Abstract
Parkinson’s disease (PD) is characterized by the selective degeneration of dopamine (DA) neurons of the substantia nigra pars compacta (SN), while the neighboring ventral tegmental area (VTA) is relatively spared. The mechanisms underlying this selectivity are not fully understood. Here, we demonstrate a vital role for subregional astrocytes in the protection of VTA DA neurons. We found that elimination of astrocytes in vitro exposes a novel vulnerability of presumably protected VTA DA neurons to the PD mimetic toxin MPP+, as well as exacerbation of SN DA neuron vulnerability. Conversely, VTA astrocytes protected both VTA and SN DA neurons from MPP+ toxicity in a dose dependent manner, and this protection was mediated via a secreted molecule. RNAseq analysis of isolated VTA and SN astrocytes demonstrated a vast array of transcriptional differences between these two closely related populations demonstrating regional heterogeneity of midbrain astrocytes. We found that GDF15, a member of the TGFβ superfamily which is expressed 230-fold higher in VTA astrocytes than SN, recapitulates neuroprotection of both rat midbrain and iPSC-derived DA neurons, whereas its knockdown conversely diminished this effect. Neuroprotection was likely mediated through the GRFAL receptor expressed on DA neurons. Together; these results suggest that subregional differences in astrocytes underlie the selective degeneration or protection of DA neurons in PD.
Keywords: Astrocyte, Parkinson’s Disease, Neuroprotection, Neurodegeneration, Trophic Factors
Introduction
Parkinson’s Disease (PD) is a well-studied neurodegenerative disease which is characterized by the selective degeneration of dopaminergic (DA) neurons of the substantia nigra pars compacta (SN), while the DA neurons of the neighboring ventral tegmental area (VTA) are relatively spared (Hirsch, Graybiel, & Agid, 1988). Many studies have focused on these two subregions to better understand why one subset of DA neurons is so much more vulnerable to PD than the other (Chung et al., 2005; Phani, Gonye, & Iacovitti, 2010; Thuret, Bhatt, O’Leary, & Simon, 2004; Yao et al., 2005). The use of PD mimetic toxins, such as 1-Methyl-4-phenylpyridinium (MPP+), has facilitated much of this work to selectively target degeneration in DA neurons (Javitch & Snyder, 1984; Kopin, 1992; Langston, 1985). However, less attention has been paid to other cells, such as astrocytes, within these subregions to understand their role in the disease process.
Astrocytes are often considered bystanders or support cells of the brain (H. K. Kimelberg & Nedergaard, 2010; Harold Kimelberg, 2007). However, recently more attention has focused on their contribution to a number of cell processes, such as potassium buffering and calcium signaling waves, and thus a greater appreciation for their active role in the brain is emerging (Allen & Barres, 2009). Classically, astrocytes are known to release a variety of neurotrophic factors, such as GDNF, BDNF and MANF among others, that aid in neuronal development and provide some degree of neuroprotection in vivo and in vitro (Anderson et al., 2016; Cekanaviciute et al., 2014; Diniz et al., 2017; H Kimelberg & Norenberg, 1989; Liddelow & Barres, 2017; Lin, Doherty, Lile, Bektesh, & Collins, 1993; Petrova et al., 2003; Wiese, Karus, & Faissner, 2012; Zamanian et al., 2012). Furthermore, previous studies have examined the numbers of neurons and astrocytes within the midbrain and found intriguing differences between these cell types (Damier, Hirsch, Zhang, & Agio, 1993; Nair-Roberts et al., 2008). The VTA is characterized by more astrocytes than the neighboring SN subregion. These facts raise interesting questions about not only the role astrocytes play in regional DA neuron susceptibility, but whether the ratios of these cell types further impacts PD vulnerability.
Additionally, differences in the transcriptional profile of astrocytes from one region to another remains another important consideration. Indeed, others have previously demonstrated regional variability in gene expression of astrocytes of the cortex, cerebellum, brainstem and hypothalamus (Datta, Ganapathy, Razdan, & Bhonde, 2017; Khakh & Sofroniew, 2015; Schitine, Nogaroli, Costa, & Hedin-Pereira, 2015; Y. Zhang & Barres, 2010) It is not yet known whether midbrain astrocytes from neighboring VTA and SN nuclei similarly exhibit subregional specificity and whether this affects midbrain DA neuronal susceptibility in PD.
Therefore in this study, we utilize our recently developed TH-GFP reporter rat (Iacovitti et al., 2014) to investigate the role of midbrain astrocytes in the subregional vulnerability/resistance of DA neurons in PD. This animal model provides us with the tools necessary to sub-dissect the midbrain into the vulnerable SN and protected VTA to determine if astrocytes of these subregions are different and if they play a role in neuroprotection through the release of neuroprotective factors. We demonstrate that astrocytes from adjacent VTA and SN nuclei in the midbrain are surprisingly different at the transcriptional and functional levels. Additionally, we demonstrate increased toxicity of SN and a novel vulnerability of VTA DA neurons when astrocyte populations are diminished in vitro. Furthermore, greater numbers of VTA, but not SN, astrocytes mediate neuroprotection and secrete factors that protect SN, VTA and iPS-derived DA neurons from MPP+ toxicity. We further show that the growth factor GDF15, a member of the TGFβ superfamily, present in much higher levels in VTA than SN astrocytes, mimics these effects while its transcriptional knockdown in VTA astrocytes significantly reduces it. Taken together, these data suggest that VTA astrocytes play a vital role in the selective and subregional protection of VTA DA neurons from PD toxicity by releasing neuroprotective factors like GDF15. Since GDF15 also protects PD-vulnerable SN, as well as iPSC-derived DA neurons and since these cells express the GDF15 receptor GFRAL, the growth factor and its receptor may represent important therapeutic targets for PD.
Materials and Methods
Animals and IACUC policies
All animals used in this study were maintained in accordance with the Office of Animal Resources at Thomas Jefferson University. The protocols were approved by the Institutional Animal Care & Use Committee (IACUC) at Thomas Jefferson University, protocol #457K/01499.
Tissue Culture – Embryonic
Timed pregnancies were performed using TH-GFP+ female rats mated with TH-GFP+ males (Iacovitti et al., 2014). Embryonic day zero (E0) was determined as the first day a vaginal plug was visible. On E14.5, embryos were harvested and placed in ice cold DPBS (Gibco #14190–144; no CaCl2 or MgCl2). GFP+ pups were visualized under a dissection microscope (Nikon SMZ1500; adjustable 1–11.5x objective) by using a high pressure mercury lamp with a 495nm filter attachment. The midbrain was surgically extracted, cleaned of non-GFP+ tissues and the VTA and SN were carefully separated along the oculomotor nerve rootlet running through the midbrain (Supplemental Figure 1). Tissues were collected and placed in 2.5mL of enzymatic dissociation solution containing 5mg DNAse I (Sigma #10104159001), 5mg Papain (Sigma #10108014001), and 50mg L-cysteine (Sigma) for up to 30 minutes. Solution was gently agitated every 5 minutes to aid in dissociation. NEP Basal media (DMEM/F12, B-27 & N2 Supplements (Gibco), 1mg/mL BSA, and Penn/Strep) was added to terminate the enzymatic reaction. Tissues were then briefly spun at 1000rpm for 2 minutes and the supernatant media was aspirated. Tissues were re-suspended in culture media (NEP Basal media + 5% FBS) for 5 minutes prior to mechanical dissociation. For mechanical dissociation, a 1mL pippetor was used with low retention tips and tissues were gently triturated 8–10 times. Cells were then pelleted for 5 minutes at 1000rpm, re-suspended in culture media and counted on an automated hemocytometer (Countess FLII Invitrogen). Cells were plated in a microdrop on 96-well tissue culture plates or 8-well chamber slides pre-coated with poly-D-lysine (0.5mg/mL, overnight at 37°C) at a density of 5–7×104 cells in approximately 20μL of culture media. Cells were allowed to attach to the substrate before media was added to final volume of 100–200μL per well.
Tissue Culture – Astrocytes
Astrocytes were collected using previously published methods (Schildge, Bohrer, Beck, & Schachtrup, 2013; Weinstein, 2001). Briefly, postnatal day 1–5 GFP+ pups were anesthetized on ice and quickly decapitated. Brains were removed and placed in ice cold DPBS (Gibco). GFP+ brains were visualized under a dissection microscope (Nikon SMZ1500). The midbrain and cortices were carefully dissected away from other brain regions and non-GFP+ tissues of the midbrain were cut away. The SN and VTA regions were carefully separated and collected in ice cold PBS. Whole cortices were collected and dissected into smaller pieces prior to enzymatic digestion. Tissues were then subjected to enzymatic digestion using a Trypsin(0.1%)/DNAse I(0.5mg/mL) mixture for 5 minutes at 37°C. The supernatant enzyme was aspirated carefully and tissues washed twice in DPBS. Tissues were then mechanically dissociated in 0.5mg/mL DNAse I and gently triturated 8–10 times. Cells were then pelleted for 5 minutes at 1000rpm, re-suspended in astrocyte media (DMEM/F12 + 10% FBS and Penn/Strep) and plated on tissue culture flasks. Cultures were maintained for 2–4 weeks with media changes every other day. To ensure astrocyte purity flasks were shaken at 220rpm for 48 hours to eliminate any contaminating cells, such as microglia, neurons or oligodendrocytes. Samples of cells were stained for glial fibrillary acidic protein (GFAP; Rabbit Polyclonal - Dako #Z0334 RRID:AB_10013382, Mouse monoclonal - Cell Signaling Technologies #3670 RRID:AB_561049), S100 calcium binding protein B (s100β; mouse monoclonal – Sigma #S2532 RRID:AB_477499), allograft inflammatory factor 1 (Iba1, Wako #019–19741, RRID: RRID:AB_2665520), CD11b/c (BD Pharmingen # 554859 RRID:AB_395560), Tubulin beta-III (βIII Tubulin, Millipore MAB1637, RRID:AB_2210524), neuron-specific nuclear protein (NeuN, Millipore MAB377, RRID:AB_2210524), Chondroitin sulfate proteoglycan (NG2, Millipore AB5320, RRID:AB_11213678), myelin basic protein (MBP, rat hybridoma supernatant; gift of Virginia Lee, University of Pennsylvania, Philadelphia, PA). Species appropriate secondary antibodies were used at a concentration of 1:200 (Alexa Fluor, Thermo Fischer Scientific).
Tissue Culture – Astrocyte Conditioned Media (ACM) generation
Astrocytes generated with the above method were grown to confluence in uncoated 75cm2 tissue culture flasks. Media was changed to serum free NEP Basal media (described above) and incubated for 48 hours to generate region specific ACM. For molecular weight fractionation studies, samples of ACM were subjected to fractionation using Amicon Ultra-4 centrifugal filters of 30 and 100kDa cutoffs. These filters result in retentate samples above and flow through samples below their respective cutoffs. The retentate was then resuspended in the original starting volume of ACM, to return the protein concentration to the unconcentrated level. Samples of unfractionated, as well as retentate and flow through were tested for their neuroprotective capability to MPP+ toxin challenge. We found that the fraction in the <30kD flow through exhibited the greatest protection compared to other samples (data not shown).
Tissue Culture – iPSC
IMR90–4 cells (Passage 35–50) were purchased from Wicell Research Institute and maintained according to the supplier’s instructions. Briefly, cells were grown as feeder free in mTesR1 medium (Stem Cell Technologies). Cell propagation was achieved through manual dissection and transfer of cell colonies once every 4–6 days. The differentiation process was initiated by treating with DMEM/F12 media (Life Tech) supplemented with 20% Knockout Serum Replacer™ (KOSR; Life Tech), 1% Non-Essential Amino Acids (Life Tech), 0.1 mM 2-mercaptoethanol (Life Tech), 4 ng/ml bFGF (R&D systems), two TGF/BMP inhibitors SB431542 (SB, Tocris, 10 μM) and LDN-193189 (LDN, Stemgent, 200nM), and SHH (C24II) (SHH, R&D systems, 100 ng/ml), Purmorphamine (Pur, Stemgent, 2 μM) for 1 week. Neural progenitors (NPs) were subsequently generated in N2/B27 NEP-basal medium supplemented with 100ng/ml FGF8 (R&D system) and 200nM Pur. Rosettes were expanded in NEP-basal medium supplemented with 20 ng/ml bFGF (R&D systems) every other day. For further differentiation down the DA pathway, cells were incubated for 1 week in NEP-basal medium supplemented with 1 mM dibutyryl cAMP (dbcAMP, Sigma), as described in Cai et al., 2013. Staining for tyrosine hydroxylase (TH) was performed using sheep anti-TH antibodies from Abcam (ab113 RRID:AB_297905), secondary antibody used was donkey anti-sheep Alexa Fluor 488 (ab150177). For quantification, full well image scans were performed using a Nikon Eclipse TI-e fitted with a Photometrics Coolsnap ES2 camera. Acquisition software was NIS Elements Advanced Research using the High Content analysis package which allows for automated full well imaging. The absolute number of TH positive cells per well was counted and compared to appropriate controls.
MPP+ Studies
To create PD-like damage, cultures of SN or VTA derived cells were plated at 5–7×104 cells/well and grown for 7 days in defined media. Cultures were then treated with PBS or varying doses of MPP+ (5–100μM; Sigma: Cat# D048) for 48 hours to determine the IC50. Control and MPP+-treated cultures were then fixed and the full well was imaged using a Nikon Eclipse TI-e fitted with a Photometrics Coolsnap ES2 camera. Images were analyzed blinded to condition in ImageJ using a custom designed macro for cell quantification. Briefly, images were converted to 8-bit and subjected to a background subtraction (25 pixels, rolling sliding paraboloid). Images were then inverted and subjected to similar auto-contrast leveling and auto-thresholding to create image of black signal on a white background. Images were then made binary and put through a watershed function to separate contiguous cells. All images were then processed with an analyze particles function with a size minimum-maximum of 90–1000 pixels, and circularity of 0.1–1.0. The outlines of all quantified cells as well as total count and characteristics of the analyzed particles were saved. Raw and processed images were manually scanned side by side to confirm accuracy of quantification. Cells lacking a clearly defined process extending from them, as well as any large debris puncta, were excluded from the count. All cell counts were compared to appropriate non-treated controls.
Co-culture studies: astrocyte:neuron ratio
Neuron-astrocyte co-cultures were prepared similarly to previously published methods(Smeyne, Jiao, Shepherd, & Smeyne, 2005). Briefly, isolated regional astrocytes were trypsinized, dissociated to a single cell suspension and quantified. Increasing numbers of astrocytes (30,000–120,000) were plated onto 48-well tissue culture plates. Cells were allowed to adhere and grow for 48 hours prior to further experimentation. Subsequently, embryonic neuron preparations were carried out to obtain TH-GFP+ neurons. Neurons were plated onto regional astrocytes in a homo- and heterotypic manner at 1:1, 2:1, and 4:1 (astrocyte:neuron) ratios. 24 hours after plating, all cultures were treated with 10μM AraC to halt astrocytic proliferation. Cultures were then allowed to mature for 6 more days in vitro prior to MPP+ treatment. After MPP+ treatment all cells were fixed and GFP+ cells were quantified using the Nikon Eclipse camera system and ImageJ macro described above.
Lentiviral shRNA knockdown
GDF-15 lentiviral shRNA plasmid was purchased from Origene (catalog# TL710232, pGFP-C-shLenti). Lentiviral particles were prepared following manufacturers protocols (shRNA lentiviral packaging kit catalog# TR30037). Lentiviral particles were concentrated using Origene Lenti Concentrator (catalog# TR30025) and isolated astrocytes were transduced at a MOI sufficient to result in >80% transduction 72 hours post exposure.
RNA isolation and cDNA synthesis
Total RNA was isolated directly from freshly collected cells in TRIzol (Invitrogen), a modification of the guanidine isothiocyanate-phenol-chloroform extraction method. cDNA was synthesized by using at least 250ng total RNA in a 20μl reaction with Superscript IV (Invitrogen) and oligo (dT)12–18 (Invitrogen). One microliter of RNase H (Invitrogen) was added to each reaction tube, and the tubes were incubated for 20min at 37°C before proceeding to real-time PCR.
RNAseq Analysis
Total RNA was isolated as above and 5μg of total RNA was sent to the Jefferson RNAseq core facility (Jefferson Cancer Genomics Laboratory). RNAseq was carried out according to manufacturer’s instructions: (https://support.illumina.com/downloads/truseq_stranded_total_rna_sample_preparation_guide_15031048.html). All subsequent read analysis was performed using Illumina recommended software including: Cufflinks Assembly & DE version 2.0.0 (BaseSpace Workflow), Isis version 2.6.25.12 (Analysis Software), STAR version STAR_2.5.0a (Alignment software), BEDTools version 2.17.0, Cufflinks version 2.2.1, BLAST version 2.2.26+.
Real-time PCR analysis
Real-time PCR was carried out on the 7500 Real Time PCR System using SYBR green PCR master mix (both from Applied Biosystems). GAPDH was used as an internal control. PCR analyses were conducted in triplicate for each sample. The reaction mix consisted of 6.35ng cDNA, 0.5μM forward and reverse primer mix, 1x SYBR green PCR master mix. Reactions were run according to manufacturer protocols for at least 40 cycles. All primer sequences used can be found in Supplemental Table 1.
GDF-15 ELISA
Mouse/Rat GDF-15 Quantikine ELISA kit was purchased from R&D Systems (catalog #MGD150). Astrocytes isolated from either the SN or VTA as described above were plated at 1.5×104 cells/cm2 on 75cm2 tissue culture flasks and allowed to grow to confluency. Media was changed to NEP-basal media for 48 hours and collected. Samples of conditioned media were measured for GDF15 protein concentration according to manufacturer’s instructions.
FACS Sorting
All preparation and transport procedures were carried out on ice. Dissociated cells, generated as described above were pelleted and re-suspended in 1mL DPBS with 5% FBS. Cell suspensions were passed through a 100μm cell strainer to eliminate any large clumps or debris. A small aliquot (approximately 30μL) of both control (GFP-) and GFP+ cell suspension were plated down before sorting. A GFP+ calibration control was prepared for the FACS sorter by removal for 100μL of GFP+ cell suspension added to 900μL DPBS+5% FBS. The same procedure was used for GFP- cells to create a GFP- control sample for calibration. To the remainder of the GFP+ sample, 100uL DPBS+5%FBS and 1μL Propidium Iodide (PI) was added as an index of cell viability and separation of PI+/GFP+ (dead) and PI-/GFP+ (live) cells. Cells were sorted on a BD Biosciences FACSAria gated to collect the PI-/GFP+ fraction and collected in culture media. After sorting, cells were spun down for 5 minutes at 1000rpm and the cell pellet was re-suspended in culture media and counted with a hemocytometer prior to plating. Sorted cells (2.5–3.5×104 cells/20μL) were plated onto pre-coated 8-well chamber slides as above.
Magnetic bead separation of primary astrocytes
Preparations of primary astrocytes were obtained similarly as described above in “Tissue culture – astrocytes” with some modifications. After the enzymatic step, samples were subjected to magnetic bead separation using the commercially available Anti-GLAST (ASCA-1) microbead kit from Miltenyi Biotec (Catalog # 130–095-826) following manufacturer’s instructions with MS Columns (Catalog # 130–041-301). Bead/column bound cells were eluted in either PBS +1% triton for western blotting or Trizol for RNA isolation (see above). Samples of midbrain (VTA/SN) as well as cortical and striatal bead bound astrocytes were examined by qPCR and western blot to confirm absence of non-astrocytic contamination (Supplemental Figure 2).
Western blot
Cells were lysed in PBS + 1% triton and protein concentration determined using the BIO-RAD DCtm Protein Assay (Catalog # 5000111) according to manufacturer’s instructions. Protein samples of 20–30ug total protein were loaded into NuPAGE 4–12% Bis-Tris protein gels (Invitrogen catalog # NP0335BOX) and run in NuPAGE MES Buffer (Invitrogen catalog # NP0002) with Novex Sharp Pre-stained protein standard (Invitrogen catalog #LC5800). Gels were transferred to nitrocellulose membranes using the Invitrogen iBlot transfer system. Membranes were then blocked in 5% non-fat dry milk in tris-buffered saline with 0.1% Tween. Blots were then incubated with primary antibodies (Supplemental Table 2) overnight at 4˚C. Blots were then incubated with species appropriate HRP-Conjugated secondary antibodies were used at manufacturer’s recommended concentrations for 1 hour at room temperature. Bands were then visualized using an ECL chemiluminescent substrate on the Bio-Rad ChemiDoc.
Experimental design and statistical analysis
All data are presented as the mean ± SEM. GraphPad was used for all statistical analysis. The statistical significance of the mean difference was calculated using either a repeated measures ANOVA with appropriate post-hoc analysis of significance or a two-tailed Student’s t-test. A p-value < 0.05 was considered significant.
Results
Loss of astrocytes exacerbates MPP+ toxicity of SN DA neurons and exposes a novel vulnerability of VTA DA neurons to toxin
To determine the role that astrocytes play in the potential neuroprotection of VTA neurons from PD toxins, we utilized the TH-GFP reporter rat previously generated by our lab (Iacovitti et al., 2014). This strain allowed us to isolate embryonic VTA and SN subregions and culture them separately to generate subregionally specific neuron-astrocyte cultures (Fig. 1). As expected, SN cultures contained fewer GFP+ TH neurons compared to VTA cultures (Fig. 1a, h). Nonetheless, we observed a similar astrocytic bed in all cultures at 7 days in vitro (Fig. 1b, e, i, l). We then determined the IC50 of 50 μM for MPP+ in vulnerable SN cultures (Fig. 1d, g); while the same dosage had no effect in protected VTA cultures (Fig. 1k, n). Higher doses (100μM) resulted in significant death in both SN and VTA cultures, suggesting general toxicity of MPP+ at this dosage (Fig. 1g, n - orange bars). With this system in place, we were then able to virtually eliminate the number of GFAP+ astrocytes from both SN and VTA cultures using Ara-C (Supplemental Fig. 3). We found that the removal of astrocytes had no effect on the survival of either SN or VTA neurons under control conditions (Fig. 2a, d, Supplemental Fig. 4). However, cultures of SN neurons exhibited greater susceptibility to MPP+ toxicity in cultures lacking astrocytes [~50% survival in astrocyte containing (Fig. 1g), versus ~12% in astrocyte diminished conditions (Fig. 2c)]. Interestingly, VTA neurons, which are typically protected from MPP+ toxicity, were vulnerable to the same MPP+ toxic insult (Fig. 2e, f) when astrocytes were removed (compare Fig. 1n with Fig. 2f, red bars), revealing a novel susceptibility of VTA neurons to PD toxin. The results of these experiments suggest that astrocytes of both SN and VTA subregions may play a role in the protection of DA neurons from MPP+ toxicity.
Figure 1: Selective toxicity of SN neurons by MPP+ treatment.
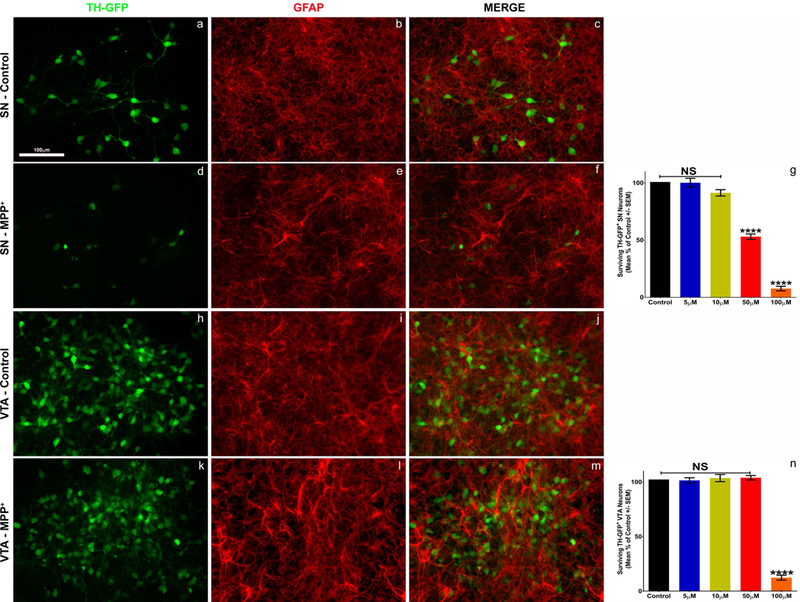
Isolated cultures of SN and VTA hTH-GFP neurons (green) were treated with MPP+ (5–100uM) or PBS (control) for 48 hours. Confluent monolayers of GFAP+ astrocytes (red) are present in both regional cultures (b, e, i, l) regardless of toxin treatment. Treatment of SN cultures with MPP+ results in a dose dependent toxicity of DA neurons (a, d, g), whereas VTA cultures are robustly protected from toxicity (h, k, n). General toxicity of both regions is seen at high MPP+ (100uM) concentrations (g, n, orange bars). SN (g) - One-way ANOVA; **** = p<0.0001 F (4, 25) = 250.9 post-hoc Bonferroni’s multiple comparisons test; VTA (n) – One-way ANOVA; **** = p<0.0001 F (4,25) = 223.9 post-hoc Bonferroni’s multiple comparisons test.
Figure 2: Manipulation of astrocyte number suggests a role for astrocytes in neuroprotection.
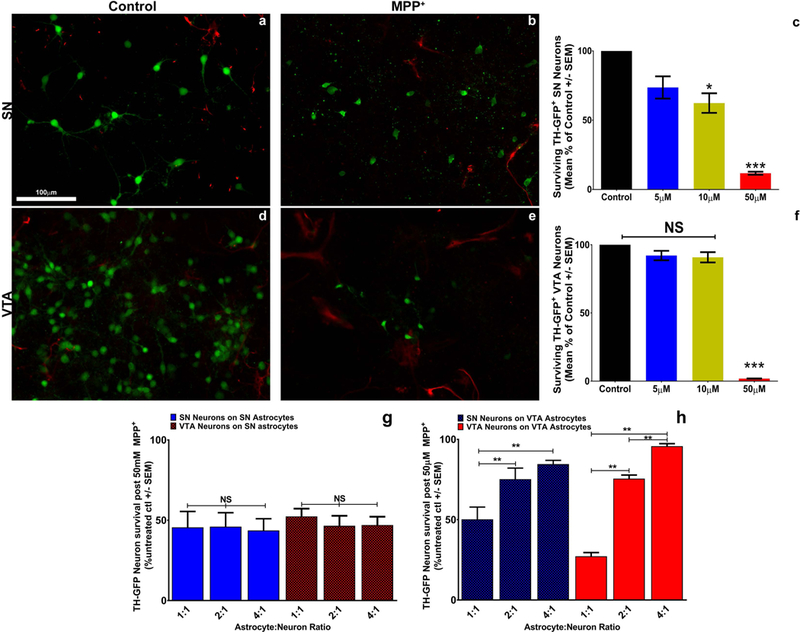
Treatment of VTA and SN cultures with Ara-C (10uM) for 48 hours results in significant decreases in the number of GFAP+ (red, a-e; Supplemental figure 1) astrocytes present, but has no impact on green TH-GFP+ DA neurons (a, d, Supplemental figure 2). MPP+ treatment of SN cultures exhibits increased susceptibility of these neurons when astrocytes are nearly absent from the cultures (b, c; One-way ANOVA; *** = p<0.0001 F (3, 56) = 1120, post-hoc Bonferroni’s multiple comparisons test). VTA cultures are susceptible to the same toxin treatment when astrocytes are removed (e, f; One-way ANOVA; *** = p<0.0001 F (3, 56) = 4833, post-hoc Bonferroni’s multiple comparisons test). Regardless of the number of SN astrocytes present in co-cultures of SN neurons (homotypic) (g, blue bars; one-way ANOVA; p=0.9794 F (2, 24) = 0.02082) and VTA neurons (heterotypic) (g, red hatched bars; one-way ANOVA; p=0.7015 F (2,24) = 0.3599), we observe no greater than 50% cell survival after MPP+ treatment. Interestingly, VTA astrocytes show a ratio-dependent increase in protection of both SN (heterotypic) (h, blue hatched bars; one-way ANOVA; p=0.0016 F (2, 24) = 8.47 post-hoc Bonferroni’s multiple comparison test) and VTA (homotypic) (h, red bars; one-way ANOVA; p<0.0001 F (2, 24) = 285.8 post-hoc Bonferroni’s multiple comparison test) neurons from MPP+ toxicity. Fields shown are representative images from raw data quantification.
Isolated VTA, but not SN, astrocytes provide protection to both SN and VTA DA neurons
Since the removal of astrocytes exposed an increased vulnerability to MPP+ in both SN and VTA cultures, we sought to determine if this phenomenon was dependent simply on the ratio of astrocytes to neurons or on their subregion of origin. Previous in vivo studies have shown that the VTA contains a greater number of astrocytes compared to the SN (Damier et al., 1993). Therefore, we established homo- and heterotypic astrocyte-neuron co-cultures utilizing subregionally isolated SN and VTA astrocytes in various proportions to SN and VTA neurons. The astrocytic cell population was determined to be homogeneous as evidenced by the absence of microglial (Iba1 and CD11b/c), neuronal (βIII tubulin and NeuN) or oligodendrocyte (NG2 and MBP) staining in cultures whereas all cells robustly stained for the astrocytic markers GFAP and S100β (Supplemental Fig. 5). Similar results were observed with PCR analysis of isolated astrocyte cultures (Supplemental Fig. 6). We tested whether increasing the ratio of SN astrocytes provided greater protection to SN or VTA neurons from MPP+ toxicity. We found that increasing ratios (1:1, 2:1 or 4:1) of SN astrocytes were unable to protect SN neurons in homotypic co-cultures or VTA neurons in heterotypic co-cultures (Fig. 2g). VTA astrocytes, on the other hand, robustly protected both SN and VTA neurons from MPP+ toxicity (Fig. 2h) at a ratio of 2:1 and 4:1 but interestingly not at the lower ratio of 1:1. These findings demonstrate that it is not simply a matter of greater proportions of astrocytes providing greater protection, as SN astrocytes lack greater than 50% protection of DA neurons at any ratio tested. Rather, VTA astrocytes appear to provide some protective factor not found in SN astrocytes that affords greater protection from PD toxicity to all midbrain DA neurons.
VTA astrocyte conditioned media demonstrates that released factors mediate protection of DA neurons
Following our findings that removal of astrocytes from SN and VTA cultures increases the neuronal susceptibility to MPP+, and that increasing numbers of VTA but not SN astrocytes are protective for both SN and VTA DA neurons, we sought to determine whether VTA astrocytes secrete a factor which is neuroprotective that is deficient in SN astrocytes. We used homogeneous SN or VTA astrocytes to collect subregionally-specific conditioned media (ACM). As a non-dopaminergic regional control, we also collected media conditioned by cortical (CTX) astrocytes. We found that the addition of VTA ACM (Fig. 3a, blue bar) robustly protected SN neurons from MPP+ toxicity when SN astrocytes were absent from the cultures. Furthermore, we found that although SN and CTX ACM were able to provide some protection from MPP+ (Fig. 3a, purple and pink bars), both regions were significantly less effective than VTA ACM. Additionally, we also examined whether VTA ACM protected VTA neurons from toxicity (Fig. 3b), similarly to increased ratios of VTA astrocytes. We found that VTA ACM was significantly more effective than SN ACM or CTX ACM in protecting VTA neurons from MPP+ toxicity in the absence of their regional astrocytes (Fig. 3b). These findings confirm that VTA astrocytes secrete neuroprotective factor(s) that are either lacking or present in insufficient concentrations in SN and CTX astrocytes.
Figure 3: VTA astrocyte conditioned media, but not GDNF alone or a growth factor cocktail, protects both SN and VTA DA neurons from MPP+ toxicity.
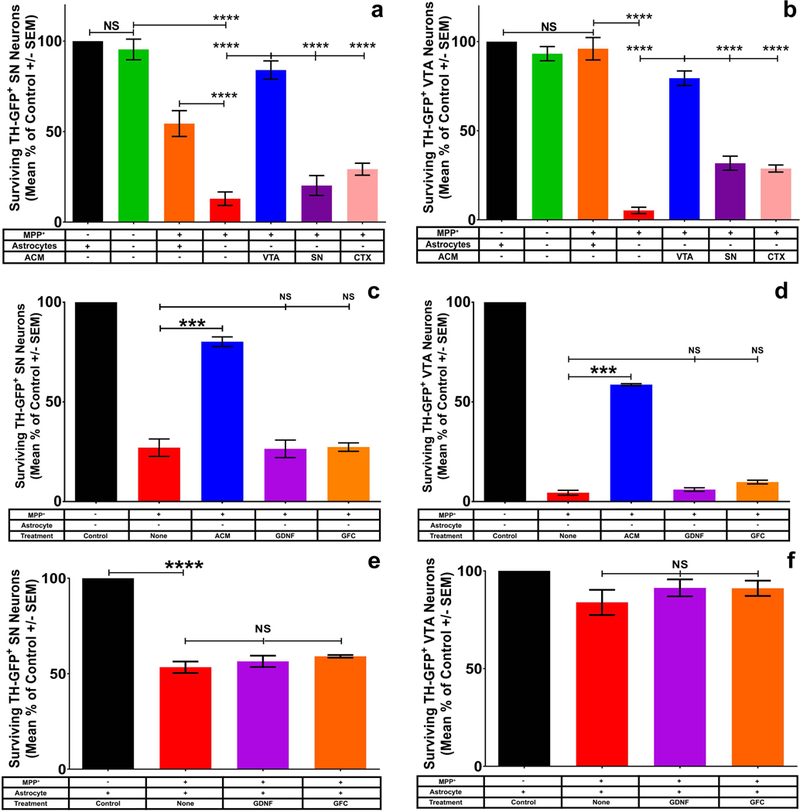
Virtual elimination of astrocytes from regional SN (a) or VTA (b) cultures does not result in DA neuron death in control cultures (green bars; supplemental figure 2). The addition of astrocyte conditioned media from VTA (a, b, blue bars) but not SN (purple bars) or cortex (CTX, pink bars) affords protection of both SN (a; One-way ANOVA; **** = p<0.0001 F (6, 25) = 57.29 post hoc Tukey’s multiple comparisons test) and VTA (b; One-way ANOVA; **** = p<0.0001 F (6, 28) = 112.3 post hoc Tukey’s multiple comparisons test) DA neurons from MPP+ toxicity. To investigate potential ACM derived protective molecules, we tested the ability of GDNF (purple bars, c-f) and a growth factor cocktail (orange bars, c-f: GFC = 50ng/mL GDNF, ARTN, NTN, PSPN, BDNF, Igf1, Igf2, GDF5, GDF8, CNTF, Fgf1, Fgf2, Fgf8, EGF, NGF, TGFβ1,TGFβ3, NT3, and 150μg/mL glutathione) to protect SN (c, One-way ANOVA; *** = p<0.0001 F (4, 10) = 54.49 post-hoc Bonferroni’s multiple comparisons test) and VTA (d, One-way ANOVA; *** = p<0.0001 F (4, 10) = 809.7 post-hoc Bonferroni’s multiple comparisons test) neurons from MPP+ toxicity in the absence of astrocytes. Similarly when astrocytes are present neither GDNF nor GFC is able to mediate increased protection of SN (e, One-way ANOVA; **** = p<0.0001 F (3, 12) = 87.81) or VTA (f, One-way ANOVA; p=0.1992 F (3, 12) =1.809) neurons in culture.
Known trophic factors do not mimic VTA ACM protection of midbrain DA neurons
To examine further the possibility that VTA astrocytes provide vital support that is deficient in SN astrocytes, we attempted to parse out what factor(s) might be responsible for the protective effect of VTA astrocytes. We first performed fractionation of VTA-ACM based on molecular weight. We found that the protective effect was greatest in the fraction <30kD (described in Methods). This molecular weight is consistent with a growth factor, cytokine, or other small molecule-mediated effect. Since GDNF is a well-characterized trophic factor (Björklund, Rosenblad, Winkler, & Kirik, 1997) of the appropriate size and has been shown to be protective in in vivo studies of DA neurons (Hoffer et al., 1994; Kearns, Cass, Smoot, Kryscio, & Gash, 1997; Kearns & Gash, 1995), we tested its efficacy in protecting SN and VTA neurons grown in culture without astrocytes. We found that GDNF alone is insufficient to recapitulate the protective effect of VTA ACM on either SN (Fig. 3c, purple bar) or VTA (Fig. 3d, purple bar) neurons. To further pursue possible protective molecules within the <30kD range, we created a growth factor cocktail (GFC) containing a number of known trophic molecules in the appropriate MW range (Fig. 3, legend). We found that the addition of this cocktail, similar to GDNF alone, is ineffective at protecting SN (Fig. 3c, orange) and VTA (Fig. 3d, orange) DA neurons from MPP+ toxicity in the absence of astrocytes. To test the possibility that these factors were working through astrocytes, thereby explaining the lack of protection in our system, we added either GDNF alone or GFC to homotypic cultures of SN and VTA neurons and astrocytes. Surprisingly, these trophic factors were not able to mediate protection of SN (Fig. 3e) or VTA (Fig. 3f) neurons even in the presence of subregional astrocytes. These findings further support the hypothesis that VTA astrocytes secrete a factor(s) that mediates protection of DA neurons in both SN and VTA subregions which is potentially novel.
RNAseq analysis of isolated VTA and SN astrocytes provides new targets for neuroprotection
Using RNAseq which allows for a comprehensive, unbiased examination of the transcriptional profile of isolated RNA, we next sought to examine subregional astrocytes to determine what factor(s) were differentially expressed in VTA and SN. As expected, we found that there were many significant differences between astrocytes of these two midbrain nuclear subregions (Fig. 4a). In total, 6,421 genes were differentially expressed between VTA and SN regions (Fig. 4b) which cover a wide variety of cellular functions and pathways (Fig. 4c).
Figure 4: RNAseq analysis of isolated midbrain astrocytes reveals potentially novel astrocytic neuroprotective factors.
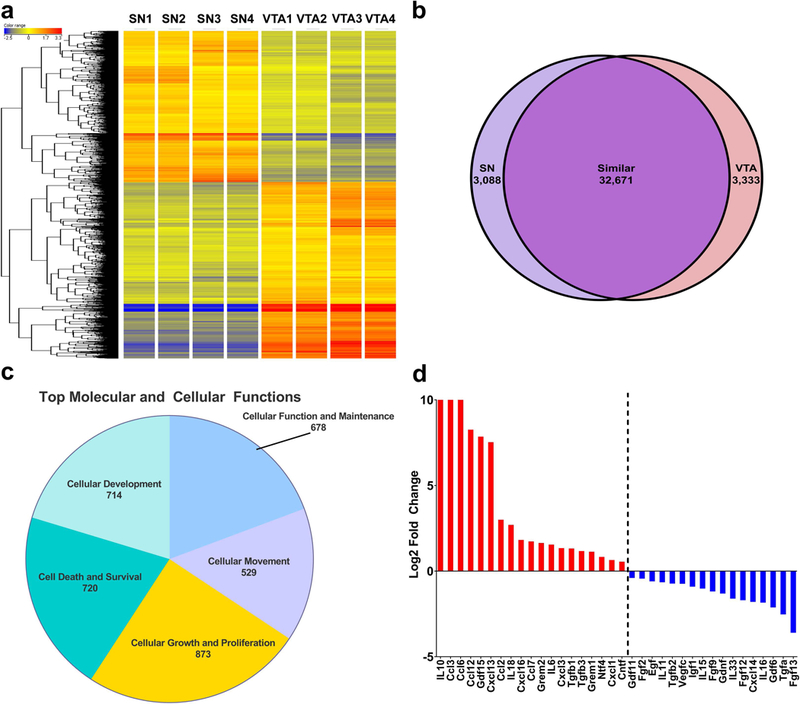
RNAseq transcriptional profiles of isolated SN and VTA astrocytes (a, Supplemental file 1) demonstrate a wide variety of transcriptional differences between regional astrocytes. Overall (b), despite a vast number of transcripts (32,671 genes) in common, the SN (3,088 genes) and VTA (3,333 genes) also exhibit stark differences within their transcriptional profile. Ingenuity pathway analysis of the overall transcriptional profile (c) demonstrates a number of interesting top molecular and cellular functions within VTA astrocytes including: cellular function and maintenance, cellular movement, cellular growth and proliferation, cell death and survival, and cellular development. Due to conditioned media fractionation experiments (data not shown) our scope was narrowed to small (<30kD), secreted, potentially neuroprotective factors which results in a list of 17 genes expressed at higher levels in the SN than the VTA, and 19 genes expressed at higher levels in the VTA than the SN (d, SN>VTA-blue bars, VTA>SN-red bars).
As RNAseq datasets are vast, we utilized our previous findings and narrowed the scope to factors that were secreted (containing a signal sequence) and fell in the <30kD range. Indeed, a number of factors within the correct MW range were differentially expressed by VTA and SN astrocytes (Fig. 4d). Due to the protective effect of VTA ACM, we further parsed the list to examine the six secreted factors that were most abundantly expressed in VTA astrocytes compared to SN astrocytes. These included: interleukin 10 (IL10), chemokine (C-C motif) ligand 3, 6 & 12 (Ccl3, 6, & 12), growth and differentiation factor 15 (GDF15), and C-X-C Motif chemokine ligand 13 (Cxcl13). Utilizing recombinant proteins for each potential target, we tested whether the addition of these proteins in a dose dependent manner was sufficient to recapitulate the effect seen using VTA ACM. We found that, of the six potential targets, GDF15 was the only factor that mediated a dose-dependent protective effect from MPP+ toxicity in both SN (Fig. 5a) and VTA (Fig. 5b) neurons. However, the GDF15 protective effect did not reach levels similar to that of VTA ACM (Fig. 5a, b blue bars). Additionally, RT-PCR and ELISA examination of isolated SN and VTA astrocytes, and ACM, confirmed significantly lower expression of GDF15 mRNA (Supplemental Fig. 7a) and protein (Supplemental Fig. 7b) by SN astrocytes, similar to that found by RNAseq. Furthermore, to examine if the differences seen in GDF15 expression in vitro between SN and VTA astrocytes are conserved in vivo, samples of astrocytes were isolated using magnetic bead separation and subjected to RT-PCR and western blotting. We found that VTA astrocytes consistently express greater levels of GDF15 mRNA (Supplemental Fig. 7c) and protein (Supplemental Fig. 7d & d’) than SN astrocytes. Taken together, these findings suggest that GDF15 may be one of the factors responsible for the VTA astrocyte specific protection of both SN and VTA DA neurons.
Figure 5: Addition of recombinant GDF15 partially recapitulates the effect of VTA ACM, whereas VTA astrocyte knockdown of GDF15 results in decreased ACM protection from MPP+ toxicity.
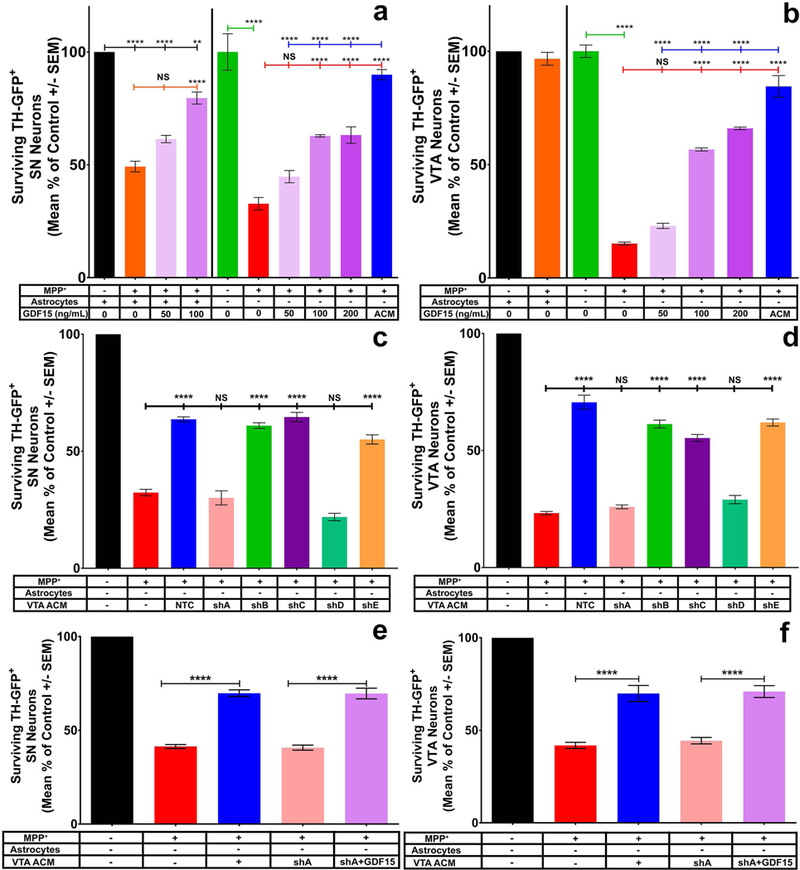
Of the top 6 candidate molecules determined by RNAseq, only GDF15 was able to recapitulate the effect of VTA ACM (a & b). Dose response of GDF15 on SN (a, One-way ANOVA; **** = p<0.0001 F (11, 25) = 54.83) and VTA (b, One-way ANOVA; **** = p<0.0001 F (9, 21) = 161) DA neurons in the absence of their regional astrocytes demonstrates increased protection from MPP+ toxicity at 100–200ng/mL (light purple and deep purple bars). These concentrations were significantly less effective than full VTA ACM in protection of SN (a) and VTA (b) DA neuron cultures, suggesting other factors exist in VTA ACM that are neuroprotective of SN and VTA DA neurons. Furthermore, the addition of GDF15 in SN cultures with regional astrocytes present (a, faint and light purple) demonstrated significant increases in protection at the 100ng/mL concentration, suggesting that the lack of GDF15 in SN astrocytes can be countered by exogenous addition of this factor. Using a commercially available lentiviral shRNA we demonstrate approximately 3–4 fold knockdown of VTA astrocyte GDF15 (Supplemental figure 6). Addition of GDF15 KD ACM from constructs shA & shD, but not shB, C, E to isolated SN (c, One-way ANOVA; **** = p<0.0001 F (7, 16) = 84.87, post-hoc Tukey’s multiple comparisons test) or VTA (d, One-way ANOVA; **** = p<0.0001 F (7, 40) = 155.1, post-hoc Tukey’s multiple comparisons test) cultures in the absence of astrocytes results in significantly decreased protection when compared to non-transfected (NTC) VTA ACM. Rescue of VTA ACM neuroprotective effect was observed for both SN (e, One-way ANOVA; **** = p<0.0001 F (4, 42) = 127.2, post-hoc Tukey’s multiple comparisons test) and VTA (f, One-way ANOVA; **** = p<0.0001 F (4, 42) = 91.08) cultures in the absence of astrocytes when exogenous GDF15 (100ng/mL) was added to KD ACM.
GDF15 knockdown decreases the efficacy of VTA ACM in protecting DA neurons
To confirm GDF15’s role in neuroprotection, we sought to determine whether knockdown of GDF15 expression in VTA astrocytes is sufficient to decrease the protective effect of VTA-derived ACM. Using commercially available lentiviral shRNA constructs for GDF15, we were able to transfect homogeneous cultures of VTA astrocytes and demonstrate significant mRNA expression decreases of GDF15 by RT-PCR (Supplemental Fig. 8). We then collected ACM from GDF15 knockdown (KD) and non-transfected astrocytes (NTC). Utilizing a culture preparation of SN and VTA DA neurons lacking their subregional astrocytes (Ara-C treated cultures), we showed that the addition of GDF15 KD ACM exhibited a significant decline in protection of both SN (Fig. 5c, pink and teal bars) and VTA (Fig. 5d, pink and teal) DA neurons in comparison to ACM from non-transfected or scramble control astrocytes (Fig. 5c & d blue and orange bars) or astrocytes transfected by constructs where no significant knockdown was observed (Fig. 5d, green and purple bars; Supplemental Fig. 8). To examine if the exogenous addition of GDF15 into KD ACM was sufficient to rescue the neuroprotective effect of ACM for SN and VTA neurons, similar cultures were established and subjected to MPP+ treatment in the presence of NTC and KD ACM with and without GDF15 addition and after knockdown with shA-GDF15. We demonstrated that the addition of GDF15 (100ng/mL) sufficiently rescues the neuroprotective effects of VTA ACM for both SN (Fig. 5e pink vs light purple bars) and VTA (Fig. 5f pink vs light purple bars) neurons after GDF15 knockdown. These findings further support the potential role of GDF15 as a VTA astrocyte derived neuroprotective factor.
GDF15 receptor expression is similar between SN and VTA DA neurons
Recent studies have demonstrated a distinct receptor interaction for GDF15 (Emmerson et al., 2017; Mullican et al., 2017). An orphan member of the GDNF family of receptors, GDNF Family Receptor Alpha Like (GFRAL), and its subsequent downstream signaling, is activated by the binding of GDF15. Furthermore, previous studies examining the expression of GFRAL within the brain demonstrated high levels of expression within the SN (Z. Li et al., 2005). However, the expression level within the VTA has not been examined. Therefore, we sought to examine the expression of GFRAL within isolated samples, as well as cultures, of SN and VTA DA neurons to confirm the presence of this putative receptor for GDF15. We found that similar to previous findings, SN, as well as VTA, DA neurons express GFRAL at a high level (Fig. 6a, b). Striatal and whole CTX samples, used as negative and positive controls, respectively show expected absence/presence of GFRAL signal (Fig. 6a, b). Furthermore, immunostaining of FACS isolated midbrain TH-GFP DA neurons demonstrates strong receptor expression on these neurons after culture (Fig. 6c, d), whereas striatal cultures lack expression of the receptor (Fig 6f-h) demonstrating antibody selectivity. These findings demonstrate that the receptor for GDF15 is indeed present within SN and VTA neurons, suggesting that the lack of GDF15 production by SN astrocytes may explain the absence of protection in this subregion and the failure to find an increased vulnerability in SN DA neurons in GDF15 knockout mice (Machado, Gilsbach, et al., 2016). In contrast, VTA astrocytes may be able to protect both VTA and SN DA neurons due to their high level of GDF15 expression coupled with the presence of the GFRAL receptor.
Figure 6: SN and VTA DA neurons strongly express the putative GDF15 receptor GFRAL.
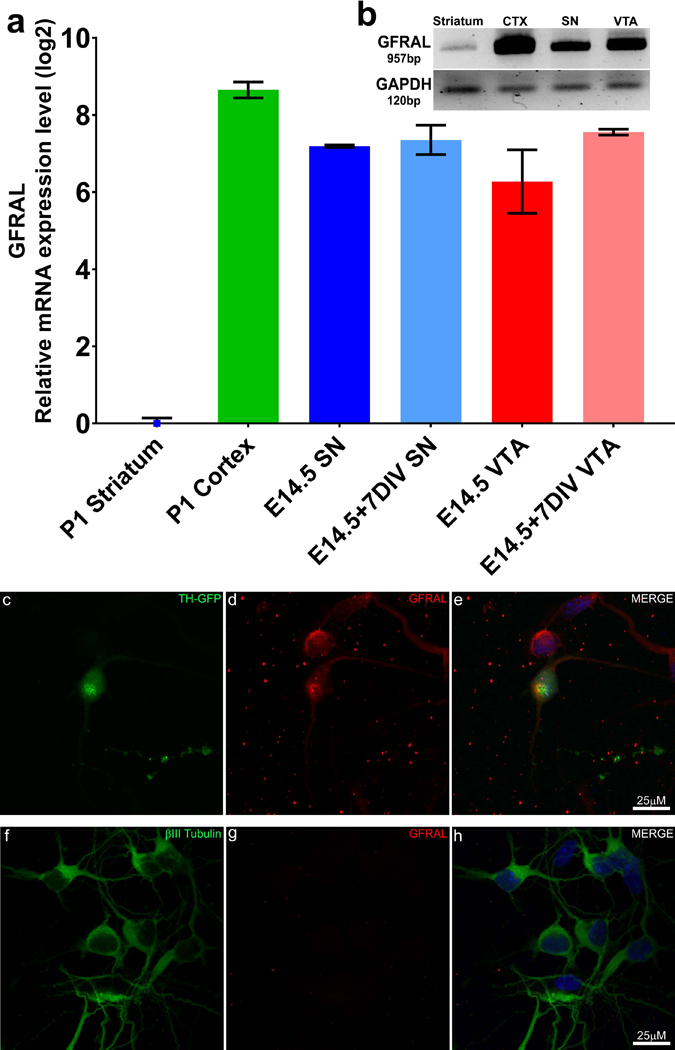
SN and VTA neurons were examined for the expression of the GDF15 receptor GFRAL. PCR examination (a & b) of GFRAL expression was performed with isolated P1 striatum (negative control, blue square), P1 cortex (CTX, positive control, green bar), as well as freshly isolated (dark blue and dark red bars) and 7 day old cultures (light blue and light red bars) of SN (blue) and VTA (red) DA neurons confirming strong expression of the receptor within the SN and VTA region and that culture conditions does not modulate the expression of this receptor. Immunofluorescent staining of FACS isolated TH-GFP DA neurons (c) confirms the presence of GFRAL (d, e) on midbrain neurons, whereas cultures of striatal neurons (f-h) clearly lack expression of GFRAL (g).
iPSC-derived DA neurons are protected from MPP+ toxicity by GDF15 and express the GFRAL receptor
To further our understanding of the potential role of GDF15 in the protection of DA neurons, we utilized iPSC-derived DA neurons to test whether GDF15 is a possible protective factor for human cells. Cultures of iPSC-derived DA neurons were treated with MPP+ in the presence of increasing concentrations of recombinant GDF15. We found that, similar to isolated rat SN and VTA DA neurons, iPSC-derived DA neurons are robustly protected from MPP+ toxicity by GDF15 in a dose dependent fashion (Fig. 7a). Furthermore, immunostaining of iPSC DA neurons demonstrates robust expression of the GDF15 receptor GFRAL (Fig. 7b). These findings further support GDF15 as a DA neuron protective molecule.
Figure 7: Recombinant GDF15 can protect iPSC derived dopaminergic neurons from MPP+ toxicity.
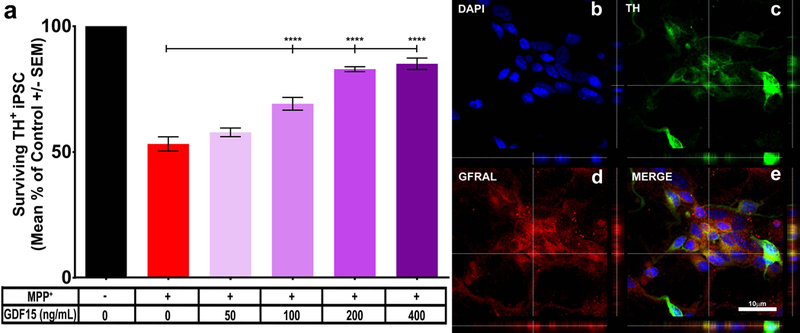
To further test whether GDF15 can be protective in a relevant human cellular system we utilized iPSC-derived DA neurons and tested a similar dose response of recombinant GDF15 coupled with MPP+ treatment. As expected MPP+ causes significant decreases in surviving TH+ cells (a, red bar). The addition of increased dosages of GDF15 (gradient purple bars, 50–400ng/mL) resulted in significantly greater survival of TH+ DA neurons after MPP+ treatment (One-way ANOVA; **** = p<0.0001 F (5, 36) = 74.49, post-hoc Tukey’s multiple comparisons test). iPSC-derived DA neurons were also examined for expression of GFRAL (b-DAPI, c-TH, d-GFRAL, e-merge) to explore their ability to respond to exogenous GDF15.
Discussion
Historically, many studies have focused on understanding the intrinsic workings of SN DA neurons to answer the question of why these neurons specifically die in PD while neighboring VTA DA neurons are relatively spared (Chung et al., 2005; Phani et al., 2010; Thuret et al., 2004; Yao et al., 2005). We demonstrate that the selective susceptibility of midbrain DA neurons may depend on factors beyond the inherent differences between the SN and VTA neurons themselves. Rather, subregional astrocytes may play a significant role in the vulnerability or resistance of DA neurons to PD and the PD mimetic toxin MPP+.
In this study, we showed that the removal of astrocytes exposes a novel vulnerability of VTA DA neurons to MPP+ and further exacerbates toxicity in SN cultures. Additionally, we found that astrocytes isolated from VTA, but not SN or CTX, are able to secrete factor(s) that are neuroprotective to both SN and VTA DA neurons. RNAseq analysis of subregional astrocytes highlighted GDF15 as a possible protective molecule robustly expressed in VTA astrocytes. Indeed, GDF15 is able to recapitulate the protective effect achieved using VTA ACM, albeit to a lesser extent, and GDF15 knockdown demonstrated decreased protective effects of VTA ACM. On the whole, our findings underscore the regional differences within astrocytes of these neighboring midbrain nuclei and the pivotal role that they play in the selective vulnerability of midbrain DA neurons to PD.
Many previous studies have investigated the susceptibility of DA neurons, however most of these studies utilized cultures where the ventral mesencephalon as a whole was dissociated and grown as mixed neurons and astrocytes rather than subregionally isolated VTA and SN (Hou, Lin, & Mytilineou, 1996; Huang, Hong, & Chuang, 2009; Jaumotte, Wyrostek, Zigmond, & Smith, 2016; Jaumotte & Zigmond, 2014; Krieglstein & Unsicker, 1994; Park & Mytilineou, 1992). These cultures necessarily mask important differences between subregions, such as the VTA houses significantly more DA neurons than the SN (Nair-Roberts et al., 2008), thereby diluting effects on vulnerable SN neurons with predominantly protected VTA neurons. Furthermore, as previous studies demonstrated a difference in the number of astrocytes present in these subregions (Damier et al., 1993), it is likely that mixed mesencephalic cultures contain many more astrocytes from the VTA which could further confound interpretation of the data. These important differences may account for much of the conflicting results in the literature.
Additionally, some studies have utilized neuron enriched mesencephalic cultures where astrocytes have been removed prior to toxin treatment (Y. Y. Li, Lu, Li, Zhao, & Pu, 2008; Wang et al., 2010; Yang, Kaul, Zhang, Anantharam, & Kanthasamy, 2004; Zhai, Zhu, Wang, Chen, & Wang, 2013). However, inconsistencies in experimental design and Ara-C dosage resulted in many discrepant findings among studies. Incomplete elimination of astrocytes could result in greater protective effects, just as saturation with VTA astrocytes would presumably bias data in the opposite direction. Our study eliminates these possible confounding factors by approaching the SN and VTA separately and examining the relationship between neurons and astrocytes in a subregionally specific fashion using homotopic and heterotopic co-cultures, as well as verifying the elimination of nearly all astrocytes through tightly controlled Ara-C dosages.
Besides removal of astrocytes, perturbations of astrocytes may also affect neuroprotective capacity. Indeed, Gegg et al.(2005) showed that depletion of glutathione, a well-studied antioxidant molecule within astrocytes, results in astrocytes that are ineffective at neuroprotection in response to nitric oxide treatment. Additionally, Drukarch et al. (1997) found that depletion of glutathione from astrocytes results in decreased trophic support by astrocytes and diminishes astrocyte capacity to be neuroprotective from hydrogen peroxide treatment (Benjamin Drukarch, Schepens, Stoof, Langeveld, & Van Muiswinkel, 1998). Furthermore, a recent paper by Zhang et al. (2016) demonstrated that knockout of an astrocyte specific water channel, aquaporin 4, in mice results in greater susceptibility of the SN and VTA DA neurons to MPTP. These findings support the possibility that astrocytes are vital to neuroprotection within the midbrain such that removal or perturbations of their function can result in exacerbation of toxic insult.
The critical role of astrocytes in neuroprotection has been recognized for many years. Early work by Bronstein et al (1995) demonstrated that the addition of mixed mesencephalic glia (mainly astrocytes) afforded protection of mesencephalic neurons. However, our studies are the first, to our knowledge, to regionally sub-dissect the VTA and SN through the use of our transgenic TH-GFP reporter rat (Iacovitti et al., 2014). This has allowed us to examine these subregions separately and parse out the subtle differences between astrocytes. The notion of regional differences between astrocytes is not new; many studies have demonstrated that all astrocytes are not the same (Khakh & Sofroniew, 2015; Schitine et al., 2015; Y. Zhang & Barres, 2010). More recent studies by Lidelow et al. (2017) and John Lin et al. (2017) have suggested that even within a specific region there can be up to five distinct types of astrocytes and that these astrocyte subtypes can take on characteristic “reactive” states similar to those seen in microglia and macrophages (Orihuela, McPherson, & Harry, 2016; Tang & Le, 2016). Our work highlights that even in closely associated contiguous subregions like the VTA and SN astrocytes differ in number and in other important and under-appreciated ways.
Thus, despite their close geographic proximity, our RNAseq data suggests a wide variety of transcriptional differences between the SN and VTA astrocytes. Included in these, are genes relating to cellular function and maintenance (678 genes), cellular movement (529 genes), cellular growth and proliferation (873 genes), cell death and survival (720 genes), and cellular development (714 genes). Our studies focused on a limited portion of this large data set. Based on the active fraction of VTA ACM, gene lists were parsed to include proteins that fell below 30kD molecular weight and contained a signal sequence for secretion, focusing on secreted growth factors and cytokines.
Many canonical growth/trophic factors are secreted by astrocytes and have been shown to be trophic for DA neurons (Strelau et al., 2000), as well as other neuronal types (Golden, Milbrandt, & Johnson, 2003; Gouin, Bloch-Gallego, Tanaka, Rosenthal, & Henderson, 1996; Huber, Krieglstein, & Unsicker, 1995; Minnich et al., 2010). Previous studies have utilized trophic molecules to demonstrate their neuroprotective effects on DA neurons in vitro (Hartikka, Staufenbiel, & Lubbert, 1992; Hou et al., 1996; Huang et al., 2009; Jaumotte et al., 2016; Jaumotte & Zigmond, 2014; Krieglstein & Unsicker, 1994; Otto & Unsicker, 1993; Park & Mytilineou, 1992; Spina, Squinto, Miller, Lindsay, & Hyman, 1992; Strelau et al., 2000) however in these studies, astrocytes were always present within the culture systems (ie. no Ara-C treatment). Therefore, in the current study, we examined the possible neuroprotective effects of growth factors in cultures essentially free of astrocytes. Somewhat surprisingly, we found that many well-known DA trophic factors, such as GDNF, BDNF, TFGβ1&−3, and Fgf-1, −2,& −8 added either individually or in a cocktail, did not provide protection to MPP+ treated SN neurons, nor did it recapitulate the effect of VTA ACM. In support of these findings, our RNAseq data further demonstrated that despite the enhanced expression of DA trophic factors (GDNF, TGFβ2, and Fgf9) in SN astrocytes, scant protection was provided by these glia to DA neurons treated with MPP+.
Indeed, of the many trophic/neuroprotective factors tested only GDF15, a distant member of the TGFβ superfamily (Bootcov et al., 1997; Fairlie et al., 1999) which was expressed at 230-fold higher mRNA levels in VTA than SN astrocytes, mimicked the neuroprotection from MPP+ toxicity seen with VTA ACM. Furthermore, we demonstrated that GFRAL, the newly described receptor for GDF15 (Emmerson et al., 2017; Mullican et al., 2017), was robustly expressed on both VTA and SN DA neurons, consistent with the fact that exogenous GDF15 rescues SN DA neurons from PD toxins in our experiments. Whether or not GDF15, which is expressed at much lower levels in SN astrocytes in vivo and in vitro, is responsible for the basal protective effect (ie. ~50% survival after MPP+) seen in homotypic SN neuron-astrocyte co-cultures remains to be proven. Arguing against this notion is the fact that increasing the ratio of SN astrocytes to neurons (4:1; Fig. 2g) as well as secretion into ACM (Fig. 3a) does not provide further protection to SN DA neurons. Alternatively, this basal effect could be mediated by other factors not examined in this study. Similarly, the protective effects of VTA astrocytes cannot be attributed entirely to GDF15. Thus, the addition of GDF15 alone is inadequate to recapitulate the full effect of VTA ACM and the knockdown of GDF15 expression decreases but does not eliminate protection of SN and VTA DA neurons from MPP+ toxicity. Therefore, while GDF15 is an important neuroprotective factor found in astrocytes, there are likely other crucial neuroprotective factors yet to be discovered.
A role for GDF15 in PD is supported by the recent discovery that greater levels of GDF15 in the cerebrospinal fluid of PD patients are correlated with a later age of disease onset (Maetzler et al., 2016). Likewise, early studies showed that exogenous GDF15 is trophic to embryonic midbrain DA neurons in vitro and pretreatment with GDF15 decreases motor dysfunction in a 6-hydroxydopamine rat model (Strelau et al., 2000). In these studies, however, GDF15, also known as macrophage inhibitory cytokine 1, was conjectured to work by decreasing the number of damaging microglia despite the fact that a reduced (not an amplified) microglial response was seen in GDF15 knockout mice (Machado, Haas, et al., 2016). Rather, our studies would indicate that GDF15 secreted by selective astrocytes, even in the total absence of microglia, mediates the neuroprotection of DA neurons and amelioration of motor deficits in PD
In summary, data in this paper suggests that differences in susceptibility of midbrain DA neurons to the PD toxin MPP+ may be dependent on protective factors supplied exogenously by subregional astrocytes. We demonstrate a novel vulnerability of VTA and exacerbation of SN DA neurons to MPP+ toxicity when their subregional astrocytes are removed. Indeed, RNAseq analysis confirmed the subregional specificity of VTA and SN astrocytes and demonstrated marked differences in their transcriptional profiles. In particular, GDF15 which is significantly expressed by VTA astrocytes but deficient in SN astrocytes, both in vitro and in vivo, provides neuroprotection from MPP+ toxicity, likely via the GFRAL receptor present on both VTA and SN DA neurons. Thus, GDF15 and its receptor GFRAL may represent important therapeutic targets in PD. Moreover, these findings could expand our current way of thinking about the neurodegenerative disease process, raising the possibility that the vulnerability of specific populations of neurons to disease risk factors may not stem entirely from neuron-intrinsic susceptibilities but also from differences in support from their astrocytic microenvironments.
Supplementary Material
Supplemental File 1: RNAseq comparison of isolated SN and VTA astrocytes. RNA was isolated from regionally specific cultures of astrocytes from the SN (4) and VTA (4) and subjected to RNAseq. The readouts demonstrate the specific gene, locus, mean FPKM, fold change and significance between samples. All samples were further subjected to Ingenuity pathway analysis.
Supplemental Figure 1: Representative dissection of TH-GFP+ ventral mesencephalon. The ventral mesencephalon of embryonic day 14.5 hTH-GFP rats was surgically dissected from the embryo similar to our previously established methods and the majority of the non-transgenic tissues were cleaned from the samples. Mesencephalic tissues can be easily observed under a dissecting microscope (a). The presumptive lateral SN tissue was sub-dissected apart from the medial VTA (denoted by red lines) and regional tissues were pooled and collected from each animal prior to enzymatic digestion and subsequent culture (b).
Supplemental Figure 2: Magnetic bead separated primary astrocytes lack neuronal and microglial markers. Samples of VTA (a) and SN (b) magnetic bead (ASCA1/GLAST) separated astrocytes were subjected to qRT-PCR analysis of astrocytic (GFAP) and neuronal (NeuN and β3Tubulin) expression. Both regions demonstrate strong expression of GFAP, greater gene expression is associated with low ΔCT values, compared to the neuronal transcripts. Additionally, western blot analysis of isolated cortical and striatal bead separated astrocytes (c) demonstrate expression of the astrocytic marker GFAP with absence of the microglial marker Iba1.
Supplemental Figure 3: Cytosine arabinoside (Ara-C) treatment results in robust elimination of astrocytes from SN and VTA cultures. Embryonic day 14.5 SN (a-c) and VTA (d-f) cultures were treated with 0 (a & d), 5 (b & e) and 10μM (c & f) Ara-C for 48 hours post plating. We found greatly decreased numbers of GFAP+ (red) astrocytes within both regional cultures consistent with increase dosing of Ara-C. Subsequent experiments were performed using the 10μM dose as it resulted in the greatest decrease of GFAP+ astrocytes, SN; 111±11, VTA; 117±10 (Two-tailed unpaired t-test, t=0.3803, df=8, p=0.7136) GFAP+ cells per well post AraC compared to a confluent bed of astrocytes in the untreated condition, while not affecting dopamine neuron health (Supplemental fig 4).
Supplemental Figure 4: Astrocyte elimination from isolated SN and VTA cultures does not result in decreased dopamine neuron viability. Total TH-GFP+ cell number was quantified with and without Ara-C treatment. Neither SN (Two-tailed Unpaired t-test, t=0.08805, df=10, p=0.9316) nor VTA (Two-tailed Unpaired t-test, t=0.8446, df=10, p=0.4181) neuronal viability was affected by the elimination of astrocytes from the culture.
Supplemental Figure 5: Astrocyte isolation results in homogeneous culture of regional astrocytes. Regionally isolated astrocytes were examined for non-astrocyte cellular contamination through histological methods. Cultures stain robustly for GFAP (a) and S100β (b) whereas no signal was evident for the microglial markers Iba1 (c) or CD11b/c (d); neuronal markers β3Tubulin (e) and NeuN (f); as well as immature (NG2, g) or mature oligodendrocytes (MBP, h).
Supplemental Figure 6: Cultures of regional isolated astrocytes exhibit low expression of non-astrocyte cell markers. qPCR analysis of 3 independent samples of SN (a), VTA (b) and CTX (c) were examined for the expression of markers of astrocytic (GFAP), microglial (Iba1, Cd11b), oligodendrocyte (Pdgfra, NG2, MBP), and neuronal gene expression (NeuN, β3Tubulin). Samples were normalized to the housekeeping gene GAPDH and the ΔCT from GAPDH was calculated. Greater gene expression is associated with low ΔCT values. **** = p<0.0001 compared to GFAP; One-way ANOVA with Dunnett’s multiple comparisons test: SN F (7, 61) = 132.1, VTA F (7, 62) = 130.9, CTX F (7, 50) = 41.13.
Supplemental Figure 7: SN astrocytes express and secrete GDF15 at significantly lower levels than VTA. To confirm the findings from our RNAseq, we examined isolated samples of VTA and SN astrocytes using RT-PCR and ELISA to examine the expression of GDF15. RT-PCR on two separate samples of cultured SN astrocytes were used to demonstrate consistently lower expression of GDF15 compared to VTA astrocytes (a). Similarly, ELISA measurement of secreted GDF15 in ACM from VTA and SN astrocytes (b) demonstrated significantly lower levels of GDF15 in media conditioned by SN (μ = 64pg/mL) compared to VTA (μ = 984pg/mL); Two-tailed unpaired t test, t=6.55, df=4, p=0.0028. Furthermore, examination of in vivo GDF15 expression, from astrocyte specific magnetic beads, demonstrated a lower level of GDF15 mRNA (c) and protein expression (d & d’) in SN astrocytes compared to VTA from two separate isolations.
Supplemental Figure 8: VTA astrocyte GDF15 expression is decreased using lentiviral shRNA. A commercially available lentiviral shRNA was added to isolated VTA astrocytes combined with the transfection reagent Polybrene (8μg/mL). Four GDF15 shRNA (shA-D) constructs and one scramble control (shE) construct were used to transduce astrocytes. We observed >80% efficiency for all tested constructs 3 days after lentiviral addition (not shown). Samples were examined with RT-PCR for GDF15 expression. Only constructs shA and shD resulted in decreased expression of GDF15 within VTA astrocytes when compared to non-transfected cells. As expected, scramble control (shE) did not change GDF15 expression.
Main points:
In vitro elimination of astrocytes exacerbates PD mimetic toxicity. Subregional astrocytes from the midbrain exhibit vastly different transcriptional profiles. VTA astrocyte secreted GDF15 mediates neuroprotection of SN, VTA, and iPSC dopamine neurons.
Acknowledgements
We thank the following funding agencies for their support: The NIH (2 RO1 NS075839) to L.I. and The Parkinson Council. We thank the Jefferson Cancer Genomics Laboratory for their expertise in performing the RNAseq analysis. Additionally, we thank the Jefferson Flow Cytometry core for their expertise in performing the FACS sorting.
Footnotes
Competing interests
The authors disclose no competing interests
References
- Allen NJ, & Barres BA (2009). Neuroscience: Glia - more than just brain glue. Nature, 457(February), 675–677. 10.1038/457675a [DOI] [PubMed] [Google Scholar]
- Anderson MA, Burda JE, Ren Y, Ao Y, O’Shea TM, Kawaguchi R, … Sofroniew MV (2016). Astrocyte scar formation aids central nervous system axon regeneration. Nature, 532(7598), 195–200. 10.1038/nature17623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björklund A, Rosenblad C, Winkler C, & Kirik D (1997). Studies on neuroprotective and regenerative effects of GDNF in a partial lesion model of Parkinson’s disease. Neurobiology of Disease, 4(3–4), 186–200. 10.1006/nbdi.1997.0151 [DOI] [PubMed] [Google Scholar]
- Bootcov MR, Bauskin AR, Valenzuela SM, Moore AG, Bansal M, He XY, … Breit SN (1997). MIC-1, a novel macrophage inhibitory cytokine, is a divergent member of the TGF-beta superfamily. Proceedings of the National Academy of Sciences of the United States of America, 94(21), 11514–9. 10.1073/pnas.94.21.11514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronstein DM, Perez-Otano I, Sun V, Mullis Sawin SB, Chan J, Wu GC, … McMillian MK (1995). Glia-dependent neurotoxicity and neuroprotection in mesencephalic cultures. Brain Research, 704(1), 112–116. 10.1016/0006-8993(95)01189-7 [DOI] [PubMed] [Google Scholar]
- Cai J, Schleidt S, Pelta-Heller J, Hutchings D, Cannarsa G, & Iacovitti L (2013). BMP and TGF-Β pathway mediators are critical upstream regulators of Wnt signaling during midbrain dopamine differentiation in human pluripotent stem cells Developmental Biology (Vol. 376). Elsevier; 10.1016/j.ydbio.2013.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cekanaviciute E, Dietrich HK, Axtell RC, Williams AM, Egusquiza R, Wai KM, … Buckwalter MS (2014). Astrocytic TGF-β signaling limits inflammation and reduces neuronal damage during central nervous system Toxoplasma infection. Journal of Immunology (Baltimore, Md. : 1950), 193(1), 139–49. 10.4049/jimmunol.1303284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung CY, Seo H, Sonntag KC, Brooks A, Lin L, & Isacson O (2005). Cell type-specific gene expression of midbrain dopaminergic neurons reveals molecules involved in their vulnerability and protection. Human Molecular Genetics, 14(13), 1709–25. 10.1093/hmg/ddi178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damier P, Hirsch EC, Zhang P, & Agio Y (1993). Glutathione Peroxidase , Glial Cells and Parkinson’s Disease. Neuroscience, 52(1), 1–6. [DOI] [PubMed] [Google Scholar]
- Datta I, Ganapathy K, Razdan R, & Bhonde R (2017). Location and Number of Astrocytes Determine Dopaminergic Neuron Survival and Function Under 6-OHDA Stress Mediated Through Differential BDNF Release. Molecular Neurobiology. 10.1007/s12035-017-0767-0 [DOI] [PubMed] [Google Scholar]
- Diniz LP, Tortelli V, Matias I, Morgado J, Bérgamo Araujo AP, Melo HM, … Gomes FCA (2017). Astrocyte Transforming Growth Factor Beta 1 Protects Synapses against Aβ Oligomers in Alzheimer’s Disease Model. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 37(28), 6797–6809. 10.1523/JNEUROSCI.3351-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drukarch B, Schepens E, Jongenelen C. a M., Stoof JC, & Langeveld CH (1997). Astrocyte-mediated enhancement of neuronal survival is abolished by glutathione deficiency. Brain Research, 770(1–2), 123–130. 10.1016/S0006-8993(97)00790-7 [DOI] [PubMed] [Google Scholar]
- Drukarch B, Schepens E, Stoof JC, Langeveld CH, & Van Muiswinkel FL (1998). Astrocyte-enhanced neuronal survival is mediated by scavenging of extracellular reactive oxygen species. Free Radical Biology and Medicine, 25(2), 217–220. 10.1016/S0891-5849(98)00050-1 [DOI] [PubMed] [Google Scholar]
- Emmerson PJ, Wang F, Du Y, Liu Q, Pickard RT, Gonciarz MD, … Wu X (2017). The metabolic effects of GDF15 are mediated by the orphan receptor GFRAL. Nature Medicine, 23(10). 10.1038/nm.4393 [DOI] [PubMed] [Google Scholar]
- Fairlie WD, Moore a G., Bauskin a R., Russell PK, Zhang HP, & Breit SN (1999). MIC-1 is a novel TGF-beta superfamily cytokine associated with macrophage activation. Journal of Leukocyte Biology, 65(1), 2–5. [DOI] [PubMed] [Google Scholar]
- Gegg ME, Clark JB, & Heales SJR (2005). Co-culture of neurones with glutathione deficient astrocytes leads to increased neuronal susceptibility to nitric oxide and increased glutamate-cysteine ligase activity. Brain Research, 1036(1–2), 1–6. 10.1016/j.brainres.2004.11.064 [DOI] [PubMed] [Google Scholar]
- Golden JP, Milbrandt J, & Johnson EM (2003). Neurturin and persephin promote the survival of embryonic basal forebrain cholinergic neurons in vitro. Experimental Neurology, 184(1), 447–55. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/14637114 [DOI] [PubMed] [Google Scholar]
- Gouin A, Bloch-Gallego E, Tanaka H, Rosenthal A, & Henderson CE (1996). Transforming growth factor-beta 3, glial cell line-derived neurotrophic factor, and fibroblast growth factor-2, act in different manners to promote motoneuron survival in vitro. Journal of Neuroscience Research, 43(4), 454–64. [DOI] [PubMed] [Google Scholar]
- Hartikka J, Staufenbiel M, & Lubbert H (1992). Cyclic AMP, but not basic FGF, increases the in vitro survival of mesencephalic dopaminergic neurons and protects them from MPP+-induced degeneration. Journal of Neuroscience Research, 32, 190–201. 10.1002/jnr.490320208 [DOI] [PubMed] [Google Scholar]
- Hirsch E, Graybiel AM, & Agid YA (1988). Melanized dopaminergic neurons are differentially susceptible to degeneration in Parkinson’s disease. Nature, 334(6180), 345–348. 10.1038/334345a0 [DOI] [PubMed] [Google Scholar]
- Hoffer BJ, Hoffman A, Bowenkamp K, Huettl P, Hudson J, Martin D, … Gerhardt G. a. (1994). Glial cell line-derived neurotrophic factor reverses toxin-induced injury to midbrain dopaminergic neurons in vivo. Neuroscience Letters, 182, 107–111. 10.1016/0304-3940(94)90218-6 [DOI] [PubMed] [Google Scholar]
- Hou JG, Lin LF, & Mytilineou C (1996). Glial cell line-derived neurotrophic factor exerts neurotrophic effects on dopaminergic neurons in vitro and promotes their survival and regrowth after damage by 1-methyl-4-phenylpyridinium. Journal of Neurochemistry, 66(1), 74–82. 10.1046/j.1471-4159.1996.66010074.x [DOI] [PubMed] [Google Scholar]
- Huang JY, Hong YT, & Chuang JI (2009). Fibroblast growth factor 9 prevents MPP+-induced death of dopaminergic neurons and is involved in melatonin neuroprotection in vivo and in vitro. Journal of Neurochemistry, 109, 1400–1412. 10.1111/j.1471-4159.2009.06061.x [DOI] [PubMed] [Google Scholar]
- Huber KA, Krieglstein K, & Unsicker K (1995). The neurotrophins BDNF, NT-3 and −4, but not NGF, TGF-beta 1 and GDNF, increase the number of NADPH-diaphorase-reactive neurons in rat spinal cord cultures. Neuroscience, 69(3), 771–9. [DOI] [PubMed] [Google Scholar]
- Iacovitti L, Wei X, Cai J, Kostuk EW, Lin R, Gorodinsky A, … Dave KD (2014). The hTH-GFP reporter rat model for the study of Parkinson’s disease. PloS One, 9(12), e113151. 10.1371/journal.pone.0113151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaumotte JD, Wyrostek SL, Zigmond MJ, & Smith Y (2016). Protection of cultured dopamine neurons from MPP+ requires a combination of neurotrophic factors. European Journal of Neuroscience, 44(1), 1691–1699. 10.1111/ejn.13252 [DOI] [PubMed] [Google Scholar]
- Jaumotte JD, & Zigmond MJ (2014). Comparison of GDF5 and GDNF as neuroprotective factors for postnatal dopamine neurons in ventral mesencephalic cultures. Journal of Neuroscience Research, 92(11), 1425–1433. 10.1002/jnr.23425 [DOI] [PubMed] [Google Scholar]
- Javitch JA, & Snyder SH (1984). Uptake of MPP(+) by dopamine neurons explains selectivity of parkinsonism-inducing neurotoxin, MPTP. European Journal of Pharmacology, 106(2), 455–456. 10.1016/0014-2999(84)90740-4 [DOI] [PubMed] [Google Scholar]
- John Lin C-C, Yu K, Hatcher A, Huang T-W, Lee HK, Carlson J, … Deneen B (2017). Identification of diverse astrocyte populations and their malignant analogs. Nature Neuroscience, 20(3). 10.1038/nn.4493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearns CM, Cass W. a, Smoot K, Kryscio R, & Gash DM (1997). GDNF protection against 6-OHDA: time dependence and requirement for protein synthesis. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 17(18), 7111–7118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearns CM, & Gash DM (1995). GNDF protects nigral dopamine neurons against 6-hydroxidopamine in vivo. Brain Res, 672, 104–111. [DOI] [PubMed] [Google Scholar]
- Khakh BS, & Sofroniew MV (2015). Diversity of astrocyte functions and phenotypes in neural circuits. Nature Neuroscience, 18(7), 942–952. 10.1038/nn.4043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimelberg H (2007). Supportive or information-processing functions of the mature protoplasmic astrocyte in the mammalian CNS? A critical appraisal. Neuron Glia Biology, 3(3), 181–9. 10.1017/S1740925X08000094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimelberg HK, & Nedergaard M (2010). Functions of Astrocytes and their Potential As Therapeutic Targets. Neurotherapeutics, 7(4), 338–353. 10.1016/j.nurt.2010.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimelberg H, & Norenberg MD (1989). Astrocytes. Scientific American, 260(4), 66–72, 74, 76. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/2564697 [DOI] [PubMed] [Google Scholar]
- Kopin IJ (1992). Features of the Dopaminergic Neurotoxin MPTP. Annals of the New York Academy of Sciences, 648(1), 96–104. 10.1111/j.1749-6632.1992.tb24527.x [DOI] [PubMed] [Google Scholar]
- Krieglstein K, & Unsicker K (1994). Transforming growth factor-beta promotes survival of midbrain dopaminergic neurons and protects them against N-methyl-4-phenylpyridinium ion toxicity. Neuroscience, 63(4), 1189–96. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/7700516 [DOI] [PubMed] [Google Scholar]
- Langston JW (1985). MPTP and parkinson’s disease. Trends in Neurosciences, 8(C), 79–83. 10.1016/0166-2236(85)90031-1 [DOI] [Google Scholar]
- Li YY, Lu JH, Li Q, Zhao YY, & Pu XP (2008). Pedicularioside A from Buddleia lindleyana inhibits cell death induced by 1-methyl-4-phenylpyridinium ions (MPP+) in primary cultures of rat mesencephalic neurons. European Journal of Pharmacology, 579(1–3), 134–140. 10.1016/j.ejphar.2007.10.052 [DOI] [PubMed] [Google Scholar]
- Li Z, Wang B, Wu X, Cheng S-Y, Paraoan L, & Zhou J (2005). Identification, expression and functional characterization of the GRAL gene. Journal of Neurochemistry, 95(2), 361–376. 10.1111/j.1471-4159.2005.03372.x [DOI] [PubMed] [Google Scholar]
- Liddelow SA, & Barres BA (2017). Reactive Astrocytes: Production, Function, and Therapeutic Potential. Immunity, 46(6), 957–967. 10.1016/j.immuni.2017.06.006 [DOI] [PubMed] [Google Scholar]
- Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, … Barres BA (2017). Neurotoxic reactive astrocytes are induced by activated microglia. Nature, 541(7638), 481–487. 10.1038/nature21029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin LF, Doherty DH, Lile JD, Bektesh S, & Collins F (1993). GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science (New York, N.Y.), 260, 1130–1132. 10.1126/science.8493557 [DOI] [PubMed] [Google Scholar]
- Machado V, Gilsbach R, Das R, Schober A, Bogatyreva L, Hauschke D, … Spittau B (2016). Gdf-15 deficiency does not alter vulnerability of nigrostriatal dopaminergic system in MPTP-intoxicated mice. Cell and Tissue Research, 365(2), 209–223. 10.1007/s00441-016-2406-x [DOI] [PubMed] [Google Scholar]
- Machado V, Haas SJP, von Bohlen und Halbach O, Wree A, Krieglstein K, Unsicker K, & Spittau B (2016). Growth/differentiation factor-15 deficiency compromises dopaminergic neuron survival and microglial response in the 6-hydroxydopamine mouse model of Parkinson’s disease. Neurobiology of Disease, 88, 1–15. 10.1016/j.nbd.2015.12.016 [DOI] [PubMed] [Google Scholar]
- Maetzler W, Deleersnijder W, Hanssens V, Bernard A, Brockmann K, Marquetand J, … Berg D (2016). GDF15/MIC1 and MMP9 cerebrospinal fluid levels in Parkinson’s disease and Lewy body dementia. PLoS ONE, 11(3), 1–12. 10.1371/journal.pone.0149349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minnich JE, Mann SL, Stock M, Stolzenbach KA, Mortell BM, Soderstrom KE, … Kozlowski DA (2010). Glial cell line-derived neurotrophic factor (GDNF) gene delivery protects cortical neurons from dying following a traumatic brain injury. Restorative Neurology and Neuroscience, 28(3), 293–309. 10.3233/RNN-2010-0528 [DOI] [PubMed] [Google Scholar]
- Mullican SE, Lin-Schmidt X, Chin C-N, Chavez JA, Furman JL, Armstrong AA, … Rangwala SM (2017). GFRAL is the receptor for GDF15 and the ligand promotes weight loss in mice and nonhuman primates. Nature Medicine, (August). 10.1038/nm.4392 [DOI] [PubMed] [Google Scholar]
- Nair-Roberts RG, Chatelain-Badie SD, Benson E, White-Cooper H, Bolam JP, & Ungless MA (2008). Stereological estimates of dopaminergic, GABAergic and glutamatergic neurons in the ventral tegmental area, substantia nigra and retrorubral field in the rat. Neuroscience, 152(4), 1024–1031. 10.1016/j.neuroscience.2008.01.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orihuela R, McPherson CA, & Harry GJ (2016). Microglial M1/M2 polarization and metabolic states. British Journal of Pharmacology, 173(4), 649–665. 10.1111/bph.13139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto D, & Unsicker K (1993). FGF-2-mediated protection of cultured mesencephalic dopaminergic neurons against MPTP and MPP+: specificity and impact of culture conditions, non-dopaminergic neurons, and astroglial cells. Journal of Neuroscience Research, 34, 382–393. 10.1002/jnr.490340403 [DOI] [PubMed] [Google Scholar]
- Park TH, & Mytilineou C (1992). Protection from 1-methyl-4-phenylpyridinium (MPP+) toxicity and stimulation of regrowth of MPP(+)-damaged dopaminergic fibers by treatment of mesencephalic cultures with EGF and basic FGF. Brain Research, 599(1), 83–97. 10.1016/0006-8993(92)90855-4 [DOI] [PubMed] [Google Scholar]
- Petrova P, Raibekas A, Pevsner J, Vigo N, Anafi M, Moore MK, … Commissiong JW (2003). MANF: a new mesencephalic, astrocyte-derived neurotrophic factor with selectivity for dopaminergic neurons. Journal of Molecular Neuroscience : MN, 20(2), 173–88. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12794311 [DOI] [PubMed] [Google Scholar]
- Phani S, Gonye G, & Iacovitti L (2010). VTA neurons show a potentially protective transcriptional response to MPTP. Brain Research, 1343, 1–13. 10.1016/j.brainres.2010.04.061 [DOI] [PubMed] [Google Scholar]
- Schildge S, Bohrer C, Beck K, & Schachtrup C (2013). Isolation and culture of mouse cortical astrocytes. Journal of Visualized Experiments : JoVE, (71). 10.3791/50079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schitine C, Nogaroli L, Costa MR, & Hedin-Pereira C (2015). Astrocyte heterogeneity in the brain: from development to disease. Frontiers in Cellular Neuroscience, 9(March), 76 10.3389/fncel.2015.00076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeyne M, Jiao Y, Shepherd KR, & Smeyne RJ (2005). Glia cell number modulates sensitivity to MPTP in mice. Glia, 52(2), 144–152. 10.1002/glia.20233 [DOI] [PubMed] [Google Scholar]
- Spina MB, Squinto SP, Miller J, Lindsay RM, & Hyman C (1992). Brain-derived neurotrophic factor protects dopamine neurons against 6-hydroxydopamine and N-methyl-4-phenylpyridinium ion toxicity: involvement of the glutathione system. Journal of Neurochemistry, 59, 99–106. 10.1111/j.1471-4159.1992.tb08880.x [DOI] [PubMed] [Google Scholar]
- Strelau J, Sullivan A, Böttner M, Lingor P, Falkenstein E, Suter-Crazzolara C, … Unsicker K (2000). Growth/differentiation factor-15/macrophage inhibitory cytokine-1 is a novel trophic factor for midbrain dopaminergic neurons in vivo. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 20(23), 8597–8603. http://doi.org/20/23/8597 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, & Le W (2016). Differential Roles of M1 and M2 Microglia in Neurodegenerative Diseases. Molecular Neurobiology, 53(2), 1181–1194. 10.1007/s12035-014-9070-5 [DOI] [PubMed] [Google Scholar]
- Thuret S, Bhatt L, O’Leary DDM, & Simon HH (2004). Identification and developmental analysis of genes expressed by dopaminergic neurons of the substantia nigra pars compacta. Molecular and Cellular Neurosciences, 25(3), 394–405. 10.1016/j.mcn.2003.11.004 [DOI] [PubMed] [Google Scholar]
- Wang P, Niu L, Guo XD, Gao L, Li WX, Jia D, … Gao GD (2010). Gypenosides protects dopaminergic neurons in primary culture against MPP+-induced oxidative injury. Brain Research Bulletin, 83(5), 266–271. 10.1016/j.brainresbull.2010.06.014 [DOI] [PubMed] [Google Scholar]
- Weinstein DE (2001). Isolation and purification of primary rodent astrocytes. Current Protocols in Neuroscience, Chapter 3, Unit 3.5. 10.1002/0471142301.ns0305s00 [DOI] [PubMed] [Google Scholar]
- Wiese S, Karus M, & Faissner A (2012). Astrocytes as a source for extracellular matrix molecules and cytokines. Frontiers in Pharmacology, 3, 120 10.3389/fphar.2012.00120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Kaul S, Zhang D, Anantharam V, & Kanthasamy AG (2004). Suppression of caspase-3-dependent proteolytic activation of protein kinase C?? by small interfering RNA prevents MPP+-induced dopaminergic degeneration. Molecular and Cellular Neuroscience, 25(3), 406–421. 10.1016/j.mcn.2003.11.011 [DOI] [PubMed] [Google Scholar]
- Yao F, Yu F, Gong L, Taube D, Rao DD, & MacKenzie RG (2005). Microarray analysis of fluoro-gold labeled rat dopamine neurons harvested by laser capture microdissection. Journal of Neuroscience Methods, 143(2), 95–106. 10.1016/j.jneumeth.2004.09.023 [DOI] [PubMed] [Google Scholar]
- Zamanian JL, Xu L, Foo LC, Nouri N, Zhou L, Giffard RG, & Barres BA (2012). Genomic analysis of reactive astrogliosis. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 32(18), 6391–410. 10.1523/JNEUROSCI.6221-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai A, Zhu X, Wang X, Chen R, & Wang H (2013). Secalonic acid A protects dopaminergic neurons from 1-methyl-4-phenylpyridinium (MPP(+))-induced cell death via the mitochondrial apoptotic pathway. European Journal of Pharmacology, 713(1–3), 58–67. 10.1016/j.ejphar.2013.04.029 [DOI] [PubMed] [Google Scholar]
- Zhang J, Yang B, Sun H, Zhou Y, Liu M, Ding J, … Hu G (2016). Aquaporin-4 deficiency diminishes the differential degeneration of midbrain dopaminergic neurons in experimental Parkinson’s disease. Neuroscience Letters, 614, 7–15. 10.1016/j.neulet.2015.12.057 [DOI] [PubMed] [Google Scholar]
- Zhang Y, & Barres BA (2010). Astrocyte heterogeneity: An underappreciated topic in neurobiology. Current Opinion in Neurobiology, 20(5), 588–594. 10.1016/j.conb.2010.06.005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental File 1: RNAseq comparison of isolated SN and VTA astrocytes. RNA was isolated from regionally specific cultures of astrocytes from the SN (4) and VTA (4) and subjected to RNAseq. The readouts demonstrate the specific gene, locus, mean FPKM, fold change and significance between samples. All samples were further subjected to Ingenuity pathway analysis.
Supplemental Figure 1: Representative dissection of TH-GFP+ ventral mesencephalon. The ventral mesencephalon of embryonic day 14.5 hTH-GFP rats was surgically dissected from the embryo similar to our previously established methods and the majority of the non-transgenic tissues were cleaned from the samples. Mesencephalic tissues can be easily observed under a dissecting microscope (a). The presumptive lateral SN tissue was sub-dissected apart from the medial VTA (denoted by red lines) and regional tissues were pooled and collected from each animal prior to enzymatic digestion and subsequent culture (b).
Supplemental Figure 2: Magnetic bead separated primary astrocytes lack neuronal and microglial markers. Samples of VTA (a) and SN (b) magnetic bead (ASCA1/GLAST) separated astrocytes were subjected to qRT-PCR analysis of astrocytic (GFAP) and neuronal (NeuN and β3Tubulin) expression. Both regions demonstrate strong expression of GFAP, greater gene expression is associated with low ΔCT values, compared to the neuronal transcripts. Additionally, western blot analysis of isolated cortical and striatal bead separated astrocytes (c) demonstrate expression of the astrocytic marker GFAP with absence of the microglial marker Iba1.
Supplemental Figure 3: Cytosine arabinoside (Ara-C) treatment results in robust elimination of astrocytes from SN and VTA cultures. Embryonic day 14.5 SN (a-c) and VTA (d-f) cultures were treated with 0 (a & d), 5 (b & e) and 10μM (c & f) Ara-C for 48 hours post plating. We found greatly decreased numbers of GFAP+ (red) astrocytes within both regional cultures consistent with increase dosing of Ara-C. Subsequent experiments were performed using the 10μM dose as it resulted in the greatest decrease of GFAP+ astrocytes, SN; 111±11, VTA; 117±10 (Two-tailed unpaired t-test, t=0.3803, df=8, p=0.7136) GFAP+ cells per well post AraC compared to a confluent bed of astrocytes in the untreated condition, while not affecting dopamine neuron health (Supplemental fig 4).
Supplemental Figure 4: Astrocyte elimination from isolated SN and VTA cultures does not result in decreased dopamine neuron viability. Total TH-GFP+ cell number was quantified with and without Ara-C treatment. Neither SN (Two-tailed Unpaired t-test, t=0.08805, df=10, p=0.9316) nor VTA (Two-tailed Unpaired t-test, t=0.8446, df=10, p=0.4181) neuronal viability was affected by the elimination of astrocytes from the culture.
Supplemental Figure 5: Astrocyte isolation results in homogeneous culture of regional astrocytes. Regionally isolated astrocytes were examined for non-astrocyte cellular contamination through histological methods. Cultures stain robustly for GFAP (a) and S100β (b) whereas no signal was evident for the microglial markers Iba1 (c) or CD11b/c (d); neuronal markers β3Tubulin (e) and NeuN (f); as well as immature (NG2, g) or mature oligodendrocytes (MBP, h).
Supplemental Figure 6: Cultures of regional isolated astrocytes exhibit low expression of non-astrocyte cell markers. qPCR analysis of 3 independent samples of SN (a), VTA (b) and CTX (c) were examined for the expression of markers of astrocytic (GFAP), microglial (Iba1, Cd11b), oligodendrocyte (Pdgfra, NG2, MBP), and neuronal gene expression (NeuN, β3Tubulin). Samples were normalized to the housekeeping gene GAPDH and the ΔCT from GAPDH was calculated. Greater gene expression is associated with low ΔCT values. **** = p<0.0001 compared to GFAP; One-way ANOVA with Dunnett’s multiple comparisons test: SN F (7, 61) = 132.1, VTA F (7, 62) = 130.9, CTX F (7, 50) = 41.13.
Supplemental Figure 7: SN astrocytes express and secrete GDF15 at significantly lower levels than VTA. To confirm the findings from our RNAseq, we examined isolated samples of VTA and SN astrocytes using RT-PCR and ELISA to examine the expression of GDF15. RT-PCR on two separate samples of cultured SN astrocytes were used to demonstrate consistently lower expression of GDF15 compared to VTA astrocytes (a). Similarly, ELISA measurement of secreted GDF15 in ACM from VTA and SN astrocytes (b) demonstrated significantly lower levels of GDF15 in media conditioned by SN (μ = 64pg/mL) compared to VTA (μ = 984pg/mL); Two-tailed unpaired t test, t=6.55, df=4, p=0.0028. Furthermore, examination of in vivo GDF15 expression, from astrocyte specific magnetic beads, demonstrated a lower level of GDF15 mRNA (c) and protein expression (d & d’) in SN astrocytes compared to VTA from two separate isolations.
Supplemental Figure 8: VTA astrocyte GDF15 expression is decreased using lentiviral shRNA. A commercially available lentiviral shRNA was added to isolated VTA astrocytes combined with the transfection reagent Polybrene (8μg/mL). Four GDF15 shRNA (shA-D) constructs and one scramble control (shE) construct were used to transduce astrocytes. We observed >80% efficiency for all tested constructs 3 days after lentiviral addition (not shown). Samples were examined with RT-PCR for GDF15 expression. Only constructs shA and shD resulted in decreased expression of GDF15 within VTA astrocytes when compared to non-transfected cells. As expected, scramble control (shE) did not change GDF15 expression.


