SUMMARY
The pathogenesis of idiopathic pulmonary fibrosis (IPF), an intractable interstitial lung disease, is unclear. Recessive mutations in some genes implicated in Hermansky-Pudlak syndrome (HPS) cause HPS-associated interstitial pneumonia (HPSIP), a clinical entity that is similar to IPF. We previously reported that HPS1−/−embryonic stem cell-derived 3D lung organoids showed fibrotic changes. Here, we show that the introduction of all HPS mutations associated with HPSIP promotes fibrotic changes in lung organoids, while the deletion of HPS8, which is not associated with HPSIP, does not. Genome-wide expression analysis revealed the upregulation of interleukin-11 (IL-11) in epithelial cells from HPS mutant fibrotic organoids. IL-11 was detected predominantly in type 2 alveolar epithelial cells in end-stage IPF, but was expressed more broadly in HPSIP. Finally, IL-11 induced fibrosis in WT organoids, while its deletion prevented fibrosis in HPS4−/− organoids, suggesting IL-11 as a therapeutic target. hPSC-derived 3D lung organoids are, therefore, a valuable resource to model fibrotic lung disease.
Graphical Abstract

In Brief
Pulmonary fibrosis is an intractable disease that can be familial or idiopathic. Strikoudis et al. modeled pulmonary fibrosis in lung organoids generated from embryonic stem cells with mutations in Hermansky-Pudlak syndrome genes that strongly predispose to this disease and demonstrate an essential role for interleukin-11 in the fibrotic process.
INTRODUCTION
The development of human pluripotent stem cell (hPSC)-derived organoids (McCauley and Wells, 2017) has allowed the modeling of several diseases affecting, among others, the CNS (Cugola et al., 2016; Dang et al., 2016; Garcez et al., 2016; Lancaster et al., 2013; Qian et al., 2016), the liver (Coll et al., 2018), the intestine (Spence et al., 2011), and the stomach (McCracken et al., 2014). Organoids derived from adult stem cells are also used for modeling disease, including cancer (Drost and Clevers, 2017) and infectious disease (Heo et al., 2018). In the lung, genetic defects affecting specific lineages, such as those associated with cystic fibrosis (McCauley et al., 2017) or surfactant deficiencies (Jacob et al., 2017), have been recapitulated using hPSC-derived spheroids containing the involved cell types. Modeling pathogenetic processes that affect lung structure and involve complex interactions between different cell types, such as those occurring in interstitial lung diseases (ILDs), has been more challenging, however. The most lethal ILD is idiopathic pulmonary fibrosis (IPF), which is characterized by the fibrotic obliteration of lung alveoli, leading to respiratory failure (Lederer and Martinez, 2018; Noble et al., 2012; Ryu et al., 2014). The median survival is 3 to 4 years, and the yearly mortality in the United States is ~40,000. Although recent trials showed that two drugs slow disease progression to some extent (King et al., 2014; Richeldi et al., 2014), the only definitive treatment is lung transplantation, an intervention that is hampered by the low availability of donor organs and severe surgical, medical, and immunological complications (McCurry et al., 2009). Insight into pathogenetic mechanisms and discovery of potential drug targets is therefore critical.
The etiology and pathogenesis of IPF are unclear (Steele and Schwartz, 2013). Genetic predisposition, age, and environmental exposure play a role (Lederer and Martinez, 2018; Noble et al., 2012; Ryu et al., 2014; Wolters et al., 2014). At least 5% of cases are inherited in an autosomal dominant fashion (Noble et al., 2012), but up to 20% of patients report familial incidence (Loyd, 2003). The nature of some mutations associated with IPF, such as those in the genes encoding surfactant proteins (SFTPs) A2 (Wang et al., 2009) and SFTPC (Lawson et al., 2004; Nogee et al., 2001; Nureki et al., 2018; Thomas et al., 2002), suggests that injury to type II alveolar epithelial (ATII) cells, the surfactant-producing cells of the alveoli, is critical to pathogenesis (Fingerlin et al., 2013; Seibold et al., 2011; Yang et al., 2015; Zhang et al., 2011). Eight percent to 15% of patients with familial IPF have heterozygous mutations in the reverse transcriptase (hTERT) or RNA component (hTERC) of telomerase (Alder et al., 2008, 2011, 2015a; Armanios, 2012a, 2012b, 2007). Furthermore, several susceptibility loci have been identified through exome sequencing that affect telomere length (Stuart et al., 2015). The association between telomeropathy and IPF also suggests a role for ATII cells, as these or a subset thereof can self-renew and replace damaged ATI cells to restore alveolar integrity after injury in the mouse (Barkauskas et al., 2013; Desai et al., 2014; Nabhan et al., 2018; Zacharias et al., 2018) and as telomere dysfunction causes the failure of ATII cells as stem cells (Alder et al., 2015b).
The notion that defects in ATII cells underlie IPF is further supported by the fact that some patients with Hermansky-Pudlak syndrome (HPS) show a high incidence of pulmonary fibrosis, also called HPS-associated interstitial pneumonia (HPSIP) (Mulugeta et al., 2015; Vicary et al., 2016). IPF and HPSIP are now considered similar clinical entities, albeit with distinct etiologies (American Thoracic Society and European Respiratory Society, 2002). HPS is an autosomal recessive disease caused by abnormal biogenesis and trafficking of lysosome-related organ-elles (LROs) and characterized by pigmentation abnormalities and bleeding diathesis associated with dysfunction of melanosomes and platelet delta granules, respectively. These are, similar to the lamellar bodies of ATII cells, where surfactant is stored and recycled, LROs. The mutations causing HPS affect four distinct protein complexes involved in LRO trafficking and biogenesis (Huizing et al., 2008; Luzio et al., 2014): biogenesis of LRO complex (BLOC)-1 (HPS7,8,9), BLOC-2 (HPS3,5,6), BLOC-3 (HPS1,4), and AP-3 (AP3B1 subunit of AP-3 in HPS2). Of the nine known mutations, only three (HPS1 and HPS4, affecting BLOC-3, and HPS2, disabling AP-3) are associated with early-onset ILD (Hengst et al., 2018; Huizing et al., 2008; Luzio et al., 2014; Vicary et al., 2016). In HPS1, the incidence of pulmonary fibrosis is 100%, making this the most penetrant ILD mutation (Seward and Gahl, 2013). Although mice with single deletion of HPS genes do not show fibrosis, studies in mice with mutations in both HPS1 and HPS2, which develop some degree of fibrosis, have suggested that epithelial injury is critical to this process (Mahavadi et al., 2012; Young et al., 2012). The fact that only some HPS mutations are associated with ILD offers a unique opportunity to search for features of epithelial cells that are shared by those with mutations that predispose to HPSIP. To this end, we used lung organoids derived from human embryonic stem cells (ESCs) with engineered mutations in several HPS genes.
We have previously reported the development of 3D lung organoids from hPSCs. These were staged at the second trimester of human development and contained a large fraction of ATII cells as well as some mesenchyme. ESCs with a mutation in HPS1, introduced using CRISPR/Cas9 gene editing, generated structurally abnormal organoids with an increased accumulation of mesenchymal cells and enhanced deposition of collagens (COLs) and fibronectin, suggestive of a fibrotic phenotype. A strong argument that an organoid model truly recapitulates disease is a correlation between phenotypes observed in the affected organoids and the clinical features of patients, however. In the present study, we additionally generated ESCs with mutations in HPS2, HPS4, and HPS8, in which HPS8 mutant organoids serve as an HPS disease control that is not associated with fibrosis. We found that fibrotic features only arose after the deletion of HPS1, HPS2, or HPS4, and were not detectable in organoids with HPS8 deletion, indicating that the in vitro phenotype correlates with the clinical incidence of HPSIP. Through genome-wide expression analysis of purified epithelial cells, we extracted an expression signature shared by fibrotic organoids that partially overlapped with publicly available genome-wide expression data from the lung tissue of IPF patients. One of the upregulated genes was interleukin-11 (IL-11), which we show here to be essential for the induction of fibrosis in HPS mutant fibrotic organoids, thus indicating IL-11 as a potential therapeutic target and supporting the utility of these organoids to study fibrotic lung disease.
RESULTS
Generation of Gene-Edited hESC Lines
We chose to genetically modify bona fide ESC lines to model HPSIP. Using this approach, all lines are co-isogenic, thus eliminating the variation caused by the genetic background associated with patient-specific induced pluripotent stem cells (iPSCs) (Inoue et al., 2014; Rouhani et al., 2014) and providing coisogenic parental cells as controls. Furthermore, bias and variability due to incomplete reprogramming and epigenetic memory (defined as a propensity of iPSCs to preferentially differentiate into the tissue from which they were derived), if these would exist (Hiler et al., 2015; Inoue et al., 2014; Kim et al., 2010), are eliminated. In addition to the HPS1−/−RUES2 cells we reported previously (Chen et al., 2017), we used CRISPR/Cas9 to introduce frameshift mutations in HPS2, HPS4, and HPS8 in RUES2 ESCs.
HPS1 is part of BLOC-3, and mutation in HPS1 is highly penetrant for HPSIP (Huizing et al., 2008; Seward and Gahl, 2013). At least 31 mutations, most of which cause a frameshift and a premature stop codon, have been described. There is a frameshift hotspot at codons 321–322 (Corral et al., 2004; Oh et al., 1998), and we targeted this region in the previously reported HPS1−/−line (Chen et al., 2017). Mutation in HPS4 affects the same complex and is also associated with HPSIP (Anderson et al., 2003). We generated three clones in which protein expression was eliminated. HPS2 mutations destabilize the AP-3 complex and predispose to fibrosis (Hengst et al., 2018). Deletions and frameshifts in AP3B1 cause nonsense-mediated mRNA decay (Feng et al., 2002; Huizing et al., 2002). We therefore generated three clones in which, similar to patients, the protein was absent. Mutations in HPS8, part of the BLOC-1 complex, are not associated with HPSIP (Vicary et al., 2016). The initial mutation described was a 1-bp frameshift deletion that theoretically gives rise to an abnormal 244-amino acid (aa) protein, as nonsense-mediated mRNA decay was not observed (Morgan et al., 2006). In rl mice, the protein is truncated to 79 aa, indicating that the Cterminus is critical for function. Another human mutation, however, did show nonsense-mediated mRNA decay (Cullinane et al., 2012). We therefore generated two clones in which protein production was eliminated. These lines serve as non-HPSIP-prone HPS controls. All of the mutants were verified for normal karyotype and confirmed by Sanger sequencing and western blot for inactivation of the targeted gene and loss of the encoded protein (Figure S1).
Fibrotic Changes in HPS Mutant Organoids Correlate with Clinical Incidence of HPSIP
Next, we differentiated each clone of the mutant lines and the parental wild-type (WT) RUES2 line in our previously published lung organoid protocol (Figure S2A). In this protocol, cells are differentiated into definitive endoderm and subsequently into anterior foregut endoderm and ventral anterior foregut endoderm, where the lung buds arise during lung development. Cells are then grown in suspension, where they form spherical structures consisting of folded epithelial strands interspersed with mesenchymal cells (Figure S2B). RNA sequencing (RNA-seq) studies showed that these spherical structures were consistent with lung buds, and were therefore called lung bud organoids (LBOs). Plating of the LBOs in Matrigel 3D cultures resulted in outwardly branching organoids showing proximodistal specification. These branching organoids were staged at the second trimester of human gestation by genome-wide expression analysis (Chen et al., 2017).
At the LBO (suspension culture) stage, no major differences were observed in the morphology and expression patterns among the mutant organoids, although quantification revealed minor increases in COL 1 and COL 3 in HPS4−/− LBOs (Figures S2B, S2C, and S3). After plating in Matrigel and culturing up to day 40, HPS1−/−, HPS2−/−, and HPS4−/− but not HPS8−/− organoids showed abnormal, compacted morphologies (Figure 1A). Compared to parental WT cells, immunofluorescent staining for mesenchymal markers revealed increased deposition of COL 1 and COL 3 and FIBONECTIN (FN), and increased presence of cells expressing mesenchymal markers (platelet-derived growth receptor-α [PDGRα], PDGFRβ, VIMENTIN, SLUG, and smooth muscle actin [SMA]) in HPS1−/−, HPS2−/−, and HPS4−/− organoids, but not in HPS8−/− organoids (Figure 1B). These observations were confirmed by quantification of the immunofluorescence (IF) images (Figure 2A) and by western blot (Figure 2B). In contrast to HPS1, HPS2, and HPS4 mutant lines, no differences were observed in HPS8−/− organoids compared to WT organoids, a finding that is in accordance with the fact that HPSIP has not been reported in patients with the HPS8 mutation. Trichrome staining (Figure 2C) showed more densely packed cells in HPS1−/− organoids that, in contrast to HPS8−/− organoids, did not form clear lumens. The high background of extracellular matrix (ECM) staining from the Matrigel, however, prevented us from clearly documenting specific ECM deposition in these samples, although this matrix appeared more disorganized in HPS1−/− organoids than in HPS8−/− organoids. Furthermore, RT-qPCR confirmed the upregulation of mRNAs encoding collagens and markers of mesenchymal cells (Figure 2D). Finally, using flow cytometry, we observed an increased fraction of THY1+ mesenchymal cells and a relative decrease in EPCAM+ epithelial cells (Figures 2E and S4A). These quantitative studies also indicated that some changes were less pronounced in HPS2 mutant lines compared to HPS1 and HPS4 mutant lines. For example, in contrast to HPS1−/− and HPS4−/− lines, SLUG, PDGRb, and COL 3 were not significantly increased in HPS2−/− cells by IF (Figure 2A), while their upregulation as measured by RT-qPCR was less pronounced (Figure 2D). Flow cytometry for THY1 did not indicate a significant increase in the HPS2−/− lines of THY1+ cells (Figures 1E and S4A), while western blot for VIMENTIN, PDGRα, PDGFRβ, and FN also showed less pronounced increases in HPS2−/− than in HPS1−/− and HPS4−/− organoids (Figure 2B). These findings may suggest that HPS2 mutation is less penetrant for HPSIP than BLOC-3 mutations. While penetrance of BLOC-3 mutations is 100%, the exact penetrance and rate of progression of HPS2-associated HPSIP are less clear (Vicary et al., 2016). These observations indicate that the presence or absence of a fibrotic phenotype in HPS mutant organoids corresponds to the clinical association of HPSIP with specific HPS mutations.
Figure 1. Generation 3D Lung Organoids Harboring Hermansky-Pudlak Syndrome (HPS)-Associated Mutations.
(A) Bright-field images of day 40 Matrigel-embedded 3D lung organoid cultures generated from parental wild-type (WT) ESCs or from ESC with CRISPR/Cas9-induced mutations in HPS genes. Protein complexes affected by each mutation are indicated at top. Scale bar: 1 mm (representative of n = 3 independent experiments).(B) Immunofluorescence (IF) analysis of day 40 Matrigel-embedded 3D lung organoids generated from parental WT ESCs or from ESC with CRISPR/Cas9-induced mutations in HPS genes. Red and green: corresponding IF markers indicated for each stain. Blue: DAPI stain. Scale bar: 100 mm (representative of n = 3 independent experiments).
Figure 2. Characterization of HPS Mutant Lung Organoids.
(A) Fluorescent area over DAPI area for indicated ECM and mesenchymal markers in cryosections of day 40 organoids. Boxplot lines indicate medians; whiskers represent minimum and maximum of all data. n = 18 independent images from n = 3 independent experiments. Unpaired two-tailed Student’s t test. n.s., non-significant (p > 0.05). *p % 0.05, **p % 0.01, ***p % 0.001, and ****p % 0.0001. Error bars indicate mean ± SEM.
(B) Western blot analysis for ECM and mesenchymal markers of day 40 WT or HPS mutant organoids.
(C) Trichrome staining of WT and HPS1−/− organoids.
(D) Expression by qRT-PCR of mRNA for ECM and mesenchymal markers relative to WT control. n = 5 independent experiments. Unpaired two-tailed Student’s t test. n.s., non-significant (p > 0.05). *p % 0.05, **p % 0.01, ***p % 0.001, and ****p % 0.0001. Error bars indicate mean ± SEM.
(E) Frequency of epithelial (EPCAM+THY1−) and mesenchymal (EPCAM−THY1+) as measured by flow cytometry. n = 3. Unpaired two-tailed Student’s t test. n.s., non-significant (p > 0.05). *p % 0.05 and **p % 0.01. Error bars indicate mean ± SEM.
Genome-wide Expression Analysis Reveals Shared Signature Associated with Fibrosis
As the pathogenesis of IPF and HPSIP involves epithelial injury, we performed RNA-seq on purified EPCAM+ epithelial cells from organoids generated from parental WT cells and from each clone of HPS8−/−, HPS1−/−, and HPS2−/− cells to search for gene expression signatures that differed from WT and were shared by the epithelial cells in the fibrotic (HPS1−/− and HPS2−/−) organoids. Cross-referencing the genome-wide expression of the organoids with the KeyGenes database (Roost et al., 2015) demonstrated the best match with second-trimester human fetal lung, similar to WT organoids (Figure 3A) and similar to our previously published data on organoids from RUES2 ESC and three iPS lines (Chen et al., 2017). These findings indicate that, despite the abnormal morphology of HPS1−/− and HPS2−/− organoids, mutation in HPS genes did not appreciably affect the developmental fate of the organoids. A total of 56 genes were found to be significantly upregulated in both HPS1 and HPS2, but not in HPS8 compared to WT, while 28 genes were significantly downregulated in both HPS1 and HPS2, but not in HPS8 (Figures 3B–3D; Table S1). We confirmed a subset of these differentially regulated genes by RT-qPCR (Figure S4B). Gene Ontology analysis for common differentially regulated genes between HPS1−/− and HPS2−/− indicated that secretory pathways and ECM organization were affected by these mutations (Figure 3E).
Figure 3. Genome-wide Expression Analysis of HPS Mutant Day 40 Lung Organoids.
(A) Comparison of genome-wide expression in EPCAM+ fluorescence-activated cell sorting (FACS)-sorted epithelial cells from day 40 WT and HPS mutant organoids with the KeyGenes database, showing matching with second-trimester human lung. Each group corresponds to three individual biological replicates.
(B) Venn diagrams indicating significant differentially upregulated or downregulated genes, respectively, following gene expression analysis of each group of HPS mutations compared to WT control.
(C) Heatmap representation of significant differentially expressed genes extracted from RNA-seq genome-wide expression analysis of EPCAM+ epithelial cells from HPS mutant and WT control organoids. Each group represents the mean of three individual biological replicates.
(D) Volcano plot showing log2 fold-change and −log10 of p value for each individual gene in epithelial cells with HPS1 mutation compared to WT control. Red: significant genes with a false-discovery rate (FDR) < 0.05. Gray: non-significant genes. Black: common HPS1 and HPS2 significant genes compared to WT control.
(E) Gene Ontology analysis of common HPS1 and HPS2 significant genes compared to WT control using DAVID.
To examine to what extent the findings in our HPS mutant organoid model would also be applicable to IPF, we re-analyzed publicly available datasets of human IPF patient samples, with the caveat that while our studies were performed on purified epithelial cells, genome-wide expression profiling in IPF is performed on tissue samples containing both epithelium and abundant mesenchymal cells. The shared upregulated genes in HPS1−/− and HPS2−/− organoids included SERPINE1 (also known as PAI-1), a well-known biomarker of IPF (Kotani et al., 1995) and of fibrosis in mice treated with bleomycin (Fernandez et al., 2016), and FN, an ECM component. We confirmed increased FN (Figures 2B and 2D) and PAI-1 (Figure S4B) expression in HPS1−/− and HPS2−/− organoids, whereas no increased expression was observed in HPS8−/− organoids. Furthermore, among a “translational set” of 12 genes that were shown to be upregulated in both human IPF lungs and in rat lungs after treatment with bleomycin (Bauer et al., 2015), two (NELL1, which was also confirmed by RT-qPCR [Figure S4B], and PLA2G2A) were also upregulated in HPS1−/− and HPS2−/− and not in HPS8−/− EPCAM+ cells. Next, we compared differentially expressed genes between 20 IPF and 19 healthy control samples from the Lung Tissue Research Consortium (LTRC) (Schafer et al., 2017a). Shared among the significantly differentially regulated genes from this study were 17 of our significantly upregulated genes and 12 of our significantly downregulated genes (Figure S5A). Comparison of our dataset with the transcriptomic profiles from 10 samples from normal-appearing IPF regions and 8 samples from scarred regions of IPF lung, each compared to 8 healthy control samples (Luzina et al., 2018), showed that 9 of our identified genes are also differentially expressed in lung tissue from healthy controls and the fibrotic lung tissue of IPF patients (Figure S5B). Six of these genes are also differentially expressed in normal-appearing lung tissue from IPF patients compared to lung tissue from healthy controls (Figure S5C), suggesting that genes identified using our 3D organoids potentially include markers of early-onset fibrosis. Finally, an additional comparison between eight IPF and seven control lung tissue samples (Nance et al., 2014) revealed seven genes shared among the significant genes from this study and ours (Figure S5D). We note that although transforming growth factor b (TGF-b) signaling has been implicated in pulmonary fibrosis (Lederer and Martinez, 2018; Wolters et al., 2014), neither TGF-β signaling nor TGF-β ligands were differentially regulated in the aforementioned genome-wide expression studies (Figures S5A–S5D). Consistent with these findings, RT-qPCR for TGF-β ligands in HPS mutant organoids showed similar expression of TGFB1 and TGFB2 mRNAs (TGFB3 mRNA was not detectable) (Figures S5E and S5F). We do note that there is an increase in TGF-β2 mRNA expression in all mutants compared to WT, although not always reaching statistical significance (Figure S5F). Since there is no clear correlation with the phenotype of the organoids, the significance of this finding is at present unclear.
These observations indicate that important aspects of IPF can be modeled in HPS mutant hESC-derived lung organoids.
Critical Role of IL-11 in Fibrotic Changes
Among the upregulated genes, IL11 was of particular interest, as IL-11 is a soluble cytokine (Schwertschlag et al., 1999) and as our analysis revealed the upregulation of IL11 mRNA in 3 publicly available genome-wide expression datasets of IPF lung samples, including a dataset generated from the unaffected lungs of IPF patients (Figures S5A–S5C). The overexpression of IL11 in HPS1−/− and HPS2−/− but not in HPS8−/− organoids was confirmed by RT-qPCR (Figure S4B) and IF (Figure S4C). Furthermore, increased IL-11 protein was detected in the supernatant of HPS1−/− organoid cultures (Figure S4D). Although the RNA-seq studies were performed on purified EPCAM+ epithelial cells, expression of IL11 (and of PAI-1 and FN1) was also observed in purified THY1+ mesenchymal cells from WT cells (Figure S4E). To assess whether IL-11 could affect the phenotype of mesenchymal cells, we isolated THY1+ mesenchymal cells from WT organoids and cultured them in the presence of IL-11. In these conditions, increased expression of PDGFRa and SMA, two markers associated with myofibroblast infiltration in IPF, was observed (Figures S4F and S4G). To further verify expression in patients, we performed in situ hybridization in sections of lungs explanted pretransplantation from HPS1 and IPF patients. In HPSIP, there was widespread expression of IL11, in particular in SFTPC+ ATII cells, which expressed IL-11. In IPF, IL-11 expression appeared limited to SFTPC+ cells (Figure 4).
Figure 4. Expression of IL-11 in HPSIP and IPF.
Representative images (from 3 HPSIP and 2 IPF patients) of expression of EPCAM, SFTPC and IL11 in explanted lungs from end-stage patients pre-transplantation as measured by in situ hybridization. The corresponding H&E stains are shown on the left (scale bar: 100 mM).
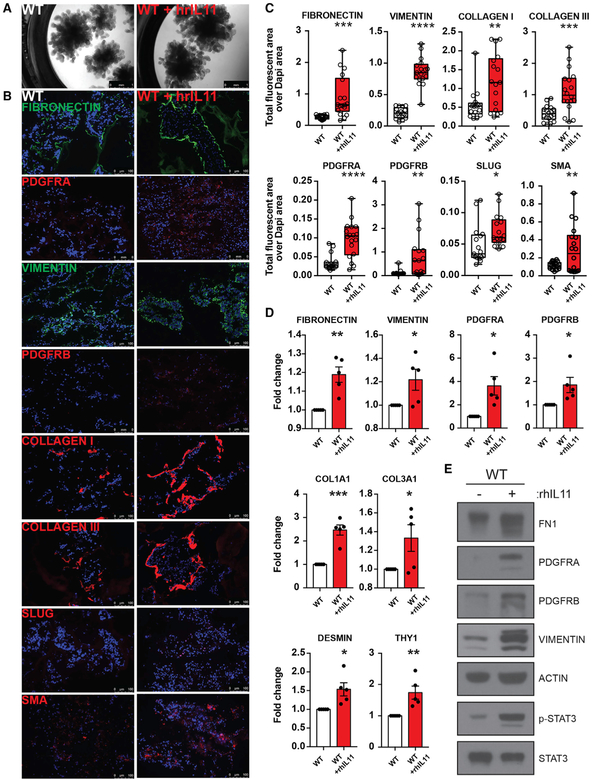
To examine whether IL-11 drives fibrosis in this model, we added recombinant human IL-11 to Matrigel-embedded organoids derived from WT ESCs from day 25. At day 40, although overall morphology did not appear affected, organoids treated with IL-11 showed increased expression of mesenchymal markers (FN, PDGFRα, PDGFRβ, VIMENTIN, SLUG, COL 1 and COL 3, and SMA) by IF (Figures 5A and 5B). A direct comparison with HPS mutant organoids after staining for PDGFRaα, PDGFRα, EPCAM, and FN is shown in Figure 6. Quantification of the IF images showed all of the differences to be statistically significant, and these findings were confirmed by RT-qPCR for the same markers (Figures 5C and 5D). These findings were further confirmed by western blot analysis (Figure 5E), which also showed the increased phosphorylation of Stat3, a downstream signaling molecule of the IL-11 receptor (Schwertschlag et al., 1999) (Figure 5E). We note that IL-11 did not significantly induce TGF-β ligands (Figures S4E and S4F). Next, to examine whether IL-11 plays a critical role in the initiation of the fibrotic process, we deleted IL-11 from the HPS4−/− ESCs using CRISPR/Cas9. Deletion was verified by RT-qPCR and Sanger sequencing of the IL-11 locus (Figures S1L–S1N). In contrast to HPS4−/− organoids, HPS4−/− IL-11−/− double knockout organoids (DKOs) displayed a morphology that is comparable to that of WT organoids (Figure 7A). IF and RT-qPCR revealed the disappearance of all of the fibrotic markers tested in HPS4−/− IL-11−/− DKOs (Figures 7B–7D). These data indicate a critical role for IL-11 in the induction of fibrosis.
Figure 5. Effect of IL-11 on Fibrosis in WT Day 40 Lung Organoids.
(A) Bright-field images of day 40 WT lung organoids cultured in the presence or absence of recombinant human IL-11 (rhIL-11) from day 25. Scale bar: 1 mm.
(B) IF images from cryosection stains from day 40 WT lung organoids cultured in the presence or absence of rhIL-11. Red and green: corresponding IF markers indicated for each stain. Blue: DAPI stain. Scale bar: 100 mm.
(C) Fluorescent area over DAPI area for indicated ECM and mesenchymal markers in cryosections of day 40 WT organoids cultured in the presence or absence or rhIL-11. Boxplot lines indicate medians; whiskers represent minimum and maximum of all data. n = 18 independent images from n = 3 independent experiments. Unpaired two-tailed Student’s t test. *p % 0.05, **p % 0.01, ***p % 0.001, and ****p % 0.0001. Error bars indicate mean ± SEM.
Figure 6. Effect of IL-11 on Fibrosis in WT Day 40 Lung Organoids Compared to HPS Mutant Organoids.
IF images from cryosection stains from day 40 WT lung organoids cultured in the presence or absence or rhIL-11, as well as HPS1−/−, HPS2−/−, HPS4−/−, and HPS8−/− organoids. Red and green: corresponding IF markers indicated for each stain. Blue: DAPI stain. Scale bar: 100 μm.
Figure 7. Characterization of HPS4−/− IL11−/− Lung Organoids.
(A) Bright-field images of HPS4−/− day 40 lung organoids compared to organoids generated from HPS4−/− IL11−/− ESCs. Scale bar: 1 mm.
(B) IF images from cryosection stains from day 40 HPS4−/− and HPS4−/− IL11−/− lung organoids. Red and green: corresponding IF markers indicated for each stain. Blue: DAPI stain. Scale bar: 100 mm.
(C) Fluorescent area over DAPI area for indicated ECM and mesenchymal markers in cryosections of day 40 HPS4−/− and HPS4−/− IL11−/− lung organoids. Boxplot lines indicate medians; whiskers represent minimum and maximum of all data. n = 18 independent images from n = 3 independent experiments. Unpaired two-tailed Student’s t test. *p % 0.05, **p % 0.01, ***p % 0.001, and ****p % 0.0001. Error bars indicate mean ± SEM.
(D) Expression by qRT-PCR of mRNA for ECM and mesenchymal markers in d40 HPS4−/− and HPS4−/− IL11−/− lung organoids, normalized to expression in HPS4−/− organoids. n = 5 independent experiments. Unpaired two-tailed Student’s t test. *p % 0.05, **p % 0.01, ***p % 0.001, and ****p % 0.0001. Error bars indicate mean ± SEM.
DISCUSSION
We have shown here that gene-edited ESC-derived lung organoids can recapitulate important features of HPSIP, that the pheno-type of the organoids matches the incidence of HPSIP in patients with corresponding mutations, and that IL-11 plays a crucial role in the fibrotic process. The overlap in expression signatures between organoids with fibrosis-prone HPS mutations and lung samples from IPF patients, including the overexpression of IL-11, indicates that this HPSIP model also recapitulates features of IPF pathogenesis, a notion that is consistent with the consensus that HPSIP and IPF are similar clinical entities (American Thoracic Society and European Respiratory Society, 2002). While our findings indicate that hPSC-derived lung organoids can be used to model pulmonary disease and identify pathogenic mechanisms that, given the correlating findings in patients, are likely clinically relevant, this model has limitations as well. As this is an in vitro developmental organoid model at a fetal stage of development, although associated with fibrotic changes as determined by IF, western blot and genome-wide expression analysis, the organoids may not necessarily reproduce all of the histological features of IPF as they occur in the adult lung. Furthermore, although we opted to generate genome-edited ESCs to avoid the confounding effects of genetic background, it cannot be excluded that different genetic backgrounds would affect phenotypes. The finding of fibrotic changes in organoids with mutations that are also associated with pulmonary fibrosis in HPS patients indicates that this approach is valid. We also note that the penetrance of pulmonary fibrosis in HPS1 patients is 100% (Seward and Gahl, 2013), indicating that at least for this subset, genetic background variation is less relevant. Furthermore, it is unlikely that every lung disease can be modeled using organoids, although we have previously shown that the morphological features of infection with respiratory syncytial virus are also faithfully recapitulated (Chen et al., 2017).
An important question in this model is the origin of the mesenchymal cells, both in WT and fibrotic organoids. The generation of the organoids recapitulates development. Thus, to generate lung and airway epithelial cells from hPSCs in 2D definitive endoderm, anterior foregut endoderm, ventral anterior foregut endoderm, and lung progenitors are sequentially specified followed by further differentiation into a mixture of alveolar and airway cells (Chen et al., 2017; Dye et al., 2015, 2016; Firth et al., 2014; Gotoh et al., 2014; Huang et al., 2014, 2015; Jacob et al., 2017; Konishi et al., 2016; McCauley et al., 2017; Mou et al., 2012; Wong et al., 2012). In our 3D protocol, spheres are generated from anterior foregut endoderm by culturing in non-tissue culture-treated plates in conditions that specify ventral anterior foregut endoderm. In these spheres, the endoderm uniformly expressed NKX2.1, FOXA1, SOX2, and SHH, which are markers of early lung bud endoderm. Mesoderm that expressed TBX4, HOXB5, and transcriptional targets of Sonic Hedgehog signaling, a constellation of markers compatible with pulmonary mesoderm, was also present (Chen et al., 2017). However, at the definitive endoderm stage of the protocol, >99% of cells expressed EPCAM, and the mesodermal marker THY1 was undetectable by flow cytometry (data not shown). It is possible that the mesoderm arises from a very small population of contaminating mesodermally specified cells. Alternatively, it cannot be excluded that what we interpret to be definitive endoderm in fact still retains characteristics of early mesendoderm. Lineage tracing studies, for which the tools in hPSCs are not available, could answer this question. It is also not known where the mesenchymal cells in IPF originate. Epithelial to mesenchymal transition (EMT) from ATII cells (Tanjore et al., 2011; Zhong et al., 2011) has been suggested, but this remains controversial (Kage and Borok, 2012; Rock et al., 2011). Lineage tracing studies in bleomycin-treated mice have excluded the involvement of EMT (Rock et al., 2011) and indicated that mesenchymal cells may arise from Gli1+PDGFRα+ perivascular pericytes (Kramann et al., 2015). It is therefore possible that, as blood vessels are absent from this in vitro model, the origin of the mesenchymal cells in the organoids differs from their physiological origin in vivo, but that the effect of mutant or injured epithelium on mesenchymal cells is not dependent on the developmental origin of the latter. A caveat, however, is that bleomycin administration in the mouse, while commonly used as model for fibrosis, is controversial as a model for IPF as it causes acute inflammation and patchy fibrosis that resolves over time. Bleomycin therefore likely models an acute inflammatory response, rather than progressive IPF (Matute-Bello et al., 2008; Moeller et al., 2008; Moore and Hogaboam, 2008).
The fibrotic features of HPS1−/−, HPS2−/−, and HPS4−/− organoids primarily arose after plating in Matrigel, where branching organoids begin to express markers of mature lung cells, in particular, ATII cells (Chen et al., 2017). This finding is consistent with genetic and cell biological evidence that ATII injury or dysfunction is involved in the initiation of fibrosis (Mulugeta et al., 2015). Furthermore, we previously reported that mixing experiments using WT and HPS1−/− LBOs indicated that the presence of the mutation in the epithelial compartment was critical for the fibrotic phenotype, while its presence in the mesodermal fraction was irrelevant. It is also interesting to note that although HPS1−/−, HPS2−/−, and HPS4−/− organoids are morphologically abnormal, genome-wide expression analysis revealed no differences in the expression of pulmonary epithelial markers. Furthermore, similar to WT organoids derived from hESCs and hiPSCs (Chen et al., 2017), all mutant organoids matched with second-trimester human fetal lung. The development of fibrosis in the organoids therefore does not appear to affect epithelial development. In HPS patients, lung development is also not known to be abnormal. In adult patients with IPF and HPSIP, however, the alveolar epithelium is remodeled and shows abnormal co-expression of proximal markers (Xu et al., 2016). This model therefore likely represents early-stage IPF.
It may also appear remarkable that fetal-stage lung organoids can model an age-associated disease to at least some extent. The additional stress of tissue culture may promote the early appearance of disease features—in particular, features of a disease such as IPF, in which both age and telomeropathy are prime risk factors (Alder et al., 2008, 2011, 2015a; Armanios et al, 2007, 2012a, 2012b; Ryu et al., 2014). Tissue culture-induced senescence and stress may therefore cause the premature appearance of fibrosis in organoids with mutations that predispose to fibrosis in patients. Although HPSIP appears earlier in life than in sporadic and other forms of familial IPF, the first signs of HPSIP are usually not detected until the third decade of life (Hengst et al., 2018; Seward and Gahl, 2013; Vicary et al., 2016). Further supporting the notion that tissue culture conveys additional stress is the finding that we could not carry hTERT+/− ESCs, generated using CRIPSR/Cas9 editing, to the Matrigel stage (days 25–40) of this differentiation protocol (data not shown), despite the fact that patients with such mutations are healthy overall until the fifth or sixth decade of life.
The identification of an expression signature that overlaps with that of IPF and IL-11 as a key driver of fibrotic changes in the organoids is compelling. IL-11 is a secreted protein, which we could detect in the supernatants of HPS1−/− organoids, further supporting and confirming the increased IL-11 expression we extensively demonstrated in all of the genotypes using IF, qPCR, and RNA-seq. IL-11 overexpression was also observed in previous genome-wide expression studies in IPF, including in normally appearing lung tissue from patients. The latter finding may suggest that IL-11 expression occurs early in the pathogenetic process. The complete prevention of fibrosis after the deletion of IL-11 indicates that IL-11 or its receptor may be therapeutic targets in HPSIP and in IPF. It is interesting to note, however, that while in HPSIP, IL11 mRNA expression was widespread but included virtually all SFTPC+ cells, and in IPF, IL11 mRNA appeared restricted mainly to SFTPC+ cells. This may indicate that while IL-11 may be involved in both clinical entities, its pathogenetic role may differ somewhat. The identification of the involvement of IL-11 in fibrotic changes in the lung organoids also corroborates other findings on the role of IL-11 in fibrosis and, therefore, further supports the fidelity with which these organoids can recapitulate features of fibrotic lung disease. Although the limited set of patients analyzed were male, RUES2 ESCs are female, suggesting that the role of IL-11 is not sex dependent. Administration or fibroblast-specific overexpression of IL-11 caused cardiovascular fibrosis in mice by mediating at least some effects of TGF-β and acting as an autocrine activator of mesenchymal cells (Schafer et al., 2017b). We did not observe a TGF-β signature in our genome-wide expression studies of HPS mutant epithelial cells or evidence of TGF-β overexpression in the fibrotic organoids. We cannot exclude differences in TGF-b activation, however. We also identified IL-11 in HPS mutant mesenchymal cells and showed that exposure of WT mesenchymal cells to IL-11 induced the expression of PDGRα and SMA, features of myofibroblasts, which are abundantly present among the heterogeneous mesenchymal cells in IPF lungs (Rock et al., 2011). However, we initially discovered IL-11 overexpression in the purified epithelial cells of HPS mutant organoids, suggesting an additional role for IL-11 produced in epithelial cells in the initiation of fibrosis. This notion is supported by previous reports showing that transgenic overexpression of IL-11 in club cells was associated with peribronchial fibrosis in mice (Ray et al., 1997; Tang et al., 1996). These findings raise the question of how epithelial injury or dysfunction induces IL-11 production. This organoid model will offer a unique opportunity to elucidate the mechanisms underlying these processes. Furthermore, this organoid model will allow the identification of upstream regulators of IL-11 and of other genes differentially expressed in the epithelial cells of fibrotic and normal organoids, which will yield mechanistic insight and may lead to the discovery of drug targets.
In conclusion, our studies validate hPSC-derived lung organoids as a tool to model fibrotic lung disease, to identify potential drug targets, and to pre-clinically test innovative medical treatment, and indicate a therapeutic target in this universally lethal disease.
STAR★METHODS
LEAD CONTACT AND MATERIALS AVAILABILITY
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Hans-Willem Snoeck (hs2680@coliumbia.edu).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Embryonic stem cells
Rockefeller University Embryonic Stem Cell Line 2 (RUES2, NIH approval number NIHhESC-09–0013, Registration number 0013, passage 17–28) were used. This is a female line.
Human subjects
Archival and deidentified frozen sections from 2 HPSIP (ages 39 and 51) and 2 IPF (ages 76 and 71) patients from explanted lung prior to lung transplantation were used for in situ hybridization studies. Patients were randomly chosen by the pathologist (A. Saqi) and were male. As these were proof-of-principle experiments on a limited set of samples, no conclusions can be drawn on sex as a biological variable. The experiments were performed in accordance with protocol approved by Columbia University Institutional Review Board protocol number AAAS1091.
METHOD DETAILS
Reagents and Media
Reagents and Media formulations used have been previously described (Chen et al., 2017) and are listed in the Key Resources table. Briefly, hESC maintenance media consisted of DMEM/F12 (1:1) supplemented with 20% knockout serum replacement, 0.1 mM β-mercaptoethanol, 1 mL Primocin, 5 mL Non-essential amino acids, 5 mL GlutaMax, and 20 μg/ml FGF-2. Serum-free differentiation (SFD) media consisted of IMDM/Ham’s F12 (3:1) supplemented with N2, B27, 0.05% bovine serum albumin, 1% penicillin-streptomycin, 50 mg/ml ascorbic acid, 2mM Glutamax, 0.4 μM monothioglycerol and different growth factor cocktails as indicated in Table S2.
Genome-editing using CRISPR/Cas9
Guide-RNAs against coding sequences of the genes AP3B1 (HPS2), BLOC3S2 (HPS4), BLOC1S3 (HPS8) and IL-11 were designed using the MIT CRISPR Design tool (http://zlab.bio/guide-design-resources). The RUES2-HPS2, RUES2-HPS4, RUES2-HPS8 knockout lines as well as the RUES2-HPS4-IL-11 double knockout line were generated in the following way: Briefly, RUES2 cells were cultured in six-well plates on MEF feeders to 70%–80% confluence. RUES2 cells were treated with Accutase/EDTA, washed and nucleofected using the Amaxa P3 Primary Cell 4-D Nucleofector X Kit L (Lonza) according to manufacturer’s specifications with 20 mg of pX300 vector (Addgene Plasmid Number: 42230) (Cong et al., 2013) following the cloning of each corresponding guide-RNA (Table S2). Twenty-four hours post-nucleofection, cells were passaged into a 10cm dish on MEF feeders. Single colonies were picked 7–10 days post passaging. Genomic DNAs from individual clones were isolated and genotyping was done using gene-specific PCR primers (Table S2). Indels were confirmed using Sanger sequencing (Genewiz, NJ).
hPSC Maintenance and endoderm induction
were maintained on mouse embryonic fibroblasts (MEFs) plated at 15,000–18,000 cells/cm2. Cells were cultured in hPSC maintenance media and medium was changed daily. hPSCs were passaged with Accutase/EDTA, washed and replated at a dilution of 1:48. Cultures were maintained in a humidified 5% CO2 atmosphere at 37°C Induction of endoderm was carried as previous described (Chen et al., 2017). Briefly, MEFs were depleted by passaging onto Matrigel for 24 h supplied with hPSC maintenance media and maintained in a humidified 5% CO2 atmosphere at 37°C. After MEF depletion, primitive streak and embryoid body induction was performed in embryoid bodies/primitive streak formation media (Table S3) in low attachment plates for 12–16 h followed by switching to endoderm induction media (Table S3) for 36–40 h. Embryoid bodies were fed every day and maintained in a humidified 5% CO2/5% O2 atmosphere at 37°C. Endoderm yield was determined by the expression of CXCR4 and c-KIT. For iPS lines, endodermal cells were purified using human CD184 (CXCR4) MicroBead kit. Cells used in all experiments had > 90% endoderm yield.
Anterior foregut endoderm induction
Anterior foregut endoderm was induced as previous described (Huang et al., 2015). On day 4, embryoid bodies were dissociated with0.05% Trypsin/EDTA and plated on fibronectin-coated multiple well plates with a density at 80,000–105,000 cells/cm2. Cells were incubated in Anteriorization media-1 for 24 h followed by switching to Anteriorization media-2 for another 24h.
Formation of lung bud organoids
At the end of anterior foregut endoderm induction, cells were treated with Ventralization media (Branching media) for 48 h and three-dimensional clump formation was observed. The clumps were then suspended by gently pipetting around the wells. The suspended clumps are called lung bud organoids (LBOs) hereafter. LBOs were maintained in non-tissue culture treated multiple-well plates submerged in Branching media and were fed every other day until d25.
Branching morphogenesis in Matrigel
The d25 LBOs were embedded in 100% Matrigel in 24-well transwell inserts and incubated in incubator until the Matrigel solidified. Branching media were added to the well, after which the transwell was inserted, branching media added into the transwell insert as well. Media were changed every other day.
Immunofluorescence staining
Branching Matrigel-embedded 3D lung organoid cultures were freshly embedded in Optimal Cutting Temperature (OCT). Samples were sectioned at 8 mm, and frozen at −80°C. The sections were fixed with 4% paraformaldehyde for 20 minutes at room temperature (RT) and washed with DPBS for 5 minutes. The sections were permeabilized with 0.3% Triton X-100/PBS for 30 minutes followed by blocking in 5% donkey serum for 1 hour. Primary antibodies (Table S4) were incubated at 4°C overnight. The next day, sections were washed with DPBS 3 × 5 minutes followed by secondary antibody (Table S4) incubation for 2 hours at RT, washed 3 × 10 minutes with DPBS then mounted with DAPI contained fluorescent mounting medium.
Isolation of EPCAM+ and THY1+ population from 3D lung organoids
Day40 3D lung organoids were dissociated using Dispase (Corning) at 37 °C. For isolating epithelial cells, or both epithelial and mesenchymal populations, the cells were stained with APC-conjugated EPCAM alone, or with a combination of PerCP-conjugated EPCAM and APC/Cy7-CD90 (THY1), respectively, for 20 minutes at 4 °C. EPCAM+ and THY1+ populations were isolated by Fluorescence activated cell sorting (FACS) using a BD Influx Cell Sorter (San Jose, CA).
RNA-seq
Total RNA from FACS-sorted cells was purified using Direct-zol™ RNA MicroPrep kit. RNA concentration and RNA integrity number (RIN) were determined using an Agilent microfluidic RNA 6000 Nano Chip kit (Agilent Technologies, Santa Clara, CA) on the 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA). Those samples with RIN greater than 9 were used for RNA-seq. Poly-A-pull-down was used to enrich mRNAs from total RNA samples. Libraries were prepared using Illumina TruSeq RNA prep kit (Illumina, San Diego, CA). Libraries were then sequenced using the Illumina HiSeq2000 (Illumina, San Diego, CA) at the Columbia Genome Center. Samples were multiplexed in each lane, yielding a targeted number of single-end 100bp reads for each sample.
Addition of recombinant IL-11 in 3-D organoid cultures
Recombinant human IL-11 (rhIL-11) (R&D Systems) was added to 3-D branching WT organoid cultures from d25 until d40 every other day at a final concentration of 5ng/mL.
Detection of IL-11 by ELISA
Day 40 WT and HPS1−/− culture supernatants were collected and human IL-11 protein concentration was measured by Enzyme-Linked Immunosorbent Assay (ELISA) method according to manufacturer’s protocol (#EHIL11: Invitrogen, Frederick, MD). Levels of IL-11 were normalized to the total cell culture protein content as measured by Pierce BCA Protein assay (Thermo Fisher Scientific, Rockford, IL).
In situ hybridization
For RNAscope in situ hybridization, 8-μm FFPE tissue sections were incubated with probes specific for IL11 (ACD, 425281), SFPTC (ACD, 452561-C2), and EpCAM (ACD, 310281-C3) after proper treatment of the slides with H2O2, target retrieval, and pro-tease digestion according to manufacturer’s instructions of the RNAscope Multiplex Fluorescent Reagent Kit v2 (ACD, 323100). Opal Multiplex IHC Kit (PerkinElmer, NEL811001KT) was used to develop RNA signals, using Opal 520 for IL11, Opal 570 for SFPTC, and Opal 690 for EpCAM, respectively. After counter staining with DAPI, the slides were imaged using the Vectra 3 automated multispectral microscope (PerkinElmer) and the Phenochart 1.0 software (PerkinElmer) at the Human Immune Monitoring Core (HIMC) of Columbia University Irving Medical Center (RRID:SCR_016740). Images were then processed and analyzed in the inForm 2.4.2 software (PerkinElmer) for quantification of signal levels of each target and their location and co-location in the tissue.
Imaging and quantification of immunofluorescence
Samples were imaged using motorized DMi8 (Leica Microsystems, Buffalo Grove, IL) inverted microscopes. Images for each marker were quantified using ImageJ. Briefly, images were converted to 8-bit images and the threshold was adjusted to correspond with the nuclear stain, which allows for measurement of total area. The total area was analyzed by the “Analyze Particles” function of ImageJ. Percentage of positive cells were calculated by dividing the total area of positive cells over the total area of DAPI.
QUANTIFICATION AND STATISTICAL ANALYSIS
Statistical analysis
Statistical analysis was done using unpaired two-tailed Student’s t test using Prism 7 software, where in all cases each was compared to parallel wt control. Results are shown mean ± s.e.m., p values < 0.05 were considered statistically significant. N-values are indicated in the legends to each figure and refer to biologically independent replicates, unless noted otherwise.
RNaseq analysis
RTA (Illumina, San Diego, CA) was used for base calling and bc12fastq (version 1.8.4) for converting BCL to fastq format, coupled with adaptor trimming. Reads were mapped to a reference genome (NCBI/build37.2) using Tophat (version 2.0.4) (Trapnell et al., 2012) with 4 mismatches and 10 maximum multiple hits. To tackle the mapping of reads that are from exon-exon junctions, Tophat infers novel exon-exon junctions ab initio, and combines them with junctions from known mRNA sequences as the reference annotation. We estimated the relative abundance of genes and splice isoforms using Cufflinks (version 2.0.2) with default settings. We tested for differentially expressed genes using Cuffdiff and identified significant genes (Table S1). Interleukin-11 (IL-11) and other important genes was confirmed as significantly differentially expressed using DESeq2, an R package based on a negative binomial distribution that models number of reads from RNaseq experiments and tests for differential expression, and plotted using ggplot2, an R package. Gene ontology analysis was performed using DAVID and Metascape (Table S1). Samples represent n = 3 individual biological replicates for each group.
Comparative analysis using KeyGenes
RNA-seq data obtained from WT, HPS1-, HPS2-, or HPS8 mutant EPCAM+ epithelial cells from d40 3D organoids, were compared to different first and second trimesters and adult organs, including the lungs, using KeyGenes (Roost et al., 2015). Hierarchical clustering of 12 samples of the 3D lung organoids and 75 samples from 19 organs from second trimester was performed using Cluster 3.0 and viewed by TreeView.
Analysis of public available datasets
Differential expression analysis of normal lung samples and IPF lung samples was calculated from public available datasets from the GEO database with the following accession numbers: GSE99621, GSE92592 and GSE52463. In all 3 cases, counts matrix tables, provided as processed files, were analyzed using DESeq2, and plotted using ggplot2 and ggrepel packages using R. We treated each published dataset as a separate experiment, and did not normalize all datasets together as it is challenging to normalize RNA-seq data that have been generated using different Illumina platforms and compare them together. We used the normalized raw counts tables from each study, which can be found in the GEO website database under each unique corresponding GSE number for each study. We compared Normal (Control) or IPF patients samples using the package DESeq2 in R and plotted the log2-fold change ad −log10 p values to get the characteristic Volcano plot.
DATA AND CODE AVAILABILITY
The accession number of the RNAseq reported in this paper is GEO:GSE121999. The accession numbers of the publicly available RNAseq data used in this paper are GEO:GSE92592 (Figure S5A), GEO:GSE99621 (Figure S5B,C), and GEO:GSE52463.
Supplementary Material
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| PDGFRA | Cell Signaling Technology | Cat#5241, RRID:AB_10692773 |
| PDGFRB | Cell Signaling Technology | Cat#3169, RRID:AB_2162497 |
| VIMENTIN | Cell Signaling Technology | Cat#5741, RRID:AB_10695459 |
| COLLAGEN I | Abcam | Cat#ab34710, RRID:AB_731684 |
| COLLAGEN III | Abcam | Cat#ab7778, RRID:AB_306066 |
| SMA | Abcam | Cat#ab32575, RRID:AB_722538 |
| FIBRONECTIN | Abcam | Cat#ab6328, RRID:AB_305428 |
| SLUG | Cell Signaling Technology | Cat#9585, RRID:AB_305428 |
| AP3B1 (HPS2) | Proteintech Group | Cat#13384–1-AP, RRID:AB_2056499 |
| HPS4 | Bethyl | Cat#A305–513A, RRID:AB_2755125 |
| BLOC1S3 (HPS8) | Bethyl | Cat#A304–612A, RRID:AB_2620807 |
| ACTIN | EMD-Millipore | Cat#MAB1501, RRID:AB_2223041 |
| STAT3 | Cell Signaling Technology | Cat#9139, RRID:AB_331757 |
| PHOSPHO-STAT3 | Cell Signaling Technology | Cat#9145, RRID:AB_2491009 |
| Biological Samples | ||
| Frozen lung sections from IPS and HPS patient | Columbia University Department of Pathology | n/a |
| Chemicals, Peptides, and Recombinant Proteins | ||
| RNAscope Probe - Hs-IL11 | Advanced Cell Diagnostics | 425281 |
| RNAscope Probe - Hs-SFTPC-C2 | Advanced Cell Diagnostics | 452561-C2 |
| RNAscope Probe - Hs-EPCAM-C3 | Advanced Cell Diagnostics | 310281-C3 |
| RNAscope® Multiplex Fluorescent Reagent Kit V2 | Advanced Cell Diagnostics | 323100 |
| RNAscope® 3-plex Positive Control Probe_Hs | Advanced Cell Diagnostics | 320861 |
| Opal Multiplex IHC Kit | PerkinElmer | NEL811001KT |
| Recombinant Human IL-11 protein | R&D Systems | 218-IL-005 |
| Critical Commercial Assays | ||
| Human IL 11 ELISA Kit | Fisher Scientific | EHIL11 |
| Deposited Data | ||
| Raw and analyzed data | This paper | GEO:GSE121999 |
| Experimental Models: Cell Lines | ||
| Rockefeller University Embryonic Stem Cell Line 2 (RUES2, NIH approval number NIHhESC-09–0013, Registration number 0013 | Dr. Ali Brivanlou | n/a |
| Oligonucleotides | ||
| HPS2-targeting guide-RNA sequence primer 5′-caccgGCTGAAAAGTGCTTATGGAC-3′ | This paper | n/a |
| HPS4–1-targeting guide-RNA sequence primer 5′- caccgTGTCAGCTGCAAGCGGTTTC-3′ | This paper | n/a |
| HPS4–2-targeting guide-RNA sequence primer 5′- caccgAACCGCTTGCAGCTGACATC-3′ | This paper | n/a |
| HPS4–3-targeting guide-RNA sequence primer 5′- caccgCGGTTTCTGGATCAGCTAGT-3′ | This paper | n/a |
| HPS8-targeting guide-RNA sequence primer 5′- caccgCTCTGCGTCCTCGTCGGAGG-3′ | This paper | n/a |
| IL11-targeting guide-RNA sequence primer 5′- caccgGCTCTCTCCTGGCGGACACG-3′ | This paper | n/a |
| Recombinant DNA | ||
| pX330-U6-Chimeric_BB-CBh-hSpCas9 | Cong et al., 2013 | Addgene Plasmid #42230 |
| Software and Algorithms | ||
| R project (DESeq2, ggplot2, ggrepel) | R project | n/a |
| Keygenes | Roost et al., 2015 | n/a |
| Leica X Software | Leica | n/a |
| ImageJ | NIH | https://imagej.nih.gov/ij/ |
| Phenochart 1.0 software | PerkinElmer | n/a |
| the inForm 2.4.2 software | PerkinElmer | n/a |
| TopHat2 | Trapnell et al., 2012 | http://ccb.jhu.edu/software/tophat/index.shtml |
| Cufflinks | Trapnell et al., 2012 | http://cole-trapnell-lab.github.io/cufflinks/ |
| Other | ||
| Re-analyzed data | Luzina et al., 2018 | GEO: GSE99621 |
| Re-analyzed data | Schafer et al., 2017a | GEO: GSE92592 |
| Re-analyzed data | Nance et al., 2014 | GEO: GSE52463 |
Highlights.
HPS-associated pulmonary fibrosis can be modeled in ESC-derived lung organoids
Interleukin-11 is required for fibrosis in lung organoids
Interleukin expression is elevated in lungs of pulmonary fibrosis patients
ACKNOWLEDGMENTS
This work was supported by the NIH (1U01HL134760 to H.-W.S.) and the Thomas R Kully IPF Research Fund (to H.-W.S.). Flow cytometry was performed in the CCTI Flow Cytometry Core, supported in part by the Office of the Director, NIH, under awards S10RR027050 and S10OD020056. A. Strikoudis is a New York Stem Cell Foundation Druckenmiller Fellow. We thank the Human Immune Monitoring Core (HIMC) at Columbia University Irving Medical Center (RRID: SCR_016740) for technical advice and support with the in situ hybridization studies.
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental Information can be found online at https://doi.org/10.1016/j.celrep.2019.05.077.
DECLARATION OF INTERESTS
The authors declare no competing interests.
REFERENCES
- Alder JK, Chen JJ, Lancaster L, Danoff S, Su SC, Cogan JD, Vulto I, Xie M, Qi X, Tuder RM, et al. (2008). Short telomeres are a risk factor for idiopathic pulmonary fibrosis. Proc. Natl. Acad. Sci. USA 105, 13051–13056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alder JK, Cogan JD, Brown AF, Anderson CJ, Lawson WE, Lansdorp PM, Phillips JA 3rd, Loyd JE, Chen JJ, and Armanios M (2011). Ancestral mutation in telomerase causes defects in repeat addition processivity and manifests as familial pulmonary fibrosis. PLoS Genet. 7, e1001352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alder JK, Stanley SE, Wagner CL, Hamilton M, Hanumanthu VS, and Armanios M (2015a). Exome sequencing identifies mutant TINF2 in a family with pulmonary fibrosis. Chest 147, 1361–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alder JK, Barkauskas CE, Limjunyawong N, Stanley SE, Kembou F, Tuder RM, Hogan BL, Mitzner W, and Armanios M (2015b). Telomere dysfunction causes alveolar stem cell failure. Proc. Natl. Acad. Sci. USA 112, 5099–5104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Thoracic Society; European Respiratory Society (2002). American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am. J. Respir. Crit. Care Med 165, 277–304. [DOI] [PubMed] [Google Scholar]
- Anderson PD, Huizing M, Claassen DA, White J, and Gahl WA (2003). Hermansky-Pudlak syndrome type 4 (HPS-4): clinical and molecular characteristics. Hum. Genet 113, 10–17. [DOI] [PubMed] [Google Scholar]
- Armanios M (2012a). Telomerase and idiopathic pulmonary fibrosis. Mutat. Res 730, 52–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armanios M (2012b). Telomerase mutations and the pulmonary fibrosis-bone marrow failure syndrome complex. N. Engl. J. Med 367, 384. [DOI] [PubMed] [Google Scholar]
- Armanios MY, Chen JJ, Cogan JD, Alder JK, Ingersoll RG, Markin C, Lawson WE, Xie M, Vulto I, Phillips JA 3rd., et al. (2007). Telomerase mutations in families with idiopathic pulmonary fibrosis. N. Engl. J. Med 356, 1317–1326. [DOI] [PubMed] [Google Scholar]
- Barkauskas CE, Cronce MJ, Rackley CR, Bowie EJ, Keene DR, Stripp BR, Randell SH, Noble PW, and Hogan BL (2013). Type 2 alveolar cells are stem cells in adult lung. J. Clin. Invest 123, 3025–3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer Y, Tedrow J, de Bernard S, Birker-Robaczewska M, Gibson KF, Guardela BJ, Hess P, Klenk A, Lindell KO, Poirey S, et al. (2015). A novel genomic signature with translational significance for human idiopathic pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol 52, 217–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YW, Huang SX, de Carvalho ALRT, Ho SH, Islam MN, Volpi S, Notarangelo LD, Ciancanelli M, Casanova JL, Bhattacharya J, et al. (2017). A three-dimensional model of human lung development and disease from pluripotent stem cells. Nat. Cell Biol 19, 542–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coll M, Perea L, Boon R, Leite SB, Vallverdu J, Mannaerts I, Smout A, El Taghdouini A, Blaya D, Rodrigo-Torres D, et al. (2018). Generation of Hepatic Stellate Cells from Human Pluripotent Stem Cells Enables In Vitro Modeling of Liver Fibrosis. Cell Stem Cell 23, 101–113.e7. [DOI] [PubMed] [Google Scholar]
- Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, Hsu PD, Wu X, Jiang W, Marraffini LA, and Zhang F (2013). Multiplex genome engineering using CRISPR/Cas systems. Science 339, 819–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corral J, González-Conejero R, Pujol-Moix N, Domenech P, and Vicente V (2004). Mutation analysis of HPS1, the gene mutated in Hermansky-Pudlak syndrome, in patients with isolated platelet dense-granule deficiency. Haematologica 89, 325–329. [PubMed] [Google Scholar]
- Cugola FR, Fernandes IR, Russo FB, Freitas BC, Dias JL, Guimaraães KP, Benazzato C, Almeida N, Pignatari GC, Romero S, et al. (2016). The Brazilian Zika virus strain causes birth defects in experimental models. Nature 534, 267–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullinane AR, Curry JA, Golas G, Pan J, Carmona-Rivera C, Hess RA, White JG, Huizing M, and Gahl WA (2012). A BLOC-1 mutation screen reveals a novel BLOC1S3 mutation in Hermansky-Pudlak Syndrome type 8. Pigment Cell Melanoma Res. 25, 584–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang J, Tiwari SK, Lichinchi G, Qin Y, Patil VS, Eroshkin AM, and Rana TM (2016). Zika Virus Depletes Neural Progenitors in Human Cerebral Organoids through Activation of the Innate Immune Receptor TLR3. Cell Stem Cell 19, 258–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai TJ, Brownfield DG, and Krasnow MA (2014). Alveolar progenitor and stem cells in lung development, renewal and cancer. Nature 507, 190–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drost J, and Clevers H (2017). Translational applications of adult stem cell-derived organoids. Development 144, 968–975. [DOI] [PubMed] [Google Scholar]
- Dye BR, Hill DR, Ferguson MA, Tsai YH, Nagy MS, Dyal R, Wells JM, Mayhew CN, Nattiv R, Klein OD, et al. (2015). In vitro generation of human pluripotent stem cell derived lung organoids. eLife 4, e05098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dye BR, Dedhia PH, Miller AJ, Nagy MS, White ES, Shea LD, and Spence JR (2016). A bioengineered niche promotes in vivo engraftment and maturation of pluripotent stem cell derived human lung organoids. eLife 5, e19732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng L, Novak EK, Hartnell LM, Bonifacino JS, Collinson LM, and Swank RT (2002). The Hermansky-Pudlak syndrome 1 (HPS1) and HPS2 genes independently contribute to the production and function of platelet dense granules, melanosomes, and lysosomes. Blood 99, 1651–1658. [PubMed] [Google Scholar]
- Fernandez IE, Amarie OV, Mutze K, Königshoff M, Yildirim AO, and Eickelberg O (2016). Systematic phenotyping and correlation of biomarkers with lung function and histology in lung fibrosis. Am. J. Physiol. Lung Cell. Mol. Physiol 310, L919–L927. [DOI] [PubMed] [Google Scholar]
- Fingerlin TE, Murphy E, Zhang W, Peljto AL, Brown KK, Steele MP, Loyd JE, Cosgrove GP, Lynch D, Groshong S, et al. (2013). Genome-wide association study identifies multiple susceptibility loci for pulmonary fibrosis. Nat. Genet 45, 613–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firth AL, Dargitz CT, Qualls SJ, Menon T, Wright R, Singer O, Gage FH, Khanna A, and Verma IM (2014). Generation of multiciliated cells in functional airway epithelia from human induced pluripotent stem cells. Proc. Natl. Acad. Sci. USA 111, E1723–E1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcez PP, Loiola EC, Madeiro da Costa R, Higa LM, Trindade P, Delvecchio R, Nascimento JM, Brindeiro R, Tanuri A, and Rehen SK (2016). Zika virus impairs growth in human neurospheres and brain organoids. Science 352, 816–818. [DOI] [PubMed] [Google Scholar]
- Gotoh S, Ito I, Nagasaki T, Yamamoto Y, Konishi S, Korogi Y, Matsumoto H, Muro S, Hirai T, Funato M, et al. (2014). Generation of alveolar epithelial spheroids via isolated progenitor cells from human pluripotent stem cells. Stem Cell Reports 3, 394–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hengst M, Naehrlich L, Mahavadi P, Grosse-Onnebrink J, Terheggen-Lagro S, Skanke LH, Schuch LA, Brasch F, Guenther A, Reu S, et al. (2018). Hermansky-Pudlak syndrome type 2 manifests with fibrosing lung disease early in childhood. Orphanet J. Rare Dis 13, 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heo I, Dutta D, Schaefer DA, Iakobachvili N, Artegiani B, Sachs N, Boonekamp KE, Bowden G, Hendrickx APA, Willems RJL, et al. (2018). Modelling Cryptosporidium infection in human small intestinal and lung organoids. Nat. Microbiol 3, 814–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiler D, Chen X, Hazen J, Kupriyanov S, Carroll PA, Qu C, Xu B, Johnson D, Griffiths L, Frase S, et al. (2015). Quantification of Retinogenesis in 3D Cultures Reveals Epigenetic Memory and Higher Efficiency in iPSCs Derived from Rod Photoreceptors. Cell Stem Cell 17, 101–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang SX, Islam MN, O’Neill J, Hu Z, Yang YG, Chen YW, Mumau M, Green MD, Vunjak-Novakovic G, Bhattacharya J, and Snoeck HW (2014). Efficient generation of lung and airway epithelial cells from human pluripotent stem cells. Nat. Biotechnol 32, 84–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang SX, Green MD, de Carvalho AT, Mumau M, Chen YW, D’Souza SL, and Snoeck HW (2015). The in vitro generation of lung and airway progenitor cells from human pluripotent stem cells. Nat. Protoc 10, 413–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huizing M, Scher CD, Strovel E, Fitzpatrick DL, Hartnell LM, Anikster Y, and Gahl WA (2002). Nonsense mutations in ADTB3A cause complete deficiency of the beta3A subunit of adaptor complex-3 and severe Herman-sky-Pudlak syndrome type 2. Pediatr. Res 51, 150–158. [DOI] [PubMed] [Google Scholar]
- Huizing M, Helip-Wooley A, Westbroek W, Gunay-Aygun M, and Gahl WA (2008). Disorders of lysosome-related organelle biogenesis: clinical and molecular genetics. Annu. Rev. Genomics Hum. Genet 9, 359–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue H, Nagata N, Kurokawa H, and Yamanaka S (2014). iPS cells: a game changer for future medicine. EMBO J. 33, 409–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob A, Morley M, Hawkins F, McCauley KB, Jean JC, Heins H, Na CL, Weaver TE, Vedaie M, Hurley K, et al. (2017). Differentiation of Human Pluripotent Stem Cells into Functional Lung Alveolar Epithelial Cells. Cell Stem Cell 21, 472–488.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kage H, and Borok Z (2012). EMT and interstitial lung disease: a mysterious relationship. Curr. Opin. Pulm. Med 18, 517–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K, Doi A, Wen B, Ng K, Zhao R, Cahan P, Kim J, Aryee MJ, Ji H, Ehrlich LI, et al. (2010). Epigenetic memory in induced pluripotent stem cells. Nature 467, 285–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King TE Jr., Bradford WZ, Castro-Bernardini S, Fagan EA, Glaspole I, Glassberg MK, Gorina E, Hopkins PM, Kardatzke D, Lancaster L, et al. ; ASCEND Study Group (2014). A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N. Engl. J. Med 370, 2083–2092. [DOI] [PubMed] [Google Scholar]
- Konishi S, Gotoh S, Tateishi K, Yamamoto Y, Korogi Y, Nagasaki T, Matsumoto H, Muro S, Hirai T, Ito I, et al. (2016). Directed Induction of Functional Multi-ciliated Cells in Proximal Airway Epithelial Spheroids from Human Pluripotent Stem Cells. Stem Cell Reports 6, 18–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotani I, Sato A, Hayakawa H, Urano T, Takada Y, and Takada A (1995). Increased procoagulant and antifibrinolytic activities in the lungs with idiopathic pulmonary fibrosis. Thromb. Res 77, 493–504. [DOI] [PubMed] [Google Scholar]
- Kramann R, Schneider RK, DiRocco DP, Machado F, Fleig S, Bondzie PA, Henderson JM, Ebert BL, and Humphreys BD (2015). Perivascular Gli1+ progenitors are key contributors to injury-induced organ fibrosis. Cell Stem Cell 16, 51–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster MA, Renner M, Martin CA, Wenzel D, Bicknell LS, Hurles ME, Homfray T, Penninger JM, Jackson AP, and Knoblich JA (2013). Cerebral organoids model human brain development and microcephaly. Nature 501, 373–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson WE, Grant SW, Ambrosini V, Womble KE, Dawson EP, Lane KB, Markin C, Renzoni E, Lympany P, Thomas AQ, et al. (2004). Genetic mutations in surfactant protein C are a rare cause of sporadic cases of IPF. Thorax 59, 977–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederer DJ, and Martinez FJ (2018). Idiopathic Pulmonary Fibrosis.N. Engl. J. Med 379, 797–798. [DOI] [PubMed] [Google Scholar]
- Loyd JE (2003). Pulmonary fibrosis in families. Am. J. Respir. Cell Mol. Biol 29 (Suppl), S47–S50. [PubMed] [Google Scholar]
- Luzina IG, Salcedo MV, Rojas-Peña ML, Wyman AE, Galvin JR, Sachdeva A, Clerman A, Kim J, Franks TJ, Britt EJ, et al. (2018). Transcriptomic evidence of immune activation in macroscopically normal-appearing and scarred lung tissues in idiopathic pulmonary fibrosis. Cell. Immunol 325, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzio JP, Hackmann Y, Dieckmann NM, and Griffiths GM (2014). The biogenesis of lysosomes and lysosome-related organelles. Cold Spring Harb. Perspect. Biol 6, a016840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahavadi P, Guenther A, and Gochuico BR (2012). Hermansky-Pudlak syndrome interstitial pneumonia: it’s the epithelium, stupid!. Am. J. Respir. Crit. Care Med 186, 939–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matute-Bello G, Frevert CW, and Martin TR (2008). Animal models of acute lung injury. Am. J. Physiol. Lung Cell. Mol. Physiol 295, L379–L399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCauley HA, and Wells JM (2017). Pluripotent stem cell-derived organoids: using principles of developmental biology to grow human tissues in a dish. Development 144, 958–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCauley KB, Hawkins F, Serra M, Thomas DC, Jacob A, and Kotton DN (2017). Efficient Derivation of Functional Human Airway Epithelium from Pluripotent Stem Cells via Temporal Regulation of Wnt Signaling. Cell Stem Cell 20, 844–857.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCracken KW, Catá EM, Crawford CM, Sinagoga KL, Schumacher M, Rockich BE, Tsai YH, Mayhew CN, Spence JR, Zavros Y, and Wells JM (2014). Modelling human development and disease in pluripotent stem-cell-derived gastric organoids. Nature 516, 400–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCurry KR, Shearon TH, Edwards LB, Chan KM, Sweet SC, Vala-pour M, Yusen R, and Murray S (2009). Lung transplantation in the United States, 1998–2007. Am. J. Transplant 9, 942–958. [DOI] [PubMed] [Google Scholar]
- Moeller A, Ask K, Warburton D, Gauldie J, and Kolb M (2008). The bleomycin animal model: a useful tool to investigate treatment options for idiopathic pulmonary fibrosis? Int. J. Biochem. Cell Biol 40, 362–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore BB, and Hogaboam CM (2008). Murine models of pulmonary fibrosis. Am. J. Physiol. Lung Cell. Mol. Physiol 294, L152–L160. [DOI] [PubMed] [Google Scholar]
- Morgan NV, Pasha S, Johnson CA, Ainsworth JR, Eady RA, Dawood B, McKeown C, Trembath RC, Wilde J, Watson SP, and Maher ER (2006). A germline mutation in BLOC1S3/reduced pigmentation causes a novel variant of Hermansky-Pudlak syndrome (HPS8). Am. J. Hum. Genet 78, 160–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mou H, Zhao R, Sherwood R, Ahfeldt T, Lapey A, Wain J, Sicilian L, Izvolsky K, Musunuru K, Cowan C, and Rajagopal J (2012). Generation of multipotent lung and airway progenitors from mouse ESCs and patient-specific cystic fibrosis iPSCs. Cell Stem Cell 10, 385–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulugeta S, Nureki S, and Beers MF (2015). Lost after translation: insights from pulmonary surfactant for understanding the role of alveolar epithelial dysfunction and cellular quality control in fibrotic lung disease. Am. J. Physiol. Lung Cell. Mol. Physiol 309, L507–L525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabhan AN, Brownfield DG, Harbury PB, Krasnow MA, and Desai TJ (2018). Single-cell Wnt signaling niches maintain stemness of alveolar type 2 cells. Science 359, 1118–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nance T, Smith KS, Anaya V, Richardson R, Ho L, Pala M, Mostafavi S, Battle A, Feghali-Bostwick C, Rosen G, and Montgomery SB (2014). Transcriptome analysis reveals differential splicing events in IPF lung tissue. PLoS One 9, e92111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble PW, Barkauskas CE, and Jiang D (2012). Pulmonary fibrosis: patterns and perpetrators. J. Clin. Invest 122, 2756–2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogee LM, Dunbar AE 3rd, Wert SE, Askin F, Hamvas A, and Whitsett JA (2001). A mutation in the surfactant protein C gene associated with familial interstitial lung disease. N. Engl. J. Med 344, 573–579. [DOI] [PubMed] [Google Scholar]
- Nureki SI, Tomer Y, Venosa A, Katzen J, Russo SJ, Jamil S, Barrett M, Nguyen V, Kopp M, Mulugeta S, and Beers MF (2018). Expression of mutant Sftpc in murine alveolar epithelia drives spontaneous lung fibrosis.J. Clin. Invest 128, 4008–4024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh J, Ho L, Ala-Mello S, Amato D, Armstrong L, Bellucci S, Carakushansky G, Ellis JP, Fong CT, Green JS, et al. (1998). Mutation analysis of patients with Hermansky-Pudlak syndrome: a frameshift hot spot in the HPS gene and apparent locus heterogeneity. Am. J. Hum. Genet 62, 593–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian X, Nguyen HN, Song MM, Hadiono C, Ogden SC, Hammack C, Yao B, Hamersky GR, Jacob F, Zhong C, et al. (2016). Brain-Region-Specific Organoids Using Mini-bioreactors for Modeling ZIKV Exposure. Cell 165, 1238–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray P, Tang W, Wang P, Homer R, Kuhn C 3rd, Flavell RA, and Elias JA (1997). Regulated overexpression of interleukin 11 in the lung. Use to dissociate development-dependent and -independent phenotypes. J. Clin. Invest 100, 2501–2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richeldi L, du Bois RM, Raghu G, Azuma A, Brown KK, Costabel U, Cottin V, Flaherty KR, Hansell DM, Inoue Y, et al. ; INPULSIS Trial Investigators (2014). Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N. Engl. J. Med 370, 2071–2082. [DOI] [PubMed] [Google Scholar]
- Rock JR, Barkauskas CE, Cronce MJ, Xue Y, Harris JR, Liang J, Noble PW, and Hogan BL (2011). Multiple stromal populations contribute to pulmonary fibrosis without evidence for epithelial to mesenchymal transition. Proc. Natl. Acad. Sci. USA 108, E1475–E1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roost MS, van Iperen L, Ariyurek Y, Buermans HP, Arindrarto W, Devalla HD, Passier R, Mummery CL, Carlotti F, de Koning EJ, et al. (2015). KeyGenes, a Tool to Probe Tissue Differentiation Using a Human Fetal Transcriptional Atlas. Stem Cell Reports 4, 1112–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouhani F, Kumasaka N, de Brito MC, Bradley A, Vallier L, and Gaffney D (2014). Genetic background drives transcriptional variation in human induced pluripotent stem cells. PLoS Genet. 10, e1004432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu JH, Moua T, Daniels CE, Hartman TE, Yi ES, Utz JP, and Limper AH (2014). Idiopathic pulmonary fibrosis: evolving concepts. Mayo Clin. Proc 89, 1130–1142. [DOI] [PubMed] [Google Scholar]
- Schafer MJ, White TA, Iijima K, Haak AJ, Ligresti G, Atkinson EJ, Oberg AL, Birch J, Salmonowicz H, Zhu Y, et al. (2017a). Cellular senescence mediates fibrotic pulmonary disease. Nat. Commun 8, 14532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer S, Viswanathan S, Widjaja AA, Lim WW, Moreno-Moral A, DeLaughter DM, Ng B, Patone G, Chow K, Khin E, et al. (2017b). IL-11 is a crucial determinant of cardiovascular fibrosis. Nature 552, 110–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwertschlag US, Trepicchio WL, Dykstra KH, Keith JC, Turner KJ, and Dorner AJ (1999). Hematopoietic, immunomodulatory and epithelial effects of interleukin-11. Leukemia 13, 1307–1315. [DOI] [PubMed] [Google Scholar]
- Seibold MA, Wise AL, Speer MC, Steele MP, Brown KK, Loyd JE, Fingerlin TE, Zhang W, Gudmundsson G, Groshong SD, et al. (2011). A common MUC5B promoter polymorphism and pulmonary fibrosis. N. Engl. J. Med 364, 1503–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seward SL Jr., and Gahl WA (2013). Hermansky-Pudlak syndrome: health care throughout life. Pediatrics 132, 153–160. [DOI] [PubMed] [Google Scholar]
- Spence JR, Mayhew CN, Rankin SA, Kuhar MF, Vallance JE, Tolle K, Hoskins EE, Kalinichenko VV, Wells SI, Zorn AM, et al. (2011). Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature 470, 105–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele MP, and Schwartz DA (2013). Molecular mechanisms in progressive idiopathic pulmonary fibrosis. Annu. Rev. Med 64, 265–276. [DOI] [PubMed] [Google Scholar]
- Stuart BD, Choi J, Zaidi S, Xing C, Holohan B, Chen R, Choi M, Dharwadkar P, Torres F, Girod CE, et al. (2015). Exome sequencing links mutations in PARN and RTEL1 with familial pulmonary fibrosis and telomere shortening. Nat. Genet 47, 512–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W, Geba GP, Zheng T, Ray P, Homer RJ, Kuhn C 3rd, Flavell RA, and Elias JA (1996). Targeted expression of IL-11 in the murine airway causes lymphocytic inflammation, bronchial remodeling, and airways obstruction. J. Clin. Invest 98, 2845–2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanjore H, Cheng DS, Degryse AL, Zoz DF, Abdolrasulnia R, Lawson WE, and Blackwell TS (2011). Alveolar epithelial cells undergo epithelial-tomesenchymal transition in response to endoplasmic reticulum stress. J. Biol. Chem 286, 30972–30980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas AQ, Lane K, Phillips J 3rd, Prince M, Markin C, Speer M, Schwartz DA, Gaddipati R, Marney A, Johnson J, et al. (2002). Heterozygosity for a surfactant protein C gene mutation associated with usual interstitial pneumonitis and cellular nonspecific interstitial pneumonitis in one kindred. Am. J. Respir. Crit. Care Med 165, 1322–1328. [DOI] [PubMed] [Google Scholar]
- Trapnell C, Roberts A, Goff L, Pertea G, Kim D, Kelley DR, Pimentel H, Salzberg SL, Rinn JL, and Pachter L (2012). Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat. Protoc 7, 562–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicary GW, Vergne Y, Santiago-Cornier A, Young LR, and Roman J (2016). Pulmonary Fibrosis in Hermansky-Pudlak Syndrome. Ann. Am. Thorac. Soc 13, 1839–1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Kuan PJ, Xing C, Cronkhite JT, Torres F, Rosenblatt RL, Di-Maio JM, Kinch LN, Grishin NV, and Garcia CK (2009). Genetic defects in surfactant protein A2 are associated with pulmonary fibrosis and lung cancer. Am. J. Hum. Genet 84, 52–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolters PJ, Collard HR, and Jones KD (2014). Pathogenesis of idiopathic pulmonary fibrosis. Annu. Rev. Pathol 9, 157–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong AP, Bear CE, Chin S, Pasceri P, Thompson TO, Huan LJ, Ratjen F, Ellis J, and Rossant J (2012). Directed differentiation of human pluripotent stem cells into mature airway epithelia expressing functional CFTR protein. Nat. Biotechnol 30, 876–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Mizuno T, Sridharan A, Du Y, Guo M, Tang J, Wikenheiser-Brokamp KA, Perl AT, Funari VA, Gokey JJ, et al. (2016). Single-cell RNA sequencing identifies diverse roles of epithelial cells in idiopathic pulmonary fibrosis. JCI Insight 1, e90558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang IV, Fingerlin TE, Evans CM, Schwarz MI, and Schwartz DA (2015). MUC5B and Idiopathic Pulmonary Fibrosis. Ann. Am. Thorac. Soc 12 (Suppl 2), S193–S199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young LR, Gulleman PM, Bridges JP, Weaver TE, Deutsch GH, Blackwell TS, and McCormack FX (2012). The alveolar epithelium deter mines susceptibility to lung fibrosis in Hermansky-Pudlak syndrome. Am. J. Respir. Crit. Care Med. 186, 1014–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacharias WJ, Frank DB, Zepp JA, Morley MP, Alkhaleel FA, Kong J, Zhou S, Cantu E, and Morrisey EE (2018). Regeneration of the lung alveolus by an evolutionarily conserved epithelial progenitor. Nature 555, 251–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Noth I, Garcia JG, and Kaminski N (2011). A variant in the promoter of MUC5B and idiopathic pulmonary fibrosis. N. Engl. J. Med 364, 1576–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Q, Zhou B, Ann DK, Minoo P, Liu Y, Banfalvi A, Krishnaveni MS, Dubourd M, Demaio L, Willis BC, et al. (2011). Role of endoplasmic reticulum stress in epithelial-mesenchymal transition of alveolar epithelial cells: effects of misfolded surfactant protein. Am. J. Respir. Cell Mol. Biol 45, 498–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The accession number of the RNAseq reported in this paper is GEO:GSE121999. The accession numbers of the publicly available RNAseq data used in this paper are GEO:GSE92592 (Figure S5A), GEO:GSE99621 (Figure S5B,C), and GEO:GSE52463.