Abstract
Human amygdalae are involved in various behavioral functions such as affective and stress processing. For these behavioral functions, as well as for psychophysiological arousal including cortisol release, sex differences are reported.
Here, we assessed cortisol levels and resting-state functional connectivity (rsFC) of left and right amygdalae in 81 healthy participants (42 women) to investigate potential modulation of amygdala rsFC by sex and cortisol concentration.
Our analyses revealed that rsFC of the left amygdala significantly differed between women and men: Women showed stronger rsFC than men between the left amygdala and left middle temporal gyrus, inferior frontal gyrus, postcentral gyrus and hippocampus, regions involved in face processing, inner-speech, fear and pain processing. No stronger connections were detected for men and no sex difference emerged for right amygdala rsFC. Also, an interaction of sex and cortisol appeared: In women, cortisol was negatively associated with rsFC of the amygdalae with striatal regions, mid-orbital frontal gyrus, anterior cingulate gyrus, middle and superior frontal gyri, supplementary motor area and the parietal-occipital sulcus. Contrarily in men, positive associations of cortisol with rsFC of the left amygdala and these structures were observed. Functional decoding analyses revealed an association of the amygdalae and these regions with emotion, reward and memory processing, as well as action execution.
Our results suggest that functional connectivity of the amygdalae as well as the regulatory effect of cortisol on brain networks differ between women and men. These sex-differences and the mediating and sex-dependent effect of cortisol on brain communication systems should be taken into account in affective and stress-related neuroimaging research. Thus, more studies including both sexes are required.
Keywords: Task-independent, resting-state, cortisol, striatum, hippocampus, frontal cortex
1 Introduction
The amygdalae are involved in a wide range of different functions such as affective (Derntl et al., 2008; Goldin et al., 2009; Ochsner et al., 2004) and stress processing (Veer et al., 2011). The amygdalae contain corticoid receptors that may be activated by cortisol (De Kloet et al., 1998), a steroid hormone involved in affective and stress-related behavior. It is expressed by the adrenal glands following stressful or arousing situations (Dickerson and Kemeny, 2004) and cortisol patterns are associated with amygdala activity, e.g. while regulating emotions (Urry et al., 2006).
Sex differences have been reported during stress and affective processing on behavioral, physiological as well as neural levels: Women tend to report more frequent and more intense emotions (Grossman and Wood, 1993), report to be more stressed in self-reports (Kudielka et al., 1998) and show more facial expressions to emotional movies (Kring and Gordon, 1998). Compared to women, men usually show higher cortisol reactions to stress situations, which seem to decrease the recall of unpleasant emotional memory (Buchanan and Tranel, 2008; Kajantie and Phillips, 2006; Kirschbaum et al., 1992). These reports point to divergent patterns in subjective and physiological stress reactions in women and men. BOLD-based amygdala activity also reveals specific patterns in women and in men during affective (Derntl et al., 2009; Domes et al., 2010; McRae et al., 2008; Stevens and Hamann, 2012) and stress (Kogler et al., 2015a) reactions. Women show a stronger amygdala response to negative emotions (Stevens and Hamann, 2012) and stress processing (Kogler et al., 2015a), while in men amygdala activity is increased when processing positive emotions (Stevens and Hamann, 2012). Thus for amygdala activity as well as for cortisol release and their related behavioral domains, sex differences are reported.
However, brain regions, and therefore also the amygdala, do not perform in isolation. Recently, the communication and interaction between them and anatomically separated neural regions came into focus of affective and stress-related neuroimaging research. Temporally dependent activation patterns in spontaneous activity of two different areas might indicate an exchange of information between functionally linked neural regions (Eickhoff and Müller, 2015; Friston et al., 1993). The amygdala interacts with multiple brain regions involved in the regulation of social, affective and stress-related information (Robinson et al., 2011; Roy et al., 2010; Stein et al., 2007), including lateral and medial frontal areas, posterior cingulate cortex, hippocampus, hypothalamus and brainstem regions. These regions also express corticoid receptors (Diorio et al., 1993; Ulrich-Lai and Herman, 2009) and hormones freely fluctuating throughout the brain potentially contribute to connectivity between regions not anatomically connected. Indeed, cortisol has been shown to modify the functional connectivity of the amygdala (Henckens et al., 2010; Vaisvaser et al., 2013; Veer et al., 2012; Vogel et al., 2015). During emotional tasks, exogenous cortisol strengthens the connectivity between the amygdala and the medial prefrontal cortex (Henckens et al., 2010) and a stress-induced increase in connectivity between the amygdala and the striatum is dependent on the availability of corticoid receptors (Vogel et al., 2015). During resting-state, a positive correlation between endogenous cortisol and the coupling of the amygdala with the medial prefrontal cortex as well as with the anterior cingulate cortex (ACC) (Veer et al., 2012) emerged. Moreover, a negative correlation between cortisol levels and the coupling of the amygdala with hippocampus (Vaisvaser et al., 2013) has been reported. Thus, the steroid hormone and its receptor’s distribution seem to modify the functional connectivity of the amygdala, and may play a mediating effect in building functional connections between brain regions. However, so far these reports are based on male-only samples (Henckens et al., 2010; Vaisvaser et al., 2013; Veer et al., 2012; Vogel et al., 2015), while specific effects of cortisol in women and possible interactions with sex differences are still lacking.
There is some evidence that the amygdalae show sex specific connectivity patterns, with higher local connectivity in women than in men (Lopez-Larson et al., 2011), and sex differences in resting-state functional connectivity (rsFC) appearing during development in adolescence (Alarcón et al., 2015). One recent study reports sex differences in rsFC of subnuclei of the amygdala in healthy adults, with higher connectivity of the basolateral amygdala to lateral-frontal and striatal regions in women and to medial-frontal regions in men (Engman et al., 2016). Thus, rsFC of amygdala subnuclei with other brain regions involved in stress and emotion processing seems to be sex-specific.
Evidence has accumulated supporting the view that sex differences exist concerning behavioral domains and (stress-related) cortisol release. Therefore, it would be of interest to assess amygdala’s rsFC in association with cortisol to determine general and sex-specific neural patterns underlying these phenomena. As shown previously, the connectivity between the amygdala and the ACC may be mediated by cortisol (Veer et al., 2012). Cortisol is thought to regulate neural network responses to adapt behavior to arousing situations (Henckens et al., 2012; Kloet et al., 2005) and sex differences in cortisol stress response (Kajantie and Phillips, 2006; Kirschbaum et al., 1992) suggest that these regulatory mechanisms may differ in women and men. Thus, the inhibitory effect of the ACC on the amygdala (Stein et al., 2007) may be influenced by cortisol and therefore depend on sex.
It is so far unknown, whether the same association appears in women and men or whether specific mediating effects on amygdala connectivity contribute to sex differences during negative emotional or stress related processing. Thus, differences in rsFC of the amygdala in association with cortisol may underlie reported sex differences in behavioral domains (Kogler et al., 2015a; Stevens and Hamann, 2012).
Notably, no direct comparison of the functional connectivity of the amygdala and its relationship to the steroid hormone in women and men has been published. However, the amygdala’s strong involvement in modulating affective and stress-related functions, its association with cortisol and previous reports of sex differences in rsFC of the amygdala warrant further investigation. Therefore, the aims of the current study are 1) to assess and analyze rsFC of the amygdala in women and men; and 2) to investigate whether women and men express different patterns of amygdala rsFC in association with cortisol. Based on previous findings, we hypothesize that 1) women and men differ in rsFC of the amygdala: Given increased amygdala activity during negative affective and stress related processing in females (Stevens and Hamann, 2012), we assume that women compared to men show higher connectivity of the amygdala with other limbic regions associated with affective and stress processing. 2) Furthermore, we hypothesize that higher cortisol levels are associated with increased connectivity of the amygdala with medial and ventrolateral prefrontal regions in men (Henckens et al., 2010; Veer et al., 2012). Due to the lack of studies on the impact of cortisol on the functional connectivity of the amygdala in women, and based on reports of sex differences in behavior, psychophysiology and amygdala activity, however, we can only assume sex differences in the association of cortisol and amygdala rsFC.
2 Material and Methods
2.1 Sample
Resting-state data and anatomical scans were obtained in ninety-four right-handed non-smoking university students. All participants were screened for the following exclusion criteria: history of neurological and psychiatric disorders, chronic illnesses, drug or hormone intake, night shift working, competitive sports, recent or current pregnancy, premenstrual dysphoric disorder, allergic asthma and the common factors of MR-incompatibility. Only naturally cycling women without intake of oral contraception were included.
Participants were asked to refrain from exercise or alcohol consumption for 24h prior to the session, medication, caffeine and drug intake on the test day, and food or drinks other than water for two hours before the session. The measurement sessions took place at the MR Center of Excellence at the Medical University of Vienna, Vienna, Austria, and were scheduled between 2:30 pm and 5:30 pm. Data assessment in all subjects was scheduled in the afternoon to control for circadian cortisol rhythm. Upon arrival, participants received detailed instructions, gave subjective mood ratings (positive and negative affect scale, PANAS) (Watson et al., 1988) and provided saliva samples for hormone analyses (approx. 15 min after arrival).
Written informed consent was obtained from all subjects. The study was approved by the local Institutional Review Board (Ethik Kommission, Medizinische Universität Wien) and participants were treated according to the Declaration of Helsinki (1964).
Due to missing hormone data (n=1), outliers in hormone data (mean +/− 2 standard deviations; n=8), sickness (1 subject vomited in the scanner) and as a result of scanner movement matching between the groups (n=3; groups were matched for the following scanner movement parameters: FD, DVARS, RMS (Power et al., 2012; Satterthwaite et al., 2013)), in total thirteen subjects had to be excluded. Women and men did not differ regarding the three movement parameters (all ps>0.689). Furthermore, no significant correlation between cortisol and any of the movement parameter emerged (all ps>0.504).
The final sample consisted of eighty-one participants (42 women; see Table 1 for sample description).
Table 1.
Sample description.
| Women (n=42) | Men (n=39) | ||||
|---|---|---|---|---|---|
| Mean | STD | Mean | STD | p value | |
| Age | 24.31 | 3.9 | 24.00 | 3.1 | 0.695 |
| Positive affect1 | 26.802 | 7.3 | 26.56 | 6.2 | 0.878 |
| Negative affect1 | 12.633 | 3.5 | 11.95 | 2.2 | 0.8785 |
| Cortisol4 | 0.99 | 0.1 | 0.92 | 0.2 | 0.082 |
Note.
raw scores (PANAS) (Watson et al., 1988);
n=40;
n=41;
pg/ml (log-transformed);
U-test (negative affect was not normally distributed) ; STD=standard deviation.
2.2 Saliva samples
To obtain hormone concentration, saliva samples were obtained and stored at −20°C at least until shipping to the analysis laboratory (SwissHealthMed, Aying, Germany), where they were frozen at −20°C over-night, then thawed and centrifuged. Competitive luminescence immunoassay kits (LUMI) were used to obtain cortisol concentrations. These kits have minimal cross reactivity to other steroid-hormones and achieved reliable measurements (cortisol: intra-assay CV < 4% and inter-assay CV < 5%). Cortisol data were normalized using a log transformation (y=log10(x+1)) prior to statistical analyses.
2.3 Data and statistical analysis of behavioral and hormone data
Statistical analyses of sex differences in demographic and cortisol data were conducted using IBM SPSS statistics for Windows, Version 20.0 (IBM Corp., Armonk, NY, USA). In cases where assumptions for parametric testing did not apply, non-parametric tests were performed (see Table 1, “negative affect”). The level of significance was set at p<.05 for all tests.
2.4 Resting-state functional connectivity analysis
Acquisition and preprocessing
Images were acquired on a 3T TIM Trio scanner (Siemens Medical Systems, Erlangen, Germany) using BOLD contrast (gradient-echo EPI-sequence with distortion correction; 23 interleaved slices, TE/TR=38/1800ms, voxel size 1.5×1.5×3mm, 90° flip angle; bandwidth = 1446 Hz/pixel, 1.8 mm slice gap) in an axial plane with 167 images per subject. Using a high spatial resolution at 3T, in particular a lower slice thickness, avoids signal dephasing especially in the ventral brain including the amygdala (Robinson et al., 2009, 2004, 2008). As a consequence, brain coverage is limited and a larger gap is needed. This should not be a critical issue as smoothing is applied in preprocessing (see below). Hence, this approach provides high sensitivity and specificity particularly around the amygdala. Data was processed using SPM8 (Wellcome Trust Centre for Neuroimaging, London, http://www.fil.ion.ucl.ac.uk/spm/software/spm8/) implemented in Matlab (Version R20112b; Mathworks Inc., Sherborn, MA, USA). The first four volumes were discarded from each subject prior to further analyses. EPI images were corrected for head movement by affine registration using a two-pass procedure. In a first step, images were aligned to the initial volumes and subsequently to the mean of all volumes. Next, the mean EPI image was spatially normalized to the non-linear MNI152 template for each subject (Holmes et al., 1998) by using the “unified segmentation” approach (Ashburner and Friston, 2005). Ensuing deformation was applied to the individual EPI volumes. Images were smoothed by a 5mm full-width-at-half-maximum Gaussian kernel to improve signal-to-noise ratio and to compensate for residual anatomical variations. Time-series of each voxel were processed as follows (Weissenbacher et al., 2009): Variance was excluded to reduce spurious correlations by the following nuisance variables: (1) the six motion parameters derived from image realignment; (2) their first derivatives. All nuisance variables entered the model as first and also as second order nuisance terms (Satterthwaite et al., 2013). These corrections have been shown to increase specificity and sensitivity of the analyses and to detect valid correlation and anti-correlations during rest (Satterthwaite et al., 2013). In turn, given recent reports of spurious effects specifically in between group comparisons due to global signal removal (Murphy et al., 2009; Saad et al., 2012; Weissenbacher et al., 2009), we did not employ global signal regression. Finally, data was band-pass filtered (cut-off frequencies of 0.01 and 0.08Hz). Furthermore and prior to the 2nd level analyses, rsFC of each subject was adjusted for the effect of age via a regression analysis.
Functional connectivity analyses
Left and right amygdala regions of interest as seed regions were provided from AnatomyToolbox v2.0 (Eickhoff et al., 2005) implemented in SPM8. For each subject, time-courses of all voxels within the seeds were extracted and expressed as the first eigenvariate. Linear (Pearson) correlation coefficients were computed between the ensuing characteristic time series of the seed regions and the time series of all other gray matter voxels of the brain to quantify rsFC. The voxel-wise correlation coefficients of each subject and seed were transformed into Fisher’s Z-scores. Then, these scores for the left and right amygdala were fed into a second-level GLM, including an appropriate non-sphericity correction as implemented in SPM8. The GLM included the factor sex as well as cortisol as a covariate, to test for group differences and effects of the steroid hormone. Firstly, we tested for sex differences in the rsFC of the left and right amygdala on a whole-brain level. Secondly, we tested for correlations between cortisol and rsFC of the left and right amygdala for the whole group (women and men). Then, we tested for differences between women and men in the association of cortisol and rsFC of the left and right amygdala on a whole-brain level. For all analyses testing for associations with cortisol and group differences in these associations we conducted conjunctions with the main effect of the amygdala’s (positive or negative) rsFC [cortisol ∩ amygdala]. This conjunction approach restricts the analyses in association with cortisol to regions having significant functional connectivity with the amygdala. Furthermore and in addition to the conjunction with the main effect of the amygdala, sex differences in the pattern of the covariate in association with rsFC of the amygdala [cortisol X sex ∩ amygdala] were masked with the effects of cortisol for each sex: positive or negative correlations in women as well as positive or negative correlations in men. This approach a) restricts the analyses to regions that are significantly functionally connected with the amygdala and b) shows whether rsFC is positively or negatively associated with the cortisol within women or men.
Results were thresholded at a cluster-level FWE corrected threshold of p<.05 (k=80; cluster-forming threshold at voxel-level p<.001) (e.g., Müller et al., 2013). Cytoarchitectonic assignment was based on AnatomyToolbox v2.0 (Eickhoff et al., 2005). In order to visualize and investigate the direction of sex differences in correlations of cortisol and rsFC, the rsFC of the amygdala with regions that appeared to be significant on a whole-brain level were extracted and further investigated.
2.5 Functional characterization
BrainMap (http://brainmap.org/) is a large database of published functional neuroimaging experiments with coordinate-based results, also including information of meta-data across these experiments. The meta-data are useful to characterize functions of a specific brain region (e.g., being involved in emotion, cognition or perception, amongst others). For the current study we used the database to identify behavioral phenomena underlying the simultaneous activation of the derived brain region with the amygdala. Although these regions may not mandatorily be related to the same functions during task-dependent and task-independent processing, an understanding of task-related functions objectively supports the interpretation underlying a specific cluster of a brain region (which is potentially included in various functions). We performed a functional characterization for a) the regions derived from the current rsFC analyses combined with b) either left or right amygdala seed. Therefore, we used meta-data categories that classify each single experimental contrast from the BrainMap database according to the assessed “behavioral domain” (BD) (such as emotion, cognition or perception) and the “paradigm class” (PC) (such as flanker task or reward task) (Turner and Laird, 2012) (complete list for BD and PC: http://brainmap.org/scribe/). The forward and reverse inference approach were calculated for the analyses as described in previous papers (Kogler et al., 2015b; Müller et al., 2013) and only results of BD and PC that appear to be significant in both approaches (forward and reverse) will be reported in this manuscript. The forward inference approach determines the probability of observing activity in a brain region when a mental process (such as BD emotion, cognition or perception or PC flanker task, mental rotation, reward task) is present. Thus, it tests whether the conditional probability of activation given a particular task [P(Activation|Task)] is higher than the baseline probability of activation [P(Activation)]. The baseline denotes the probability of finding a (random) activation from BrainMap in the region of interest. Significance was tested using a binominal test (p<.05, corrected for multiple comparisons [FDR]). Additionally, the reverse inference approach tests the probability of the presence of a mental process given knowledge of activation in a particular region of interest. This likelihood [P(Task|Activation)] can be derived from P(Activation|Task) as well as P(Task) and P(Activation) using Bayes’ rule. Significance was assessed by means of a chi-square test (p<.05, corrected for multiple comparisons [FDR]).
2.6 Correlational analyses
We performed correlation analyses across the whole group as well as separately for women and men between positive and negative affect and the significant rsFC of the amygdala to investigate associations between subjective mood and amygdala’s rsFC. Correlations were corrected for multiple comparisons where appropriate.
2.7 Voxel-based-morphometry (VBM) analysis
To account for potential sex differences in amygdala volume, a high-resolution anatomical image using an MPRAGE sequence (3-D Magnetization Prepared Rapid Gradient Echo: 160 sagittal slices, TR=2300ms, TE=4.21ms, 1×1×1.1mm resolution, flip angle 9°, inversion time 900ms) was acquired from each participant. The anatomical scans were preprocessed with VBM8 toolbox (dbm.neuro.uni-jena.de/vbm) in SPM8 using standard settings (DARTEL normalization, spatially adaptive non-linear means denoising) (see also Müller et al., 2015). Within a unified segmentation model (Ashburner and Friston 2005), images were corrected for biasfield inhomogeneity. The brain tissue was classified into gray matter, white matter and cerebrospinal fluid, adjusted for partial volume effects and spatially normalized to the Montreal Neurological Institute (MNI) template. The segmented images were non-linearly modulated to adjust them to the amount of expansion and contraction, which was applied during normalization. We computed the volume of the left and right amygdala by integrating the (non-linearly) modulated voxel-wise gray matter probabilities for each subject. Age was included as nuisance variable. As we did not multiply the segmented images by linear components but rather modulated the images by the non-linear components only, the calculated gray matter volume represents the amount of gray matter corrected for individual brain size.
We tested for sex differences in gray matter volume of left and right amygdala, first. Then, we investigated the associations between cortisol and left or right amygdala volume and further, we tested for sex differences in the correlations of cortisol with left or right amygdala volume. Statistical significance was evaluated at p<0.05, and Bonferroni-corrected for multiple comparisons.
3 Results
3.1 Mood and hormones
Female and male groups did not differ in age (p=.695), positive and negative affect (PANAS) (both ps=.878), nor in cortisol level (p=.082). Cortisol levels were not correlated with positive or negative affect, neither for the whole group nor in sex-specific analyses (all ps>.119). See Table 1 for sample description.
3.2 Sex differences in the resting-state functional connectivity of the amygdalae
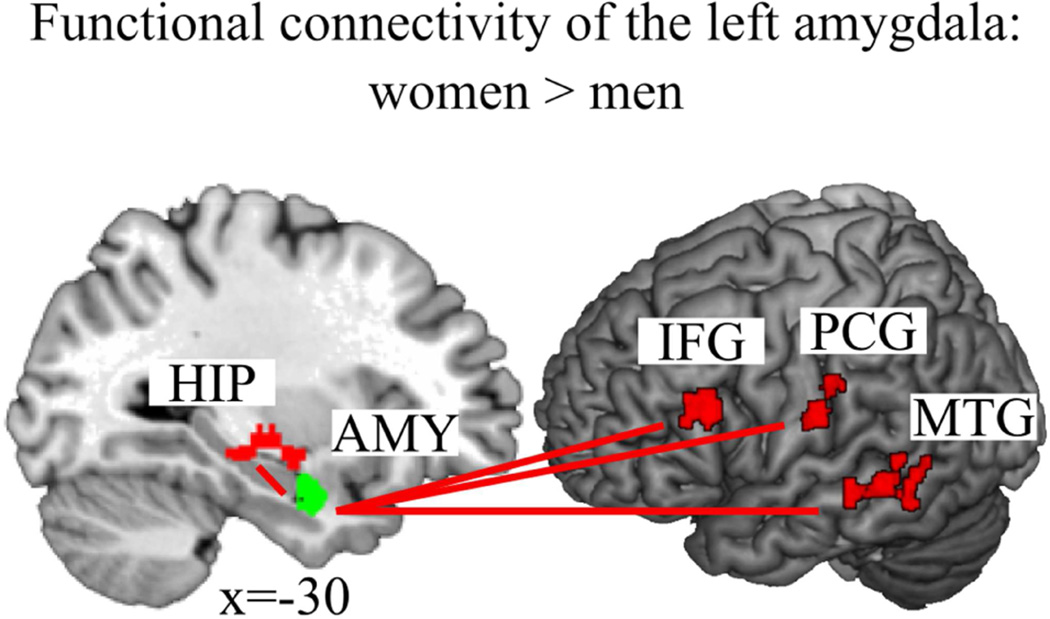
Analysis of sex differences in amygdala connectivity revealed significantly higher rsFC of the left amygdala with the left middle temporal gyrus (MTG), left inferior frontal gyrus (IFG) (triangularis), left hippocampus, and left postcentral gyrus in women compared to men (Figure 1 and Table 2). Given our threshold, no sex difference for right amygdala connectivity emerged, and also no stronger connectivity of either left or right amygdala appeared in men as compared to women.
Figure 1.
(1 column). Women had a stronger functional connectivity of the left amygdala (AMY, green) to the left hippocampus (HIP), the left inferior frontal gyrus (IFG), the left postcentral gyrus (PCG) and the left middle temporal gyrus (MTG), all in red, than men.
Table 2.
Sex differences in the resting-state functional connectivity (rsFC) of the left amygdala.
| t value | p value | X | Y | Z | Macroanatomical location |
Cytoarchitectonic location |
|
|---|---|---|---|---|---|---|---|
| Women > men | |||||||
| Cluster 1 (k=163) | 3.98 | 0.001 | −58 | −40 | −6 | L Middle Temporal Gyrus |
|
| Cluster 2 (k=118) | 4.77 | 0.008 | −46 | 20 | 24 | L IFG (p. Triangularis) |
|
| Cluster 3 (k=105) | 4.98 | 0.014 | −32 | −16 | −8 | L Hippocampus | |
| Cluster 4 (k=104) | 4.67 | 0.015 | −56 | −16 | 22 | L Postcentral Gyrus/Rolandic Operculum/ SupraMarginal Gyrus |
Area OP 1/PFt (IPL)/OP 4 |
Note. No stronger rsFC appeared for men nor for the right amygdala. Only the maximum peak of each cluster is reported. Degrees of freedom (1, 154). Macroanatomical and cytoarchitectonical locations indicate the extensions of each cluster. L=left. Coordinates x, y, z of local maxima refer to Montreal Neurological Institute space (MNI). k = number of voxels in cluster. References for histological assignments: PFt (Caspers et al., 2006); OP1-4 (Eickhoff et al., 2006a, 2006b).
The functional decoding analyses (BrainMap, http://brainmap.org/), to characterize behavioral phenomena (BD and PC) associated with the derived clusters, revealed the following results: Left MTG and left amygdala co-activation were found to be associated with the PC [face monitoring and discrimination]. No BD emerged to be significant. Left IFG and left amygdala co-activation were significantly linked to the PC [face monitoring and discrimination as well as semantic monitoring and discrimination]. No BD emerged to be significant. Co-activation of the left hippocampus and the left amygdala was significantly associated with the BD [fear, happiness, emotion, memory, explicit memory] and the PC [affective words and pictures, face monitoring and discrimination, emotion induction, encoding, passive viewing]. Left postcentral gyrus and left amygdala co-activation was found to be related to BD [pain perception] and PC [classical conditioning].
3.3 Association of amygdala’s functional connectivity with cortisol
Whole group
For the whole group no significant association emerged in association with cortisol for rsFC of the left and right amygdala.
Interaction with sex
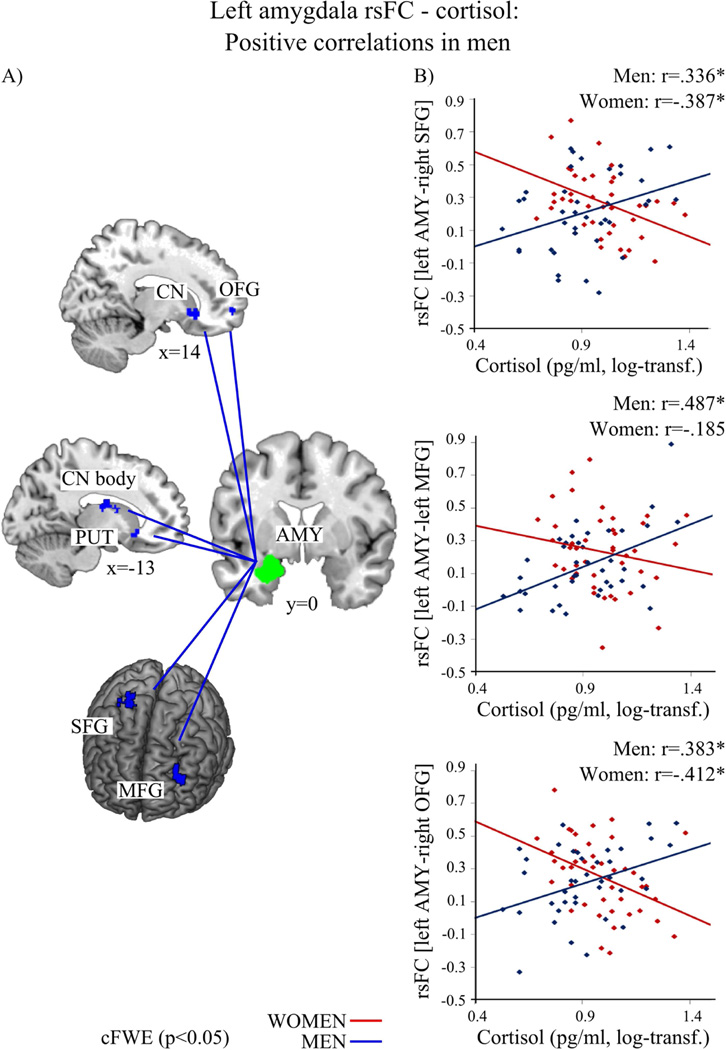
Sex differences appeared for the association of cortisol with rsFC of both amygdala with striatal, frontal and parietal regions (Figure 2–4 and Table 3 & 4). While in men higher cortisol levels were associated with a stronger rsFC of those regions with amygdala, women showed the opposite pattern, i.e., less connectivity went along with higher cortisol concentrations.
Figure 2.
In men (blue) higher cortisol levels were associated with stronger resting-state functional connectivity (rsFC) of the left amygdala (AMY, green) with bilateral caudate nucleus (CN), left putamen (PUT), right mid-orbital frontal gyrus (OFG), left middle frontal gyrus (MFG), right superior frontal gyrus (SFG). A) depicts the regions; B) shows the association between cortisol level and rsFC of the left amygdala and SFG, MFG as well as OFG in men (blue, positive) and women (red). (log-transf.=log-transformed). Significant correlations of the whole-brain analysis (p<0.05) are marked with an asterisk.
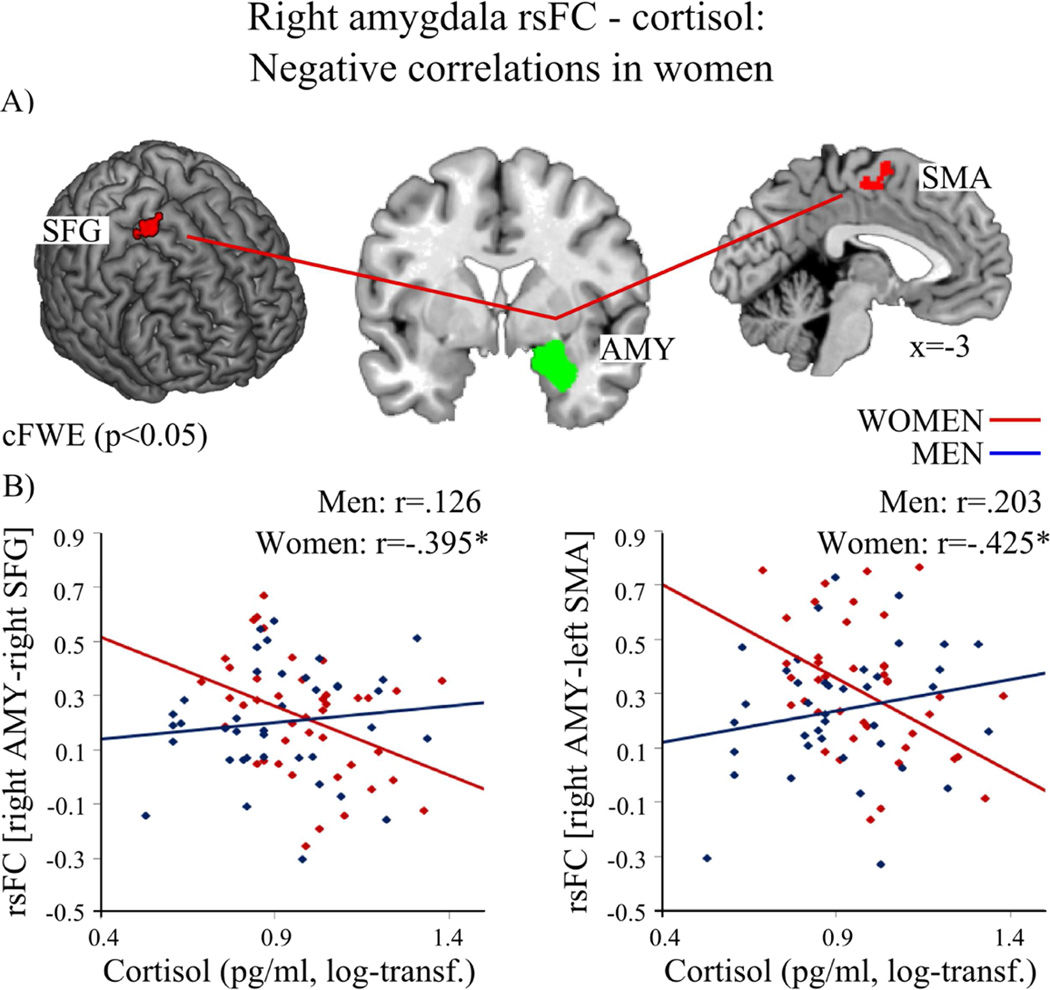
Figure 4.
(2 columns). In women (red) higher cortisol levels were associated with decreased resting-state functional connectivity (rsFC) of the right amygdala (AMY, green) with left supplementary motor area (SMA) and right superior frontal gyrus (SFG). A) depicts the regions; B) shows the association between cortisol level and rsFC of the right amygdala and SFG as well as SMA in women (red, negative) and men (blue). (log-transf.=log-transformed). Significant correlations of the whole-brain analysis (p<0.05) are marked with an asterisk.
Table 3.
Positive correlations between resting-state functional connectivity (rsFC) of the left amygdala and cortisol in men.
| t value | p value | X | Y | Z | Macroanatomical location |
Cytoarchitectonic location |
|
|---|---|---|---|---|---|---|---|
| Cluster 1 (k=161) | 4.78 | 0.001 | 14 | 22 | −8 | R Caudate Nucleus/ Mid Orbital Gyrus |
|
| Cluster 2 (k=131) | 4.49 | 0.004 | −18 | 12 | −8 | L Putamen/ Caudate Nucleus |
|
| Cluster 3 (k=108) | 4.47 | 0.013 | −8 | −2 | 6 | L Caudate Nucleus/ Pallidum |
Thal: Temporal |
| Cluster 4 (k=98) | 4.59 | 0.020 | 14 | 58 | −6 | R Mid Orbital Gyrus/Superior Medial Gyrus |
Area Fp1/FP2 |
| Cluster 5 (k=85) | 3.99 | 0.038 | −30 | 46 | 32 | L Middle Frontal Gyrus |
|
| Cluster 6 (k=80) | 4.03 | 0.050 | 24 | −8 | 62 | R Superior Frontal Gyrus/Precentral Gyrus |
Note. Sex differences in the association of cortisol with rsFC of the left amygdala: Positive correlations in men. Degrees of freedom (1, 154). R=right, L=left. Coordinates x, y, z of local maxima refer to Montreal Neurological Institute space (MNI). k = number of voxels in cluster. Only the maximum peak of each cluster is reported. Macro- and cytoarchitectonical locations indicate the clusters’ extensions. References for histological assignments: Thalamus (Behrens et al., 2003); Fp1, Fp2 (Bludau et al., 2014).
Table 4.
Negative correlations between resting-state functional connectivity (rsFC) of left and right amygdala and cortisol in women.
| t value | p value | X | Y | Z | Macroanatomical location |
Cytoarchitectonic location |
|
|---|---|---|---|---|---|---|---|
| Left amygdala: Negative correlations in women | |||||||
| Cluster 1 (k=215) | 4.78 | < 0.001 |
14 |
22 | −8 | R Caudate Nucleus /Mid Orbital Gyrus /Pallidum |
|
| Cluster 2 (k=210) | 4.49 | < 0.001 | −18 | 12 | −8 | L Putamen/ Caudate Nucleus |
|
| Cluster 3 (k=169) | 4.46 | 0.001 | −4 | 40 | −2 | L ACC/Mid Orbital Gyrus |
Area Fp2 |
| Cluster 4 (k=132) | 4.59 | 0.004 | 14 | 58 | −6 | R Mid Orbital Gyrus/Superior Medial Gyrus |
Area Fp1/Fp2 |
| Cluster 5 (k=102) | 4.47 | 0.017 | −8 | −2 | 6 | L Caudate Nucleus/ Pallidum |
Thal: Temporal |
| Cluster 6 (k=91) | 4.03 | 0.029 | 24 | −8 | 62 | R Superior Frontal Gyrus/Precentral Gyrus |
|
| Cluster 7 (k=91) | 4.01 | 0.029 | −4 | −68 | 22 | L Calcarine Gyrus/ Cuneus/Precuneus |
|
| Cluster 8 (k=86) | 4.08 | 0.037 | 34 | 46 | 26 | R Middle Frontal Gyrus |
|
| Cluster 9 (k=85) | 4.06 | 0.038 | 24 | 28 | 30 | R Superior Frontal Gyrus/Middle Frontal Gyrus |
|
| Right amygdala: Negative correlations in women | |||||||
| Cluster 1 (k=96) | 4.47 | 0.022 | −2 | −4 | 64 | L Supplementary Motor Area/MCC |
|
| Cluster 2 (k=87) | 4.72 | 0.035 | 24 | −8 | 62 | R Superior Frontal Gyrus/Posterior- medial frontal |
|
Note. Negative correlations of cortisol with rsFC of the left and right amygdala in women. Degrees of freedom (1, 154). R=right, L=left. Coordinates x, y, z of local maxima refer to Montreal Neurological Institute space (MNI). k = number of voxels in cluster. Only the maximum peak of each cluster is reported. Macro- and cytoarchitectonical locations indicate the clusters’ extensions. References for histological assignments: Thalamus (Behrens et al., 2003); Fp1, Fp2 (Bludau et al., 2014).
Positive associations in men vs. women
Investigation of these interaction effects for the left amygdala indicated significantly positive correlations of cortisol with bilateral striatal regions (caudate nucleus, putamen), right mid-orbitofrontal gyrus (OFG), left middle frontal gyrus (MFG) and right superior frontal gyrus (SFG) in men in contrast to women (see Figure 2 and Table 3). For men, neither a negative association was found for cortisol with rsFC of the left amygdala, nor any significant positive or negative association for rsFC of the right amygdala.
Functional decoding analyses revealed the following results for these clusters: Co-activation of right caudate nucleus and left amygdala was associated with the BD [emotion, cognition] and the PC [reward, face monitoring and discrimination]. Concurrent activation of left caudate nucleus and left amygdala was particularly related to the BD [emotion, sadness, cognition] and the PC [reward, film viewing]. Neither BD nor PC emerged to be significant for co-activation of left putamen and left amygdala applying our threshold. Lowering the threshold (p<.05, uncorr.), the BD [emotion, cognition, speech execution] and the PC [reward, face monitoring and discrimination] emerged. Concurrent activation of right OFG and left amygdala was associated with the BD [emotion] and the PC [passive viewing, face monitoring and discrimination]. Co-activation of left MFG and left amygdala was associated with the PC [passive viewing, face monitoring and discrimination]. No BD emerged to be significant. Co-activation of the right SFG and left amygdala was associated with the BD [explicit memory]. No PC emerged to be significant.
Negative associations in women vs. men
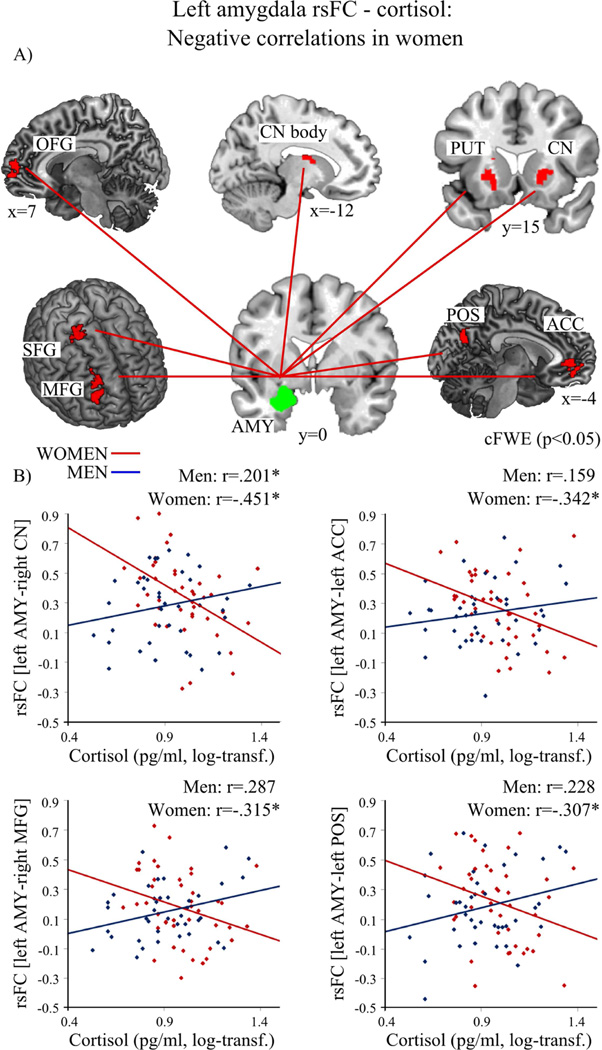
For women, cortisol was negatively associated with rsFC of the left amygdala and bilateral striatal regions (caudate nucleus, putamen), left ACC, right OFG, right MFG, right SFG as well as adjacent medial cortical areas of the parietal-occipital sulcus (POS) (precuneus, cuneus) (see Figure 3 and Table 4). Furthermore, cortisol correlated negatively with rsFC of the right amygdala with left supplementary motor area (SMA) as well as with right SFG (see Figure 4 and Table 4). No positive association between cortisol and left or right amygdala emerged in women.
Figure 3.
(2 columns). In women (red) higher cortisol levels were associated with decreased resting-state functional connectivity (rsFC) of the left amygdala (AMY, green) with bilateral caudate nucleus (CN), left putamen (PUT), left anterior cingulate gyrus (ACC), right mid-orbital frontal gyrus (OFG), right middle frontal gyrus (MFG), right superior frontal gyrus (SFG) and left parietal-occipital sulcus (POS) with adjacent cortical regions precuneus and cuneus. A) depicts the regions; B) shows the association between cortisol level and rsFC of the left amygdala and CN, ACC, MFG, and POS in women (red, negative) and men (blue). (log-transf.=log-transformed). Significant correlations of the whole-brain analysis (p<0.05) are marked with an asterisk.
Functional decoding analyses for the derived clusters revealed the following results: Co-activation of right caudate nucleus and left amygdala was associated with the BD [emotion, cognition] and the PC [reward, face monitoring and discrimination]. Concurrent activation of left caudate nucleus and left amygdala was particularly related to the BD [emotion]. No PC emerged to be significant. Co-activation of left putamen and left amygdala was linked to the BD [explicit memory]. No PC emerged to be significant. Concurrent activation of right OFG and left amygdala was associated with the BD [emotion, sadness, interoception sexuality, perception olfaction, cognition] and the PC [reward, olfactory monitoring and discrimination, film viewing]. No BD or PC emerged to be significant applying our threshold for right MFG. Lowering the threshold (p<.05, uncorr.), right MFG and left amygdala co-activation was associated with the BD [cognition, explicit memory] and the PC [reward]. Concurrent activation of left ACC and left amygdala was associated with the BD [emotion, fear] and the PC [face monitoring and discrimination, passive viewing]. Right SFG and left amygdala co-activation was linked to the BD [emotion, fear] and PC [face monitoring and discrimination, passive viewing]. Co-activation of the left POS and left amygdala was particularly related to the BD [emotion] and the PC [face monitoring and discrimination, cued explicit recognition]. Co-activation of SMA and amygdala was found to be associated with the BD [action execution]. No PC emerged to be significant.
In summary, cortisol and rsFC of amygdala with striatal regions, right OFG, and right SFG were significantly positively associated in men and significantly negatively in women, whereas the association of cortisol and rsFC of the amygdala with left MFG was significant only in men and rsFC of ACC, right MFG, SMA and CAL was significant only in women (Figure 2–4 and Table 3 & 4).
3.4 Correlational analyses with subjective mood
In women, negative affect was negatively correlated with the rsFC of the amygdala and the IFG (Spearman rs=−0.377; p=0.015). No other significant association appeared for negative mood nor any with positive mood, neither in women nor in men nor across the whole group.
3.5 Gray matter volume of the amygdala in association with cortisol
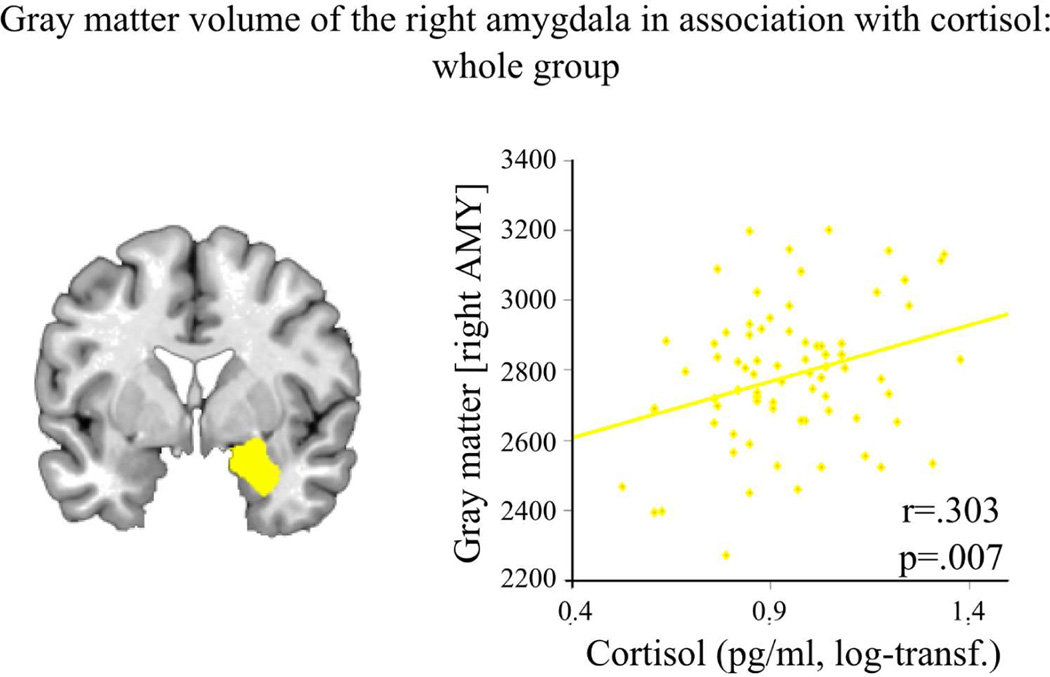
For VBM analyses three subjects had to be excluded due to outlying data in amygdala gray matter volume (mean +/− 2STD, 2 women, 1 men). No sex differences emerged for gray matter volume of left or right amygdala (both ps>.105, see supplemental material and Figure S1). Performing correlation analyses revealed a positive association of gray matter volume of the right amygdala with cortisol levels across the whole group (Figure 5), while no such correlation occurred for the left amygdala. No significant sex differences emerged for correlation analyses of amygdala-volume with cortisol.
Figure 5.
(1 column). Higher cortisol levels are associated with higher gray matter volume of the right amygdala (AMY, yellow) in both, women and men (log-transf.=log transformed).
4 Discussion
The aim of the current study was to assess sex differences in the functional connectivity of the amygdala, a brain region associated with emotional and stress-related behavior, and its association with cortisol. Sex differences are reported for certain functions (such as stress and affective processing) as well as in mental health issues including diverging prevalence rates for psychiatric disorders (e.g., attention deficit hyperactivity disorder, depression, anxiety disorder) (Cahill, 2006; McCarthy et al., 2012). Also, for cortisol release sex differences are reported. Unfortunately, studies investigating associations with hormones often focus either on female- or male-only samples. However, conclusions that are based on results of only one sex have limited value in understanding the same phenomena in the other sex (McCarthy et al., 2012). The current results therefore crucially contribute to the knowledge of sex specific effects of cortisol and its underlying neural networks.
In accordance with previous literature (Quaedflieg et al., 2015; Veer et al., 2011), our results show that steroid hormone levels are associated with the amygdala’s functional connectivity. Furthermore, these associations differ between women and men particularly regarding the functional connectivity of the amygdala with frontal and striatal regions. The coupling between the amygdala and the frontal cortex as well as the basal ganglia in interaction with hormone levels may drive sex specific differences in a variety of behaviors, which are discussed in the following.
4.1 Stronger functional connectivity of the amygdala in women than in men
Our results revealed stronger rsFC of the amygdala in women compared to men, while no stronger rsFC in men vs. women was observed. One of the regions showing increased functional connectivity with the amygdala in women compared to men was the posterior MTG. Based on our functional decoding analysis co-activation of both regions is associated with face monitoring and discrimination. More specifically, studies investigating processing faces under different circumstances (e.g., emotion induction or imitation, rating of attractiveness; infant facial cues (Habel et al., 2005; Lee et al., 2006; Strathearn et al., 2008; Winston et al., 2007)) report activation of both brain regions. There are various reports on sex differences in emotional face processing (for a review see e.g., Forni-Santos and Osório, 2015) with women outperforming men in accuracy and identification (Megreya et al., 2011). Increased connectivity between amygdala and a multimodal area like the MTG may contribute to a superior performance in women compared to men during face processing, independent of the specific task requirements. Taken together, stronger coupling between amygdala and MTG in women may contribute to the reported sex differences in face processing.
Furthermore, in the current study the left IFG was more strongly coupled with the left amygdala in women than in men. Our functional characterization revealed that the IFG and the amygdala are associated with tasks involved in semantic processing such as judging semantics in different languages (Longe et al., 2007; Luke et al., 2002). The IFG is also involved in self-referenced inner speech (Morin and Hamper, 2012) as well as in rumination, which is a perseverative focus on perceived threats, negative events and emotions, thus kind of a negative afflicted inner speech (Kühn et al., 2014). Interestingly, the connectivity between the amygdala and the IFG is reported to be higher in healthy, trauma-exposed controls compared to trauma-exposed subjects with posttraumatic-stress disorder (Brown et al., 2014). This may indicate that successful coping and the use of positive self-referenced inner speech goes along with higher amygdala-IFG coupling. Women tend to use self-referenced positive speech as well as rumination more frequently than men (Nolen-Hoeksema, 2012; Tamres et al., 2002; Trapnell and Campbell, 1999). The amygdala-IFG coupling may underlie coping mechanisms related to self-referenced speech and may result in a health-preserving mechanism such as a positive, self-referenced speech to cope with stressful situations, but also in negatively biased rumination. In concert with this we observed a negative correlation between negative affect and amygdala-IFG rsFC: Higher rsFC was associated with reduced negative affect. Thus, higher coupling between the amygdala and the IFG may indicate a stronger positive self-related talk thereby reducing negative affect probably serving as a coping mechanism. Therewith, the amygdala-IFG coupling may subserve coping mechanisms, related to inner-speech.
Furthermore, our results show a stronger coupling between the left hippocampus and the left amygdala in women than in men. The hippocampus is often associated with fear conditioning, extinction and memory (Hartley and Phelps, 2010; Maren and Holmes, 2015; Myers and Davis, 2007; Pape and Pare, 2010), and also the current functional decoding analysis of the hippocampus in association with the amygdala shows its involvement in fear and memory processing. An increased coupling between two regions processing fear and memory may lead to a disproportionate consolidation of fearful experiences in women. Indeed, women give higher subjective fear ratings in fear learning and retrieval than men (Lonsdorf et al., 2015) and women have a higher prevalence rate of anxiety and panic disorder (McCarthy et al., 2012; World Health Organization, 2004). Although the current results are based on healthy subjects, a higher coupling of hippocampus and amygdala in women may act as a neural contributing factor to sex-specific prevalence rates in anxiety and panic disorders.
Furthermore, women show stronger functional connectivity between the amygdala and a part of the inferior postcentral gyrus. This region has previously been shown to be part of a network of higher order somatosensory processing (Eickhoff et al., 2010) and found to be activated during somatosensory and pain perception (Bingel et al., 2006). Our functional decoding analyses also revealed the association of postcentral gyrus and amygdala co-activation with painful stimulation. In female pain patients, an increased functional connectivity is reported for inferior parietal regions also to other brain regions than the amygdala (Napadow et al., 2010). Furthermore, there is some evidence for higher chronic pain perception and increased reports of pain intensity in women compared to men (Mogil, 2012). The coupling between a subcortical region driving emotional and stress reactions, such as the amygdala, and a cortical area associated with somatosensory pain processing, may be associated with the increased reports in pain sensitivity and chronic pain in women (Mogil, 2012).
4.2 Sex specific association of cortisol with functional connectivity of the amygdalae
The analyses of the amygdala’s functional connectivity in association with cortisol revealed a significant interaction with sex. In men, cortisol is positively associated with the functional connectivity of the amygdala with striatal and frontal regions, whereas in women this association is negative. Our results support previous findings showing a stress-induced increase in connectivity between the amygdala and the striatum (Vogel et al., 2015), as well as with frontal regions (Veer et al., 2011) in male-only samples. Also, they support previous reports on functional connectivity of the amygdala in association with cortisol: Exogenous cortisol administration increases the functional coupling of amygdala and the prefrontal cortex in men (Henckens et al., 2010), and a positive association between circadian cortisol decrease and a decrease in amygdala-ACC connectivity was shown in previous studies for healthy men (Veer et al., 2012). In addition, the current results crucially extend those previous studies by demonstrating that cortisol-connectivity associations are sex dependent.
Our functional decoding analyses demonstrate a tight coupling of cortisol with regions that are associated with emotion, reward and face processing. Indeed, cortisol has been shown to be associated with reward processing (Montoya et al., 2014) as well as emotion regulation (Urry et al., 2006). In particular, in a male sample, exogenous cortisol decreased reward preference and activity in the striatum (Montoya et al., 2014), and circadian cortisol decrease was associated with activity in the amygdala during emotion regulation (Urry et al., 2006). In the current study, increased cortisol levels in men were associated with increased connectivity between the left amygdala and regions of the reward and limbic system, such as caudate nucleus, putamen, OFG and MFG. Aversive stimuli (Domes et al., 2010), monetary loss (Beck et al., 2009), decision-making in learners in a card betting game (Schönberg et al., 2007), as well as processing pleasant or infant faces (Jabbi et al., 2007; Strathearn et al., 2008) increases activity in the amygdala as well as caudate nucleus, putamen and MFG. Contrarily when retrieving stressful events, the OFG as well as the amygdala seems to be deactivated in healthy subjects (Britton et al., 2005). Taken together with the current results on rsFC , it seems that cortisol may increase connectivity of amygdala and these specific regions in men, while in women the connectivity may decrease with increasing cortisol. Cortisol is thought to regulate neural network responses that underlie behavioral adaption to arousing situations (Henckens et al., 2012; Kloet et al., 2005). However, the current results indicate sex-dependent regulatory effects of cortisol on regions associated with emotion and reward processing.
Particularly, the pattern of cortisol in association with amygdala-ACC coupling seems to be sex-dependent. In men, this association was reported to be positive (Veer et al., 2012). This previous study, however, did only test men and could thus not provide any information how cortisol may affect amygdala-ACC connectivity in women. Our study now demonstrate an interaction between sex and cortisol in amygdala-ACC connectivity, with a negative association in women, while men showed an opposite (though not significant) relationship. The ACC is thought to down-regulate amygdala activity (Stein et al., 2007; Veer et al., 2012), playing an important role in emotion regulation and adapted stress response (Diorio et al., 1993; Ulrich-Lai and Herman, 2009; Veer et al., 2012). Our results in addition with the literature indicate that the mediating effect of cortisol on ACC activity, and consequently its influence on amygdala activity, differs between women and men.
Another sex-specific effect was shown for the connectivity between amygdala and SFG in association with cortisol. Our functional decoding analysis revealed that concurrent activation of the SFG and the amygdala is associated with explicit memory such as recognition of objects (e.g., Achim and Lepage, 2005). Sex differences in association with stressful situations are already reported for memory retrieval: Stress impairs memory more strongly in men than in women, and memory performance is also correlated negatively with cortisol in men (Hidalgo et al., 2015; Wolf et al., 2001). This together with our current results indicates that men feature an altered ability to retrieve information during stressful situations which is possibly mediated by a stronger coupling of the SFG and the amygdala with increased cortisol levels. In contrast, in women, increased cortisol levels going along with decreased SFG-amygdala connectivity may indicate less emotional interference during explicit memory processing, shown by the decoupling of an area associated with emotion processing such as the amygdala and an area associated with memory such as the SFG with higher cortisol concentrations in women. Thus, sex differences in association of cortisol and functional connectivity of amygdala and SFG as found in our study may underlie sex specific effects that cortisol exerts on memory performance in women and men.
In addition, the connectivity of the left amygdala and the left POS, including adjacent precuneus and cuneus, demonstrated sex-specific associations with cortisol. Increased connectivity between precuneus and the amygdala following stress in a male-only sample has been reported previously (Veer et al., 2011), and additionally in the current study a negative association was found in women in association with cortisol. Interestingly, in women with post-traumatic stress disorder compared to healthy women the connectivity between the medial parietal area and the amygdala is decreased (Bluhm et al., 2009). Besides being functionally associated with emotion and face processing (Schilbach et al., 2012), as also revealed by the current decoding analysis, the medial parietal area is often associated with “default mode”, mind-wandering and self-generated thoughts (Cavanna and Trimble, 2006; Fox et al., 2015) that appear during resting-states (Mason et al., 2007; Tusche et al., 2014). Furthermore, cortisol release is associated with self-generated thoughts: While future oriented social thoughts are associated with decreased cortisol levels, an increase in cortisol is positively correlated with negative thoughts (Engert et al., 2014). Thus, increased cortisol concentrations in association with decreased connectivity of the amygdala and the POS in women may indicate a higher engagement of women in future oriented thoughts, whereas an increased connectivity of POS and amygdala with higher cortisol levels in men suggests a focus on negative thoughts during resting-state. Hence, one could speculate that in the scanner environment, aroused women distract themselves with thinking about the future, while aroused men tend to concentrate on negative thoughts. Whether women and men deal with different contents during mind-wandering, and whether this is directly related to cortisol release, remains to be tested in future research.
In woman, contrarily to men, cortisol was negatively associated with the coupling of the amygdala and the SMA, two regions which co-activation has been associated with action execution. Interestingly, theories on stress reactions claim that neuroendocrine mechanisms regulate stress responses in women and men differently (Taylor et al., 2000): In women a predominant “tend-and-befriend” reaction to arousing situations is assumed (Taylor et al., 2000) suggesting that in arousing states the need for action in women may be decreased. This pattern was not seen in men. The results thus further support the notion that cortisol exerts different functions in women and men.
4.3 Positive association of gray matter volume and cortisol levels
Analyses of gray matter volume revealed a significant association between right amygdala and cortisol levels, with greater amygdala volumes going along with higher cortisol concentrations across women and men. Notably, no sex differences in amygdala volume emerged. Previously, larger volumes in men than in women have been reported for bilateral subcortical clusters, including the amygdala, for an age range of 7–80 years (Ruigrok et al., 2014). Differences to the current results might be explained by the relatively homogenous age range of the sample used in the current study. Furthermore, sex hormones are known to have an influence on amygdala volume (Giedd et al., 2012; Neufang et al., 2009; Pletzer et al., 2010). Given that we only included naturally cycling women in the current study, sex hormone release throughout the menstrual cycle may also contribute to the missing sex differences here. Furthermore, the observed positive association between amygdala volume and cortisol levels is in line with the assumption that the amygdala has an excitatory effect on the hypothalamus-pituitary-adrenal gland axis, leading to increased corticoid secretion (Ulrich-Lai and Herman, 2009), in a healthy sample.
Interestingly, we observed a positive association between amygdala volume and baseline cortisol levels. The amygdala, as a key brain structure in response to emotionally salient situations such as stress, rapidly responds to environmental demands by triggering physiological stress responses (Evans et al., 2015; McEwen and Gianaros, 2010) and exerts excitatory effects on the hypothalamus-pituitary-adrenal gland (HPA) axis (McEwen and Gianaros, 2010; Ulrich-Lai and Herman, 2009). Increased life stress during childhood leads to larger amygdala volumes in children and adults (Evans et al., 2015; Lupien et al., 2011; Tottenham et al., 2010) emphasizing the association of amygdala volume and stress reactivity. The positive correlation between amygdala volume and baseline cortisol levels in our data underlines the excitatory effect of the amygdala on HPA-axis leading to increased corticoid secretion. Furthermore, our data show that this is already apparent in healthy subjects under baseline conditions.
4.4 Limitations
There are some suggestions for future research that may be seen as limitations of the current study. Interestingly, previous reports partly support the current findings on sex differences in amygdala rsFC, however they appeared to be right lateralized: Higher coupling of right basolateral amygdala with right IFG and right postcentral gyrus were reported in women than in men (Engman et al., 2016). Differences in lateralization between studies may be explained by the area used as seed region (subnuclei vs. whole amygdala). Due to the current scanning parameters, which were not optimized to assess small amygdala subnuclei, and the ubiquitous smoothing factor, we decided to use the whole amygdala as seed region for the current analyses (e.g., Robinson et al., 2004). Furthermore, differences in preprocessing approaches may influence the data (Weissenbacher et al., 2009). Global signal regression is suspected to rather create than reveal relationships between brain regions (Saad et al., 2012), which further may suggest non-existent group differences. To avoid false positive results, we did not include global signal as nuisance variable. However, replication studies are needed to confirm the results.
Importantly, it has to be considered that amygdala connectivity measures might be confounded by signal from nearby veins, especially when using spatial smoothing. While direct assessment of venous influences has been investigated in short-TR (temporal resolution) datasets (Boubela et al., 2015) and corrections might be implemented with these types of data, it is not yet clear how to appropriately account for vascular effects in a dataset with long TR, where multiple high-frequency fluctuations present in the amygdala region are not critically sampled. The cleansing of long TR data with respect to venous effect is a topic for future investigation, but at present the possibility of potential distortion of results by venous effects should be considered when interpreting results.
Hormone levels vary across the female menstrual cycle and cortisol levels are reported to differ between follicular and luteal cycle phase in response to arousing or stressful situations (Andreano et al., 2008; Duchesne and Pruessner, 2013; Kirschbaum et al., 1999). For exploratory reasons we directly compared cortisol levels between our naturally cycling women with independent t-tests (15 follicular vs. 5 ovulation vs. 22 luteal) and did not find significant group differences (ovulation vs. follicular p=.716; ovulation vs. luteal: p=.980; follicular vs. luteal: p=.514) (menstrual cycle phase was assessed via self-reports surveying the last three cycles and the onset of the following menses). In the current study we did not induce a stressful, arousing situation and the missing difference between sexes and also between menstrual cycle phases in baseline cortisol are consistent with previous reports (Chung et al., 2016; Duchesne and Pruessner, 2013; Kirschbaum et al., 1999; Maki et al., 2015). Furthermore, sex hormones seem to critically modulate cortisol response in emotion and stress regulation during different stages of the menstrual cycle (Albert et al., 2015; Andreano and Cahill, 2010; Duchesne and Pruessner, 2013). Thus, we cannot exclude that diverging levels of sex steroids may interact with the reported findings regarding cortisol, specifically as we included females from different menstrual cycle phases with different sex hormone concentrations. Hence, future studies might want to specifically analyze the interaction between sex hormones and cortisol across the menstrual cycle and further investigate the combined impact on functional connectivity. Moreover, oral contraception seems to have an impact on brain networks of rsFC (Petersen et al., 2014). Here we only included naturally cycling women, but the impact of oral contraceptives on functional connectivity of the amygdala has to be elucidated in upcoming studies.
The current study is based on correlational analyses, thus we cannot deduce causal effects between cortisol and functional connectivity. Furthermore, we can only speculate about the direction of the described connections, and did this based on previous reports on effective or anatomical neural connections. Additionally, the current study mainly focused on associations between physiological arousal and rsFC and many important variables, e.g. personality traits, stress-related behavior, etc. were not included in the analyses but would be important to further strengthen our assumptions.
Also, we wish to note that the current results reflect states of sex differences in healthy young adults in association with cortisol. We do not assume that the reported sex and cortisol differences are determined by biological sex only (Miller and Halpern, 2014). They may also be a result of socialization and gender roles. A longitudinal study of the functional connectivity of the amygdala in association with steroid hormones and gender roles remains to be tested.
4.5 Summary and conclusion
Taken together, resting-state functional connectivity of the amygdala differs between women and men. Women showed stronger connections of the amygdala with the MTG, IFG, hippocampus and the postcentral gyrus. This indicates a stronger coupling in women compared to men of regions associated with face processing, inner-speech, fear and pain processing with a region of emotion processing. These sex-specific connectivities may underlie sex differences in those behavioral phenomena.
Furthermore, an interaction of sex and cortisol levels was apparent for rsFC of the amygdala: Higher levels of cortisol were positively correlated with functional connectivity in men and negatively in women. Cortisol seems to enhance the crosstalk of regions included in emotion, reward, memory and action processing in men, while the coupling of these regions is reduced with increasing cortisol levels in women. Our results thus indicate that the regulatory mechanism of cortisol on neural networks to adapt behavior in arousing situations differs between women and men. Given the amount of literature based on male-only samples, the current results significantly extend our knowledge and should lead to a more sensitive way of dealing with biological sex and hormonal influence on functional connectivity and stress research. Therefore, sex differences in functional connectivity and interactions with hormones should be taken into account much stronger in future research.
Supplementary Material
Highlights.
rsFC of the amygdala differs between women and men
Women show higher amygdala rsFC with hippocampus, IFG, MTG and postcentral gyrus
Cortisol is sex-dependently associated with rsFC of the amygdala
In women, cortisol is negatively correlated with rsFC of the amygdala
In men, cortisol is positively associated with rsFC of the amygdale
Acknowledgments
This study was supported by the Deutsche Forschungsgemeinschaft (DFG; IRTG-1328 [LK, RCG, UH, BD]; EI 816/4-1 [SBE]; EI 816/6-1 [SBE]; LA 3071/3-1 [SBE]), Jülich-Aachen-Research-Alliance, Translational Brain Medicine (LK, UH, BD), the National Institute of Mental Health (R01-MH074457) (SBE), the European EFT program (Human Brain Project) (SBE) and Austrian Science Fund (FWF Project P-23533 [RB, KK, EMS, EM, RCG, UH, BD]). The funding sources had no involvement in study design, collection, analysis or interpretation of the data, in writing the article and in decision for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
All authors declare no conflict of interest in relation to the manuscript.
BD, UH, RCG, EMS, EM designed research; EMS, RB, KK performed research; VIM, SBE contributed analytic tools; LK, VIM, SBE, BD analyzed data; LK, VIM, SBE, BD wrote and EMS, RB, KK, UH, RCG, EM revised and approved the paper.
Preliminary results of parts of this study were presented at the annual Meeting of the Organization of Human Brain Mapping (OHBM) in Honolulu 2015.
References
- Achim M, Lepage M. Neural correlates of memory for items and for associations: An event-related functional magnetic resonance imaging study. J. Cogn. Neurosci. 2005;14:652–667. doi: 10.1162/0898929053467578. [DOI] [PubMed] [Google Scholar]
- Alarcón G, Cservenka A, Rudolph MD, Fair DA, Nagel BJ. Developmental sex differences in resting state functional connectivity of amygdala sub-regions. Neuroimage. 2015;115:235–244. doi: 10.1016/j.neuroimage.2015.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert K, Pruessner J, Newhouse P. Estradiol levels modulate brain activity and negative responses to psychosocial stress across the menstrual cycle. Psychoneuroendocrinology. 2015;59:14–24. doi: 10.1016/j.psyneuen.2015.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreano JM, Arjomandi H, Cahill L. Menstrual cycle modulation of the relationship between cortisol and long-term memory. Psychoneuroendocrinology. 2008;33:874–882. doi: 10.1016/j.psyneuen.2008.03.009. [DOI] [PubMed] [Google Scholar]
- Andreano JM, Cahill L. Menstrual cycle modulation of medial temporal activity evoked by negative emotion. Neuroimage. 2010;53:1286–1293. doi: 10.1016/j.neuroimage.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Beck A, Schlagenhauf F, Wüstenberg T, Hein J, Kienast T, Kahnt T, Schmack K, Hägele C, Knutson B, Heinz A, Wrase J. Ventral striatal activation during reward anticipation correlates with impulsivity in alcoholics. Biol. Psychiatry. 2009;66:734–742. doi: 10.1016/j.biopsych.2009.04.035. [DOI] [PubMed] [Google Scholar]
- Behrens TE, Johansen-Berg H, Woolrich MW, Smith SM, Wheeler-Kingshott CA, Boulby PA, Barker GJ, Sillery EL, Sheehan K, Ciccarelli O, Thompson AJ, Brady JM, Matthews PM. Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nat. Neurosci. 2003;6:750–757. doi: 10.1038/nn1075. [DOI] [PubMed] [Google Scholar]
- Bingel U, Lorenz J, Schoell E, Weiller C, Büchel C. Mechanisms of placebo analgesia: rACC recruitment of a subcortical antinociceptive network. Pain. 2006;120:8–15. doi: 10.1016/j.pain.2005.08.027. [DOI] [PubMed] [Google Scholar]
- Bludau S, Eickhoff SB, Mohlberg H, Caspers S, Laird AR, Fox PT, Schleicher A, Zilles K, Amunts K. Cytoarchitecture, probability maps and functions of the human frontal pole. Neuroimage. 2014;93:260–275. doi: 10.1016/j.neuroimage.2013.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluhm RL, Williamson PC, Osuch EA, Frewen PA, Stevens TK, Boksman K, Neufeld RWJ, Théberge J, Lanius RA. Alterations in default network connectivity in posttraumatic stress disorder related to early-life trauma. J. Psychiatry Neurosci. 2009;34:187–194. [PMC free article] [PubMed] [Google Scholar]
- Boubela RN, Kalcher K, Huf W, Seidel E-M, Derntl B, Pezawas L, Nasel C, Moser E. fMRI measurements of amygdala activation are confounded by stimulus correlated signal fluctuation in nearby veins draining distant brain regions. Sci. Rep. 2015;5:10499. doi: 10.1038/srep10499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton JC, Phan KL, Taylor SF, Fig LM, Liberzon I. Corticolimbic blood flow in posttraumatic stress disorder during script-driven imagery. Biol. Psychiatry. 2005;57:832–840. doi: 10.1016/j.biopsych.2004.12.025. [DOI] [PubMed] [Google Scholar]
- Brown VM, LaBar KS, Haswell CC, Gold AL, McCarthy G, Morey RA Mid-Atlantic-MIRECC-Workgroup. Altered resting-state functional connectivity of basolateral and centromedial amygdala complexes in posttraumatic stress disorder. Neuropsychopharmacology. 2014;39:351–359. doi: 10.1038/npp.2013.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill L. Why sex matters for neuroscience. Nat. Rev. Neurosci. 2006;7:477–484. doi: 10.1038/nrn1909. [DOI] [PubMed] [Google Scholar]
- Caspers S, Geyer S, Schleicher A, Mohlberg H, Amunts K, Zilles K. The human inferior parietal cortex: cytoarchitectonic parcellation and interindividual variability. Neuroimage. 2006;33:430–448. doi: 10.1016/j.neuroimage.2006.06.054. [DOI] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. 2006:564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Chung KC, Peisen F, Kogler L, Radke S, Turetsky BI, Freiherr J, Derntl B. The influence of menstrual cycle and androstadienone on female stress reaction: an fMRI study. Front. Hum. Neurosci. 2016 doi: 10.3389/fnhum.2016.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Kloet ER, Vreugdenhil E, Oitzl MS, Joëls M. Brain corticosteroid receptor balance in health and disease*. Endocr. Rev. 1998;19:269–301. doi: 10.1210/edrv.19.3.0331. [DOI] [PubMed] [Google Scholar]
- Derntl B, Habel U, Windischberger C, Robinson S, Kryspin-Exner I, Gur RC, Moser E. General and specific responsiveness of the amygdala during explicit emotion recognition in females and males. BMC Neurosci. 2009;10:1–14. doi: 10.1186/1471-2202-10-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derntl B, Windischberger C, Robinson S, Lamplmayr E, Kryspin-exner I, Gur RC. Facial emotion recognition and amygdala activation are associated with menstrual cycle phase. 2008 doi: 10.1016/j.psyneuen.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychol. Bull. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Diorio D, Viau V, Meaney J. The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamic-pituitary-adrenal responses to stress. J. Neurosci. 1993;13:3839–3847. doi: 10.1523/JNEUROSCI.13-09-03839.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domes G, Schulze L, Böttger M, Grossmann A, Hauenstein K, Wirtz PH, Heinrichs M, Herpertz SC. The neural correlates of sex differences in emotional reactivity and emotion regulation. Hum. Brain Mapp. 2010;31:758–769. doi: 10.1002/hbm.20903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchesne A, Pruessner JC. Association between subjective and cortisol stress response depends on the menstrual cycle phase. Psychoneuroendocrinology. 2013;38:3155–3159. doi: 10.1016/j.psyneuen.2013.08.009. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Amunts K, Mohlberg H, Zilles K. The human parietal operculum. II. Stereotaxic maps and correlations with functional imaging results. Cereb. Cortex. 2006a;16:268–279. doi: 10.1093/cercor/bhi106. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Jbabdi S, Caspers S, Laird AR, Fox PT, Zilles K, Behrens TEJ. Anatomical and functional connectivity of cytoarchitectonic areas within the human parietal operculum. J. Neurosci. 2010;30:6409–6421. doi: 10.1523/JNEUROSCI.5664-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Müller VI. Brain Mapping - an Encyclopedic Reference. Vol. 2. Anatomy and Physiology, Systems; 2015. Functional connectivity; pp. 187–201. [Google Scholar]
- Eickhoff SB, Schleicher A, Zilles K, Amunts K. The human parietal operculum. I. Cytoarchitectonic mapping of subdivisions. Cereb. Cortex. 2006b;16:254–267. doi: 10.1093/cercor/bhi105. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage. 2005;25:1325–1335. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Engert V, Smallwood J, Singer T. Mind your thoughts: Associations between self-generated thoughts and stress-induced and baseline levels of cortisol and alpha-amylase. Biol. Psychol. 2014;103:283–291. doi: 10.1016/j.biopsycho.2014.10.004. [DOI] [PubMed] [Google Scholar]
- Engman J, Linnman C, Van Dijk KRA, Milad MR. Amygdala subnuclei resting-state functional connectivity sex and estrogen differences. Psychoneuroendocrinology. 2016;63:34–42. doi: 10.1016/j.psyneuen.2015.09.012. [DOI] [PubMed] [Google Scholar]
- Evans GW, Swain JE, King AP, Wang X, Javanbakht A, Ho SS, Angstadt M, Phan KL, Xie H, Liberzon I. Childhood cumulative risk exposure and adult amygdala volume and function. J. Neurosci. Res. 2015 doi: 10.1002/jnr.23681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forni-Santos L, Osório FL. Influence of gener in the recognition of basic facial expression: A critical literature review. World J. Psychiatry. 2015;5:342–351. doi: 10.5498/wjp.v5.i3.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox KCR, Spreng RN, Ellamil M, Andrews-Hanna JR, Christoff K. The wandering brain : Meta-analysis of functional neuroimaging studies of mind-wandering and related spontaneous thought processes. Neuroimage. 2015;111:611–621. doi: 10.1016/j.neuroimage.2015.02.039. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Frith CD, Liddle PF, Frackowiak RSJ. Functional connectivity: The principal-component analysis of large (PET) data sets. J. Cereb. blood flow Metab. 1993;13:5–14. doi: 10.1038/jcbfm.1993.4. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Raznahan A, Mills KL, Lenroot RK. Review: magnetic resonance imaging of male / female differences in human adolescent brain anatomy. Biol. Sex Differ. 2012;3:1. doi: 10.1186/2042-6410-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin PR, Manber-ball T, Werner K, Heimberg R, Gross JJ. Neural Mechanisms of Cognitive Reappraisal of Negative Self-Beliefs in Social Anxiety Disorder. BPS. 2009;66:1091–1099. doi: 10.1016/j.biopsych.2009.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habel U, Klein TM, Kellermann T, Shah NJ, Schneider F. Same or different? Neural correlates of happy and sad mood in healthy males. Neuroimage. 2005;26:206–214. doi: 10.1016/j.neuroimage.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Hartley CA, Phelps EA. Changing fear: the neurocircuitry of emotion regulation. Neuropsychopharmacol. Rev. 2010;35:136–146. doi: 10.1038/npp.2009.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henckens MJAG, van Wingen GA, Joëls M, Fernández G. Time-dependent effects of cortisol on selective attention and emotional interference: a functional MRI study. Front. Integr. Neurosci. 2012;6:66. doi: 10.3389/fnint.2012.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henckens MJAG, Wingen GA, Van Joe M. Time-dependent effects of corticosteroids on human amygdala processing. J. Neurosci. 2010;30:12725–12732. doi: 10.1523/JNEUROSCI.3112-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidalgo V, Pulopulos MM, Puig-Perez S, Espin L, Gomez-Amor J, Salvador A. Acute stress affects free recall and recognition of pictures differently depending on age and sex. Behav. Brain Res. 2015;292:393–402. doi: 10.1016/j.bbr.2015.07.011. [DOI] [PubMed] [Google Scholar]
- Holmes CJ, Hoge R, Collins L, Woods R, Toga AW. Enhancement of MR images using registration for signal averaging. J. Comput. Assist. Tomogr. 1998;22:324–333. doi: 10.1097/00004728-199803000-00032. [DOI] [PubMed] [Google Scholar]
- Jabbi M, Swart M, Keysers C. Empathy for positive and negative emotions in the gustatory cortex. 2007;34:1744–1753. doi: 10.1016/j.neuroimage.2006.10.032. [DOI] [PubMed] [Google Scholar]
- Kajantie E, Phillips DIW. The effects of sex and hormonal status on the physiological response to acute psychosocial stress. Psychoneuroendocrinology. 2006;31:151–178. doi: 10.1016/j.psyneuen.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Kudielka BM, Gaab J, Schommer NC, Hellhammer DH. Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituitary-adrenal axis. Psychosom. Med. 1999;61:154–162. doi: 10.1097/00006842-199903000-00006. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Wüst S, Hellhammer D. Consistent sex differences in cortisol responses to psychological stress. Psychosom. Med. 1992;54:648–657. doi: 10.1097/00006842-199211000-00004. [DOI] [PubMed] [Google Scholar]
- Kloet ER, De Joëls M, Holsboer F. Stress and the brain: From adaption to disease. Nat. Rev. Neurosci. 2005;6:463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- Kogler L, Gur RC, Derntl B. Sex differences in cognitive regulation of psychosocial achievement stress: Brain and behavior. Hum. Brain Mapp. 2015a;36:1028–1042. doi: 10.1002/hbm.22683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogler L, Müller VI, Chang A, Eickhoff SB, Fox PT, Gur RC, Derntl B. Psychosocial versus physiological stress - Meta-analyses on the deactivations and activations of the neural correlates of stress reactions. Neuroimage. 2015b doi: 10.1016/j.neuroimage.2015.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn S, Vanderhasselt M-A, De Raedt R, Gallinat J. The neural basis of unwanted thoughts during resting state. Soc. Cogn. Affect. Neurosci. 2014;9:1320–1324. doi: 10.1093/scan/nst117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T, Josephs O, Dolan RJ, Critchley HD. Imitating expressions: Emotion-specific neural substrates in facial mimicry. Soc. Cogn. Affect. Neurosci. 2006:122–135. doi: 10.1093/scan/nsl012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longe O, Randall B, Stamatakis EA, Tyler LK. Grammatical categories in the brain: The role of morphological structure. Cereb. Cortex. 2007;17:1812–1820. doi: 10.1093/cercor/bhl099. [DOI] [PubMed] [Google Scholar]
- Lonsdorf TB, Haaker J, Schümann D, Sommer T, Bayer J, Brassen S, Bunzeck N, Gamer M, Kalisch R. Sex differences in conditioned stimulus discrimination during context-dependent fear learning and its retrieval in humans: the role of biological sex, contraceptives and menstrual cycle phases. J. psychiatry Neurosci. 2015;40:140336. doi: 10.1503/140336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Larson MP, Anderson JS, Ferguson MA, Yurgelun-Todd D. Local brain connectivity and association with gender and age. Dev. Cogn. Neurosci. 2011;1:187–197. doi: 10.1016/j.dcn.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luke K-K, Liu H-L, Wai Y-Y, Wan Y-L, Tan LH. Functional anatomy of syntactic and semantic processing in language comprehension. Hum. Brain Mapp. 2002;16:133–145. doi: 10.1002/hbm.10029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, Parent S, Evans AC, Tremblay RE, David P, Corbo V. Larger amygdala but no change in hippocampal volume in 10-year-old children exposed to maternal depressive symptomatology since birth i. 2011 doi: 10.1073/pnas.1105371108. doi:10.1073/pnas.1105371108/-/DCSupplemental.www.pnas.org/cgi/doi/10.1073/pnas.1105371108. [DOI] [PMC free article] [PubMed]
- Maki PM, Mordecai KL, Rubin LH, Sundermann E, Savarese A, Eatough E, Drogos L. Menstrual cycle effects on cortisol responsivity and emotional retrieval following a psychosocial stressor. Horm. Behav. 2015;74:201–208. doi: 10.1016/j.yhbeh.2015.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Holmes A. Stress and fear extinction. Neuropsychopharmacol. Rev. 2015:1–22. doi: 10.1038/npp.2015.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN. Wandering minds: The default network and stimulus-independent thoughts. Science. 2007;315:393–395. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM, Arnold AP, Ball GF, Blaustein JD, De Vries GJ. Sex differences in the brain: The not so inconvenient truth. J. Neurosci. 2012;32:2241–2247. doi: 10.1523/JNEUROSCI.5372-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Gianaros PJ. Central role of the brain in stress and adaption: Links to socioeconomic status, health, and disease. Ann N Y Acad Sci. 2010;118611:190–222. doi: 10.1111/j.1749-6632.2009.05331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae K, Ochsner KN, Mauss IB, Gabrieli JJD, Gross JJ. Gender differences in emotion regulation: an fMRI study of cognitive reappraisal. Gr. Process. Intergr. Relations. 2008;11:143–162. doi: 10.1177/1368430207088035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megreya AM, Bindemann M, Havard C. Sex differences in unfamiliar face identification: Evidence from matching tasks. Acta Psychol. (Amst) 2011;137:83–89. doi: 10.1016/j.actpsy.2011.03.003. [DOI] [PubMed] [Google Scholar]
- Miller DI, Halpern DF. The new science of cognitive sex differences. Trends Cogn. Sci. 2014;18:37–45. doi: 10.1016/j.tics.2013.10.011. [DOI] [PubMed] [Google Scholar]
- Mogil JS. Sex differences in pain and pain inhibition: multiple explanations of a controversial phenomenon. Nat. Rev. Neurosci. 2012;13:859–866. doi: 10.1038/nrn3360. [DOI] [PubMed] [Google Scholar]
- Montoya ER, Bos PA, Terburg D, Rosenberger LA, Van Honk J. Cortisol administration induces global down-regulation of the brain’s reward circuitry. Psychoneuroendocrinology. 2014;47:31–42. doi: 10.1016/j.psyneuen.2014.04.022. [DOI] [PubMed] [Google Scholar]
- Morin A, Hamper B. Self-reflection and the inner voice: activation of the left inferior frontal gyrus during perceptual and conceptual self-referential thinking. Open Neuroimag. J. 2012;6:78–89. doi: 10.2174/1874440001206010078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller VI, Cieslik EC, Laird AR, Fox PT, Eickhoff SB. Dysregulated left inferior parietal activity in schizophrenia and depression: functional connectivity and characterization. Front. Hum. Neurosci. 2013;7:268. doi: 10.3389/fnhum.2013.00268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller VI, Langner R, Cieslik EC, Rottschy C, Eickhoff SB. Interindividual differences in cognitive flexibility: influence of gray matter volume, functional connectivity and trait impulsivity. Brain Struct. Funct. 2015;220:2401–2414. doi: 10.1007/s00429-014-0797-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA. The impact of global signal regression on resting state correlations: Are anti-correlated networks introduced? Neuroimage. 2009;44:893–905. doi: 10.1016/j.neuroimage.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers KM, Davis M. Mechanisms of fear extinction. Mol. Psychiatry. 2007;12:120–150. doi: 10.1038/sj.mp.4001939. [DOI] [PubMed] [Google Scholar]
- Napadow V, LaCount L, Park K, As-Sanie S, Clauw DJ, Harris RE. Intrinsic brain connectivity in fibromyalgia is associated with chronic pain intensity. Arthritis Rheum. 2010;62:2545–2555. doi: 10.1002/art.27497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufang S, Specht K, Hausmann M, Konrad K. Sex differences and the impact of steroid hormones on the developing human brain. Cereb. Cortex. 2009;19:464–473. doi: 10.1093/cercor/bhn100. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S. Emotion regulation and psychopathology: the role of gender. Annu. Rev. Clin. Psychol. 2012;8:161–187. doi: 10.1146/annurev-clinpsy-032511-143109. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli JDE, Gross JJ. For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. Neuroimage. 2004;23:483–499. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Pape H-C, Pare D. Plastic synaptic networks of the amygdala for the acquisition, expression, and extinction of conditioned fear. Physiol. Rev. 2010;90:419–463. doi: 10.1152/physrev.00037.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen N, Kilpatrick LA, Goharzad A, Cahill L. Oral contraceptive pill use and menstrual cycle phase are associated with altered resting state functional connectivity. Neuroimage. 2014;90:24–32. doi: 10.1016/j.neuroimage.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pletzer B, Kronbichler M, Aichhorn M, Bergmann J, Ladurner G, Kerschbaum HH. Menstrual cycle and hormonal contraceptive use modulate human brain structure. Brain Res. 2010;1348:55–62. doi: 10.1016/j.brainres.2010.06.019. [DOI] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaedflieg CWEM, van de Ven V, Meyer T, Siep N, Merckelbach H, Smeets T. Temporal dynamics of stress-induced alternations of intrinsic amygdala connectivity and neuroendocrine levels. PLoS One. 2015;10:e0124141. doi: 10.1371/journal.pone.0124141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson JL, Laird AR, Glahn DC, Lovallo WR, Fox PT. Meta-analytic connectivity modeling: Delineating the functional of the human amygdala. Hum. Brain Mapp. 2011;31:173–184. doi: 10.1002/hbm.20854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson S, Moser E, Peper M. fMRI of Emotion. FMRI Techniques and Protocols, Neuromethods. Neuromethods. 2009:411–456. [Google Scholar]
- Robinson S, Windischberger C, Rauscher A, Moser E. Optimized 3 T EPI of the amygdalae. Neuroimage. 2004;22:203–210. doi: 10.1016/j.neuroimage.2003.12.048. [DOI] [PubMed] [Google Scholar]
- Robinson SD, Pripfl J, H B, Moser E. The impact of EPI voxel size on SNR and BOLD sensitivity in the anterior medio-temporal lobe: a comparative group study of deactivation of the Default Mode. Magn. Reson. Mater. physics, Biol. Med. 2008;21:279–290. doi: 10.1007/s10334-008-0128-0. [DOI] [PubMed] [Google Scholar]
- Roy AK, Shehzad Z, Margulies DS, Kelly AMC, Uddin LQ, Gotimer K, Biswal BB, Castellanos FX, Milham MP. Functional connectivity of the human amygdala using resting state fMRI. Neuroimage. 2010;45:614–626. doi: 10.1016/j.neuroimage.2008.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruigrok AN, Salimi-Khorshidi G, Lai MC, Baron-Cohen S, Lombardo MV, Tait RJ, Suckling J. A meta-analysis of sex differences in human brain structure. Neurosci. Biobehav. Rev. 2014;39:34–50. doi: 10.1016/j.neubiorev.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad ZS, Gotts SJ, Murphy K, Chen G, Jo HJ, Martin A, Cox RW. Trouble at rest: How correlation patterns and group differences become distorted after global signal regression. Brain Connect. 2012;2:25–32. doi: 10.1089/brain.2012.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite TD, Elliott M, Gerraty R, Ruparel K, Loughead J, Calkin ME, Eickhoff SB, Hakonarson H, Gur RC, Gur RE, Wolf DH. An improved framework for confound regression and filtering for control of motion artifact in the preprocessing of resting-state functional connectivity data. Neuroimage. 2013;64:240–256. doi: 10.1016/j.neuroimage.2012.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilbach L, Bzdok D, Timmermans B, Fox PT, Laird AR, Vogeley K, Eickhoff SB. Introspective minds: using ALE meta-analyses to study commonalities in the neural correlates of emotional processing, social & unconstrained cognition. PLoS One. 2012;7:e30920. doi: 10.1371/journal.pone.0030920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schönberg T, Daw ND, Joel D, O’Doherty JP. Reinforcement learning signals in the human striatum distinguish learners from nonlearners during reward-based decision making. J. Neurosci. 2007;27:12860–12867. doi: 10.1523/JNEUROSCI.2496-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein JL, Wiedholz LM, Bassett DS, Weinberger DR, Zink CF, Mattay VS, Meyer-Lindenberg A. A validated network of effective amygdala connectivity. Neuroimage. 2007;36:736–745. doi: 10.1016/j.neuroimage.2007.03.022. [DOI] [PubMed] [Google Scholar]
- Stevens JS, Hamann S. Sex differences in brain activation to emotional stimuli: a meta-analysis of neuroimaging studies. Neuropsychologia. 2012;50:1578–1593. doi: 10.1016/j.neuropsychologia.2012.03.011. [DOI] [PubMed] [Google Scholar]
- Strathearn L, Li J, Fonagy P, Montague PR. What’s in a smile? Maternal brain responses to infant facial cues. Pediatrics. 2008;122:40–51. doi: 10.1542/peds.2007-1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamres L, Janicki D, Helgeson V. Sex differences in coping behavior: A meta-analytic review and an examination of relative coping. Personal. Soc. Psychol. Rev. 2002;6:2–30. [Google Scholar]
- Taylor SE, Klein LC, Lewis BP, Gruenewald TL, Gurung RAR, Updegraff JA. Biobehavioral responses to stress in females: tend-and-befriend, not fight-or-flight. Psychol. Rev. 2000;107:411–429. doi: 10.1037/0033-295x.107.3.411. [DOI] [PubMed] [Google Scholar]
- Tottenham N, Hare TA, Quinn BT, McCarry TW, Nurse M, Gilhooly T, Milner A, Galvan A, Davidson MC, Eigsti I-M, Thomas KM, Freed P, Booma ES, Gunnar M, Altemus M, Aronson J, Casey BJ. Prolonged institutional rearing is associated with atypically larger amygdala volume and difficulties in emotion regulation. Dev Sci. 2010;13:1–26. doi: 10.1111/j.1467-7687.2009.00852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapnell PD, Campbell JD. Private self-consciousness and the five-factor model of personality: distinguishing rumination from reflection. J. Pers. Soc. Psychol. 1999;76:284–304. doi: 10.1037//0022-3514.76.2.284. [DOI] [PubMed] [Google Scholar]
- Turner J, Laird A. The cognitive paradigm ontology: design and application. Neuroinformatics. 2012;10:57–66. doi: 10.1007/s12021-011-9126-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tusche A, Smallwood J, Bernhardt BC, Singer T. Classifying the wandering mind: Revealing the affective content of thoughts during task-free rest periods. Neuroimage. 2014;97:107–116. doi: 10.1016/j.neuroimage.2014.03.076. [DOI] [PubMed] [Google Scholar]
- Ulrich-Lai Y, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat. Rev. Neurosci. 2009;10:397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urry HL, van Reekum CM, Johnstone T, Kalin NH, Thurow ME, Schaefer HS, Jackson Ca, Frye CJ, Greischar LL, Alexander AL, Davidson RJ. Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. J. Neurosci. 2006;26:4415–4425. doi: 10.1523/JNEUROSCI.3215-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaisvaser S, Lin T, Admon R, Podlipsky I, Greenman Y, Stern N, Fruchter E, Wald I, Pine DS, Tarrasch R, Bar-Haim Y, Hendler T. Neural traces of stress: cortisol related sustained enhancement of amygdala-hippocampal functional connectivity. Front. Hum. Neurosci. 2013;7:313. doi: 10.3389/fnhum.2013.00313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veer IM, Oei NYL, Spinhoven P, van Buchem Ma, Elzinga BM, Rombouts Sa R.B. Beyond acute social stress: increased functional connectivity between amygdala and cortical midline structures. Neuroimage. 2011;57:1534–41. doi: 10.1016/j.neuroimage.2011.05.074. [DOI] [PubMed] [Google Scholar]
- Veer IM, Oei NYL, Spinhoven P, Van Buchem MA, Elzinga BM, Rombouts SARB. Endogenous cortisol is associated with functional connectivity between the amygdala and medial prefrontal cortex. Psychoneuroendocrinology. 2012;37:1039–1047. doi: 10.1016/j.psyneuen.2011.12.001. [DOI] [PubMed] [Google Scholar]
- Vogel S, Klumpers F, Krugers HJ, Fang Z, Oplaat KT, Oitzl MS, Joëls M, Fernández G. Blocking the mineralocorticoid receptor in humans prevents the stress-induced enhancement of centromedial amygdala connectivity with the dorsal striatum. Neuropsychopharmacology. 2015;40:947–956. doi: 10.1038/npp.2014.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. J. Pers. Soc. Psychol. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Weissenbacher A, Kasess C, Gerstl F, Lanzenberger R, Moser E, Windischberger C. Correlations and anticorrelations in resting-state functional connectivity MRI: a quantitative comparison of preprocessing strategies. Neuroimage. 2009;47:1408–1416. doi: 10.1016/j.neuroimage.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Winston JS, Doherty JO, Kilner JM, Perrett DI, Dolan RJ. Brain systems for assessing facial attractiveness. Neuropsychologia. 2007;45:195–206. doi: 10.1016/j.neuropsychologia.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Wolf OT, Schommer NC, Hellhammer DH, McEwen BS, Kirschbaum C. The relationship between stress induced cortisol levels and memory differs between men and women. Psychoneuroendocrinology. 2001;26:711–720. doi: 10.1016/s0306-4530(01)00025-7. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Gender in mental health research. Geneva: World Heal. Organ; 2004. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.