Abstract
This study was conducted to identify the influence of ambulatory status prior to treatment on survival of patients with spinal metastases. Two investigators independently retrieved relevant electronic literature in PubMed, Embase, and Cochrane Library databases, to identify eligible studies. Effect estimates for hazard risk (HR) were extracted and synthesized through fixed‐effects or random‐effects models as appropriate. A total of 17 eligible studies were identified, with an accumulated number of 3962 participants. HR from 14 studies regarding comparison between ambulatory versus non‐ambulatory groups were pooled using a random‐effects model, and statistical significance was presented for the pooled HR (HR = 1.96; 95% confidence interval [CI], 1.65–2.34). In subgroups of mixed primary tumor and lung cancer, ambulatory status was considered to be a significant prognostic factor (P < 0.05), while in the subgroup of prostate cancer it was not (HR = 1.72; 95% CI, 0.79–3.74). HR from 4 studies related to comparison between Frankel E versus Frankel C–D were pooled using a fixed‐effects model, which revealed statistical significance (HR = 1.73; 95% CI, 1.27–2.36). Ambulatory status is a significant prognostic factor in patients with spinal metastases. However, in patients with primary prostate cancer, the prognostic effect of ambulatory status has not yet been confirmed to be significant.
Keywords: Ambulatory status, Overall survival, Prognostic factor, Spinal metastasis
Introduction
With notable improvements in systemic treatment options and diagnostic techniques, cancer patients’ overall survival has increased obviously over the past three decades. The likelihood of spinal metastasis, however, has grown, impacting patients’ quality of life and treatment outcomes. As many as 70% of advanced cancer patients develop spinal metastases and approximately 10% of all malignant tumor cancer patients are treated for metastatic spinal cord compression (MSCC)1, 2. In general, 20% of the patients with spinal metastases suffer from neurological deficits3, 4, 5. These patients are more likely to benefit from aggressive surgical intervention.
However, patients with a limited life expectancy sometimes benefit little from surgery. For instance, Rades et al. report that elderly patients with MSCC did not benefit from surgery in addition to radiotherapy in terms of functional outcome, local control of MSCC, or survival6. In addition, complications and death can follow surgical treatment. Hence, to select the optimal therapeutic modality for patients with spinal metastases, prognostic factors associated with the postoperation life expectancy should be taken into consideration. Many studies have attempted to identify the prognostic factors for predicting survival of patients with MSCC, such as ambulatory status, presence of visceral or extraspinal bone metastases, and number of spinal metastases involved.
The ambulatory status before treatment, as one of the prognostic factors, has commonly been cited in many previous studies. However, these studies report conflicting results for the prognostic effect on patients’ survival after treatment. We also know that Tokuhashi et al. and Sioutos et al. included the grade of neurological deficits in their prognostic scores, whereas Tomita et al., Bauer and Wedin, North et al., and Van der Linden et al. did not7, 8, 9, 10, 11, 12, 13. However, there have been very few conclusive studies to resolve this controversy. Thus, the current exploratory meta‐analysis is performed with the goal of identifying and quantifying the role of ambulatory status before treatment in predicting survival of patients with spinal metastases and the difference in the prognostic effect of ambulatory status among each subgroup of primary tumor types, to give some guidance in selecting a treatment.
Methods
Data Sources and Searches
Two individual researchers (XG Yang and DX Lun) conducted database searches in the PubMed, Embase, and Cochrane Library using the following keywords: “spinal metastasis, overall survival, prognostic factor”. This way, studies published between 1997 and 2017 were retrieved, with the publication language restricted to English. In addition, reference studies involved in retrieved studies were hand‐searched.
Inclusion and Exclusion Criteria for Studies
Complete texts published with a cohort or case‐control study design that examine the survival and prognostic effects of ambulatory status before treatment in patients with spinal metastases from various primary tumors were included in the current study.
However, studies would be excluded for the following reasons: (i) duplicated studies; (ii) systematic reviews, literature reviews, basic research, letters to the editor, and or diagnostic studies; (iii) studies that involved fewer than 10 participants; and (iv) studies using the same patient cohorts as any other study. When several cohorts used the same population as each other, only the most recent (or thorough) study was used. There were no limitations on the participants’ nationalities and study designs applied to the search.
Data Extraction and Quality Assessment
For studies meeting the inclusion criteria, data that referred to prognostic factors of ambulatory status before treatment were extracted by the two individual reviewers independently and entered into a pre‐built Microsoft Excel spreadsheet. Collected data included general information (title, author, year of publication, country, period of the study, and study design), participants’ characteristics (age, percentage of males, number of involved patients, number of patients with MSCC, ambulatory status of patients before treatment, and primary tumor histology), therapeutic modality provided for patients, follow‐up information and patients’ overall survival time, effect sizes of hazard ratio (HR) or risk ratio (RR) combined with their 95% confidence interval (95%CI), and also associated raw data which involved some further calculations, such as survival rates at certain points in time, or diagrams, such as Kaplan–Meier survival curves to gain relevant data using the software Get Data Graph Digitizer (version 2.25, getdata-graph-digitizer.com). The calculations spreadsheet was also used to assist us in carrying out the calculations. We determined the causes of diversity in the obtained information and resolved disagreements with face‐to‐face discussion14.
The Newcastle–Ottawa Scale was used for assessment of eligible studies’ methodological quality and risk of bias by the previously mentioned two researchers independently15. This scale employs a 9‐star system that assesses three domains: patient selection, comparability of the study groups, and the ascertainment of study outcome. Studies with a score of 9 stars have a low risk of bias, whereas scores of 7–8 mean there is a medium bias risk; a score of 6 or less than 6 indicates a high chance of bias.
Data Synthesis and Analysis
Data extracted into a Microsoft Excel spreadsheet were pooled using an exploratory time‐to‐event meta‐analysis. All recorded HR combined with 95%CI (including statistically significant or non‐significant) from eligible literature, incorporating HR re‐calculated from raw data or Kaplan–Meier curves obtained from primary studies, were synthesized narratively. The pooled estimates for HR and 95%CI of ambulatory status before treatment were determined using a random‐effects or fixed‐ffects model, and heterogeneity among each involved study was tested by estimating I 2 and using the Cochrane Q‐test (significance level at P < 0.1). In case of significant heterogeneity, irrespective of the I 2 estimation, random effects models were used to allow for it. Subgroup analyses were performed according to the participants’ primary tumor histology in each study. A test for the overall effect of pooled HR by Z‐test was performed and statistical significance was defined as a two‐sided P‐value of less than 0.05. Data synthesis and analysis was carried out using Stata's metan command. Publication bias was assessed using a funnel plot and Egger's regression asymmetry test (P < 0.10 represented statistically significant publication bias)16. A sensitivity analysis was also performed when significant heterogeneity existing by omitting each individual study to check the stability of the result. The meta‐analysis, testing for publication bias, and the sensitivity analysis were performed using Stata software (version 13.0, StataCorp LLC, College Station, Texas, USA).
Results
Search Result and Study Selection
The process of eligible literature selection is presented in Fig. 1. The initial electronic literature search conducted by the two individual researchers yielded a total of 1065 studies published from 1997 to 2017. After 147 duplicates were excluded, 918 articles remained. Then, after skimming over titles and abstracts and further perusing full texts, 816 of the titles and abstracts and 79 of the full‐texts were excluded, respectively. In addition, there were 3 studies by Lei that used the same patient cohort, and only the study that identified primary tumor histology as non‐small cell lung cancer (NSCLC) was included17, 18, 19. Another 4 studies by Rades were also excluded that used the same patient cohorts as other studies6, 20, 21, 22. Finally, 17 studies containing 3962 participants met the inclusion criteria, of which 14 studies involved comparison between ambulatory and non‐ambulatory groups of patients on the influence of overall survival after treatment and 4 studies reported comparison between Frankel grade C–D and Frankel grade E12, 19, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37.
Figure 1.
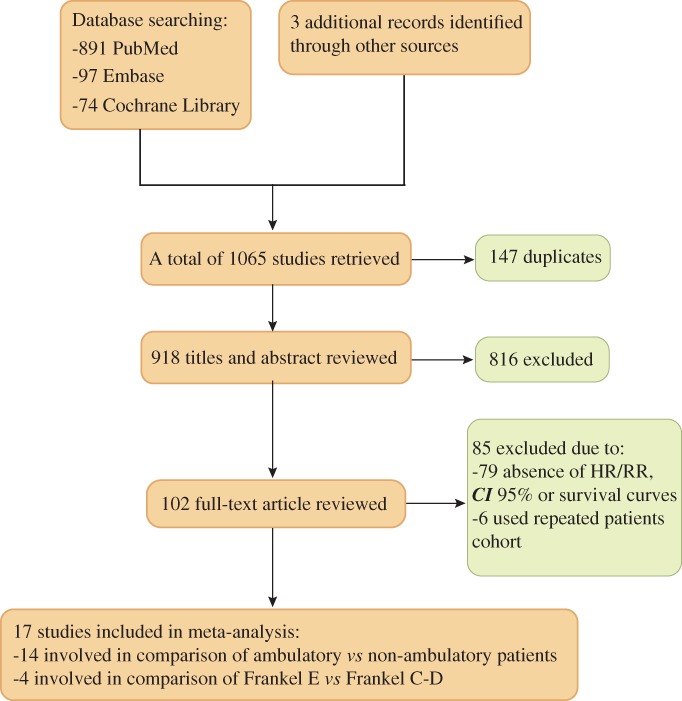
Flowchart of eligible literature selection.
General Information of Studies
A summary of individual studies is provided in Supplementary Appendix S1. Participants come from different countries: the USA in 5 studies, Germany in 4 studies, China in 2 studies, and 1 each from the UK, the Netherlands, Sweden, Austria, Korea, and Czech Republic. Primary tumor histology was various among the included studies, with six not specified, three non‐small cell lung cancer (NSCLC), three prostate cancer, two renal cell cancer, two thyroid cancer and one breast cancer. The percentage of non‐ambulatory patients before treatment in cohorts of prostate cancer was the highest, with an average percentage of 64%, while only 44%, 31%, and 21% in cohorts of NSCLC, non‐identified cancer, and other kinds of tumors, respectively. In 13 cohorts of studies, patients underwent surgery plus other adjuvant therapy, while patients in the remaining 4 studies received radiotherapy alone. The majority of the studies were of high quality, with an average score of 8.0 stars; only 1 study had a score of 6.0 stars.
Qualitative Summary and Data Synthesis
A total of 17 studies were related to influence of ambulatory before treatment on survival in patients with spinal metastases. Fourteen studies reported comparison between ambulatory and non‐ambulatory groups directly, with 8 of them having significant results. Four studies reported comparisons between Frankel grades before treatment, which provided information on neurological status as patients with Frankel grade E were neurologically complete while those with Frankel C–D were neurologically defective. Among these 4 studies, 2 had significant results. In addition, 1 study that compared patients with MRC (British Medical Research Council) scores of 0–3, which means that patients are non‐ambulatory, and those with MRC scores of 4–5, which means that patients have enough strength to walk before treatment, found no influence on overall survival after treatment.
Data extracted included 14 effect estimates of the hazard ratio (HR) for patients who were ambulatory (including Frankel D, E) versus non‐ambulatory (including Frankel A‐C) before treatment and 4 effect estimates of HR for patients without (Frankel E) versus with neurological deficit (Frankel C‐D). All these effect estimates of HR are presented in two individual forest plots and are pooled together with Stata software (version 13.0, StataCorp LLC, College Station, Texas, USA) (Fig. 2).
Figure 2.
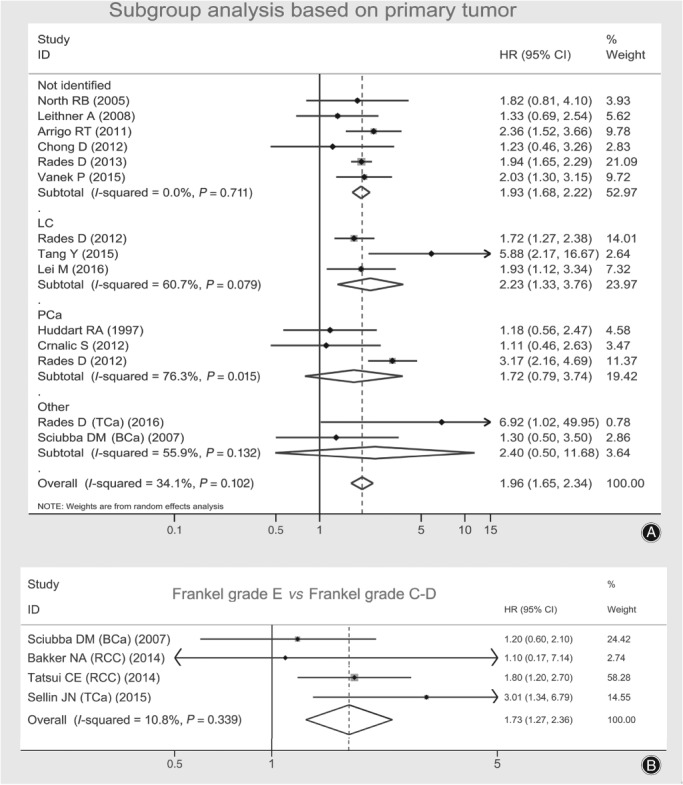
Forest plots presenting combined effect estimates (hazard ratios [HR]) of survival in spinal metastatic patients: (A) ambulatory versus non‐ambulatory, a subgroup analysis based on the histology of primary tumor; and (B) Frankel E versus Frankel C&D.
According to the histology of primary tumors, the 14 studies related to patients ambulatory versus non‐ambulatory before treatment were stratified into subgroups of mixed tumor type, NSCLC, prostate cancer and others, and an exploratory subgroup meta‐analysis was carried out in forest plot A using a random‐effects model. The overall pooled HR was 1.96 (95% CI, 1.65–2.34), I 2 = 34.1%. In the test for the overall effect by Z‐test, the pooled effect estimate of HR proved to be statistically significant (Z = 7.57, P < 0.001). In subgroups of no identified tumor type and NSCLC, pooled HR were 1.93 (95%CI, 1.68–2.22; I 2 = 0.0%) and 2.23 (95%CI, 1.33–3.76; I 2 = 60.7%), respectively, and were proved to be significant by Z‐test (Z = 9.34, P < 0.001 and Z = 3.03, P = 0.002, respectively). While in subgroups of patients with primary tumors of prostate cancer and others (one each for thyroid cancer and breast cancer), pooled HR were 1.72 (95%CI, 0.79–3.74; I 2 = 76.3%) and 2.40 (95%CI, 0.50–11.68; I 2 = 55.9%), respectively, and were considered non‐significant (Z = 1.37, P = 0.169 and Z = 1.09, P = 0.277, respectively).
The other four effect size of HR for comparison between patients with (Frankel C‐D) and without neurological deficit were pooled in forest plot B with a fixed‐effect model. The pooled HR was 1.73 (95%CI, 1.27–2.36; I 2 = 10.8%), which was considered to be statistically significant, with a Z‐value of 3.48 and a P‐value of less than 0.001 by Z‐test.
Heterogeneity and Publication Bias
In subgroups of primary lung cancer, primary prostate cancer and others (one each for thyroid cancer and breast cancer), which are presented in Fig. 2A; I 2 are greater than 50% (60.7%, 76.3% and 55.9%, respectively), which indicates obvious heterogeneity. Hence, sensitivity analyses were performed for all the studies included in Fig. 2A and subgroups of primary lung cancer and prostate cancer but not for the subgroup which included only two studies (one each for thyroid and breast cancer). The sensitivity analysis plots are presented in Fig. 3, which all demonstrated stability when omitting each individual study.
Figure 3.
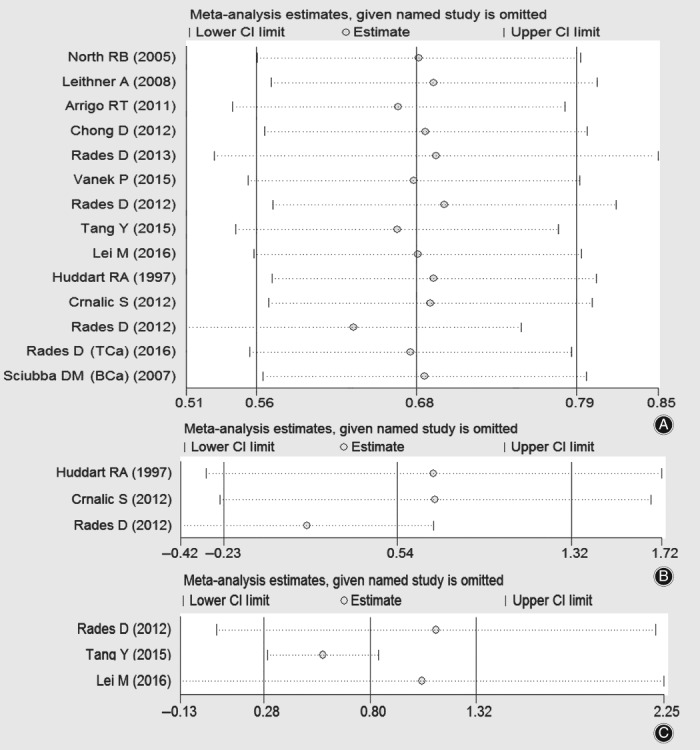
Plots of sensitivity analysis for 14 studies included in the forest plot A (A) and studies in subgroups of primary prostate cancer (B) and primary lung cancer (C).
Publication bias was assessed using a funnel plot and Egger's regression asymmetry test. The funnel plot in Fig. 4A presents the publication bias of the 14 studies included in Fig. 2A and shows good symmetry, with most studies converging at the top of the funnel. Hence, based on the funnel plot, there was no obvious publication bias. Egger's publication bias plot in Fig. 4B presents the risk of bias across 14 studies included in forest plot A, with a P‐value of 0.952, which refutes the existence of obvious publication bias. The funnel plot and Egger's publication bias plot in Fig. 4C,D present the risk of bias across 4 studies related to patients without neurological defects (Frankel grade E) versus with neurological defects (including Frankel grade C‐D) before treatment. These results are also included in forest plot B, which demonstrates symmetry; the P‐value of 0.929 also refutes the existence of obvious publication bias.
Figure 4.
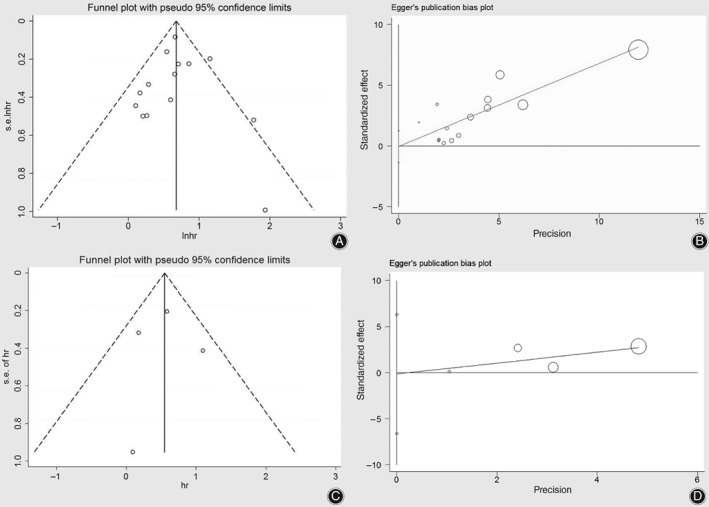
Funnel plot (A) and Egger's publication bias plot (B) presenting publication condition of 14 studies related to patients ambulatory versus non‐ambulatory before treatment, which were included in forest plot A; Funnel plot (C) and Egger's publication bias plot (D) presenting the risk of bias across 4 studies related to patients without neurological defect (Frankel grade E) versus with neurological defect (including Frankel grade C‐D) before treatment which were included in forest plot B.
Discussion
The treatment of spinal metastasis is often focused on optimal relief of symptoms of MSCC, such as severe pain and neurological deficits, to improve the quality of the remaining life span. The various individualized therapeutic options include surgery, radiotherapy, chemotherapy, and targeted therapy. To achieve optimal remission of symptoms, surgeons must consider the patients’ life expectancy and clinical outcomes when conducting treatment. The prognostic effect of ambulatory status before treatment has been evaluated in many previous studies. However, conflicting results are reported among these studies7, 8, 9, 10, 11, 12, 13. In the current study, an exploratory meta‐analysis was performed to examine the role of ambulatory status before treatment in predicting the overall survival of patients with spinal metastases. Ambulatory status before treatment has been identified to be a statistically significant prognostic factor for overall survival after treatment. It could provide some answers to the current controversy and make ambulatory status a more remarkable prognostic factor when selecting the treatment modality.
In reviewing the existing literature, it is unclear whether ambulatory status before treatment is a prognostic factor of survival in patients with spinal metastases. We know that Tokuhashi et al., Sioutos et al., and Enkaoua et al. included the grade of neurological deficit in their score systems as one of the prognostic factors7, 8, 9, 38. In the study of Arrigo et al., preoperative ambulatory status was considered as one of the most robust predictors of survival23. Rades et al. also suggested that ambulatory status pre‐treatment was significantly associated with survival, and the authors insisted that non‐ambulatory patients were more likely to suffer from major complications such as pneumonia, which will cause patients to deteriorate22. Tang et al. also conclude that preoperation ambulatory status is a significant prognostic factor in patients with spinal metastases from non‐small cell lung cancer, that patients with neurological deficit may deteriorate too much to tolerate some more aggressive surgical procedures and adjuvant therapies, and that more severe complications will arise among paraplegic patients29.
However, there were also numerous studies that were opposed to adopt pre‐treatment neurological status as a significant prognostic factor based on their cohorts. In 2001, Tomita et al. developed a score system that did not include pre‐treatment neurological status as a prognostic factor for survival10. The authors insisted that neurological deficit could be improved through appropriate treatment such as spinal cord decompression, which can bring about longer survival, even in patients with severe paraplegia. In the study of Van der Linden et al., a total of 342 patients who were free of neurological deficit with Harrington grade I and II were included, and evaluation of the prognostic effect of neurological status was not conducted13. However, the authors refused to accept it as a prognostic factor in their score system, and they speculated that symptoms of myoplegia can just reflect the location and volume of spinal metastasis lesions; this concurred with the opinion of Tomita et al.10. Yamashita et al. used the revised Tokuhashi score system to evaluate the prognostic effect in patients with spinal metastases and found that Frankel grade is not a significant prognostic factor39. Chong et al. observed that preoperative ambulatory status was a significant factor in predicting postoperative ambulation but not postoperative overall survival24.
Our exploratory meta‐analysis found a significant correlation between pre‐treatment neurological status and overall survival, with pooled overall HR 1.96 (95%CI, 1.65–2.34, P < 0.001) in comparison between patients who were ambulatory and non‐ambulatory, and 1.73 (95%CI, 1.27–2.36; P < 0.001) in comparison between patients who were Frankel grade E (without neurological deficit) and C–D (with neurological deficit). This is contrary to the results of Tomita et al., who concluded that pre‐treatment neurological status is not an effective prognostic factor as they believed that neurological deficit could be cured following special treatment procedures10. Vanek et al. report that preoperation neurology is an independent prognostic factor but that improvement in the Frankel scale is not associated with a longer survival of patients26. Moon et al. and Quraishi et al. report the same result40, 41. It follows that an improved neurological status after treatment is not a certain factor to influence overall survival through their studies, but ambulatory status pre‐treatment is an independent prognostic factor. We found that neurological status could reflect the degree of local compression and progression of local lesion in spinal metastases. Patients who were non‐ambulatory pre‐treatment usually had a spinal metastasis lesion with a progressive nature. Patients tended to live for a shorter duration after treatment when symptoms of neurological deficit were involved, despite a similar survival span between patients with and without neurological deficit being found in some individual studies with a small sample size after a series of treatment procedures. Based on our meta‐analysis, which pooled individual studies together and included 3962 participants, an unfavorable life expectancy was found among patients with neurological deficit prior to treatment, which could lead to the conclusion that although symptoms of neurological deficit can be resolved, the unfavorable nature of local spinal metastases cannot be reversed.
For the subgroup of cohorts with primary prostate cancer, the pooled effect estimate was 1.72 (95%CI, 0.79–3.74; P = 0.169), which was not statistically significant. This result was in accordance with most of the studies based on a cohort of primary prostate cancer 30, 31, 42. Crnalic et al. delivered a score system based on 68 patients with spinal metastasis from prostate cancer30. In their study, most (87%) of the patients were non‐ambulatory before treatment, and the prognostic effect of ambulatory status was non‐significant. Meng et al. analyzed the prognostic factors based on 31 patients with spinal metastasis from prostate cancer, 59% of whom were non‐ambulatory before treatment, and found that neurological status before radiotherapy was not an independent prognostic factor42. Among all the included studies, the percentages of non‐ambulatory patients before treatment in cohorts of prostate cancer were the highest, with an average percentage of 64%, with only 44%, 31% and 21% in cohorts of NSCLC, non‐identified cancer, and other types of tumors, respectively. Thus, it would be speculated that patients were composed of elderly men in prostate cancer mainly and had a poor basic condition probably. These elderly men were more likely to suffer from neurological deficits than relatively younger patients in cohorts with other types of primary tumors. Thus, some patients who had a long life expectancy and favorable biological behaviour of spinal metastases suffered from non‐ambulation before treatment, which would mean that ambulatory status does not reflect the real biological behaviour of spinal metastases significantly among this group of patients. Hence, whether ambulatory status is a significant prognostic factor cannot be confirmed yet in spinal metastases from prostate cancer.
Limitations of This Study
Our study has several limitations. Most studies included in the current systematic review and meta‐analysis were retrospective cohort studies and not prospective cohort studies. However, most of the studies included here are of relatively high quality according to the Newcastle–Ottawa Scale few prospective cohort studies have been carried out on patients with spinal metastases and. Thus, more observational studies with a prospective design are necessary.
Conclusion
The current study suggests that ambulatory status before treatment is a significant prognostic factor in patients with spinal metastases and should be considered when choosing the treatment modality. We suggested that among patients with neurological deficit or who were non‐ambulatory before treatment, the implementation of aggressive surgical procedures should proceed with caution. However, in the subgroup of patients with primary prostate cancer, ambulatory status before treatment cannot yet be confirmed to be a significant prognostic factor of survival, and further study is necessary to give some more constructive guidance.
Supporting information
Appendix S1 Summary of included studies.
Acknowledgments
I would like to express special thanks to my partners for the encouragement and support they gave me during my study.
Disclosure: This research did not receive any specific grant from funding agencies in the public, commercial, or not‐for‐profit sectors. All authors declare that they have no conflict of interest.
References
- 1. Byrne TN. Spinal cord compression from epidural metastases. N Engl J Med, 1992, 327: 614–619. [DOI] [PubMed] [Google Scholar]
- 2. Jacobs WB, Perrin RG. Evaluation and treatment of spinal metastases: an overview. Neurosurg Focus, 2001, 11: e10. [DOI] [PubMed] [Google Scholar]
- 3. Barron KD, Hirano A, Araki S, Terry RD. Experiences with metastatic neoplasms involving the spinal cord. Neurology, 1959, 9: 91–106. [DOI] [PubMed] [Google Scholar]
- 4. Sundaresan N, Digiacinto GV, Hughes JE, Cafferty M, Vallejo A. Treatment of neoplastic spinal cord compression: results of a prospective study. Neurosurgery, 1991, 29: 645–650. [DOI] [PubMed] [Google Scholar]
- 5. Schaberg J, Gainor BJ. A profile of metastatic carcinoma of the spine. Spine (Phila Pa 1976), 1985, 10: 19–20. [DOI] [PubMed] [Google Scholar]
- 6. Rades D, Huttenlocher S, Evers JN, et al Do elderly patients benefit from surgery in addition to radiotherapy for treatment of metastatic spinal cord compression?. Strahlenther Onkol, 2012, 188: 424–430. [DOI] [PubMed] [Google Scholar]
- 7. Tokuhashi Y, Matsuzaki H, Toriyama S, Kawano H, Ohsaka S. Scoring system for the preoperative evaluation of metastatic spine tumor prognosis. Spine (Phila Pa 1976), 1990, 15: 1110–1113. [DOI] [PubMed] [Google Scholar]
- 8. Tokuhashi Y, Matsuzaki H, Oda H, Oshima M, Ryu J. A revised scoring system for preoperative evaluation of metastatic spine tumor prognosis. Spine (Phila Pa 1976), 2005, 30: 2186–2191. [DOI] [PubMed] [Google Scholar]
- 9. Sioutos PJ, Arbit E, Meshulam CF, Galicich JH. Spinal metastases from solid tumors. Analysis of factors affecting survival. Cancer, 1995, 76: 1453–1459. [DOI] [PubMed] [Google Scholar]
- 10. Tomita K, Kawahara N, Kobayashi T, Yoshida A, Murakami H, Akamaru T. Surgical strategy for spinal metastases. Spine (Phila Pa 1976), 2001, 26: 298–306. [DOI] [PubMed] [Google Scholar]
- 11. Bauer HC, Wedin R. Survival after surgery for spinal and extremity metastases. Prognostication in 241 patients. Acta Orthop Scand, 1995, 66: 143–146. [DOI] [PubMed] [Google Scholar]
- 12. North RB, LaRocca VR, Schwartz J, et al Surgical management of spinal metastases: analysis of prognostic factors during a 10‐year experience. J Neurosurg Spine, 2005, 2: 564–573. [DOI] [PubMed] [Google Scholar]
- 13. Van der Linden YM, Dijkstra SP, Vonk EJ, Marijnen CA, Leer JW. Dutch Bone Metastasis Study Group. Prediction of survival in patients with metastases in the spinal column. Results based on a randomized trial of radiotherapy. Cancer, 2005, 103: 320–328. [DOI] [PubMed] [Google Scholar]
- 14. Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time‐to‐event data into meta‐analysis. Trials, 2007, 8: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M. The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta‐analyses. 2012, http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm.
- 16. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ, 1997, 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lei M, Liu Y, Yan L, Tang C, Yang S, Liu S. A validated preoperative score predicting survival and functional outcome in lung cancer patients operated with posterior decompression and stabilization for metastatic spinal cord compression. Eur Spine J, 2016, 25: 3971–3978. [DOI] [PubMed] [Google Scholar]
- 18. Lei M, Liu Y, Liu S, Wang L, Zhou S, Zhou J. Individual strategy for lung cancer patients with metastatic spinal cord compression. Eur J Surg Oncol, 2016, 42: 728–734. [DOI] [PubMed] [Google Scholar]
- 19. Lei M, Liu Y, Tang C, Yang S, Liu S, Zhou S. Prediction of survival prognosis after surgery in patients with symptomatic metastatic spinal cord compression from non‐small cell lung cancer. BMC Cancer, 2015, 15: 853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rades D, Huttenlocher S, Bajrovic A, et al Surgery followed by radiotherapy versus radiotherapy alone for metastatic spinal cord compression from unfavorable tumors. Int J Radiat Oncol Biol Phys, 2011, 81: e861–e868. [DOI] [PubMed] [Google Scholar]
- 21. Rades D, Weber A, Karstens JH, Schild SE, Bartscht T. Number of extraspinal organs with metastases: a prognostic factor of survival in patients with metastatic spinal cord compression (MSCC) from non‐small cell lung cancer (NSCLC). Anticancer Res, 2014, 34: 2503–2507. [PubMed] [Google Scholar]
- 22. Rades D, Fehlauer F, Schulte R, et al Prognostic factors for local control and survival after radiotherapy of metastatic spinal cord compression. J Clin Oncol, 2006, 24: 3388–3393. [DOI] [PubMed] [Google Scholar]
- 23. Arrigo RT, Kalanithi P, Cheng I, et al Predictors of survival after surgical treatment of spinal metastasis. Neurosurgery, 2011, 68: 674–681. [DOI] [PubMed] [Google Scholar]
- 24. Chong S, Shin SH, Yoo H, et al Single‐stage posterior decompression and stabilization for metastasis of the thoracic spine: prognostic factors for functional outcome and patients' survival. Spine J, 2012, 12: 1083–1092. [DOI] [PubMed] [Google Scholar]
- 25. Rades D, Hueppe M, Schild SE. A score to identify patients with metastatic spinal cord compression who may be candidates for best supportive care. Cancer, 2013, 119: 897–903. [DOI] [PubMed] [Google Scholar]
- 26. Vanek P, Bradac O, Trebicky F, Saur K, de Lacy P, Benes V. Influence of the pre‐operative neurological status on survival after the surgical treatment of symptomatic spinal metastases with spinal cord compression. Spine (Phila Pa 1976), 2015, 40: 1824–1830. [DOI] [PubMed] [Google Scholar]
- 27. Leithner A, Radl R, Gruber G, et al Predictive value of seven preoperative prognostic scoring systems for spinal metastases. Eur Spine J, 2008, 17: 1488–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rades D, Douglas S, Veninga T, et al Metastatic spinal cord compression in non‐small cell lung cancer patients. Prognostic factors in a series of 356 patients. Strahlenther Onkol, 2012, 188: 472–476. [DOI] [PubMed] [Google Scholar]
- 29. Tang Y, Qu J, Wu J, Li S, Zhou Y, Xiao J. Metastatic spinal cord compression from non‐small‐cell lung cancer treated with surgery and adjuvant therapies: a retrospective analysis of outcomes and prognostic factors in 116 patients. J Bone Joint Surg Am, 2015, 97: 1418–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Crnalic S, Löfvenberg R, Bergh A, Widmark A, Hildingsson C. Predicting survival for surgery of metastatic spinal cord compression in prostate cancer: a new score. Spine (Phila Pa 1976), 2012, 37: 2168–2176. [DOI] [PubMed] [Google Scholar]
- 31. Huddart RA, Rajan B, Law M, Meyer L, Dearnaley DP. Spinal cord compression in prostate cancer: treatment outcome and prognostic factors. Radiother Oncol, 1997, 44: 229–236. [DOI] [PubMed] [Google Scholar]
- 32. Rades D, Douglas S, Veninga T, et al A survival score for patients with metastatic spinal cord compression from prostate cancer. Strahlenther Onkol, 2012, 188: 802–806. [DOI] [PubMed] [Google Scholar]
- 33. Rades D, Janssen S, Käsmann L, Bolm L, Schild SE. Outcomes after irradiation of epidural spinal cord compression due to metastatic thyroid cancer. Anticancer Res, 2016, 36: 2035–2039. [PubMed] [Google Scholar]
- 34. Sciubba DM, Gokaslan ZL, Suk I, et al Positive and negative prognostic variables for patients undergoing spine surgery for metastatic breast disease. Eur Spine J, 2007, 16: 1659–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bakker NA, Coppes MH, Vergeer RA, Kuijlen JM, Groen RJ. Surgery on spinal epidural metastases (SEM) in renal cell carcinoma: a plea for a new paradigm. Spine J, 2014, 14: 2038–2041. [DOI] [PubMed] [Google Scholar]
- 36. Tatsui CE, Suki D, Rao G, et al Factors affecting survival in 267 consecutive patients undergoing surgery for spinal metastasis from renal cell carcinoma. J Neurosurg Spine, 2014, 20: 108–116. [DOI] [PubMed] [Google Scholar]
- 37. Lee BH, Park JO, Kim HS, Park YC, Lee HM, Moon SH. Perioperative complication and surgical outcome in patients with spine metastases: retrospective 200‐case series in a single institute. Clin Neurol Neurosurg, 2014, 122: 80–86. [DOI] [PubMed] [Google Scholar]
- 38. Enkaoua EA, Doursounian L, Chatellier G, Mabesoone F, Aimard T, Saillant G. Vertebral metastases: a critical appreciation of the preoperative prognostic Tokuhashi score in a series of 71 cases. Spine (Phila Pa 1976), 1997, 22: 2293–2298. [DOI] [PubMed] [Google Scholar]
- 39. Yamashita T, Siemionow KB, Mroz TE, Podichetty V. Lieberman IH. A prospective analysis of prognostic factors in patients with spinal metastases: use of the revised Tokuhashi score. Spine (Phila Pa 1976), 2011, 36: 910–917. [DOI] [PubMed] [Google Scholar]
- 40. Moon KY, Chung CK, Jahng TA, Kim HJ, Kim CH. Postoperative survival and ambulatory outcome in metastatic spinal tumors: prognostic factor analysis. J Korean Neurosurg Soc, 2011, 50: 216–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Quraishi NA, Rajagopal TS, Manoharan SR, Elsayed S, Edwards KL, Boszczyk BM. Effect of timing of surgery on neurological outcome and survival in metastatic spinal cord compression. Eur Spine J, 2013, 22: 1383–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Meng T, Chen R, Zhong N, et al Factors associated with improved survival following surgical treatment for metastatic prostate cancer in the spine: retrospective analysis of 29 patients in a single center. World J Surg Oncol, 2016, 14: 200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Summary of included studies.


