Abstract
Although prominent sex differences exist in the hypothalamic-pituitary-adrenal axis’s response to stressors, few studies of its regulation in the hypothalamic paraventricular nucleus (PVN) have compared both male and female subjects. In this study, we sought to explore sex differences in the acute regulation of PVN neuropeptide expression following glucocorticoid (GC) removal and the underlying role of gonadal hormones. We first examined the effects of short-term adrenalectomy (ADX) on PVN Crh and arginine vasopressin (Avp) expression in mice using in situ hybridization. ADX increased PVN AVP mRNA levels in both sexes. In contrast, PVN CRH mRNA was increased by 2 days after ADX in males only. Both sexes showed increases in CRH mRNA after 4 days. To determine if gonadal hormones contributed to this sex bias, we examined adrenalectomized (ADX’d) and gonadectomized (GDX’d) mice with or without gonadal hormone replacement. Unlike the pattern in intact animals, 2 days following ADX/gonadectomy, CRH mRNA levels did not increase in either sex. When males were given DHT propionate, CRH mRNA levels increased in ADX’d/GDX’d males similar to those observed following ADX alone. To determine a potential mechanism, we examined the coexpression of androgen receptor (AR) immunoreactivity and CRH neurons. Abundant colocalization was found in the anteroventral bed nucleus of the stria terminalis but not the PVN. Thus, our findings reveal a sex difference in PVN Crh expression following the removal of GC-negative feedback that may depend on indirect AR actions in males.
All mammals, when faced with any environmental or homeostatic challenge, rely on activation of the hypothalamic-pituitary-adrenal (HPA) axis to adapt and survive. This key neuroendocrine system is activated by neurons in the hypothalamus in response to stressors and culminates in the synthesis of glucocorticoids [GCs; cortisol in humans or corticosterone (CORT) in rats and mice] by the adrenal cortex. Such stressor-induced elevations in GCs ultimately serve as first responders against threats to homeostasis. In such a situation, GCs mobilize energy stores to allow for appropriate physiological and behavioral responses (1, 2). However, the same GC response to stressors that is greatly beneficial in the short term can increase risk for stress-related diseases if persistently elevated (2–4). Thus, the understanding of how GC production is regulated may have important therapeutic applications.
The HPA axis is principally controlled by neurons in the paraventricular nucleus (PVN) of the hypothalamus that release several neuropeptides during a stress response (5, 6). These neuropeptides include CRH and arginine vasopressin (AVP), which are coexpressed by some parvocellular PVN neurons and act together to stimulate the production of ACTH by corticotrophs of the anterior pituitary gland (7–13). Increased ACTH in the systemic circulation acts on the adrenal cortex to induce the synthesis and release of GCs. GCs, in turn, downregulate their own production by the inhibition of Crh and Avp gene expression in the PVN (14, 15). Therefore, PVN CRH and AVP coexpressing neurons represent an important nexus in the control of HPA activity by GCs.
Prominent sex differences exist in the activation of the HPA axis (16). Following numerous stressors, neuronal activation is greater in the PVN of female vs male rodents (17–19). Females also have greater stress-induced levels of PVN AVP and CRH mRNAs (19–21). Such sex biases in the PVN have frequently been attributed to the opposing effects of gonadal hormones in adulthood (16, 22). Accordingly, estrogens often are reported to increase, whereas androgens exclusively decrease elevations in neuropeptide mRNAs brought on by varying types of stressors (17, 18, 23–26). Thus, gonadal hormones influence sex differences in the PVN, as well as those found at all levels of the HPA axis, to produce a more robust GC response to stress in female rodents (16, 22).
Compared with the activation of the HPA axis, far less is known about sex differences in the inhibition of PVN neuropeptide expression by GCs. One study found that GC receptor (GR) expression in the PVN is important for downregulation of neuroendocrine stress responses in male but not female mice (27). Yet, other studies of GC negative feedback that use both male and female subjects are few and far between. Given that many stress-related diseases are associated with a dysregulation of GC responsiveness and exhibit a strong sex bias, it is essential that we understand HPA axis regulation in both sexes (28). Thus, the current study aimed to explore sex differences in the control of PVN neuropeptide gene expression by GCs. We first examined the effects of short-term depletion of circulating GCs by adrenalectomy (ADX) on PVN Crh and Avp expression in male vs female mice. We found that ADX more rapidly increased CRH mRNA in males. We next determined if gonadal hormones influenced this sex difference by using adrenalectomized (ADX’d) and gonadectomized (GDX’d) mice treated with or without gonadal hormones. Our findings reveal a sex difference unique to the timing of the Crh response to GC removal, which depends on androgen actions in males. To assess a potential mechanism for such androgen actions, we explored androgen receptor (AR) expression within CRH neurons in the PVN and the anteroventral bed nucleus of the stria terminalis (BNSTav), an upstream HPA-controlling region. By crossing B6(Cg)-Crhtml(cre)Zjh/J (Crh-IRES-Cre) and B6.Cg-Gt(ROSA)26Sortml4(CAG-TdTomato)Hze/J (Ai14) strains, we generated Crh-IRES-Cre;Ai14 mice in which CRH neurons are permanently tagged with a fluorescent protein. We identified abundant AR and CRH coexpression in neurons of the BNSTav, but not the PVN using these mice. Thus, our findings ultimately support a role for androgens to modulate indirectly the PVN Crh response to the removal of GC negative feedback.
Materials and Methods
Animals
Animals in these studies were housed in the laboratory animal research facility at Colorado State University. Subjects primarily included adult (2 to 4 months old) male and female C57BL/6N mice obtained from Charles River Laboratories (Wilmington, MA) or bred in-house. If purchased, animals were allowed to acclimate for at least 1 week before use in experiments. If bred in-house, mice were weaned at 21 days of age and group housed by sex. All animals were maintained in a 12-hour light/12-hour dark cycle with lights on at 6:00 am and were provided ad libitum access to food and water.
Subjects included adult male Crh-IRES-Cre;Ai14 mice. Crh-IRES-Cre;Ai14 mice were generated from our colonies by the crossing of the Crh-IRES-Cre [The Jackson Laboratory, Bar Harbor, ME; stock no. 012704; RRID: IMSR_JAX:012704 (29)] and Ai14 [The Jackson Laboratory; stock no. 007914; RRID: IMSR_JAX:007914 (30)] strains. These original stocks were generated as described previously (31, 32). Genotyping was performed at weaning using an ear punch to identify Crh-IRES-Cre and Ai14 mutants for use in the generation of heterozygous Crh-IRES-Cre;Ai14 offspring, as previously reported (33). Adult male Crh-IRES-Cre;Ai14 mice were used in subsequent experiments. These animals have previously been validated for the examination of CRH (33–35) and the expression of CRH-immunoreactivity (ir) matches that of cre-driven reporter gene expression (33). All animal surgeries and protocols were approved by the Institutional Animal Care and Use Committee at Colorado State University and were performed within National Institutes of Health and Association for Assessment and Accreditation of Lab Animal Care International guidelines.
Experiment 1. Effect of time after ADX on PVN neuropeptide gene expression in male and female mice
Intact C57BL/6N male (n = 5 to 8 per group) and female (n = 7 to 9 per group) mice were bilaterally ADX’d under isoflurane anesthesia and given 0.9% saline as drinking water to maintain osmolarity. Sham-operated males (n = 5 to 7 per group) and females (n = 7 per group) were given tap water. Two or 4 days following surgery, animals were decapitated within 2 minutes of first cage disturbance, and brains were removed, flash frozen in 2-methylbutane at −40°C, and stored at −80°C until sectioning for in situ hybridization to examine PVN gene expression. Tissue was also harvested for droplet digital PCR (ddPCR) from a separate cohort of sham (n = 8) vs 2-day ADX’d (n = 5) male mice. Upon decapitation, trunk blood was collected from all animals in chilled tubes containing 0.5 M EDTA and aprotinin (4 mg/mL; Sigma-Aldrich, St. Louis, MO). Trunk blood was then centrifuged at 4°C, and plasma was removed and stored at −20°C until it was assayed for CORT by radioimmunoassay.
To control for the influence of the estrous cycle, all females in this experiment were euthanized on a day of diestrus when circulating estradiol levels are low. Cycle day was determined using vaginal cytology, according to previously established methods (36). In brief, each female was lavaged daily with saline (0.9% NaCl) solution, and then samples were dried on glass slides and dipped in methylene blue (0.05%) for visualization using light microscopy. Estrous cycle stage was monitored daily for roughly 2 weeks before ADX to allow for habituation to handling and to enable prediction of estrous stage at time of death.
Experiment 2. Effect of gonadectomy and ADX on PVN neuropeptide gene expression in both sexes
C57BL/6N male (n = 4 to 6 per group) and female (n = 5 to 6 per group) mice were bilaterally ADX’d and GDX’d under isoflurane anesthesia and kept on 0.9% saline or were sham operated and given tap drinking water. Two or 4 days following surgery, animals were euthanized, and brains were removed, flash frozen, and stored at −80°C until sectioning for microdissection and ddPCR. Upon decapitation, trunk blood was collected and centrifuged, and plasma was removed and stored, as described in Experiment 1. Tissue harvested from sham and 2-day ADX’d/GDX’d male mice in this experiment was also examined alongside tissue from sham and 2-day ADX’d male mice from Experiment 1 using ddPCR.
Experiment 3. Effect of androgen and estrogen treatment on PVN Crh expression in ADX’d and GDX’d males
C57BL/6N male mice (n = 4 to 7 per group) were bilaterally ADX’d and GDX’d, as in Experiment 1. Starting at the time of surgery, males received daily subcutaneous (s.c.) injections of testosterone (T) propionate [TP; Sigma-Aldrich; 1 mg/kg body weight (BW)], DHT propionate (DHTP; Steraloids, Newport, RI; 1 mg/kg or 10 mg/kg BW), 5α-androstane-3β, 17β-diol dipropionate (3β-diol P; Steraloids; 1 mg/kg BW), estradiol benzoate (EB; Steraloids; 25 μg/kg or 250 μg/kg BW), or vehicle (27% hydroxypropyl-β-cyclodextrin; Cyclodextrin Technologies Development, Inc., Alachua, FL). Our objective with the delivery of DHTP was to match roughly total androgen levels of intact male mice, not just DHT levels, given that T can bind and activate the AR and can be 5α reduced to DHT in tissue (22). However, because of the broad range of androgen levels found in male mice (37, 38), the doses of DHTP were estimates of what might be seen in vivo. The lower doses for DHTP and EB have previously been shown to alter HPA activity in the male mouse (39), and the higher doses were simply ×10 the low-dose amounts. The dose for TP has previously been shown to alter HPA axis activity in rats (40) and physiological function in mice (41), and the dose for 3β-diol P was determined using an allometric scaling calculator (http://clymer.altervista.org/minor/allometry.html) to adjust a dose (5 mg/kg) shown in our previous studies to alter Avp gene expression in the rat brain to one appropriate for the mouse (42). Two days following surgery, animals were decapitated, and brains were collected and stored as above for ddPCR. Plasma was saved for CORT radioimmunoassay as described above.
CORT radioimmunoassay
Plasma levels of CORT were measured, as previously described (43), for all ADX’d animals in these studies to ensure the complete removal of the adrenal gland by ADX. In brief, samples were diluted 1:25 in PBS and then heated at 65°C to denature CORT-binding globulin, which can interfere with the assay. Diluted plasma samples (20 μL or 50 μL) were incubated overnight at 4°C with rabbit anti-CORT antiserum [1:1200; catalog no. 7120016; RRID: AB_2801269 (44); MP Biomedicals, Sonon, OH] and 3H-CORT (PerkinElmer, Boston, MA) in 0.01 M PBS containing 0.1% gelatin. Dextran-coated charcoal was used to separate antibody-bound CORT from free CORT. A standard curve, ranging from 2.5 to 750 pg/tube CORT (Steraloids), was run in every assay and used to quantify experimental samples. The interassay coefficient of variation, as measured by internal quality controls, was <10% for all assays. Animals were considered ADX’d if they had plasma CORT levels that were below the limit of detection (10 ng/mL). Only three animals had measureable levels of CORT and were removed from the study.
In situ hybridization
Brain sections 16 μm thick that contained the PVN were cut in the coronal plane using a CM3050 S cryostat (Leica, Wetzlar, Germany) into five series at −20°C, thaw mounted onto positively charged slides (Superfrost Plus; VWR Scientific, West Chester, PA), and stored dessicated at −80°C. For in situ hybridization, tissue was thawed to room temperature (RT), fixed with 4% formaldehyde, acetylated with 0.25% acetic anhydride, delipidated in chloroform, dehydrated in a series of increasing ethanols, and air dried, as previously described (45). Oligonucleotide probes (48-bp) for Crh (5′-CAGTTTCCTGTTGCTGTGAGCTTGCTGAGCTAACTGCTCTGCCCGGGC-3′) and Avp (5′-GTAGACCCGGGGCTTGGCAGAATCCACGGACTCCCGTGTCCCAGCCAG-3′) were end labeled with [35S] using terminal deoxynucleotidyl transferase (Thermo Fisher Scientific, Waltham, MA). Brain sections were then incubated at 37°C overnight in hybridization solution (50% formamide, 0.25% SDS, 50 mM dithiothreitol, 0.05% sodium thiosulfate, 600 mM NaCl, 10 mM Tris-HCl, 0.02% Denhardt solution, 1 mM EDTA, 0.01% denatured salmon testis DNA, 0.05% total yeast RNA, 0.005% yeast tRNA, 10% dextran sulfate) containing the radiolabeled probe at a concentration of 20 × 106 cpm/mL. After hybridization, sections were washed in 2× saline sodium citrate with a final wash stringency of 1× saline sodium citrate and then dehydrated in a series of ethanols ranging from 50% to 100%. To examine hybridization, slides were apposed to X-ray film (Carestream Kodak Biomax MR; Carestream, Rochester, NY) for up to 4 days (CRH) or 19 hours (AVP) to generate autoradiograms. Analysis of film autoradiograms was conducted using ImageJ software (version 1.51r). Optical density was quantified bilaterally throughout the rostral-caudal extent of the PVN in three (for CRH) or four (for AVP) adjacent and anatomically matched sections using a template of fixed size (1.8 μm2). The density of exposed pixels was measured and expressed as arbitrary density units (AdUs) for the PVN in all sections. Background activity in an adjacent area without labeling was subtracted from each measurement, and resulting AdUs were averaged to obtain a single value per animal for statistical analysis.
Microdissection, RNA isolation, and ddPCR
Frozen brains were sectioned at −16°C into 300 μm-thick sections containing the PVN using a CM3050 S cryostat (Leica). PVN punches were obtained from two atlas-matched, thick sections per animal using a micropunch fashioned from sharpened heavy wall stainless-steel type 304 tubing with an internal diameter of 0.991 ± 0.0381 mm (Small Parts Inc., Miami Lakes, FL). Tissue punches were kept frozen at all times and stored at −80°C until RNA extraction. Total RNA was isolated from PVN punches using the RNeasy mini kit (Qiagen, Germantown, MD), according to the manufacturer’s instructions. Total RNA was reverse transcribed to yield cDNA using the iScript Reverse Transcription Super Mix (Bio-Rad, Hercules, CA). ddPCR was then used to measure target cDNA copies as a number of molecules. This approach shows increased sensitivity and decreased variability compared with other quantitative PCR methods (46–49). The ddPCR reaction mix was prepared by the addition of cDNA to an EvaGreen supermix (10 μL; Bio-Rad), combined with forward (1 μL) and reverse (0.4 μL) primers and nuclease-free water, up to a total volume of 20 μL. Primers used are detailed in Table 1, and all systems and reagents used for ddPCR were obtained from Bio-Rad. To generate droplets, the 20-μL reaction mix and 70 μL droplet generation oil were added to specified wells in the DG8 Cartridge for the QX200 droplet generator, which was then inserted into the automated droplet generator. Postgeneration, droplets were transferred to a 96-well plate. The plate was sealed with foil using the PX1 PCR plate sealer, and PCR amplification of template molecules in each individual droplet was accomplished in the C1000 Touch Thermal Cycler with a 96-deep well reaction. The following thermal cycling protocol was used: 95°C for 10 minutes (one cycle), 95°C for 30 seconds, and then 60°C for 1 minute (40 cycles), 4°C for 5 minutes (one cycle), 90°C for 5 minutes (one cycle), and hold at 4°C. The ramp rate was set at 2°C/s, the sample volume at 40 µL, and the heated lid at 105°C. After PCR amplification, the plate was inserted into the QX200 Droplet Reader, and the absolute template expression in copies per microliter was quantified using QuantaSoft software. All values were normalized to the amount of cDNA loaded in each reaction, which was calculated based on cDNA concentrations quantified using the Quant-iT OliGreen single-stranded DNA assay kit (Thermo Fisher Scientific), according to the manufacturer’s instructions.
Table 1.
Primers Used for ddPCR
| Primer | Sequence (5′ to 3′) |
|---|---|
| CRH mRNA forward | ATGCTGCTGGTGGCTCTGTC |
| CRH mRNA reverse | GGATCAGAACCGGCTGAGGT |
| AVP mRNA forward | TGCTCGCCAGGATGCTCAACAC |
| AVP mRNA reverse | TTGCCGCCTCTTGGGCAGTT |
| GR mRNA forward | GCAGTGGAAGGACAGCACAA |
| GR mRNA reverse | GAGACTCCTGCAGTGGCTTG |
| MR mRNA forward | GGCAAGCACTGCAACAGGTA |
| MR mRNA reverse | GTCCTCTCTGCAGGTCCAAG |
| CRFR1 mRNA forward | CTCTTCGCTCTGGGATGTCG |
| CRFR1 mRNA reverse | CACTGCAGGCCAGAGACATT |
Abbreviations: CRFR1, CRH receptor 1; MR, mineralocorticoid receptor.
Immunohistochemistry
Adult male Crh-IRES-Cre;Ai14 mice (n = 3) were intracardially perfused with ice-cold PBS (0.01 M; pH 7.4), followed by 4% paraformaldehyde in phosphate buffer (4°C). Brains were removed from the skull, then placed in 4% buffered paraformaldehyde for 24 hours, and then infiltrated with 30% sucrose as a cryoprotectant. Four series of 35 μm-thick coronal sections were obtained using a Leica CM3050 S cryostat. Immunohistochemistry was performed on free-floating sections. Sections were washed 3 × 10 minutes in PBS and then quenched with 0.3% hydrogen peroxide in PBS, followed by a 1 × 10-minute wash in PBS. Sections were incubated in blocking solution [10% normal goat serum (NGS) in PBS] for 1 hour at RT before incubation in primary antibody solution [10% NGS in PBS with 0.1% Triton-X 100 (pH 7.4)] overnight at RT. The primary antibody used was a rabbit anti-AR [1:500; catalog no. ab133273; RRID: AB_11156085 (50); Abcam, Cambridge, MA], which has been previously validated (51, 52). Sections were then washed 3 × 10 minutes in Tris-NaCl-Tween (TNT; pH 7.4) buffer before incubation with a biotinylated goat anti-rabbit secondary antibody [1:200; Vectastain Elite ABC Kit; catalog no. PK-6101; RRID: AB_2336820 (53); Vector Laboratories, Burlingame, CA] in TNT with 1.5% NGS for 1 hour at RT. Sections were washed 3 × 10 minutes in TNT and incubated for 30 minutes in TNT with 1:50 Reagent A plus 1:50 Reagent B from the Vectastain Elite ABC Kit, according to the manufacturer’s instructions (Vector Laboratories). Following 3 × 10-minute washes in TNT, sections were developed for 6 minutes using a Tyramide Signal Amplification Plus Fluorescein System (Perkin Elmer, Waltham, MA) per the manufacturer’s instructions. Washes (3 × 10 minute) were performed in TNT, after which, sections were mounted and coverslipped using ProLong Diamond Antifade Mountant (Thermo Fisher Scientific).
Confocal imaging and colocalization quantification
Immunohistochemistry-treated sections containing the rostral (Bregma −0.83 mm), middle (Bregma −0.95 mm), or caudal (Bregma −1.07 mm) PVN or the BNSTav (Bregma 0.01 to 0.13 mm) were identified using Paxinos and Franklin’s The Mouse Brain in Stereotaxic Coordinates (54). The bed nucleus of the stria terminalis (BNST), lateral division, ventral part, and BNST, medial division, ventral part, were collectively defined as the BNSTav (55). Images were taken of each region bilaterally using laser-scanning confocal microscopy. Z-Stacks, composed of 0.83 μm-thick optical sections, spanning ∼26 μm, were created for each image. Images for CRH neurons that stably express a tdtomato fluorophore (CRH:tdtomato) with AR-ir were obtained using a Zeiss 880 laser-scanning confocal microscope and a 20× (W Plan-Apochromat 20×/1.0 differential interference contrast Vis-ir ∞/0.17) objective.
Three-dimensional images were rendered from confocal Z-stacks with Imaris v9.1 software (Bitplane Inc., Zurich, Switzerland). CRH:tdtomato-positive neurons and AR-ir-positive neurons were automatically counted using Imaris v9 and manually checked. CRH:tdtomato and AR-ir were considered to be colocalized when automatically determined cell centers were within 3 μm of each other. Colocalization was confirmed visually, three dimensionally. Percentages of CRH:tdtomato neurons containing AR-ir were determined as the number of colocalized CRH:tdtomato neurons divided by the total number of these neurons ×100. For the PVN, numbers of total and colocalized CRH:tdtomato neurons were summed from bilateral counts at each imaged level (rostral, middle, or caudal). For the BNSTav, numbers of total and colocalized CRH:tdtomato neurons were obtained from bilateral counts in one image.
Statistical methods
All data shown are mean values, and all error bars represent SEM. In studies with only two treatment groups, unpaired Student t test was used for all pairwise data comparison. Statistical comparisons among three treatment groups were made using one-way ANOVAs. Fisher least significant difference post hoc test was used where appropriate. All statistics were done using the Prism statistical program (GraphPad Software, La Jolla, CA), and results were considered statistically significant when P < 0.05. Additionally, all data were analyzed using the Extreme Studentized Deviate method (GraphPad Software) to detect significant outliers. Only three outliers were detected and excluded from further analyses.
Results
Experiment 1. Effect of time after ADX on PVN neuropeptide gene expression in male and female mice
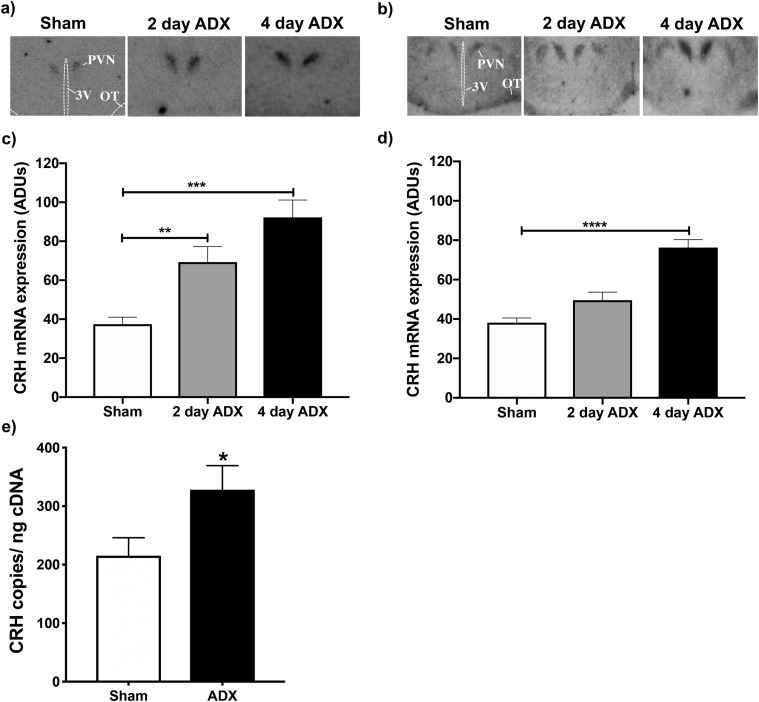
To determine if GCs similarly regulate PVN neuropeptide gene expression in male and female mice, we examined the effect of ADX after 2 and 4 days on PVN CRH (Fig. 1) and AVP (Fig. 2) mRNA expression in both sexes using in situ hybridization. ADX significantly increased CRH mRNA levels compared with those of sham-operated controls in both males (F(2,13) = 13.12; P < 0.001) and females (F(2,20) = 29.31; P < 0.0001), as determined by one-way ANOVAs. However, post hoc analyses revealed a sex difference in the effect of ADX, based on time following surgery. Compared with intact controls, ADX’d males had significant increases in CRH mRNA levels after 2 (P < 0.01) and 4 days (P < 0.001). ADX’d females, alternatively, exhibited increased CRH mRNA only after 4 days (P < 0.0001).
Figure 1.
Changes in Crh gene expression within the PVN following ADX of male and female mice. PVN CRH mRNA levels were evaluated using (a–d) in situ hybridization or (e) ddPCR in ADX’d male and female mice vs sham-operated controls. (a and b) Representative autoradiograms of CRH mRNA hybridization in sham, 2-day ADX, or 4-day ADX (a) male or (b) female mice. (c and d) AdUs calculated from autoradiograms for (c) males and (d) females. Bars represent mean ± SEM AdUs in the PVN of n = 5 to 9 individuals. **P < 0.01; ***P < 0.001; ****P < 0.0001. (e) Mean absolute levels ± SEM of PVN CRH mRNA in n = 5 to 8 male mice, 2 days after ADX or sham surgery. Absolute CRH mRNA levels were measured by ddPCR and normalized to the amount of input cDNA. *P < 0.05 for ADX’d males compared with controls. 3V, third ventricle; OT, optic tract.
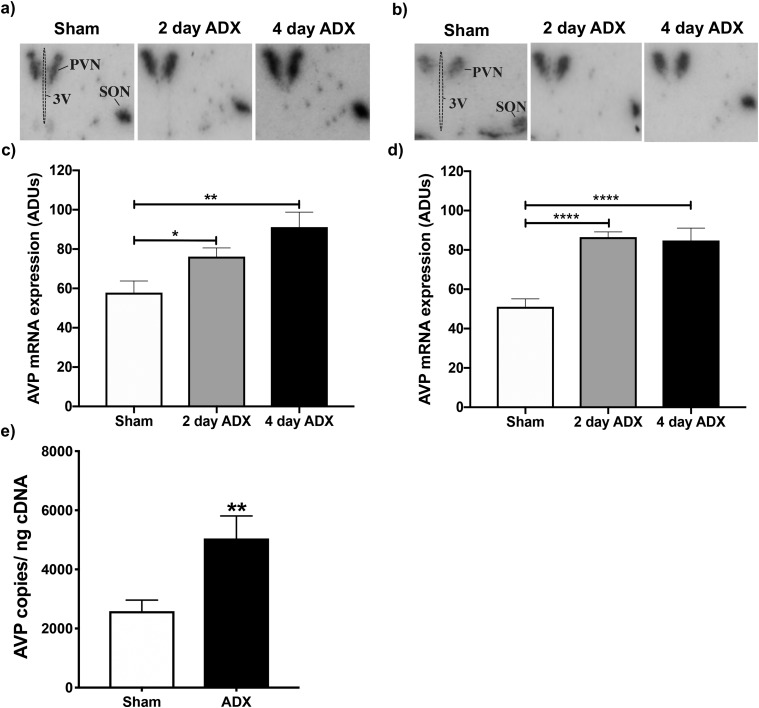
Figure 2.
Changes in Avp gene expression within the PVN following ADX of male and female mice. PVN AVP mRNA levels were evaluated using (a–d) in situ hybridization or (e) ddPCR in ADX’d male and female mice vs sham-operated controls. (a and b) Representative autoradiograms of AVP mRNA hybridization in sham, 2-day ADX, or 4-day ADX (a) male or (b) female mice. (c and d) AdUs calculated from autoradiograms for (c) males and (d) females. Bars represent the mean ± SEM AdUs in the PVN of n = 5 to 9 individuals. *P < 0.05; **P < 0.01; ****P < 0.0001. (e) Mean absolute levels ± SEM of PVN AVP mRNA in n = 5 to 8 male mice, 2 days after ADX or sham surgery. Absolute AVP mRNA levels were measured by ddPCR and expressed relative to the amount of input cDNA. **P < 0.01 for ADX’d males compared with controls. 3V, third ventricle; SON, supraoptic nucleus.
Whereas ADX increased PVN CRH mRNA in a sex-dependent fashion, it increased AVP mRNA levels in a similar pattern in both sexes (Fig. 2). One-way ANOVAs revealed significant increases in AVP mRNA in both ADX’d males (F(2,19) = 7.3; P < 0.01) and females (F(2,19) = 17; P < 0.0001) compared with sham-operated controls. Moreover, ADX’d males had significantly increased AVP mRNA after 2 (P < 0.05) and 4 days (P < 0.01) compared with intact controls. Likewise, ADX’d females showed increased AVP mRNA after 2 (P < 0.0001) and 4 days (P < 0.0001).
Experiment 2. Effects of gonadectomy on ADX-induced PVN Crh and Avp expression
We next examined whether the sex-dependent effects of ADX on PVN Crh gene expression persist when gonadal hormone production is eliminated by gonadectomy (GDX; Fig. 3a and 3b). When paired with GDX, ADX significantly increased CRH mRNA levels compared with control levels in males (F(2,13) = 5.396; P < 0.05), and there was a strong trend in females (F(2,15) = 3.437; P = 0.0591). Post hoc tests also revealed similarities between the sexes, as both males and females had significantly increased CRH mRNA expression, 4 but not 2 days following surgery (males: P < 0.01; females: P < 0.05). Ultimately, GDX eliminated the sex difference in Crh gene expression at the 2-day ADX time point that was observed in Experiment 1. To ensure that this was not a consequence of use of ddPCR rather than in situ hybridization to measure mRNA levels, we also demonstrated that 2 days after ADX, males had significantly increased CRH (P < 0.05; Fig. 1e) and AVP (P < 0.01; Fig. 2e) mRNA when assessed by ddPCR.
Figure 3.
Changes in neuropeptide gene expression within the PVN following ADX and GDX of male and female mice. PVN CRH and AVP mRNA levels were evaluated in ADX’d and GDX’d male and female mice compared with sham-operated controls using ddPCR. (a and b) Absolute levels of PVN CRH mRNA in sham, 2-day ADX and GDX, or 4-day ADX and GDX (a) male or (b) female mice. (c and d) AVP mRNA levels in (c) male or (d) female groups. All values were normalized to amount of input cDNA. Each bar represents mean normalized gene expression ± SEM of 4 to 6 mice. *P < 0.05; **P < 0.01. OVX, ovariectomized.
In contrast to the response of CRH mRNA, GDX did not alter the effect of ADX on PVN AVP mRNA levels in males or females (Fig. 3c and 3d). The ADX and GDX combination significantly increased AVP mRNA levels compared with sham-operated controls in both males (F(2,12) = 7.578; P < 0.01) and females (F(2,12) = 4.674; P < 0.05). Moreover, post hoc analysis showed increases in AVP mRNA at both 2 (males: P < 0.05; females: P < 0.05) and 4 days (males: P < 0.01; females: P < 0.05) after surgery, irrespective of sex.
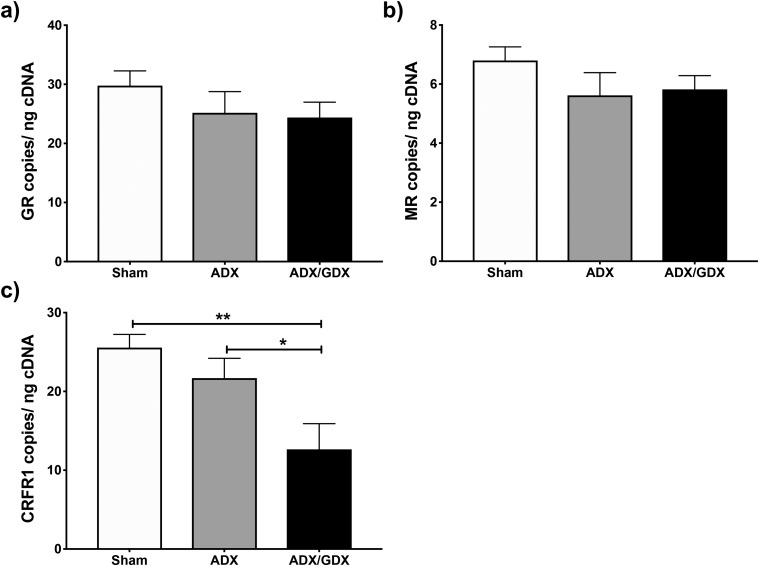
To investigate how GDX alters the Crh response to ADX after 2 days, we examined changes in GC and CRH receptor gene expression following 2-day ADX and ADX plus GDX. One-way ANOVAs revealed no effects of these procedures on GR or mineralocorticoid receptor (MR) mRNA levels (Fig. 4a and 4b). However, CRH receptor type 1 (Crfr1) expression was altered (F(2,14) = 6.915; P < 0.01), and post hoc analyses showed significant decreases in CRFR1 mRNA levels in ADX’d and GDX’d males compared with both sham-operated (P < 0.01) and ADX’d (P < 0.05) subjects (Fig. 4c).
Figure 4.
Changes in GC and CRH receptor gene expression within the PVN, 2 days following ADX or ADX plus GDX of male mice. PVN GR, MR, and CRFR1 mRNA levels were evaluated using ddPCR in ADX’d or ADX’d and GDX’d male mice and compared with those of sham-operated controls. Absolute levels of mRNA, normalized to the amount of input cDNA, are shown for PVN (a) GR, (b) MR, or (c) CRFR1. Each bar represents mean normalized gene expression ± SEM of n = 5 to 9 mice. *P < 0.05; **P < 0.01.
Experiment. 3. Effects of androgen and estrogen treatment on PVN Crh expression in ADX’d and GDX’d males
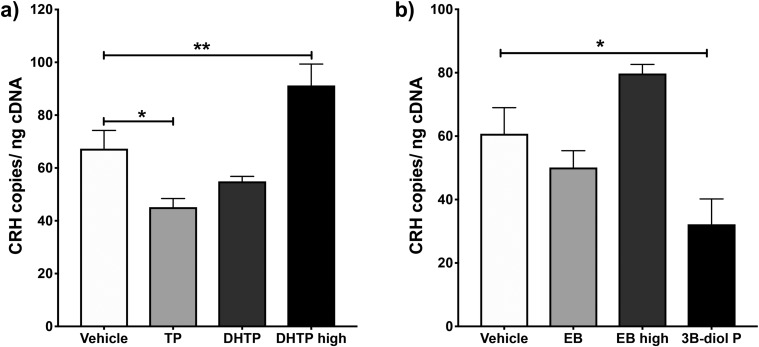
To determine if androgens facilitate the increase in PVN Crh expression observed following 2-day ADX, we examined the ability of TP and two doses of the nonaromatizable androgen DHTP to increase PVN CRH mRNA in ADX’d and GDX’d males (Fig. 5a). One-way ANOVA revealed a significant effect of androgen treatment on CRH mRNA levels overall (F(3,21) = 12.05; P < 0.0001). Specifically, TP treatment significantly decreased Crh gene expression (P < 0.05), whereas the higher dose of DHTP significantly increased it (P < 0.01), compared with that of vehicle-treated controls.
Figure 5.
Effects of androgen or estrogen treatment on Crh gene expression within the PVN of ADX’d and GDX’d males. (a) PVN CRH mRNA levels were measured in 2-day ADX’d and GDX’d male mice given TP (1 mg/kg BW, s.c.), DHTP (1 or 10 mg/kg BW, s.c.), or vehicle, daily for 2 days. (b) PVN CRH mRNA levels were measured 2 days following ADX and GDX of male mice who were also administered EB (25 or 250 μg/kg BW, s.c.), 3β-diol P (1 mg/kg BW, s.c.), or vehicle, daily for 2 days. Mean absolute levels of PVN CRH mRNA normalized to the amount of input cDNA ± SEM of n = 5 to 7 animals are shown. *P < 0.05; **P < 0.01.
We also examined the ability of EB and 3β-diol P, an androgen metabolite that binds estrogen receptor β (ERβ) (56), to alter PVN Crh expression in ADX’d and GDX’d males (Fig. 5b). Although one-way ANOVA showed a main effect of these treatments on CRH mRNA (F(3,18) = 7.05; P < 0.01), post hoc analysis revealed a significant decrease in CRH mRNA only in 3β-diol P-treated animals compared with vehicle-treated controls (P < 0.05).
AR expression in CRH neurons
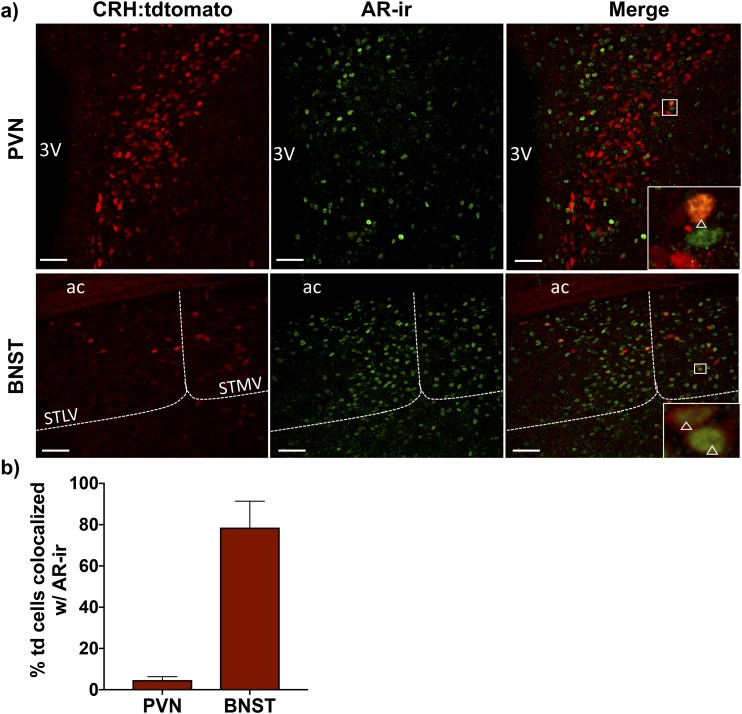
As DHTP treatment increased CRH mRNA, we next sought to determine if DHTP could act directly through ARs in PVN CRH neurons to increase Crh gene expression. We examined the expression of AR-ir in PVN CRH:tdtomato neurons (Fig. 6). tdtomato cells (4.69 ± 0.78%) coexpressed AR-ir throughout the rostral-caudal extent of the PVN (Fig. 6b). In the BNSTav; however, 78.60 ± 10.43% of tdtomato cells coexpressed AR-ir (Fig. 6b).
Figure 6.
AR expression in CRH neurons. (a) Representative photomicrographs of brain sections taken from Crh-IRES-Cre;Ai14 male mice containing the anterior PVN (Bregma −0.83 mm) and BNSTav (Bregma 0.13 mm) and immunolabeled for AR are shown. (Left) CRH:tdtomato neurons in both brain regions; (middle) AR-ir; and (right) merged images at ×20 magnification. Magnified insets highlight coexpression of CRH:tdtomato and AR-ir that is present in the BNST but not the PVN. Open triangles indicate CRH:tdtomato neurons that express AR-ir. Scale bars, 50 μm. STMV and STLV are collectively defined as the BNSTav, and dashed lines indicate the boundaries for each region. (b) Mean ± SEM percentages of CRH:tdTomato neurons (td) coexpressing AR-ir in the PVN and BNSTav of 3 mice are shown. 3V, third ventricle; ac, anterior commissure; STLV, bed nucleus of the stria terminalis, lateral division, ventral part; STMV, bed nucleus of the stria terminalis, medial division, ventral part.
Discussion
In these studies, we identified a sex difference in the response of a key HPA axis regulatory gene to the removal of circulating adrenal GCs and explored the underlying influence of gonadal hormones. Our findings indicate that whereas PVN Avp expression is upregulated following short-term ADX in both male and female mice, regardless of gonadal hormone levels, PVN Crh expression is increased in a sex-dependent fashion. Two days after ADX, CRH mRNA was upregulated in males but not females, and GDX eliminated this sex difference, such that the male pattern was now more like that of the female’s. When given the potent, nonaromatizable androgen DHTP, ADX’d and GDX’d males showed increased PVN CRH mRNA after 2 days, as observed following the 2-day, ADX-alone protocol. Taken together, these findings suggest that sex differences in the acute effects of ADX on PVN Crh arise as a result of DHT actions in males. Such effects of DHT are likely mediated by indirect actions of ARs in the PVN or in upstream brain regions, as we observed limited AR expression in CRH:tdtomato neurons of the PVN.
Previous studies examining the effects of ADX on PVN neuropeptide gene expression have reported increases in both CRH and AVP heterogeneous nuclear RNA (57), mRNA (57–59), and protein (60) levels. However, these studies have almost exclusively used male rats. The present studies fill a gap in our understanding of sex differences in the response to removal of GC-negative feedback by comparing both adult male and female ADX’d mice. In the short term, males responded more rapidly to the removal of GC-negative feedback than females, as we demonstrated that CRH mRNA increased by 2 days after ADX in males but not until 4 days in females. Yet, both sexes exhibited increased AVP mRNA levels at both 2 and 4 days following ADX. Thus, females may initially rely more on GC regulation of PVN Avp vs Crh to limit neuroendocrine stress responses. Supporting this, PVN AVP mRNA levels have been shown to be more sensitive to GC-negative feedback than are levels of CRH mRNA (58).
The more rapid increase in PVN CRH mRNA that we observed in males vs females after ADX was eliminated if mice were GDX’d at the time of ADX. Thus, gonadal hormones are important for facilitation of the CRH mRNA response to ADX in male mice. In contrast, previous studies that use ADX’d and GDX’d male rats have shown that GDX inhibits Avp, but not Crh, transcriptional responses to ADX (59, 61). A number of factors could contribute to this difference, including the species used or the duration of ADX and ADX/GDX. Viau et al. (59, 61) examined ADX’d and ADX’d/GDX’d rats, 1 to 2 weeks after surgery, whereas we studied more rapid changes occurring 2 or 4 days after surgery. Perhaps initially, PVN Crh is more sensitive to coregulation by gonadal hormones in the absence of GCs, and Avp eventually becomes more sensitive to this coregulation. A more discrete time course of the effects of ADX vs ADX/GDX on PVN Crh and Avp gene expression is necessary to assess this.
Although GDX may prevent the acute PVN Crh response to 2-day ADX in males via numerous mechanisms, one possibility is that it modifies the gene expression of GC and/or CRH receptors in the PVN to adjust the sensitivity of CRH neurons to regulation by GCs and CRH, respectively. We found no effect of short-term ADX or ADX plus GDX on expression of the GC receptors MR and GR. Yet, the combination of ADX and GDX decreased expression of the primary receptor for CRH, Crfr1, relative to levels found in sham-operated controls and in ADX’d subjects. CRFR1 expression has been identified in PVN CRH neurons, especially following stress, where it likely activates Crh transcription via its coupling to adenylate cyclase (14, 62–64). Additionally, a distinct population of CRFR1-expressing neurons has been found in the mouse PVN that inhibits the activity of nearby hypophysiotrophic CRH neurons (65–67). Thus, it is possible that GDX decreases CRFR1 mRNA in PVN CRH neurons or in a nearby population to eliminate the increase in Crh transcription found following ADX, but further investigation of the molecular mechanisms underlying these possibilities is necessary.
Our findings suggest that the potent androgen DHT is important for facilitation of the acute (2-day) Crh response to ADX in males, as treatment with DHTP increased CRH mRNA in ADX’d and GDX’d males. Notable discrepancies exist between our observed stimulatory effect of DHTP on Crh and a body of literature, suggesting that DHT suppresses ACTH and CORT secretion, partly by the decrease of Crh gene expression in the PVN (17, 23, 24, 68). Such discrepancies could reflect a dose dependency in the effects of DHT on components of the HPA axis, as a relatively lower dose of DHTP seemed to cause a slight, albeit nonsignificant (P = 0.1870), decrease in CRH mRNA in our studies, matching the conclusions of previous studies (17, 23, 24, 68). However, our findings related to treatment with a higher dose of DHTP do align well with studies demonstrating that DHT facilitates the Avp response to 1 week of ADX, as well as others that support a stimulatory or biphasic effect of DHT on the HPA axis (61). Stereotaxic placement of DHT in the BNST increases PVN Avp expression and stress-induced neuronal activation (69). Furthermore, GDX of male rats tends to decrease hypothalamic CRH content within the first week following surgery, whereas it significantly increases CRH content by week 3 (70). Although the potentially dose-dependent, excitatory effect of DHTP observed in the current studies is not unprecedented, the possibility that DHT has opposing effects, if administered at high and low doses, is certainly intriguing. There are several explanations for this apparent paradox that warrant further investigation, including the dose-dependent expression, activation, and/or recruitment of ARs and/or AR-interacting transcriptional coregulatory proteins. DHT, at lower levels, could also be converted to and act through 3β-diol to decrease PVN CRH mRNA, an effect that might be overcome at higher androgen doses.
Interestingly, DHTP increased, whereas TP decreased, PVN CRH mRNA in ADX’d and GDX’d males. Because DHT is produced following T reduction by the 5α-reductase enzyme, and T and DHT both bind ARs with high affinity (although DHT has higher affinity), T and DHT have often been reported to decrease similarly HPA activity (24, 40, 68, 71, 72). Yet, T can also be converted to estradiol in brain tissue by the aromatase enzyme and may act through estradiol to influence PVN neuropeptide expression (73). Our finding that neither a low nor a high dose of estradiol significantly altered PVN CRH mRNA in ADX’d and GDX’d males makes this scenario unlikely. Another possibility is that the differential expression and/or regulation of 5α-reductase in brain regions, known to modulate HPA activity, enable T to have distinct effects from DHT. Moreover, T, like DHT, could have dose-dependent effects on PVN CRH mRNA in these studies, but further investigation is ultimately necessary.
DHT can also influence the activity of the HPA axis through its metabolism in the brain and periphery to 3β-diol, which binds selectively to ERβ (68). However, DHTP and its dipropionated metabolite 3β-diol P had opposing effects on PVN CRH mRNA in ADX’d and GDX’d males. Such opposing influences may be a result of activation of different receptor types or activation of different receptor populations (56). 3β-Diol can act directly on ERβs in the PVN to inhibit the HPA axis response to stress, whereas DHT likely acts on AR containing brain regions upstream of the PVN, as neurons directly controlling the HPA axis largely do not express ARs (56, 74, 75). Additionally, regulation of 3β-diol-synthesizing enzymes in the PVN by GCs and/or gonadal hormones may determine whether DHT or 3β-diol has a predominate influence on PVN Crh gene expression. Our data indicate that neither 3β-hydroxysteroid dehydrogenase type 1 nor type 2 is expressed within neurons of the PVN regardless of GC or gonadal hormone status (data not shown). However, other enzymes (e.g., 3α-hydroxysteroid dehydrogenase) can act as surrogates for the production of 3β-diol and have been reported in the PVN (22, 68). Further investigation of 3β-diol synthesis in the PVN and in upstream brain regions will be important for the understanding of the opposing actions of DHT and 3β-diol in the acute regulation of the HPA axis.
In the present studies, we examined AR expression in CRH neurons by use of a transgenic mouse model (Crh-IRES-Cre;Ai14) in which CRH neurons stably express a fluorescent protein, tdtomato. This mouse model has been previously validated for the study of CRH neurons and enables accurate visualization of CRH without the need for manipulations typically used to enhance CRH-ir (33). With the use of this model, we demonstrated that ARs are found in <5% of CRH:tdtomato neurons of the mouse PVN, suggesting that DHT may act predominantly on ARs outside of CRH neurons in the PVN or in upstream brain regions to exert its unique, permissive effects on the Crh transcriptional response to ADX. A previous study conducted in rats demonstrated an absence of AR in CRH neurons of the hypothalamus, septum, BNST, and amygdala (76). However, this study likely did not examine entire populations of CRH neurons, as no method of enhancement of CRH expression, such as colchicine treatment or ADX, was used. With the use of the Crh-IRES-Cre;Ai14 mouse model, we have captured the entire population of CRH neurons to more accurately represent their coexpression of AR-ir. The coexpression of AR-ir in CRH neurons that we found in the mouse PVN, although limited, parallels the findings of a previous study showing AR colocalization in some CRH neurons of the human PVN (77). Given that we do not know the percentage of colocalization in the human PVN, whether our findings indicate an interesting species-related difference in the androgen regulation of HPA function at the level of the PVN remains to be determined (77).
Although colocalization of CRH:tdtomato and AR-ir was limited in the PVN, we observed robust AR labeling within and outside of CRH:tdtomato neurons in the BNSTav, a brain region well positioned to mediate the androgen regulation of the HPA axis. Anterior subdivisions of the BNST have neurons with AR expressing projections to the CRH containing parvocellular regions of the PVN (78). Additionally, the BNSTav includes substantial populations of PVN projecting CRH neurons that are functionally distinct from GABAergic neurons in the region and have been suggested to participate in the activation of the HPA axis (79–81). DHT may enhance the activity of these BNST CRH neurons to increase Crh gene expression in the PVN in the absence of GCs. Our findings show that GDX selectively decreases PVN Crfr1 expression when paired with ADX and support a role for CRH signaling in such androgen regulation of PVN Crh. Despite evidence for BNST to PVN CRH signaling, we cannot exclude the possibility that DHT inhibits the activity of GABAergic neurons in the BNSTav and other regions of the BNST that are not CRH expressing to increase PVN Crh expression following ADX. However, results of one study argued against such a mechanism of GABA disinhibition, as local DHT administration did not alter expression of GABA-synthesizing enzymes in the posterior BNST, a region thought to play an especially prominent role in the inhibition of the HPA axis (69). Nonetheless, our findings certainly warrant further investigation of the role that BNSTav CRH-expressing and -nonexpressing neurons may play in mediating androgen regulation of the HPA axis, particularly following the release from negative feedback.
Ultimately, the results of these studies have shown a sex difference in the regulation of PVN Crh that is revealed in the absence of GC-negative feedback and may depend on DHT actions outside of PVN CRH neurons in males. Our findings highlight the need for further systemic evaluation of central gonadal hormone effects on the HPA axis. Such studies will continue to advance our understanding of sex biases in the prevalence of stress-related pathologies.
Acknowledgments
Financial Support: This research was supported by the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health (Grant RO1-DK105826 to R.J.H.).
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- 3β-diol P
5α-androstane-3β, 17β-diol dipropionate
- AdU
arbitrary density unit
- ADX
adrenalectomy
- ADX’d
adrenalectomized
- Ai14
B6.Cg-Gt(ROSA)26Sortml4(CAG-TdTomato)Hze/J
- AR
androgen receptor
- AVP
arginine vasopressin
- BNST
bed nucleus of the stria terminalis
- BNSTav
anteroventral bed nucleus of the stria terminalis
- BW
body weight
- CORT
corticosterone
- CRFR1
CRH receptor type 1
- Crh-IRES-Cre
B6(Cg)-Crhtml(cre)Zjh/J
- Crh-IRES-Cre;Ai14
B6(Cg)-Crhtml(cre)Zjh/J crossing with B6.Cg-Gt(ROSA)26Sortml4(CAG-TdTomato)Hze/J
- CRH:tdtomato
CRH neurons that stably express a tdtomato fluorophore
- ddPCR
droplet digital PCR
- DHTP
DHT propionate
- EB
estradiol benzoate
- ERβ
estrogen receptor β
- GC
glucocorticoid
- GDX
gonadectomy
- GDX’d
gonadectomized
- GR
glucocorticoid receptor
- HPA
hypothalamic-pituitary-adrenal
- ir
immunoreactivity
- MR
mineralocorticoid receptor
- NGS
normal goat serum
- PVN
paraventricular nucleus
- RT
room temperature
- s.c.
subcutaneous
- T
testosterone
- TNT
Tris-NaCl-Tween
- TP
testosterone propionate
References and Notes
- 1. Munck A, Guyre PM, Holbrook NJ. Physiological functions of glucocorticoids in stress and their relation to pharmacological actions. Endocr Rev. 1984;5(1):25–44. [DOI] [PubMed] [Google Scholar]
- 2. Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev. 2000;21(1):55–89. [DOI] [PubMed] [Google Scholar]
- 3. Holsboer F. Stress, hypercortisolism and corticosteroid receptors in depression: implications for therapy. J Affect Disord. 2001;62(1-2):77–91. [DOI] [PubMed] [Google Scholar]
- 4. de Kloet ER, Joëls M, Holsboer F. Stress and the brain: from adaptation to disease. Nat Rev Neurosci. 2005;6(6):463–475. [DOI] [PubMed] [Google Scholar]
- 5. Herman JP, Figueiredo H, Mueller NK, Ulrich-Lai Y, Ostrander MM, Choi DC, Cullinan WE. Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Front Neuroendocrinol. 2003;24(3):151–180. [DOI] [PubMed] [Google Scholar]
- 6. Whitnall MH. Regulation of the hypothalamic corticotropin-releasing hormone neurosecretory system. Prog Neurobiol. 1993;40(5):573–629. [DOI] [PubMed] [Google Scholar]
- 7. Rivier C, Vale W. Interaction of corticotropin-releasing factor and arginine vasopressin on adrenocorticotropin secretion in vivo. Endocrinology. 1983;113(3):939–942. [DOI] [PubMed] [Google Scholar]
- 8. Schlosser SF, Almeida OF, Patchev VK, Yassouridis A, Elands J. Oxytocin-stimulated release of adrenocorticotropin from the rat pituitary is mediated by arginine vasopressin receptors of the V1b type. Endocrinology. 1994;135(5):2058–2063. [DOI] [PubMed] [Google Scholar]
- 9. Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981;213(4514):1394–1397. [DOI] [PubMed] [Google Scholar]
- 10. Lolait SJ, Stewart LQ, Jessop DS, Young WS III, O’Carroll AM. The hypothalamic-pituitary-adrenal axis response to stress in mice lacking functional vasopressin V1b receptors. Endocrinology. 2007;148(2):849–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Müller MB, Landgraf R, Preil J, Sillaber I, Kresse AE, Keck ME, Zimmermann S, Holsboer F, Wurst W. Selective activation of the hypothalamic vasopressinergic system in mice deficient for the corticotropin-releasing hormone receptor 1 is dependent on glucocorticoids. Endocrinology. 2000;141(11):4262–4269. [DOI] [PubMed] [Google Scholar]
- 12. Sawchenko PE, Swanson LW, Vale WW. Co-expression of corticotropin-releasing factor and vasopressin immunoreactivity in parvocellular neurosecretory neurons of the adrenalectomized rat. Proc Natl Acad Sci USA. 1984;81(6):1883–1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Antoni FA. Vasopressinergic control of pituitary adrenocorticotropin secretion comes of age. Front Neuroendocrinol. 1993;14(2):76–122. [DOI] [PubMed] [Google Scholar]
- 14. Aguilera G, Liu Y. The molecular physiology of CRH neurons. Front Neuroendocrinol. 2012;33(1):67–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ferrini MG, Grillo CA, Piroli G, de Kloet ER, De Nicola AF. Sex difference in glucocorticoid regulation of vasopressin mRNA in the paraventricular hypothalamic nucleus. Cell Mol Neurobiol. 1997;17(6):671–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Heck AL, Handa RJ. Sex differences in the hypothalamic-pituitary-adrenal axis’ response to stress: an important role for gonadal hormones. Neuropsychopharmacology. 2019;44(1):45–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Seale JV, Wood SA, Atkinson HC, Harbuz MS, Lightman SL. Gonadal steroid replacement reverses gonadectomy-induced changes in the corticosterone pulse profile and stress-induced hypothalamic-pituitary-adrenal axis activity of male and female rats. J Neuroendocrinol. 2004;16(12):989–998. [DOI] [PubMed] [Google Scholar]
- 18. Larkin JW, Binks SL, Li Y, Selvage D. The role of oestradiol in sexually dimorphic hypothalamic-pituitary-adrena axis responses to intracerebroventricular ethanol administration in the rat. J Neuroendocrinol. 2010;22(1):24–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Viau V, Bingham B, Davis J, Lee P, Wong M. Gender and puberty interact on the stress-induced activation of parvocellular neurosecretory neurons and corticotropin-releasing hormone messenger ribonucleic acid expression in the rat. Endocrinology. 2005;146(1):137–146. [DOI] [PubMed] [Google Scholar]
- 20. Babb JA, Masini CV, Day HE, Campeau S. Sex differences in activated corticotropin-releasing factor neurons within stress-related neurocircuitry and hypothalamic-pituitary-adrenocortical axis hormones following restraint in rats. Neuroscience. 2013;234:40–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Iwasaki-Sekino A, Mano-Otagiri A, Ohata H, Yamauchi N, Shibasaki T. Gender differences in corticotropin and corticosterone secretion and corticotropin-releasing factor mRNA expression in the paraventricular nucleus of the hypothalamus and the central nucleus of the amygdala in response to footshock stress or psychological stress in rats. Psychoneuroendocrinology. 2009;34(2):226–237. [DOI] [PubMed] [Google Scholar]
- 22. Handa RJ, Weiser MJ. Gonadal steroid hormones and the hypothalamo-pituitary-adrenal axis. Front Neuroendocrinol. 2014;35(2):197–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Viau V, Lee P, Sampson J, Wu J. A testicular influence on restraint-induced activation of medial parvocellular neurons in the paraventricular nucleus in the male rat. Endocrinology. 2003;144(7):3067–3075. [DOI] [PubMed] [Google Scholar]
- 24. Lund TD, Munson DJ, Haldy ME, Handa RJ. Androgen inhibits, while oestrogen enhances, restraint-induced activation of neuropeptide neurones in the paraventricular nucleus of the hypothalamus. J Neuroendocrinol. 2004;16(3):272–278. [DOI] [PubMed] [Google Scholar]
- 25. Lunga P, Herbert J. 17β-Oestradiol modulates glucocorticoid, neural and behavioural adaptations to repeated restraint stress in female rats. J Neuroendocrinol. 2004;16(9):776–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Paulmyer-Lacroix O, Héry M, Pugeat M, Grino M. The modulatory role of estrogens on corticotropin-releasing factor gene expression in the hypothalamic paraventricular nucleus of ovariectomized rats: role of the adrenal gland. J Neuroendocrinol. 1996;8(7):515–519. [DOI] [PubMed] [Google Scholar]
- 27. Solomon MB, Loftspring M, de Kloet AD, Ghosal S, Jankord R, Flak JN, Wulsin AC, Krause EG, Zhang R, Rice T, McKlveen J, Myers B, Tasker JG, Herman JP. Neuroendocrine function after hypothalamic depletion of glucocorticoid receptors in male and female mice. Endocrinology. 2015;156(8):2843–2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bangasser DA, Valentino RJ. Sex differences in stress-related psychiatric disorders: neurobiological perspectives. Front Neuroendocrinol. 2014;35(3):303–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. RRID:IMSR_JAX:012704, https://scicrunch.org/resolver/IMSR_JAX:012704.
- 30. RRID:IMSR_JAX:007914, https://scicrunch.org/resolver/IMSR_JAX:007914.
- 31. Taniguchi H, He M, Wu P, Kim S, Paik R, Sugino K, Kvitsiani D, Fu Y, Lu J, Lin Y, Miyoshi G, Shima Y, Fishell G, Nelson SB, Huang ZJ. A resource of Cre driver lines for genetic targeting of GABAergic neurons in cerebral cortex [published correction appears in Neuron. 2011;72(6):1091]. Neuron. 2011;71(6):995–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, Ng LL, Palmiter RD, Hawrylycz MJ, Jones AR, Lein ES, Zeng H. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci. 2010;13(1):133–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wamsteeker Cusulin JI, Füzesi T, Watts AG, Bains JS. Characterization of corticotropin-releasing hormone neurons in the paraventricular nucleus of the hypothalamus of Crh-IRES-Cre mutant mice. PLoS One. 2013;8(5):e64943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Smith JA, Wang L, Hiller H, Taylor CT, de Kloet AD, Krause EG. Acute hypernatremia promotes anxiolysis and attenuates stress-induced activation of the hypothalamic-pituitary-adrenal axis in male mice. Physiol Behav. 2014;136:91–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Walker LC, Cornish LC, Lawrence AJ, Campbell EJ. The effect of acute or repeated stress on the corticotropin releasing factor system in the CRH-IRES-Cre mouse: a validation study [published online ahead of print 26 September 2018]. Neuropharmacology. doi: 10.1016/j.neuropharm.2018.09.037. [DOI] [PubMed] [Google Scholar]
- 36. Goldman JM, Murr AS, Cooper RL. The rodent estrous cycle: characterization of vaginal cytology and its utility in toxicological studies. Birth Defects Res B Dev Reprod Toxicol. 2007;80(2):84–97. [DOI] [PubMed] [Google Scholar]
- 37. Lacombe A, Lelievre V, Roselli CE, Muller JM, Waschek JA, Vilain E. Lack of vasoactive intestinal peptide reduces testosterone levels and reproductive aging in mouse testis. J Endocrinol. 2007;194(1):153–160. [DOI] [PubMed] [Google Scholar]
- 38. Okaji Y, Tashiro Y, Gritli I, Nishida C, Sato A, Ueno Y, Del Canto Gonzalez S, Ohki-Koizumi M, Akiyama H, Nakauchi H, Hattori K, Heissig B. Plasminogen deficiency attenuates postnatal erythropoiesis in male C57BL/6 mice through decreased activity of the LH-testosterone axis. Exp Hematol. 2012;40(2):143–154. [DOI] [PubMed] [Google Scholar]
- 39. Lund TD, Munson DJ, Haldy ME, Handa RJ. Dihydrotestosterone may inhibit hypothalamo-pituitary-adrenal activity by acting through estrogen receptor in the male mouse. Neurosci Lett. 2004;365(1):43–47. [DOI] [PubMed] [Google Scholar]
- 40. Handa RJ, Kudwa AE, Donner NC, McGivern RF, Brown R. Central 5-alpha reduction of testosterone is required for testosterone’s inhibition of the hypothalamo-pituitary-adrenal axis response to restraint stress in adult male rats. Brain Res. 2013;1529:74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Blanqué R, Lepescheux L, Auberval M, Minet D, Merciris D, Cottereaux C, Clément-Lacroix P, Delerive P, Namour F. Characterization of GLPG0492, a selective androgen receptor modulator, in a mouse model of hindlimb immobilization. BMC Musculoskelet Disord. 2014;15(1):291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pak TR, Chung WCJ, Hinds LR, Handa RJ. Arginine vasopressin regulation in pre- and postpubertal male rats by the androgen metabolite 3beta-diol. Am J Physiol Endocrinol Metab. 2009;296(6):E1409–E1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Weiser MJ, Handa RJ. Estrogen impairs glucocorticoid dependent negative feedback on the hypothalamic-pituitary-adrenal axis via estrogen receptor alpha within the hypothalamus. Neuroscience. 2009;159(2):883–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. RRID:AB_2801269, https://scicrunch.org/resolver/AB_2801269.
- 45. Donner N, Handa RJ. Estrogen receptor beta regulates the expression of tryptophan-hydroxylase 2 mRNA within serotonergic neurons of the rat dorsal raphe nuclei. Neuroscience. 2009;163(2):705–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hayden RT, Gu Z, Ingersoll J, Abdul-Ali D, Shi L, Pounds S, Caliendo AM. Comparison of droplet digital PCR to real-time PCR for quantitative detection of cytomegalovirus. J Clin Microbiol. 2013;51(2):540–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hindson BJ, Ness KD, Masquelier DA, Belgrader P, Heredia NJ, Makarewicz AJ, Bright IJ, Lucero MY, Hiddessen AL, Legler TC, Kitano TK, Hodel MR, Petersen JF, Wyatt PW, Steenblock ER, Shah PH, Bousse LJ, Troup CB, Mellen JC, Wittmann DK, Erndt NG, Cauley TH, Koehler RT, So AP, Dube S, Rose KA, Montesclaros L, Wang S, Stumbo DP, Hodges SP, Romine S, Milanovich FP, White HE, Regan JF, Karlin-Neumann GA, Hindson CM, Saxonov S, Colston BW. High-throughput droplet digital PCR system for absolute quantitation of DNA copy number. Anal Chem. 2011;83(22):8604–8610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Miotke L, Lau BT, Rumma RT, Ji HP. High sensitivity detection and quantitation of DNA copy number and single nucleotide variants with single color droplet digital PCR. Anal Chem. 2014;86(5):2618–2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Pinheiro LB, Coleman VA, Hindson CM, Herrmann J, Hindson BJ, Bhat S, Emslie KR. Evaluation of a droplet digital polymerase chain reaction format for DNA copy number quantification. Anal Chem. 2012;84(2):1003–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. RRID:AB_11156085, https://scicrunch.org/resolver/AB_11156085.
- 51. Chen CV, Brummet JL, Jordan CL, Breedlove SM. Down, but not out: partial elimination of androgen receptors in the male mouse brain does not affect androgenic regulation of anxiety or HPA activity. Endocrinology. 2016;157(2):764–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Swift-Gallant A, Coome LA, Ramzan F, Monks DA. Nonneural androgen receptors affect sexual differentiation of brain and behavior. Endocrinology. 2016;157(2):788–798. [DOI] [PubMed] [Google Scholar]
- 53. RRID:AB_2336820, https://scicrunch.org/resolver/AB_2336820.
- 54. Paxinos G, Franklin K. Paxinos and Franklin’s The Mouse Brain in Stereotaxic Coordinates. Waltham, MA: Academic Press; 2013. [Google Scholar]
- 55. Gungor NZ, Paré D. Functional heterogeneity in the bed nucleus of the stria terminalis. J Neurosci. 2016;36(31):8038–8049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Handa RJ, Weiser MJ, Zuloaga DG. A role for the androgen metabolite, 5α-androstane-3β,17β-diol, in modulating oestrogen receptor β-mediated regulation of hormonal stress reactivity. J Neuroendocrinol. 2009;21(4):351–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ma XM, Aguilera G. Differential regulation of corticotropin-releasing hormone and vasopressin transcription by glucocorticoids. Endocrinology. 1999;140(12):5642–5650. [DOI] [PubMed] [Google Scholar]
- 58. Makino S, Smith MA, Gold PW. Increased expression of corticotropin-releasing hormone and vasopressin messenger ribonucleic acid (mRNA) in the hypothalamic paraventricular nucleus during repeated stress: association with reduction in glucocorticoid receptor mRNA levels. Endocrinology. 1995;136(8):3299–3309. [DOI] [PubMed] [Google Scholar]
- 59. Viau V, Chu A, Soriano L, Dallman MF. Independent and overlapping effects of corticosterone and testosterone on corticotropin-releasing hormone and arginine vasopressin mRNA expression in the paraventricular nucleus of the hypothalamus and stress-induced adrenocorticotropic hormone release. J Neurosci. 1999;19(15):6684–6693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Sawchenko PE. Adrenalectomy-induced enhancement of CRF and vasopressin immunoreactivity in parvocellular neurosecretory neurons: anatomic, peptide, and steroid specificity. J Neurosci. 1987;7(4):1093–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Viau V, Soriano L, Dallman MF. Androgens alter corticotropin releasing hormone and arginine vasopressin mRNA within forebrain sites known to regulate activity in the hypothalamic-pituitary-adrenal axis. J Neuroendocrinol. 2001;13(5):442–452. [DOI] [PubMed] [Google Scholar]
- 62. Jezova D, Ochedalski T, Glickman M, Kiss A, Aguilera G. Central corticotropin-releasing hormone receptors modulate hypothalamic-pituitary-adrenocortical and sympathoadrenal activity during stress. Neuroscience. 1999;94(3):797–802. [DOI] [PubMed] [Google Scholar]
- 63. Luo X, Kiss A, Makara G, Lolait SJ, Aguilera G. Stress-specific regulation of corticotropin releasing hormone receptor expression in the paraventricular and supraoptic nuclei of the hypothalamus in the rat. J Neuroendocrinol. 1994;6(6):689–696. [DOI] [PubMed] [Google Scholar]
- 64. Makino S, Schulkin J, Smith MA, Pacák K, Palkovits M, Gold PW. Regulation of corticotropin-releasing hormone receptor messenger ribonucleic acid in the rat brain and pituitary by glucocorticoids and stress. Endocrinology. 1995;136(10):4517–4525. [DOI] [PubMed] [Google Scholar]
- 65. Jiang Z, Rajamanickam S, Justice NJ. Local corticotropin-releasing factor signaling in the hypothalamic paraventricular nucleus. J Neurosci. 2018;38(8):1874–1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Justice NJ, Yuan ZF, Sawchenko PE, Vale W. Type 1 corticotropin-releasing factor receptor expression reported in BAC transgenic mice: implications for reconciling ligand-receptor mismatch in the central corticotropin-releasing factor system. J Comp Neurol. 2008;511(4):479–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ramot A, Jiang Z, Tian JB, Nahum T, Kuperman Y, Justice N, Chen A. Hypothalamic CRFR1 is essential for HPA axis regulation following chronic stress. Nat Neurosci. 2017;20(3):385–388. [DOI] [PubMed] [Google Scholar]
- 68. Lund TD, Hinds LR, Handa RJ. The androgen 5α-dihydrotestosterone and its metabolite 5α-androstan-3β, 17β-diol inhibit the hypothalamo-pituitary-adrenal response to stress by acting through estrogen receptor β-expressing neurons in the hypothalamus. J Neurosci. 2006;26(5):1448–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Bingham B, Myung C, Innala L, Gray M, Anonuevo A, Viau V. Androgen receptors in the posterior bed nucleus of the stria terminalis increase neuropeptide expression and the stress-induced activation of the paraventricular nucleus of the hypothalamus. Neuropsychopharmacology. 2011;36(7):1433–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Bingaman EW, Magnuson DJ, Gray TS, Handa RJ. Androgen inhibits the increases in hypothalamic corticotropin-releasing hormone (CRH) and CRH-immunoreactivity following gonadectomy. Neuroendocrinology. 1994;59(3):228–234. [DOI] [PubMed] [Google Scholar]
- 71. Viau V, Meaney MJ. The inhibitory effect of testosterone on hypothalamic-pituitary-adrenal responses to stress is mediated by the medial preoptic area. J Neurosci. 1996;16(5):1866–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Handa RJ, Nunley KM, Lorens SA, Louie JP, McGivern RF, Bollnow MR. Androgen regulation of adrenocorticotropin and corticosterone secretion in the male rat following novelty and foot shock stressors. Physiol Behav. 1994;55(1):117–124. [DOI] [PubMed] [Google Scholar]
- 73. Roselli CE, Abdelgadir SE, Resko JA. Regulation of aromatase gene expression in the adult rat brain. Brain Res Bull. 1997;44(4):351–357. [DOI] [PubMed] [Google Scholar]
- 74. Bingham B, Williamson M, Viau V. Androgen and estrogen receptor-β distribution within spinal-projecting and neurosecretory neurons in the paraventricular nucleus of the male rat. J Comp Neurol. 2006;499(6):911–923. [DOI] [PubMed] [Google Scholar]
- 75. Handa RJ, Burgess LH, Kerr JE, O’Keefe JA. Gonadal steroid hormone receptors and sex differences in the hypothalamo-pituitary-adrenal axis. Horm Behav. 1994;28(4):464–476. [DOI] [PubMed] [Google Scholar]
- 76. Bingaman EW, Baeckman LM, Yracheta JM, Handa RJ, Gray TS. Localization of androgen receptor within peptidergic neurons of the rat forebrain. Brain Res Bull. 1994;35(4):379–382. [DOI] [PubMed] [Google Scholar]
- 77. Bao AM, Fischer DF, Wu YH, Hol EM, Balesar R, Unmehopa UA, Zhou JN, Swaab DF. A direct androgenic involvement in the expression of human corticotropin-releasing hormone. Mol Psychiatry. 2006;11(6):567–576. [DOI] [PubMed] [Google Scholar]
- 78. Williamson M, Viau V. Androgen receptor expressing neurons that project to the paraventricular nucleus of the hypothalamus in the male rat. J Comp Neurol. 2007;503(6):717–740. [DOI] [PubMed] [Google Scholar]
- 79. Dong HW, Petrovich GD, Watts AG, Swanson LW. Basic organization of projections from the oval and fusiform nuclei of the bed nuclei of the stria terminalis in adult rat brain. J Comp Neurol. 2001;436(4):430–455. [DOI] [PubMed] [Google Scholar]
- 80. Choi DC, Furay AR, Evanson NK, Ostrander MM, Ulrich-Lai YM, Herman JP. Bed nucleus of the stria terminalis subregions differentially regulate hypothalamic-pituitary-adrenal axis activity: implications for the integration of limbic inputs. J Neurosci. 2007;27(8):2025–2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Radley JJ, Gosselink KL, Sawchenko PE. A discrete GABAergic relay mediates medial prefrontal cortical inhibition of the neuroendocrine stress response. J Neurosci. 2009;29(22):7330–7340. [DOI] [PMC free article] [PubMed] [Google Scholar]