Abstract
Total knee arthroplasty (TKA) often causes a significant amount of blood loss with an accompanying decline in hemoglobin and may increase the frequency of allogeneic blood transfusion rates. Unfortunately, allogeneic blood transfusions have associated risks including postoperative confusion, infection, cardiac arrhythmia, fluid overload, increased length of hospital stay, and increased mortality. Other than reducing the need for blood transfusions, reducing perioperative blood loss in TKA may also minimize intra‐articular hemorrhage, limb swelling, and postoperative pain, and increase the range of motion during the early postoperative period. These benefits improve rehabilitation success and increase patients’ postoperative satisfaction. Preoperative anemia, coupled with intraoperative and postoperative blood loss, is a major factor associated with higher rates of blood transfusion in TKA. Thus, treatment of preoperative anemia and prevention of perioperative blood loss are the primary strategies for perioperative blood management in TKA. This review, combined with current evidence, analyzes various methods of blood conservation, including preoperative, intraoperative, and postoperative methods, in terms of their effectiveness, safety, and cost. Because many factors can be controlled to reduce blood loss and transfusion rates in TKA, a highly efficient, safe, and cost‐effective blood management strategy can be constructed to eliminate the need for transfusions associated with TKA.
Keywords: Blood management, Conservation strategies, Total knee arthroplasty
Introduction
Total knee arthroplasty (TKA) is the most common elective orthopaedic surgical procedure, with more than 600 000 TKA procedures performed annually in the United States1. Although TKA can effectively improve joint functionality and a patient’s quality of life, it is often associated with substantial blood loss and a high rate of allogeneic blood transfusion. Some centers indicate that 20%–40% of all patients who underwent TKA received blood transfusions2, 3. Blood transfusion effectively corrects anemia, but also increases the economic burden placed on patients and society. Allogeneic blood transfusions are also associated with significant potential risks and complications, including infections, fluid overload, cardiac arrhythmia, prolonged hospitalization, and an increase in mortality4, 5. Minimizing blood loss and blood transfusions associated with TKA is critical to avoid unnecessary expense and complications.
Preventing blood loss around the knee during TKA also minimizes hemarthrosis, limb swelling, postoperative pain, and the use of analgesics. These outcomes facilitate better outcomes when performing functional exercise during the early postoperative period, thus improving postoperative patient satisfaction.
Perioperative blood management strategies focus on minimizing blood loss and the need for blood transfusions. Preoperative anemia and significant blood loss intraoperatively and postoperatively are common factors that increase the need for postoperative blood transfusions. There are several blood conservation methods that can be utilized during TKA. However, implementation of these methods is based on subjective judgment rather than relevant evidence‐based guidelines. The goal of this article is to explore the current scientific literature and to provide an overview of the effectiveness of blood management strategies in TKA.
Method
For this review of the literature, related reports were found through searches of PubMed, OVID Medline, and the Cochrane database using the following subject terminology: “knee arthroplasty,” “blood transfusion,” “preoperative,” “intraoperative,” and “postoperative”. In all, 589 articles were found. After excluding 75 non‐English articles, 514 English articles were browsed and assessed. As a result, 322 articles were excluded from the analysis because they were not relevant to the subject of this study, thus leaving 192 articles for final review. Each article was strictly screened for quality using the following criteria: whether there was acceptable research design and the reputability of its journal. Ninety‐three articles were screened out because the study methods were unclear or not suitable, leaving 99 research articles for inclusion in this review. This entire process is depicted in Fig. 1.
Figure 1.
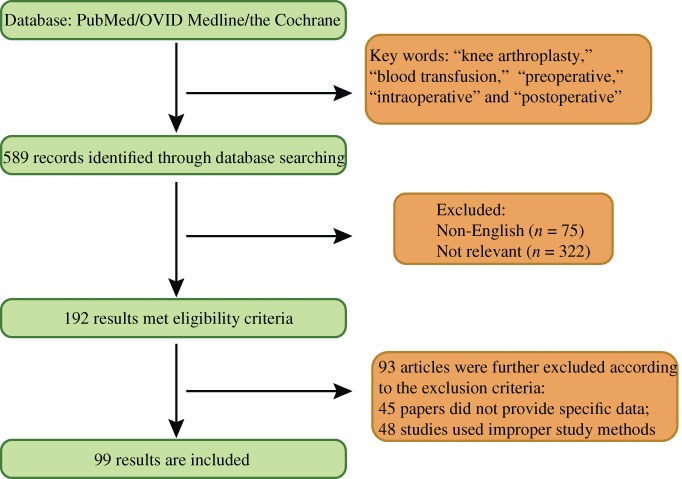
Flow‐chart of search of published reports showing the process of inclusion and exclusion.
Preoperative Strategies
Iron Therapy
Approximately one‐third of the world’s population is anemic, mainly due to iron deficiency6. Current data indicates that patients undergoing major surgery may have a higher prevalence of anemia, with rates as high as 75%7. Various studies indicate that preoperative anemia is associated with poor prognosis8, 9, 10. In addition, preoperative anemia is the strongest predictor of transfusion requirements11, 12. Therefore, preoperative correction of anemia is of great significance.
Iron supplementation is an important method to optimize hemoglobin (Hb) concentration and can be administrated orally or intravenously. Oral iron is an inexpensive way to treat anemia, but the daily absorption of oral iron is limited to 2–16 mg daily. Thus, to be effective, patients must take oral iron for 3–6 months preoperatively. Unfortunately, the malabsorption and gastrointestinal side effects of oral iron further limit its application. Intestinal resection, Helicobacter pylori infections, antacids, and phenolic compounds also decrease the absorption of oral iron, with the likelihood of patients experiencing gastrointestinal dysfunction when taking oral iron approximately 32%13, 14, 15, 16.
Routine use of oral iron supplementation during TKA has been a controversial issue. Lachance et al. found that preoperative continuous iron supplementation (300 mg, 3 times/day) lasting longer than 3 weeks increased ferritin concentrations by 25.8 ng/mL (P < 0.001), but caused a drop in Hb by 0.14 g/dL (P = 0.015)17. However, they also report many side effects, including constipation (33.3%), heartburn (13.8%), and abdominal pain (12.6%). However, other investigators have reported that the therapeutic regimen of taking ferrous sulfate (256 mg/day), vitamin C (1000 mg/day), and folic acid (5 mg/day) 30–45 days before surgery could reduce transfusion rates (5.8% vs 32%; P < 0.01) and the units of blood transfused (1.78 U vs 2.22 U; P < 0.05) in TKA when compared with a control group18. A meta‐analysis by Yang and his colleagues corroborates that oral iron can increase Hb levels (P = 0.009), but also reports that there are no significant differences in the rate and volume of transfusion (P > 0.05) between patients who receive iron therapy and those who do not19.
Many studies indicate that using intravenous iron is a safe and effective way to correct preoperative anemia20, 21. Munoz et al. reveal that intravenous iron significantly reduced allogeneic blood transfusion rates (8.9% vs 30.1%; P = 0.001), shortened hospitalization periods (8.4 days vs 10.7 days; P = 0.001), and did not increase postoperative complications (P > 0.05)21. A meta‐analysis by Litton et al. proved that intravenous iron increases the mean Hb concentration (mean difference [MD], 6.5 g/L; 95% CI, 5.1–7.9 g/L), decreasing the need for blood transfusion (risk ratio [RR], 0.74; 95% CI, 0.62–0.88), but increases the risk of infection (RR, 1.33; 95% CI, 1.10–1.64) as compared to oral iron or no iron supplementation22. Another meta‐analysis examined 103 studies and compared patients receiving intravenous iron (n = 10 390) to patients taking oral iron (n = 1329), placebo (n = 3335), and intramuscular iron (n = 155) or no iron (n = 1329)20. The results showed that intravenous iron did not increase the risk of serious adverse events (RR, 1.04; 95% CI, 0.93–1.17) and was not associated with an increased risk of infection (RR, 1.17; 95% CI, 0.83–1.65), although it led to higher rates of severe infusion reactions (RR, 2.47; 95% CI, 1.43–4.28). However, the rate of serious infusion reaction was very low (1: 263) and occurred mainly in patients infused with high‐molecular weight iron dextran. Despite the current limited evidence regarding the routine use of different iron formulations in TKA, preoperative anemia is the strongest predictor of blood transfusion in TKA; thus, we recommend iron supplementation to correct iron deficiency in anemia.
Erythropoietin
Erythropoietin is a natural glycoprotein secreted primarily in the kidney. It stimulates progenitor cells in bone marrow to produce red blood cells. Erythropoietin is widely used to treat various types of anemia, especially in patients with anemia secondary to chronic kidney disease. Erythropoietin is also commonly used in orthopaedic surgery, but currently its optimal dosage is unclear, with only two recommended treatment regimens. The first recommendation is to administer 300 U/kg 10 days before surgery, the day of surgery, and 4 days postoperatively23. The second recommendation is to inject 600 U/kg subcutaneously on days 21, 14, and 7 before surgery, and on the day of surgery24. Erythropoietin does have risks, including an increased risk of thromboembolism events25. To ensure the safety and effectiveness of erythropoietin, its use must be combined with iron to enhance the response to erythropoietin and reduce its dosage, while taking precautions to prevent thrombosis26. The use of erythropoietin should cease when the Hb concentration in men reaches 13 mg/dL and 12 mg/dL in women27.
In an unrestricted transfusion standard study, Bedair et al. demonstrate that erythropoietin could increase the postoperative Hb level (P < 0.001) and lower transfusion rates (0% vs 41%, P < 0.001) but increase healthcare costs ($2632 vs $2284)28. Another study found that erythropoietin reduced blood transfusion even under restrictive transfusion criteria29. This study demonstrated that erythropoietin reduces blood transfusion units by 29% and blood transfusion rates by 50% but increased the cost by €785. A recent meta‐analysis of 2439 TKA patients reinforced that erythropoietin can reduce the need for allogeneic blood transfusions (RR, 0.38; 95% CI, 0.27–0.53) and the amount of transfused red blood cells (MD, −0.57; 95% CI, −0.86 to −0.29), and at the same time, not increase the incidence of thrombotic and other adverse events30. Although current evidence indicates that erythropoietin can effectively reduce the transfusion rate in TKA, we do not recommend the routine use of erythropoietin due to cost. More investigation regarding lowering the costs of erythropoietin to promote its application is needed.
Preoperative Autologous Blood Donation
Preoperative autologous blood donation is often used for elective surgery. Autologous blood is collected and stored before the operation for reinfusion during surgery when required. Utilization of PABD for orthopaedic surgeries is popular, especially for joint replacements, but use of this approach is controversial. Some researchers have found that PABD can reduce the risk of allogeneic blood transfusion in TKA (19% vs 38%, P < 0.05)31. However, it can lead to 29% autologous blood wastage and increase the cost of health care ($395 vs $220; P < 0.001). A related meta‐analysis points out that PABD can reduce the absolute risk of allogeneic blood transfusions by 44% (95% CI, −0.68 to −0.21), but it also increases the risk of total blood transfusion (allogeneic or autologous transfusion) (RR, 1.24; 95% CI, 1.02–1.51)32.
Although several studies have demonstrated that PABD can reduce the risk of allogeneic blood transfusions, many researchers do not recommend PABD in TKA. Jakovina et al. found that PABD did not reduce the TKA allogeneic blood transfusion rate and caused a large quantity of autologous blood waste33. They also confirmed that PABD‐induced iatrogenic anemia would increase the need for allogeneic blood transfusion. Another study supports these findings, stating the application of PABD in hip and knee replacement would likely not benefit patients34. In this study, 461 patients using postoperative cell salvage were analyzed. Among these, 182 patients used PABD at the same time. The study found that PABD led to a greater reduction in Hb concentration (P < 0.001) and a higher transfusion rate (P < 0.01). In addition, 86.3% of PABD products were wasted.
A low utilization rate of autologous blood is an important factor that restricts the use of PABD. One study reports that the waste rate of PABD units at Mayo Clinic’s increased from 22.8% to 88.9% between 2004 and 201035. Another factor limiting the use of PABD is the dramatic increase in medical costs. A study by Etchason and his colleagues discovered that substituting autologous for allogeneic blood had little expected health benefits (0.0002–0.00044 quality‐adjusted year of life saved), and the cost per unit of autologous blood increased by 68% to $478336. Therefore, we do not recommend the use of PABD on a routine basis in TKA.
Intraoperative Strategies
Acute Normovolemic Hemodilution
Acute normovolemic hemodilution is similar to PABD where a certain amount of autologous blood is collected right before the operation. At the same time, an equal volume of colloid or crystal solution is added. If necessary, this autologous blood is transfused intraoperatively or postoperatively. ANH can reduce the erythrocyte loss when an equivalent amount of blood is lost due to dilution. ANH is widely used during major surgeries to reduce the postoperative allogeneic blood transfusion rates and can effectively reduce allogeneic transfusion rates37, 38. A meta‐analysis of 2439 patients in 29 randomized controlled trials showed that patients in the ANH group received less allogeneic transfusion units (MD = −0.79; P = 0.001), with a lower allogeneic transfusion rate (42.1% vs 56.1%; P < 0.0001), and had less blood loss (388 mL vs 450 mL; P < 0.0001)37.
Of note, there is limited literature evaluating the efficacy of ANH in TKA. A prospective randomized controlled trial of 30 patients treated with TKA demonstrated that ANH was effective in reducing allogeneic transfusion39. The study found that in comparison with the control group, the groups that had hemodiluted autologous blood infusions 2 h or 6 h after surgery received less allogeneic blood transfusions. In a retrospective study, Schmied et al. also found that ANH can reduce allogeneic blood transfusions in TKA40. However, other investigators report that the use of ANH does not decrease allogeneic transfusions but leads to an increase in total blood loss in TKA41, 42. Juelsgaard et al. found that the use of ANH significantly increased postoperative blood loss (1306 mL vs 1026 mL, P < 0.05) compared to the control group41. In a randomized, single‐blind study comparing the effects of ANH to tranexamic acid in reducing allogeneic blood transfusion in TKA, Zohar et al. found using ANH increased postoperative drainage (259 mL vs 110 mL; P < 0.0008) and the amount of allogeneic blood transfusion (19 U vs 2 U; P < 0.0008)42. The current literature regarding the use of ANH in TKA is limited and conflicted, so more research on the efficacy of this technique in TKA is needed.
Hypotensive Anesthesia
Hypotensive anesthesia reduces blood pressure by utilizing different drugs and methods during the period of anesthesia, typically maintaining the average arterial pressure at 55–60 mm Hg. The purpose of this technique is to reduce peripheral blood flow and blood loss during surgery. This technique also improves the surgical field of vision while sustaining normal central venous pressure, stroke volume, and cardiac output. Many researchers have confirmed that this technique can effectively reduce intraoperative blood loss43, 44.
Although hypotension anesthesia is commonly used in TKA, there are few reports evaluating its effectiveness in TKA. A prospective, randomized, single‐blind study of 100 patients treated with TKA showed that controlled hypotension reduced the drop in Hb levels when compared with using a tourniquet45. The results of the study showed that the Hb concentration in the hypotensive anesthesia group was higher at the end of surgery, and 5 and 6 days after surgery (P = 0.043, 0.012, 0.014 respectively). It also reduced the transfusion rate (18.4% vs 29.4%; P = 0.196) and transfusion units (19 U vs 33 U; P = 0.222). Another study found that compared with the control group, total blood loss in the controlled hypotension groups was significantly decreased (1056 vs 1826 mL; P = 0.001), blood transfusion rates were lower (42.9% vs 81.2%; P < 0.05), and the average transfusion volume was reduced (93 mL vs 775 mL; P = 0.005)46. Although current studies show that controlled hypotension can effectively reduce blood loss in TKA, more high‐quality research is required to prove the value of hypotensive anesthesia in TKA.
Tourniquet
Using a tourniquet can effectively reduce intraoperative bleeding, provide a clear surgical visual field, and shorten operation time. Thus, it has been routinely used in TKA. Some researchers support the application of a tourniquet in TKA. In a randomized controlled study, Tai et al. discovered that in 72 cases of TKA where a tourniquet was utilized, there was a reduced postoperative loss of Hb (2.6 ± 0.9 g/dL vs 3.7 ± 1.3 g/dL) and hematocrit (7.6% ± 2.8% vs 10.4% ± 4.0%), and lower increases in postoperative C‐reactive protein (139 ± 75 mg/dL vs 175 ± 55 mg/dL) and creatine phosphokinase (162 ± 104 U/L vs 214 ± 89 U/L)47.
However, a significant amount of literature does not support the application of a tourniquet. A recent article reports that tourniquet use would increase the bone cement thickness (14.2 mm vs 13 mm; P = 0.009) and blood loss postoperatively (0.9 L vs 0.6 L; P = 0.02)48. Mori and his colleagues point out that tourniquet use was also associated with higher risk of distal deep venous thrombosis (52.9% vs 23.1%; P = 0.002)49. In addition, the use of a tourniquet led to the weakness of quadriceps femoris muscle and negatively affected the recovery of knee function postoperatively50, 51. A meta‐analysis examining 13 randomized controlled study with 689 patients showed that although a tourniquet reduced the intraoperative blood loss (weighted mean difference [WMD] = −198.21; P < 0.01), it did not reduce the total blood loss (WMD = 63.20; P = 0.80) and the rate of transfusion (RR = 1.27; P = 0.47)52. It also led to a decreased range of motion (WMD = −10.41; P < 0.01), and increased the risk of thrombotic events (RR = 5.00; P = 0.02), and other related complications (RR = 2.03; P = 0.02).
Some researchers support a shorter duration of tourniquet use to minimize the incidence of postoperative complications. Wang et al. compared the difference of using a tourniquet for a short and long period of time53. They found that longer use of the tourniquet reduced total blood loss, where shorter use helped decrease pain and limb swelling, and promoted functional recovery after the operation. However, there was no significant difference in transfusion rates between the two groups. Other investigators have pointed out that earlier release of the tourniquet would prolong the operation time and increase the rate of blood transfusion, and does not benefit patients54. A meta‐analysis by Zan et al. confirmed that early release of tourniquet increased the total blood loss (MD = 184.19 mL; P < 0.00001), but may reduce the risk of various complications, including postoperative incision margin erythema, exudation, necrosis, shallow infection, and deep vein thrombosis (odds risk [OR] = 0.39; P = 0.0007), as well as other complications, including wound dehiscence, hematoma, and deep infection (OR = 0.32; P = 0.05)55. Overall, the use of tourniquets remains a controversial issue. Based on the current evidence we do not recommend the routine use of tourniquets in TKA.
Tranexamic Acid
Tranexamic acid plays an important role in blood conservation strategies during TKA. Many studies show that TXA can effectively reduce blood loss and transfusion rates in TKA56, 57, 58, 59. TXA can be administrated orally, through intravenous infusion, and by topical application. Although in the literature oral TXA has been reported to effectively reduce blood loss in TKA60, 61, TXA is mainly administrated by intravenous infusion and topical application. A meta‐analysis of 15 randomized controlled trials reported that intravenous TXA reduced blood loss by 504.9 mL (P < 0.00001) and blood transfusion units by 1.43 (P < 0.00001), and did not increase the risk of deep vein thrombosis (OR = 0.75; P = 0.48) or pulmonary embolism (OR = 0.65; P = 0.50)62.
Topical application of TXA in the joint cavity is also an effective approach to reduce blood loss in TKA. Gomez‐Barrena et al. demonstrate that 3 g of topical TXA is as effective as intravenous TXA (15 mg/kg TXA before releasing tourniquet and 3 h postoperatively)56. In a meta‐analysis including 12 studies with 1189 patients, Yue et al. found that topical TXA reduced blood loss by an average of 280.65 mL (P < 0.00001), decreased the risk of blood transfusion (risk ratio [RR], 0.26; P < 0.00001), and was not associated with an increased risk of deep venous thrombosis or pulmonary embolism (P > 0.05)63. They recommend the dosage of topical TXA to be no less than 20 mg/kg.
Intravenous TXA combined with topical TXA is effective at reducing blood loss and transfusion rates. Recently, researchers compared the effects of 1 g of intravenous TXA combined with 3 g of topical TXA to 1 g of intravenous TXA alone in reducing blood loss in TKA64. The results demonstrated that compared to the control group, the combined group had decreased blood loss on postoperative day 1 (MD, 277 mL; P = 0.002) and day 2 (MD, 373 mL; P = 0.003). A meta‐analysis compared intravenous or topical TXA alone to the combination of both, which revealed that the combination facilitated less total blood loss, hidden blood loss, drainage volume, a lower transfusion rate, and a lower decline of Hb level (P < 0.05), and was not associated with a higher risk of wound infection and deep venous thrombosis (P > 0.05)65. There is high quality evidence that favors the use of TXA in TKA. Thus, we recommend that TXA be routinely used in TKA.
Topical Hemostatic Agents
Topical hemostatic agents are primarily utilized to promote blood clotting at the surgical incision site and to reduce postoperative bleeding. Current topical hemostatic agents used to reduce bleeding in TKA include platelet‐rich plasma and fibrin sealant. Platelet‐rich plasma is rich in platelets, fibrinogen, thromboxane, platelet‐derived growth factor and transforming growth factor beta. Therefore, platelet‐rich plasma theoretically promotes hemostasis and wound healing. Mochizuki et al. conducted a clinical trial on reducing bleeding in TKA with platelet‐rich plasma66. A total of 315 patients were included in the trial, and the results showed that compared to patients who did not receive platelet‐rich plasma, platelet‐rich plasma can effectively reduce blood loss (446.9 ± 149.7 mL vs 550.7 ± 178.1 mL, P < 0.001) and improve the Hb level (P < 0.05). Aggarwal et al. and Gardner et al. also found that platelet‐rich plasma can effectively reduce blood loss and postoperative pain, and promote early postoperative function in TKA67, 68.
However, other researchers have found that platelet‐rich plasma does not reduce blood loss in TKA69, 70, 71, 72. In a randomized controlled trial, Morishita et al. found that there was no significant difference in blood loss between the platelet‐rich plasma group and a control group (826.2 mL vs 830.2 mL; P = 0.96) in TKA72. This study also demonstrated that platelet‐rich plasma did not decrease postoperative pain or help improve joint function postoperatively. A related meta‐analysis showed that platelet‐rich plasma could reduce the amount of blood loss postoperatively (RR, 0.73; 95% CI, 0.59–0.90), but the result was highly heterogeneous (P < 0.00001, I 2 = 79%)73.
Fibrin sealant promotes blood coagulation reactions through fibrinogen and thrombin to reduce postoperative bleeding. Many researchers have confirmed that fibrin sealant is associated with the reduction of blood loss in TKA74, 75. In a study of 176 patients treated with TKA, Bou Monsef et al. discovered that fibrin sealant could significantly decrease blood loss (603 mL vs 822 mL; P < 0.005) and blood transfusion rates (18% vs 38%, P < 0.05)74. A recent meta‐analysis reports that fibrinogen sealant could reduce the drop of Hb levels after surgery (MD, −0.72; P < 0.00001) and led to lower drainage volumes (MD, −354.53; P < 0.00001) and transfusion rates (rate difference, −0.27; P = 0.006), and a lower incidence of hematomas (rate difference, −0.11; P = 0.04)76. However, Aguilera et al. tested two different types of fibrin sealants in their study and found that compared with the control group, fibrin sealants did not reduce the total amount of blood loss in TKA (P > 0.05)77. Recently published studies report that fibrin sealant does not reduce blood loss and the rate of blood transfusion78, 79, 80. There is conflicting evidence about the efficacy of topical hemostatic agents in preventing blood loss in TKA. Thus, additional research is needed to ascertain the application of platelet‐rich plasma and fibrin sealant in TKA.
Postoperative Strategies
Drainage
The placement of a drainage tube followed the standard approach during TKA. Drainage tubes can prevent joint hematoma and exudation, and, thus, theoretically prevent infection and decrease limb swelling81. However, some researchers have discovered that using drainage tubes increases postoperative bleeding and transfusion rates (P < 0.05)82. A prospective randomized study by Jhurani et al. failed to find any significant difference in postoperative Hb concentrations (P = 0.38), blood loss (P = 0.33), and transfusion rates (P = 0.52) between patients using drainage tubes and those without in TKA83. Two recent meta‐analyses revealed that whether or not drainage tubes were used in TKA led to no significant differences in blood loss, infection rate, hematoma formation, deep vein thrombosis, and function recovery84, 85. Several published studies have shown that clamping the drainage tube can reduce the postoperative blood loss86, 87. A related meta‐analysis pointed out that clamping the drainage tube for 4–6 h led to a lower drop in Hb levels (WMD, −0.43; P < 0.00001) and optimized blood loss (WMD, −305.09; P < 0.00001)87. Based on the data, drainage tube use in TKA is a controversial issue. The current evidence shows that the usage of drainage lacks efficacy in preventing blood loss and reducing transfusion rates in TKA, while drainage clamping may optimize the outcome when drainage is used in TKA.
Blood Salvage Systems
Blood salvage systems may be used intraoperatively or postoperatively to collect and reinfuse shed blood (washed or unwashed) into the patient to reduce allogeneic blood transfusion. Numerous studies report that this technique can reduce blood loss volume and allogeneic blood transfusion units in TKA88, 89. Horstmann et al. found that reinfusion of autologous shed blood can optimize the Hb levels postoperatively (11.6 g/dL vs 11 g/dL; P = 0.003), and decrease blood loss (1576 mL vs 1837 mL; P = 0.03) and allogeneic blood transfusion rates (10.2% vs 19.6%; P = 0.15)88. A meta‐analysis including 43 studies with 5631 patients demonstrated that reinfusion systems can reduce the need for allogeneic blood transfusions (RR, 0.51; 95% CI, 0.39–0.68), but an analysis of studies after 2010 indicated that reinfusing the shed blood does not reduce the rate of allogeneic blood transfusions (RR, 0.91; 95% CI, 0.63–1.31)90. Recently, some studies reported that using reinfusion systems does reduce allogeneic transfusion rates but instead increases the costs of health care29, 91. So‐Osman et al. researched the effect of reinfusing shed blood under strict standards of blood transfusion in TKA29. The study involving 683 patients found that reinfusing shed blood does not reduce allogeneic blood transfusions (OR, 1.3; P = 0.26) without the use of erythropoietin. However, when accompanied with erythropoietin, reinfusing shed blood significantly increases the requirement of allogeneic blood transfusions (OR, 2.2; P = 0.02). The authors also found that the use of reinfusion systems increases the medical care cost by €537 (P = 0.03). Thus, according to the current evidence, we do not recommend the routine use of blood salvage systems.
Postoperative Limb Positioning
Postoperative flexion of the knee joint is the most simple, economical, and effective measure to reduce postoperative bleeding in TKA92. A recent study reported that flexing the knee at 45° for 48 h postoperatively reduces the total blood loss (1008.4 ± 102.6 mL vs 1212.0 ± 113.9 mL; P < 0.05) and hidden blood loss (505.1 ± 28.0 mL vs 617.5 ± 52.4 mL; P < 0.05), and increases the Hb level (10.8 ± 1.1 mL vs 10.0 ± 1.3 mL; P = 0.04)93. Many researchers reiterate that the postoperative flexion of the knee can reduce the hidden blood loss and limb swelling, and promote the function recovery in their studies94, 95. A meta‐analysis, including 10 randomized controlled studies, supported these findings by reporting that flexing the knee postoperatively led to lower total blood loss (MD, −130.66 mL; P = 0.0002) hidden blood loss (MD, −73.27 mL; P = 0.001) and Hb level (MD, 0.73; P < 0.00001), increased the range of motion (MD, 3.79; P = 0.002), and was not associated with higher risk of deep venous thrombosis and wound infection (P > 0.05)94. Thus, we view flexing knee postoperatively as a simple and cost‐effective method to decrease blood loss in TKA.
Restrictive Transfusion Thresholds
Strict transfusion thresholds are important measures of blood management in TKA, which may significantly reduce the rate of blood transfusion. In a retrospective cohort study, Loftus et al. demonstrated that restrictive transfusion algorithms could effectively reduce blood transfusions96. The study including 19 950 patients showed that patients with Hb levels higher than 7 g/dL, or who were hemostatically stable with systolic pressures no less than 100 mm Hg and heart rates no higher than 100 bpm, did not receive blood transfusions. The restrictive transfusion thresholds implemented in this study decreased the rate of blood transfusion by 44% (11.7% vs 20.9%, P < 0.0001) and the transfused blood units per 1000 patients by 41.3% (262.51 U vs 447.48 U, P < 0.0001), and decreased postoperative complications, mortality rates, the hospital length of stay and 30‐day readmission rates (P < 0.05). The American Association of Blood Banks clinical application guidelines recommend that orthopaedic surgeons use the transfusion standard of Hb less than 8 g/dL97. In a meta‐analysis including 31 studies with 12 587 patients, the authors found that compared with liberal transfusion strategies, a restrictive transfusion strategy (Hb level, 7–8 g/dL) resulted in a 43% decrease in transfusion rates and was not associated with a higher risk of 30‐day mortality or other complications, including myocardial infarction, stroke, pneumonia, infection, and mortality98. Another meta‐analysis suggests that the implementation of restrictive transfusion thresholds for non‐cardiac surgery in patients with cardiovascular disease increases the risk of acute coronary syndromes (RR, 1.78; P = 0.01; I 2 = 0%)99. The present study supports the application of a more liberal transfusion threshold (Hb level > 8 g/dL) for patients with acute or chronic cardiovascular disease. Thus, we recommend adhering to restrictive transfusion thresholds in TKA (Hb level, 7–8 g/dL), but for patients with cardiovascular disease, utilizing more liberal transfusion thresholds (Hb level > 8 g/dL).
Summary
Perioperative blood management strategies for TKA aim to reduce allogeneic blood transfusion rates and associated risks, while reducing associated healthcare costs (Fig. 2). Although various blood management strategies are available, the efficacy and cost–benefit of commonly used methods are debatable and further research is required. For PABD and tourniquet, current evidence does not support their routine use in TKA. The most common strategies used in hospitals is treating iron‐deficiency anemia with intravenous iron preoperatively and tranexamic acid intraoperatively, and implementing restrictive transfusion standards postoperatively. There are many factors that, when managed appropriately, help reduce the rates of allogenic transfusions in TKA. Therefore, it is possible to establish a highly efficient, safe, and cost‐effective blood management protocol eliminating the need for blood transfusions in TKA. Building a blood management algorithm founded on evidence‐based medicine is the ideal direction for improving perioperative blood management for the future.
Figure 2.
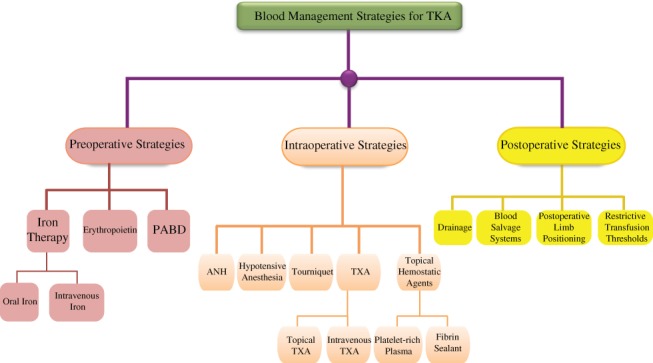
Flow chart showing perioperative blood management strategies for total knee arthroplasty.
Disclosure: This research was funded by the key research and development plan of GuangXi (No. GuiKe AB16380230). All authors report no conflicts of interest.
References
- 1. Melvin JS, Stryker LS, Sierra RJ. Tranexamic acid in hip and knee arthroplasty. J Am Acad Orthop Surg, 2015, 23: 732–740. [DOI] [PubMed] [Google Scholar]
- 2. Gombotz H, Rehak PH, Shander A, Hofmann A. The second Austrian benchmark study for blood use in elective surgery: results and practice change. Transfusion, 2014, 54: 2646–2457. [DOI] [PubMed] [Google Scholar]
- 3. Hart A, Khalil JA, Carli A, Huk O, Zukor D, Antoniou J. Blood transfusion in primary total hip and knee arthroplasty. Incidence, risk factors, and thirty‐day complication rates. J Bone Joint Surg Am, 2014, 96: 1945–1951. [DOI] [PubMed] [Google Scholar]
- 4. Friedman R, Homering M, Holberg G, Berkowitz SD. Allogeneic blood transfusions and postoperative infections after total hip or knee arthroplasty. J Bone Joint Surg Am, 2014, 96: 272–278. [DOI] [PubMed] [Google Scholar]
- 5. Maempel JF, Wickramasinghe NR, Clement ND, Brenkel IJ, Walmsley PJ. The pre‐operative levels of haemoglobin in the blood can be used to predict the risk of allogenic blood transfusion after total knee arthroplasty. Bone Joint J, 2016, 98: 490–497. [DOI] [PubMed] [Google Scholar]
- 6. Auerbach M, Adamson JW. How we diagnose and treat iron deficiency anemia. Am J Hematol, 2016, 91: 31–38. [DOI] [PubMed] [Google Scholar]
- 7. Munoz M, Gomez‐Ramirez S, Campos A, Ruiz J, Liumbruno GM. Pre‐operative anaemia: prevalence, consequences and approaches to management. Blood Transfus, 2015, 13: 370–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Baron DM, Hochrieser H, Posch M, et al Preoperative anaemia is associated with poor clinical outcome in non‐cardiac surgery patients. Br J Anaesth, 2014, 113: 416–423. [DOI] [PubMed] [Google Scholar]
- 9. Musallam KM, Tamim HM, Richards T, et al Preoperative anaemia and postoperative outcomes in non‐cardiac surgery: a retrospective cohort study. Lancet, 2011, 378: 1396–1407. [DOI] [PubMed] [Google Scholar]
- 10. Smilowitz NR, Oberweis BS, Nukala S, et al Association between anemia, bleeding, and transfusion with long‐term mortality following noncardiac surgery. Am J Med, 2016, 129: 315–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Clevenger B, Richards T. Pre‐operative anaemia. Anaesthesia, 2015, 70 (Suppl. 1): 20–28, e6–8. [DOI] [PubMed] [Google Scholar]
- 12. Guinn NR, Guercio JR, Hopkins TJ, et al How do we develop and implement a preoperative anemia clinic designed to improve perioperative outcomes and reduce cost? Transfusion, 2016, 56: 297–303. [DOI] [PubMed] [Google Scholar]
- 13. Lopez A, Cacoub P, Macdougall IC, Peyrin‐Biroulet L. Iron deficiency anaemia. Lancet, 2016, 387: 907–916. [DOI] [PubMed] [Google Scholar]
- 14. Munoz M, Garcia‐Erce JA, Cuenca J, Bisbe E, Naveira E, AWGE (Spanish Anaemia Working Group) . On the role of iron therapy for reducing allogeneic blood transfusion in orthopaedic surgery. Blood Transfus, 2012, 10: 8–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Low MS, Speedy J, Styles CE, De‐Regil LM, Pasricha SR. Daily iron supplementation for improving anaemia, iron status and health in menstruating women. Cochrane Database Syst Rev, 2016, 4: D9747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Moore RA, Gaskell H, Rose P, Allan J. Meta‐analysis of efficacy and safety of intravenous ferric carboxymaltose (Ferinject) from clinical trial reports and published trial data. BMC Blood Disord, 2011, 11: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lachance K, Savoie M, Bernard M, et al Oral ferrous sulfate does not increase preoperative hemoglobin in patients scheduled for hip or knee arthroplasty. Ann Pharmacother, 2011, 45: 764–770. [DOI] [PubMed] [Google Scholar]
- 18. Cuenca J, Garcia‐Erce JA, Martinez F, Cardona R, Perez‐Serrano L, Munoz M. Preoperative haematinics and transfusion protocol reduce the need for transfusion after total knee replacement. Int J Surg, 2007, 5: 89–94. [DOI] [PubMed] [Google Scholar]
- 19. Yang Y, Li H, Li B, Wang Y, Jiang S, Jiang L. Efficacy and safety of iron supplementation for the elderly patients undergoing hip or knee surgery: a meta‐analysis of randomized controlled trials. J Surg Res, 2011, 171: e201–e207. [DOI] [PubMed] [Google Scholar]
- 20. Avni T, Bieber A, Grossman A, Green H, Leibovici L, Gafter‐Gvili A. The safety of intravenous iron preparations: systematic review and meta‐analysis. Mayo Clin Proc, 2015, 90: 12–23. [DOI] [PubMed] [Google Scholar]
- 21. Munoz M, Gomez‐Ramirez S, Cuenca J, et al Very‐short‐term perioperative intravenous iron administration and postoperative outcome in major orthopedic surgery: A pooled analysis of observational data from 2547 patients. Transfusion, 2014, 54: 289–299. [DOI] [PubMed] [Google Scholar]
- 22. Litton E, Xiao J, Ho KM. Safety and efficacy of intravenous iron therapy in reducing requirement for allogeneic blood transfusion: systematic review and meta‐analysis of randomised clinical trials. BMJ, 2013, 347: f4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Alsaleh K, Alotaibi GS, Almodaimegh HS, Aleem AA, Kouroukis CT. The use of preoperative erythropoiesis‐stimulating agents (ESAs) in patients who underwent knee or hip arthroplasty: a meta‐analysis of randomized clinical trials. J Arthroplasty, 2013, 28: 1463–1472. [DOI] [PubMed] [Google Scholar]
- 24. Munoz M, Gomez‐Ramirez S, Kozek‐Langeneker S, et al ‘Fit to fly’: overcoming barriers to preoperative haemoglobin optimization in surgical patients. Br J Anaesth, 2015, 115: 15–24. [DOI] [PubMed] [Google Scholar]
- 25. Lin DM, Lin ES, Tran MH. Efficacy and safety of erythropoietin and intravenous iron in perioperative blood management: a systematic review. Transfus Med Rev, 2013, 27: 221–234. [DOI] [PubMed] [Google Scholar]
- 26. Munoz M, Gomez‐Ramirez S, Kozek‐Langeneker S. Pre‐operative haematological assessment in patients scheduled for major surgery. Anaesthesia, 2016, 71 (Suppl. 1): 19–28. [DOI] [PubMed] [Google Scholar]
- 27. Goodnough LT, Maniatis A, Earnshaw P, et al Detection, evaluation, and management of preoperative anaemia in the elective orthopaedic surgical patient: NATA guidelines. Br J Anaesth, 2011, 106: 13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bedair H, Yang J, Dwyer MK, McCarthy JC. Preoperative erythropoietin alpha reduces postoperative transfusions in THA and TKA but may not be cost‐effective. Clin Orthop Relat Res, 2015, 473: 590–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. So‐Osman C, Nelissen RG, Koopman‐van GA, et al Patient blood management in elective total hip‐ and knee‐replacement surgery (Part 1): a randomized controlled trial on erythropoietin and blood salvage as transfusion alternatives using a restrictive transfusion policy in erythropoietin‐eligible patients. Anesthesiology, 2014, 120: 839–851. [DOI] [PubMed] [Google Scholar]
- 30. Voorn VM, van der Hout A, So‐Osman C, et al Erythropoietin to reduce allogeneic red blood cell transfusion in patients undergoing total hip or knee arthroplasty. Vox Sang, 2016, 111: 219–225. [DOI] [PubMed] [Google Scholar]
- 31. Kapadia BH, Banerjee S, Issa K, McElroy MJ, Harwin SF, Mont MA. Preoperative blood management strategies for total knee arthroplasty. J Knee Surg, 2013, 26: 373–377. [DOI] [PubMed] [Google Scholar]
- 32. Henry DA, Carless PA, Moxey AJ, et al Pre‐operative autologous donation for minimising perioperative allogeneic blood transfusion. Cochrane Database Syst Rev, 2002, 2: D3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jakovina BS, Bicanic G, Hrabac P, Tripkovic B, Delimar D. Pre‐operative autologous blood donation versus no blood donation in total knee arthroplasty: a prospective randomised trial. Int Orthop, 2014, 38: 341–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Perazzo P, Vigano M, De Girolamo L, et al Blood management and transfusion strategies in 600 patients undergoing total joint arthroplasty: an analysis of pre‐operative autologous blood donation. Blood Transfus, 2013, 11: 370–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Su LL, Adamski J, Gilman EA, Cusick R, Hernandez JS. Decreasing preoperative autologous blood donation: collaboration between a hospital and a blood center to prompt change in physician ordering behavior. Lab Med, 2015, 46: 74–78. [DOI] [PubMed] [Google Scholar]
- 36. Etchason J, Petz L, Keeler E, et al The cost effectiveness of preoperative autologous blood donations. N Engl J Med, 1995, 332: 719–724. [DOI] [PubMed] [Google Scholar]
- 37. Barile L, Fominskiy E, Di Tomasso N, et al Acute normovolemic hemodilution reduces allogeneic red blood cell transfusion in cardiac surgery: a systematic review and meta‐analysis of randomized trials. Anesth Analg, 2017, 124: 743–752. [DOI] [PubMed] [Google Scholar]
- 38. Guo JR, Shen HC, Liu Y, et al Effect of acute normovolemic hemodilution combined with controlled low central venous pressure on blood coagulation function and blood loss in patients undergoing resection of liver cancer operation. Hepatogastroenterology, 2015, 62: 992–996. [PubMed] [Google Scholar]
- 39. Olsfanger D, Fredman B, Goldstein B, Shapiro A, Jedeikin R. Acute normovolaemic haemodilution decreases postoperative allogeneic blood transfusion after total knee replacement. Br J Anaesth, 1997, 79: 317–321. [DOI] [PubMed] [Google Scholar]
- 40. Schmied H, Schiferer A, Sessler DI, Meznik C. The effects of red‐cell scavenging, hemodilution, and active warming on allogenic blood requirements in patients undergoing hip or knee arthroplasty. Anesth Analg, 1998, 86: 387–391. [DOI] [PubMed] [Google Scholar]
- 41. Juelsgaard P, Moller M, Larsen U. Preoperative acute normovolaemic hemodilution (ANH) in combination with hypotensive epidural anaesthesia (HEA) during knee arthroplasty surgery. No effect on transfusion rate. A randomized controlled trial [ISRCTN87597684]. BMC Anesthesiol, 2002, 2: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zohar E, Fredman B, Ellis M, Luban I, Stern A, Jedeikin R. A comparative study of the postoperative allogeneic blood‐sparing effect of tranexamic acid versus acute normovolemic hemodilution after total knee replacement. Anesth Analg, 1999, 89: 1382–1387. [DOI] [PubMed] [Google Scholar]
- 43. Lin S, McKenna SJ, Yao CF, Chen YR, Chen C. Effects of hypotensive anesthesia on reducing intraoperative blood loss, duration of operation, and quality of surgical field during orthognathic surgery: a systematic review and meta‐analysis of randomized controlled trials. J Oral Maxillofac Surg, 2017, 75: 73–86. [DOI] [PubMed] [Google Scholar]
- 44. Freeman AK, Thorne CJ, Gaston CL, et al Hypotensive epidural anesthesia reduces blood loss in pelvic and sacral bone tumor resections. Clin Orthop Relat Res, 2017, 475: 634–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kiss H, Raffl M, Neumann D, Hutter J, Dorn U. Epinephrine‐augmented hypotensive epidural anesthesia replaces tourniquet use in total knee replacement. Clin Orthop Relat Res, 2005, 436: 184–189. [DOI] [PubMed] [Google Scholar]
- 46. Juelsgaard P, Larsen UT, Sorensen JV, Madsen F, Soballe K. Hypotensive epidural anesthesia in total knee replacement without tourniquet: reduced blood loss and transfusion. Reg Anesth Pain Med, 2001, 26: 105–110. [DOI] [PubMed] [Google Scholar]
- 47. Tai TW, Chang CW, Lai KA, Lin CJ, Yang CY. Effects of tourniquet use on blood loss and soft‐tissue damage in total knee arthroplasty: a randomized controlled trial. J Bone Joint Surg Am, 2012, 94: 2209–2215. [DOI] [PubMed] [Google Scholar]
- 48. Pfitzner T, von Roth P, Voerkelius N, Mayr H, Perka C, Hube R. Influence of the tourniquet on tibial cement mantle thickness in primary total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc, 2016, 24: 96–101. [DOI] [PubMed] [Google Scholar]
- 49. Mori N, Kimura S, Onodera T, Iwasaki N, Nakagawa I, Masuda T. Use of a pneumatic tourniquet in total knee arthroplasty increases the risk of distal deep vein thrombosis: a prospective, randomized study. Knee, 2016, 23: 887–889. [DOI] [PubMed] [Google Scholar]
- 50. Dennis DA, Kittelson AJ, Yang CC, Miner TM, Kim RH, Stevens‐Lapsley JE. Does tourniquet use in TKA affect recovery of lower extremity strength and function? A randomized trial. Clin Orthop Relat Res, 2016, 474: 69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Guler O, Mahirogullari M, Isyar M, et al Comparison of quadriceps muscle volume after unilateral total knee arthroplasty with and without tourniquet use. Knee Surg Sports Traumatol Arthrosc, 2016, 24: 2595–2605. [DOI] [PubMed] [Google Scholar]
- 52. Zhang W, Li N, Chen S, Tan Y, Al‐Aidaros M, Chen L. The effects of a tourniquet used in total knee arthroplasty: a meta‐analysis. J Orthop Surg Res, 2014, 9: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wang K, Ni S, Li Z, et al The effects of tourniquet use in total knee arthroplasty: a randomized, controlled trial. Knee Surg Sports Traumatol Arthrosc, 2017, 25: 2849–2857. [DOI] [PubMed] [Google Scholar]
- 54. Hafeez S, Amin MS, Ameen J, Adnan R. Early release of tourniquet in total knee arthroplasty: is it worthwhile? J Pak Med Assoc, 2015, 65: S77–S81. [PubMed] [Google Scholar]
- 55. Zan PF, Yang Y, Fu D, Yu X, Li GD. Releasing of tourniquet before wound closure or not in total knee arthroplasty: a meta‐analysis of randomized controlled trials. J Arthroplasty, 2015, 30: 31–37. [DOI] [PubMed] [Google Scholar]
- 56. Gomez‐Barrena E, Ortega‐Andreu M, Padilla‐Eguiluz NG, Perez‐Chrzanowska H, Figueredo‐Zalve R. Topical intra‐articular compared with intravenous tranexamic acid to reduce blood loss in primary total knee replacement: a double‐blind, randomized, controlled, noninferiority clinical trial. J Bone Joint Surg Am, 2014, 96: 1937–1944. [DOI] [PubMed] [Google Scholar]
- 57. Lin SY, Chen CH, YC F, Huang PJ, Chang JK, Huang HT. The efficacy of combined use of intraarticular and intravenous tranexamic acid on reducing blood loss and transfusion rate in total knee arthroplasty. J Arthroplasty, 2015, 30: 776–780. [DOI] [PubMed] [Google Scholar]
- 58. Pitta M, Zawadsky M, Verstraete R, Rubinstein A. Intravenous administration of tranexamic acid effectively reduces blood loss in primary total knee arthroplasty in a 610‐patient consecutive case series. Transfusion, 2016, 56: 466–471. [DOI] [PubMed] [Google Scholar]
- 59. Serrano ML, Goudarz MK, Caceres L, Lee YY, Gonzalez Della Valle A. Topical tranexamic acid may improve early functional outcomes of primary total knee arthroplasty. J Arthroplasty, 2016, 31: 1449–1452. [DOI] [PubMed] [Google Scholar]
- 60. Alipour M, Tabari M, Keramati M, Zarmehri AM, Makhmalbaf H. Effectiveness of oral Tranexamic acid administration on blood loss after knee artroplasty: a randomized clinical trial. Transfus Apher Sci, 2013, 49: 574–577. [DOI] [PubMed] [Google Scholar]
- 61. Irwin A, Khan SK, Jameson SS, Tate RC, Copeland C, Reed MR. Oral versus intravenous tranexamic acid in enhanced‐recovery primary total hip and knee replacement: results of 3000 procedures. Bone Joint J, 2013, 95: 1556–1561. [DOI] [PubMed] [Google Scholar]
- 62. Yang ZG, Chen WP, Wu LD. Effectiveness and safety of tranexamic acid in reducing blood loss in total knee arthroplasty: a meta‐analysis. J Bone Joint Surg Am, 2012, 94: 1153–1159. [DOI] [PubMed] [Google Scholar]
- 63. Yue C, Pei F, Yang P, Xie J, Kang P. Effect of topical tranexamic acid in reducing bleeding and transfusions in TKA. Orthopedics, 2015, 38: 315–324. [DOI] [PubMed] [Google Scholar]
- 64. Nielsen CS, Jans O, Orsnes T, Foss NB, Troelsen A, Husted H. Combined intra‐articular and intravenous tranexamic acid reduces blood loss in total knee arthroplasty: a randomized, double‐blind, placebo‐controlled trial. J Bone Joint Surg Am, 2016, 98: 835–841. [DOI] [PubMed] [Google Scholar]
- 65. Yuan ZF, Yin H, Ma WP, Xing DL. The combined effect of administration of intravenous and topical tranexamic acid on blood loss and transfusion rate in total knee arthroplasty: combined tranexamic acid for TKA. Bone Joint Res, 2016, 5: 353–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Mochizuki T, Yano K, Ikari K, et al Platelet‐rich plasma for the reduction of blood loss after total knee arthroplasty: a clinical trial. Eur J Orthop Surg Traumatol, 2016, 26: 901–905. [DOI] [PubMed] [Google Scholar]
- 67. Aggarwal AK, Shashikanth VS, Marwaha N. Platelet‐rich plasma prevents blood loss and pain and enhances early functional outcome after total knee arthroplasty: a prospective randomised controlled study. Int Orthop, 2014, 38: 387–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Gardner MJ, Demetrakopoulos D, Klepchick PR, Mooar PA. The efficacy of autologous platelet gel in pain control and blood loss in total knee arthroplasty. An analysis of the haemoglobin, narcotic requirement and range of motion. Int Orthop, 2007, 31: 309–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Bernasek TL, Burris RB, Fujii H, Levering MF, Polikandriotis JA, Patterson JJ. Effect on blood loss and cost‐effectiveness of pain cocktails, platelet‐rich plasma, or fibrin sealant after total knee arthroplasty. J Arthroplasty, 2012, 27: 1448–1451. [DOI] [PubMed] [Google Scholar]
- 70. Diiorio TM, Burkholder JD, Good RP, Parvizi J, Sharkey PF. Platelet‐rich plasma does not reduce blood loss or pain or improve range of motion after TKA. Clin Orthop Relat Res, 2012, 470: 138–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Guerreiro JP, Danieli MV, Queiroz AO, Deffune E, Ferreira RR. Platelet‐rich plasma (PRP) applied during total knee arthroplasty. Rev Bras Ortop, 2015, 50: 186–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Morishita M, Ishida K, Matsumoto T, Kuroda R, Kurosaka M, Tsumura N. Intraoperative platelet‐rich plasma does not improve outcomes of total knee arthroplasty. J Arthroplasty, 2014, 29: 2337–2341. [DOI] [PubMed] [Google Scholar]
- 73. Carless PA, Rubens FD, Anthony DM, O’Connell D, Henry DA. Platelet‐rich‐plasmapheresis for minimising peri‐operative allogeneic blood transfusion. Cochrane Database Syst Rev, 2011, 3: D4172. [DOI] [PubMed] [Google Scholar]
- 74. Bou Monsef J, Buckup J, Waldstein W, Cornell C, Boettner F. Fibrin sealants or cell saver eliminate the need for autologous blood donation in anemic patients undergoing primary total knee arthroplasty. Arch Orthop Trauma Surg, 2014, 134: 53–58. [DOI] [PubMed] [Google Scholar]
- 75. Notarnicola A, Moretti L, Martucci A, et al Comparative efficacy of different doses of fibrin sealant to reduce bleeding after total knee arthroplasty. Blood Coagul Fibrinolysis, 2012, 23: 278–284. [DOI] [PubMed] [Google Scholar]
- 76. Li ZJ, Fu X, Tian P, et al Fibrin sealant before wound closure in total knee arthroplasty reduced blood loss: a meta‐analysis. Knee Surg Sports Traumatol Arthrosc, 2015, 23: 2019–2025. [DOI] [PubMed] [Google Scholar]
- 77. Aguilera X, Martinez‐Zapata MJ, Bosch A, et al Efficacy and safety of fibrin glue and tranexamic acid to prevent postoperative blood loss in total knee arthroplasty: a randomized controlled clinical trial. J Bone Joint Surg Am, 2013, 95: 2001–2007. [DOI] [PubMed] [Google Scholar]
- 78. Maheshwari AV, Korshunov Y, Naziri Q, Pivec R, Mont MA, Rasquinha VJ. No additional benefit with use of a fibrin sealant to decrease peri‐operative blood loss during primary total knee arthroplasty. J Arthroplasty, 2014, 29: 2109–2112. [DOI] [PubMed] [Google Scholar]
- 79. Randelli F, D’Anchise R, Ragone V, Serrao L, Cabitza P, Randelli P. Is the newest fibrin sealant an effective strategy to reduce blood loss after total knee arthroplasty? A randomized controlled study. J Arthroplasty, 2014, 29: 1516–1520. [DOI] [PubMed] [Google Scholar]
- 80. Skovgaard C, Holm B, Troelsen A, et al No effect of fibrin sealant on drain output or functional recovery following simultaneous bilateral total knee arthroplasty: a randomized, double‐blind, placebo‐controlled study. Acta Orthop, 2013, 84: 153–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Ares O, Seijas R, Hernandez A, Castellet E, Sallent A. Knee arthroplasty and bleeding: when to remove drainages. Knee Surg Sports Traumatol Arthrosc, 2013, 21: 393–397. [DOI] [PubMed] [Google Scholar]
- 82. Abolghasemian M, Huether TW, Soever LJ, Drexler M, MacDonald MP, Backstein DJ. The use of a closed‐suction drain in revision knee arthroplasty may not be necessary: a prospective randomized study. J Arthroplasty, 2016, 31: 1544–1548. [DOI] [PubMed] [Google Scholar]
- 83. Jhurani A, Shetty GM, Gupta V, Saxena P, Singh N. Effect of closed suction drain on blood loss and transfusion rates in simultaneous bilateral total knee arthroplasty: a prospective randomized study. Knee Surg Relat Res, 2016, 28: 201–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Quinn M, Bowe A, Galvin R, Dawson P, O’Byrne J. The use of postoperative suction drainage in total knee arthroplasty: a systematic review. Int Orthop, 2015, 39: 653–658. [DOI] [PubMed] [Google Scholar]
- 85. Si HB, Yang TM, Zeng Y, Shen B. No clear benefit or drawback to the use of closed drainage after primary total knee arthroplasty: a systematic review and meta‐analysis. BMC Musculoskelet Disord, 2016, 17: 183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Chareancholvanich K, Siriwattanasakul P, Narkbunnam R, Pornrattanamaneewong C. Temporary clamping of drain combined with tranexamic acid reduce blood loss after total knee arthroplasty: a prospective randomized controlled trial. BMC Musculoskelet Disord, 2012, 13: 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Li T, Zhuang Q, Weng X, Zhou L, Bian Y. Non‐continuous versus continuous wound drainage after total knee arthroplasty: a meta‐analysis. Int Orthop, 2014, 38: 361–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Horstmann W, Kuipers B, Ohanis D, Slappendel R, Kollen B, Verheyen C. Autologous re‐transfusion drain compared with no drain in total knee arthroplasty: a randomised controlled trial. Blood Transfus, 2014, 12 (Suppl. 1): S176–S181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Li B, Liu ZT, Shen P, Zhou BZ, Bai LH. Comparison of therapeutic effects between drainage blood reinfusion and temporary clamping drainage after total knee arthroplasty in patients with rheumatoid arthritis. Clinics (Sao Paulo), 2015, 70: 202–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. van Bodegom‐Vos L, Voorn VM, So‐Osman C, et al Cell salvage in hip and knee arthroplasty: a meta‐analysis of randomized controlled trials. J Bone Joint Surg Am, 2015, 97: 1012–1021. [DOI] [PubMed] [Google Scholar]
- 91. Thomassen BJ, den Hollander PH, Kaptijn HH, Nelissen RG, Pilot P. Autologous wound drains have no effect on allogeneic blood transfusions in primary total hip and knee replacement: a three‐arm randomised trial. Bone Joint J, 2014, 96: 765–771. [DOI] [PubMed] [Google Scholar]
- 92. Banerjee S, Kapadia BH, Issa K, et al Postoperative blood loss prevention in total knee arthroplasty. J Knee Surg, 2013, 26: 395–400. [DOI] [PubMed] [Google Scholar]
- 93. Liu J, Li YM, Cao JG, Wang L. Effects of knee position on blood loss following total knee arthroplasty: a randomized, controlled study. J Orthop Surg Res, 2015, 10: 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Fu X, Tian P, Li ZJ, Sun XL, Ma XL. Postoperative leg position following total knee arthroplasty influences blood loss and range of motion: a meta‐analysis of randomized controlled trials. Curr Med Res Opin, 2016, 32: 771–778. [DOI] [PubMed] [Google Scholar]
- 95. Yang Y, Yong‐Ming L, Pei‐jian D, Jia L, Ying‐ze Z. Leg position influences early blood loss and functional recovery following total knee arthroplasty: a randomized study. Int J Surg, 2015, 23: 82–86. [DOI] [PubMed] [Google Scholar]
- 96. Loftus TJ, Spratling L, Stone BA, Xiao L, Jacofsky DJ. A patient blood management program in prosthetic joint arthroplasty decreases blood use and improves outcomes. J Arthroplasty, 2016, 31: 11–14. [DOI] [PubMed] [Google Scholar]
- 97. Carson JL, Guyatt G, Heddle NM, et al Clinical practice guidelines from the AABB: red blood cell transfusion thresholds and storage. JAMA, 2016, 316: 2025–2035. [DOI] [PubMed] [Google Scholar]
- 98. Carson JL, Stanworth SJ, Roubinian N, et al Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion. Cochrane Database Syst Rev, 2016, 10: D2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Docherty AB, O’Donnell R, Brunskill S, et al Effect of restrictive versus liberal transfusion strategies on outcomes in patients with cardiovascular disease in a non‐cardiac surgery setting: systematic review and meta‐analysis. BMJ, 2016, 352: i1351. [DOI] [PMC free article] [PubMed] [Google Scholar]


