Abstract
Objective
Infection of total knee arthroplasty (TKA) is a rare but devastating complication. Two‐stage revision is an effective treatment for late infected TKA. This study aimed to assess the short‐term results of two‐stage revision using articulating antibiotic‐loaded spacers.
Methods
Twenty‐five patients (10 men and 15 women) were diagnosed with late infections after TKA and treated with two‐stage revision from April 2006 to August 2010; 19 of these patients had TKA for osteoarthritis and 6 for rheumatoid arthritis. Median age was 64.9 (range, 56–83) years. In the first‐stage surgery, the prosthesis and all bone cement was removed. After thorough debridement, bone cement with vancomycin and tobramycin was put into a die cavity and made into temporary femoral and tibial spacers, respectively. In the cases of good knee range of motion, the temporary spacers were affixed to the bone surface using the same antibiotic bone cement. In the second surgery, gentamycin Refobacin Bone Cement with vancomycin was used to fix the prosthesis. After two‐stage revision, patients were followed up clinically and radiologically at 1, 3, and 6 months, and then annually. Knee Society Score (KSS), knee function score, knee pain score, and knee range of motion (ROM) were assessed.
Results
Among the group, all spacers were easily removed, and bone defect degree showed no obvious change compared with pre‐implant, 24 (96%) patients had been debrided once, and 1 patient had been debrided twice before reimplant prosthesis. Mean follow‐up was 64.2 (range, 52–89) months. There was no infection recurrence at final follow‐up. Compared with preoperative data, the KSS (66 [59, 71], 83 [80, 88] vs 46 [43, 57], P < 0.01), knee function score (43 [42, 49], 78 [73, 82] vs 32 [25, 37], P < 0.01), knee pain score (34 [33, 37], 42 [40, 45] vs 18 [16, 23], P < 0.01), and knee ROM (92° [86°, 96°], 94° [90°, 98°] vs 78° [67°, 86°], P < 0.01) were all improved during follow‐up and at final visit. Three patients experienced complications in the interval period: one case had knee dislocation, one had knee instability, and one had a chip in the femoral component of the spacer.
Conclusion
Using articulating antibiotic‐loaded spacers showed benefits for treating infected TKA in selected patients. No infection recurrence was observed during follow‐up.
Keywords: Articulating spacer, Bone cement, Delayed infection, Total knee arthroplasty, Two‐stage revision
Introduction
Total knee arthroplasty (TKA) is an effective treatment for end‐stage osteoarthritis and rheumatoid arthritis of the knee. With the elderly population expanding, demand for knee arthroplasty is continuing to increase. By the end of 2030, the number of TKA programs performed is expected to reach approximately 3.5 million annually in the United States1. Peri‐prosthesis joint infection (PJI) is a leading cause of TKA failure. The reported incidence of PJI after TKA was from 0.5% to 1.8%2, 3. The incidence of postoperative infection is on the rise with the increasing volume of TKA.
Treating infected TKA is often challenging and peri‐prosthesis joint infection after TKA is a serious condition that can lead to a lot of complications. Two‐stage revision using a temporary antibiotic‐impregnated cement spacer is the gold standard for the treatment of infected TKA as this allows early knee joint activity, a shorter hospital stay and potentially a decreased rate of reinfection4, 5, 6, 7, 8. The first stage involves the removal of the prosthesis, extensive debridement, the insertion of an antibiotic‐eluting spacer, and intravenous antibiotic therapy. After the infection has been cured, a second‐stage operation is performed to remove the spacer and accomplish the joint reconstruction with revision prosthesis. These spacers can be either static or articulating. Static spacers are used as a block, keeping the knee motionless, which may lead to contraction of joint soft tissue, making difficult the subsequent exposure for the second revision9, 10. Articulating spacers could be used in preference to static spacers to attain the same level of infection clearance rate while preserving range of motion to prevent soft‐tissue contracture and improve the daily activity of patients10.
Some studies have examined the outcomes of using antibiotic‐impregnated articulated spacers, but data are still limited. Van Thiel et al. report a series of 60 patients treated with antibiotic‐loaded articulated spacers and 53 (88.3%) achieved good results11. Chiang et al. report a series of 23 patients that underwent antibiotic‐loaded articulated spacer implantation, with a success rate of 91.3% and an infection recurrence rate of 4.5%10. Emerson et al. showed that a mobile spacer had good efficacy and an infection recurrence rate of 9%9. Garg et al. report that all 36 knees they re‐operated on with antibiotic‐loaded articulated spacers achieved good results in terms of infection control and mobility6. However, there is much controversy in many aspects relating to treatment of infected TKA, including the choice of spacers, application of perioperative antibiotics, surgical interval time, and the occasion of implanting the prosthesis again12. To minimize complications and optimize clinical outcomes, it is necessary to further research the treatment effects for infected TKA using articulating antibiotic‐loaded cement spacer technology.
To provide additional data about the benefits of antibiotic‐loaded articulated spacers, the present study aimed to: (i) evaluate the clinical results and complications of two‐stage revision for infected TKA using articulating antibiotic‐loaded cement spacer (AALCS) technology; (ii) explore the technical points and preliminary experience of the AALCS technology to treat PJI after TKA; and (iii) observe the curative effects of individualized antibiotics usage during the whole treatment.
Patients and Methods
Study Design
This was a retrospective study of patients treated for infected TKA at our hospital between April 2006 and August 2010. The study was approved by the ethics committee of our hospital.
Inclusion and Exclusion Criteria
The inclusion criteria were: (i) primary TKA infection; (ii) no previous history of TKA infection; (iii) knee pain; (iv) knee movement limitation; and (v) treatment with two‐stage revision surgery with AALCS. Patients were excluded who had diseases known to induce severe immunity deficiency, such as malignant tumor, nephrotic syndrome, systemic lupus erythematosus (SLE), human immunodeficiency virus (HIV) infection, chronic hepatitis virus infection, and organ transplantation. Patients with rheumatoid arthritis or diabetes were not excluded.
Diagnosis of Primary Total Knee Arthroplasty Infections
All cases were reviewed and the diagnosis of TKA infection was confirmed based on the criteria by Parvizi et al.13. After knee replacement surgery, patients were considered suspect for infection with symptoms of persistent pain, wound swelling, increased skin temperature, exudate, oozing pus, or skin induration. Infection was confirmed by pyorrhea, sinus tract formation, or positive bacterial culture. X‐rays showing false surrounding translucent bands indicated possible infection. All patients were first treated with antibiotics. If the symptoms persisted for 1 week, the diagnosis was confirmed by knee puncture performed 2 weeks after antibiotic discontinuation. The knee joint cavity puncture and bacteria culture were performed 2 weeks after ceasing administration of antibiotics. A 2‐week delay was used because of the risk of a false‐negative if the puncture was done during antibiotics administration or shortly after. Bacterial culture had to be positive. Patients with negative bacteria culture were diagnosed based on the erythrocyte sedimentation rate (ESR), C‐reactive protein (CRP) levels, synovial fluid examination and histopathological examination. According to Tsukayama et al.14, delayed TKA infection was considered when it occurred more than 1 month after TKA.
First‐stage Surgery
All 25 cases were operated on by four senior orthopaedic surgeons and a chief orthopaedic surgeon with more than 15 years of experience in the diagnosis and treatment of orthopaedic diseases, together with three assistants, a chief physician, an attending physician, and a resident. The two‐stage operations were performed in an operating room with vertical laminar airflow, and all surgeries were performed by the same surgeon. Intraoperative antibiotics based on the preoperative cultures (vancomycin in patients with negative culture) were used 30 min before the first skin incision.
The prosthesis and all bone cement were removed. After debridement, joint fluid and obvious inflammatory tissues were taken for bacterial culture and quick‐frozen sections were examined. Each respective verification required at least three specimens, and two specimens with the same positive result were considered clinically significant. Bone cement (Zimmer, Warsaw, IN, USA) was used to make the tibial and femoral spacers (Fig. 1). For every 40 g of bone cement, 4 g of vancomycin and 2.4 g of tobramycin was added. Bone cement was stirred manually, put into the die cavity, and trimmed according to the bone bed size. After temporary installation of the prosthesis and verifying that flexion and extension clearance were satisfactory, the spacer was affixed to the bone surface at the end of its polymerization time using the same proportion of antibiotic bone cement. Using the appropriate technique, the spacer was pressed to adjust the bone cement thickness to maintain flexion and extension gap balance and to ensure a normal joint level. Vancomycin powder was put in the articular area and drainage was not used. Absorbable suture material was used to close the wound, along with a compression bandage. After the operation, X‐ray examination of the knee was performed (Fig. 2).
Figure 1.

Articulating cement spacer. The femoral spacer was made using a metal prosthesis mold (left). The tibial bone cement spacer was molded with the metal prosthesis (center). The installed cement spacer (right).
Figure 2.
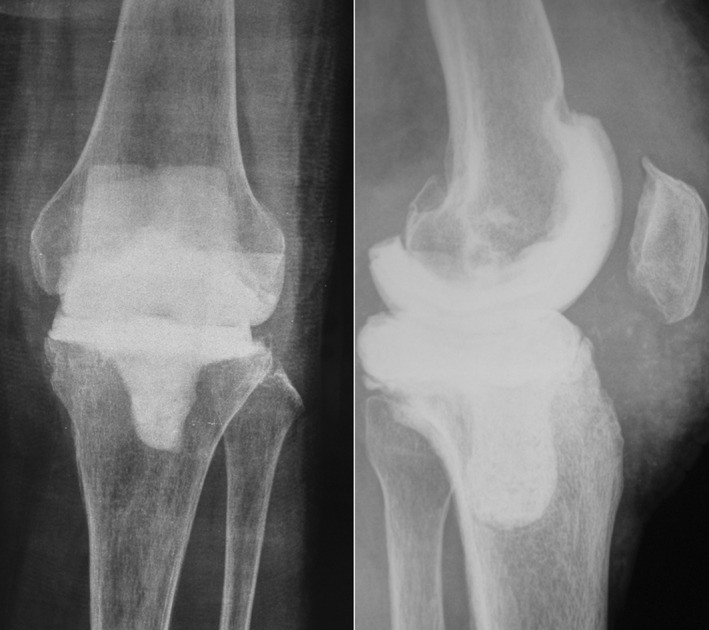
X‐ray after the first surgery. This was a female patient aged 66 years. Infection occurred in the left knee after arthroplasty. The infected prosthesis was removed and an antibiotic‐impregnated bone cement spacer was implanted.
According to the preoperative drug sensitivity results, intravenous non‐vancomycin antibiotics were used in patients with positive cultures. For patients with negative preoperative cultures, vancomycin and other antibiotics based on intraoperative culture results were used. Antibiotics were discontinued when patients’ body temperature returned to normal for at least 2 weeks, local symptoms and signs had disappeared, CRP returned to normal, and ESR showed progressive decline. In special discretionary cases, antibiotic treatment time was extended.
Second Stage Surgery
After the antibiotics had been stopped for 2 weeks and synovial fluid culture (tested twice), ESR, and CRP were normal, the second surgery was performed. Gentamycin Refobacin Bone Cement (Biomet, Warsaw, IN, USA) was used to fix the prosthesis (Fig. 3). For every 40 g of cement, 1 g of vancomycin was added. The wound was dressed with a negative pressure drainage dressing, and the antibiotic use principle was the same as for the first surgery. If the intraoperative culture results were negative a few days later, the antibiotics were discontinued. If suspected infection was still present, and if each high‐power field showed neutrophils in ≥5 frozen sections or if infectious organism was not found, but every high‐power field showed ≥10 neutrophils, wound debridement was repeated.
Figure 3.
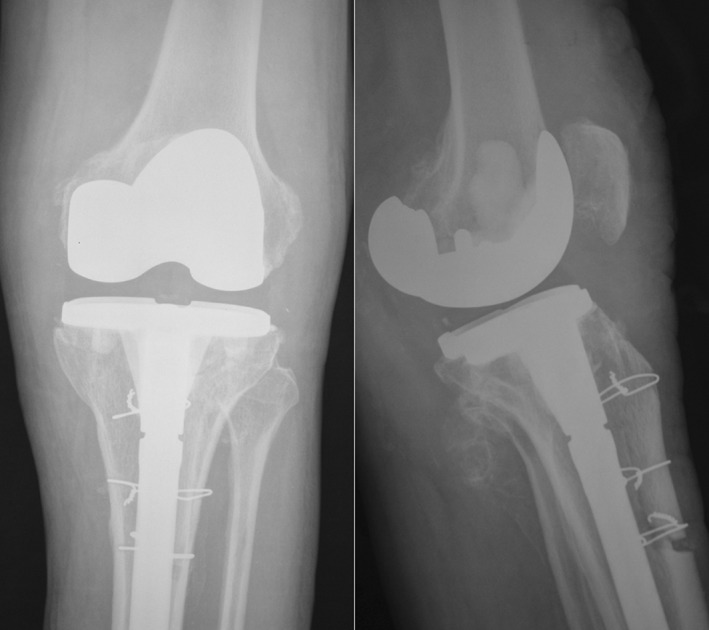
Second operation using the extended tibial tubercle osteotomy and PS prosthesis with tibia extended handle. This patient was the same as in Fig. 2. The second surgery was performed 3 months after the first one.
Follow‐up
After two‐stage revision, all 25 patients were followed up clinically and radiologically at 1, 3, and 6 months, and then annually. The Knee Society Score (KSS) of the American Knee Society was used15. Knee function score16, knee pain score17, and knee range of motion (ROM)18 were assessed. All patients completed follow‐up, without any infection recurrence during the mean follow‐up of 64.2 (52 to 89) months.
Statistical Analysis
Skewed distributed data were expressed as median (interquartile range). The Wilcoxon test was used to compare repeated measures. SPSS 21.0 (IBM, Armonk, NY, USA) was used for analysis. Two‐sided P‐values <0.01 were considered statistically significant.
Results
Characteristics of the Patients
The group included 10 men and 15 women, with a median age of 64.9 years (ranging from 56 to 83 years). Indications for TKA included osteoarthritis (n = 19) and rheumatoid arthritis (n = 6). Among the patients, 8 had diabetes and 4 underwent incision or arthroscopic cleaning operations after infection. The time interval from the initial infection outbreak to revision surgery was 8 to 26 months (mean of 12.9 months). Nineteen patients had elevated local skin temperature. A fistula developed in 7 patients. X‐ray showed a bright band around the prosthesis in 16 patients, and showed periosteal reaction in 4 patients. Bacterial cultures were positive in 19 patients, including 11 cases of low‐toxic coagulase‐negative staphylococcus (CNS) (4 cases were methicillin‐resistant), 3 cases of Staphylococcus aureus (1 case was methicillin‐resistant), and 5 cases of Streptococcus agalactiae. Bacterial culture was positive in 76.0% of cases.
Knee Scores
Table 1 presents the knee scores before surgery, between the two surgeries, and at the last follow‐up. All four scores improved with time (all P < 0.01).
Table 1.
Assessment results of knee function at different stages (n = 25)
| Assessment | Pre‐operation | Interval period | Terminal follow‐up | χ2 | P |
|---|---|---|---|---|---|
| KSS | 46 (43, 57) | 66 (59, 71) | 83 (80, 88) | 43.818 | <0.01 |
| Knee function score | 32 (25, 37) | 43 (42, 49) | 78 (73, 82) | 50.000 | <0.01 |
| Pain score | 18 (16, 23) | 34 (33, 37) | 42 (40, 45) | 41.705 | <0.01 |
| ROM (°) | 78 (67, 86) | 92 (86, 96) | 94 (90, 98) | 31.608 | <0.01 |
Results are presented as median (interquartile range).
KSS, Knee Society Score; ROM, range of motion.
Characteristics of the First Surgery
During the first surgery, all patients had obvious inflammation and necrotic tissues. Four patients had visible purulent exudate. Frozen sections of all patients showed >10 neutrophils in each high‐power field. The intraoperative culture results of 6 patients were negative.
Characteristics of the Interval Period
The mean interval time was 11.5 (range, 6 to 32) weeks. During the interval period, 5 patients showed knee joint instability, among whom 3 patients experienced spacer‐related complications: shank strands of joint dislocation in 1 patient and subluxation in another. In 1 patient, the spacer broke on the femoral side 27 weeks postoperatively. We did not find space prolapse related to the bone cement spacer around spacer fractures, knee extensor device damage, or patellofemoral joint instability.
Characteristics of the Second Surgery
During the second surgery, pus and other signs of infection were absent in all patients. Frozen sections and general pathology examinations did not show any signs of acute inflammation, and bacterial cultures were negative. All spacers were removed, and bone defect degree showed no obvious change compared with the baseline. Twenty‐four (96%) patients were debrided once, and 1 patient had to be debrided twice before implant revision implantation. Articulating spacers were used to maintain the motion range of the knee joint during the interval period between the two surgeries. Nevertheless, some patients did not perform the regular rehabilitation training following the surgeons’ advice, and they lost some motion range (i.e. flexion motion could not reach 90°). Because of the multiple operations, the elasticity of the soft tissue was low. Therefore, to avoid the complications of knee extension apparatus injury (such as patellar tendon avulsion), expanded exposure was applied. During the second surgery, 13 patients needed extension exposure, including 2 cases of quadriceps snip and 11 patients opting for tibial tubercle osteotomy; 7 patients used intramedullary stem prostheses, 5 used condylar‐restricted type prostheses, and 1 used a rotating hinge prosthesis.
Complications
During the whole course of treatments (from the first to the second surgery), no patients showed obvious liver and/or renal abnormalities or other complications such as poor wound healing, deep vein thrombosis, pulmonary embolism, or cardio‐cerebral vascular accident. Meanwhile, during follow‐up, no sinus tract recurrence occurred, and infection manifestations such as knee‐joint swelling and rest pain were absent. Joint instability, limitation of motion, and periprosthetic fracture were not observed either.
Discussion
The standard technique used to treat late TKA infection is two‐stage revision4, 6, 8, 11, 19. Compared with a static spacer, AALCS is closer to normal human knee‐joint anatomical structure and preserves joint mobility10, but relevant data are still limited. Therefore, this study aimed to assess the short‐term results of two‐stage revision using articulating antibiotic‐loaded spacers. There was no infection recurrence at final follow‐up. Compared with preoperative data, KSS, knee function score, knee pain score, and knee ROM were improved during follow‐up and at final visit. During the interval period, 5 patients showed knee joint instability and 3 of them experienced complications: 1 had knee dislocation, 1 patient had knee instability, and 1 had a chip in the femoral component of the spacer. Using articulating antibiotic‐loaded spacers to treat infected TKA seems to have some benefits in selected patients. No infection recurrence was observed during follow‐up.
Antibiotics Choice
The antibiotics were selected according to the results of bacterial culture. Because coagulase‐negative staphylococci, a common pathogen in prosthesis infection, are often resistant to β‐lactam antibiotics, vancomycin has to be used as a first‐line treatment drug, and fluoroquinolone antibiotics can also be used 20. Therefore, vancomycin was chosen for the patients with negative culture. Rifampicin has extremely strong tissue penetration and can damage the sessile bacteria in bacterial biofilms (including methicillin‐resistant Staphylococcus aureus and methicillin‐resistant Staphylococcus epidermidis)21, 22. Here, combination of rifampicin was used to reduce the reoccurrence of infection. Vancomycin and tobramycin were chosen for local application because both have efficient large‐spectrum bactericidal effects on Gram‐positive/negative bacteria. In addition, they also have high affinity with bone tissues and high stability, thus meeting the requirements of a sustained release system5, 23, 24, 25. Previous studies reported the use of a variety of antibiotics to load the cement5, 10, 24, 26, 27. Which antibiotics are optimal for use should still be studied, but it is our opinion that the choice of antibiotics should be made based on the culture results. In the present study, 19 cases showed positive culture and 6 showed negative results. Among the positive cases, 3 had methicillin‐sensitive Staphylococcus aureus (MSSA), 1 had methicillin‐resistant Staphylococcus aureus (MRSA), 4 had methicillin sensitive Staphylococcus epidermidis (MSSE), 5 had methicillin‐resistant Staphylococcus epidermidis (MRSE), and 6 had other infections. There was no case of poly‐bacterial infection. The present study was not designed to determine the exact causes of the high success rate (100%). Possible explanations lie in the fact that a high proportion of the patients were infected by coagulase‐negative staphylococci with low virulence, and by the low frequency of drug‐resistant strains (only 1 case). Furthermore, the local application of vancomycin and tobramycin, combined with the systemic application of rifampin, could decrease the postoperative recurrence, due to their wide antibacterial spectrum. Further study is necessary to assess the exact causes of recurrence after two‐stage surgery.
Operative Technique
In the present study, all patients achieved improvements in knee function and pain, and there was no recurrence. Most previous studies reported good outcomes, with success rates ranging from 88.3% to 100.0%6, 9, 10, 11, 26 and recurrence rates from 4.5% to 9.0%9, 10. Taken together, these results strongly suggest that the use of AALCS is associated with good benefits for patients.
At our center, we usually select the original incision for debridement, and we remove the antrum, foreign bodies, and necrotic tissues. We pay special attention to cleaning the back joint capsule. Residual bone cement in the medullary cavity and bone hole are cleaned by curettage, periosteum elevator, and high‐speed grinding drill. After debridement, a large number of fixed bacteria are released into the joint and form planktonic bacteria28. Checking the effects of debridement after relaxing the tourniquet helps to judge the inactivation of infectious agents accurately. The antibiotics with proved efficacy based on preoperative samples can then permeate the remaining microorganisms and enhance the antibacterial effect. The spacer releases the highest concentration of antibiotics in the early postoperative phase29; therefore, drainage should not be used after the first‐stage debridement. In the second‐stage surgery, the residual microorganisms are reassessed. Repeat debridement offers the opportunity to culture bone and soft‐tissue bacteria and to evaluate the early therapeutic effect.
Spacer Design
The constant release of antibiotics from a large number of pores in the cement spacer may cause the spacer's mechanical strength to gradually wane29. With greater use, the spacer becomes more vulnerable to fatigue fracture, so excessive weight on the knee should be avoided in the interval between the two surgeries. In the present study, an obese patient refused second‐stage treatment because of fear of surgery and perceived improved knee joint function in the interval period, but the spacer eventually created a partial femoral fracture, leading to worsening knee pain and repetitive effusion. After 32 weeks, the patient had an overhaul prosthesis implanted again.
The present study showed improved pain, knee function, and ROM from pre‐operation to the last follow‐up, as supported by previous studies of two‐stage surgery using articulated antibiotics‐loaded spacers26, 27, 30. Because of the knee's flexion and extension movements, the back stabilizing column composed of bone cement often bears a large amount of stress and is easily fractured. Therefore, we do not use the common back stabilizing design19. The tibial spacers of the 5 early patients were planar, and knee joint stability was poor during the interval period: 1 patient had shank strands joint dislocation and 1 patient had shank strands of joint subluxation when the knees flexed more than 90°. The spacer was then designed as a double concave disk with the condyle slightly lifted to improve spacer shape and stability. Obvious knee instability did not recur in the following patients. Postoperative knee joint activity is not very good after the first surgery, and the second surgery is needed to guarantee optimal results.
Research Limitations
The present study is not without limitations. This was a retrospective analysis of a small sample from a single center. In addition, a single chief surgeon performed all surgeries. Finally, the lack of a control group prevented assessing the efficacy and safety of this approach. Additional prospective studies are still necessary.
In conclusion, using articulating antibiotic‐loaded spacers to treat infected TKA seems to have some benefits in selected patients. No infection recurrence was observed during follow‐up.
Disclosure: This study was supported by the Science and Technology Foundation of Guizhou (Grant nos. [2015] 3044 and [2015] 2089).
References
- 1. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am, 2007, 89: 780–785. [DOI] [PubMed] [Google Scholar]
- 2. Namba RS, Inacio MC, Paxton EW. Risk factors associated with deep surgical site infections after primary total knee arthroplasty: an analysis of 56,216 knees. J Bone Joint Surg Am, 2013, 95: 775–782. [DOI] [PubMed] [Google Scholar]
- 3. Pruzansky JS, Bronson MJ, Grelsamer RP, Strauss E, Moucha CS. Prevalence of modifiable surgical site infection risk factors in hip and knee joint arthroplasty patients at an urban academic hospital. J Arthroplasty, 2014, 29: 272–276. [DOI] [PubMed] [Google Scholar]
- 4. Jacobs C, Christensen CP, Berend ME. Static and mobile antibiotic‐impregnated cement spacers for the management of prosthetic joint infection. J Am Acad Orthop Surg, 2009, 17: 356–368. [DOI] [PubMed] [Google Scholar]
- 5. Cui Q, Mihalko WM, Shields JS, Ries M, Saleh KJ. Antibiotic‐impregnated cement spacers for the treatment of infection associated with total hip or knee arthroplasty. J Bone Joint Surg Am, 2007, 89: 871–882. [DOI] [PubMed] [Google Scholar]
- 6. Garg P, Ranjan R, Bandyopadhyay U, Chouksey S, Mitra S, Gupta SK. Antibiotic‐impregnated articulating cement spacer for infected total knee arthroplasty. Indian J Orthop, 2011, 45: 535–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Antoci V, Phillips MJ, Antoci V Jr, Krackow KA. Using an antibiotic‐impregnated cement rod‐spacer in the treatment of infected total knee arthroplasty. Am J Orthop (Belle Mead NJ), 2009, 38: 31–33. [PubMed] [Google Scholar]
- 8. Regis D, Sandri A, Rizzo A, Bartolozzi P. A preformed temporary antibiotic‐loaded cement spacer for the treatment of destructive septic hip arthritis: a case report. Int J Infect Dis, 2010, 14: e259–e261. [DOI] [PubMed] [Google Scholar]
- 9. Emerson RH Jr, Muncie M, Tarbox TR, Higgins LL. Comparison of a static with a mobile spacer in total knee infection. Clin Orthop Relat Res, 2002, (404 ): 132 – 138. [DOI] [PubMed] [Google Scholar]
- 10. Chiang ER, Su YP, Chen TH, Chiu FY, Chen WM. Comparison of articulating and static spacers regarding infection with resistant organisms in total knee arthroplasty. Acta Orthop, 2011, 82: 460–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Van Thiel GS, Berend KR, Klein GR, Gordon AC, Lombardi AV, Della Valle CJ. Intraoperative molds to create an articulating spacer for the infected knee arthroplasty. Clin Orthop Relat Res, 2011, 469: 994–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Burnett RS, Kelly MA, Hanssen AD, Barrack RL. Technique and timing of two‐stage exchange for infection in TKA. Clin Orthop Relat Res, 2007, 464: 164–178. [DOI] [PubMed] [Google Scholar]
- 13. Parvizi J, Adeli B, Zmistowski B, Restrepo C, Greenwald AS. Management of periprosthetic joint infection: the current knowledge: AAOS exhibit selection. J Bone Joint Surg Am, 2012, 94: e104. [DOI] [PubMed] [Google Scholar]
- 14. Tsukayama DT, Goldberg VM, Kyle R. Diagnosis and management of infection after total knee arthroplasty. J Bone Joint Surg Am, 2003, 85: S75–S80. [DOI] [PubMed] [Google Scholar]
- 15. Scuderi GR, Bourne RB, Noble PC, Benjamin JB, Lonner JH, Scott WN. The new knee society knee scoring system. Clin Orthop Relat Res, 2012, 470: 3–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Collins NJ, Misra D, Felson DT, Crossley KM, Roos EM. Measures of knee function: International Knee Documentation Committee (IKDC) subjective knee evaluation form, Knee Injury And Osteoarthritis Outcome Score (KOOS), Knee Injury And Osteoarthritis Outcome Score Physical Function Short Form (KOOS‐PS), Knee Outcome Survey Activities of Daily Living Scale (KOS‐ADL), Lysholm Knee Scoring Scale, Oxford Knee Score (OKS), Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC), Activity Rating Scale (ARS), and Tegner Activity Score (TAS). Arthritis Care Res (Hoboken), 2011, 63: S208–S228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shelbourne KD, Trumper RV. Preventing anterior knee pain after anterior cruciate ligament reconstruction. Am J Sports Med, 1997, 25: 41–47. [DOI] [PubMed] [Google Scholar]
- 18. Dennis DA, Komistek RD, Stiehl JB, Walker SA, Dennis KN. Range of motion after total knee arthroplasty: the effect of implant design and weight‐bearing conditions. J Arthroplasty, 1998, 13: 748–752. [DOI] [PubMed] [Google Scholar]
- 19. Meek RM, Masri BA, Dunlop D, et al Patient satisfaction and functional status after treatment of infection at the site of a total knee arthroplasty with use of the PROSTALAC articulating spacer. J Bone Joint Surg Am, 2003, 85: 1888–1892. [DOI] [PubMed] [Google Scholar]
- 20. Betriu C, Redondo M, Boloix A, Gomez M, Culebras E, Picazo JJ. Comparative activity of linezolid and other new agents against methicillin‐resistant Staphylococcus aureus and teicoplanin‐intermediate coagulase‐negative staphylococci. J Antimicrob Chemother, 2001, 48: 911–913. [DOI] [PubMed] [Google Scholar]
- 21. Tote K, Berghe DV, Deschacht M, de Wit K, Maes L, Cos P. Inhibitory efficacy of various antibiotics on matrix and viable mass of Staphylococcus aureus and Pseudomonas aeruginosa biofilms. Int J Antimicrob Agents, 2009, 33: 525–531. [DOI] [PubMed] [Google Scholar]
- 22. Zimmerli W, Widmer AF, Blatter M, Frei R, Ochsner PE. Role of rifampin for treatment of orthopedic implant‐related staphylococcal infections: a randomized controlled trial. Foreign‐Body Infection (FBI) study group. JAMA, 1998, 279: 1537–1541. [DOI] [PubMed] [Google Scholar]
- 23. Howlin RP, Brayford MJ, Webb JS, Cooper JJ, Aiken SS, Stoodley P. Antibiotic‐loaded synthetic calcium sulfate beads for prevention of bacterial colonization and biofilm formation in periprosthetic infections. Antimicrob Agents Chemother, 2015, 59: 111–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chen AF, Parvizi J. Antibiotic‐loaded bone cement and periprosthetic joint infection. J Long Term Eff Med Implants, 2014, 24: 89–97. [DOI] [PubMed] [Google Scholar]
- 25. Gonzalez Della Valle A, Bostrom M, Brause B, Harney C, Salvati EA. Effective bactericidal activity of tobramycin and vancomycin eluted from acrylic bone cement. Acta Orthop Scand, 2001, 72: 237–240. [DOI] [PubMed] [Google Scholar]
- 26. Vecchini E, Micheloni GM, Perusi F, et al. Antibiotic‐loaded spacer for two‐stage revision of infected Total knee arthroplasty. J Knee Surg, 2017, 30: 231–237. [DOI] [PubMed] [Google Scholar]
- 27. Drexler M, Dwyer T, Kuzyk PR, et al The results of two‐stage revision TKA using Ceftazidime‐Vancomycin‐impregnated cement articulating spacers in Tsukayama Type II periprosthetic joint infections. Knee Surg Sports Traumatol Arthrosc, 2016, 24: 3122–3130. [DOI] [PubMed] [Google Scholar]
- 28. Costerton JW. Biofilm theory can guide the treatment of device‐related orthopaedic infections. Clin Orthop Relat Res, 2005, 437: 7–11. [DOI] [PubMed] [Google Scholar]
- 29. Anagnostakos K, Kelm J, Regitz T, Schmitt E, Jung W. In vitro evaluation of antibiotic release from and bacteria growth inhibition by antibiotic‐loaded acrylic bone cement spacers. J Biomed Mater Res B Appl Biomater, 2005, 72: 373–378. [DOI] [PubMed] [Google Scholar]
- 30. Veltman ES, Moojen DJ, Glehr M, Poolman RW. Similar rate of infection eradication for functional articulating, prefabricated and custom‐made spacers in 2‐stage revision of the infected total hip: a literature review. Hip Int, 2016, 26: 319–326. [DOI] [PubMed] [Google Scholar]


