Abstract
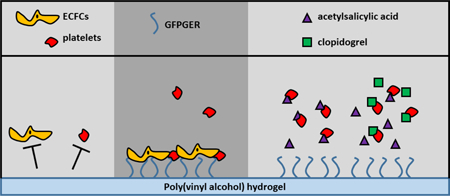
Poly(vinyl alcohol) (PVA) has shown promise as a biomaterial for cardiovascular application. However, its antifouling properties prevent in vivo endothelialization. This work examined the endothelialization and thrombogenicity of modified PVA with different concentrations of proteins and adhesion peptides: collagen, laminin, fibronectin, GFPGER, YIGSR, and cRGD. Material surface properties were quantified, and the endothelialization potential was determined with human endothelial colony forming cells. Additionally, platelet attachment was assessed in vitro with human platelet rich plasma, and promising samples were tested in an ex vivo shunt model. This well-established arteriovenous shunt model was used with and without clinically-relevant antiplatelet therapies, specifically acetylsalicylic acid (ASA) with and without clopidogrel to examine the minimum necessary treatment to prevent thrombosis. Collagen, laminin, and GFPGER biomolecules increased endothelialization, with GFPGER showing the greatest effect at the lowest concentrations. GFPGER-PVA tubes tested under whole blood did exhibit an increase in platelet (but not fibrin) attachment compared to plain PVA and clinical controls. However, application of ASA monotherapy reduced the thrombogenicity of GFPGER-PVA below the clinical control with the ASA. This work is significant in developing cardiovascular biomaterials—increasing endothelialization potential while reducing bleeding side effects by using an antiplatelet monotherapy, typical of clinical patients.
Keywords: Endothelial cell, thrombosis, vascular graft, extracellular matrix, hydrogel
Graphical Abstract
1. Introduction
There is a great clinical need for biosynthetic vascular grafts with the increased prevalence of occlusive arterial disease. Autologous vein grafting is considered superior to synthetic grafting; however, nearly one-third of patients who require bypass procedures lack suitable autologous veins for grafting.[1] The currently available clinical standard synthetic grafts are made from expanded polytetrafluoroethylene (ePTFE) and polyethylene terephthalate (Dacron). These materials are desirable for their antithrombotic and hydrophobic properties; however, they have poor long-term outcomes with the success rate dropping from a primary patency rate of 88% at 12mths to 56% at 24mths post-implant.[2] In a recent retrospective review of femorofemoral grafts comparing ePTFE to autologous or cryopreserved tissues, the autologous femoral veins had superior patency at 1, 2, and 3yrs post-transplant compared to PTFE.[3] Moreover, ePTFE is specifically vulnerable to the loss of patency at diameters less than 6mm, as reviewed previously.[4] Suspected reasons for failure are the mechanical mismatch with native tissues, a lack of endothelium to promote a healthy, blood-contacting surface, thrombosis leading to occlusion, and stenosis of the vessel from intimal hyperplasia.[5–7]
One proposed biomaterial for vascular grafting is poly(vinyl alcohol) (PVA), a synthetic, hydrogelling, nontoxic[8] polymer with widely studied applications. PVA has modifiable mechanical properties, which can more closely resemble the elastic modulus (250–500kPa for PVA[9,10] and 230kPa in the rabbit femoral artery[11]) and compliance (3–7% in PVA[9,10] and 6% in the rabbit femoral artery[11]) of native vasculature, making it suitable for implantation in multiple animal models.[9,10,12] However, PVA does not inherently support in situ endothelialization, which is the clinical goal of this biomaterial.[13] Thus, there is a need to modify the PVA surface to improve endothelialization without affecting material hemocompatibility. Prior work has modified PVA for improved cell attachment, specifically with the incorporation of protein-(fibronectin), peptide-(RGDS and cRGD) and polysaccharide-(heparin) based biochemical cues.[14]
As reviewed previously, the surfaces of cardiovascular biomaterials are frequently modified to improve biointegration and endothelialization.[15] Surface modifiers include antibodies, nucleic acid aptamers, cytokines, and extracellular matrix (ECM) proteins or peptides. ECM proteins of vascular tissue support endothelial cell (EC) growth and functions. Fibronectin is an ECM protein shown to improve EC attachment.[16] Cutiongco et al. showed the incorporation of cRGD, a cell-binding peptide sequence in fibronectin, significantly improved human vascular EC viability on the PVA surface.[14] Similarly, other ECM proteins such as collagen and laminin have also improved EC attachment on hydrogel surfaces.[17–19] Recently, groups have identified peptide sequences that promote EC attachment and proliferation without increasing thrombogenicity. GFPGER, a peptide sequence within collagen type I, is an example that has shown promise in vitro.[20] Similarly, YIGSR, a peptide sequence within laminin, has similar promising results showing improved cellular attachment.[21] We hypothesized that surface modification of PVA with short peptide sequences of the vascular ECM would increase EC growth without compromising graft hemocompatibility.
Clinically, CABG for multivessel coronary artery disease, lower extremity bypass for peripheral vascular disease, and arteriovenous fistulas for hemodialysis are common applications for vascular grafting. It has been shown that acetylsalicylic acid (ASA) therapy after CABG improves vein graft patency and reduces major adverse cardiovascular events such as cardiovascular-related death, myocardial infarction, or stroke.[22,23] ASA has been commonly combined with clopidogrel, labeled dual antiplatelet therapy (DAPT), in the clinical context as it is thought that the combination inhibits platelet aggregation via different mechanisms.[24] Several randomized clinical trials have been unable to reach a consensus regarding graft patency when comparing DAPT and ASA alone.[25,26] However, clinical practice after grafting continues to use DAPT for 6–12mths post-surgery. While some antiplatelet therapy is important for maintaining graft patency, these therapies carry a bleeding risk that increases with multiple drugs.[27,28]
In this study, we modified PVA surfaces with ECM proteins and their corresponding peptide sequences shown to improve cell attachment. Each group - collagen type I, GFPGER, fibronectin, cRGD, laminin, and YIGSR – was examined at multiple concentrations. We characterized material hydrophilicity and cell attachment and proliferation of human endothelial colony forming cells (ECFCs). ECFCs are an outgrowth product of circulating endothelial progenitors, obtained from blood leukopheresis,[29] with potential use in regenerative medicine.[30] This type of cell has been studied extensively and explained in greater detail in the review by Medina et al.[32] Thrombosis on these materials was a particular emphasis of these studies, since some of these ECM proteins and peptides are expected to be prothrombotic. Toward this end, the thrombotic potential was quantified from platelet attachment after incubation with platelet rich plasma (PRP). Samples with promising cell attachment and minimal thrombotic potential were manufactured as tubes and tested in a non-human primate ex vivo shunt model using whole blood. Samples were tested with and without standard clinical antiplatelet therapy (ASA or DAPT) to determine a minimal therapeutic approach for using these samples to promote endothelialization and reduce thrombosis in vivo. We further hypothesized that a minimal level of antiplatelet therapy can be found to negate any increases in platelet attachment seen by the biomaterial modification.
2. Materials and Methods
2.1. Film manufacturing
PVA samples were made as previously described.[31] Autoclaved 10% or 15% aqueous PVA (Sigma) was mixed with sterile-filtered 15% (w v−1) sodium trimetaphosphate (STMP, Sigma) followed by 30% (w v−1) NaOH. The solution was cast into sterile tissue culture-treated well plates at a 1.5:1 volume:surface area ratio (µL mm−2). Films were crosslinked in a sterile, room temperature incubator at 95–99% humidity followed by storage in a biosafety cabinet until films reached a stable mass. Films were sterilely rehydrated in 10X phosphate buffered saline (PBS), rinsed in 1X PBS, and stored in deionized water. To biochemically modify the PVA, collagen I (BD Biosciences), GFPGER (Sigma), fibronectin (Sigma), cyclic RGD (Genscript), laminin (Sigma), or YIGSR (Sigma) was added during the STMP step described above. The amount of additive was based on the final concentration (30, 90, 180, or 360 µg of biomolecule/g PVA).
2.2. Material characterization
Films were rehydrated, rinsed, and re-dried for material characterization. 1µL of deionized water was deposited on the surface of the film and a picture was captured. The static water contact angle was measured by a trained, blinded user with ImageJ (NIH).
2.3. Cell isolation
Human mononuclear cells (MNCs) were isolated using a procedure modified from Taljaard et al.[29] MNCs were obtained from a healthy subject using an Optia leukopheresis platform (Key Biologics). The MNC product processed through a density gradient (Ficoll-Paque Premium, GE Healthcare) and rinsed repeatedly with PBS. After counting, cells were plated at 415,000cells cm−2 in endothelial growth media (EGM-2, Lonza) with 20% human serum (Sigma) and onto human fibronectin (Roche) coated-tissue culture flasks (1.6µg fibronectin cm−2). Cells were incubated at 37°C in 5% CO2 and media exchanged every 2–3days. After 14days, media was removed from the flasks, rinsed with PBS, and incubated with 0.08mL cm−2 TrypLE (Invitrogen) to passage the cells. Cells from 3 flasks were combined onto 1 flask without fibronectin coating. After sufficient outgrowth of ECFCs, cells were sorted using magnetic CD31- Dynabeads (Invitrogen), according to the manufacturer’s protocol. The CD31+ population was grown to confluence and frozen in 50% EGM-2, 40% fetal bovine serum (FBS, Hyclone), and 10% dimethyl sulfoxide. After thawing, the ECFCs were maintained in complete endothelial growth medium with 10% FBS and used at P4 or P5 for seeding experiments.
2.4. Cell seeding and quantification
Rehydrated films were incubated with FBS for 1hr at 37°C. Human ECFCs were seeded onto the PVA films at a density of 200cells mm−2 in 10% FBS EGM-2. After 48hrs, films were rinsed and frozen dry to promote cell lysing. To quantify cell attachment, the cells were lysed with 0.02% sodium dodecyl sulphate in sodium citrate buffer (pH=7.0). The DNA was quantified from the lysate using the PicoGreen assay™ (Invitrogen), according to the manufacturer’s instructions. Attachment was calculated as a percent coverage relative to positive controls of cell-matched tissue culture treated plastic wells.
2.5. Fluorescent staining
After 48hrs of cell incubation, samples were stained using standard immunofluorescence procedures, as described previously.[33] Briefly, films were fixed and stained with Alexa Fluor ® 568 phalloidin, a primary VE-Cadherin antibody and secondary IgG1 Alexa-488, and DAPI. Films were mounted onto glass slides and imaged with a Nikon Ellipse TE-200U fluorescent microscope.
2.6. Platelet adhesion quantification
Static platelet adhesion was quantified with a method modified from Yim et al.[34] Blood was obtained from a healthy, human donor in 3.8% sodium citrate and centrifuged at 100G for 15min to isolate the PRP. PRP was incubated on PVA on a shaker at 60rpm for 1hr at 37°C. Samples were rinsed to remove non-attached platelets and incubated with 1% Triton-X for 1hr at 37°C to lyse the attached platelets. The lysate was mixed with the assay reaction for 30min, mixed with the stop solution, and quantified with light absorbance according to the lactate dehydrogenase (LDH) assay (ThermoFisher) instructions.
2.7. Whole blood platelet and fibrin attachment quantification
PVA tubes (inner diameter=4mm) were manufactured, assembled, and tested as described previously.[14] Briefly, PVA tubes with or without 30µg GFPGER peptide/g PVA were dip cast into tubes. Rehydrated tubes were connected to silicone tubing that extended from a chronic, femoral arteriovenous baboon shunt (Figure 1). Platelets were labelled with 111In and fibrinogen was labelled with 125I. Whole blood flowed through the tubes at 100mL min−1, and platelet attachment was dynamically quantified from the 111In signal on a Brivo NM615 nuclear imaging camera (GE) for 60min. End point fibrin was quantified from the 125I signal on a 1480 Wizard Gamma Counter (PerkinElmer, Waltham, MA). Clinical grade ePTFE grafts (inner diameter=4mm) were tested as a clinical control, and collagen-coated ePTFE samples (1mg mL−1 equine collagen type I, Chrono-log Corp.) were used as a positive control. Samples were tested without antiplatelet therapies, with ASA monotherapy, or with DAPT (ASA and clopidogrel). For ASA and DAPT trials, ASA (Bayer Healthcare) was given orally at 10 mg kg−1 at least 4hrs before the start of each experiment. For DAPT trials, clopidogrel (Torrent Pharmaceuticals) was also given twice orally at 2mg kg−1 on the afternoon before and the morning of each study. Light transmission platelet aggregometry was used to confirm the antiplatelet response of the animal to each drug condition at least once per condition using a Model 440 Dual Aggregometer (Chrono-Log Corporation), as described previously.[35] Aggregometry was not used to assess platelet reactivity to the biomaterials.
Figure 1. Ex vivo shunt model set up.
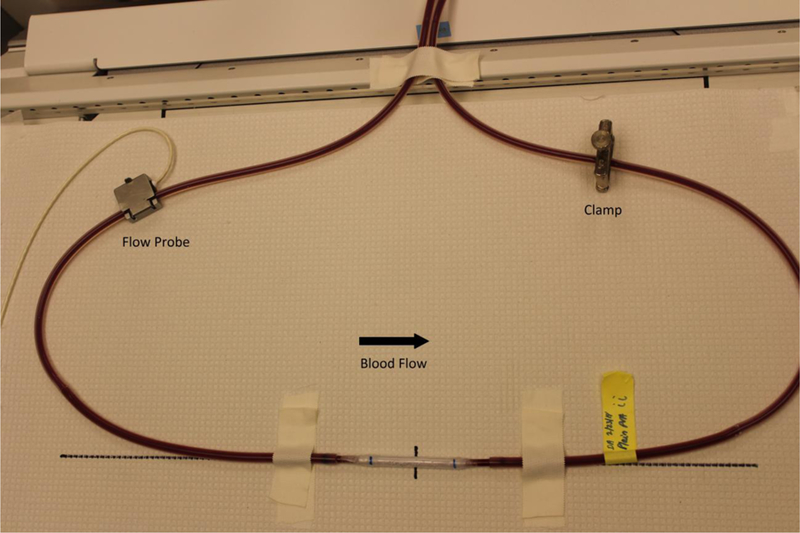
Graft samples were placed in an ex vivo shunt loop and exposed to whole, flowing blood for 60min. The arrow in the image indicates the direction of blood flow. A flow probe was included upstream to measure flow rate and a clamp was applied downstream to control the flow at a constant 100mL/min. Samples were tested without any antiplatelet therapies, with ASA monotherapy, or with DAPT.
Male baboons (papio anubus) were housed and cared for by the Oregon National Primate Research Center staff according to the National Institutes of Health ‘‘Guide to the Care and Use of Laboratory Animals’’ by the Committee on Care & Use of Laboratory Animals of the Institute of Laboratory Animal Resources, National Research Council (NIH Publications No. 8023, revised 1978) and as approved by the Oregon National Primate Research Center Institutional Animal Care and Use Committee (IP00000300).
2.8. Statistics
All statistics were calculated using R (R Foundation for Statistical Computing, Vienna, Austria, version 3.5.1). Data are presented as mean ± standard deviation. All statistical analyses were considered significant when p<0.05. Water contact angle data were analyzed with a 1-way analysis of variance (ANOVA, n=2–6 per group, specifically, n=2 for L180 and Y180, n=3 for C30, F180, and F90, n=5 for C90, G180, G30, and cRGD30, n=6 for C180, 0, and R90, and n=4 for the rest of the samples). ECFC attachment (n≥6 per group) was analyzed with a 2-way ANOVA within each ECM family (collagen- GFPGER, laminin-YIGSR, or fibronectin-cRGD). Factors were biomolecule and concentration. Static platelet attachment data were analyzed with a 1-way ANOVA and Dunnett’s post hoc with plain PVA as the reference group.
Platelet and fibrin data from the ex vivo shunt studies were natural log-transformed in order to approximate a normal distribution. Platelet deposition data were analyzed using multi-way within-drug- groups linear mixed effects models (LME, package nlme version 3.1–137). LMEs included fixed effect coefficients for device material, time, time × ln(time), interactions, and a random effect term for trial ID. The time × ln(time) term and its interactions are included to more closely fit observed data. Post-hoc contrasts were manually constructed and p-values were corrected using Tukey contrasts (package multcomp version 1.4–8). Fibrin accumulation was analyzed using within-drug-group 1-way ANOVAs against surface modification with Tukey’s post-hoc tests when relevant. While the collagen-coated ePTFE controls used to confirm expected reactivity are displayed in figures as references, collagen was not included in statistical models due to low sample size (n=2 without drugs, n=1 with ASA or DAPT).
3. Results
3.1. Material characterization
Water contact angle data are shown in Table 1. While no significant differences were observed, there was a trend toward decreased hydrophilicity for the collagen samples and the lower concentrations of laminin- and fibronectin-modified PVA.
Table 1.
Hydrophilicity of biomolecule-modified PVA was unchanged
| Collagen | GFPGER | Laminin | YIGSR | Fibronectin | cRGD | |
|---|---|---|---|---|---|---|
| 0 µg/g PVA | 45±6 | |||||
| 30 µg/g PVA | 54±9 | 43±6 | 55±13 | 41±6 | 53±15 | 44±11 |
| 90 µg/g PVA | 54±9 | 44±9 | 48±10 | 44±17 | 53±5 | 42±11 |
| 180 µg/g PVA | 55±9 | 49±6 | 33±1 | 50±6 | 58±10 | 49±10 |
| 360 µg/g PVA | 53±12 | 46±9 | 51±9 | 43±8 | 48±7 | |
Water contact angle of PVA films demonstrated no significant differences between the groups compared to the plain PVA control (ANOVA F23,75=1.311, p=0.189).
3.2. Cell-material interactions
Picogreen quantification (Figure 2) and fluorescent staining (Figure 3) showed similar results with GFPGER and laminin modifications increasing ECFC attachment. Collagen modification also increased cell attachment at the highest concentration, while GFPGER-containing samples were confluent at all concentrations. Laminin samples in particular showed increasing cell attachment with increasing concentration; however, it was cost prohibitive to find the saturation point for this molecule. GFPGER had significantly higher cell quantity than collagen (p<0.0001), and laminin was significantly greater than YIGSR (p=0.0006). Fibronectin, cRGD, and YIGSR had little cell attachment, and comparing fibronectin to cRGD showed no significant difference (p=0.083).
Figure 2. ECFC adhesion increases on biomolecule-modified PVA.
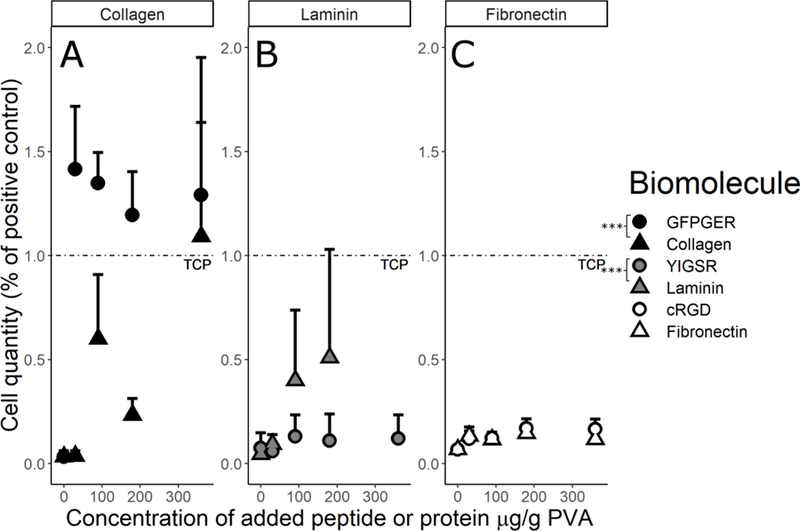
ECFC quantification after 48hrs on PVA films for the (A) collagen, (B) laminin, and (C) fibronectin families demonstrated that cell attachment increased on the biomolecule-modified PVA compared to the plain PVA. Data were analyzed with a 2-way ANOVA to compare each peptide to its corresponding protein. Factors were biomolecule and concentration. GFPGER showed a significant increase (as indicated by * in the figure) in ECFC binding compared to collagen modification (ANOVA F1,56=27.04, p=2.91×10−6), and laminin showed a significant increase from YIGSR (ANOVA F1,75=12.84, p=0.0006). Fibronectin and cRGD were not significantly different (ANOVA F1,88=3.07, p=0.0831). As a factor, increasing concentration significantly increased cell attachment in the collagen (ANOVA F1,56=22.29, p=1.61×10−5) and fibronectin (F1,88=13.04, p=0.0005) families, but not in the laminin family (F1,75=1.51, p=0.224).
Figure 3. ECFCs formed confluent monolayers on GFPGER-, collagen-, and laminin-modified PVA.
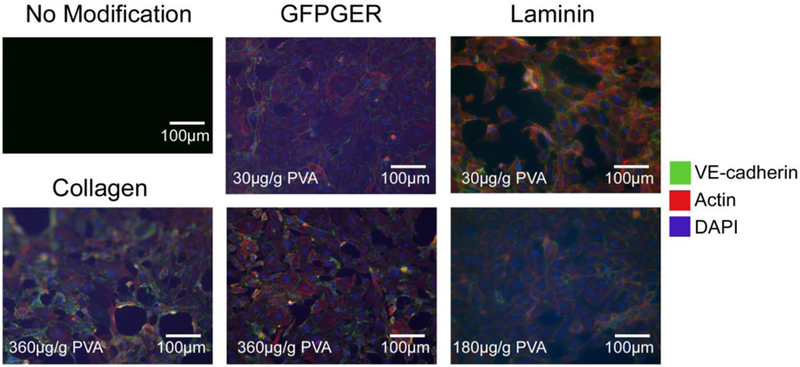
Representative staining of human ECFCs on PVA films after 48hrs. While these samples showed islands of confluent monolayers, staining supported the quantification data of cell attachment. The addition of the GFPGER peptide sequence showed a near confluent EC monolayer even at the lowest concentration.
3.3. Material thrombosis potential
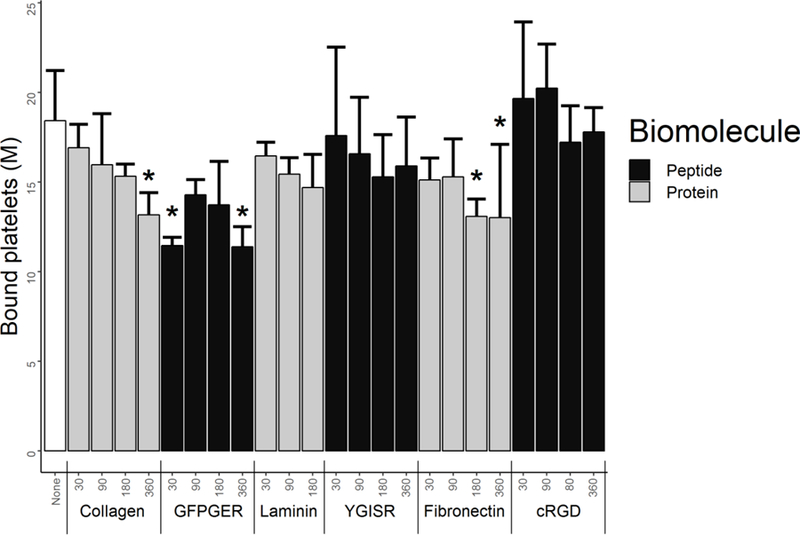
Static platelet attachment quantification (Figure 4) on PVA films showed either statistically equivalent or lower platelet binding to the modified PVA compared to plain PVA. Collagen at 360µg/g PVA, GFPGER at 30µg/g PVA and 360µg/g PVA, and fibronectin at 180µg/g PVA and 360µg/g PVA all demonstrated significantly lower platelet attachment in vitro (p<0.05). Base platelet count of the PRP was calculated as 848M/mL.
Figure 4. Biomolecule-modified PVA films had the same or decreased static platelet attachment.
Quantification of human platelets bound to PVA films using LDH assay after 1hr of static incubation. Static platelet attachment was statistically unchanged or decreased from the plain PVA according to this assay. * indicates statistical significance from plain PVA (ANOVA Dunnett’s t-test post hoc F23,72=3.775 p=8.3×10−6, n=4 per group).
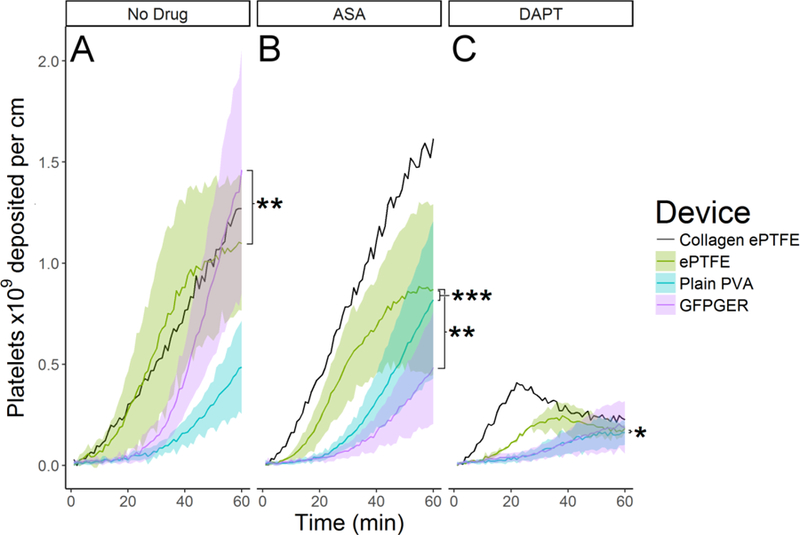
During ex vivo testing without antiplatelet therapies, GFPGER-PVA accumulated significantly more platelets than ePTFE grafts (Figure 5A, p=0.0035). However under ASA monotherapy, GFPGER and plain PVA devices accumulated significantly fewer platelets than ePTFE (Figure 5B, p=0.001, p<0.001 respectively). While platelet deposition was minimal across all devices under DAPT, a significant reduction in platelet deposition was seen on plain PVA relative to ePTFE (Figure 5C, p=0.031).
Figure 5. Ex vivo platelet adhesion increase on GFPGER-PVA was reversed with platelet monotherapy.
Tubes of PVA with or without 30µg GFPGER/g PVA were tested in an ex vivo shunt model. Platelet accumulation was quantified over 60min exposure to whole blood (n=2 for collagen-ePTFE and N=5–6 for other groups). (A) In the absence of antiplatelet drugs, ePTFE devices accumulated significantly more platelets than GFPGER devices (LME ANOVA F2, 11=12.755 p=0.001, post hoc: ePTFE vs. plain PVA, p=1.0, ePTFE vs. GFPGER p=0.0035, plain PVA vs. GFPGER p=0.0834). (B) Under ASA monotherapy, ePTFE devices accumulated significantly more platelets than either PVA device. (LME ANOVA F2,12=13.86 p=8×10−4, post hoc: ePTFE vs. plain PVA p=0.0008, ePTFE vs. GFPGER p=0.0013, plain PVA vs. GFPGER p=1.0). (C) Under DAPT, plain PVA devices accumulated significantly fewer platelets than ePTFE devices (RMA F2,11=3.24 p=0.079, post hoc: ePTFE vs. plain PVA p=0.0310, ePTFE vs. GFPGER p=0.261, plain PVA vs. GFPGER p=0.681).
Significantly more fibrin was seen on ePTFE devices than plain PVA grafts (Figure 6A, p=0.017) in the absence of antiplatelet therapy. Under ASA monotherapy, there were no significant differences (Figure 6B). Under DAPT, ePTFE showed significantly greater fibrin accumulation than either plain PVA or GFPGER-PVA (Figure 6C, p=0.044, p=0.019 respectively). Blood analysis quantified before and after shunt studies yielded an average platelet count of 332±65M/mL and hematocrit of 35%±2%.
Figure 6. Ex vivo fibrin accumulation was decrease for PVA compared to ePTFE.
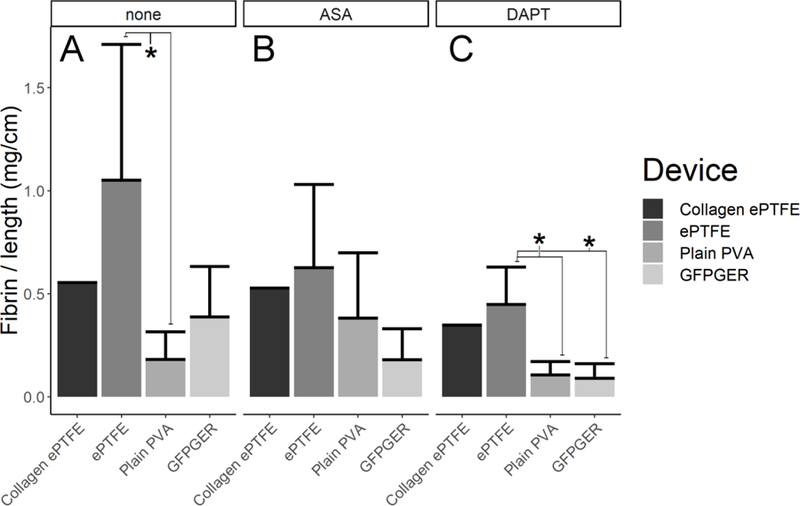
Fibrin data were quantified and normalized per centimeter of axial length for all grafts at 60min. Results showed that (A) without antiplatelet therapy, ePTFE devices accumulated more fibrin than either PVA device (ANOVA F2, 11=5.57, p=0.02, post hoc: ePTFE vs. plain PVA, p=0.0168*, ePTFE vs. GFPGER p=0.180, plain PVA vs. GFPGER p=0.235). (B) No statistically significant differences were seen in fibrin deposition under ASA monotherapy (ANOVA F2,12=3.18, p=0.078). (C) ePTFE devices accumulated significantly more fibrin than either PVA device under DAPT (ANOVA F2,11=5.449, p=0.0227, post hoc: ePTFE vs. plain PVA, p=0.0444*, ePTFE vs. GFPGER p=0.019*, plain PVA vs. GFPGER p=0.768).
4. Discussion
Generating an off-the-shelf vascular graft capable of in situ endothelialization remains an unachieved goal of cardiovascular translational research. Promoting EC attachment frequently means modification of a biomaterial surface to encourage protein adsorption—enabling integrin attachment and EC spreading on a surface. Unfortunately, many of the modifications which promote this attachment also promote platelet attachment. Furthermore, the attachment and activation of platelets on biomaterial surfaces occurs more rapidly than the attachment of circulating ECs or migrating ECs from the native tissue.[36] The development of a synthetic material which can support an endothelium without first promoting thrombosis would be a substantial contribution for cardiovascular devices, particularly small- diameter vascular grafts. Despite the many years of research by multiple groups toward this goal, patients continue to see synthetic vascular graft technology that was developed decades ago. This lack of new clinical options suggests that a paradigm shift for preclinical research of artificial materials is necessary. The work presented here demonstrates a large survey of ECM proteins and peptides at different concentrations to find the best approach for promoting EC adhesion. It further strives to find an appropriate antiplatelet regimen, which prevents platelet adhesion while also minimizing bleeding risk.[27,28,37,38] Specifically, these studies support the use of ASA monotherapy, which would reduce the risk of bleeding side effects, rather than the more typical DAPT used after clinical grafting procedures.
This work modified PVA hydrogel films with collagen type I, fibronectin, and laminin proteins, as well as their respective cell adhesion peptides, GFPGER, cRGD, and YIGSR, to systematically study ECM components at several concentrations. These modifications were selected to examine the various ECM groups relevant to vascular biology at both the protein and peptide level. Concentrations were selected to explore a broad range in hopes of finding a threshold of endothelialization benefit which was not examined in initial, previous work.[14] This study did not quantify changes in mechanical properties of the PVA with additives, because previous work did not see significant differences except with very large concentrations of gelatin.[13] Qualitatively, no changes were observed in the mechanical integrity or physical characteristics of the films or tubes when the biomolecules were added to the test materials. Prior study of the mechanical properties of PVA have shown that large differences occur based on the environmental conditions during cross-linking, which were kept constant for all groups in these experiments.[9]
Previous work showed variable results for contact angle when adding molecules to PVA. One study demonstrated a significant decrease in hydrophilicity when additives of cRGD, fibronectin, or heparin were added to PVA,[14] while another study showed a significant increase in hydrophilicity with large concentrations of gelatin.[13] Overall, contact angles ranged from 40° to 60° with the addition of biomolecules, which reflects the results obtained here. Characterizing the PVA after modification with the biomolecules used in this study showed no significant difference in water contact angle suggesting that changes in cell attachment were not strictly a surface energy protein attachment phenomenon, but more likely due to direct protein or peptide binding. Surface roughness can also influence serum protein adsorption and cellular attachment, but previous work on cRGD and fibronectin with this same manufacturing technique did not observe any physical changes in the PVA surface, so it in unlikely that the surface roughness was changed in the current studies.
ECFC attachment was substantially increased on GFPGER peptide-modified PVA resulting in a confluent monolayer at the lowest GFPGER concentration. ECFC attachment also increased on laminin and collagen with increasing concentrations of these proteins; however, a confluent layer of cells was only observed at the highest concentration of collagen. Previous work found that GFPGER and collagen both bind to the integrin α1 and α2 domains on ECs resulting in similar attachment.[20] Our work reflected GFPGER supporting EC attachment, and supported the hypothesis that the peptide would improve EC binding compared to the whole protein. This was expected and likely due to the increased concentration of sites available for binding on the hydrogel surface. The density of available binding sites for the same quantity of peptide is greater than the density for the same quantity of protein, allowing for greater cell binding. Cell attachment was nearly unchanged for fibronectin, YIGSR, and cRGD, which did not reflect our hypothesis that peptides would universally improve cell attachment compared to their native protein family. This is consistent with prior work showing no significant difference between cell attachment on fibronectin and cRGD modified PVA at 28µg/g PVA, which is near the lowest concentration used for this work.[14] While all these biomolecules would be expected to promote cell adhesion based on previous work, which incorporated peptides and proteins into hydrogels using more complicated binding,[16,18] the process of mixing these biomolecules into PVA likely resulted in the sequestration of their binding domains, preventing interaction with EC integrins. Overall, the unaltered hydrophilicity and the specific attachment of cells with some biomolecules, suggest that cell attachment was related to the specific ECM molecule used. Given the data and the cost advantage of GFPGER over the very expensive laminin protein or even collagen type I, GFPGER was used in the manufacturing of PVA tubes for whole blood thrombosis testing.
The lack of cell attachment on fibronectin, YIGSR, or RGD groups suggests that future work may benefit from manufacturing techniques beyond simple mixing. While confocal imaging of fluorescently-labeled collagen samples (data not shown) indicated that the manufacturing technique used here lead to uniform distribution of the biomolecules, exploration of specific binding chemistries may present a more stable approach for biomolecule attachment. Previous work with antibody-cell binding showed substantial improvements in EC attachment under flow when antibodies were oriented to present their binding domain away from a surface.[39] Similar exploration to orient protein binding domains on a surface may result in better cell attachment with less protein needed.
While numerous researchers have explored the use of ECM proteins or peptides to modify cardiovascular biomaterials for endothelialization, often limited attention is given to the effects that these modifications have on coagulation. Researchers frequently perform benchtop studies on PRP in static conditions. To provide a comparable metric, the work presented here also used a static, PRP assay. This LDH assay showed no increase in platelet adhesion, even on molecules which would typically support platelet attachment. Seo et al.[20] found that collagen I-coated wells would increase platelet attachment while the GFPGER coating did not when tested with light transmission platelet aggregometry using PRP. This may be attributed to the difference between static platelet adhesion and platelet attachment under flow.[40,41] To more thoroughly assess platelet adhesion, we used a well- established, ex vivo, non-human primate, arteriovenous shunt model to examine thrombosis using whole, flowing blood pumped entirely by the animal’s heart. Data collected from these experiments did not reflect what was seen in the static LDH assay. Rather, a significant increase in platelet attachment was seen with the addition of GFPGER to the PVA tubes. This suggests that this static platelet adhesion assay may not be a good representation of the translatability of cardiovascular biomaterials to a clinical setting.
In the whole blood ex vivo model without antiplatelet drugs, the plain PVA trended to the lowest platelet accumulation, supporting previous work that PVA has potential as a non-thrombogenic cardiovascular biomaterial.[14,33] An increase in platelet adhesion was seen for GFPGER-modified PVA compared to ePTFE, yet ePTFE had significantly more fibrin deposition when no antiplatelet drugs were used. To study the translatability of PVA with the endothelialization-promoting GFPGER modification, we tested the thrombogenicity of this material using clinically relevant antiplatelet therapies: ASA or DAPT. Both PVA and GFPGER-PVA had significantly decreased platelet attachment compared to ePTFE with ASA alone. Furthermore, the ePTFE showed little change in platelet attachment between the conditions of with and without ASA monotherapy. This suggests that the thrombogenicity of the GFPGER may be linked to the anti-platelet mechanism of ASA (via cyclooxygenase (COX)-1 and COX-2 inhibition) and is sufficient for platelet inhibition. All samples had decreased platelet attachment due to DAPT, although platelet attachment to plain PVA was still significantly decreased from attachment to ePTFE. However, DAPT has been shown to significantly increase the risk of major bleeding events compared to ASA alone, particularly in CABG.[37,38] Overall, this suggests that the GFPGER-PVA may promote endothelialization without increasing platelet attachment through antiplatelet monotherapy, ASA, a typical lifetime requirement of a heart disease patient.
Supporting an endothelium without increasing thrombosis may give substantially longer patency rates than current, clinical cardiovascular biomaterials, which have limited endothelialization potential.[42] Furthermore, eliminating clopidogrel from post-implant care, while maintaining graft patency, can reduce bleeding incidents. The ability to have ASA-only follow-up care would also eliminate the unpredictability of variable patient responses to clopidogrel, owing to common polymorphisms affecting the activity of cytochrome P450 CYP2C19.[43] While ASA is a typical treatment for heart disease patients and post-implant care, previous work has found that ASA can limit the mobilization of circulating endothelial cells.[44] While it is difficult to envision a post-surgical plan that does not include anticoagulant therapy, it is important to consider the typical drug load of a cardiovascular surgical patient when performing biointegration research. Future work will explore the in vivo response of PVA in our non-human primate model using implanted bilateral aortoiliac bypass grafts[45,46], as initial success has been shown in small animal models with an end-to-end implant of 2mm, 1mm, and even submillimeter inner diameters.[9,10]
Ultimately, this research is a significant step toward the development of a synthetic vascular graft that will be a suitable alternative to autologous vein grafting. The work presented here supported the hypothesis that a limited antiplatelet regimen could be used to prevent platelet adhesion on a biomaterial that was modified to support endothelialization. While it is important to study thrombosis in the absence of antiplatelet therapy to observe the clotting phenomenon,[14,45,47] translational work should also include examination of clinically relevant antiplatelet therapies to provide the most useful information for bench-to-bedside preclinical research.
Statement of Significance.
We modified the endothelialization potential of synthetic, hydrogel vascular grafts with proteins and peptides of the vascular tissue matrix. Cell attachment was dramatically increased with the GFPGER peptide, and while some additional platelet attachment was seen under flow with whole blood, this was completely knocked down using clinical antiplatelet monotherapy. This indicates that long-term patency of this biomaterial could be improved without the associated bleeding risk of multiple platelet therapies.
Acknowledgements
This work was supported by the NIH [grant numbers R01HL130274; R01HL144113; R01DE026170]. The authors are grateful for the critical technical assistance of Mses. Grace Pohan and Yuan Yao in PVA manufacturing. We thank the staff at ONPRC and the technical assistance of Ms. Jennifer Johnson and Ms. Tiffany Burch. We would like to thank Dr. Patrick Jurney and Ms. Novella Bates for their help in collecting contact angle data. We appreciate W.L. Gore for supplying ePTFE graft material for scientific study. We also thank Dr. David Courtman and Ms. Ashley Rammeloo for their critical help in developing a protocol for human ECFC isolations. We appreciate the help of Ms. Anh Ngo in collecting PRP.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
Authors have no conflicts of interest to disclose.
References
- [1].Sayers RD, Raptis S, Berce M, Miller JH, Long-term results of femorotibial bypass with vein or polytetrafluoroethylene., Br. J. Surg 85 (1998) 934–8. doi: 10.1046/j.1365-2168.1998.00765.x. [DOI] [PubMed] [Google Scholar]
- [2].Uzun A, Diken AI, Yalçinkaya A, Hanedan O, Ciçek ÖF, Lafçi G, Altintaş G, Cağli K, Long-term patency of autogenous saphenous veins vs. PTFE interposition graft for prosthetic hemodialysis access, Anadolu Kardiyol. Dergisi/The Anatol. J. Cardiol 14 (2014) 542–546. doi: 10.5152/akd.2014.4910. [DOI] [PubMed] [Google Scholar]
- [3].Nguyen KP, Moneta G, Landry G, Venous Conduits Have Superior Patency Compared with Prosthetic Grafts for Femorofemoral Bypass, Ann. Vasc. Surg (2018). doi: 10.1016/j.avsg.2018.03.024. [DOI] [PubMed]
- [4].Pashneh-Tala S, MacNeil S, Claeyssens F, The Tissue-Engineered Vascular Graft—Past, Present, and Future, Tissue Eng. Part B Rev (2015) 10.1089/ten.teb.2015.0100. [DOI] [PMC free article] [PubMed]
- [5].Sarkar S, Salacinski HJ, Hamilton G, Seifalian AM, The Mechanical Properties of Infrainguinal Vascular Bypass Grafts: Their Role in Influencing Patency, Eur. J. Vasc. Endovasc. Surg 31 (2006) 627–636. doi: 10.1016/j.ejvs.2006.01.006. [DOI] [PubMed] [Google Scholar]
- [6].Braune S, Sperling C, Maitz MFF, Steinseifer U, Clauser J, Hiebl B, Krajewski S, Wendel HPP, Jung F, Evaluation of platelet adhesion and activation on polymers: Round-robin study to assess inter-center variability, 158 (2017) 416–422. doi: 10.1016/j.colsurfb.2017.06.053. [DOI] [PubMed] [Google Scholar]
- [7].de Mel A, Jell G, Stevens MM, Seifalian AM, Biofunctionalization of biomaterials for accelerated in situ endothelialization: a review, Biomacromolecules 9 (2008) 2969–2979. doi: 10.1021/bm800681k. [DOI] [PubMed] [Google Scholar]
- [8].DeMerlis CC, Schoneker DR, Review of the oral toxicity of polyvinyl alcohol (PVA)., Food Chem. Toxicol 41 (2003) 319–26. http://www.ncbi.nlm.nih.gov/pubmed/12504164 (accessed December 6, 2018). [DOI] [PubMed] [Google Scholar]
- [9].Cutiongco MFA, Kukumberg M, Peneyra JL, Yeo MS, Yao JY, Rufaihah AJ, Le Visage C, Ho JP, Yim EKF, Submillimeter Diameter Poly(Vinyl Alcohol) Vascular Graft Patency in Rabbit Model, Front. Bioeng. Biotechnol 4 (2016) 44. doi: 10.3389/fbioe.2016.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Cutiongco MFA, Goh SH, Aid-Launais R, Le Visage C, Low HY, Yim EKF, Planar and Tubular Patterning of Micro and Nano-topographies on Poly(vinyl alcohol) Hydrogel for Improved Endothelial Cell Responses, Biomaterials (2016). doi: 10.1016/j.biomaterials.2016.01.036. [DOI] [PubMed]
- [11].Uchida N, Emoto H, Kambic H, Harasaki H, Chen JF, Hsu SH, Murabayashi S, Nosé Y, Compliance effect on patency of small diameter vascular grafts., ASAIO Trans 35 (1989) 556–558. http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=2597533&retmode=ref&cmd=prlinks. [DOI] [PubMed] [Google Scholar]
- [12].Chaouat M, Le Visage C, Baille WE, Escoubet B, Chaubet F, Mateescu MA, Letourneur D, A Novel Cross-linked Poly(vinyl alcohol) (PVA) for Vascular Grafts, Adv. Funct. Mater 18 (2008) 2855–2861. doi: 10.1002/adfm.200701261. [DOI] [Google Scholar]
- [13].Ino JM, Sju E, Ollivier V, Yim EKF, Letourneur D, Le Visage C, Evaluation of hemocompatibility and endothelialization of hybrid poly(vinyl alcohol) (PVA)/gelatin polymer films., J. Biomed. Mater. Res. B. Appl. Biomater (2013). doi: 10.1002/jbm.b.32977. [DOI] [PubMed]
- [14].Cutiongco MFA, Anderson DEJ, Hinds MT, Yim EKF, In vitro and ex vivo hemocompatibility of off-the-shelf modified poly(vinyl alcohol) vascular grafts., Acta Biomater 25 (2015) 97–108. doi: 10.1016/j.actbio.2015.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Liu T, Liu S, Zhang K, Chen J, Huang N, Endothelialization of implanted cardiovascular biomaterial surfaces: The development from in vitro to in vivo, J. Biomed. Mater. Res. Part A 102 (2014) 3754–3772. doi: 10.1002/jbm.a.35025. [DOI] [PubMed] [Google Scholar]
- [16].Nuttelman CR, Mortisen DJ, Henry SM, Anseth KS, Attachment of fibronectin to poly(vinyl alcohol) hydrogels promotes NIH3T3 cell adhesion, proliferation, and migration., J. Biomed. Mater. Res 57 (2001) 217–23. http://www.ncbi.nlm.nih.gov/pubmed/11484184 (accessed August 6, 2018). [DOI] [PubMed] [Google Scholar]
- [17].Kuo K-C, Lin R-Z, Tien H-W, Wu P-Y, Li Y-C, Melero-Martin JM, Chen Y-C, Bioengineering vascularized tissue constructs using an injectable cell-laden enzymatically crosslinked collagen hydrogel derived from dermal extracellular matrix., Acta Biomater 27 (2015) 151–166. doi: 10.1016/j.actbio.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ali S, Saik JE, Gould DJ, Dickinson ME, West JL, Immobilization of Cell-Adhesive Laminin Peptides in Degradable PEGDA Hydrogels Influences Endothelial Cell Tubulogenesis, 2 (2013) 241–249. doi: 10.1089/biores.2013.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Schnittler HJ, Franke RP, Akbay U, Mrowietz C, Drenckhahn D, Improved in vitro rheological system for studying the effect of fluid shear stress on cultured cells., Am. J. Physiol 265 (1993) C289–98. doi: 10.1152/ajpcell.1993.265.1.C289. [DOI] [PubMed] [Google Scholar]
- [20].Seo N, Russell BH, Rivera JJ, Liang X, Xu X, Afshar-Kharghan V, Höök M, An Engineered α1 Integrin-binding Collagenous Sequence, J. Biol. Chem 285 (2010) 31046–31054. doi: 10.1074/jbc.M110.151357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Jung JP, Moyano JV, Collier JH, Multifactorial optimization of endothelial cell growth using modular synthetic extracellular matrices, Integr. Biol 3 (2011) 185–96. doi: 10.1039/c0ib00112k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Johnson WD, Kayser KL, Hartz AJ, Saedi SF, Aspirin use and survival after coronary bypass surgery., Am. Heart J 123 (1992) 603–8. http://www.ncbi.nlm.nih.gov/pubmed/1539511 (accessed August 6, 2018). [DOI] [PubMed] [Google Scholar]
- [23].Goldman S, Copeland J, Moritz T, Henderson W, Zadina K, Ovitt T, Doherty J, Read R, Chesler E, Sako Y, Improvement in early saphenous vein graft patency after coronary artery bypass surgery with antiplatelet therapy: results of a Veterans Administration Cooperative Study., Circulation 77 (1988) 1324–32. http://www.ncbi.nlm.nih.gov/pubmed/3286040 (accessed August 6, 2018). [DOI] [PubMed] [Google Scholar]
- [24].Gurbuz AT, Zia AA, Vuran AC, Cui H, Aytac A, Postoperative clopidogrel improves mid-term outcome after off-pump coronary artery bypass graft surgery: a prospective study., Eur. J. Cardiothorac. Surg 29 (2006) 190–5. doi: 10.1016/j.ejcts.2005.11.033. [DOI] [PubMed] [Google Scholar]
- [25].Gao C, Ren C, Li D, Li L, Clopidogrel and aspirin versus clopidogrel alone on graft patency after coronary artery bypass grafting., Ann. Thorac. Surg 88 (2009) 59–62. doi: 10.1016/j.athoracsur.2009.04.024. [DOI] [PubMed] [Google Scholar]
- [26].Kulik A, Le May MR, Voisine P, Tardif J-C, Delarochelliere R, Naidoo S, Wells GA, Mesana TG, Ruel M, Aspirin plus clopidogrel versus aspirin alone after coronary artery bypass grafting: the clopidogrel after surgery for coronary artery disease (CASCADE) Trial., Circulation 122 (2010) 2680–7. doi: 10.1161/CIRCULATIONAHA.110.978007. [DOI] [PubMed] [Google Scholar]
- [27].Hansen ML, Sørensen R, Clausen MT, Fog-Petersen ML, Raunsø J, Gadsbøll N, Gislason GH, Folke F, Andersen SS, Schramm TK, Abildstrøm SZ, Poulsen HE, Køber L, Torp-Pedersen C, Risk of Bleeding With Single, Dual, or Triple Therapy With Warfarin, Aspirin, and Clopidogrel in Patients With Atrial Fibrillation, Arch. Intern. Med 170 (2010) 1433–41. doi: 10.1001/archinternmed.2010.271. [DOI] [PubMed] [Google Scholar]
- [28].Pickard AS, Becker RC, Schumock GT, Frye CB, Clopidogrel-Associated Bleeding and Related Complications in Patients Undergoing Coronary Artery Bypass Grafting, Pharmacotherapy 28 (2008) 376–392. doi: 10.1592/phco.28.3.376. [DOI] [PubMed] [Google Scholar]
- [29].Taljaard M, Ward MR, Kutryk MJB, Courtman DW, Camack NJ, Goodman SG, Parker TG, Dick AJ, Galipeau J, Stewart DJ, Rationale and design of Enhanced Angiogenic Cell Therapy in Acute Myocardial Infarction (ENACT-AMI): The first randomized placebo-controlled trial of enhanced progenitor cell therapy for acute myocardial infarction, Am. Heart J 159 (2010) 354–360. doi: 10.1016/j.ahj.2009.12.021. [DOI] [PubMed] [Google Scholar]
- [30].Glynn JJ, Hinds MT, Endothelial Outgrowth Cells: Function and Performance in Vascular Grafts, Tissue Eng Part B Rev (2013). [DOI] [PMC free article] [PubMed]
- [31].Cutiongco MFA, Choo RKT, Shen NJXN, Chua BBMX, Sju E, Choo AAWL, Le Visage C, Yim EKF, Composite scaffold of poly(vinyl alcohol) and interfacial polyelectrolyte complexation fibers for controlled biomolecule delivery., Front. Bioeng. Biotechnol 3 (2015) 3. doi: 10.3389/fbioe.2015.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Medina RJ, Barber CL, Sabatier F, Dignat-George F, Melero-Martin JM, Khosrotehrani K, Ohneda O, Randi AM, Chan JKY, Yamaguchi T, Van Hinsbergh VWM, Yoder MC, Stitt AW, Endothelial Progenitors: A Consensus Statement on Nomenclature, Stem Cells Transl. Med 6 (2017) 1316–1320. doi: 10.1002/sctm.16-0360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Jurney PL, Anderson DE, Pohan G, Yim EK, Hinds MT, Reactive Ion Plasma Modification of Poly(Vinyl-Alcohol) Increases Primary Endothelial Cell Affinity and Reduces Thrombogenicity, Macromol. Biosci In Press (2018). [DOI] [PMC free article] [PubMed]
- [34].Yim EKF, Liao I, Leong KW, Tissue Compatibility of Interfacial Polyelectrolyte Complexation Fibrous Scaffold: Evaluation of Blood Compatibility and Biocompatibility, Tissue Eng 13 (2007) 423–433. doi: 10.1089/ten.2006.0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Hagen MW, Girdhar G, Wainwright J, Hinds MT, Thrombogenicity of flow diverters in an ex vivo shunt model: effect of phosphorylcholine surface modification., J. Neurointerv. Surg 9 (2017) 1006–1011. doi: 10.1136/neurintsurg-2016-012612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Ratner JE, B.D., Hoffman AS, Schoen FJ, and Lemons, Biomaterials Science: An Introduction to Materials in Medicine - Ratner Buddy D., Hoffman Allan S., Schoen Frederick J., Lemons Jack E. - Google Books, 2nd ed., Elsevier Academic Press, San Diego, CA, 2004. https://books.google.com/books?hl=en&lr=&id=9PMU1iYGe34C&oi=fnd&pg=PR5&dq=biomaterials+science+ratner&ots=gnUarcnAEy&sig=ogbXZTOLD03kD3kAguLEw5Nxe50#v=onepage&q=biomaterialsscienceratner&f=false. [Google Scholar]
- [37].Deo SV, Dunlay SM, Shah IK, Altarabsheh SE, Erwin PJ, Boilson BA, Park SJ, Joyce LD, Dual Anti-platelet Therapy After Coronary Artery Bypass Grafting: Is There Any Benefit? A Systematic Review and Meta-Analysis, J. Card. Surg 28 (2013) 109–116. doi: 10.1111/jocs.12074. [DOI] [PubMed] [Google Scholar]
- [38].Nocerino AG, Achenbach S, Taylor AJ, Meta-analysis of effect of single versus dual antiplatelet therapy on early patency of bypass conduits after coronary artery bypass grafting., Am. J. Cardiol 112 (2013) 1576–9. doi: 10.1016/j.amjcard.2013.07.017. [DOI] [PubMed] [Google Scholar]
- [39].Markway BD, McCarty OJT, Marzec UM, Courtman DW, Hanson SR, Hinds MT, Capture of flowing endothelial cells using surface-immobilized anti-kinase insert domain receptor antibody., Tissue Eng. Part C. Methods 14 (2008) 97–105. doi: 10.1089/ten.tec.2007.0300. [DOI] [PubMed] [Google Scholar]
- [40].Ruggeri ZM, Mendolicchio GL, Adhesion Mechanisms in Platelet Function, Circ. Res 100 (2007) 1673–1685. doi: 10.1161/01.RES.0000267878.97021.ab. [DOI] [PubMed] [Google Scholar]
- [41].Horbett TA, Fibrinogen adsorption to biomaterials, J. Biomed. Mater. Res. Part A (2018). doi: 10.1002/jbm.a.36460. [DOI] [PMC free article] [PubMed]
- [42].Clowes AW, Gown AM, Hanson SR, Reidy MA, Mechanisms of arterial graft failure. 1. Role of cellular proliferation in early healing of PTFE prostheses., Am. J. Pathol 118 (1985) 43–54. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1887858&tool=pmcentrez&rendertype=abstract (accessed August 29, 2013). [PMC free article] [PubMed] [Google Scholar]
- [43].Yamamoto K, Hokimoto S, Chitose T, Morita K, Ono T, Kaikita K, Tsujita K, Abe T, Deguchi M, Miyagawa H, Saruwatari J, Sumida H, Sugiyama S, Nakagawa K, Ogawa H, Impact of CYP2C19 polymorphism on residual platelet reactivity in patients with coronary heart disease during antiplatelet therapy, J. Cardiol 57 (2011) 194–201. doi: 10.1016/J.JJCC.2010.10.007. [DOI] [PubMed] [Google Scholar]
- [44].de Boer HC, Hovens MM, van Oeveren-Rietdijk AM, Snoep JD, de Koning EJP, Tamsma JT, Huisman MV, Rabelink AJ, van Zonneveld AJ, Human CD34+/KDR+ cells are generated from circulating CD34+ cells after immobilization on activated platelets., Arterioscler. Thromb. Vasc. Biol 31 (2011) 408–15. doi: 10.1161/ATVBAHA.110.216879. [DOI] [PubMed] [Google Scholar]
- [45].Anderson DEJ, Glynn JJ, Song HK, Hinds MT, Engineering an endothelialized vascular graft: A rational approach to study design in a non-human primate model, PLoS One 9 (2014) e115163. doi: 10.1371/journal.pone.0115163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Anderson DEJ, Pohan G, Raman J, Konecny F, Yim EKF, Hinds MT, Improving Surgical Methods for Studying Vascular Grafts in Animal Models., Tissue Eng. Part C. Methods 24 (2018) 457–464. doi: 10.1089/ten.TEC.2018.0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Anderson DEJ, McKenna KA, Glynn JJ, Marzec U, Hanson SR, Hinds MT, Thrombotic responses of endothelial outgrowth cells to protein-coated surfaces., Cells Tissues Organs 199 (2014) 238–48. doi: 10.1159/000368223. [DOI] [PMC free article] [PubMed] [Google Scholar]


