Abstract
This paper reports the application of endoscopic light scattering spectroscopy (LSS) with light gating to detect malignancies in the biliary and pancreatic ducts, and also reviews the application of endoscopic LSS for differentiating cystic neoplasms in the pancreas and detecting invisible dysplasia in Barrett’s esophagus. Information about tissue structure within the superficial epithelium where malignancy starts is present within the spectra of reflected light. Fortunately, this component of the reflected light is not yet randomized. However multiple scattering randomizes the signal from the underlying connective tissue which obscures the desired signal. In order to extract diagnostic information from the reflected signal the multiple scattering component related to connective tissue scattering and absorption must be removed. This is accomplished using described here spatial or polarization gating implemented with endoscopically compatible fiber optic probes.
Keywords: Optical spectroscopy, light scattering, light gating, cancer, cellular scale
Introduction
Spectra of light reflected from tissue in the gastrointestinal tract depend strongly on the elastic scattering from the connective tissue fibers and extracellular matrix and on hemoglobin absorption in the capillary network. However, to reach connective tissue, light has to penetrate the superficial cellular layer called epithelium, which lines both outer and inner surfaces of organs throughout the body. Intracellular structures with sizes smaller than optical wavelengths are responsible for the larger part of the epithelial scattering signal, with the component from epithelial cell nuclei also present. Relative contribution of the nuclear component is increasing in the backscattering direction. As enlarged, crowded, and dense epithelial cell nuclei are the primary histological features of pre-cancer and cancer, a technique which relates the spectroscopic characteristics of light reflected from tissue to epithelial cell nuclear morphology can be used for pre-cancer diagnosis. Such a technique is called biomedical light scattering spectroscopy, or LSS [1], [2].
As light propagates through tissue, multiple scattering events randomize information about the tissue structure. However, within the superficial epithelium, where malignancy starts, the light is not yet randomized. In order to extract the diagnostic information from the reflected signal the multiple scattering component related to the connective tissue scattering and absorption needs to be removed. This can be accomplished by several light gating approaches [3], with polarization gating and spatial gating well suited for endoscopy applications [4]–[7].
Polarization gating employs the fact that light scattered back from the epithelial cells located at the surface retains the incoming polarization, whereas multiple scattering from the deeper tissue regions is depolarized (see Fig. 1(a)). Subtracting parallel and perpendicular signals cancels the deeper tissue effect. The result is then due only to the contribution of the epithelial cells [4]–[6].
Fig. 1.

Light gating approaches. (a) Polarization gating. Epithelial tissue is illuminated with polarized light emitted from the polarized scanning fiber optic probe. The backscattered light from the cells in the superficial layer of epithelium is polarized parallel to the incoming light, while the light reflected from the deeper tissues becomes depolarized, containing equal amounts of parallel and perpendicular polarizations. Subtracting the two polarizations cancels out the contribution of deeper tissues and the resulting signal is proportional to the signal from the epithelium, which contains the information about early precancerous changes. (b) Spatial gating. Epithelial tissue is illuminated with the left fiber while two other fibers collect backscattered light at very small source-detector separations. Subtraction of signals collected by those fibers with the appropriate weighting coefficients cancels contribution from deeper tissue regions, with the result related to the contribution of the epithelial cells.
Spatial gating utilizes the fact that contribution of light scattered from the superficial layers increases with the decrease in source-detector significantly faster than that scattered from the deeper tissue regions. Therefore, by collecting light signals at two different small source-detector separations and subtracting these signals with appropriate weighting coefficients, contribution from deeper tissue regions can be canceled. The result is once again related to the contribution of the epithelial cells [7].
In this paper, we describe the use of LSS with both techniques of light gating for early cancer detection in several organs of the gastrointestinal tract. Each of these applications requires the use of endoscopy and, therefore, an endoscopic LSS clinical instrument and several fiber optic gating probes have been developed. The paper reviews the application of endoscopic LSS for diagnosing dysplasia in Barrett’s esophagus, differentiation of cystic neoplasms in the pancreas, and reports new results of detecting dysplasia and malignancy in the biliary and pancreatic ducts.
II. Light Gating LSS Fiber Optics Probes
The endoscopic LSS clinical instrument compatible with polarization and spatial light gating is shown in Fig. 2. A multi-channel spectrometer, broadband bright LED light source, and power supply are installed in the bottom part of the case (see Fig. 2(b)). A computer for system control is also located in the bottom part of the case and is used for data analysis and visualization of the diagnostic information. The middle shelf houses fiber connector for light delivery connected to the light source and two fiber connectors for light detection connected to two spectrometer channels. This shelf also houses the probe control box (Fig. 2(c)) employed when the instrument is used in combination with the polarization gating scanning fiber probe. In that case the spectrometer channels are connected to the parallel and perpendicular polarization fibers of the polarization gating fiber probe. This easily accessible middle shelf is also used for the safe storage of the probes. A hinged door installed on the side wall of the case allows convenient extension of the probe from the case for its insertion in the working channel of the commercial endoscope, without the need to disconnect and reconnect the probe. The upper shelf of the instrument has a built-in keyboard, mouse and a slot for probe calibration. The slot provides probe access to a light-tight enclosure housing an attached Spectralon reflectance standard. The case’s cover houses a flat touch screen.
Fig. 2.

Endoscopic LSS clinical instrument compatible with polarization and spatial light gating. (a) The endoscopic LSS clinical instrument on a cart.
(b) The internal structure of the instrument with the upper shelf lifted.
(c) Details of the control box.
The instrument employs either polarization or spatial gating probes. Polarization gating is well suited for rapid non-contact scanning of large epithelial surfaces, such as esophageal or colonic wall, and requires the probe to be inserted into the working channel of an endoscope, ranging from 2.0 to 4.2 mm [8]. Therefore, these types of probes can afford more complex optics which includes polarizers, mirrors, and lenses. At the same time, certain applications in the gastrointestinal tract require fitting the probe into very tight sub-millimeter spaces, such as a 1.2 mm cholangiopancreatoscopy system working channel or even a 0.5 mm internal diameter aspiration needle. Here spatial gating, which can be implemented in a much more compact package, is the way to go.
The polarization gating scanning fiber probe is presented in Fig. 3(a). Here a 400 µm core delivery fiber (NA = 0.22) is attached to a 200 µm thick, 1.5 mm diameter cylindrical linear polarizer/analyzer with two orthogonal polarization components. The delivery fiber is adjacent to two 200 µm core collection fibers (NA = 0.22), each behind a linear polarizer which is shaped to ensure that one fiber collects light with the same polarization as the delivery fiber and the other collects perpendicularly polarized light. It is necessary to mount the polarizers at the distal tip since multimode fibers do not maintain polarization. At the probe distal tip a parabolic mirror collimates the illumination beam and collects light from the 2 mm illuminated spot. This compensates for peristaltic and other motions of the esophagus or other GI organ. Collimation ensures that the spectrum is not affected by distance or orientation. In the esophagus for instance, gentle air insufflation of the gastrointestinal lumen helps maintain a relatively stable tubular shape and prevents the optical probe from touching the esophageal walls. However, even if the probe comes in contact with the wall, the overlap of the illumination and collection fields at the probe window is 75% which is adequate for evaluating the backscattering component. With the increase of the distance the overlap is also increasing and becomes optimal at 11 mm distance from the axis of the probe, although the degree of overlap remains adequate up to approximately 20 mm from the probe axis. To avoid specular reflection, light is directed approximately 70° to the axis of the probe. The probe is protected by the parylene coated stainless steel torque tube connected to two stepper motors of the control box, providing rotary and axial scanning. To increase scanning accuracy the data collection is performed only during the counterclockwise rotational scan. After the data collection is complete, the probe rotates 1.25 revolutions clockwise and then revolution counterclockwise to minimize hysteresis of the torque tube and ensure the same angular position for the start of the next rotational scan.
Fig. 3.

Light gating LSS fiber optics probes. (a) The exploded view of the polarization gating scanning fiber probe distal tip. When assembled the parabolic mirror is opposite the quartz window. (b) Distal tip of the spatial gating fiber probe consisting of seven fibers with the delivery fiber in the outer ring marked with yellow color. (c) Polarization gating scanning fiber probe inserted into the working channel of an endoscope. (d) The spatial gating fiber probe inserted in the endoscopic ultrasound aspiration needle. Three SMA connectors at the proximal end for coupling groups of fibers with 120 µm, 220 µm and 240 µm distal end source-detector separations with three individual spectrometers and another SMA connector for coupling delivery fiber with the broadband light source. (e) Spatial gating fiber probe inserted into cholangiopancreatoscopy system.
The 450 µm outer diameter spatial gating fiber probe is presented in Fig. 3(b) and consists of seven 100 µm core diameter fibers (NA = 0.21). A fiber in the outer ring of the probe is selected as the delivery fiber and is connected to a dedicated SMA connector, while three groups of collection fibers are selected to provide source-detector separations of 120, 220 and 240 µm and are terminated in three SMA connectors coupled to individual spectrometers. All of the fiber trunks are connected to a metal ferrule. The probe jacket is made of a robust medical grade biocompatible polyimide.
We used multispectral endoscopy with light gating to detect dysplasia in Barrett’s esophagus, pancreatic cystic neoplasms, and malignancy of biliary and pancreatic ducts (see Fig. 4). In the case of Barrett’s esophagus, a polarization gating scanning fiber probe is inserted directly into the working channel of a standard gastroscope. In order to evaluate a pancreatic cyst, or the biliary and pancreatic ducts, the spatial gating fiber probes are inserted in the endoscopic ultrasound aspiration needle, or a cholangiopancreatoscopy system respectively, which in turn are inserted into the working channel of either an echoendoscope or standard gastroendoscope. The details of each of those studies are found below.
Fig. 4.

Multispectral endoscopy with light gating in the esophagus, pancreas, and biliary and pancreatic ductal systems. Upper inset shows a clinical procedure in the esophagus. The fiber probe performs rapid automated rotational/longitudinal scanning of the entire Barrett’s esophagus segment. Middle inset shows a clinical procedure in a pancreatic cyst. The echoendoscope is advanced to the stomach or duodenum and the cyst is punctured under ultra-sound guidance with an FNA needle. The probe tip is extended from the needle, illuminating a location of the internal cyst surface. Bottom inset shows a clinical procedure in the biliary and pancreatic ductal systems.
III. Dysplasia in Barrett’s Esophagus
Esophageal cancer is increasing in frequency in the United States faster than any other cancer. Barrett’s esophagus, a precancerous complication of esophageal reflux, affects approximately three million Americans and precedes almost all cases of esophageal cancer. In cases when Barrett’s esophagus progresses to higher risk ‘high-grade dysplasia’, it can be definitively treated to prevent esophageal cancer. However, dysplasia may not be detected by standard-of-care screening endoscopy which uses visual endoscopy and a prescribed pattern of biopsy. This procedure, in which a tiny fraction of the affected tissue is selected for pathological examination, has a low probability of dysplasia detection because dysplasia is highly focal and is often not distinguishable by endoscopic appearance alone.
Several new imaging approaches have been explored clinically to visualize dysplasia: high resolution endoscopy (HRE) combined with narrow band imaging (NBI) [9], autofluorescence imaging (AFI) [10], trimodal imaging [11], which is a combination of the previous three, confocal laser endomicroscopy (CLE) [12], and optical coherence tomography (OCT) [13]-[15]. These techniques showed promise in increased detection of dysplasia in Barrett’s esophagus, and are used at specialized referral centers, but are not yet part of routine clinical practice.
A clinically viable dysplasia detecting technique in Barrett’s esophagus has to overcome the challenge of combining conflicting goals of (1) macroscopic large area imaging and (2) microscopic subcellular sensitivity. Our approach achieves both by combining (1) rapid endoscopically compatible scanning of the entire esophageal surface with (2) LSS that uses polarization gating to remove the contribution of the deeper tissues.
To date we have employed the endoscopic LSS clinical instrument in 76 Barrett’s esophagus subjects at the Center for Advanced Endoscopy These evaluations were performed during routine endoscopic procedures for patients with suspected dysplasia and individuals undergoing radiofrequency ablation (RFA) or cryoablation treatment of Barrett’s esophagus for histologically confirmed high grade dysplasia. The 57 subjects from the first group reporting to the Center for Advanced Endoscopy underwent initial screening at other institutions and were referred with confirmed Barrett’s esophagus and suspicion of dysplasia. In the treatment group, confirmatory biopsies had typically already been obtained, and patients were beginning endoscopic treatment. Therefore, in the first group endoscopic biopsies were collected according to the standard-of-care protocol [16], while in the second group of 19 subjects, in which biopsy diagnoses had been made previously, and the endoscopic procedure was focused on treatment [17], repeat biopsies were not obtained the LSS data was retained for the future longitudinal study of treatment efficacy.
During endoscopy spectroscopy of the entire Barrett’s segment is performed by scanning adjacent sections, 2 cm in length, with the polarized scanning probe. The probe is extended 2 cm beyond the endoscope tip, placing it at the distal boundary of a Barrett’s esophagus region chosen for examination. One complete rotary scan, collecting 30 data points on the esophageal wall, is completed followed by a linear 2 mm backward withdrawal into the endoscope. This is repeated for 10 rotary scans, so that an entire 2 cm length of Barrett’s esophagus is scanned; then, the endoscope tip is withdrawn 2 cm and the next length of BE is examined. Three hundred data points in 30 sec for each 2 cm segment of Barrett’s esophagus are collected.
We validated the capabilities of the method by comparing The LSS data with subsequent pathology at each site where biopsies were taken. We recorded the locations of biopsied tissue sites by their distances from the upper incisors and their angles relative to the start of the LSS scan. The results are presented as pseudocolor maps (see Fig. 5(a)).
Fig. 5.

In vivo optical spectroscopic detection of pre-cancer in esophagus. (a) (a) Typical pseudo-color maps highlighting areas suspicious for dysplasia in six Barrett’s esophagus subjects, with maps produced from in vivo LSS data overlaid with circles indicating biopsy sites and confirmed pathology. Red and pink regions of the map indicate areas suspicious for dysplasia, with diagnostic parameter below 0.05 colored dark green, 0.05–0.10 colored light green, 0.10–0.15 colored pink, and above 0.15 as red. The green circles indicate the locations of biopsies histologically diagnosed as non-dysplastic and red circles – as high-grade dysplasia. (b) Receiver operating characteristic (ROC) analyses. ROC curve shows the performance of the LSS endoscopic imaging in identifying dysplastic sites in subjects with BE by comparing the LSS results with the definitive histopathology findings for all values of D in our data set. The area under the ROC curve (AUC) is 0.95 (95% confidence interval: 0.926–0.974, p < 0.001). Diagnostic cut-offs 0.10 along with 0.09 and 0.11 are shown for illustration purposes.
The performance of LSS with polarization light gating was evaluated in two separate double-blind studies, emphasizing (1) biopsy-based diagnosis and (2) patient-based diagnosis. The former approach, which characterizes the LSS potential for performing guided biopsy, is based on double-blind comparison of the LSS maps with the biopsy reports and identifying true positive, false-positive, true-negative and false-negative sites for every biopsy location. The latter approach, which characterizes the LSS ability to serve as a screening tool, emphasizes identifying presence of dysplasia in a particular Barrett’s esophagus patient rather than localizing the dysplastic sites. The rationale for that approach is that radiofrequency ablation and cryoablation therapies are applied to the entire Barrett’s esophagus segment, rather than specific sites where biopsies diagnosed as high-grade dysplasia were collected [17].
The patient based diagnosis study is characterized by the sensitivity of 96% with a 95% confidence interval (CI) of 82–99% and the specificity of 97% (95% CI: 83–99%), exceeding the respective characteristics of 90% and 80% required by the American Society for Gastrointestinal Endoscopy (ASGE) for a new biopsy guidance technique to replace or supplement the current standard of care [18].
A smaller biopsy based diagnosis study is characterized by a sensitivity of 88% (95% CI: 79–93%) and a specificity of 91% (95% CI: 87–93%) in detecting individual locations of high-grade dysplasia, with the area under the ROC curve (see Fig. 5(b)) being 0.95 (95% CI: 93–97%). This means when that dysplasia is present in Barrett’s esophagus, the LSS guided biopsy procedure has a 99.5% probability to locate it with just 4 guided biopsies, while the standard-of-care Seattle protocol has approximately 43% chance of detection with 20 biopsies [19].
Pancreatic Cystic Neoplasms
Pancreatic cancer is the third leading cause of cancer-related death in the United States, surpassing breast cancer. With a median survival of three months it has the highest mortality rate of all major cancers [20]. The poor prognosis of pancreatic cancer is due in large part to the inability to detect this cancer at an early stage, when the option of a curative surgical resection is still possible [21]. About one fifth of pancreatic cancers arise from cystic lesions that can be discovered in early, treatable stages using non-invasive imaging techniques such as CT and MRI. However, not all pancreatic cystic lesions are precancerous, and differentiating between precancerous and benign cyst types is both crucially important and very difficult. It is estimated that 8 million Americans have pancreatic cystic lesions [22], many being found incidentally during routine cross-sectional imaging in older adults. While CT and MRI could be used to screen for cystic lesions, they have poor accuracy with regard to distinguishing cancerous and pre-cancerous cysts from benign cysts. It should therefore come as no surprise that cystic lesions account for a disproportionate one third of all pancreatic surgeries [23]. 287 The decision for surgical resection is usually based on cross-sectional imaging results, in combination with the minimally invasive endoscopic ultrasound-guided fine needle aspiration (EUS-FNA). Unfortunately, cyst fluid often contains few cells, and fluid chemical analysis lacks accuracy. Misdiagnosis can lead to dire consequences, including the development of cancer in cysts thought to be benign, or unnecessary pancreatic surgery for benign cysts, often with significant morbidity and mortality. Thus, there is a critical need for a new diagnostic approach that accurately identifies those pancreatic cysts that require surgical intervention and those that do not.
We performed clinical measurements using endoscopic LSS instrument with spatial light gating on freshly resected pancreatoduodenectomy and distal pancreatectomy samples from 11 subjects, followed by in vivo measurements during routine EUSFNA procedures in 14 consecutively enrolled subjects with pancreatic cysts. A total of 27 cysts in 25 subjects were evaluated. To ensure proper co-registration with the subsequent histopathology examination in the measurements on freshly resected samples, the measured sites were marked with India ink, and photographed. To differentiate cystic neoplasms, we employed the same diagnostic parameter used in Barrett’s esophagus studies described in Section III, with cystic lesions with diagnostic parameter below 0.1 classified as benign, in the range from 0.1 to 0.2 as low-grade dysplasia, and above 0.2 as high-grade dysplasia and adenocarcinoma.
In vivo measurements using spatial gating LSS during routine EUS-FNA procedure were performed in 14 consecutively enrolled subjects with pancreatic cysts. Before the procedure, the spatial gating probe was inserted in the 22-gauge endoscopic ultrasound aspiration needle and secured with a fixed-length tube and probe latching mechanism to ensure that its distal end was completely inside the FNA needle. The subject was administered sedation and supplemental oxygen was used. The echoendoscope was introduced through the mouth and advanced to the stomach or duodenum. After pancreatic EUS examination, the FNA needle was inserted into the echoendoscope, and the cyst was punctured under ultrasound guidance. The spatial gating probe was then extended 2 mm beyond the tip of the needle with the probe latching mechanism and locked in that position with the locking button and multiple locations covering a portion of the forward hemisphere of the internal cyst surface were measured under EUS guidance. The total LSS measurement time was less than 2 minutes. The spatial gating probe was then removed, a 10 ml syringe was attached to the proximal end of the needle and the aspirated fluid was collected in the standard fashion. After the procedure, the aspirated fluid was sent for cytological and biochemical analysis.
The results of double-blind prospective study in 25 patients, with 14 cysts measured in vivo and 13 postoperatively are presented in Fig. 6. The technique achieved an overall accuracy of 95%, with a 95% confidence interval of 78–99%, in cysts with definitive diagnosis.
Fig. 6.

Optical spectroscopic differentiation of pancreatic cystic neoplasms in 25 subjects. Cysts 1 and 2 are from the first subject, and cysts 10 and 11 are from the ninth subject. Colors represent diagnoses by histopathology, when available, or survival with follow-ups, showing no indication of cancer development, when histopathology is not available. Red color represents malignancy (adenocarcinoma, high-grade dysplasia, and one cystic neuroendocrine tumor), blue representing low-grade dysplasia and green representing benign cysts.
IV. Malignancy of Biliary and Pancreatic Ducts
Bile duct cancer or cholangiocarcinoma is a relatively rare and lethal form of adenocarcinoma with most people having advanced stage, inoperable disease at presentation. Detecting cholangiocarcinoma at an early stage, when the option of a curative surgical resection is still possible, is a key priority in the field of gastroenterology.
We performed clinical measurements during endoscopic retrograde cholangiopancreatography (ERCP) procedures on patients with suspected dysplasia and malignancy of biliary and pancreatic ducts. When the duodenal papilla is located during endoscopy, a sphincterotome is inserted through the endoscope operating channel and guided through the papillary opening. A wire is then passed into the bile duct or pancreatic duct, and, when a concerning-appearing stricture was identified, standard brush cytology was performed to evaluate for malignancy. A smaller scope, called a cholangioscope, was then advanced over the wire and into the duct of interest. Then the spatial gating fiber-optic probe was inserted into the biliary or pancreatic duct through the cholangioscope working channel. When the probe is placed in contact with the tissue, a set of three spectra are collected and stored for future processing. Biopsies were taken at the same locations, with cholangiopancreatoscopy system video (Fig. 7(a)) and X-ray cholangiogram video (Fig. 7(b)) used to ensure co-registration of the spectroscopy and biopsy locations.
Fig. 7.
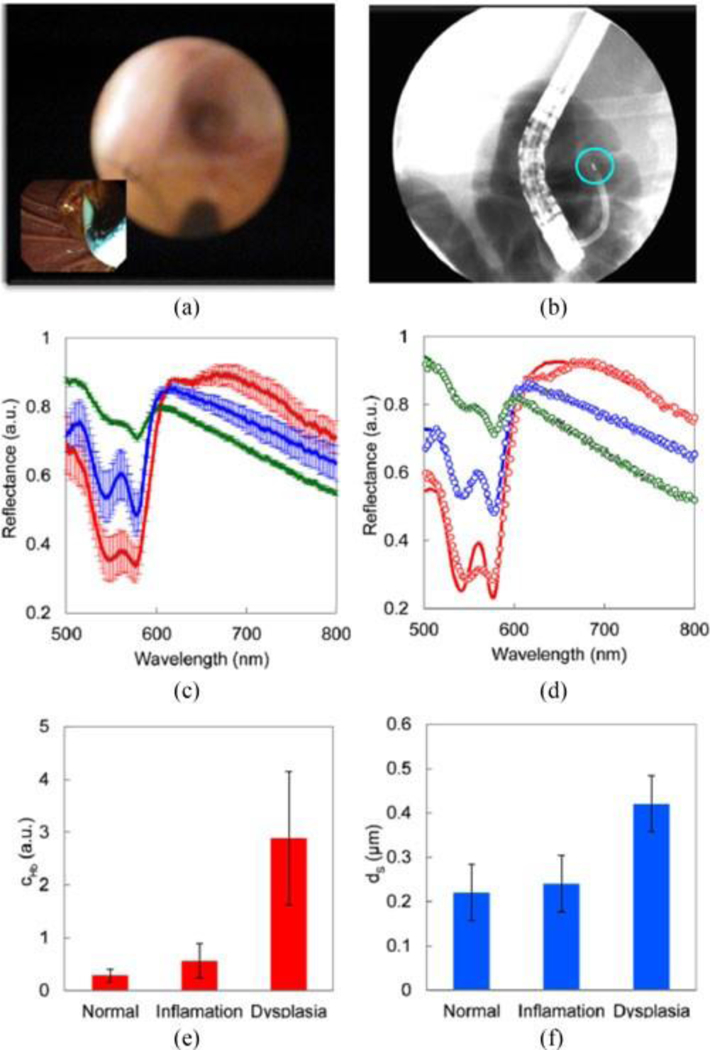
In vivo optical spectroscopic detection of early precancerous changes in pancreatico-biliary system in 6 subjects in vivo. (a) Cholangiopancreatoscopy system view during clinical data collection in the pancreatico-biliary system. The inset shows view via duodenoscope. (b) Cholangiogram of the probe during clinical data collection clearly shows the X-ray image of the probe’s distal tip marked with radiopaque marker (highlighted with the blue circle). (c) Mean reflectance spectra and standard deviations for normal (green), inflammatory (blue), and dysplastic (red) sites for 120 µm source-detector separation of the fiber probe. (d) Typical fits for each of the three pathology diagnosis, model - solid lines, experimental data – dots. (e) Mean hemoglobin concentration and standard deviation for each of the three pathology diagnosis. (f) Same for the mean effective scatterer size.
We collected spectra from 24 sites in pancreaticobiliary system in 6 patients. Fig. 7(c) shows mean reflectance spectra and standard deviations for normal, inflammatory, and dysplastic sites, as reported by pathology, for 120 µm source-detector separation of the fiber probe. The spectra show clear differentiation between dysplasia (red), inflammation (blue) and normal (green) sites. By taking into account all three source-detector separations for each of the sites we modeled the spectra and extracted four morphological and biochemical tissue parameters: effective scatterer size and effective scatterer density, hemoglobin concentration, and hemoglobin oxygen saturation, the model fits were excellent, as can be seen from three typical fits, presented in Fig. 7(d) for each of the three pathology diagnosis. From four parameters employed by the model, two – hemoglobin concentration (Fig. 7(e)) and effective scatterer size (Fig. 7(f)) – appear to provide the best differentiation between sites. This makes sense taking into account the onset of the angiogenesis and changes in the cellular morphology, typical of dysplastic transformation in epithelial tissues.
CONCLUSION
We conclude that multispectral endoscopy with light gating offers great promise for detecting precancerous and early cancerous conditions in various organs of the gastrointestinal tract. For organs with large epithelial surfaces, such as the esophagus, polarization gating provides rapid non-contact interrogation of the entire epithelial area. For organs located deep in the abdomen which are difficult to access, spatial gating, which can be implemented in a much more compact package, is the preferred approach. Multispectral endoscopy with light gating is rapid, inexpensive, and accurate. We have shown that this technique can help identify early precancerous lesions and early cancers of the GI tract accurately. This approach may enable earlier and more effective treatments for patients with these conditions, and may also reduce the risk of unnecessary surgery for benign lesions.
Acknowledgment
We would like to thank Ram Chuttani, Eric Yee, Yunping Li, Shweta Shinagare, and Fen Wang for help in data acquisition.
This work was supported in part by the U.S. National Insti-tutes of Health under Grants R01 EB003472, R01 EB025173, R01 CA205431, and R01 CA218382 and in part by U.S. National Science Foundation under Grants EFRI-1240410, CBET-1402926 and CBET-1605116.
Biography

Le Qiu received the B.S degree in physics from Beijing University, Beijing, China, in 2003, the Ph.D. degree in medical physics from Boston University, Boston, MA, USA, in 2009 and the M.S. degree in medical sciences from Harvard University, Boston, MA, USA, in 2013. He is an Assistant Professor of Medicine with Harvard University and a Group Leader with the Center for Advanced Biomedical Imaging and Photonics, Beth Israel Deaconess Medical Center, Boston, MA, USA. He published in Nature family journals as first, co-first, and co-senior author, and also published four book chapters as the first author. His interests include application of light scattering spectroscopy and imaging techniques in the fields of biology and medicine.
Dr. Qiu was the recipient of the Harvard Catalyst Advanced Imaging Concept Development Award.

Lei Zhang Joined the Center for Advanced Biomedical Imaging and Photonics in 2013 as a Postdoctoral Fellow. He is currently working on the interface of genetics, biomedical optics and bioengineering developing optically controlled epigenetic and optogenetic tools and light scattering spectroscopic methods for early cancer detection. He is an Instructor in medicine with Harvard University, Boston, MA, USA.
He has published multiple papers and chapters, including papers in Nature Biomedical Engineering as a first author, Light: Science and Applications, Endocrinology, and others. Originally trained as a geneticist.

Vladimir Turzhitsky received the B.S. degree in biomedical engineering from Rensselaer Polytechnic Institute, Troy, NY, USA, in 2004, the M.S. and Ph. D. degrees in biomedical engineering from Northwestern University, Evanston, IL, USA, in 2006 and 2009. He is currently an Instructor in Medicine with Harvard University, Boston, MA, USA and a Group Leader with the Center for Advanced Biomedical Imaging and Photonics, Beth Israel Deaconess Medical Center, Boston, MA, USA. He has authored more than 40 peer-reviewed journal publications including papers in Nature Communications and Nature Biomedical Engineering, 4 patents, and 3 book chapters. His research interests include the application of light scattering and imaging techniques in the fields of biology and medicine.

Umar Khan is Research Technician with the Center for Advanced Biomedical Imaging and Photonics.
He has co-authored papers in Nature Biomedical Engineering, Light: Science and Applications and chapter in the book Barrett’s Esophagus: Emerging Evidence for Improved Clinical Practice. He is an expert in computer graphics, software development, and 3D printing based microfabrication.

Yuri Zakharov received the M.S. degree in radio physics and electronics in 1981 and Ph.D. degree in physics in 1988 both from Lobachevsky State University, Nizhni Novgorod, Russia, where he was an Assistant Professor in Physics from 1998 to 2013. He is a Research Fellow in Medicine with Harvard University, Boston, MA, USA, and a Research Scientist with the Center for Advanced Biomedical Imaging and Photonics. He has authored more than 50 papers and chapters on holography, microscopy, and cancer detection. His research interests include digital holography, microscopy, and the application of light scattering and imaging techniques in the fields of biology and medicine.

Douglas K. Pleskow received the B.A degree in neuroscience from the University of Rochester, Rochester, NY, USA, in 1978 and M.D degree in 1982 from the State University of New York at Buffalo, Buffalo, NY, USA.
He is an Associate Professor of Medicine with Harvard University, Boston, MA, USA and a Chief of Clinical Gastroenterology with the Beth Israel Deaconess Medical Center, Boston, MA, USA. He is also the Director of the Center for Advanced Endoscopy and the Director of the Colorectal Cancer Center. He has authored multiple book chapters and papers, including publications in Nature Medicine, Nature Biomedical Engineering, Annals of Internal Medicine, and Gastroenterology. He has also edited a book Barrett’s Esophagus: Emerging Evidence for Improved Clinical Practice. He is an expert in complex gastrointestinal problems that require therapeutic endoscopic procedures, all aspects of biliary endoscopy, endoscopic ultrasound, and pancreatic diseases. His major research interests have been application of optics and spectroscopy to early detection of precancerous changes in Barrett’s esophagus, study of serologic markers in pancreatic disease, therapeutic pancreaticobiliary endoscopy and endoscopic ultrasound.
Dr. Pleskow is a Fellow of American Gastroenterological Association. In 2009, he was elected the Man of the Year by the National Pancreas Foundation.

Mandeep Sawhney received the MD degree from the University College of Medical Sciences, New delhi, India, in 1993 and MS degree in clinical research from the University of Minnesota, Minneapolis, MN, USA, in 2006. He is an Associate Professor of Medicine, Harvard University, BOston, MA, USA and co-Director of GI Endoscopy and the Director of Endoscopy Research, Beth Israel Deaconess Medical Center, Boston, MA, USA.
He has published more than 80 manuscripts in peer reviewed journals. His clinical areas of interests are endoscopy for pancreas and biliary diseases, achalasia, Barrett’s esophagus and gastrointestinal malignancy.
Dr. Sawhney is a Fellow of the American Society for Gastrointestinal Endoscopy. He has served on several national committees, including a combined American Society for Gastrointestinal Endoscopy and the American College of Gastroenterology task-force that set national quality indicators for gastrointestinal endoscopy.

Tyler M. Berzin received the ScB degree in neuroscience from Brown University, Providence, RI, USA, in 1999 and the MD degree form Brown Medical School, Providence, RI, USA, in 2003. He is an Assistant Professor of Medicine with Harvard University, Boston, MA, USA and the co-Director of GI Endoscopy and the Director of the Advanced Endoscopy Fellowship with Beth Israel Deaconess Medical Center, Boston, MA, USA.
He has authored more than 60 articles and chapters on endoscopic ultrasound, ERCP, and quality and safety in endoscopy.
Dr. Berzin is a Fellow of the American Society of Gastrointestinal Endoscopy and a member of the American Gastroenterologic Association. At Harvard, he has been the recipient of numerous clinical and classroom teaching Awards, including a Harvard Medical School Academy Certificate of Excellence in Teaching for 2010, and the Z. Myron Falchuk Award for Mentoring and Clinical Teaching in 2016.

Jeffrey D. Goldsmith received the B.S degree in biology from the University of Michigan, Arbor, MI, USA, in 1992 and the MD degree from Wayne State University School of Medicine, Detroit, MI, USA, in 1996.
He is an Associate Professor of Pathology with Harvard University, Boston, MA, USA and the Director of Gastrointestinal Pathology with Boston Children’s Hospital, Boston, MA, USA. He has authored more than 90 articles, reviews and chapters mostly pertaining to the pathology of gastrointestinal disease and soft tissue neoplasms. His research interests include medical diseases of the gastotintestinal tract that occur in the pediatric population such as idiopathic inflammatory bowel disease and celiac disease.
Dr. Goldsmith was the President of Rodger C. Haggitt Gastrointestinal Pathology Society in 2016–2017 and a Chair of the Immunohistochemistry Committee for the College of American Pathologists in 2009–2012. At Harvard, he has been the recipient of numerous teaching Awards, most recently the Betty and Ernie Singer Prize for Sustained Excellence in Teaching.

Kanchan Kantekure received the MD degree from Vaishampayan Memorial Government Medical College, Solapur, India, in 2004. She is an Instructor in Pathology with Harvard University, Boston, MA, USA and a Staff Pathologist with Beth Israel Deaconess Medical Center, Boston, MA, USA.
Her current research interests include pathology of gastrointestinal cancers and their precursor lesions.

Edward Vitkin is a Lecturer in Medicine with Harvard University, Boston, MA, USA and a Senior Scientist with the Center for Advanced Biomedical Imaging and Photonics.
He led the effort to find a straightforward analytical method for solving the diffusion approximation near the point of entry, a long-standing problem in photon transport in turbid media. He has authored multiple papers in Nature Medicine, Nature Biomedical Engineering, Nature Communications, PNAS, Cancer Cell, and other journals. His areas of interest include modeling of light transport in biological tissue and its applications to cell biology.

Irving Itzkan is Lecturer in Medicine at Harvard University and Senior Scientist at the Center for Advanced Biomedical Imaging and Photonics. From 1988 to 2000 he served as Senior Scientist at MIT. Prior to that he was the Chairman of Optics Research at the Avco-Everett Research Lab (AERL) which designed and developed the first intra-aortic balloon pump, a temporary cardiac assist device which has been used worldwide on three million people. He also served as an Engineering and Laser Research Section Head at Sperry Rand.He and L.T. Perelman explained ablation stress confinement, a basic mechanism of short pulse laser surgery widely used now in ophthalmology, neurosurgery, dentistry, oral and maxillofacial surgery, and general surgery. He also spearheaded the efforts to develop confocal light scattering microscopy for label-free functional imaging of live cells. His present research interests involve application of optics to medicine and cell biology.
Dr. Itzkan is a Founding Fellow of American Society for Laser Medicine and Surgery.

Lev T. Perelman is Professor of Medicine and Biological and Biomedical Sciences faculty at Harvard University and Director of the BIDMC Center for Advanced Biomedical Imaging and Photonics. Prior to that, he was Principal Scientist and Group Head at MIT. His research interests are pri-marily focused on application of optics to medicine and biology. His group pioneered biomedical light scattering spectroscopy for noninvasive detection of early precancerous changes in epithelial tissues and tissue characterization on a subcellular scale and developed label-free confocal light scattering microscopy, recently employed to demonstrate that subcellular exosomes promote tumorigenesis. He also collaborated with I. Itzkan to explain laser ablation stress confinement, a basic mechanism of short pulse laser surgery widely used now in ophthalmology, neurosurgery, dentistry, oral and maxillofacial surgery, and general surgery. His nonmedical related work includes demonstration with K. Kneipp of single molecule detection with SERS, and development with J. Marshall of the first nonhydrostatic model of the ocean, the MIT General Circulation Model.
Prof. Perelman was a member of the U.S. Department of Health and Human Services Joint Working Group charged with setting up funding priorities in oncologic imaging in the U.S. for the past decade. He was elected to serve as the General Chair of the 2012 Optical Society of America Biophotonics Congress. He was also the recipient of the National Science Foundation Emerging Frontiers in Research and Innovation Award given to global leaders in interdisciplinary research.
Contributor Information
Le Qiu, Email: lqiu@bidmc.harvard.edu, Center for Advanced Biomedical Imaging and Photonics, Division of Gastroenterology, Department of Medicine, Beth Israel Deaconess Medical Center, Harvard University, Boston, MA 02215 USA.
Lei Zhang, Email: lzhang11@bidmc.harvard.edu, Center for Advanced Biomedical Imaging and Photonics, Division of Gastroenterology, Department of Medicine, Beth Israel Deaconess Medical Center, Harvard University, Boston, MA 02215 USA.
Vladimir Turzhitsky, Email: vturzhit@bidmc.harvard.edu, Center for Advanced Biomedical Imaging and Photonics, Division of Gastroenterology, Department of Medicine, Beth Israel Deaconess Medical Center, Harvard University, Boston, MA 02215 USA.
Umar Khan, Email: ukhan1@bidmc.harvard.edu, Center for Advanced Biomedical Imaging and Photonics, Division of Gastroenterology, Department of Medicine, Beth Israel Deaconess Medical Center, Harvard University, Boston, MA 02215 USA.
Yuri Zakharov, Email: yzakharo@bidmc.harvard.edu, Center for Advanced Biomedical Imaging and Photonics, Division of Gastroenterology, Department of Medicine, Beth Israel Deaconess Medical Center, Harvard University, Boston, MA 02215 USA.
Kanchan Kantekure, Email: kkanteku@bidmc.harvard.edu, Center for Advanced Biomedical Imaging and Photonics, Division of Gastroenterology, Department of Medicine, Beth Israel Deaconess Medical Center, Harvard University, Boston, MA 02215 USA; Department of Pathology, Beth Israel Deaconess Medical Center, Harvard University, Boston, MA 02215 USA.
Edward Vitkin, Email: evitkin@bidmc.harvard.edu, Center for Advanced Biomedical Imaging and Photonics, Division of Gastroenterology, Department of Medicine, Beth Israel Deaconess Medical Center, Harvard University, Boston, MA 02215 USA.
Irving Itzkan, Email: iitzkan@bidmc.harvard.edu, Center for Advanced Biomedical Imaging and Photonics, Division of Gastroenterology, Department of Medicine, Beth Israel Deaconess Medical Center, Harvard University, Boston, MA 02215 USA.
Douglas K. Pleskow, Email: dpleskow@bidmc.harvard.edu, Center for Advanced Endoscopy, Division of Gastroenterology, Department of Medicine, Beth Israel Deaconess Medical Center, Harvard University, Boston, MA 02215 USA.
Mandeep Sawhney, Email: msawhney@bidmc.harvard.edu, Center for Advanced Endoscopy, Division of Gastroenterology, Department of Medicine, Beth Israel Deaconess Medical Center, Harvard University, Boston, MA 02215 USA.
Tyler M. Berzin, Email: tberzin@bidmc.harvard.edu, Center for Advanced Endoscopy, Division of Gastroenterology, Department of Medicine, Beth Israel Deaconess Medical Center, Harvard University, Boston, MA 02215 USA.
Jeffrey D. Goldsmith, Email: jgoldsmi@bidmc.harvard.edu, Department of Pathology, Boston Children’s Hospital, Harvard University, Boston, MA 02115 USA.
Lev T. Perelman, Biological and Biomedical Sciences Program, and also with Center for Advanced Biomedical Imaging and Photonics, Division of Gastroenterology, Department of Medicine, Beth Israel Deaconess Medical Center, Harvard University, Boston, MA 02115 USA
References
- [1].Perelman LT et al. , “Observation of periodic fine structure in reflectance from biological tissue: A new technique for measuring nuclear size distribution,” Phys. Rev. Lett, vol. 80, pp. 627–630, January 1998. [Google Scholar]
- [2].Backman V et al. , “Detection of preinvasive cancer cells,” Nature, vol. 406, pp. 35–36, July 2000. [DOI] [PubMed] [Google Scholar]
- [3].Perelman LT, “Optical diagnostic technology based on light scattering spectroscopy for cancer,” Expert Rev. Med. Devices, vol. 3, pp. 787–803, November 2006. [DOI] [PubMed] [Google Scholar]
- [4].Backman V et al. , “Polarized light scattering spectroscopy for quantitative measurement of epithelial cellular structures in situ,” IEEE J. Sel. Topics Quantam Electron, vol. 5, pp. 1019–1027, July 1999. [Google Scholar]
- [5].Qiu L et al. , “Multispectral scanning during endoscopy guides biopsy of dysplasia in Barrett’s esophagus,” Nature Med, vol. 16, pp. 603–606, May 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Qiu L et al. , “Multispectral light scattering endoscopic imaging of esophageal precancer” Light Sci. Appl, vol. 7, April 2018, Art. no. e17174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Zhang L et al. , “Light scattering spectroscopy identifies malignant potential of pancreatic cysts during endoscopy,” Nature Biomed. Eng, vol. 1, p. 0040, March 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Varadarajulu S et al. , “GI endoscopes,” Gastrointest. Endosc, vol. 74, pp. 1–6, July 2011. [DOI] [PubMed] [Google Scholar]
- [9].Kara MA, Ennahachi M, Fockens P, ten Kate FJ, and Bergman JJ, “Detection and classification of the mucosal and vascular patterns (mucosal morphology) in Barrett’s esophagus by using narrow band imaging,” Gastrointest. Endosc, vol. 64, pp. 155–166, August 2006. [DOI] [PubMed] [Google Scholar]
- [10].Kara MA et al. , “Endoscopic video autofluorescence imaging may improve the detection of early neoplasia in patients with Barrett’s esophagus,” Gastrointest. Endosc, vol. 61, pp. 679–685, May 2005. [DOI] [PubMed] [Google Scholar]
- [11].Curvers WL et al. , “Endoscopic tri-modal imaging for detection of early neoplasia in Barrett’s oesophagus: A multi-centre feasibility study using high-resolution endoscopy, autofluorescence imaging and narrow band imaging incorporated in one endoscopy system,” Gut, vol. 57, pp. 167–172, January 2008. [DOI] [PubMed] [Google Scholar]
- [12].Pohl H et al. , “Miniprobe confocal laser microscopy for the detection of invisible neoplasia in patients with Barrett’s oesophagus,” Gut, vol. 57, pp. 1648–1653, November 2008. [DOI] [PubMed] [Google Scholar]
- [13].Cobb MJ et al. , “Imaging of subsquamous Barrett’s epithelium with ultrahigh-resolution optical coherence tomography: A histologic correlation study,” Gastrointest. Endosc, vol. 71, pp. 223–230, January 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Liang K et al. , “Ultrahigh speed en face oct capsule for endoscopic imaging,” Biomed. Opt. Exp, vol. 6, pp. 1146–1163, March 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Yun SH et al. , “Comprehensive volumetric optical microscopy in vivo,” Nature Med vol. 12, pp. 1429–1433, November 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Levine DS et al. , “An endoscopic biopsy protocol can differentiate high-grade dysplasia from early adenocarcinoma in Barrett’s esophagus,” Gastroenterology, vol. 105, pp. 40–50, July 1993. [DOI] [PubMed] [Google Scholar]
- [17].Shaheen NJ et al. , “Radiofrequency ablation in Barrett’s esophagus with dysplasia,” New Engl. J. Med, vol. 360, pp. 2277–88, May 2009. [DOI] [PubMed] [Google Scholar]
- [18].Sharma P et al. , “The American society for gastrointestinal endoscopy PIVI (preservation and incorporation of valuable endoscopic innovations) on imaging in Barrett’s esophagus,” Gastrointest. Endosc, vol. 76, pp. 252–254, August 2012. [DOI] [PubMed] [Google Scholar]
- [19].Levine DS, “Management of dysplasia in the columnar-lined esophagus,” Gastroenterol. Clin. North Am, vol. 26, pp. 613–634, September 1997. [DOI] [PubMed] [Google Scholar]
- [20].Cancer Facts & Figures 2016 Atlanta, GA, USA: American Cancer Society, 2016. [Google Scholar]
- [21].Brand RE et al. , “Advances in counselling and surveillance patients at risk for pancreatic cancer,” Gut, vol. 56, pp. 1460–1469, September 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ryan DP, Hong TS, and Bardeesy N, “Pancreatic adenocarcinoma,” New Engl. J. Med vol. 371, pp. 1039–1049, September 2014. [DOI] [PubMed] [Google Scholar]
- [23].Brugge WR, Lauwers GY, Sahani D, Fernandez-del Castillo C, and Warshaw AL, “Cystic neoplasms of the pancreas,” New Engl. J. Med, vol. 351, pp. 1218–1226, September 2004. [DOI] [PubMed] [Google Scholar]


