Abstract
We present a modified method of embedded bioprinting, which allows maintaining freestanding three-dimensional (3D) printed structures in cell culture conditions for extended periods of time. This method, termed CLASS (constructs laid in agarose slurry suspension), was tested using cell-laden alginate and gelatin methacrylate (GelMa)-based bioinks. A direct comparison of 3D printed constructs, supported by gelatin and agarose hydrogel slurries, revealed several advantages, including slurry stability across different print temperatures and blending times, increased slurry homogeneity, and the ability of CLASS to support freestanding constructs for an extended time in cell culture. We conclude that CLASS is a straightforward and cost-efficient way to print and support freestanding cell-laden biomaterials.
Keywords: cell-laden bioinks, agarose slurry, hydrogels
Introduction
Recent advances in bioprinting have made the engineering of complex tissues and organs a closer reality.1–3 Compared with three-dimensional (3D) printing using plastics and metals, bioprinting has a number of additional requirements. Among these requirements are (1) bioink materials that support cell interactions, growth, and spreading; (2) physiologically compatible print conditions, which are defined by printing with the biocompatible temperature, bioink pH, extrusion pressure, and osmolarity; and (3) sustained cell nourishment for 3D printed constructs. It is also important to consider how encapsulated cells interact with their surrounding matrix and how that matrix might be compromised by cellular enzymatic activity. The structure, stability, and composition of multicellular bioprinted constructs can significantly change during long-term cell culture or implantation.
Cell-laden bioinks cannot be too stiff without inhibiting cell growth and spreading.4–6 This lack of stiffness imposes major limitations on the height, complexity, and stability of printed constructs due to the effects of gravity. Therefore, most articles explore new materials or approaches by printing 3D constructs consisting of only a few layers.3–5
One way to prevent the collapse of macroscopic 3D constructs made from soft biomaterials is to provide a support bath that can hold them in place. In 2015, three different laboratories described such an approach—each giving it a different name. Highley et al.7 described what they called guest–host writing using a modified, hyaluronic acid-based support bath. Bhattacharjee et al.8 referred to their method as writing in granular gels and used carbopol as their support bath material. Hinton et al.9 described free-form reversible embedding of suspended hydrogels (abbreviated as FRESH) using gelatin slurry as the main support bath ingredient.
The above three studies include a secondary cross-linking step for cell-laden constructs printed in support baths. Constructs can be cross-linked chemically or by using photopolymerization; but, it is only after cross-linking that 3D constructs can be freed from their support material and placed in cell culture conditions.9 However, for cell-laden bioinks, even after cross-linking, it can be beneficial to maintain 3D printed constructs in their suspended form for extended periods of time, allowing cells to deposit extracellular matrix (ECM) on their own. Many bioinks that include naturally occurring ECM proteins such as collagen tend to form a more physiologically suitable matrix by means of slow self-polymerization rather than rapid chemical or photocross-linking.10 For this, one needs to be able to feed the cells within a 3D printed construct without removing the support bath material.
To address the need for a cell culture-friendly and thermally stable support material, we developed a simple and affordable modification of the FRESH technique.9 We called it CLASS, an acronym for constructs laid in agarose slurry suspension. It enabled us to print high-definition 3D constructs that can be kept in standard cell culture conditions for extended periods of time.
Materials and Methods
Hydrogel slurry development
For alginate-containing bioinks, slurries were made in an aqueous solution of 10 mM CaCl2 to induce alginate polymerization. For cell-laden collagen or gelatin methacrylate (GelMa)-containing bioinks, calcium chloride solution (No. 83H08615; Sigma Cell Culture) was replaced with Tyrode solution and phenol-free cell culture media (1% penicillin–streptomycin No. K99520; VWR and amphotericin B 0.1% No. A2942; Sigma). In a 16-oz Ball Mason jar, 5.0% (w/v) gelatin or 1% agarose (No. X174-25G; VWR) was dissolved in 150 mL of warm media. Jars with gelatin or agarose solutions were refrigerated at 4°C until they formed solid gel pucks. A few milliliters of chilled media were added to cover the surface of the formed pucks, and a spatula was used to detach the pucks’ edges from the walls of their jars. Each jar was then filled to the brim with CaCl2 solution or Tyrode solution (depending on the type of bioink) and placed in a −20°C freezer for 20 min thereafter. Once removed from the freezer, gel pucks were blended in pulse mode from 30 to 120 s using a household blender (Osterizer, 564A). The jars’ contents were then aliquoted into 50-mL conical tubes, resuspended, and centrifuged at 4°C at 4200 rpm for 2 min. After centrifugation, liquid supernatant was removed to leave hydrogel slurry at the bottom of the tube. Adding more media to the pelleted slurry and subsequent centrifugation made for more refined slurry.
Bioinks
Among the tested bioinks were 2% alginate (Alginic Acid, No. A2158; Sigma), 2% alginate/0.1–1% gelatin, alginate–collagen I (collagen type I, No. 354249; Corning), and 5% GelMA (Allevi). Coomassie Brilliant Blue (G-250 No. 161-0406, 0.1–1% w/v; Bio-Rad) powder was added to alginate bioinks to make prints visible in their support baths. HEK293 RFP cells, permanently expressing red fluorescent protein (Cat. No. SC007; Gen Target, Inc.), were used at a 5 × 106/mL final cell concentration. The cell-laden bioink was made based on Dulbecco's modified media supplemented with 10% bovine serum (heat inactivated, New Zealand origin No. 26170-043; Gibco) and included 5% GelMA, 0.15% Biokey (Allevi), 10% Matrigel (No. 354234; Corning), 3% fibronectin (No. AK8350-0005, stock solution of 1 mg/mL; Akron Biotech), and 1% penicillin–streptomycin (No. K99520; VWR). Cell-laden constructs printed in agarose-filled cell strainers were transferred to new wells with fresh media every 48 h.
Grid CAD model, Sli3r, and bioprinting
Extrusion-based bioprinting was performed using a pneumatic 3D bioprinter (Allevi). A 3D grid model was created using SolidWorks and exported as an STL file to Slic3r for further optimization. In Slic3r, the file was tailored to single-layer printing, 100% infill, and a 12-mm/s print speed. The file was then exported as G-code and uploaded to a bioprinting platform. Pneumatic pressure was adjusted using Allevi's bioprinting control panel. Qualitative conclusions and representative images were made based on at least three independent experiments.
Pressure, syringe volume, gauge, and ink extrusion rate
Experiments to test the effects of extrusion pressure and syringe volume were performed by loading 2% alginate into 10-mL BD syringes fitted with 30-gauge needles (Jensen Global). For each pressure setting, the volume of extruded material was measured by weighing the mass of collected material. For cell-laden prints, HEK293 RFP cells were encapsulated in 5% GelMa, and prints were made using a 23-gauge needle at an extrusion pressure of 3 psi.
Results
Main steps involved in preparing and culturing 3D printed constructs in agarose slurry
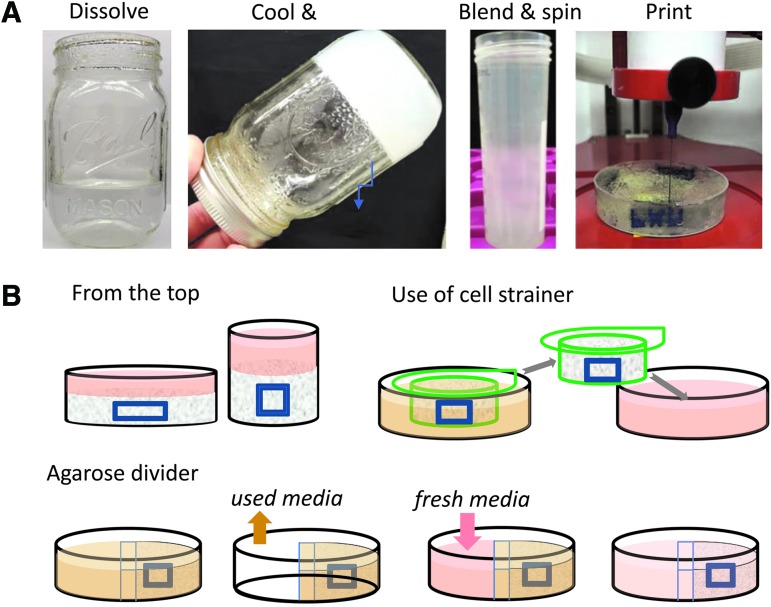
The CLASS technique is a modified version of the previously reported FRESH technique.9 The main difference between the two techniques is the use of agarose as a base material instead of gelatin for the slurry. The main protocol of agarose slurry development involves dissolving 1% w/v agarose in warm media, followed by cooling, blending, and centrifugation (Fig. 1A). The CLASS method to develop agarose slurry takes about 30 min from start to finish, which is much quicker than the overnight FRESH protocol.9
FIG. 1.
CLASS method outline. (A) Main steps involved in preparing agarose slurry. (B) Ways to change media for long-term culturing using the CLASS platform. CLASS, constructs laid in agarose slurry suspension. Color images are available online.
Several ways to change the media around embedded prints can be used (Fig. 1B). They include adding media on top of the agarose slurry; placing agarose in a cell culture insert, which can be subsequently placed in a freshly filled well; or creating agarose dividers that can be filled with agarose slurry on one side and cell culture media on the other. Using the latter technique, the media can be suctioned from the nonslurry part of the well and replaced with a fresh medium. Since agarose gel is permeable to medium components, this leads to a diffusion-based medium exchange near the construct without disturbing the slurry that supports it.
Calculating optimal bioink flow rates for pneumatic printers
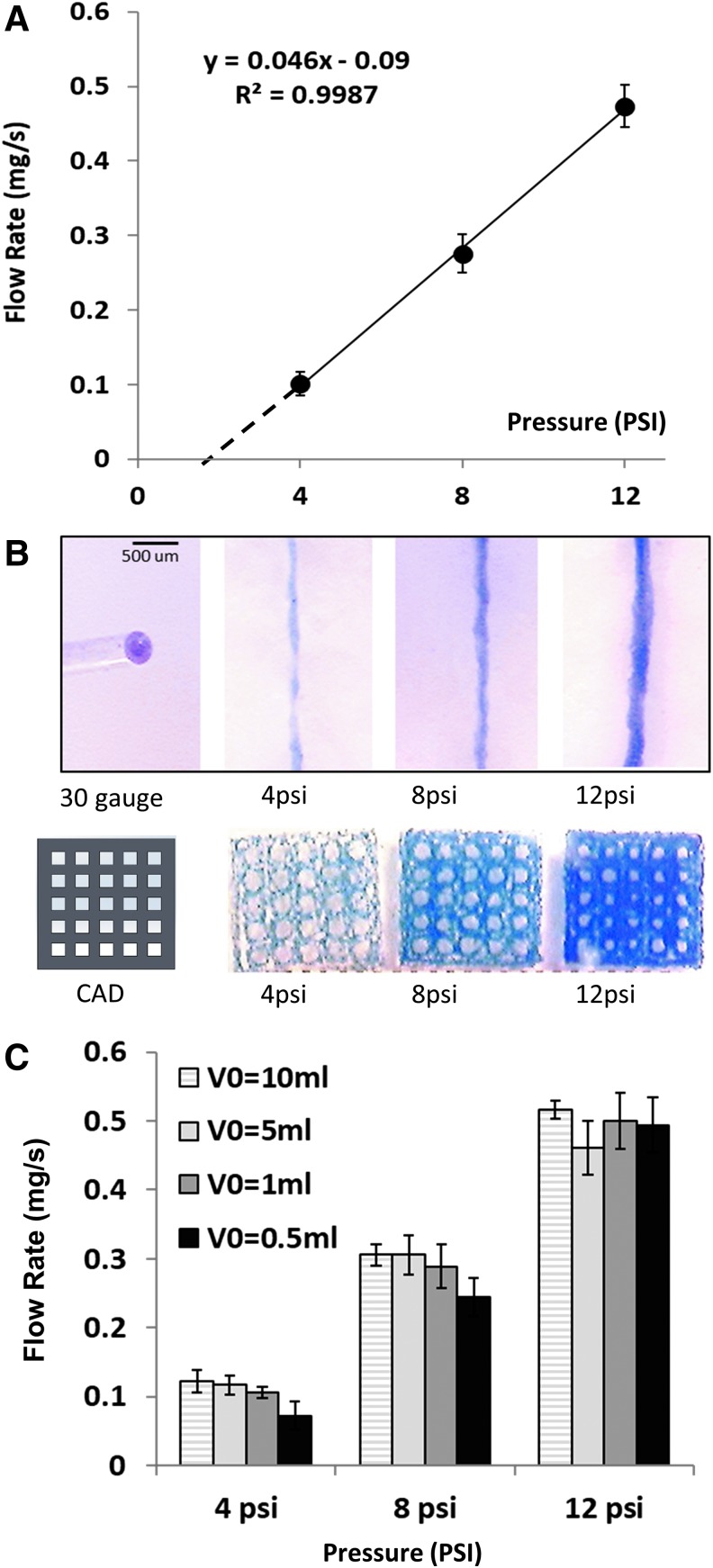
Since FRESH studies were performed using a mechanical printer, we first had to optimize the pneumatic-based extrusion process itself before we could directly compare the quality of prints made inside agarose and gelatin slurries. With pneumatic printers, several variables can affect the extrusion rate of a bioink, including temperature, needle gauge, or viscosity. Therefore, to predict the speed of needle movement that will produce struts of expected thickness, we performed a weight-based calibration of the extruded volume from the needle tip. At our tested pressures, the bioprinter yielded a nearly linear relationship between volume of extruded bioink (R2 = 0.99) and flow rates with 4, 8, and 12 psi yielding 0.101 ± 0.016, 0.275 ± 0.025, and 0.473 ± 0.028 mg/s extrusion volumes, respectively (Fig. 2A). The data also indicated 2 psi as the minimum pressure required to extrude 2% alginate through a 30-gauge needle. We then used the relationship shown in Figure 2A to calculate the speed necessary to produce a continuous alginate filament through a specified needle gauge. For example, for a 30-gauge needle, with an inner nominal diameter of 160 microns and printer extruder pressure of 8 psi, one will need to move the extruder at ≈12 mm/s linear speed to make a strut that is neither discontinuous nor too bulky. In first approximation, this can be calculated by dividing the rate of extruded volume with the cross-sectional area of the needle. In our case, the formula will be 0.275 μg/s = 0.275 mm3/s/(3.14 × 80 × 80 μm) = 13.6 mm/s. The effects of different extrusion pressures on the width of printed lines are shown in Figure 2A. If the correct speed of alginate extrusion is selected, a defined geometry can be obtained by printing in either gelatin or agarose slurry. Examples of calibration grids printed using different alginate extrusion rates are shown in Figure 2B.
FIG. 2.
Flow rate and printability of 2% alginate bioink across different pneumatic pressures. (A) A linear relationship empirically established between pneumatic pressure (psi) and measured flow rate (mg/s). The minimum threshold of alginate extrusion was extrapolated to be 2 psi (dotted line). (B) Filaments and printed constructs using specified pneumatic pressure settings at a travel speed of 12 mm/s. (C) Effect of syringe volume on the bioink flow rate. Two percent alginate was extruded using a 30-gauge needle from a syringe loaded with the specified initial volumes. No difference between the flow rate and the initial material syringe loading volume was observed. Color images are available online.
Potential effect of bioink loading volume
For cell-laden bioinks, the syringe volume can vary significantly based on cell amount and/or cost of involved materials. Therefore, it was important to clarify the extent by which this variable must be considered. From a physics point of view, a larger initial bioink volume implies the added effect of gravity to the extrusion pressure created by the printer pump. In our case, the 10-mL volume of fluid within a 10-mL BD syringe yields about 6.2 cm of fluid height or 0.09 psi. This comprises <3% of the lowest recommended extrusion pressure of 4 psi. The measurements of the amount of extruded material at different starting bioink volumes have confirmed the absence of statistical differences between syringes loaded with different initial volumes of 2% alginate bioink (Fig. 2C). This suggests that even very small volumes of 0.5 mL or less can be efficiently printed using the calibration curve established in Figure 2A. The effect of syringe volume might nevertheless become significant for extralow-viscosity bioinks or low-gauge needles, which require printing pressures of 2 psi or less.
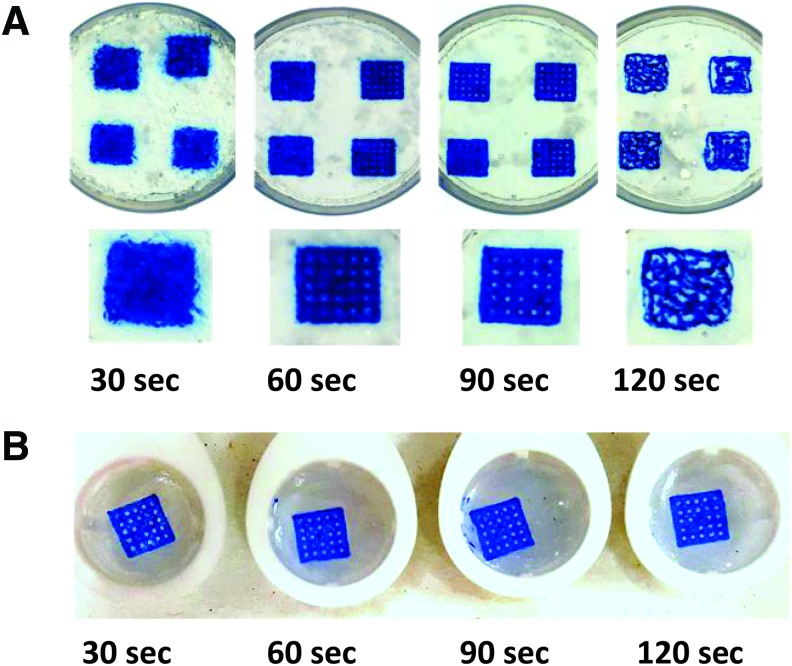
Effect of slurry blending time
Next, we examined how printability is affected by the duration of blending. When we used the type of blender and the Mason jar specified in the Materials and Methods section, 60 and 90-s blending times of gelatin slurry consistently produced anticipated prints. On the other hand, the use of gelatin slurry blended for either 30 or 120 s yielded morphed or defective prints (Fig. 3A). Notably, prints made in agarose slurry were much less sensitive to the duration of blending time (Fig. 3B). One explanation of this difference is the lower melting temperature of gelatin compared with that of agarose. As a result, gelatin microparticles can be affected by heating the slurry during extended blending times, effectively melting them. Blending times are of course specific to the equipment one uses, so it is advised to test several blending times to reach the optimal, Bingham plastic-like consistency when other types of blenders or volumes of slurry are used.
FIG. 3.
Effect of slurry blending time. (A) Outcomes of different blending times on printability of 2% alginate in gelatin slurry (FRESH). Either shortening or prolonging slurry blend time leads to grid deformation. (B) Outcomes of different blending times on printability of 2% alginate in agarose slurry (CLASS) showing no impact of slurry blending time on print fidelity. FRESH, free-form reversible embedding of suspended hydrogels. Color images are available online.
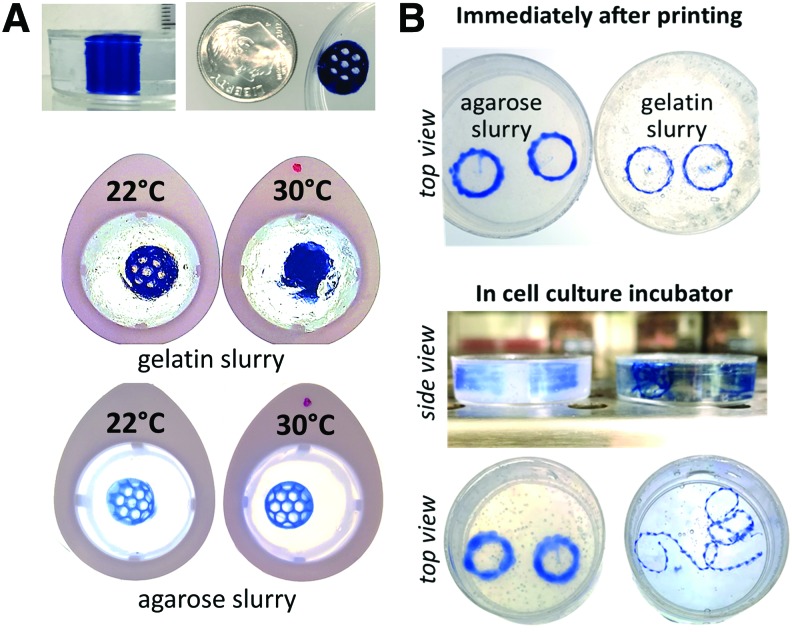
Temperature sensitivity of gelatin versus agarose slurry platforms
Once the printing parameters have been optimized, finely defined constructs can be printed using agarose bath support (Fig. 4A, top). However, it is important to note one more technical detail to print consistent 3D constructs. Temperature differences between gelatin and agarose melting points can affect not only the consistency of the slurry but also the 3D printing process itself. More specifically, if the temperature of the room is higher than 20°C, or if there is accidental heating by extraneous sources (such as a lamp, air pump, or other equipment near the 3D printer), consistency of the slurry can be compromised. This is particularly true for gelatin slurry, which, in warm environments, reverts back to its unprintable gel form. To show this effect directly, we prewarmed both platforms to either 22°C or 30°C, followed by cooling and subsequent 3D printing. The gelatin platform that was prewarmed to 30°C and then cooled back to room temperature resulted in a morphed print (Fig. 4A, middle). In contrast, under the same conditions, no changes in printability were observed for 3D prints made in agarose slurry (Fig. 4B, bottom).
FIG. 4.
Effect of temperature on slurry printability and postprinting integrity. (A) Top: appearance of 3D printed honeycomb construct after removal of support slurry. Middle: the same shape printed in gelatin slurry that was transiently heated to 30°C and then cooled back to room temperature. Bottom: the same experiment using agarose slurry. (B) Top: two helixes printed in agarose and gelatin slurries. Middle: when placed in cell culture incubator for 1 h at 37°C, gelatin slurry melts, releasing the helixes. 3D, three-dimensional. Color images are available online.
Maintenance of 3D printed constructs in a cell culture environment
The biggest advantage of the agarose slurry platform is that it can support complex, freestanding 3D constructs in cell culture conditions, namely a 37°C environment. These very same constructs could not be supported in the gelatin-based FRESH platform since gelatin melts in a cell culture incubator. This is illustrated in Figure 4B, which shows, side by side, the appearance of a freestanding spiral construct before and after 1 h in a cell culture incubator, printed in gelatin versus agarose slurries.
Retention of Coomassie Blue dye in alginate constructs
To monitor print quality and make prints visible in either gelatin or agarose slurry, we included a protein dye, 1% Coomassie Blue G-250 stain (CB250), in the 2% alginate ink. Although CB250 does not bind to alginate, the prints were nicely outlined and remained intensely blue days after they had been removed from the gelatin slurry and placed in aqueous media. The likely explanation for this effect is that a small amount of dissolved gelatin penetrates alginate prints and gets trapped due to the presence of an ionic interaction between negatively charged alginate moieties and positively charged amine groups.11 When the same bioink formulation was used to print in agarose slurry, CB250 rapidly diffused out since both the alginate bioink material and slurry material were made of polysaccharides. When the agarose melted away, the fine structure of prints reappeared, although not much dye was left and so the prints became essentially translucent. To make our alginate prints more visible, we included a small amount of gelatin (anywhere from 0.1% to 1%) in the 2% alginate formulation. This led to stably stained alginate prints that remained stained for prolonged periods of time. Notably, both CB250 alginate bioink formulations, with and without the gelatin, can be useful. If one needs to create alginate prints that are initially visible, but subsequently cleared from stain, one can use gelatin-free CB250 alginate ink. Conversely, in cases where one needs to monitor print shape continuously in the slurry suspension, adding a small amount of gelatin to CB250-stained alginate can be a solution. Considering that alginate tends to hinder cell growth,12 most studies include alginate bioink protein components anyway, namely collagen, Matrigel, or fibronectin.
Printing other types of bioinks and long-term culturing
To demonstrate the applicability of CLASS printing using different bioinks and its capacity for long-term cell culturing, we printed different 3D shapes using cell-laden GelMA bioinks. Instead of 10 mM CaCl2, phenol-free media or Tyrode solution were used to make the slurry. The slurry was then sterilized before being placed at 4°C for cooling. HEK293 RFP cells were mixed with a bioink that included GelMa, Matrigel, and fibronectin to support cell growth. Immediately after printing, constructs laden in the agarose slurry were subjected to ultraviolet (UV) light for 60 s to polymerize GelMa and then placed in the cell incubator (Fig. 5A). During the subsequent days, confocal imaging of embedded constructs allowed us to visualize highly defined, interconnected cell networks, which continued to develop in all planes while retaining the original printed shape (Fig. 5B).
FIG. 5.
Use of the CLASS platform for long-term culturing of 3D cell-laden constructs. (A) Confocal images of newly printed, cell-laden GelMa constructs. (B) Close-up images of cell-laden constructs showing cell proliferation and spreading during several days in cell culture conditions. GelMa, gelatin methacrylate.
Discussion
Bioprinting has significantly advanced the tissue engineering discipline, addressing an increasing demand for direct assembly of biologically relevant materials with a prescribed 3D hierarchical organization.13
The study by Hinton et al.9 demonstrated the validity of a gelatin-based FRESH approach when extruding bioinks using a mechanical printer. Mechanical-based extrusion printers often have better control of material flow compared with pneumatic-based printers. However, pneumatic printers have simpler drive mechanisms that make them more versatile and affordable, particularly when using multiple printer heads.2 To print with high precision using pneumatic printers, additional efforts are required. In this study, we demonstrate that by optimizing extruder pressure, the flow rate of bioink can yield high-fidelity 3D constructs with a resolution analogous to those made by mechanical printers.
A major component of bioprinting is the bioink. Today, most bioinks can be divided into two main categories. The first category encompasses biomaterials that are mechanically robust, but have low potential for cell biocompatibility. These materials include curable polymers and ceramics. The second category includes bioinks that are less mechanically robust, but have better cell biocompatibility. Examples of these materials include hydrogels and naturally derived proteins. To help support 3D printed hydrogel constructs, several laboratories have suggested the use of a support bath.7–9 It is our understanding that neither of these reports focused on how soft 3D constructs can be cultured after they have been printed and removed from their support baths. At the same time, being able to maintain the shape of cell-laden 3D constructs for extended periods in cell culture could be critical for cells to form a tissue-like construct. To form a tissue, cells have to grow, spread, and produce enough ECM proteins to build their own niches and structural support. Once at this stage, prints can be removed from their agarose support baths by a simple dilution of agarose slurry with excess media.
Most of our tuning experiments involved alginate bioink. Alginate has often been used as a carrying vector to control the spatial location of cells within a printed construct. While alginate is a reliable and affordable bioink, it does not support cell spreading and growth unless modified with RGD residues.14–16 Alginate-based bioinks can be also enriched with Matrigel, fibronectin, collagen, GelMa, fibrinogen, or other cell growth-supporting materials. Alternatively, alginate-based constructs can be degraded using calcium chelators in a controlled manner.17 After degradation, cells can once again grow and divide.
Agarose is a natural polysaccharide found in the cell walls of red algae. Because of its versatility, a number of studies have used low concentrations of agarose to create constructs for tissue engineering applications using either pneumatic- or mechanical extrusion-based printers.18–20 Agarose has also been used for cell microencapsulation21 and as a sacrificial material.22 In bone tissue engineering applications, it was found to be useful for modulating the thermoresponsive properties of collagen-based bioinks.23 To the best of our knowledge, our laboratory is the first to report the use of agarose slurry as a support bath for 3D printing of freestanding cell-laden constructs.
Once the validity of our CLASS approach was confirmed using alginate-based bioinks, we then printed freestanding cell-laden constructs using other common bioink formulations, such as GelMa (Fig. 5) and collagen (data not shown). Notably, depending on the type of bioink, one must be conscious as to which media to use when making the slurry. For alginate-based inks, the slurry must contain high concentrations of calcium ions for alginate to form a gel. For GelMa, blue or UV light has to be applied to initiate cross-linking of the photoinitiator within the bioink. Since agarose slurry is translucent, photocross-linking of GelMa can be done without removing the support bath. For collagen, it is mainly time, temperature, and physiological pH that lead to the stiffening of printed constructs. Importantly, when the bioink contains cells, slurry should be made based on a physiological solution such as Tyrode or culture media. After printing, fresh media can be added either on top or to the side of the agarose slurry to avoid disturbing the printed construct (Fig. 1B). Alternatively, one can use cell strainers, transwells, or any other type of mesh-based container that can be filled with agarose slurry. These containers then can be transferred in and out of cell culture wells to feed cells with new media.
We nicknamed our method CLASS to distinguish it from an earlier, gelatin slurry-based approach called FRESH.9 In this study, we employed CLASS to print freestanding 3D constructs using relatively simple designs. More complex geometries that include multiple compartments can encase the slurry, creating slurry pockets. Since it is not clear how these pockets might affect long-term tissue growth or tissue contraction, complex designs may benefit from having drainage points for slurry removal at later time points when a construct is more mature. Future studies are needed to not only address the efficacy of the CLASS approach for more complex structures but to also establish the method's applicability to other cell types and longer cultivation times.
Conclusions
We describe a new bioprinting support platform that offers a simple yet effective way to 3D print soft biomaterials. The use of agarose slurry has several advantages over gelatin slurry, including a shorter blending time and insensitivity to temperature variations during the print process. The main advantage of agarose slurry is its superior performance in supporting freestanding soft tissue constructs in cell culture conditions. The latter allows suspended cells to stay alive for extended periods of time, enabling formation of intercellular connections within the constraints of 3D printed constructs.
Acknowledgments
Financial support from the National Institutes of Health (R21 HL122882) and intramural Cross-Disciplinary Research Fund is gratefully acknowledged.
Author Disclosure Statement
No competing financial interests exist.
References
- 1. Markstedt K, Mantas A, Tournier I, et al. 3D bioprinting human chondrocytes with nanocellulose-alginate bioink for cartilage tissue engineering applications. Biomacromolecules 2015;16:1489–1496 [DOI] [PubMed] [Google Scholar]
- 2. Murphy SV, Atala A. 3D bioprinting of tissues and organs. Nat Biotechnol 2014;32:773–785 [DOI] [PubMed] [Google Scholar]
- 3. Shin SR, Aghaei-Ghareh-Bolagh B, Gao X, et al. Layer-by-layer assembly of 3D tissue constructs with functionalized graphene. Adv Funct Mater 2014;24:6136–6144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Webb B, Doyle BJ. Parameter optimization for 3D bioprinting of hydrogels. Bioprinting 2017;8:8–12 [Google Scholar]
- 5. Carletti E, Motta A, Migliaresi C. Scaffolds for tissue engineering and 3D cell culture. Methods Mol Biol 2011;695:17–39 [DOI] [PubMed] [Google Scholar]
- 6. Lee J-H, Kim H-W. Emerging properties of hydrogels in tissue engineering. J Tissue Eng 2018;9:2041731418768285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Highley CB, Rodell CB, Burdick JA. Direct 3D printing of shear-thinning hydrogels into self-healing hydrogels. Adv Mater 2015;27:5075–5079 [DOI] [PubMed] [Google Scholar]
- 8. Bhattacharjee T, Zehnder SM, Rowe KG, et al. Writing in the granular gel medium. Sci Adv 2015;1:e1500655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hinton TJ, Jallerat Q, Palchesko RN, et al. Three-dimensional printing of complex biological structures by freeform reversible embedding of suspended hydrogels. Sci Adv 2015;1:e1500758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kreger ST, Bell BJ, Bailey J, et al. Polymerization and matrix physical properties as important design considerations for soluble collagen formulations. Biopolymers 2010;93:690–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhang YS, Khademhosseini A. Advances in engineering hydrogels. Science. 2017;356:eaaf3627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hunt NC, Shelton RM, Grover LM. Reversible mitotic and metabolic inhibition following the encapsulation of fibroblasts in alginate hydrogels. Biomaterials 2009;30:6435–6443 [DOI] [PubMed] [Google Scholar]
- 13. Dababned BA, Ozbolar TI. Bioprinting technology: A current state-of-the-art review. J Manuf Sci Eng 2014;136:061016 [Google Scholar]
- 14. Wang L, Shelton R, Cooper P, et al. Evaluation of sodium alginate for bone marrow cell tissue engineering. Biomaterials 2003;24:3475–3481 [DOI] [PubMed] [Google Scholar]
- 15. Rowley JA, Mooney DJ. Alginate type and RGD density control myoblast phenotype. J Biomed Mater Res 2002;60:217–223 [DOI] [PubMed] [Google Scholar]
- 16. Yu J, Gu Y, Du TK, et al. The effect of injected RGD modified alginate on angiogenesis and left ventricular function in a chronic rat infarct model. Biomaterials 2009;30:751–756 [DOI] [PubMed] [Google Scholar]
- 17. Wu Z, Su X, Xu Y, et al. Bioprinting three-dimensional cell-laden tissue constructs with controllable degradation. Sci Rep 2016;6:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Suntornnond R, An J, Chua CK. Bioprinting of thermoresponsive hydrogels for next generation tissue engineering: A review. Macromol Mater Eng 2017;302:1600266 [Google Scholar]
- 19. Malda J, Visser J, Melchels FP, et al. 25th Anniversary article: Engineering hydrogels for biofabrication. Adv Mater 2013;25:5011–5028 [DOI] [PubMed] [Google Scholar]
- 20. Fedorovich NE, De Wijn JR, Verbout AJ, et al. Three-dimensional fiber deposition of cell-laden, viable, patterned constructs for bone tissue printing. Tissue Eng Part A 2008;14:127–133 [DOI] [PubMed] [Google Scholar]
- 21. Gasperini L, Mano JF, Reis RL. Natural polymers for the microencapsulation of cells. J R Soc Interface 2014;11:20140817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bertassoni LE, Cecconi M, Manoharan V, et al. Hydrogel bioprinted microchannel networks for vascularization of tissue engineering constructs. Lab Chip 2014;14:2202–2211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Duarte Campos DF, Blaeser A, Buellesbach K, et al. Bioprinting organotypic hydrogels with improved mesenchymal stem cell remodeling and mineralization properties for bone tissue engineering. Adv Healthc Mater 2016;5:1336–1345 [DOI] [PubMed] [Google Scholar]