Abstract
Background
In human epidermal growth factor receptor 2 (HER2+) breast cancers, neoadjuvant trials of chemotherapy plus anti-HER2 treatment consistently showed lower pathologic complete response (pCR) rates in hormone receptor (HR) positive versus negative tumors. The PerELISA study was aimed to evaluate the efficacy of a de-escalated, chemotherapy-free neoadjuvant regimen in HR+/HER2+ breast cancer patients selected on the basis of Ki67 inhibition after 2-week letrozole.
Patients and methods
PerELISA is a phase II, multicentric study for postmenopausal patients with HR+/HER2+ operable breast cancer. Patients received 2-week letrozole, and then underwent re-biopsy for Ki67 evaluation. Patients classified as molecular responders (Ki67 relative reduction >20% from baseline) continued letrozole and started trastuzumab-pertuzumab for five cycles. Patients classified as molecular non-responders started weekly paclitaxel for 13 weeks combined with trastuzumab-pertuzumab. Primary aim was breast and axillary pCR. According to a two-stage Simon’s design, to reject the null hypothesis, at least 8/43 pCR had to be documented.
Results
Sixty-four patients were enrolled, 44 were classified as molecular responders. All these patients completed the assigned treatment with letrozole-trastuzumab-pertuzumab and underwent surgery. A pCR was observed in 9/44 cases (20.5%, 95% confidence interval 11.1% to 34.5%). Among molecular non-responders, 16/17 completed treatment and underwent surgery, with pCR observed in 81.3% of the cases. PAM50 intrinsic subtype was significantly associated with Ki67 response and pCR. Among molecular responders, the pCR rate was significantly higher in HER2-enriched than in other subtypes (45.5% versus 13.8%, P = 0.042).
Conclusions
The primary end point of the study was met, by reaching the pre-specified pCRs. In patients selected using Ki67 reduction after short-term letrozole exposure, a meaningful pCR rate can be achieved without chemotherapy. PAM50 intrinsic subtyping further refines our ability to identify a subset of patients for whom chemotherapy might be spared.
EUDRACT number
2013-002662-40
ClinicalTrials.gov Identifier
Keywords: early breast cancer, HER2-positive breast cancer, pertuzumab, trastuzumab, neoadjuvant
Key Message
PerELISA is the first clinical trial to show that postmenopausal HR+/human epidermal growth factor receptor 2 (HER2+) breast cancer patients with Ki67 reduction (molecular responders) after 2-week letrozole can achieve a meaningful pCR rate without chemotherapy. The combined use of PAM 50 intrinsic subtype and Ki67 inhibition might further improve a more personalized treatment, with 45% pCR rate in molecular responders with HER2-E subtype.
Introduction
The human epidermal growth factor receptor 2 (HER2) is over-expressed in 15% to 20% of breast cancers and is generally associated with a highly aggressive behavior. However, HER2-positive cancers are heterogeneous, and the co-expression of hormone receptor has a significant impact on tumor behavior and response to therapy. Indeed, neoadjuvant trials of chemotherapy plus anti-HER2 treatment consistently showed lower pathologic complete response (pCR) rates in patients with hormone receptor-positive versus hormone receptor-negative tumors [1]. Difference in terms of pCR rate according to hormone receptor expression has been shown even in the context of studies of chemotherapy and dual HER2 blockade with trastuzumab plus either lapatinib or pertuzumab [2–5]. Preclinical and clinical data suggest the existence of a complex cross talk between HER2 and hormone receptor signaling pathways, therefore, targeting both hormone receptors and EGFR signaling pathways represents an attractive therapeutic option for HER2-positive/hormone receptor positive breast cancer (BC) patients, with the aim of optimizing endocrine therapy and possibly overcoming the occurrence of resistance [6]. In this perspective, the efficacy of dual HER2 blockade allows to explore the possibility of reducing the burden of anticancer treatment. Neoadjuvant trials investigating dual HER2 blockade (±endocrine therapy) without chemotherapy were associated with a pCR rate of approximately 20% to 30%.[4, 7, 8] Therefore, the question whether chemotherapy can be avoided in selected patients with HER2-positive/hormone receptor-positive disease deserves further investigations.
In hormone receptor-positive disease, studies of preoperative endocrine treatment suggested that Ki67 decrease after short-term treatment might be used to identify patients more likely to respond to preoperative hormonal therapy [9]. On these premises, we designed a neoadjuvant study to evaluate if a de-escalated chemotherapy-free treatment can be a valuable option in HER2-positive/hormone receptor-positive patients selected on the basis of Ki67 response after 2-week letrozole.
Methods
PerELISA is an investigator-driven, open label, phase II neoadjuvant study conducted at eight Italian Institutions. The trial was approved by local ethical Committees of the participating Institutions and was conducted in compliance with the principles of Good Clinical Practice and the Declaration of Helsinki.
Patients were eligible if they met the following criteria: previously untreated, histologically confirmed, infiltrating, HER2-positive [immunohistochemistry (IHC) 3+ or in situ hybridization amplification [10]), hormone receptor-positive (estrogen receptor ≥ 10% and/or progesterone receptor ≥ 10%) BC. Other inclusion criteria were as follows: stage II–IIIA, age ≥ 18 years, postmenopausal status, cardiac ejection fraction within institutional normal range, normal organ and marrow function, availability of tumor tissue suitable for biological/molecular examination before starting treatment. Major exclusion criteria were stage IV disease, prior or concurrent cytotoxic therapy, uncontrolled hypertension or clinically significant cardiovascular disease, known infection with HIV, HBV, HCV. All patients provided written informed consent.
Procedures
After diagnostic core biopsy including baseline Ki67 evaluation, confirmation of eligibility and informed consent signature, the patients started letrozole 2.5 mg p.o. daily for 2 weeks followed by a core-biopsy for Ki67 evaluation. Ki67 was evaluated by IHC on formalin-fixed paraffin-embedded (FFPE) tissue sections from diagnostic core biopsy and after 2-week letrozole at local laboratories of participating institutions. Assignment to molecular response cohort was based on local assessment. Patients defined as molecular responders (Ki67 relative reduction >20% from baseline) received the combination of letrozole, trastuzumab and pertuzumab, according to the following schedule: letrozole 2.5 mg p.o. daily, trastuzumab 8 mg/kg i.v. loading dose in the first cycle, then 6 mg/kg every 3 weeks, pertuzumab 840 mg i.v. loading dose in the first cycle, then 420 mg i.v. every 3 weeks. Trastuzumab and pertuzumab were administered for five courses, letrozole was continued until surgery.
Patients defined as molecular non-responders discontinued letrozole and received weekly paclitaxel 80 mg/sqm for 13 weeks, combined with pertuzumab and trastuzumab (same dose and schedule as molecular responding cohort) (supplementary Figure S1, available at Annals of Oncology online).
Criteria for dose modifications, physical and instrumental examinations (including left ventricular ejection fraction and radiologic tumor assessment) are detailed in the protocol (supplementary Material S2, available at Annals of Oncology online).
Surgery was performed within 3 weeks from last dose of i.v. treatment. Treatment after surgery included radiotherapy per local standards, endocrine treatment for at least 5 years, trastuzumab up to 1 year. Postoperative chemotherapy was at discretion of treating physician. The majority of patients with residual disease were offered chemotherapy plus trastuzumab up to 1 year.
End points
Primary end point of the study was pCR rate in the molecular responder cohort, defined as complete absence of infiltrating tumor cells in the breast and in axillary lymph nodes. Residual in situ disease only was included in the pCR definition.
Secondary end points were as follows: clinical response rate in the breast (comparing before and after treatment, as assessed by ultrasound examination, according to RECIST criteria), breast conservative surgery rate (conservative procedures, and conversion from mastectomy to conservative surgery), and safety [incidence and severity of adverse events; graded using National Cancer Institute Common Toxicity Criteria for Adverse Events (NCI CTCAE) v. 4.0].
Correlative science analyses
FFPE tumor blocks from core biopsies (diagnostic and after 2-week letrozole) and from surgical specimens were centralized and reviewed by BC-dedicated pathologists for quality and tumor content. Fresh-frozen samples from diagnostic core biopsies were centralized for genomic studies. Residual cancer burden (RCB) was calculated in molecular responders patients not achieving pCR [11].
Intrinsic subtype [according to 50-gene prediction analysis of microarray (PAM50)], tumor infiltrating lymphocytes (TILs) and PIK3CA mutational status were assessed as detailed in supplementary Material S3, available at Annals of Oncology online.
Statistics
Sample size was estimated using Simon’s two-stage design, on the basis of H0: 0.10; H1: 0.25. Setting α = 0.05 and β = 0.20, a total of 43 patients were required. If at least eight pCRs were observed, further investigation of the combination of letrozole, trastuzumab and pertuzumab was warranted. Assuming 70% of molecular responding patients, a total of 68 subjects had to be included to ensure 43 patients in the letrozole plus trastuzumab and pertuzumab cohort, with 10% drop off rate. Patients not achieving a molecular response were evaluated as internal control for all study end points. No comparison between the two cohorts was foreseen. Association of variables with molecular response and pCR was studied using the chi-square test or the Mann–Whitney U non-parametric test for categorical or continuous variables, respectively. Individual changes in TILs were assessed by Wilcoxon paired rank sum test.
Results
From January 2014 to July 2017, 64 patients were enrolled and 61 were evaluable for molecular response after 2 weeks of letrozole (supplementary Figure S4, available at Annals of Oncology online). Forty-four patients were classified as molecular responders (72%), and 17 patients as molecular non-responders (28%). Mean absolute change of Ki67 after 2-week letrozole was −13.6% (SD ± 17.6) overall, −20.5% (SD ± 13.3) in molecular responders, and +4.2% (SD ± 15.0) in molecular non-responders.
Patients and tumor characteristics at baseline, overall and according to molecular response are detailed in Table 1. The majority of the patients had stage IIA disease, ductal histology, and grade 3 tumors (Elston and Ellis classification). A higher baseline estrogen and progesterone receptors expression was significantly associated with molecular response.
Table 1.
Baseline patients’ and tumor characteristics
| Overall | Molecular responders | Molecular non-responders | P value | |
|---|---|---|---|---|
| N = 61 | N = 44 | N = 17 | ||
| Median age, y (range) | 64 (49–83) | 66 (50–83) | 60 (49–78) | 0.209 |
| Clinical stage, n (%) | 0.606 | |||
| IIA | 41 (67) | 31 (70) | 10 (59) | |
| IIB | 16 (26) | 10 (23) | 6 (35) | |
| IIIA | 4 (6) | 3 (7) | 1 (6) | |
| Histology, n (%) | 0.682 | |||
| Ductal | 56 (92) | 40 (91) | 16 (94) | |
| Lobular/other | 5 (8) | 4 (9) | 1 (6) | |
| Histologic grade, n (%) | 0.054 | |||
| G2 | 14 (24) | 13 (30) | 1 (6) | |
| G3 | 45 (76) | 30 (70) | 15 (94) | |
| Median ER expression % (range) | 90 (10–100) | 90 (25–100) | 60 (10–100) | 0.014 |
| Median PgR expression % (range) | 20 (0–100) | 20 (0–95) | 0 (0–100) | 0.013 |
| Median Ki67 expression % (range) | 30 (7–90) | 28 (7–80) | 34 (15–90) | 0.081 |
Significant P-values in bold.
Surgery and responses
All 44 molecular responders completed assigned treatment and underwent surgery. Nine out of 44 patients achieved pCR in breast and axillary nodes [20.5%, 95% confidence interval (CI) 11.1% to 34.5%]. Clinical response rate in breast was 74%. Rates of breast conserving surgery and of conversion from mastectomy to breast conservative surgery were 65.9% and 54.5%, respectively. For patients not achieving pCR, median Ki67 assessed on surgical specimen was 8% (range 1% to 80%). RCB was evaluable for 34 out of 35 molecular responder patients with residual disease: RCB-I, N = 11 (32%); RCB-II N = 21 (62%); RCB-III, N = 2 (6%). Overall, 20 of the 44 molecular responder patients (45.5%) achieved pCR or RCB-I.
Among 17 molecular non-responders, one patient prematurely discontinued treatment due to a cardiac adverse event. Sixteen patients underwent surgery, and 13 patients achieved pCR (81%, 95% CI 57.0% to 93.4%). Clinical response rate in the breast was 94%. Rates of breast conserving surgery and of conversion from mastectomy to breast conservative surgery were 63% and 57%, respectively.
Safety
No grade 4 toxicity was reported. The most frequently reported AEs in the molecular responder cohort were fatigue and fever. The most frequently reported AEs in the molecular non-responder cohort were neuropathy, skin reactions, neutropenia and diarrhea. Incidence of grade ≥2 AEs is reported in Table 2.
Table 2.
Incidence of grade ≥2 adverse events according to molecular response
| Molecular responders | N = 44 (%) | |
|---|---|---|
|
|
||
| G2 | G3 | |
| Fatigue | 7 | – |
| Fever | 7 | – |
| Hypertension | 2 | 2 |
| Muscoloskeletal symptoms | 2 | 2 |
| Diarrhea | 5 | – |
| Dyspnea | 5 | – |
| Skin reactions | 5 | – |
| GGT Increase | 5 | – |
| Infections | 2 | – |
| Allergic reaction | – | 2 |
| Conjunctivitis | 2 | – |
| Neutropenia | 2 | – |
|
| ||
| Molecular non-responders |
N = 17 (%) |
|
| G2 | G3 | |
|
| ||
| Neuropathy | 12 | 12 |
| Skin reactions | 18 | 6 |
| Neutropenia | 18 | 6 |
| Diarrhea | 24 | – |
| Fatigue | 18 | – |
| Anemia | 18 | – |
| Nausea | 12 | – |
| Infections | 12 | – |
| Heart failure | – | 6 |
| Abdominal pain | – | 6 |
| GGT Increase | – | 6 |
| Muscoloskeletal symptoms | 6 | – |
| Gastric pyrosis | 6 | – |
Correlative science studies
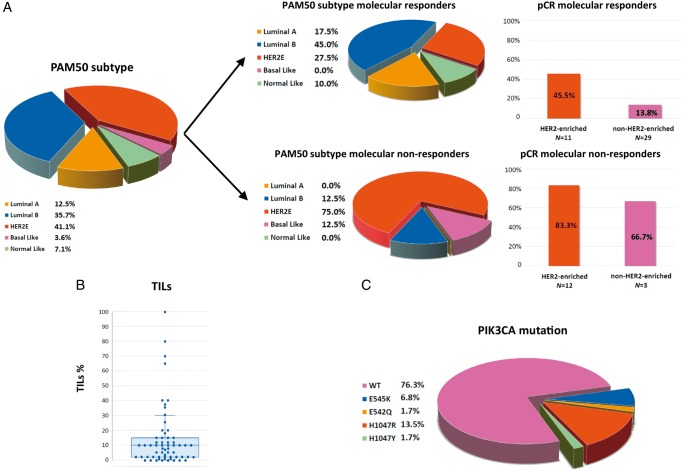
PAM50 subtype distribution is reported in Figure 1A. Intrinsic subtype was significantly associated with ki67 reduction after 2-week letrozole, being molecular responders 92% among luminal A + B versus 44% among HER2-enriched+Basal-like (P < 0.001). Among molecular responders, pCR rate was significantly higher in HER2-enriched tumors than in other subtypes (45.5% versus 13.8%; P = 0.042). Among molecular non-responders, pCR rate was numerically higher in HER2-enriched tumors than in other subtypes (83.3% versus 66.7%; P = 0.527).
Figure 1.
Correlative science results: tumor-infiltrating lymphocytes (TILs), PIK3CA status and PAM50 intrinsic subtype at baseline (all patients). (A) PAM50 intrinsic subtype distribution according to molecular response and pathologic complete response (pCR) rates according to PAM50 and molecular response; (B) distribution of TILs and (C) PIK3CA mutations at baseline in all patients.
All 61 cases were evaluable for TILs at baseline (median 10%; range 0% to 100%; Figure 1B). There was no significant correlation between baseline TILs and molecular response, nor with pCR rate according to molecular response. Median TILs after 2-week letrozole was 10% (range 0% to 85%), with no difference between molecular responders and non-responders. supplementary Table S5, available at Annals of Oncology online summarizes correlation between TILs at different time points with molecular response and pCR. Individual TILs changes are depicted in supplementary Figure S6, available at Annals of Oncology online.
Mutations in the PIK3CA gene were detected in 23.7% (N = 14) of the 59 genotyped patients (Figure 1C). Among molecular responders, pCRs were numerically higher in PIK3CA wild-type tumors (24.2% versus 10% in PIK3CA mutant, P = 0.332). No difference in pCR rate was observed in molecular non-responders according to PIK3CA mutational status (supplementary Table S5, available at Annals of Oncology online).
Discussion
Preoperative therapy represents the ideal model to study more personalized treatment strategies. Indeed, patients with different treatment sensitivities can be identified on the basis of clinical, molecular or pathologic response. The PerELISA study was designed to evaluate if a Ki67 inhibition after 2-week letrozole allows to identify HER2-positive/hormone receptor-positive patients eligible for a chemotherapy-free regimen with dual anti-HER2 blockade. Ki67 inhibition is a validated marker of endocrine sensitivity in hormone receptor-positive disease, while its value in HER2-positive disease is less clear. In our study, we applied the arbitrary cutoff of more than 20% Ki67 relative reduction from baseline to define molecular response, with the intent to capture an early signal of endocrine treatment effect on tumor proliferation, and, more importantly, to exclude those cases with paradoxical Ki67 increases, which are described even in the context of pure Luminal A tumors[12]. Indeed, with this cutoff we observed 70% of molecular responders, while in the cohort of molecular non responders, the mean absolute change of Ki67 was a 4% increase from baseline values. The probability of achieving a molecular response was associated with a higher hormone receptor expression at baseline. In large adjuvant trials, mainly including HER2-negative patients, higher expression for estrogen and progesterone receptor correlates with better prognosis and is also predictive of benefit from endocrine therapy [13]. In order to enrich the study population for endocrine sensitive tumors and to limit the risk of progression during the 2 weeks of letrozole treatment, a higher cutoff of 10% expression was used as inclusion criteria. Indeed, our data suggest that, also in HER2-positive BC, the degree of hormone receptor expression might directly correlate with endocrine sensitivity and supports the evaluation of higher hormone receptor expression cutoffs in de-escalation trials in HER2+ BC.
Ki67 modulation after 2-week letrozole allowed to identify patients with a 20% pCR rate following letrozole-pertuzumab-trastuzumab and patients with more than 80% pCR rate with 13 weeks of paclitaxel combined with trastuzumab and pertuzumab. Overall, these results favorably compare with the pCR rates observed with chemotherapy plus anti-HER2 treatment in hormone receptor-positive disease, also in view of the good safety profile reported in both treatment cohorts. Indeed, in the NeoSphere trial the pCR rate in this subgroup was 26% after four courses of docetaxel-trastuzumab-pertuzumab [4].
In the TRYPHAENA trial, hormone receptor-positive tumors had a pCR rate below 50% after six courses of sequential anthracycline-taxanes or carboplatin-docetaxel, plus trastuzumab and pertuzumab [14]. In the KRISTINE trial, which included 17% of locally advanced patients, pCR rate in the hormone receptor positive subgroup was 38% after six courses of T-DM1 plus pertuzumab and 46% after six courses of Docetaxel, carboplatin, trastuzumab and pertuzumab [15].
In the ADAPT trial, only enrolling patients with HER2-positive/hormone receptor-positive BC, 41% of the patients achieved a pCR with T-DM1 administered for four courses. The addition of hormonal therapy did not increase the rate of pCR. Interestingly, trastuzumab plus hormonal therapy for 12 weeks resulted in 15% pCR rate. However, almost half of the patients enrolled in the ADAPT trial had clinical stage I [16]. These patients were not eligible in the PerELISA study, where patient population consisted of almost 70% of patients with stage IIA, and 30% had stages IIB–III. In the PerELISA trial, correlative science studies get further insight in the molecular heterogeneity of HER2-positive/hormone receptor-positive BC.
PAM50 intrinsic subtype further refines the possibility of selecting patients with hormone receptor-positive/HER2-positive more likely to benefit from hormonal therapy plus dual anti-HER2 therapy. In the PAMELA trial, investigating the role of lapatinib and trastuzumab without chemotherapy, the pCR rate (breast only) in the cohort of hormone receptor-positive/HER2-enriched after endocrine therapy plus dual HER2 blockade was 32% [7]. In the PerELISA study, HER2-enriched patients with a molecular response achieved a notably 45% pCR rate, suggesting that the combined use of intrinsic subtype and Ki67 inhibition might further improve a more personalized treatment.
We have previously reported that the presence of PIK3CA mutation predicts for a reduced pCR rate after chemotherapy plus trastuzumab and lapatinib [17, 18]. A worse pCR rate in case of PIK3CA mutation was reported with pertuzumab plus trastuzumab, irrespectively from ER status [19]. In our study, we observed a numerically higher pCR rate for PIK3CA wild-type patients in molecular responders only.
Our observations are promising but limited by small sample size and by the absence of controls. Another limitation of the trial is the use of local Ki67 testing to define molecular response, especially in the context of the open debate regarding Ki67 reproducibility. However, Ki67 change, and not a predefined cutoff, was used and the same laboratory evaluated both samples, thus limiting interlaboratory reproducibility issues.
Ki67 response after short-term endocrine treatment is currently been used to allocate patients to treatment in several neoadjuvant hormone receptor-positive BC trials. This is the first trial testing this strategy in the context of HER2-positive BC. We observed a clear correlation between Ki67 change, hormone receptor expression and PAM50-subtyping, thus supporting its ability to distinguish tumors with different biology, which might be used in future trials.
In conclusion, the primary end point of the study was met, by reaching the pre-specified numbers of pCRs. A Ki67 reduction after short term letrozole exposure allows to identify patients achieving a meaningful pCR rate without chemotherapy. PAM50 intrinsic subtyping further refines our ability to identify a subset of patients for whom chemotherapy might be spared. Indeed, patients with less than pCR can receive standard adjuvant chemotherapy after surgery. Last, but not least, patients without a molecular response in our study achieved a remarkable 81% pCR rate with 13 weeks of paclitaxel plus dual HER2 blockade. Although observed in a limited number of patients, this pCR rate is one of the highest ever reported, suggesting that lack of ki67 inhibition after 2-week letrozole can help identify patients benefiting particularly from the use of chemotherapy in addition to anti-HER2 treatment. Based on these data, we believe that this approach warrants further investigations in larger series.
Funding
This work was supported by Roche Italy which provided study drugs and financial support, but had no role in study design, data collection, data analysis, data interpretation, or writing the report. Correlative science studies were partly supported by grants from Italian Ministry of Health (GR-2013-02356771 to VG); Instituto de Salud Carlos III (ISCIII) through the Plan Estatal de Investigacion Cientifica y Tecnica y de Innovacion 2013–2016 (PI16/00904 to AP), Banco Bilbao Vizcaya Argentaria Foundation to AP (no grant number applies), Pas a Pas to AP (no grant number applies), Save the Mama to AP (no grant number applies); and the Breast Cancer Research Foundation to AP (no grant number applies). The authors and the PerELISA Investigators maintained sole responsibility for collection, management, monitoring, and analysis of the data. Data were collected by physicians, study nurses, clinical research coordinators and other study-center staff.
Disclosure
VG has received fees from EliLilly for participation on advisory boards; fees from AstraZeneca; fees from Novartis (speakers bureau); travel grants from Novartis, Celgene and Tesaro; research grant (Institution) from Roche. MVD has received: fees from EliLilly for consultancy role and participation on advisory boards; fees from Genomic Health for consultancy role; fees from Celgene for participation on advisory boards; travel grants from Pfizer and Celgene. AF has received fees for lectures from Pfizer, AstraZeneca, Eisai and Novartis; travel grants from Roche, Novartis, AstraZeneca, Pfizer, Celgene, EliLilly. GG has received travel grants from Novartis. AP has received: fees from Novartis, Lilly Spain, Pfizer, Roche, Bristol-Myers Squibb and Amgen for advisory role and fees from Oncolytics Biotech for scientific advisory role; research grant (Institution) from Roche, Novartis, Sysmex Europe, Medical Scientia Inno. Research, Celgene, Astellas Pharma and Nanostring Technologies. PFC has received lecture fees and honoraria for participation on advisory boards from EliLilly, Novartis, Roche, AstraZeneca; travel grants from Celgene and Tesaro; research grant (Institution) from Merck KGaA, Bristol-Myers Squibb, Novartis. All remaining authors have declared no conflicts of interest.
Supplementary Material
Note: This study was previously presented at 2018 ASCO annual meeting (oral session, 4 June 2018).
References
- 1. Cortazar P, Zhang L, Untch M. et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 2014; 384(9938): 164–172. [DOI] [PubMed] [Google Scholar]
- 2. Baselga J, Bradbury I, Eidtmann H. et al. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): a randomised, open-label, multicentre, phase 3 trial. Lancet 2012; 379(9816): 633–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Guarneri V, Frassoldati A, Bottini A. et al. Preoperative chemotherapy plus trastuzumab, lapatinib, or both in human epidermal growth factor receptor 2-positive operable breast cancer: results of the randomized phase II CHER-LOB study. J Clin Oncol 2012; 30(16): 1989–1995. [DOI] [PubMed] [Google Scholar]
- 4. Gianni L, Pienkowski T, Im Y-H. et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol 2012; 13(1): 25–32. [DOI] [PubMed] [Google Scholar]
- 5. Nahta R, O’Regan RM.. Therapeutic implications of estrogen receptor signaling in HER2-positive breast cancers. Breast Cancer Res Treat 2012; 135(1): 39–48. [DOI] [PubMed] [Google Scholar]
- 6. Giuliano M, Trivedi MV, Schiff R.. Bidirectional crosstalk between the estrogen receptor and human epidermal growth factor receptor 2 signaling pathways in breast cancer: molecular basis and clinical implications. Breast Care (Basel) 2013; 8(4): 256–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Llombart-Cussac A, Cortés J, Paré L. et al. HER2-enriched subtype as a predictor of pathological complete response following trastuzumab and lapatinib without chemotherapy in early-stage HER2-positive breast cancer (PAMELA): an open-label, single-group, multicentre, phase 2 trial. Lancet Oncol 2017; 18(4): 545–554. [DOI] [PubMed] [Google Scholar]
- 8. Rimawi MF, Mayer IA, Forero A. et al. Multicenter phase II study of neoadjuvant lapatinib and trastuzumab with hormonal therapy and without chemotherapy in patients with human epidermal growth factor receptor 2-overexpressing breast cancer: tBCRC 006. JCO 2013; 31(14): 1726–1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dowsett M, Smith IE, Ebbs SR. et al. Prognostic value of Ki67 expression after short-term presurgical endocrine therapy for primary breast cancer. J Natl Cancer Inst 2007; 99(2): 167–170. [DOI] [PubMed] [Google Scholar]
- 10. Wolff AC, Hammond MEH, Hicks DG. et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Update. JCO 2013; 31(31): 3997–4013. [DOI] [PubMed] [Google Scholar]
- 11. Symmans WF, Peintinger F, Hatzis C. et al. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. JCO 2007; 25(28): 4414–4422. [DOI] [PubMed] [Google Scholar]
- 12. Ellis MJ, Suman VJ, Hoog J. et al. Randomized phase II neoadjuvant comparison between letrozole, anastrozole, and exemestane for postmenopausal women with estrogen receptor-rich stage 2 to 3 breast cancer: clinical and biomarker outcomes and predictive value of the baseline PAM50-based intrinsic subtype–ACOSOG Z1031. J Clin Oncol 2011; 29(17): 2342–2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brouckaert O, Paridaens R, Floris G. et al. A critical review why assessment of steroid hormone receptors in breast cancer should be quantitative. Ann Oncol 2013; 24(1): 46–53. [DOI] [PubMed] [Google Scholar]
- 14. Schneeweiss A, Chia S, Hickish T. et al. Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: a randomized phase II cardiac safety study (TRYPHAENA). Ann Oncol 2013; 24(9): 2278–2284. [DOI] [PubMed] [Google Scholar]
- 15. Hurvitz SA, Martin M, Symmans WF. et al. Neoadjuvant trastuzumab, pertuzumab, and chemotherapy versus trastuzumab emtansine plus pertuzumab in patients with HER2-positive breast cancer (KRISTINE): a randomised, open-label, multicentre, phase 3 trial. Lancet Oncol 2018; 19(1): 115–126. [DOI] [PubMed] [Google Scholar]
- 16. Harbeck N, Gluz O, Christgen M. et al. De-escalation strategies in human epidermal growth factor receptor 2 (HER2)-positive early breast cancer (BC): final analysis of the west German study group adjuvant dynamic marker-adjusted personalized therapy trial optimizing risk assessment and therapy response prediction in early BC HER2- and hormone receptor-positive phase II randomized trial-efficacy, safety, and predictive markers for 12 weeks of neoadjuvant trastuzumab emtansine with or without endocrine therapy (ET) versus trastuzumab plus ET. J Clin Oncol 2017; 35(26): 3046–3054. [DOI] [PubMed] [Google Scholar]
- 17. Guarneri V, Dieci MV, Frassoldati A. et al. Prospective biomarker analysis of the randomized CHER-LOB study evaluating the dual anti-HER2 treatment with trastuzumab and lapatinib plus chemotherapy as neoadjuvant therapy for HER2-positive breast cancer. Oncologist 2015; 20(9): 1001–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Loibl S, Majewski I, Guarneri V. et al. PIK3CA mutations are associated with reduced pathological complete response rates in primary HER2-positive breast cancer: pooled analysis of 967 patients from five prospective trials investigating lapatinib and trastuzumab. Ann Oncol 2016; 27(8): 1519–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bianchini G, Kiermaier A, Bianchi GV. et al. Biomarker analysis of the NeoSphere study: pertuzumab, trastuzumab, and docetaxel versus trastuzumab plus docetaxel, pertuzumab plus trastuzumab, or pertuzumab plus docetaxel for the neoadjuvant treatment of HER2-positive breast cancer. Breast Cancer Res 2017; 19(1): 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.