Abstract
Objective
To compare mid‐term clinical results of total hip arthroplasty (THA) with metal‐on‐metal (MoM) and metal‐on‐polyethylene (MoP) bearings and to evaluate the biological safety of the two kinds of prostheses.
Methods
Thirty‐two patients who received a primary THA with an MoM articulation between January 2008 and December 2010 were selected to form the MoM group retrospectively. The MoP group consisted of 32 patients who received a THA with an MoP prosthesis during the same period. Clinical assessments, imaging examinations, laboratory tests, and metal ion concentration detections were conducted on each patient. Another 32 healthy volunteers were recruited as the control group.
Results
Twenty‐seven patients in the MoM group and 28 patients in the MoP group completed the follow‐up, with a mean follow‐up time of 74.6 and 75.9 months, respectively. The mean Harris score at the latest follow‐up was 91.5 ± 5.1 in the MoM group versus 88.9 ± 4.0 in the MoP group (P = 0.22). The MoM group showed a better range of motion in flexion, abduction, and external rotation. Co and Cr levels in the MoM group were 2.5‐fold and 2.0‐fold of these in the MoP group. A mild change of liver function was observed in both groups, while the values of renal function and humoral immunity stayed static. Elevated proportions of Th1 and Th17 cells and decreased proportion of Th2 cells were observed in the MoM group. The occurrence rate of pseudotumors in the MoM and MoP groups was 40.74% ± 9.45% and 14.28% ± 6.61%, respectively (P < 0.05).
Conclusion
At the mid‐term follow‐up, clinical results were satisfied in both groups. MoM prosthesis could result in elevated serum metal ion levels and there is a higher risk of pseudotumor. Long follow‐up is needed to evaluate the safety of MoM prostheses.
Keywords: Metal ions, Metal‐on‐metal hip arthroplasty, Metal‐on‐polyethylene hip arthroplasty, Pseudotumor, Total hip arthroplasty
Introduction
Total hip arthroplasty (THA) has been considered the most successful way to treat advanced arthritis since the late 20th century1. Due to the great improvement in the quality of life of patients, more younger patients, with high activity demands, have received THA in recent years2. However, the revision of failed THA has remained a challenge, especially in young patients3, 4. Research and modifications in wear surface were conducted to extend the service time and to reduce the revision risk of the implant.
Traditional THA with a metal‐on‐polyethylene (MoP) articulation has proved to have favorable early‐term outcomes5. However, the osteolysis and aseptic loosening induced by polyethylene wear particles limited the longer survivorship of the implant6. To improve the joint function and implant longevity, large‐diameter metal‐on‐metal (MoM) articulations were designed as an alternative option with less bearing wear and dislocation risk7, 8. Although this was supposed to provide better stability and mobility theoretically9, by the end of a median follow‐up of 5.7 years, the MoM and MoP implants achieved similar improvements in Harris hip score (HHS)10. Some authors assert that the early revision rates of MoM THA are higher than for conventional THA11, 12, while other studies report satisfying early results with low revision rates following MoM THA13, 14.
Concerns about biological exposure to potentially cytotoxic wear products, such as chromium (Cr), cobalt (Co), titanium (Ti), nickel, and vanadium, have long existed. Elevated serum Co, Cr, and Ti ion levels were observed in postoperative patients with MoM implants after a follow‐up of over 10 years15. Similar results are reported in other published studies16. The accumulation of excessive metal in the kidney and liver over time was harmful to hepatic and renal functions17, 18. In an in vitro study, Co and Cr ions could function as haptens to activate the immune response or as anti‐chemokines to suppress certain signaling pathways19. They could also function as lymphotoxin factors to induce lymphocyte apoptosis20.
Many recent articles have reported peri‐articular local soft tissue lesions or a soft mass in post‐THA patients with an MoM implant21. These soft tissue lesions could also be described as adverse local tissue reactions (ALTR)21, adverse reactions to metal debris (ARMD)22, pseudotumors23, aseptic lymphocytic vasculitis associated lesions (ALVAL)24, and metallosis25. Pseudotumors were often described as invasive and destructive. A pseudotumor would cause pain in the region of the hip, and limit the range of motion (ROM) of the affected hip, which would impact the postoperative clinical results. In some severe cases, a revision surgery was needed. The etiology of pseudotumors remained incompletely understood. Excessive bearing wear was considered responsible. However, recent reports revealed that pseudotumors could also be found in patients using MoP prostheses26, 27, 28, 29, 30, which was considered to be related with the wear and corrosion at modular THA junctions. The true risk of pseudotumors in MoM and non‐MoM THA remains unknown and needs to be further explored.
In our early study with a mean follow‐up of 2 years, the clinical outcomes of MoM and MoP implants resembled each other31. Elevated Co and Cr levels in patients with MoM implants were observed, while the liver and renal function of patients was unimpaired. Although results indicated a generalized lymphatic suppressive effect on patients with MoM implants, the subsequent effect on the immune system from different types of hip implants remained unclear.
The main purpose of our present study was to compare the mid‐term clinical results and metal ion concentrations of patients who received a primary arthroplasty with MoM and MoP prostheses. The potential impairment of kidney and liver was evaluated by comparing the postoperative and latest hepatic and renal functions. Humoral immunity and lymphocyte subgroup analysis were used to evaluate mid‐term effects from different implants on the immune system. Besides routine X‐ray examinations to detect component migration and osteolysis, metal artifact reduction sequence magnetic resonance imaging (MARS‐MRI) was used to detect the pseudotumor in both groups, to reveal the impact of different implants on pseudotumor formation.
Materials and Methods
Implants
The Durom cup (Zimmer, Warsaw, IN, USA) is a non‐modular acetabular component with a chromium–cobalt alloy bearing and a titanium plasma‐sprayed exterior surface for bone ingrowth. The implant is a 165° truncated hemisphere with an elliptical shape providing a 2‐mm press fit circumferentially for initial fixation.
Patients in the MoM group were treated with an acetabulum (46–54 mm) and a large‐diameter Co‐Cr alloy head (40–48 mm) component. The Trilogy cup (Zimmer) is a modular acetabular component made of titanium–aluminum–vanadium alloy with an exterior surface of titanium fiber‐metal mesh for bone ingrowth. The implant is a full 180° hemisphere and allows for additional screw fixation. It uses an extra high‐crosslinked polyethylene liner to form a metal‐on‐polyethylene bearing.
Patients in the MoP group were treated with an acetabulum (44–56 mm) and a 28‐mm Co‐Cr alloy head using a highly cross‐linked polyethylene liner. The Trabecular Metal Primary Hip Stem (Zimmer, Warsaw, IN, USA) is a tapered straight femoral prosthesis made up of titanium alloy. It loads the femur proximally with a biological fixation, which was enhanced by a titanium‐alloy wire coat. The VerSys Fiber Metal Taper Hip Stem (Zimmer, Warsaw, IN, USA) is also a tapered straight titanium alloy femoral prosthesis, with a biological fixation proximally enhanced by a porous titanium wire coat.
All patients in the MoM group were treated with a VerSys stem, the size of which ranged from 11 to 15, while 5 patients in the MoP group received the surgery with a Trabecular stem; the others were treated with a VerSys stem. The stem size of the MoP group also ranged from 11 to 15.
Inclusion and Exclusion Criteria
Inclusion criteria: (i) patients with osteoarthritis, femoral neck fracture, and osteonecrosis; (ii) patients treated with a unilateral primary THA; and (iii) patients aged over 18 years. Exclusion criteria: (i) poor general condition to receive the examinations; (ii) hepatic and renal dysfunction; (iii) a medical history of immunological diseases or allergic diseases; and (iv) a medical history of more than one metal implantation including a second arthroplasty or revision surgery.
Patients’ Data
We retrospectively analyzed all 43 patients who underwent a primary total hip arthroplasty with a Durom large‐head MoM non‐modular acetabular component hip implant between January 2008 and December 2010; 32 among them were chosen to form the MoM group. The indications for the implantation of the Durom prosthesis were osteoarthritis in 4 patients, osteonecrosis in 16, and femoral neck fracture in 12. Among 200 patients who underwent THA and received a Trilogy metalon polyethylene prosthesis implant during the same period, 32 were chosen to form the Trilogy group. The indications for the implantation of the Trilogy prosthesis were osteoarthritis in 19 patients, femoral neck fracture in 12, and osteonecrosis in 1.
A control group was made up of 32 healthy volunteers chosen from 80 healthy volunteers without a metal implantation history. The three groups were matched based on age, gender, and body mass index (BMI), and compared using analysis of variance (ANOVA) and the χ2‐test to ensure sample homogeneity.
Operative Technique
All the surgeries were performed by two senior surgeons using a modified Harding approach. The desired acetabular position was 45° of abduction and 15° of anteversion.
Ethical Approval
Before the follow‐up began, all aspects of the study were approved by the ethical committee of the institution. Each patient was fully informed about the study and the use of data. All patients provided their signed consent before undergoing the tests.
Clinical Evaluation
All patients in both groups underwent a physical examination and the results were evaluated using HHS and Medical Outcome Study Short Form‐36 scores (SF‐36).
Serum ion Level Measurement
All blood samples were collected from the median cubital veins using a 22‐gauge stainless steel needle (BD Medical, Sandy, UT, USA). The first 5 mL of blood was eliminated to avoid contamination. The serum was prepared from a 10‐mL whole blood sample after a 4‐h static duration and a 10‐min 1062 g centrifugation. The serum concentrations of chromium and cobalt ion of the three groups were tested by inductively coupled plasma mass spectrometry (ICP‐MS, PE, ELAN 9000, Perkin Elmer, Waltham, MA, USA). The detection limits were 0.001 μg/L for Cr and Co.
Hepatorenal Function
The circulating levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), blood urea nitrogen (BUN), and serum creatinine (Cr) of the three groups were determined at the latest follow‐up evaluation.
Humoral Immunity
The circulating C3, C4, IgA, IgG, and IgM levels of the three groups were detected through the turbidimetric immunoassay.
Lymphocyte Subgroup Analysis
The lymphocytes in the peripheral venous blood were counted and sorted by flow cytometry. A 20‐mL sample of venous blood was collected from each patient in all three groups and added to the anticoagulant heparin. After stimulation and cultivation with Leukocyte Activation Cocktail (BD Biosciences) in a 37°C humidified CO2 incubator for 6 h, the blood samples were stained with monoclonal antibodies against the surface markers CD3, CD4, and CD8 (BD Biosciences). Next, lysis buffer (BD Biosciences) was added to lyse the red blood cells. After the procedure of permeabilization and fixation, the lymphocytes were stained with monoclonal antibodies (BD Biosciences) against the intracellular markers Th1, Th2, and Th17. Finally, the samples were counted and sorted using a Gallios flow cytometer (Beckman Coulter, Indianapolis, IN, USA).
Cytokine Levels
Plasma from each patient was obtained from venous blood centrifuged at 1062 g for 5 min and stored at −80°C for the measurement of cytokines. All the plasma samples were thawed at room temperature before detection. Interleukin 4 (IL‐4) and interferon‐γ (INF‐γ) levels were assayed using the enzyme‐linked immunosorbent assay (ELISA) technique.
Radiographic Evaluation
Each patient received an anteroposterior supine radiograph of the pelvis and anteroposterior radiograph of the affected hip joint. All radiographs were evaluated by two observers. The migration or radiolucent lines of the acetabular part were evaluated using the standards described by Hartley et al.32, while the femoral part was evaluated using the standards from Martell et al.33. All the MARS‐MRI scans of the hip were taken using one MRI machine (GE, 1.5T Signa HDx, USA). The parameters of the MRI and diagnostic criteria of pseudotumors were as described in a study by Anderson et al.34.
Statistical Analysis
Student’s t‐test, Fisher’s exact test, and one‐way ANOVA were applied in this study. The results are expressed as mean ± SD. A level of significance was set at P = 0.05 for all statistical tests. All analyses were performed using the SPSS software (Version 23.0, SPSS, IBM, Armonk, NY, USA).
Results
Basic Patient Data
Twenty‐seven patients in the MoM group and 28 patients in the MoP group completed the follow‐up, with a mean follow‐up time of 74.6 and 75.9 months, respectively. There was no difference in mean age, sex ratio, and mean BMI for all three groups (Table 1) .
Table 1.
General characteristics of the three groups
| Groups | Number (recruited) | Number (completed) | Females (cases (%)) | Age at surgery (mean ± SD, years) | Follow‐up time (months) | Age at latest follow‐up (mean ± SD, years) | BMI (mean ± SD, kg/m2) |
|---|---|---|---|---|---|---|---|
| MoM | 32 | 27 | 18 (66.67) | 57.44 ± 12.25 | 80.6 (74–92) | 62.67 ± 11.51 | 23.26 ± 2.80 |
| MoP | 32 | 28 | 19 (67.85) | 59.16 ± 13.97 | 81.9 (72–106) | 62.36 ± 14.81 | 22.40 ± 3.65 |
| Control | 32 | 32 | 20 (62.5) | — | — | 63.03 ± 10.23 | 23.76 ± 2.72 |
Percentage of females: χ2 = 0.2126, P = 0.90. Age at latest follow‐up: F = 0.0206, P = 0.98. BMI: F = 0.8361, P = 0.44. BMI, body mass index; MoM, metal‐on‐metal; MoP, metal‐on‐polyethylene
Clinical Results
The mean HHS at the latest follow‐up in the MoM group was 91.5 ± 5.1, while in the MoP group this was 88.9 ± 4.0 (P = 0.2242). The MoM group showed better outcomes for flexion, abduction, and external rotation (Table 2).
Table 2.
The ROM of affected hips in MoM and MoP groups (mean ± SD, °)
| Groups | Flexion | Abduction | Adduction | External rotation | Internal rotation |
|---|---|---|---|---|---|
| MoM | 106.60 ± 11.21 | 33.33 ± 8.35 | 14.33 ± 3.72 | 41.25 ± 8.27 | 21.70 ± 4.55 |
| MoP | 91.92 ± 8.79 | 31.36 ± 7.78 | 11.67 ± 3.26 | 34.62 ± 8.03 | 20.94 ± 5.99 |
| P‐value | <0.01 | 0.03 | 0.57 | 0.04 | 0.38 |
| t‐value | 3.841 | 2.300 | 0.584 | 2.177 | 0.897 |
MoM, metal‐on‐metal; MoP, metal‐on‐polyethylene; ROM, range of motion.
The SF‐36 score in the MoM group at the latest follow‐up was significantly higher than that in the MoP group (654.6 ± 101.9 vs 569.1 ± 81.64, P = 0.02).
Metal Ion Levels
The serum Co levels in the three groups were 1.983 ± 0.899, 0.799 ± 0.371, and 0.332 ± 0.221 μg/L, while the serum Co levels were 3.613 ± 1.476, 1.789 ± 0.636, and 0.583 ± 0.332, respectively. The Co and Cr levels in the MoM group were 9.1 ± 4.1 and 6.2 ± 2.5 times those in the control group (both P < 0.01), while the Co and Cr levels in the MoP group were 3.7 ± 1.7 and 3.9 ± 1.1 times those in the control group (both P < 0.01). The Co and Cr levels in the MoM group were 2.5 ± 1.1 and 2.0 ± 0.8 times those in the MoP group at the latest follow‐up (both P < 0.01, Fig. 1, Table 3).
Figure 1.

Distribution of cobalt and chromium ion levels in metal‐on‐metal (MoM) and metal‐on‐polyethylene (MoP) groups.
Table 3.
The serum metal ion levels of three groups (mean ± SD, μg/L)
| Groups | Co | Cr |
|---|---|---|
| MoM | 1.983 ± 0.899 | 3.613 ± 1.476 |
| MoP | 0.799 ± 0.371 | 1.789 ± 0.636 |
| Control | 0.332 ± 0.221 | 0.583 ± 0.332 |
MoM, metal‐on‐metal; MoP, metal‐on‐polyethylene.
Hepatic and Renal Function
The values of ALT, AST, BUN, and creatinine of the MoM and MoP groups had no difference preoperatively. Comparing the values at the latest follow‐up in all three groups, a significant difference was only found in AST (P = 0.01), while no difference was found in ALT, BUN or creatinine (P = 0.50, 0.21, 0.09). AST values in MoM and MoP groups were higher than in the control group (P < 0.01, P = 0.03), but no difference was found between the former two (P = 0.74, Table 4).
Table 4.
The hepatic and renal functions of three groups (mean ± SD)
| Groups | ALT (IU/L) | AST (IU/L) | BUN (mmol/L) | Creatinine (μmol/L) |
|---|---|---|---|---|
| MoM | ||||
| Pre‐surgery | 20.48 ± 8.77 | 21.00 ± 5.30 | 4.27 ± 1.56 | 64.24 ± 16.33 |
| Final follow‐up | 21.77 ± 10.97 | 28.07 ± 6.11 | 4.36 ± 0.78 | 55.33 ± 13.64 |
| MoP | ||||
| Pre‐surgery | 20.03 ± 10.39 | 24.55 ± 4.71 | 5.08 ± 1.82 | 62.01 ± 7.81 |
| Final follow‐up | 21.53 ± 14.38 | 26.76 ± 9.50 | 5.72 ± 1.91 | 67.25 ± 10.46 |
| Control | 16.87 ± 8.41 | 19.45 ± 5.19 | 4.72 ± 1.07 | 57.48 ± 11.10 |
ALT: MoM (final follow‐up) vs MoP (final follow‐up) vs control, F = 0.7156, P = 0.50. AST: MoM (final follow‐up) vs MoP (final follow‐up) vs control, F = 5.168 P = 0.01. BUN: MoM (final follow‐up) vs MoP (final follow‐up) vs control, F = 2.000, P = 0.16. Creatinine: MoM (final follow‐up) vs MoP (final follow‐up) vs control, F = 1.989, P = 0.16. ALT, alanine aminotransferase; AST, aspartate aminotransferase; BUN, blood urea nitrogen; MoM, metal‐on‐metal; MoP, metal‐on‐polyethylene.
Humoral Immunity
There were no differences in C3, C4, IgA, IgG, and IgM levels in the three groups at the latest follow‐up (MoM vs MoP, P = 0.64, 0.69, 0.64, 0.63, 0.37; Table 5).
Table 5.
The humoral immune functions of the three groups (mean ± SD, g/L)
| Groups | C3 | C4 | IgA | IgG | IgM |
|---|---|---|---|---|---|
| MoM | 1.12 ± 0.15 | 0.27 ± 0.09 | 2.70 ± 0.79 | 13.37 ± 2.61 | 1.13 ± 0.63 |
| MoP | 1.15 ± 0.29 | 0.29 ± 0.04 | 2.51 ± 0.18 | 13.98 ± 1.78 | 0.81 ± 0.29 |
| Control | 1.06 ± 0.10 | 0.27 ± 0.08 | 1.97 ± 0.58 | 11.58 ± 1.63 | 0.76 ± 0.44 |
| F‐value | 2.511 | 0.1139 | 3.07 | 1.285 | 2.833 |
| P‐value | 0.10 | 0.89 | 0.07 | 0.30 | 0.08 |
MoM, metal‐on‐metal; MoP, metal‐on‐polyethylene.
Lymphocyte Subgroups and Cytokine Levels
We took the number of CD3+ cells to indicate the number of T‐lymphocytes in peripheral venous blood and found a significant decrease both in the MoM group and the MoP group compared with controls (MoM vs control, P = 0.01; MoP vs control, P = 0.03). However, there was no significant difference in the CD3+ proportion between the former two groups (P = 0.73). The proportions of the CD4+ and CD8+ in CD3+ cells, and CD4+/CD8+ ratios were counted, respectively. A significant decrease of the CD4+ proportion was observed in both MoM and MoP groups when compared with the control (MoM vs control, P < 0.01; MoP vs control, P < 0.01), and still no significant difference was found between the former two (P = 0.99). Correspondingly, there was a significant increase of CD8+ proportion in both MoM and MoP groups compared with the control (MoM vs control, P < 0.01; MoP vs control, P < 0.01), and no difference between the former two (P = 0.7641). Both the MoM and MoP group showed a decrease of the CD4+/CD8+ ratio compared with the control (MoM vs control, P < 0.01; MoP vs control, P < 0.01) but there was no difference between the former two groups (P = 0.89, Table 6).
Table 6.
The proportions of CD3+, CD4+ and CD8+, and CD4+/CD8+ ratio of the three groups (mean ± SD)
| Groups | CD3+ (%) | CD4+ (%) | CD8+ (%) | CD4+/CD8+ |
|---|---|---|---|---|
| MoM | 50.88 ± 6.49 | 48.41 ± 11.98 | 46.69 ± 10.37 | 1.13 ± 0.54 |
| MoP | 49.67 ± 5.01 | 48.50 ± 10.42 | 48.46 ± 10.29 | 1.09 ± 0.53 |
| Control | 60.19 ± 9.15 | 65.13 ± 8.13 | 48.46 ± 10.29 | 2.12 ± 0.69 |
| F‐value | 5.351 | 8.968 | 12.12 | 8.945 |
| P‐value | 0.01 | <0.01 | <0.01 | <0.01 |
MoM, metal‐on‐metal; MoP, metal‐on‐polyethylene.
The proportion of Th1 cells in the MoM group was highest, while this value in the control group was the lowest (MoM vs MoP: P < 0.01; MoM vs control: P < 0.01; MoP vs control: P < 0.01). The proportion of Th2 cells in the MoM group was lower than in the MoP group or the control group. No significant difference was found in the latter two groups. (MoM vs MoP: P < 0.01; MoM vs control: P = 0.02; MoP vs control: P = 0.13). The proportion of Th17 cells in the MoM group was higher than in the MoP group or the control group. Still, no significant difference was found in the latter two groups. (MoM vs MoP: P < 0.01; MoM vs control: P < 0.01; MoP vs control: P = 0.71). The MoM group showed the highest Th1/Th2 ratio and no significant difference was found between the MoP group and the control group (MoM vs MoP: P < 0.01; MoM vs control: P < 0.01; MoP vs control: P = 0.52; Table 7).
Table 7.
The proportions Th1, Th2, Th17, and Th1/Th2 ratios of the three groups (mean ± SD)
| Groups | Th1 (%) | Th2 (%) | Th17 (%) | Th1/Th2 |
|---|---|---|---|---|
| MoM | 6.0 ± 2.1 | 1.5 ± 0.3 | 0.9 ± 0.2 | 4.1 ± 1.7 |
| MoP | 2.8 ± 1.1 | 2.3 ± 1.1 | 0.4 ± 0.1 | 1.4 ± 0.7 |
| Control | 1.9 ± 0.5 | 1.9 ± 0.8 | 0.5 ± 0.2 | 1.3 ± 1.0 |
| F‐value | 68.05 | 6.451 | 20.12 | 36.96 |
| P‐value | <0.01 | <0.01 | <0.01 | <0.01 |
MoM, metal‐on‐metal; MoP, metal‐on‐polyethylene.
The MoM group showed a lower INF‐γ level than the other two groups, while no difference was found between the MoP group and the control group (MoM vs MoP: P = 0.02; MoM vs control: P < 0.01; MoP vs control: P = 0.66). The MoM group showed the lowest IL‐4 level, while no significant difference was found between the MoP group and the control group (MoM vs MoP: P<0.01; MoM vs control: P < 0.01; MoP vs control: P = 0.09; Table 8).
Table 8.
The interferon (IFN)‐γ and interleukin 4 (IL‐4) levels of the three groups (mean ± SD, pg/mL)
| Groups | INF‐γ | IL‐4 |
|---|---|---|
| MoM | 327.30 ± 92.07 | 367.20 ± 67.41 |
| MoP | 234.40 ± 50.80 | 231.90 ± 128.70 |
| Control | 135.10 ± 51.13 | 189.20 ± 49.19 |
| F‐value | 61.31 | 4.555 |
| P‐value | <0.01 | <0.01 |
MoM, metal‐on‐metal; MoP, metal‐on‐polyethylene.
Radiological Evaluation
There was no significant difference in acetabular abduction between MoM and MoP groups on anteroposterior pelvic X‐rays (MoM: 47.30° ± 6.51° vs MoP: 48.25° ± 5.613°; P = 0.7829). No radiolucent line was observed in the MoM group but was found in 3 patients in the MoP group (2 on the top of the acetabular bone and 1 at the greater trochanter; Figs 2 and 3).
Figure 2.

A, Anteroposterior X‐ray of hip joint at 2 days after the surgery. B, 58 months after the surgery, we found that the acetabular component was migrated slightly to the upper and medial side and a radiolucent line could be observed at the rim of the acetabulum.
Figure 3.

A, Anteroposterior X‐ray of hip joint 3 days after the surgery. B, 58 months after the surgery, a radiolucent line could be observed at the lateral side of the greater trochanter.
In the MoM group, 11 patients developed a peri‐prosthesis pseudotumor, including 2 C3 level cases, 6 C2 level cases, and 3 C1 level cases. In the MoP group, 4 patients developed pseudotumors according to the same diagnostic criteria, including 1 C3 level case, 2 C2 level cases, and 1 C1 level case. The occurrence rate of pseudotumors in the MoM and MoP groups was, respectively, 40.74% ± 9.45% and 14.28% ± 6.61% (u = 2.2032, P < 0.05; Figs 4 and 5).
Figure 4.

A metal artifact reduction sequence (MARS)‐MRI scan showing a pseudotumor in 1 patient 74 months after the primary total hip arthroplasty with a metal‐on‐metal (MoM) prosthesis.
Figure 5.
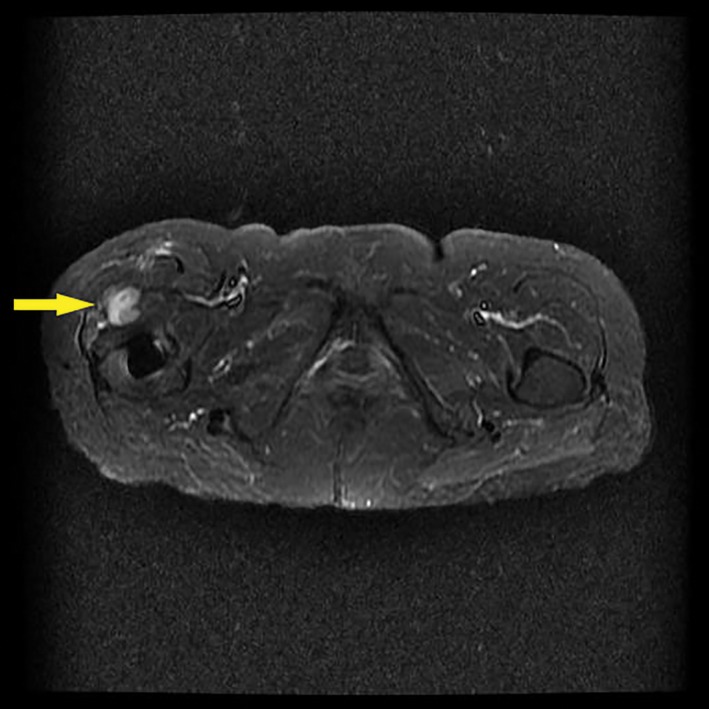
A metal artifact reduction sequence (MARS)‐MRI scan showing a pseudotumor in 1 patient 66 months after the primary total hip arthroplasty with a metal‐on‐polyethylene (MoP) prosthesis.
Discussion
Clinical Outcomes
At the mid‐term follow‐up, the HHS of MoM and the MoP groups were similar, with little decrease compared with the results of our former follow‐up in 2011. Compared with the MoP group, patients in the MoM group had a better range of motion in flexion, abduction, and external rotation. This may explain why the SF‐36 scores in the MoM group were higher than in the MoP group. In a multi‐centered follow‐up of over 20 000 primary total hip arthroplasties covering over 10 years, Furnes et al. found that the survival rate of MoM prostheses was similar to that for the MoP prostheses at 2 years postoperatively. However, the survival rates for both gradually decreased thereafter and the survival rate of the MoM prosthesis dropped faster. At 6 years postoperatively, the survival rate for MoM prostheses was only 93%, compared to 96% for MoP prostheses35. Compared with other published studies, the survival rate in our follow‐up was higher. Besides the impact of the small sample size, a more cautious postoperative lifestyle of Chinese patients may prolong the use of the prosthesis.
Imaging Results
Compared to traditional polyethylene, highly cross‐linked polyethylene (HXLPE) improved the wearing of the liner greatly5. However, in our study, osteolysis could still be found in the MoP group. Comparing the X‐ray images taken shortly after the operation and at follow‐up, we observed that 2 patients in the MoP group had a radiolucent line at the acetabular bone and 1 patient in the MoP group had a radiolucent line at the lateral wall of the femoral cavity, while no osteolysis was found in the MoM group.
Metal Ion Concentration
At the mid‐term follow‐up, an elevated metal ion level was observed in both MoM and MoP groups, which only appeared in the MoM group at the early follow‐up. Along with the wear between the head and the acetabular component, the bio‐chemical corrosion occurring around the prosthesis and the mechanical wear between the taper junction may have also contributed to this result. According to Kwon et al., edge‐loading caused much higher stress between the acetabular rim and the head, thus resulting in the production of more wear particles 36.
Hepatic and Renal Function
The increased AST levels in MoM and MoP groups indicated that the elevated Co and Cr ion levels had already impaired the liver function, while the effect on renal function may need longer follow‐up.
Immunological Changes
At the latest follow‐up, all evidence proved that no humoral immunity deficiency occurred in post‐THA patients. However, the T lymphocyte counts and the CD4+/CD8+ ratios in the MoM and MoP groups decreased significantly compared to the control group, which suggested a general suppressive effect on cellular immunity. An in vivo test conducted by Briggs et al. demonstrated that early non‐lethal and lethal chromosomal aberrations followed both MoM and MoP THA, which may lead to the necrosis and apoptosis of lymphocytes 37.
Interferon (IFN)‐γ induces the Th0 cells to differentiate into Th1 cells and inhibits the proliferation of Th2 cells, while IL‐4 induces Th0 to differentiate into Th2 cells and inhibits Th1 cells. The dynamic balance and mutual restraint of IFN‐γ and IL‐4 is of great importance in maintaining homeostasis of the immune system. Th1 cells can secrete IFN‐γ and IL‐2 and mainly participate in cellular immunity with delayed hypersensitivity reaction. Th2 cells mainly secrete IL‐4, IL‐6, and IL‐10, which assist B lymphocytes to secrete antibodies. Th17 cells secrete IL‐17, which induces inflammatory reactions and autoimmune responses, including graft rejection. From the changes in subpopulations of Th cells, we could learn how the metal ions affect the immune system and induce inflammation. In our study, the MoM group showed a significant increase in Th1/Th2 ratio and the proportion of Th17 cells was also elevated. The IFN‐γ level was elevated, while the IL‐4 levels decreased in the MoM group. According to Lawrence et al., the binding of Co ions to TLR‐4 can activate the secretion of IL‐8 and CXCL‐10. These cytokines induce the inflammatory response related with T lymphocytes 38. Caicedo et al. revealed that metal ions, including Co2+ and Cr3+, played a role in regulating some important signal pathways to secrete inflammatory mediators and induce inflammation19. The evidence suggested that an inflammation‐related immune response occurred in patients who received an MoM arthroplasty. In the MoP group, we also found an elevated IFN‐γ level. Lin et al. proved in their study that polyethylene particles only promote the secretion of IFN‐γ and have no effect on IL‐439. Goodman presented an opinion on the factor that induces different immune responses. Polyethylene particles were found to be larger in diameter and to induce an initial immune response, while metal particles were found in a much larger amount and to induce both initial and specific immune responses 40.
Pseudotumor
From the systematic review of the literature by Wiley et al., the occurrence of pseudotumors or ALVAL in different studies was as high as 6.5%, with a pooled estimate of 0.6% (95% CI, 0.3% to 1.2%)24. Matharu et al. revealed that the risk of ARMD revision surgery in all implanted primary hip replacements was 0.38% (3,340/873,188; 95% CI, 0.37%–0.40%). The risk of ARMD revision surgery in all non‐MoM hip replacements recorded in the NJR was 0.032% (249/789,397; 95% CI, 0.028%–0.036%), compared to 3.7% (3,091/83,791; 95% CI, 3.6%–3.8%) in MoM hip replacements41. In our study, the occurrence rate of pseudotumors in the MoM group seemed higher compared with other published studies, and we found no variance in the occurrence rate of pseudotumors between MoM and MoP groups. As well as the limited sample size possibly affecting the final result, differences in detection methods (e.g. ultrasound, computed tomography, and MARS‐MRI) could affect the detection rate of pseudotumors42, 43.
The limitations of our study are as follows. First, patients withdrew from the mid‐term follow‐up for different reasons, including lost contact and death. A withdraw bias may affect the final results. Second, this was a retrospective follow‐up study and, thus, lacked preoperative data, including HHS scores, metal ion levels, immunological functions, and lymphocyte counts. Third, the sample size was small, and the data for individuals varied. Large sample, prospective, randomized controlled studies are recommended to achieve more accurate results. Finally, except for the only revision surgery in the MoP group, all the diagnoses of pseudotumors were based on non‐invasive examinations. Although we ruled out the effect of periprothetic joint infection on our results, it was still hard to distinguish the pseudotumor from the scar tissue. A synovial fluid test and a tissue biopsy were recommended in each hip involved in this study to acquire a precise diagnosis and to help better investigate the pathogenesis between pseudotumor and inflammation. In conclusion, through a mid‐term follow‐up, similar clinical results were observed in MoM and MoP prostheses. Both kinds of prosthesis resulted in an elevated metal ion concentration, which had a more severe effect in patients with an MoM articulation. The effect of metal ions on the immune system and pseudotumors in post‐THA patients should be monitored continuously and further investigated.
Disclosure: No funds were received in support of this work.
References
- 1. Learmonth ID, Young C, Rorabeck C. The operation of the century: total hip replacement. Lancet, 2007, 370: 1508–1519. [DOI] [PubMed] [Google Scholar]
- 2. Daras M, Macaulay W. Total hip arthroplasty in young patients with osteoarthritis. Am J Orthop (Belle Mead NJ), 2009, 38: 125–129. [PubMed] [Google Scholar]
- 3. Mäkelä KT, Eskelinen A, Pulkkinen P, Paavolainen P, Remes V. Results of 3,668 primary total hip replacements for primary osteoarthritis in patients under the age of 55 years. Acta Orthop, 2011, 82: 521–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Philpott A, Weston‐Simons JS, Grammatopoulos G, et al Predictive outcomes of revision total hip replacement–a consecutive series of 1176 patients with a minimum 10‐year follow‐up. Maturitas, 2014, 77: 185–190. [DOI] [PubMed] [Google Scholar]
- 5. Hanna SA, Somerville L, McCalden RW, Naudie DD, MacDonald SJ. Highly cross‐linked polyethylene decreases the rate of revision of total hip arthroplasty compared with conventional polyethylene at 13 years’ follow‐up. Bone Joint J, 2016, 98: 28–32. [DOI] [PubMed] [Google Scholar]
- 6. Harris WH. The problem is osteolysis. Clin Orthop Relat Res, 1995, 311: 46–53. [PubMed] [Google Scholar]
- 7. Dowson D, Hardaker C, Flett M, Isaac GH. A hip joint simulator study of the performance of metal‐on‐metal joints: Part II: design. J Arthroplasty, 2004, 19: 124–130. [DOI] [PubMed] [Google Scholar]
- 8. Lombardi AV Jr, Skeels MD, Berend KR, Adams JB, Franchi OJ. Do large heads enhance stability and restore native anatomy in primary total hip arthroplasty?. Clin Orthop Relat Res, 2011, 469: 1547–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Burroughs BR, Hallstrom B, Golladay GJ, Hoeffel D, Harris WH. Range of motion and stability in total hip arthroplasty with 28‐, 32‐, 38‐, and 44‐mm femoral head sizes. J Arthroplasty, 2005, 20: 11–19. [DOI] [PubMed] [Google Scholar]
- 10. Lombardi AV Jr, Mallory TH, Cuckler JM, Williams J, Berend KR, Smith TM. Mid‐term results of a polyethylene‐free metal‐on‐metal articulation. J Arthroplasty, 2004, 19: 42–47. [DOI] [PubMed] [Google Scholar]
- 11. Fabi D, Levine B, Paprosky W, et al Metal‐on‐metal total hip arthroplasty: causes and high incidence of early failure. Orthopedics, 2012, 35: e1009–e1016. [DOI] [PubMed] [Google Scholar]
- 12. Smith AJ, Dieppe P, Vernon K, Porter M, Blom AW. Failure rates of stemmed metal‐on‐metal hip replacements: analysis of data from the National Joint Registry of England and Wales. Lancet, 2012, 379: 1199–1204. [DOI] [PubMed] [Google Scholar]
- 13. Innmann MM, Gotterbarm T, Kretzer JP, et al Minimum ten‐year results of a 28‐mm metal‐on‐metal bearing in cementless total hip arthroplasty in patients fifty years of age and younger. Int Orthop, 2014, 38: 929–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Meding JB, Meding LK, Keating EM, Berend ME. Low incidence of groin pain and early failure with large metal articulation total hip arthroplasty. Clin Orthop Relat Res, 2012, 470: 388–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Levine BR, Hsu AR, Skipor AK, et al Ten‐year outcome of serum metal ion levels after primary total hip arthroplasty: a concise follow‐up of a previous report. J Bone Joint Surg Am, 2013, 95: 512–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Smeekes C, Ongkiehong B, van der Wal B, Wolterbeek R, Henseler JF, Nelissen R. Large fixed‐size metal‐on‐metal total hip arthroplasty: higher serum metal ion levels in patients with pain. Int Orthop, 2015, 39: 631–638. [DOI] [PubMed] [Google Scholar]
- 17. Cheung AC, Banerjee S, Cherian JJ, et al Systemic cobalt toxicity from total hip arthroplasties: review of a rare condition part 1 ‐ history, mechanism, measurements, and pathophysiology. Bone Joint J, 2016, 98: 6–13. [DOI] [PubMed] [Google Scholar]
- 18. Zywiel MG, Cherian JJ, Banerjee S, et al Systemic cobalt toxicity from total hip arthroplasties: review of a rare condition part 2. Measurement, risk factors, and step‐wise approach to treatment. Bone Joint J, 2016, 98: 14–20. [DOI] [PubMed] [Google Scholar]
- 19. Caicedo MS, Desai R, McAllister K, Reddy A, Jacobs JJ, Hallab NJ. Soluble and particulate Co‐Cr‐Mo alloy implant metals activate the inflammasome danger signaling pathway in human macrophages: a novel mechanism for implant debris reactivity. J Orthop Res, 2009, 27: 847–854. [DOI] [PubMed] [Google Scholar]
- 20. Chattopadhyay S, Dash SK, Tripathy S, et al Toxicity of cobalt oxide nanoparticles to normal cells; an in vitro and in vivo study. Chem Biol Interact, 2015, 226: 58–71. [DOI] [PubMed] [Google Scholar]
- 21. Chalmers BP, Perry KI, Taunton MJ, Mabry TM, Abdel MP. Diagnosis of adverse local tissue reactions following metal‐on‐metal hip arthroplasty. Curr Rev Musculoskelet Med, 2016, 9: 67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Langton DJ, Jameson SS, Joyce TJ, Hallab NJ, Natu S, Nargol AV. Early failure of metal‐on‐metal bearings in hip resurfacing and larger‐diameter total hip replacement: a consequence of excess wear. J Bone Joint Surg Br, 2010, 92: 38–46. [DOI] [PubMed] [Google Scholar]
- 23. Pandit H, Glyn‐Jones S, McLardy‐Smith P, et al Pseudotumours associated with metal‐on‐metal hip resurfacings. J Bone Joint Surg Br, 2008, 90: 847. [DOI] [PubMed] [Google Scholar]
- 24. Wiley KF, Ding K, Stoner JA, Teague DC, Yousuf KM. Incidence of pseudotumor and acute lymphocytic vasculitis associated lesion (ALVAL) reactions in metal‐on‐metal hip articulations: a meta‐analysis. J Arthroplasty, 2013, 28: 1238–1245. [DOI] [PubMed] [Google Scholar]
- 25. Neumann DR, Thaler C, Hitzl W, Huber M, Hofstädter T, Dorn U. Long term results of a contemporary metal‐on‐metal total hip arthroplasty. J Arthroplasty, 2010, 25: 700–708. [DOI] [PubMed] [Google Scholar]
- 26. Leigh W, O’Grady P, Lawson EM, Hung NA, Theis JC, Matheson J. Pelvic pseudotumor: an unusual presentation of an extra‐articular granuloma in a well‐fixed total hip arthroplasty. J Arthroplasty, 2008, 23: 934–938. [DOI] [PubMed] [Google Scholar]
- 27. Mao X, Tay GH, Godbolt DB, Crawford RW. Pseudotumor in a well‐fixed metal‐on‐polyethylene uncemented hip arthroplasty. J Arthroplasty, 2012, 27: e13–e17. [DOI] [PubMed] [Google Scholar]
- 28. Murgatroyd SE. Pseudotumor presenting as a pelvic mass: a complication of eccentric wear of a metal on polyethylene hip arthroplasty. J Arthroplasty, 2012, 27: e1–e4. [DOI] [PubMed] [Google Scholar]
- 29. Sporer SM, Bernini PM. Extensive periacetabular osteolysis presenting as a mass on rectal examination. A case report. J Bone Joint Surg Am, 2002, 84: 1439–1441. [DOI] [PubMed] [Google Scholar]
- 30. Walsh AJ, Nikolaou VS, Antoniou J. Inflammatory pseudotumor complicating metal‐on‐highly cross‐linked polyethylene total hip arthroplasty. J Arthroplasty, 2012, 27: e5–e8. [DOI] [PubMed] [Google Scholar]
- 31. Chen Z, Wang Z, Wang Q, Cui W, Liu F, Fan W. Changes in early serum metal ion levels and impact on liver, kidney, and immune markers following metal‐on‐metal total hip arthroplasty. J Arthroplasty, 2014, 29: 612–616. [DOI] [PubMed] [Google Scholar]
- 32. Hartley WT, McAuley JP, Culpepper WJ, Engh CA Jr, Engh CA Sr. Osteonecrosis of the femoral head treated with cementless total hip arthroplasty. J Bone Joint Surg Am, 2000, 82: 1408–1413. [DOI] [PubMed] [Google Scholar]
- 33. Martell JM, Pierson RH 3rd, Jacobs JJ, Rosenberg AG, Maley M, Galante JO. Primary total hip reconstruction with a titanium fiber‐coated prosthesis inserted without cement. J Bone Joint Surg Am, 1993, 75: 554–571. [DOI] [PubMed] [Google Scholar]
- 34. Anderson H, Toms AP, Cahir JG, Goodwin RW, Wimhurst J, Nolan JF. Grading the severity of soft tissue changes associated with metal‐on‐metal hip replacements: reliability of an MR grading system. Skeletal Radiol, 2011, 40: 303–307. [DOI] [PubMed] [Google Scholar]
- 35. Furnes O, Paxton E, Cafri G, et al Distributed analysis of hip implants using six national and regional registries: comparing metal‐on‐metal with metal‐on‐highly cross‐linked polyethylene bearings in cementless total hip arthroplasty in young patients. J Bone Joint Surg Am, 2014, 96: 25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kwon YM, Mellon SJ, Monk P, Murray DW, Gill HS. In vivo evaluation of edge‐loading in metal‐on‐metal hip resurfacing patients with pseudotumours. Bone Joint Res, 2012, 1: 42–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Briggs TW, Hanna SA, Kayani B, et al Metal‐on‐polyethylene versus metal‐on‐metal bearing surfaces in total hip arthroplasty: a prospective randomised study investigating metal ion levels and chromosomal aberrations in peripheral lymphocytes. Bone Joint J, 2015, 97: 1183–1191. [DOI] [PubMed] [Google Scholar]
- 38. Lawrence H, Deehan D, Holland J, Kirby J, Tyson‐Capper A. The immunobiology of cobalt: demonstration of a potential aetiology for inflammatory pseudotumours after metal‐on‐metal replacement of the hip. Bone Joint J, 2014, 96: 1172–1177. [DOI] [PubMed] [Google Scholar]
- 39. Lin TH, Kao S, Sato T, et al Exposure of polyethylene particles induces interferon‐gamma expression in a natural killer T lymphocyte and dendritic cell coculture system in vitro: a preliminary study. J Biomed Mater Res A, 2015, 103: 71–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Goodman SB. Wear particles, periprosthetic osteolysis and the immune system. Biomaterials, 2007, 28: 5044–5048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Matharu GS, Pandit HG, Murray DW, Judge A. Adverse reactions to metal debris occur with all types of hip replacement not just metal‐on‐metal hips: a retrospective observational study of 3340 revisions for adverse reactions to metal debris from the National Joint Registry for England, Wales, Northern Ireland and the Isle of Man. BMC Musculoskelet Disord, 2016, 17: 495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kwon YM. Cross‐sectional imaging in evaluation of soft tissue reactions secondary to metal debris. J Arthroplasty, 2014, 29: 653–656. [DOI] [PubMed] [Google Scholar]
- 43. Parsons TM, Satchithananda K, Berbe R, Siddiqui IA, Robinson E, Hart AJ. MRI investigations in patients with problems due to metal‐on‐metal implants. Orthopade, 2013, 42: 629–636. [DOI] [PubMed] [Google Scholar]


