolic factors including lipid management [23, 34]. These children also require dietary interventions and support to improve nutrition and to control weight while maximizing the opportunity for exercise [23]. The protracted use of glucocorticoids also requires careful evaluation in each case.
Although this study has several strengths, including longitudinal follow-up and the standardization of testing measures, there are limitations that should be considered. The sample size of the FSGS group was relatively small compared to the rest of the cohort and thus the study may not be powered to be generalizable to the pediatric FSGS population. In addition, FSGS is a histologic lesion with heterogeneous etiologies; genetic data and information on primary vs. secondary causes of FSGS was lacking. Another potential limitation was the comparison of FSGS with two diagnostic categories that have markedly different pathologies and disease courses, particularly the congenital renal abnormalities. Indeed, the burden of years with CKD was much longer for those with congenital diagnoses compared to those with FSGS. Lastly, given the lack of data about the duration and cumulative dose of immunosuppressive/steroid therapy, we are unable to discern whether the excess in CVD risk factors is due to diagnosis alone or the cumulative burden of these therapies.
Conclusion
Our study provides evidence that children with FSGS are at significantly increased CVD risk due to a combination of medication exposure, poorly controlled hypertension over time, masked hypertension, LVH and dyslipidemia. Of importance, children with FSGS and mild-moderate CKD have greater CVD risk when compared to other CKD related diagnoses. Our findings strongly support the notion that children with FSGS should undergo screening for CVD risk factors and that these should be monitored on a regular basis. Treatment needs to be aimed at minimizing the impact that these risk factors have on the children’s cardiovascular well-being. Future longitudinal studies with longer term follow-up (i.e. > 5 or 10 years) are needed to determine what interventions best reduce adverse cardiovascular outcomes for children with FSGS.
Supplementary Material
Acknowledgements
The CKiD study is funded by the National Institute of Diabetes and Digestive and Kidney Diseases with additional funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Heart, Lung, and Blood Institute (UO1-DK-66143, UO1-DK-66174, U01-DK-082194, and UO1-DK-66116).
Support
This work was supported by American Heart Association Grant #15GRNT25360029 / Christine B. Sethna/2015–2018.
Footnotes
Disclosures
None
References
- 1.Kitiyakara C, Eggers P, Kopp JB (2004) Twenty-one-year trend in ESRD due to focal segmental glomerulosclerosis in the United States. Am J Kidney Dis 44:815–825. [PubMed] [Google Scholar]
- 2.Korbet SM, Schwartz MM, Lewis EJ (1994) Primary focal segmental glomerulosclerosis: clinical course and response to therapy. Am J Kidney Dis 23:773–783. [DOI] [PubMed] [Google Scholar]
- 3.Studies NAPRTaC (2014) NAPRTCS Annual Report.
- 4.Ihm CG (2015) Hypertension in Chronic Glomerulonephritis. Electrolyte Blood Press 13:41–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ng DK, Robertson CC, Woroniecki RP, Limou S, Gillies CE, Reidy KJ, Winkler CA, Hingorani S, Gibson KL, Hjorten R, Sethna CB, Kopp JB, Moxey-Mims M, Furth SL, Warady BA, Kretzler M, Sedor JR, Kaskel FJ, Sampson MG (2017) APOL1-associated glomerular disease among African-American children: a collaboration of the Chronic Kidney Disease in Children (CKiD) and Nephrotic Syndrome Study Network (NEPTUNE) cohorts. Nephrol Dial Transplant 32:983–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Woroniecki RP, Ng DK, Limou S, Winkler CA, Reidy KJ, Mitsnefes M, Sampson MG, Wong CS, Warady BA, Furth SL, Kopp JB, Kaskel FJ (2016) Renal and Cardiovascular Morbidities Associated with APOL1 Status among African-American and Non-African-American Children with Focal Segmental Glomerulosclerosis. Front Pediatr 4:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ito K, Bick AG, Flannick J, Friedman DJ, Genovese G, Parfenov MG, Depalma SR, Gupta N, Gabriel SB, Taylor HA Jr., Fox ER, Newton-Cheh C, Kathiresan S, Hirschhorn JN, Altshuler DM, Pollak MR, Wilson JG, Seidman JG, Seidman C Increased burden of cardiovascular disease in carriers of APOL1 genetic variants. Circ Res 114:845–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wong CJ, Moxey-Mims M, Jerry-Fluker J, Warady BA, Furth SL CKiD (CKD in Children) prospective cohort study: a review of current findings. Am J Kidney Dis 60:1002–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brady TM, Schneider MF, Flynn JT, Cox C, Samuels J, Saland J, White CT, Furth S, Warady BA, Mitsnefes M Carotid intima-media thickness in children with CKD: results from the CKiD study. Clin J Am Soc Nephrol 7:1930–1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kupferman JC, Aronson Friedman L, Cox C, Flynn J, Furth S, Warady B, Mitsnefes M BP control and left ventricular hypertrophy regression in children with CKD. J Am Soc Nephrol 25:167–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mitsnefes MM, Barletta GM, Dresner IG, Chand DH, Geary D, Lin JJ, Patel H (2006) Severe cardiac hypertrophy and long-term dialysis: the Midwest Pediatric Nephrology Consortium study. Pediatr Nephrol 21:1167–1170. [DOI] [PubMed] [Google Scholar]
- 12.Schwartz GJ, Schneider MF, Maier PS, Moxey-Mims M, Dharnidharka VR, Warady BA, Furth SL, Munoz A (2012) Improved equations estimating GFR in children with chronic kidney disease using an immunonephelometric determination of cystatin C. Kidney Int 82:445–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Furth SL, Cole SR, Moxey-Mims M, Kaskel F, Mak R, Schwartz G, Wong C, Munoz A, Warady BA (2006) Design and methods of the Chronic Kidney Disease in Children (CKiD) prospective cohort study. Clin J Am Soc Nephrol 1:1006–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National High Blood Pressure Education Program Working Group on High Blood Pressure in C, Adolescents (2004) The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics 114:555–576. [PubMed] [Google Scholar]
- 15.Urbina E, Alpert B, Flynn J, Hayman L, Harshfield GA, Jacobson M, Mahoney L, McCrindle B, Mietus-Snyder M, Steinberger J, Daniels S (2008) Ambulatory blood pressure monitoring in children and adolescents: recommendations for standard assessment: a scientific statement from the American Heart Association Atherosclerosis, Hypertension, and Obesity in Youth Committee of the council on cardiovascular disease in the young and the council for high blood pressure research. Hypertension 52:433–451. [DOI] [PubMed] [Google Scholar]
- 16.Soergel M, Kirschstein M, Busch C, Danne T, Gellermann J, Holl R, Krull F, Reichert H, Reusz GS, Rascher W (1997) Oscillometric twenty-four-hour ambulatory blood pressure values in healthy children and adolescents: a multicenter trial including 1141 subjects. J Pediatr 130:178–184. [DOI] [PubMed] [Google Scholar]
- 17.Sorof JM, Cardwell G, Franco K, Portman RJ (2002) Ambulatory blood pressure and left ventricular mass index in hypertensive children. Hypertension 39:903–908. [DOI] [PubMed] [Google Scholar]
- 18.Lai WW, Geva T, Shirali GS, Frommelt PC, Humes RA, Brook MM, Pignatelli RH, Rychik J (2006) Guidelines and standards for performance of a pediatric echocardiogram: a report from the Task Force of the Pediatric Council of the American Society of Echocardiography. J Am Soc Echocardiogr 19:1413–1430. [DOI] [PubMed] [Google Scholar]
- 19.Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, Reichek N (1986) Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol 57:450–458. [DOI] [PubMed] [Google Scholar]
- 20.de Simone G, Daniels SR, Devereux RB, Meyer RA, Roman MJ, de Divitiis O, Alderman MH (1992) Left ventricular mass and body size in normotensive children and adults: assessment of allometric relations and impact of overweight. J Am Coll Cardiol 20:1251–1260. [DOI] [PubMed] [Google Scholar]
- 21.Khoury PR, Mitsnefes M, Daniels SR, Kimball TR (2009) Age-specific reference intervals for indexed left ventricular mass in children. J Am Soc Echocardiogr 22:709–714. [DOI] [PubMed] [Google Scholar]
- 22.Doyon A, Kracht D, Bayazit AK, Deveci M, Duzova A, Krmar RT, Litwin M, Niemirska A, Oguz B, Schmidt BM, Sozeri B, Querfeld U, Melk A, Schaefer F, Wuhl E, Consortium CS (2013) Carotid artery intima-media thickness and distensibility in children and adolescents: reference values and role of body dimensions. Hypertension 62:550–556. [DOI] [PubMed] [Google Scholar]
- 23.Expert Panel on Integrated Guidelines for Cardiovascular H, Risk Reduction in C, Adolescents, National Heart L, Blood I (2011) Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: summary report. Pediatrics 128 Suppl 5:S213–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, Wei R, Curtin LR, Roche AF, Johnson CL (2002) 2000 CDC Growth Charts for the United States: methods and development. Vital Health Stat 11:1–190. [PubMed] [Google Scholar]
- 25.Denburg MR, Kalkwarf HJ, de Boer IH, Hewison M, Shults J, Zemel BS, Stokes D, Foerster D, Laskin B, Ramirez A, Leonard MB (2013) Vitamin D bioavailability and catabolism in pediatric chronic kidney disease. Pediatr Nephrol 28:1843–1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kambham N, Markowitz GS, Valeri AM, Lin J, D’Agati VD (2001) Obesity-related glomerulopathy: an emerging epidemic. Kidney Int 59:1498–1509. [DOI] [PubMed] [Google Scholar]
- 27.Kavey RE, Allada V, Daniels SR, Hayman LL, McCrindle BW, Newburger JW, Parekh RS, Steinberger J, American Heart Association Expert Panel on P, Prevention S, Council on Cardiovascular Disease in the Y, Council on E, Prevention, Council on N, Council on Physical A, Metabolism, Council on High Blood Pressure R, Council on Cardiovascular N, Council on the Kidney in Heart D, Interdisciplinary Working Group on Quality of C, Outcomes R (2007) Cardiovascular risk reduction in high-risk pediatric patients: a scientific statement from the American Heart Association Expert Panel on Population and Prevention Science; the Councils on Cardiovascular Disease in the Young, Epidemiology and Prevention, Nutrition, Physical Activity and Metabolism, High Blood Pressure Research, Cardiovascular Nursing, and the Kidney in Heart Disease; and the Interdisciplinary Working Group on Quality of Care and Outcomes Research. J Cardiovasc Nurs 22:218–253. [DOI] [PubMed] [Google Scholar]
- 28.Ray EC, Rondon-Berrios H, Boyd CR, Kleyman TR (2015) Sodium retention and volume expansion in nephrotic syndrome: implications for hypertension. Adv Chronic Kidney Dis 22:179–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.(1978) Nephrotic syndrome in children: prediction of histopathology from clinical and laboratory characteristics at time of diagnosis. A report of the International Study of Kidney Disease in Children. Kidney Int 13:159–165. [DOI] [PubMed] [Google Scholar]
- 30.Sethna CB, Meyers KEC, Mariani LH, Psoter KJ, Gadegbeku CA, Gibson KL, Srivastava T, Kretzler M, Brady TM (2017) Blood Pressure and Visit-to-Visit Blood Pressure Variability Among Individuals With Primary Proteinuric Glomerulopathies. Hypertension 70:315–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frishberg Y, Feinstein S, Rinat C, Becker-Cohen R, Lerer I, Raas-Rothschild A, Ferber B, Nir A (2006) The heart of children with steroid-resistant nephrotic syndrome: is it all podocin? J Am Soc Nephrol 17:227–231. [DOI] [PubMed] [Google Scholar]
- 32.Pruette CS, Fivush BA, Flynn JT, Brady TM (2013) Effects of obesity and race on left ventricular geometry in hypertensive children. Pediatr Nephrol 28:2015–2022. [DOI] [PubMed] [Google Scholar]
- 33.Brady TM (2016) The Role of Obesity in the Development of Left Ventricular Hypertrophy Among Children and Adolescents. Curr Hypertens Rep 18:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Agrawal S, Zaritsky JJ, Fornoni A, Smoyer WE (2018) Dyslipidaemia in nephrotic syndrome: mechanisms and treatment. Nat Rev Nephrol 14:57–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brady TM, Schneider MF, Flynn JT, Cox C, Samuels J, Saland J, White CT, Furth S, Warady BA, Mitsnefes M (2012) Carotid intima-media thickness in children with CKD: results from the CKiD study. Clin J Am Soc Nephrol 7:1930–1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matteucci MC, Chinali M, Rinelli G, Wuhl E, Zurowska A, Charbit M, Pongiglione G, Schaefer F, Group ET (2013) Change in cardiac geometry and function in CKD children during strict BP control: a randomized study. Clin J Am Soc Nephrol 8:203–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.