Abstract
Objectives
As promising alternative to current metallic biomaterials, the porous Mg scaffold with a 3‐D open‐pore framework has drawn much attention in recent years due to its suitable biodegradation, biocompatibility, and mechanical properties for human bones. This experiment's aim is to study the mechanical properties, biosafety, and osteogenesis of porous Mg‐Zn alloy.
Methods
A porous Mg‐2Zn‐0.3Ca (wt%) alloy was successfully prepared by infiltration casting, and the size of NaCl particles was detected by a laser particle size analyzer. The microstructure of the Mg‐2Zn‐0.3Ca alloy was characterized by the stereoscopic microscope and Sirion Field emission scanning electron microscope. X‐ray computerized tomography scanning (x‐CT) was used to create the 3‐D image. The degradation rate was measured using the mass loss method and the pH values were determined together. The engineering stress–strain curve, compressive modulus, and yield strength were tested next. The bone marrow stromal cells (BMSC) were cultured in vitro. The CCK‐8 method was used to detect the proliferation of the BMSC. Alkaline phosphatase (ALP) and alizarin red staining were used to reflect the differentiation effects. After co‐culturing, cell growth on the material's surface was observed by scanning electron microscope (SEM). The cell adhesion was tested by confocal microscopy.
Results
The obtained results showed that by using near‐spherical NaCl filling particles, the porous Mg alloy formed complete open‐cell foam with a very uniform size of pores in the range of 500–600 μm. Benefitting from the small size and uniform distribution of pores, the present porous alloy exhibited a very high porosity, up to 80%, and compressive yield strength up to 6.5 MPa. The degradation test showed that both the pH and the mass loss rate had similar change tendency, with a rapid rise in the early stage for 1–2 day's immersion and subsequently remaining smooth after 3 days. In vitro cytocompatibility trials demonstrated that in comparison with Ti, the porous alloy accelerated proliferation in 1, 3, 5, and 7 days (P < 0.001), and the osteogenic differentiation test showed that the ALP activity in the experimental group was significantly higher (P = 0.017) and has more osteogenesis nodules. Cell adhesion testing showed good osteoconductivity by more BMSC adhesion around the holes. The confocal microscopy results showed that cells in porous Mg‐based alloy had better cytoskeletal morphology and were larger in number than in titanium.
Conclusions
These results indicated that this porous Mg‐based alloy fabricated by infiltration casting shows great mechanical properties and biocompatibilities, and it has potential as an ideal bone tissue engineering scaffold material for bone regeneration.
Keywords: Cytocompatibility, Degradation behavior, Mechanical property, Mg‐Zn‐Ca, Porous alloy
Introduction
To repair bone defects, especially in large bones, various materials have been tried around the world (e.g. autografts and allograft)1, 2. However, there are still some imperfections in clinical practice for these methods, such as supply limitation, high failure rate, and body rejection3. Therefore, bone tissue engineering (BTE) has been proposed and developed in recent years, which could supply a special porous scaffold environment for osteoblasts to be seeded for the bone growth and healing; it is considered to be a potential substitution for bone grafts4.
At present, there are some kinds of scaffold materials that are utilized in BTE, such as polymers, bioactive ceramics, and composites5. Polymers like chitosan display favorable biocompatibility and biodegradability, and bioactive ceramics exhibit high hardness and compressive strength6, 7. However, defects also occur, including low mechanical performance, brittleness, and biodegradation. Compared with the abovementioned commercial materials, metals have been proved to be ideal implant materials with excellent mechanical properties, such as toughness (e.g. titanium alloy and stainless steel). However, permanent metal materials such as titanium and stainless steel cannot be degraded in vivo. They also can result in a stress shielding effect in the course of treatment, and need to be removed in a second surgery. Therefore, over the past few decades, magnesium alloys have aroused much attention from researchers worldwide due to their satisfying biodegradability when used as as scaffold materials8, which could prevent the need for further surgery and, hence, relieve patients’ pain.
Owing to the relatively low modulus of elasticity, similar density to human bone and appropriate compressive strength, porous alloys have received much attention and some corresponding progress has been achieved4. Lu et al. investigated the influencing factors of the microstructure and the mechanical properties of porous titanium prepared by metal injection molding9. Jiang et al. developed a novel approach to fabricate porous alloys by using the titanium wire space holder (TWSH) method10. However, for the porous alloy prepared using frequently‐used methods there still exist some deficiencies, including low porosity, large pore size, high degradation rate, even high cost, which restrict further applications of porous alloys to a large extent11, 12. Hence, porous biodegradable medical alloys with suitable pores are needed urgently. When porous magnesium‐based alloy is implanted in the human body, the human body's blood vessels and muscles can grow into their holes, which can facilitate the delivery of blood and human tissue nutrition. By adjusting the porosity and pore size of the materials, the elastic modulus can be the same or similar to that of human bone. The bone tissues and the materials can be stressed at the same time after being implanted in the human body, and would not be affected by the difference of the elastic modulus. Finally, the implant materials and the bone tissues can achieve the ideal combination. Therefore, in this study, infiltration casting was adopted because of not only its simple procedures and low cost, but high porosity of the products13. One of the aims of our study is to verify whether low cost methods can produce excellent magnesium alloys with suitable pores and degradation rate.
The degradation rate of pure magnesium metals is too fast, and the surface area increases in porous structures which results in a faster degradation rate. It is not suitable for clinical usage. Therefore, adding other metals can effectively reduce the degradation rate of magnesium alloys so that they can meet the actual needs in the human body. It is reported that a small amount of Zn, in quantities of less than 3%, can promote the corrosion resistance and the mechanical performances of Mg scaffolds, and Ca possesses the same effects as Zn14. To increase the melt fluidity, and promote the mechanical properties and corrosion resistance of this porous material as much as possible, 2 wt% Zn and 0.3 wt% Ca were added in the Mg melt in our study. Then the microstructure, mechanical properties, degradation behavior, and cytocompatibility of this porous Mg‐2Zn‐0.3Ca alloy were systematically investigated. The obtained results could provide a better understanding on the relationship between porous structures and the mechanical and biological properties of the promising Mg scaffold. In fact, the porous magnesium material is composed of the pore portion and the skeleton portion, which means that the material is inferior in mechanical properties to the pure metal materials. In addition to alloying treatment, the different porosities and pore sizes can also change the mechanical properties of the metal; therefore, another aim of this study is to verify that this porous magnesium‐based alloy can promote bone ingrowth and is also appropriate for the human body in terms of mechanical properties. In addition, we know that magnesium has good biocompatibility, but the biocompatibility of this alloy containing zinc and calcium remains to be determined. The final aim of this study is to verify the biosafety of this porous Mg‐based alloy.
Finally, this study attempts to verify the good mechanical properties and biocompatibilities of the porous Mg‐2Zn‐0.3Ca (wt%) alloy made by infiltration casting.
Materials and Methods
Materials Preparation
The infiltration casting method was used to prepare the porous Mg‐2Zn‐0.3Ca alloy. NaCl particles with near‐spherical shape were sieved and pre‐placed in the bottom of the casting mold as space holder materials. To improve the melt fluidity in space holder materials, a negative pressure was set by evacuating the mold from the bottom. When melted at 740°C, the Mg‐2Zn‐0.3Ca melt was poured into a preheated cylindrical mold with inner diameter of 50 mm. After demolding, porous Mg‐2Zn‐0.3Ca rods can be obtained by dissolving NaCl particles in water. For microstructure characterization and mechanical testing, rectangular samples with dimension of 6 mm × 6 mm × 16 mm were then cut from the central part of the porous ingot by wire cut electrical discharge machining. Moreover, some disc‐shaped samples that were 10 mm in diameter and 1.5 mm in thickness were also cut for degradation and cytocompatibility evaluations.
Microstructure Characterization and Mechanical Test
Microstructure of the Mg‐2Zn‐0.3Ca alloy was characterized using a stereoscopic microscope (ZOOM645S) and a Sirion Field emission scanning electron microscope (SEM). To examine the pore structure of porous alloys, the size of NaCl particles was also detected by a laser particle size analyzer (LPSA). Moreover, X‐ray computerized tomography scanning (x‐CT) was used to create the 3‐D image of the rectangular block samples and to calculate their porosity. To evaluate the mechanical properties of this porous alloy, compression testing was performed using a CMT5105 electronic universal testing machine with a ram speed of 1 mm/min at room temperature, according to the GB/T 7314–2005. The specimens used for compression tests exhibit rectangular parallelepiped with dimension of 6 mm × 6 mm × 16 mm. After the compression test, the engineering stress–strain curve, compressive modulus, and yield strength were obtained for analysis.
Degradation Behavior
To evaluate the degradation behavior of the present porous alloy, the disk samples were immersed in Hank's solution at 37°C for 5 days. The change of mass loss and pH value were recorded regularly. In addition, the macroscopic and microscopic observations of the surface morphologies after degradation were conducted by stereo microscope and SEM, respectively.
The C57 mouse bone marrow stromal cells (BMSC) were cultured in MUBMX‐90011 medium, which was supplemented with 10% qualified FBS, 1% penicillin–streptomycin, and 1% glutamine, were used to investigate the cytocompatibility of porous alloys. When the confluency of stem cells reached 80%–90%, the cells were trypsinized and cultured for 1:2 passage. After that, BMSC were induced to differentiate in the medium. To determine the cytocompatibility of porous alloys, three aspects including proliferation, osteogenic differentiation and adhesion of the cells were analyzed and compared with a control group of pure titanium biomaterial, which performed particularly well in clinical treatments13. Then the two metals were irradiated with a UV lamp for 30 min. In a humidified atmosphere containing 5% CO2 at 37°C for 24 h (ISO 10993–5), the extract was made from the extraction medium with a ratio of extraction medium/sample surface area of approximately 1.25 cm2/mL.
Cytocompatibility
To evaluate the cytocompatibility of the biomaterial accurately, proliferation of stem cells was examined using CCK‐8 assays. BMSC were inoculated at a density of 2 × 103 per well in 96‐well plates. After cell attachment, the cells were incubated for 1, 3, 5, and 7 days with the extract, followed by adding CCK‐8 reagent and incubating BMSC for 4 h. Then optical density (OD value) of formazan was quantified by metering the absorbance at 450 nm to reflect the number of active cells.
In osteogenic differentiation trials, the cells were seeded at a density of 5 × 104 per well in 6‐well plates. The rest of the procedures were identical to those for the proliferation examination mentioned above, except for changing the extract every 2 days. To evaluate the differentiation of stem cells objectively, alkaline phosphatase (ALP) activity was measured using the microplate method after 7 days, as well as mineralized nodules stained with alizarin red after 14 days and 21 days.
To observe the cell adhesion on the scaffold materials, the cells were co‐cultured with the two groups of materials for 24 h with 1 × 105 cells. Then the samples were shaken twice in phosphate buffer saline (PBS) for 1 min, followed by fixing cells with 2% glutaraldehyde. After that, the cells were gradient dehydrated with ethanol solution ranging from 45% to 100%, air seasoned, and, finally, treated with gold spray. Finally, SEM was used to observe the surface morphologies of the sample disks.
Cytoskeleton staining is another effective method to study the cell adhesion. In the present study, 2 × 103 cells were inoculated on the petri dishes, and were cultured for 24 h in extract made from each group. After that, the cells were fixed for 15 min by 3.7% formaldehyde solution and immersed in PBS contain 0.1% Triton X‐100 for 5 min. Then, bull serum albumin was used to immerse cells. Finally, the cytoskeletal protein was dyed with phalloidin for 40 min and the cell nucleus was dyed by Hoechst for 3 min. The cytoskeletal proteins were observed under laser scanning confocal microscopy.
Statistical Analysis
Data in this study were analyzed by SPSS 18.0 software and there was a statistical difference when the P‐value less than 0.05. t test, variance analysis, or rank sum test were used if necessary.
Results
Microstructure and Mechanical Property of Porous Alloy
To determine the structures of the porous alloy studied, the shape and size of NaCl particles were first examined. The stereomicrograph of NaCl particles is shown in Fig. 1A, and it is apparent that the shape of these particles is more like a rounded cube. Their dimensions are relatively uniform, with an average size of approximately 500 μm. Moreover, the detailed sizes of NaCl particles were detected by LPSA, and the size distribution curves are shown in Fig. 1B. All particles were in the range of 400–700 μm and the mean size was estimated to be 520 μm.
Figure 1.

The stereomicrograph (A) of NaCl particles shows that the space holder is like a rounded cube, with dimensions almost in the range of 400–700 μm, exhibited by the distribution curves of NaCl particles (B).
Figure 2A shows the stereomicrograph of the porous alloy. The sample exhibits a typical porous structure with uniform distribution of near‐spherical pores. Most of the pore sizes were 500–600 μm in diameter, and this is consistent with the above particle size distribution of NaCl particles. More detailed pore structures were also observed by SEM and are shown in Fig. 2B. It is obvious that these spherical pores are interconnected through smaller holes with a size range of 100–200 μm and form complete open‐cell foam.
Figure 2.

The stereomicrograph (A) indicates that there is a uniform distribution of near‐spherical pores on the porous alloy. Scanning electron microscope micrograph (B) of the microstructures of porous alloy showed substantial interconnected spherical pores.
Further confirmation was given by x‐CT, and the corresponding 3‐D image of the block‐shaped sample is shown in Fig. 3. Based on analysis of the 3‐D structure, the porosity of this porous alloy was calculated to be 80%, and the alloy exhibited almost 100% pore connectivity.
Figure 3.

The 3‐D image of the block‐shaped porous alloy sample is created by X‐ray computerized tomography scanning, and the porosity of this porous alloy is up to 80%.
Fig. 4 displays the engineering stress–strain curve of the porous alloy. It can be calculated that the elastic modulus and compressive yield strength of the alloy are 0.56 GPa and 6.5 MPa, respectively. In terms of the relative research (i.e. Trinidad et al.13), this porous alloy actually displayed eminent anti‐compression performance, although exhibiting a very high porosity of 80%.
Figure 4.

The compressive curve of porous alloy displays that the elastic modulus and compressive yield strength are 0.56 GPa and 6.5 MPa, respectively.
Degradation Behavior of Porous Alloy
To evaluate the degradation (corrosion) property of the porous alloy, in vitro degradation testing was performed by immersion of porous alloy disks in Hank's solution for 5 days. Figure 5 shows the changes in pH value of solution and the mass loss rate of samples with increasing immersion time. As an important reference for the corrosion rate, both pH and mass loss rates had similar change tendency, with a rapid rise in the early stages for 1–2 day's immersion and subsequently remaining smooth after 3 days. This result suggests that the corrosion will gradually slow down, with prolonged immersion time under the protection of corrosion products.
Figure 5.

The changes in mass loss rate and the pH value with immersion time are exhibited during degradation of porous alloy. The mass loss rate and pH value indicated that there is a rapid rise in the early stage, and subsequently both remain smooth after 3 days.
After removing the corrosion products using chromic acid, the macro and micro surface morphologies of corroded discs were observed for 1–5 days by stereo microscope and SEM, respectively, as shown in Fig. 6. In the macroscopic scale, the corrosion first appeared on the edge of the disc (Fig. 6A for 1‐day immersion) due to bigger surface area exposed in solution, and then the entire disc was gradually corroded along with localized damaged on the edge (Fig. 6B) after 3‐day immersion. With increased immersion time to 5 days, there was some serious corrosion, even fractures, on the edge region (Fig. 6C). From the corresponding magnified SEM micrographs of pore structure (Fig. 6D‐F), it was evident that the thin hole wall between pores first corroded, and those small holes expanded and gradually connected into bigger holes; simultaneously metal frameworks were also corroded, resulting in break or reduction of pore structures.
Figure 6.

The stereomicrographs and SEM micrographs of porous alloy after degradation for 1 day (A, D), 3 days (B, E), and 5 days (C, F), respectively. The degradation first appeared on the edge of the disc, and with increasing immersion time to 5 days, some fracture emerged on the edge region due to the severe corrosion.
Evaluation of Bone Marrow Stromal Cell Functions
To identify the proliferation of BMSC on the porous alloy, the results of CCK‐8 assay are depicted in Fig. 7A, where the OD values of experimental and control groups are obviously different. The proliferating rate of cells in the porous alloy extract was superior to that in pure Ti, at more than 10% (P < 0.001) on the first day, indicating that the alloy had a better effect in promoting cell proliferation than the control. As the particular structure of the porous material further increased the contact area with extracts, magnesium ions, which have been proved to accelerate cell proliferation15, were released heavily in the degradation process.
Figure 7.
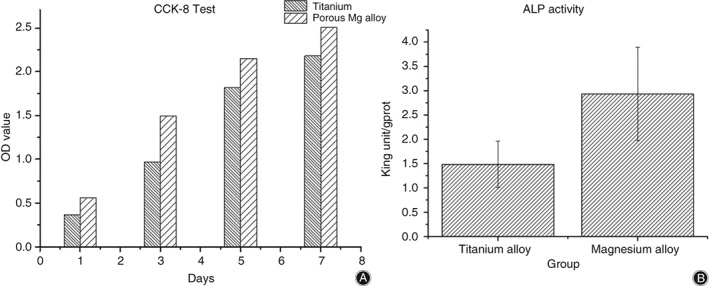
The histograms of (A) CCK‐8 test after culturing for 1, 3, 5, and 7 days. Although the number of bone marrow stromal cells is growing sustainably during the period in both groups, the magnesium group does better than the titanium group. The histogram of (B) is alkaline phosphatase (ALP) activity after culturing for 7 days. The ALP activity in the magnesium group is superior to the titanium group obviously.
In cell differentiation trials, osteoblast differentiation potential can be mainly reflected by the results of ALP activity and calcium deposition16. On the one hand, ALP is considered as an early marker of osteogenic differentiation and bone formation, and a high level of ALP means that the material has strong osteogenic activity. As presented in Fig. 7B, the ALP activity in the experimental group was significantly higher (P = 0.017). It has obvious osteogenic effects in Magnesium alloy group because the ratio of ALP quantities with the intracellular total protein quantities is higher than the Titanium group, which reflects the ALP activities.
The formation of calcium nodules is an indicator of differentiation and important evidence for mineralization of osteoblasts. The contrast between Fig. 8A with (B) and (C) with (D) clearly showed that there were more calcium nodules in the experimental group than in the control, at 14 and 21 days, indicating that the porous alloy can promote the osteogenic differentiation of BMSC effectively compared with Ti.
Figure 8.

The micrographs of (A) the experimental group and (B) the control group after alizarin red staining at 14 days. (C) Experimental group and (D) control group after alizarin red staining at 21 days. The red dots in the figures are osteogenesis nodules, which are greater in the magnesium group than in the titanium group both at 14 and 21 days.
The outcomes of adhesion and binding response of these cells on porous alloy and pure Ti are displayed in Fig. 9A,B, respectively. After co‐cultivation for 24 h, there were more cells adhering on the porous alloy and the cells displayed a spherical shape (Fig. 9A), while there were almost no cells on Ti. Compared with Ti, the porous alloy exhibited superior advantages on osteoblast adhesion, and there was no any apoptosis or necrosis on porous biomaterial. Moreover, these cells adhered around the holes implied that porous structures are favorable for ingrowth of osteoblasts in the implanted scaffolds and will provide porous alloy good osteoconductivity17.
Figure 9.
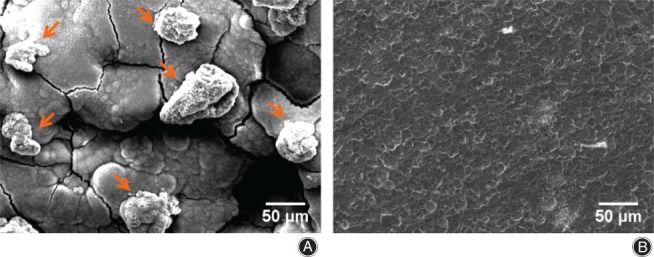
The scanning electron microscope micrographs of (A) the experimental group and (B) the control group after co‐culturing. The arrows point to bone marrow stromal cells, which are attached on the materials’ surface. The result shows that there are more cells on the magnesium alloys’ surface than the titanium.
The cytoskeleton staining indicated that the number of cells in the experimental group is more than in the control group, which means the environment contain Mg ions is beneficial to cell proliferation (Fig. 10A,B). In addition, after culturing with extract containing Mg ions, cells show clearer and more complete cytoskeletal protein in the cells, and cellular morphology was better. Therefore, this biodegradation magnesium alloy has a good effect on cell adhesion.
Figure 10.

The laser scans confocal micrographs of (A) the experimental group and (B) the control group after cytoskeleton staining. The bright circles are the cell nucleus and are surrounded by the skeletal protein which is light red. The figure shows that the number of the magnesium group is larger than the titanium group.
Discussion
Mechanical Property of Porous Alloy
From the stereomicrograph and X‐CT results, we can see that the pore sizes of the porous alloy studied were also in good agreement with that of human cancellous bones, which are in the range of 400 to 600 μm18, 19. It is apparent from the above examinations of NaCl particles and pore structures that the Mg melt had a very good fluidity and almost filled all gaps between particles under the present casting process. All these results for pores are totally in agreement with those for human cancellous bone, demonstrating that the prepared porous Mg‐2Zn‐0.3Ca alloy could provide a very good structural condition for the proliferation and differentiation of osteoblasts.
In comparison with the porosity of porous alloy fabricated by powder metallurgy method, approximately 35%–55%20, infiltration casting can provide higher porosity. Trinidad et al. (2013) report a high porosity of 69% for infiltration casting ZXM200 (Mg‐2Zn‐Mn) alloy13. However, there is still a limitation in the porosity by infiltration casting due to its processing feature, similar to the limitation for the density of crystal structures. For all the space holder materials with same perfect spheres, the highest porosity was only 74%; that is, the density of face‐centered cubic (fcc) or hexagonal close‐packed (hcp) structure. However, for near‐spherical NaCl particles (Fig. 1A) in this study, there are more areas contacting each other, which could increase the porosity of the porous alloy. Furthermore, the different sizes of particles could further improve the porosity as for some topologically close‐packed structures having higher density than fcc or hcp21. Based on the above considerations, a porous Mg alloy with relatively high porosity of 80% was successfully fabricated using the infiltration casting method.
From the compressive curve of the porous alloy it is evident that although exhibiting a very high porosity of 80%, the present porous alloy still showed a relatively high mechanical property (with compressive yield stress of 6.5 MPa) compared with those in other works. In the research of Trinidad et al., the porous samples with porosity in the range of 67%–69% only had a compressive yield stress of 2.31–3.62 MPa13. It could be concluded that the good mechanical property of the porous alloy in this study is attributed to the very uniform distribution of the spherical pores, as shown in Fig. 2.
According to the present degradation results, it seems that the corrosion rate of the porous alloy studied was too fast to satisfy the demand for bone healing. However, it should be noted that there is usually a significant difference between in‐vitro and in‐vivo testing. The in‐vivo degradation period of the porous alloy needs to be determined in future works. In contrast, surface modification, including anodizing, micro‐arc oxidation, and chemical conversion, is a good method for substantially improving the corrosion resistance of Mg alloy. Further related works are well underway and will be published in future.
Biosecurity of Porous Alloy
Magnesium is one of the most important elements in the human skeleton, and now there are a large number of studies proving that degradable magnesium plays an important role in promoting bone proliferation and differentiation. Fei et al. found that magnesium alloy is non‐toxic to osteoblasts, and can promote proliferation22. Other studies also pointe out that magnesium alloy can promote cell migration and adhesion 23, 24, thus having a clear advantage for bone healing. Fiocco et al. 25 and Cheng et al.26 also found that porous Mg alloy has good biocompatibilities and can lead to higher bone mass formation. In addition, porous Mg alloys can be easily adjusted in relation to mechanical properties and degradation rate to satisfy the demand of different bones through changing the size of pores and porosity as well as suitable surface modification techniques, hence showing great potential as BTE scaffold materials.
Compared with the technology of laser drilling, powder metallurgy or 3‐D printing, the infiltration casting method is much simpler and more efficient, and is apt to control the size and shape of pores. Using infiltration casting, the porous Mg‐2Zn‐0.3Ca alloy showed good pore distribution and mechanical properties. In addition, cell studies have also indicated that the scaffold has a good effect on bone formation and is better than imporous Mg‐based metals, which may be attributed to the uniform pore distribution and high porosity. However, in comparison with the fluorinated porous Mg scaffold27, there is still considerable room for improvement of the degradation rate and cell adhesion on the present bare scaffolds. Further works on surface treatments to improve the corrosion resistance and bioactivity of porous Mg alloy are under way.
In addition, the cytoskeleton staining indicated that this porous Mg‐based alloy can promote BMSC adhesion and also cause cells to have better cytoskeletal morphology as well as to be larger in number. All the results above indicate that our alloys have good biosecurity and can promote cell proliferation, differentiation, and adhesion.
Conclusions
For the application of bone tissue engineering (BTE) scaffolds, the biomaterial is required to present a 3‐D open‐pore framework. Because degradation Mg alloys are regarded as very promising alternatives to current metallic biomaterials due to suitable biodegradation, biocompatibility, and mechanical properties for human bones, the porous Mg scaffold has also drawn much attention from researchers.
In this study, the porous Mg alloy was prepared by infiltration casting, and its pore structure, mechanical properties, degradation behavior and cytocompatibility were studied.
On the one hand, the mechanical studies’ results showed that this porous Mg‐2Zn‐0.3Ca (wt%) alloy BTE scaffold for bone regeneration was successfully prepared by infiltration casting in this study. Using near‐spherical NaCl filling particles, the porous Mg alloy formed complete open‐cell foam with a very uniform size of pores in the range of 500 to 600 μm. Benefitting from the small size and uniform distribution of pores, the present porous alloy exhibited a very high porosity, up to 80%, and compressive yield strength up to 6.5 MPa.
On the other hand, from the biological studies we can see that in comparison with Ti, the porous alloy accelerated proliferation and osteogenic differentiation of stem cells and possessed good osteoconductivity, with more BMSC adhesion around the holes. The cytocompatibility evaluation also showed that the present porous Mg has better cytocompatibility and osteoconductivity compared with Ti. These results indicated that the porous Mg is a very promising BTE scaffold material for bone regeneration.
In vitro mechanical and biological trials demonstrated that most of the pore sizes were 500–600 μm in diameter and the porosity was up to 80%, providing a high compressive yield strength and excellent structural condition for the proliferation and differentiation of stem cells. Although further works on improving the degradation rate and cell adhesion are still needed, the porous Mg alloy fabricated by infiltration casting shows great potential as an ideal scaffold of BTE.
Disclosure: The authors declare no conflict of interest. This work was supported by the National Key Research and Development Program of China (No. 2016YFC1102402), the National Natural Science Foundation of China (No. 31570961; No. 51501039; No.81472508), and the Natural Science Foundation of Jiangsu Province of China (BK20160869; BK20151411; BK20161385; BK20141376).
Contributor Information
Jian‐ning Zhao, Email: zhaojianning.0207@163.com.
Guang‐xin Zhou, Email: oxis@163.com.
References
- 1. Decoster TA, Gehlert RJ, Mikola EA, Pirela‐Cruz MA. Management of posttraumatic segmental bone defects. J Am Acad Orthop Surg, 2004, 12: 28–38. [DOI] [PubMed] [Google Scholar]
- 2. Garbuz DS, Masri BA, Czitrom AA. Biology of allografting. Orthop Clin North Am, 1998, 29: 199–204. [DOI] [PubMed] [Google Scholar]
- 3. Agarwal R. Biomaterial strategies for engineering implants for enhanced osseointegration and bone repair. Adv Drug Deliv Rev, 2015, 94: 53–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Seyedraoufi ZS, Mirdamadi S. In vitro biodegradability and biocompatibility of porous Mg‐Zn scaffolds coated with nano hydroxyapatite via pulse electrodeposition. Trans Nonferrous Met Soc Chin, 2015, 25: 4018–4027. [Google Scholar]
- 5. Roseti L, Parisi V, Petretta M, et al Scaffolds for bone tissue engineering: state of the art and new perspectives. Mater Sci Eng C Mater Biol Appl, 2017, 78: 1246–1262. [DOI] [PubMed] [Google Scholar]
- 6. Dhandayuthapani B, Yoshida Y, Maekawa T, Kumar DS. Polymeric scaffolds in tissue engineering application: a review. Int J Ploym Sci, 2011, 2011: 609–618. [Google Scholar]
- 7. Gao C, Deng Y, Feng P, et al Current progress in bioactive ceramic scaffolds for bone repair and regeneration. Int J Ploym Sci, 2014, 15: 4714–4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Peron M, Torgersen J, Berto F. Mg and its alloys for biomedical applications: exploring corrosion and its interplay with mechanical failure. Metals, 2017, 7: 252. [Google Scholar]
- 9. Lu Z, Huang Z, Jiang S, Liu W, Zhang K. Influencing factors for the microstructure and mechanical properties of micro porous titanium manufactured by metal injection molding. Metals, 2016, 6: 83. [Google Scholar]
- 10. Jiang G, Guo H. A new approach to the fabrication of porous magnesium with well‐controlled 3D pore structure for orthopedic applications. Mater Sci Eng C Mater Biol Appl, 2014, 43: 317–320. [DOI] [PubMed] [Google Scholar]
- 11. Geng F, Tan L, Zhang B, et al Study on β‐TCP coated porous mg as a bone tissue engineering scaffold material. J Mater Sci Technol, 2009, 25: 123–129. [Google Scholar]
- 12. Zhuang H, Han Y, Feng A. Preparation, mechanical properties and in vitro biodegradation of porous magnesium scaffolds. Mat Sci Eng C, 2008, 28: 1462–1466. [Google Scholar]
- 13. Trinidad J, Marco I, Arruebarrena G, et al Processing of magnesium porous structures by infiltration casting for biomedical applications. Adv Eng Mater, 2013, 16: 241–247. [Google Scholar]
- 14. Yin DC, Zhang EL, Zeng SY. Effect of Zn on mechanical property and corrosion property of extruded mg‐Zn‐Mn alloy. Chin J Non‐Ferr Met, 2008, 18: 763–768. [Google Scholar]
- 15. He LY, Zhang XM, Liu B, Tian Y, Ma WH. Effect of magnesium ion on human osteoblast activity. Braz J Med Biol Res, 2016, 49: pii:: S0100‐879X2016000700604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liu C, He P, Wan P, et al The in vitro biocompatibility and macrophage phagocytosis of Mg17Al12 phase in mg‐Al‐Zn alloys. J Biomed Mater Res A, 2015, 103: 2405–2415. [DOI] [PubMed] [Google Scholar]
- 17. Husna N, Lee CC, Norbahiyah S, Sanuddin AB. Processing and characterization of porous mg alloy for biomedical applications. Aust J Basic Appl Sci, 2014, 8: 160–164. [Google Scholar]
- 18. Karageorgiou V, Kaplan D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials, 2005, 26: 5474–5491. [DOI] [PubMed] [Google Scholar]
- 19. Doktor T, Valach J, Kytýř D, Jiroušek O. Pore size distribution of human trabecular bone ‐ Comparison of intrusion measurements with image analysis. Eng Mech, 2011. [Google Scholar]
- 20. Wen CE, Yamada Y, Shimojima K, Chino Y, Hosokawa H, Mabuchi M. Compressibility of porous magnesium foam: dependency on porosity and pore size. Mater Lett, 2004, 58(3): 357–360. [Google Scholar]
- 21. Sinha AK. Topologically close‐packed structures of transition metal alloys. Prog Mater Sci, 1972, 15: 181–185. [Google Scholar]
- 22. Fei J, Wen X, Lin X, et al Biocompatibility and neurotoxicity of magnesium alloys potentially used for neural repairs. Mat Sci Eng C Mater Biol Appl, 2017, 78: 1155–1163. [DOI] [PubMed] [Google Scholar]
- 23. Ge S, Wang Y, Tian J, Lei D, Yu Q, Wang G. An in vitro study on the biocompatibility of WE magnesium alloys. J Biomed Mater Res B, 2016, 104: 482–487. [DOI] [PubMed] [Google Scholar]
- 24. Witte F, Kaese V, Haferkamp H, et al In vivo corrosion of four magnesium alloys and the associated bone response. Biomaterials, 2005, 26: 3557–3563. [DOI] [PubMed] [Google Scholar]
- 25. Fiocco L, Li S, Stevens MM, Bernardo E, Jones JR. Biocompatibility and bioactivity of porous polymer‐derived Ca‐Mg silicate ceramics. Acta Biomater, 2017, 50: 56–67. [DOI] [PubMed] [Google Scholar]
- 26. Cheng MQ, Wahafu T, Jiang GF, et al A novel open‐porous magnesium scaffold with controllable microstructures and properties for bone regeneration. Sci Rep, 2016, 6: 24134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yu W, Zhao H, Ding Z, et al In vitro and in vivo evaluation of MgF2 coated AZ31 magnesium alloy porous scaffolds for bone regeneration. Colloids Surf B Biointerfaces, 2017, 149: 330–340. [DOI] [PubMed] [Google Scholar]


