Abstract
Voluntary participation in behavioural studies offers several scientific, management, and welfare benefits to non-human primates (NHPs). Aside from the scientific benefit of increased understanding of NHP cognition, sociality, and behaviour derived from noninvasive behavioural studies, participation itself has the potential to provide functional simulations of natural behaviours, enrichment opportunities, and increased control over the captive environment, all of which enhance welfare. Despite a developing consensus that voluntary participation offers these welfare enhancements, little research has empirically investigated the ways that participation in behavioural studies may affect welfare. In the current study, we investigated potential relationships between captive chimpanzee welfare and long-term, repeated voluntary participation in noninvasive behavioural studies. We collected behavioural data on 118 chimpanzees at the National Center for Chimpanzee Care (NCCC) in Bastrop, Texas, USA between 2016 and 2018 using 15-minute focal animal samples. Additionally, we collected information on 41 behavioural studies conducted between 2010 and 2018 with the NCCC chimpanzees that involved exposure to a stimulus or manipulation. The total number of behavioural studies in which chimpanzees had participated over the approximately eight-year period was then examined in relation to levels of behavioural diversity, abnormal behaviour, rough scratching, inactivity, and locomotion using a series of regression analyses that controlled for rearing status and age of the chimpanzee at the time of data collection. Analyses revealed significant, positive relationships between the total number of studies in which chimpanzees participated and 1) behavioural diversity scores, R2adj = .21, F(3,114) = 11.25, p < 0.001; and 2) rough scratching, R2adj = .11, F(3,114) = 6.01, p = 0.001. The positive relationship between behavioural diversity scores and the total number of studies in which chimpanzees participated seems unsurprising, although we cannot draw conclusions about the directionality of this relationship. The result that rough scratching and the total number of studies in which chimpanzees participated were positively correlated is unexpected. However, rough scratching made up less than 1% of all activity in the current study, and as such, this result may not be biologically meaningful. These findings suggest that participation in behavioural studies is not likely to be detrimental to chimpanzee well-being, and may even be beneficial. Data such as these, which empirically investigate existing recommendations can help inform decisions pertaining to the participation of chimpanzees in behavioural research.
Keywords: Behaviour, Welfare, Captive Chimpanzee, Voluntary Participation, Behavioural Research
1. Introduction
Although there is a developing consensus that voluntary participation in behavioural studies provides various scientific and welfare benefits to non-human primates (NHPs), there is limited empirical data regarding the relationships between participation in behavioural studies and NHP welfare (Clark, 2011; Clark and Smith, 2013; Yamanashi & Hayashi, 2011; Reamer, Haller, Lambeth, & Schapiro, 2017; Hopkins & Latzman 2017; Hopper et al., 2016; Ross, 2010; Neal Webb, Hau, & Schapiro, 2018b). Beyond the benefit of increased understanding of NHP behaviour, sociality, and cognition, voluntary participation in behavioural studies has the potential to provide NHPs with enrichment opportunities, increased control over the captive environment, and functional simulations of natural conditions/behaviours, all of which should enhance welfare.
Voluntary participation in veterinary and behavioral management procedures is an important component of captive NHP welfare (Baker et al., 2017; Lambeth et al., 2006; Reamer et al., 2017; Reinhardt 1997). Several studies have shown that voluntary participation in veterinary- and management-related procedures results in enhanced well-being (Baker 2016; Baker, Weed, Crocket, & Bloomsmith, 2007; Bridges, Mocarski, Lambeth, & Schapiro, 2013; Laule & Whittaker 2002; Laule & Whittaker 2007; Magden et al., 2013; Graham 2017; Magden 2017; Reamer et al., 2017; Reinhardt 1997). A logical extension of this is that voluntary participation in behavioural studies results in enhanced well-being, as it is thought to be intrinsically rewarding (Tarou & Bashaw, 2007) and enriching (Bloomsmith, Ross, & Baker, 2000; Hopkins & Latzman, 2017; Ross, 2010; Yamanashi & Hayashi, 2011). NHPs repeatedly return to participate in various types of behavioural studies, and behavioural signs of distress are typically absent, implying that these experiences may be rewarding from the NHP’s perspective (Hopkins & Latzman, 2017; Tarou & Bashaw, 2007; Watanabe, 2007). There also seem to be indirect benefits of participation that extend beyond the individual that is participating. Hopper, Shender, and Ross (2016) found that even chimpanzees that did not participate in a token-exchange experiment showed increased activity during the experimental period. Furthermore, some evidence suggests that the procedures of behavioural studies can be inherently enriching. For example, the TUBE task, used to assess handedness, and behavioural and hemispheric asymmetry, provides foraging opportunities and functional simulations of natural tool-use behaviour (Hopkins & Latzman, 2017).
A growing body of literature suggests that participation specifically in cognitive studies enhances well-being (for an overview, see Ross, 2010). In rhesus macaques, interaction with a joystick cognitive testing paradigm “replaced” various abnormal behaviours (Washburn & Rumbaugh, 1992), whereas supplemental enrichment had no such effect. Furthermore, rhesus monkeys actively avoided situations in which their previous choice cost them access to the task, suggesting that the task itself is valued beyond the food reward (Washburn & Rumbaugh, 1992; Ross, 2010). Additionally, chimpanzees show a high level of interest in, and utilization of, cognitive tasks, as evidenced by continued proximity to, and/or participation in, the task (Clark & Smith, 2013; Herelko, Vick, & Buchanan-Smith, 2012; Hopper, Shender, & Ross, 2016; Ross, 2010), and have shown decreased solitary and self-directed behaviours while computerized tasks are accessible (Bloomsmith et al., 2000). The importance of participation in cognitive research is evident in the recent designs of zoo enclosures for NHPs, which now allow and encourage animals to voluntarily participate in cognitive research while on exhibit in the social group (Egelkamp, Hopper, Cronin, Jacobson, & Ross, 2016; Hopper, 2017).
Empirical data from behavioural studies are essential for refining the care of captive non-human primates (Schapiro, 2017). Improvements specifically to the care of captive chimpanzees have often been the direct result of findings from behavioural studies, including, but not limited to, improvements in social and structural housing, enrichment, rearing methods, and health care (Ross, 2010; Schapiro et al., 2017). Indeed, some apparatus and stimuli that were originally used for behavioural research projects are now provided as part of everyday enrichment (Ross, 2010). For example, providing simulated termite-mounds for termite fishing promotes species-typical tool-use behaviour, increased activity, and decreased abnormal behaviour, thereby improving psychological well-being (Maki et al., 1989). Findings regarding cognitive capacities and intelligence, trainability, tool use, dominance hierarchies, alliances, and inequity have been applied to, and are utilized in, enclosure design, enrichment development, veterinary procedures, group formation and introductions, and feeding routines (Brent, 2001; Brent, Bloomsmith, & Fisher, 1995; Brosnan et al., 2015; Lambeth, Hau, Perlman, Martino, & Schapiro, 2006; Reamer et al., 2014; Reamer et al., 2017; Ross, Schapiro, Hau, & Lukas, 2009; Russell, Lyn, Schaeffer, & Hopkins, 2011; Taglialatela et al., 2015; Vale et al., 2017a).
In 2016, the National Institutes of Health (NIH) announced that it would no longer fund research with captive chimpanzees, unless the research was consistent with the definition of “noninvasive” research as described in the “Standards of Care for Chimpanzees Held in the Federally Supported Chimpanzee Sanctuary System” (NIH, 2016). However, little research exists as to whether voluntary participation in noninvasive behavioural studies has long-lasting effects on welfare. In the current study, we aimed to provide empirical data that may help inform decisions regarding the participation of chimpanzees in behavioural research. Specifically, we aimed to examine the relationships between captive chimpanzee welfare (using established behavioural indicators of well-being) and long-term, repeated voluntary participation in noninvasive behavioural research studies. To accomplish this, we collected information on dozens of behavioural studies that had been conducted at the National Center for Chimpanzee Care in Bastrop, Texas, USA during an approximately eight-year period, and investigated potential relationships between welfare-related behaviours and the total number of studies in which each chimpanzee had participated. We predicted that participation in more studies would be related to higher levels of overall welfare, including increased affiliative and locomotive behaviour, higher levels of behavioural diversity, and lower levels of rough scratching (a possible indicator of tension and anxiety), abnormal behaviour, and inactivity.
2. Method
2.1. Subjects and Housing
Subjects included 118 captive chimpanzees (72 females and 46 males) living in 17 separate social groups at the National Center for Chimpanzee Care (NCCC), Michale E. Keeling Center for Comparative Medicine and Research (Keeling Center) of The University of Texas MD Anderson Cancer Center (UTMDACC) in Bastrop, Texas, USA. The Keeling Center has been continuously accredited by AAALAC since 1979. Chimpanzees ranged in age from 15-56 (mean age = 31 yrs). There were 76 mother-reared chimpanzees, 27 nursery-reared chimpanzees, and 15 chimpanzees with an unknown rearing history (wild-born). Of the 118 subjects, 58 had been relocated to the NCCC from the Primate Foundation of Arizona (PFA) approximately 10 years prior to data collection for the current study. Chimpanzees were housed in groups ranging from 4-10 individuals per group, in either Primadomes™ or corrals with indoor and outdoor access (Neal Webb et al., 2018a, 2019). The research conducted in this study complied with the approved protocols of the UTMDACC Institutional Animal Care and Use Committee, the legal requirements of the United States, and the International Society for Applied Ethology’s ethical guidelines.
2.2. Procedure
Behavioural observations were collected using 15-min focal animal sampling (Altmann, 1974) on a Dell Latitude E7270 laptop computer (Xiamen, China) running Noldus Observer XT (Leesburg, Virginia, USA, 2010) between August 2016 and May 2018. Focal observations were conducted between 0700 and 1600 and were counterbalanced across morning and afternoon. To be included in analyses, each chimpanzee had to have been observed for a minimum of 22 observation sessions (705.25 hrs of data total). Categories of behaviour included locomotive, affiliative, aggressive, abnormal, self-directed, sexual, object manipulation, inactivity, and other (see Supplementary Materials for ethogram; Neal Webb et al., 2018, 2019). In addition, proximity of chimpanzees to groupmates (near, distant, touching) was recorded (see Supplemental Materials).
2.3. Design
Chimpanzees at the NCCC have participated in numerous noninvasive behavioural studies over an approximately 20-year period. To be included in analyses in the current study, a past behavioural study had to meet two criteria. First, data collection for the study had to have occurred between March 2010 and May 2018. March 2010 was chosen as the beginning date for inclusion in the current study to create a starting point that would be the same for the two populations of chimpanzees housed at the NCCC. Between October 2006 and October 2009, 72 chimpanzees were relocated to the NCCC from the Primate Foundation of Arizona (PFA) in Arizona, USA (see Schapiro et al., 2012 for details). Schapiro et al. (2012) found that the effects of this transport were detectable three months after arrival at the NCCC. Therefore, we chose March of 2010 as an even starting point for behavioural studies in which the PFA and NCCC chimpanzees could participate. Welfare-related behavioural data collection was completed in May 2018. As such, this was chosen as the end date for inclusion of behavioural studies in the current investigation. The second criterion for inclusion was that the study had to involve exposure to a stimulus or manipulation (i.e., the chimpanzee had to have the opportunity to participate in the study). Therefore, observation-only studies were excluded from the present analysis. Due to the breeding moratorium put in place by the NIH in 1998, all chimpanzees at the beginning of the aforementioned study period were adults or subadults. Therefore, no chimpanzees had greater opportunity to participate than any other chimpanzee based on age, given that our investigation was limited to only the most recent eight years of participation.
2.4. Data analysis
Using the criteria listed above, 41 noninvasive behavioural studies were included in the analyses for the current investigation (see Table 1). These included studies primarily from four research areas conducted by long-time collaborative research groups (see Schapiro, 2017), including 1) behavioural laterality, cerebral hemispheric asymmetry, and evolution of language (Hopkins group; Hopkins & Latzman, 2017); 2) inequity and economic games (Brosnan group; Brosnan et al., 2015; Hall, Lambeth, Schapiro, & Brosnan, 2015); 3) social learning and cultural transmission (Whiten and Kendal group; Vale et al., 2017c; Watson et al., 2018); and 4) training, medical, and behavioural management (NCCC group; Reamer et al., 2014). For all studies, chimpanzee participation was completely voluntary. Chimpanzees that chose not to participate in a particular study were simply not subjects in that study. Each chimpanzee did not have an opportunity to participate in all 41 studies included in analyses. Generally, a research coordinator for the chimpanzee colony communicated with the researcher to select chimpanzees based on study method, availability and ease of access to social groups, and, for a limited number of studies, past participation history of the group. For some studies, the goal was to obtain data on all chimpanzees in the NCCC, while for other studies, researchers needed a smaller sample of chimpanzees. Overall, there was no significant difference in the number of studies in which chimpanzees participated based on the social group in which they were housed (one-way ANOVA: F(16,101)=1.12, p =0.35).
Table 1.
Citations by Category of study
| Behavioural Laterality, Cerebral Asymmetries, & Evolution of Language | Hopkins, Russell, & Schaeffer, 2014 | Hopkins et al., 2016 | Hopkins et al., 2017 | Leavens et al., 2001 | Hopkins, Reamer, Mareno, & Schapiro, 2014 | Hopkins, Adams, & Weiss, 2013 |
| Hopkins et al., 2014 | Hopkins, 2013 | Bogart et al., 2014 | Hopkins et al. 2015 | Pope, Mareno, Reamer, Schapiro, & Hopkins, 2014 | Hopkins, unpublished (4) | |
| Social Learning and Cultural Transmission | Watson et al., 2017 | Vale et al., 2016 | Vale et al., In Prep | Vale et al., 2017c | Vale, Flynn, Lambeth, Schapiro, & Kendal, 2014 | Vale et al. 2017b |
| Vale, Davis, Lambeth, Schapiro, & Whiten, 2017c | Claidiere et al., 2015 | Hopper, Price, Freeman, Lambeth, Schapiro, & Kendal, 2014 | Rawlings, unpublished (2) | Davis, Vale, Schapiro, Lambeth, & Whiten, 2016 | Wood, unpublished (1) | |
| Inequity and Economic Games | Hall et al., 2015 | Vale, In prep | Hopper, Lambeth, Schapiro, & Brosnan, 2014 | Hall et al., 2014 | Hopper, Lambeth, Schapiro, & Brosnan 2013 | Brosnan et al., 2015 |
| Behavioural Management, Training | Reamer et al., 2014 | Haller et al., 2016 | Schapiro, Reamer, Mareno, & Lambeth, 2014 | Neal Webb, Hau, & Schapiro, 2018 | Schapiro, unpublished (1) | |
| Other | House, Silk, Lambeth, Schapiro, 2014 | Watson, unpublished (1) |
Note. Some published manuscripts include multiple studies. Numbers in parentheses after "unpublished" denote the number of separate studies for which data were collected but results were either not published or are in preparation.
The 41 studies were categorized as having a procedure that either 1) primarily involved human interaction, or 2) primarily involved exposure only to a stimulus. For the former, the procedure often involved a human experimenter who presented stimuli to the chimpanzee subject(s) (e.g., token-exchange, cognitive test batteries, positive reinforcement training; Claidière et al., 2015; Hopper, Lambeth, Schapiro, & Brosnan, 2013; Reamer et al., 2014), whereas the latter primarily involved a human experimenter setting up and allowing the subject to interact with a stimulus or manipulanda (e.g., tool construction or use, social learning, food or tool sharing, self-recognition, handedness; Bogart et al., 2014; Hopkins et al., 2017; Hopper et al., 2014; Vale, Davis, Lambeth, Schapiro, & Whiten, 2017; House, Silk, Lambeth, & Schapiro, 2014). Using this categorization, a total of 20 studies were classified as human interaction (H) and 21 were classified as stimulus only (S). We then calculated the percentage of studies for each subject that involved primarily human interaction [(H / total number of studies) * 100] for use in later analyses.
Total durations of each welfare-related behaviour were averaged across all observations for each chimpanzee. Average durations of “out-of-view” were removed from the denominator to create a measure of “in-view” behaviour (chimpanzees were “out-of-view” for an average of less than 2% of all observation time). These durations were then converted into percentages of time [Percent Time = (Duration in seconds / 900 seconds) * 100]. A behavioural diversity score was also created for each chimpanzee, representing the average number of different, positive welfare-related behaviours exhibited by chimpanzees across all observations (Neal Webb, Hau, & Schapiro, 2019).
Prior to conducting analyses, assumptions for regression were tested. Q-Q plots showed that the data were normally distributed with the exception of abnormal behaviour, which was positively skewed; however, inspection of the data showed no significant problematic values. The predictors were uncorrelated, as tolerance levels were above 0.2; the errors had a mean of zero, and were homoscedastic as evidenced by visual inspection of scatterplots and histograms.
We first used an ANCOVA with chimpanzee age as a covariate and Bonferroni post-hoc comparisons to assess whether the number of studies in which chimpanzees participated differed by rearing status (mother-reared, nursery-reared, or unknown rearing history). A series of regression analyses were then used to examine the relationships between three predictor variables and six welfare-related behaviours, while controlling for rearing status and age of the chimpanzee at the time of data collection for the current study. Predictors included: 1) the total number of studies in which chimpanzees participated over the approximately eight-year period; 2) the number of studies in which chimpanzees participated over the two-year period that coincided with behavioural data collection for the current study; and 3) the percentage of studies that involved primarily human interaction (see Data Analysis above and Table 2 for correlation matrix). The behaviours chosen as dependent variables for the analyses in the current investigation are commonly used indicators of welfare and/or have been shown in previous work to be affected by participation in various types of behavioural studies (Bloomsmith et al., 2000; Hopper et al., 2016; Leavens, Aureli, Hopkins, & Hyatt, 2001; Ross, 2010; Tasker & Buchanan-Smith, 2016). These behaviours include rough scratching (as a potential indicator of tension and anxiety), abnormal, affiliative, inactive, and locomotive behaviour (see Supplementary Materials), as well as behavioural diversity scores. Rearing status and age were entered on the first block of each equation, and the three predictor variables were entered in a hierarchical fashion in subsequent blocks of the equation. We used Akaike’s information criterion (AIC) to assess model fit for each model. Statistics (R2, F, df, t, and β) are reported from the model with the lowest AIC. To correct for multiple comparisons, but also to avoid overcorrection and risk Type II error, alpha levels were set at p < 0.01 (Nakagawa, 2004; Perneger, 1998). All analyses were performed using SPSS 24 (IBM Corporation, Chicago, IL, USA).
Table 2.
Correlation matrix for all predictors.
| Age | Rearing Status | Total # studies | HI % | ||
|---|---|---|---|---|---|
| Rearing Status | Pearson Correlation | 0.704 | 1 | ||
| p value | 0.000 | ||||
| Total # studies | Pearson Correlation | 0.318 | 0.21 | 1 | |
| p value | 0.000 | 0.023 | |||
| HI % | Pearson Correlation | −0.165 | −0.027 | −0.286 | 1 |
| p value | 0.074 | 0.774 | 0.002 | ||
| # Studies 2 yrs | Pearson Correlation | 0.135 | −0.049 | 0.343 | −0.24 |
| p value | 0.144 | 0.595 | 0.000 | 0.009 | |
Age: Age of chimpanzee; Total # Studies: Total number of studies in which chimpanzee participated; HI %: Percentage of studies in which chimpanzees participated that involved primarily human interaction rather than interaction with stimulus only; # Studies 2 yrs: The number of studies in which chimpanzees participated in the last two years, coinciding with data collection.
3. Results
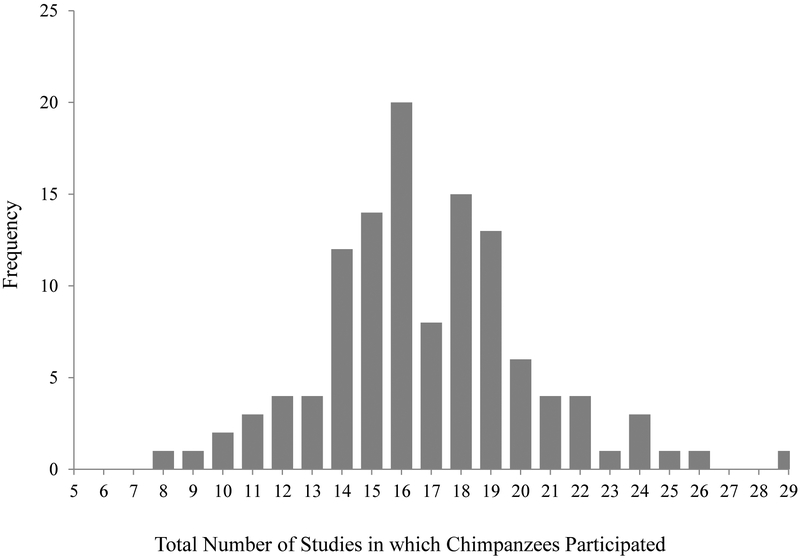
Individual chimpanzees participated in an average of 16.84 (SE = 0.32; range = 8 – 29; see Figure 1) studies over the approximately eight-year period used for the current study, approximately half of which were characterized as involving primarily human interaction (M = 55.41%, SE = 0.59; range = 40.91% - 77.78%). Over the last two years (during behavioural data collection), individual chimpanzees participated in an average of 2.42 studies (SE = 0.09; range = 2 – 14).
Figure 1.
Histogram of the total number of studies in which chimpanzees participated over the approximately 8-year period.
The ANCOVA assessing whether rearing status significantly affected the number of studies in which chimpanzees participated (with chimpanzee age as a covariate) was not significant [F(2,114) = 0.142, p = 0.087]. Mother-reared chimpanzees (M = 16.92, SE = 0.43), nursery-reared chimpanzees (M = 17.00, SE = 0.65), and chimpanzees with an unknown rearing history (i.e., chimpanzees that were wild-born) (M = 16.20, SE = 1.36) participated in a similar number of studies across the approximately eight-year period.
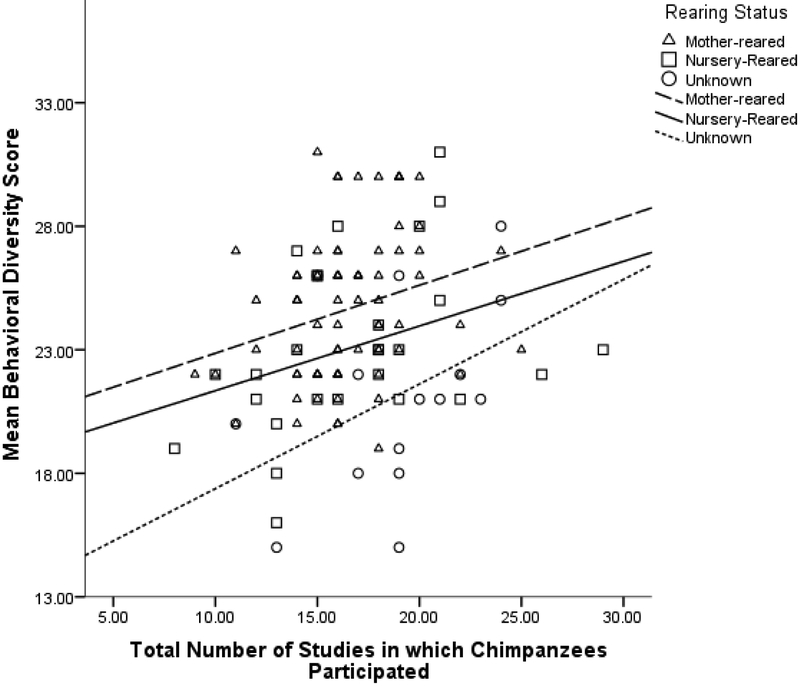
Regarding behavioural diversity, there was most support for the model in which there was an effect of the total number of studies in which chimpanzees participated while controlling for age and rearing, F(3,114) = 11.25, p < 0.001 (Table 3). The control variable of rearing status had a significant effect (β = −0.29, t = −2.47, p = 0.015), whereas age did not (p = 0.10, Table 4). A higher total number of studies in which chimpanzees participated was significantly associated with higher behavioural diversity scores (M = 16.84, SD = 3.52, N = 118, β = 0.33, t= 3.83, p < 0.001; Figure 2). The number of studies in which chimpanzees participated in the two years during behavioural data collection and the percentage of studies that involved primarily human interaction did not add uniquely to the model (p > 0.10).
Table 3.
Model definition, AICs, Delta AIC, and Adjusted R2 for each model.
| Model | df | AIC | Delta AIC |
Adjusted R2 |
|---|---|---|---|---|
| 1: Behavioral Diversity~Age + Rearing | 115 | 277.223 | 0 | 0.114 |
| 2: Behavioral Diversity~Age + Rearing + Total # of Studies | 114 | 264.925 | 12.298 | 0.208 |
| 3: Behavioral Diversity~Age + Rearing + Total # of Studies + HI % + # Studies 2 yrs | 113 | 266.807 | 10.416 | 0.202 |
| 3: Behavioral Diversity~Age + Rearing + Total # of Studies + HI % + # Studies 2 yrs | 112 | 268.27 | 8.953 | 0.198 |
| 1: Rough Scratching~Age + Rearing | 115 | −369.281 | 0 | 0.081 |
| 2: Rough Scratching~Age + Rearing + Total # of Studies |
114 | −372.571 | 3.29 | 0.114 |
| 3: Rough Scratching~ Age + Rearing + Total # of Studies + HI % |
113 | −371.161 | 1.88 | 0.11 |
| 3: Rough Scratching~ Age + Rearing + Total # Studies + HI % + # Studies Last 2 yrs | 112 | −371.273 | 1.992 | 0.118 |
Age: Age of chimpanzee; Total # Studies: Total number of studies in which chimpanzee participated; HI %: Percentage of studies in which chimpanzees participated that involved primarily human interaction rather than interaction with stimulus only; # Studies 2 yrs: The number of studies in which chimpanzees participated in the last two years, coinciding with data collection.
Table 4.
Beta, t-value, and significance level for each predictor variable from the best fitting model in Table 2.
| Dependent Variable | Predictor Variable | Beta | t | p |
|---|---|---|---|---|
| Behavioural Diversity | Age | −0.197 | −1.647 | 0.102 |
| Rearing | −0.286 | −2.466 | 0.015 | |
| Total # of Studies | 0.333 | 3.832 | 0.000 | |
| Rough Scratching | Age | 0.156 | 1.234 | 0.220 |
| Rearing | 0.113 | 0.920 | 0.360 | |
| Total # of Studies | 0.210 | 2.286 | 0.024 |
Figure 2.
Relationship between behavioural diversity scores, rearing status, and the total number of studies in which chimpanzees participated between March 2010 and May 2018.
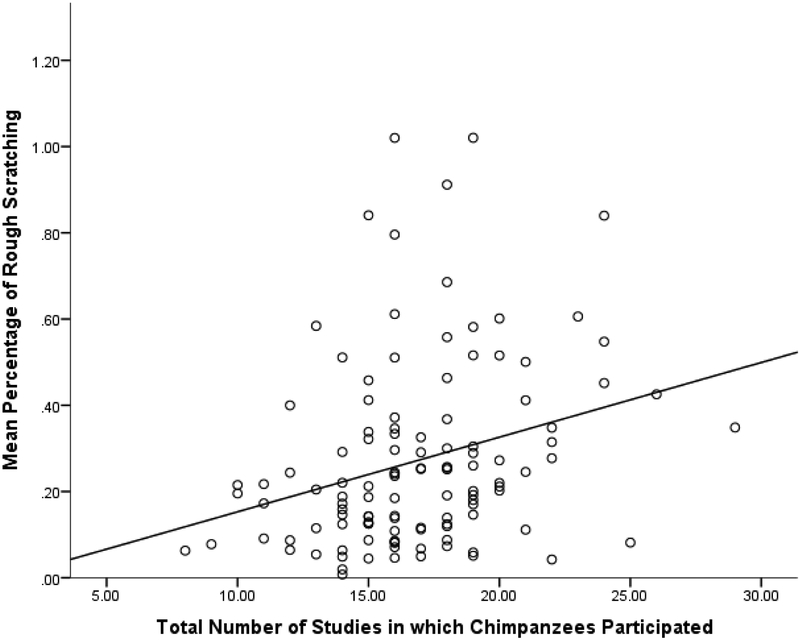
Regarding rough scratching, there was most support for the model in which there was an effect of the total number of studies in which chimpanzees participated while controlling for age and rearing status, F(3,114) = 6.01, p = 0.001 (Table 3). The control variables of rearing status and age did not have significant effects (p > 0.20, Table 4). A higher total number of studies in which chimpanzees participated was associated with a higher percentage of rough scratching (β = 0.21, t = 2.29, p = 0.024; Figure 3). The number of studies in which chimpanzees participated in the two years during behavioural data collection and the percentage of studies that involved primarily human interaction did not add uniquely to the model (p > 0.10).
Figure 3.
Relationship between percentage of rough scratching and total number of studies in which chimpanzees participated between March 2010 and May 2018.
The total number of studies, the number of studies within the last two years, and the percentage of studies that involved primarily human interaction were not significant predictors of abnormal, inactive, locomotive, or affiliative behaviour (p > 0.10).
4. Discussion
Voluntary participation in behavioural studies not only increases 1) enrichment opportunities, 2) functional simulations of natural conditions/behaviours, and 3) control within the captive environment, but also benefits science and has implications for refining management techniques that should enhance welfare. However, there is a lack of data regarding relationships between welfare and participation in behavioural studies, and these data are needed in order to empirically address relevant regulation questions, and aid in decision-making by managers, veterinarians, and governmental agencies. As such, we investigated potential relationships between welfare and long-term (over an approximately eight-year period), repeated participation in behavioural studies. Results revealed two statistically significant, positive correlations between the total number of studies in which chimpanzees participated and 1) behavioural diversity scores and 2) time spent rough scratching. We found no relationships between the number of studies in which chimpanzees participated and other welfare-related behaviours, including affiliative, abnormal, inactive, or locomotive behaviour. Given these findings, as well as the persistent and repeated voluntary participation of the chimpanzees throughout the eight-year period, we believe the results suggest that voluntary participation in behavioural studies is not detrimental to captive chimpanzees, and may have some welfare benefits.
Chimpanzees that participated in a higher number of studies over the eight-year period exhibited a higher level of diversity in their behaviour (although, it is important to note that this could also be stated in reverse: chimpanzees that exhibited a higher level of behavioural diversity participated in more behavioural studies). Behavioural diversity scores in the current study represented the average number of different, positive welfare-related behaviours exhibited by each chimpanzee over the course of the approximately two-year data collection period. Higher scores are assumed to be indicative of enhanced well-being (Neal Webb et al., 2018a, 2018b, 2019; Suomi & Novak, 1991; Tasker & Buchanan-Smith, 2016). Indeed, a refinement to captive care (increased human interaction) aimed at enhancing well-being of laboratory-housed cynomolgus macaques (Macaca fascicularis) resulted in a more varied behavioural repertoire, in addition to other behavioural and physiological enhancements (Tasker & Buchanan-Smith, 2016). It is possible that increasing the number of opportunities for chimpanzees to participate in behavioural studies may also increase behavioural diversity. Alternatively, it could be that chimpanzees with higher behavioural diversity scores have a higher proclivity to participate in behavioural studies. Both of these alternatives justify increased opportunities for participation in such studies: if the former is true, managers can enhance welfare through increased behavioural diversity; if the latter is the case, managers can increase welfare of those particular chimpanzees by providing them with more opportunities to engage in tasks that seem to be intrinsically rewarding (Tarou & Bashaw, 2007).
We found a positive correlation between rough scratching, sometimes regarded as a behavioural indicator of anxiety in chimpanzees (Baker & Aureli, 1997; Hopkins et al., 2006), and the number of studies in which chimpanzees had participated. First and foremost, it is important to note that rough scratching in the current study made up less than 1% of all activity, and the R2 was quite low (slightly above 0.1). Therefore, although the relationship may be statistically significant, this result may not be biologically meaningful (Neal Webb, Hau, & Schapiro, 2018b, 2019), and may represent a spurious correlation. Regardless, we believe the finding stimulates an interesting discussion concerning rough scratching as an indicator of anxiety. It is assumed that, if behavioural studies are enriching, behavioural indicators of anxiety should decrease rather than increase (Bloomsmith, 2000). However, some work has found increases in rough scratching with concurrent increases in positive welfare-related behaviours during certain behavioural studies and tasks. For example, Herelko, Vick, and Buchanan-Smith (2012) found that chimpanzee scratching increased during husbandry training sessions when visual access to keepers was restricted, yet interest in research activities remained high. Additionally, rough scratching in chimpanzees was higher (as was social play and problem-solving) when a novel cognitive challenge device was present (Clark & Smith, 2013). Furthermore, rates of self-directed behaviours (SDBs) in chimpanzees were higher during a computerized cognitive challenge when chimpanzees started the task at levels of low-difficulty (Leavens et al., 2001). Some suggest that a certain amount of uncertainty (and even anxiety) may be enriching for chimpanzees and other NHPs (Caine, 2017; Chamove & Moodie, 1990; Herrelko et al., 2012). Recent work also suggests that scratching may have a less clearly defined relationship with uncertainty, anxiety, and arousal than is currently assumed (Neal & Caine, 2016). As such, it may be that the increases in rough scratching found in the current study do not necessarily reflect decreases in well-being, but are more a reflection of uncertainty or anticipation. Nevertheless, if it is the case that increased scratching in the current study is related to increased anxiety, the general consensus seems to be that these behavioural studies and tasks are not stressful overall, given the positive behavioural changes seen as a result of participation (Herelko et al., 2012; Ross, 2010). As such, the benefit of behavioural research as enrichment may outweigh the cost of increased tension, uncertainty, or anxiety; emotional states that may actually be enriching for NHPs (Caine, 2017; Chamove & Moodie, 1990; Herrelko et al., 2012).
Previous studies investigating behaviour in the context of current participation in behavioural studies have found positive changes in behaviour, including increased activity and locomotion (Hopper et al., 2016), increased social play (Clark & Smith, 2013), increased presence in proximity to the tasks (Herelko et al., 2012), and decreased solitary and self-directed behaviour (Bloomsmith et al., 2000). However, the current study found no relationships between behaviour and the number of studies in which chimpanzees participated during the two-year period coinciding with behavioural data collection. It is possible that the behavioural effects of participation in behavioural studies are limited to the time period (e.g., the hours or days) during and/or directly following the participation. Indeed, Hopper et al. (2016) found that chimpanzee activity returned to baseline within two hours following a 30-minute token-exchange test session. As such, perhaps the current study did not detect these relationships because, unlike the studies mentioned above, we did not specifically examine behaviour as a function of participation in any particular study (i.e., during or immediately following participation in procedures of behavioural studies).
Lastly, we also did not find any statistically significant relationships between behaviour and the percentage of studies in which chimpanzees participated that involved primarily human interaction. This is surprising, given previous research showing the benefits of increased human interaction in several species of NHPs, including lower rates of self-directed behaviours, increased activity, and more varied behavioural repertoires (Chelluri, Ross, & Wagner, 2013; Tasker & Buchanan-Smith, 2016). It is possible that the two types of behavioural studies characterized in the current investigation (i.e., those involving primarily human interaction and those involving primarily stimulus-only interaction) are equally rewarding from the chimpanzee’s perspective. It is important to emphasize that chimpanzee participation was completely voluntary. Therefore, the finding that chimpanzees participated in an average of almost 17 studies (the majority of which, at least 75%, were composed of multiple trials) over the eight-year period suggests that these chimpanzees voluntarily returned many, many times to participate in studies. Given this repeated participation; the lack of relationship between abnormal, affiliative, inactive, and locomotive behaviour and participation; and the positive relationship between behavioural diversity and participation, the data seem to suggest that, at the least, participation is not detrimental to chimpanzees. In fact, we would suggest that this participation in noninvasive behavioural studies is not only not harmful for the animals, but is enriching and rewarding, and that the chimpanzees sought out opportunities to participate in the various types of behavioural research projects (Hopkins and Latzman, 2017; Tarou & Bashaw, 2007).
5. Conclusions
The current study found two significant relationships between welfare and voluntary participation in behavioural studies. Results showed a positive relationship between the total number of studies in which chimpanzees participated and 1) behavioural diversity scores and 2) rough scratching. Although we cannot draw conclusions about the direction of the relationships between behavioural diversity and rough scratching, and the total number of studies in which chimpanzees participated over the previous eight years, we believe that these results provide no evidence to suggest that voluntary participation in behavioural studies is harmful to chimpanzees. The evidence from the current study may, in fact, suggest that participation is positive for the animals; behavioural diversity and participation are positively related, participation was unrelated to abnormal behaviours, and animals repeatedly return to participate in the studies. Additionally, we believe the benefits of behavioural research outweigh the potential for a slight (and likely not biologically meaningful) increase in rough scratching, which may be an indication of anticipation or uncertainty, rather than a decrease in welfare (Clark & Smith, 2012; Herrelko et al., 2012; Leavens et al., 2001). Overall, the findings from the current study highlight the importance of empirical assessments of the ways that voluntary participation in behavioural studies can affect welfare, and how these types of results can be applied to regulations about the inclusion of chimpanzees in behavioural research projects.
Supplementary Material
Highlights.
Higher behavioral diversity with participation in more behavioral studies over time.
No relationship between welfare and concurrent study participation.
Acknowledgements
We would like to thank all researchers who contributed lists of chimpanzees who participated in their studies, as well as those who collected data at the NCCC between March 2010 and May 2018, including (in alphabetical order) Stephanie Bogart, Jennifer Bridges, Sarah Brosnan, Nicolas Claidière, Sarah Davis, Katie Hall, Rachel Haller, William Hopkins, Lydia Hopper, Bailey House, Rachel Kendal, Sarah Pope, Sara Price, Bruce Rawlings, Joan Silk, Jared Taglialatela, Erica Thiele, Gill Vale, Stuart Watson, Andrew Whiten, and Lara Wood. We would also like to thank the carestaff at the NCCC for their amazing care of the chimpanzees and accommodations for researchers collecting data. A special thank you to Susan (Lambeth) Pavonetti, Lisa Reamer, Mary Catherine Mareno, and Michele Mulholland for their helpful comments, suggestions, and support on this project. This work was supported by NIH U42-OD 011197 and the University of Copenhagen.
Footnotes
Conflicts of Interest
The authors have no conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Altmann J (1974). Observational study of behaviour: Sampling methods. Behaviour, 49, 227–267. [DOI] [PubMed] [Google Scholar]
- Baker KC, & Aureli F (1997). Behavioural indicators of anxiety: An empirical test in chimpanzees. Behaviour, 134(13/14), 1031–1050. [Google Scholar]
- Baker KC, Weed JL, Crockett CM, & Bloomsmith MA (2007). Survey of environmental enhancement programs for laboratory primates. American Journal of Primatology, 69(4), 377–394. 10.1002/ajp.20347 [DOI] [PubMed] [Google Scholar]
- Baker KC (2016). Survey of 2014 behavioural management programs for laboratory primates in the United States: Survey of Primate Behavioural Management Programs. American Journal of Primatology, 78(7): 780–796. 10.1002/ajp.22543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker KC, Bloomsmith MA, Coleman K, Crockett CM, Worlein J, Lutz CK, McCowan B, Pierre P, and Weed J (2017). The behavioural management consortium: A partnership for promoting consensus and best practices In Schapiro SJ (ed) Handbook of Primate Behavioural Management pp 9–23. CRC Press, Taylor & Francis Group: Boca Raton, Florida, USA [Google Scholar]
- Brent L (Ed.). (2001). Special topics in primatology, Vol. 2: The care and management of captive chimpanzees. San Antonio, TX: American Society of Primatologists. [Google Scholar]
- Brent L, Bloomsmith MA, & Fisher SD (1995). Factors determining tool-using ability in two captive chimpanzee (Pan troglodytes) colonies. Primates, 36(2), 265–274. [Google Scholar]
- Bloomsmith MA, Ross SK, & Baker KC (2000). Control over computer-assisted enrichment for socially housed chimpanzees. American Journal of Primatolology, 51(Suppl 1), 45. [Google Scholar]
- Bogart SL, Russell JL, Reamer LA, Mareno MC, Schapiro SJ, & Hopkins WD (2014). Chimpanzee social tolerance and possible genetic influences of the vasopressin V1A receptor gene. American Journal of Primatology 76(S1), 57. [Google Scholar]
- Bridges JP, Mocarski EC, Reamer LA, Lambeth SP, & Schapiro SJ (2013). Weight management in captive chimpanzees (Pan troglodytes) using a modified feeding device. American Journal of Primatology, 75(Suppl 1), 51. [Google Scholar]
- Brosnan SF, Hopper LM, Richey S, Freeman HD, Talbot CF, Gosling SD, Lambeth SP, & Schapiro SJ (2015). Personality influences responses to inequity and contrast in chimpanzees. Animal Behaviour, 101, 75–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caine NG (2017). Antipredator behaviour: Its expression and consequences in captive primates In Schapiro SJ (ed) Handbook of Primate Behavioural Management pp 127–138. CRC Press, Taylor & Francis Group: Boca Raton, Florida, USA [Google Scholar]
- Chamove AS, & Moodie EM (1990). Are alarming events good for captive monkeys? Applied Animal Behaviour Science, 27(1), 169–176. [Google Scholar]
- Chelluri GI, Ross SR, & Wagner KE (2013). Behavioural correlates and welfare implications of informal interactions between caretakers and zoo-housed chimpanzees and gorillas. Applied Animal Behaviour Science, 147(3–4), 306–315. 10.1016/j.applanim.2012.06.008 [DOI] [Google Scholar]
- Claidière N, Whiten A, Mareno MC, Messer EJE, Brosnan SF, Hopper LM, Lambeth SP, Schapiro SJ, & McGuigan N (2015). Selective and contagious prosocial resource donation in capuchin monkeys, chimpanzees and humans. Scientific Reports, 5(1). 10.1038/srep07631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark FE (2011). Space to choose: network analysis of social preferences in a captive chimpanzee community, and implications for management. American Journal of Primatology, 73(8), 748–757. 10.1002/ajp.20903 [DOI] [PubMed] [Google Scholar]
- Clark FE, & Smith LJ (2013). Effect of a Cognitive Challenge Device Containing Food and Non-Food Rewards on Chimpanzee Well-Being: Chimpanzee Cognitive Challenge. American Journal of Primatology, 75(8), 807–816. 10.1002/ajp.22141 [DOI] [PubMed] [Google Scholar]
- Graham ML (2017). Positive reinforcement training and research In Schapiro SJ (ed) Handbook of Primate Behavioural Management pp 187–200. CRC Press, Taylor & Francis Group: Boca Raton, Florida, USA [Google Scholar]
- Hall K, Lambeth SP, Schapiro SJ, & Brosnan SF (2015). Chimpanzees respond differently to inequity based on relative food values. American Journal of Primatology, 77(S1), 84. [Google Scholar]
- Herrelko ES, Vick S-J, & Buchanan-Smith HM (2012). Cognitive research in zoo-housed chimpanzees: Influence of personality and impact on welfare: chimpanzee welfare in cognitive research. American Journal of Primatology, 74(9), 828–840. 10.1002/ajp.22036 [DOI] [PubMed] [Google Scholar]
- Hopkins WD, Russell JL, Freeman H, Reynolds EA, Griffis C, & Leavens DA (2006). Lateralized scratching in chimpanzees (Pan troglodytes):. Evidence of a functional asymmetry during arousal. Emotion, 6(4), 553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD & Latzman RD (2017) Future research with captive chimpanzees in the United States: Integrating scientific programs with behavioural management In Schapiro SJ (ed) Handbook of Primate Behavioural Management pp 139–155. CRC Press, Taylor & Francis Group: Boca Raton, Florida, USA [Google Scholar]
- Hopper LM (2017). Cognitive research in zoos. Current Opinion in Behavioural Sciences, 16, 100–110. 10.1016/j.cobeha.2017.04.006 [DOI] [Google Scholar]
- Hopper LM, Lambeth SP, Schapiro SJ, & Brosnan SF (2013). When given the opportunity, chimpanzees maximize personal gain rather than “level the playing field.” PeerJ, 1, e165 10.7717/peerj.165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopper LM, Price SA, Freeman HD, Lambeth SP, Schapiro SJ, & Kendal RL (2014). Influence of personality, age, sex, and estrous state on chimpanzee problem-solving success. Animal Cognition, 17(4), 835–847. 10.1007/s10071-013-0715-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopper LM, Shender MA, & Ross SR (2016). Behavioural research as physical enrichment for captive chimpanzees: Behavioural Research as Enrichment for Chimpanzees. Zoo Biology, 35(4), 293–297. 10.1002/zoo.21297 [DOI] [PubMed] [Google Scholar]
- House BR, Silk JB, Lambeth SP, & Schapiro SJ (2014). Task design influences prosociality in captive chimpanzees (Pan troglodytes). PLoS ONE, 9(9), e103422 10.1371/journal.pone.0103422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambeth SP, Hau J, Perlman JE, Martino M, & Schapiro SJ (2006). Positive reinforcement training affects hematologic and serum chemistry values in captive chimpanzees (Pan troglodytes). American Journal of Primatology, 68(3), 245. [DOI] [PubMed] [Google Scholar]
- Laule G & Whittaker M 2002. The use of positive reinforcement techniques with chimpanzees for enhanced care and welfare In Brent L (ed) Special Topics in Primatology (Vol 2): Care and Management of Captive Chimpanzees pp 242–265. American Society of Primatologists: San Antonio, Texas, USA [Google Scholar]
- Laule G, & Whittaker M (2007). Enhancing nonhuman primate care and welfare through the use of positive reinforcement training. Journal of Applied Animal Welfare Science, 10(1), 31–38. [DOI] [PubMed] [Google Scholar]
- Leavens DA, Aureli F, Hopkins WD, & Hyatt CW (2001). Effects of cognitive challenge on self-directed behaviors by chimpanzees (Pan troglodytes). American Journal of Primatology, 55(1), 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magden ER, Haller RL, Thiele EJ, Buchl SJ, Lambeth SP, & Schapiro SJ (2013). Acupuncture as an adjunct therapy for osteoarthritis in chimpanzees (Pan troglodytes). Journal of the American Association for Laboratory Animal Science, 52(4), 6. [PMC free article] [PubMed] [Google Scholar]
- Magden ER 2017. Positive reinforcement training and health care In Schapiro SJ (ed) Handbook of Primate Behavioural Management pp 201–215. CRC Press, Taylor & Francis Group: Boca Raton, Florida, EISA [Google Scholar]
- Maki S, & Bloomsmith MA (1989). Uprooted trees facilitate the psychological well-being of captive chimpanzees. Zoo Biology, 8(1), 79–87. [Google Scholar]
- National Institutes of Health (2016). Notice of Agency Decision: NIH Research Involving Chimpanzees. Retrieved from: https://grants.nih.gov/grants/guide/notice-files/NOT-OD-16-095.html
- Neal SJ, & Caine NG (2016). Scratching under positive and negative arousal in common marmosets (Callithrix jacchus). American Journal of Primatology, 78(2), 216–226. [DOI] [PubMed] [Google Scholar]
- Neal Webb SJ, Hau J, & Schapiro SJ (2018a). Captive chimpanzee (Pan troglodytes) behaviour as a function of space per animal and enclosure type. American Journal of Primatology, 80(3), e22749 https://doi.org10.1002/ajp.22749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal Webb SJ, Hau J, & Schapiro SJ (2018b). Refinements to captive chimpanzee (Pan troglodytes) care: A self-medication paradigm. Animal Welfare, 27(4): 327–341. 10.7120/09627286.27.4.327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal Webb SJ, Hau J, & Schapiro SJ (2019). Does group size matter? Captive chimpanzee behavior as a function of group size and composition. American Journal of Primatology, 81(1), e22947 10.1002/ajp.22947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reamer LA, Haller RL, Thiele EJ, Freeman HD, Lambeth SP, & Schapiro SJ (2014). Factors affecting initial training success of blood glucose testing in captive chimpanzees (Pan troglodytes): Chimpanzee Diabetic Treatment Training. Zoo Biology, 33(3), 212–220. 10.1002/zoo.21123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reamer L, Haller R, Lambeth SP, & Schapiro SJ (2017). Behavioural management of Pan spp In Schapiro SJ (Ed.), Handbook of Primate Behavioural Management (pp. 395–407). Boca Raton, FL: CRC Press, Taylor & Francis Group. [Google Scholar]
- Reinhardt V (1997). Training nonhuman primates to cooperate during handling procedures: A review. Animal Technology, 48(2), 55–74. [Google Scholar]
- Ross SR (2010). How cognitive studies help shape our obligation for the ethical care of chimpanzees In Lonsdorf EL, Ross SR, and Matsuzawa T (Eds.), The Mind of the Chimpanzee (pp. 309–319). Chicago, IL: The University of Chicago Press. [Google Scholar]
- Ross SR, Schapiro SJ, Hau J, & Lukas KE (2009). Space use as an indicator of enclosure appropriateness: A novel measure of captive animal welfare. Applied Animal Behaviour Science, 121(1), 42–50. 10.1016/j.applanim.2009.08.007 [DOI] [Google Scholar]
- Russell JL, Lyn H, Schaeffer JA, & Hopkins WD (2011). The role of socio-communicative rearing environments in the development of social and physical cognition in apes: Development of social and physical cognition in apes. Developmental Science, 14(6), 1459–1470. 10.1111/j.1467-7687.2011.01090.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schapiro SJ (2017). Handbook of Primate Behavioural Management. Boca Raton, FL: CRC Press, Taylor & Francis Group. [Google Scholar]
- Schapiro SJ, Lambeth SP, Jacobsen KR, Williams LE, Nehete BN, & Nehete PN (2012). Physiological and welfare consequences of transport, relocation, and acclimatization of chimpanzees (Pan troglodytes). Applied Animal Behaviour Science, 137(3–4), 183–193. 10.1016/j.applanim.2011.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suomi SJ, & Novak MA (1991). The role of individual differences in promoting psychological well-bring in rhesus monkeys In Novak MA & Petto AJ (Eds.), Through the Looking Glass: Issues of Psychological Well-being in Captive Nonhuman Primates (50-56). Washington, DC: American Psychological Association. [Google Scholar]
- Taglialatela JP, Russell JL, Pope SM, Morton T, Bogart S, Reamer LA, … Hopkins WD (2015). Multimodal communication in chimpanzees: Multimodal Communication in Chimpanzees. American Journal of Primatology, 77(11), 1143–1148. 10.1002/ajp.22449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarou LR, & Bashaw MJ (2007). Maximizing the effectiveness of environmental enrichment: Suggestions from the experimental analysis of behaviour. Applied Animal Behaviour Science, 102(3–4), 189–204. 10.1016/j.applanim.2006.05.026 [DOI] [Google Scholar]
- Tasker L & Buchanan-Smith HM (2016). Linking welfare and quality of scientific output through refinement of enhanced socialization with carestaff in cynomolgus macaques (Macaca fascicidaris) used for regulatory toxicology. Presented at the Joint meeting of the International Primatological Society and the American Society of Primatologists August 21–27, 2016. [Google Scholar]
- Vale GL, Davis SJ, van de Waal E, Schapiro SJ, Lambeth SP, & Whiten A (2017a). Lack of conformity to new local dietary preferences in migrating captive chimpanzees. Animal Behaviour, 124, 135–144. 10.1016/j.anbehav.2016.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale GL, Davis SJ, Lambeth SP, Schapiro SJ, & Whiten A (2017b). Acquisition of a socially learned tool use sequence in chimpanzees: Implications for cumulative culture. Evolution and Human Behaviour, 38(5), 635–644. 10.1016/j.evolhumbehav.2017.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale GL, Flynn EG, Kendal J, Rawlings B, Hopper LM, Schapiro SJ, … Kendal RL (2017c). Testing differential use of payoff-biased social learning strategies in children and chimpanzees. Proceedings of the Royal Society B: Biological Sciences, 284(1868), 20171751 10.1098/rspb.2017.1751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washburn DA, & Rumbaugh D (1992). Comparative assessment of psychomotor performance: Target prediction by humans and macaques (Macaca mulatta). Journal of Experimental Psychology: General, 121, 305–312. [DOI] [PubMed] [Google Scholar]
- Watanabe S (2007). How animal psychology contributes to animal welfare. Applied Animal Behaviour Science, 106(4), 193–202. 10.1016/j.applanim.2007.01.003 [DOI] [Google Scholar]
- Watson SK, Vale GL, Hopper LM, Dean LG, Kendal RL, Price EE, Wood LA, Davis SJ, Schapiro SJ, Lambeth SP, & Whiten A (2018). Chimpanzees demonstrate individual differences in social information use. Animal Cognition, 21(5), 639–650. 10.1007/s10071-018-1198-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanashi Y, & Hayashi M (2011). Assessing the effects of cognitive experiments on the welfare of captive chimpanzees (Pan troglodytes) by direct comparison of activity budget between wild and captive chimpanzees. American Journal of Primatology, 73(12), 1231–1238. 10.1002/ajp.20995 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.