Abstract
Background
Helminth and protozoan infections affect more than 1 billion children globally. Improving water quality, sanitation, handwashing, and nutrition could be more sustainable control strategies for parasite infections than mass drug administration, while providing other quality of life benefits.
Methods and findings
We enrolled geographic clusters of pregnant women in rural western Kenya into a cluster-randomized controlled trial (ClinicalTrials.gov NCT01704105) that tested 6 interventions: water treatment, improved sanitation, handwashing with soap, combined water treatment, sanitation, and handwashing (WSH), improved nutrition, and combined WSH and nutrition (WSHN). We assessed intervention effects on parasite infections by measuring Ascaris lumbricoides, Trichuris trichiura, hookworm, and Giardia duodenalis among children born to the enrolled pregnant women (index children) and their older siblings. After 2 years of intervention exposure, we collected stool specimens from 9,077 total children aged 2 to 15 years in 622 clusters, including 2,346 children in an active control group (received household visits but no interventions), 1,117 in the water treatment arm, 1,160 in the sanitation arm, 1,141 in the handwashing arm, 1,064 in the WSH arm, 1,072 in the nutrition arm, and 1,177 in the WSHN arm. In the control group, 23% of children were infected with A. lumbricoides, 1% with T. trichiura, 2% with hookworm, and 39% with G. duodenalis. The analysis included 4,928 index children (median age in years: 2) and 4,149 older siblings (median age in years: 5); study households had an average of 5 people, <10% had electricity access, and >90% had dirt floors. Compared to the control group, Ascaris infection prevalence was lower in the water treatment arm (prevalence ratio [PR]: 0.82 [95% CI 0.67, 1.00], p = 0.056), the WSH arm (PR: 0.78 [95% CI 0.63, 0.96], p = 0.021), and the WSHN arm (PR: 0.78 [95% CI 0.64, 0.96], p = 0.017). We did not observe differences in Ascaris infection prevalence between the control group and the arms with the individual interventions sanitation (PR: 0.89 [95% CI 0.73, 1.08], p = 0.228), handwashing (PR: 0.89 [95% CI 0.73, 1.09], p = 0.277), or nutrition (PR: 86 [95% CI 0.71, 1.05], p = 0.148). Integrating nutrition with WSH did not provide additional benefit. Trichuris and hookworm were rarely detected, resulting in imprecise effect estimates. No intervention reduced Giardia. Reanalysis of stool samples by quantitative polymerase chain reaction confirmed the reductions in Ascaris infections measured by microscopy in the WSH and WSHN groups. Trial limitations included imperfect uptake of targeted intervention behaviors, limited power to detect effects on rare parasite infections, and that it was not feasible to blind participants and sample collectors to treatment status. However, lab technicians and data analysts were blinded to treatment status. The trial was funded by the Bill & Melinda Gates Foundation and the United States Agency for International Development.
Conclusions
Integration of improved water quality, sanitation, and handwashing could contribute to sustainable control strategies for Ascaris infections, particularly in similar settings with recent or ongoing deworming programs. Combining nutrition with WSH did not provide further benefits, and water treatment alone was similarly effective to integrated WSH. Our findings provide new evidence that drinking water should be given increased attention as a transmission pathway for Ascaris.
Trial registration
ClinicalTrials.gov NCT01704105.
Interventions for improved water and hygiene in pregnant women in Kenya resulted in fewer Ascaris infections in newborns, Amy Pickering and colleagues reveal.
Author summary
Why was this study done?
Intestinal worm and protozoan infections affect >1 billion children and are associated with growth faltering and impaired cognitive development.
High reinfection rates can prevent mass drug administration programs from eliminating transmission.
Improved water quality, sanitation, handwashing, and nutrition could interrupt environmental transmission of parasites, but few trials evaluating these interventions have measured parasite infections as an outcome.
What did the researchers do and find?
The authors conducted a randomized controlled trial among a birth cohort to test if single and combined improved drinking water quality, sanitation, handwashing, and nutrition interventions can reduce intestinal worm and Giardia infections. The authors measured parasite infections after 2 years of intervention exposure.
The authors demonstrated that water treatment alone and integrated water, sanitation, and handwashing interventions can sustainably reduce roundworm (Ascaris) infection prevalence among young children in Kenya.
Improved nutrition did not enhance the effectiveness of the water, sanitation, and handwashing interventions, and none of the interventions reduced Giardia.
What do these findings mean?
Improving water quality, sanitation, and handwashing concurrently in the household environment can protect children from infection with Ascaris.
The study also provides evidence that water treatment alone may provide a similar level of protection against Ascaris infection, suggesting that combining water, sanitation, and handwashing interventions does not yield greater health benefits than implementing single interventions.
Treating drinking water is a relatively unexplored strategy for controlling intestinal worm infections, and drinking water should be given increased attention as an ingestion exposure pathway for Ascaris eggs.
Introduction
Intestinal soil-transmitted helminth (STH) infections, including Ascaris lumbricoides, Trichuris trichiura, and hookworm, and the protozoan Giardia duodenalis are common parasitic infections among children in low-resource settings and are neglected tropical diseases. Globally, STHs are estimated to affect 1.45 billion people [1], while Giardia has been cited as the most common enteropathogen in low-income countries [2]. STH and Giardia infections can result in poor absorption of nutrients and weight loss [3,4]. There is some evidence that STH and Giardia infections, even when asymptomatic, may contribute to growth faltering and impaired cognitive development [5–8]. Longitudinal cohort studies in Bangladesh and Brazil have identified early infection with Giardia as a risk factor for stunting among children [7,9]. In Peru, children with multiple Giardia infections per year during the first 2 years of life had lower cognitive function scores at age 9 years than children with 1 or fewer Giardia infections [10]. Evidence on the effect of child STH infections on child growth, cognitive development, and school performance has been mixed and strongly debated by experts, with some suggesting additional evidence is needed [5,6,11–14].
School-based targeted mass drug administration (MDA) campaigns have been the cornerstone of the global strategy to control STH infections; however, high reinfection rates limit the ability of MDA to achieve sustained reduction in STH infection prevalence [15]. Ascaris, Trichuris, Giardia, and Ancylostoma duodenale are primarily transmitted through the fecal—oral ingestion route, although A. duodenale as well as Necator americanus can be transmitted transdermally [3]. A meta-analysis of studies from settings with medium-to-high endemic STH prevalence identified an average reinfection rate for Ascaris at 12 months of 94% of baseline prevalence, while the average 12-month reinfection rates for Trichuris and hookworm were 82% and 57%, respectively [16]. To achieve elimination of STH transmission, it has been suggested that MDA control efforts may need to be integrated with improved water, sanitation, and handwashing [17]. Control of Giardia has historically relied on drug treatment after diagnosis as well as exposure prevention by water treatment and improved sanitation, but zoonotic transmission can complicate exposure prevention: Few interventions have been developed to prevent human exposure to animal fecal contamination [18].
Recent systematic reviews suggest that improved water, sanitation, and handwashing can reduce the odds of STH and Giardia infections, though the quality of the evidence base remains poor and consists almost exclusively of observational analyses [2,19]. Two randomized controlled trials (RCTs) in rural India found no impact of community sanitation interventions on helminth infections; however, both studies reported low usage rates of toilets among intervention households [20,21]. A recent cluster-randomized trial in Timor-Leste found no additional benefit from combining improved water, sanitation, and handwashing with deworming over deworming alone [22]. We were able to identify 3 previous RCTs evaluating water and sanitation effects on Giardia [2]. Two water treatment trials in Guatemala and Rwanda with small sample sizes (n < 200 participants per arm) did not detect an effect on serological measures of Giardia [23,24], while a community-level sanitation trial detected a reduction in Giardia infection prevalence in rural India [20].
An individual’s susceptibility to STH and Giardia infection is influenced by exposure and immune response. A recent systematic review concluded that there was some evidence that nutritional supplementation decreases the risk of infection or reinfection with STHs, but studies have been of low quality [25]. Plausible mechanisms by which nutrition might reduce STH or Giardia infection are through improvements in effective immune response, including repair of cell damage caused by parasite infection, and through changes to the gut microbiome [26,27].
We conducted a cluster-randomized controlled trial (WASH Benefits) in rural Kenya to assess the effects of water, sanitation, handwashing, and nutrition interventions delivered alone and in combination on STH and Giardia infections among a birth cohort. STH and Giardia infections were prespecified as trial outcomes before the trial began [28]. In a separate paper, we reported the effects of the interventions on child growth and diarrhea [29]. The trial’s nutrition intervention was the only component that improved child growth, and none of the interventions reduced diarrhea [29]. Here, we report intervention effects on Ascaris, Trichuris, hookworm, and Giardia infections measured after 2 years of intervention exposure.
Methods
Study design
The trial protocol and detailed methods are published [28]. The trial was registered at ClinicalTrials.gov, identification number: NCT01704105. The study protocol was approved by the Committee for Protection of Human Subjects at the University of California, Berkeley (protocol number 2011-09-3654), the Institutional Review Board at Stanford University (IRB-23310), and the Scientific and Ethics Review Unit at the Kenya Medical Research Institute (protocol number SSC-2271). Innovations for Poverty Action enrolled participants, implemented the intervention delivery, and collected the data. Mothers provided written informed consent for themselves and their children.
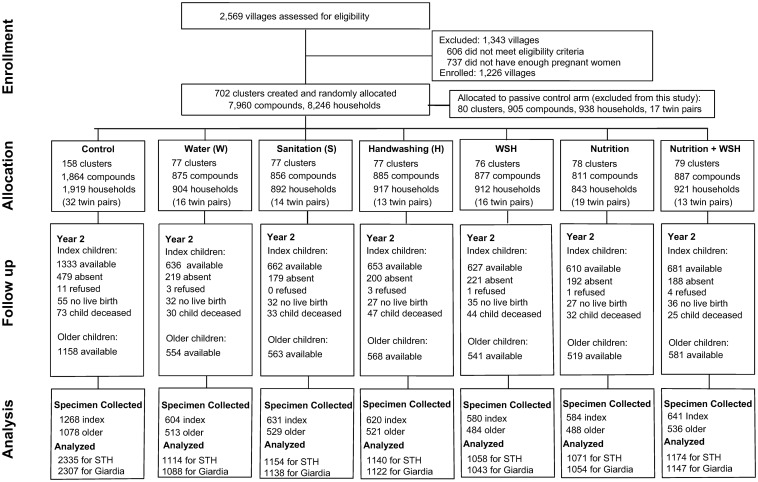
Clusters of eligible pregnant women were randomized by geographic proximal blocks into 1 of 8 study arms: water treatment (chlorine treatment of drinking water); improved sanitation(provision of toilets with plastic slabs and hardware to manage child feces); handwashing with soap; combined water treatment, sanitation, and handwashing (WSH); improved nutrition (infant and young child feeding counseling plus small-quantity lipid-based nutrient supplements [LNSs]); combined WSH and nutrition (WSHN); a double-sized active control; and a passive control. The trial included a passive control arm to test if promoter visits alone (active control) had an effect on the trial’s primary outcomes diarrhea and growth; children in the passive control arm were purposively excluded from parasitology measurement (Fig 1).
Fig 1. Trial profile and participant flow.
STH, soil-transmitted helminth.
We conducted a cluster-randomized trial because there could have been behavior and infectious disease interactions between neighboring households. Villages were eligible for selection into the study if they were rural, the majority of the population lacked access to piped water supplies, and there were no other ongoing WSH or nutrition programs. Within selected villages, a census was conducted to identify eligible pregnant women in their second or third trimester who planned to continue to live at their current residence for the next year. Since interventions were designed to reduce child exposure to pathogens through a cleaner environment and exclusive breastfeeding, we enrolled pregnant women to allow time for intervention delivery to occur prior to or as close to birth as possible. After the census, clusters were formed from 1–3 neighboring villages and had a minimum of 6 pregnant women per cluster after the enrollment survey (each village could only be assigned to 1 cluster). Enrolled study compounds were thus a small proportion of the total number of compounds residing in each cluster. Children born to enrolled pregnant women were considered “index” children. We measured parasite infections approximately 27 months post-enrollment (which equates to a minimum of 24 months of intervention exposure since intervention hardware was delivered <3 months after enrollment). Outcomes were assessed among index children, including twins, as well as among 1 older child in the index child’s compound to understand the effect of the interventions on both preschool-aged and school-aged children. The older child was selected by enrolling the youngest available child within the age range of 3–15 years old, with priority for a sibling in the index child’s household.
Baseline survey
A survey at enrollment measured household socioeconomic characteristics and demographics (including maternal age, maternal education, electricity access, type of floor, and number of people in the household), as well as water, sanitation, and handwashing infrastructure and behaviors (including type of water source, reported water treatment, defecation location, type of toilet, and presence of water and soap at a handwashing station). In addition, at study enrollment we measured Giardia, Entamoeba histolytica, and Cryptosporidium spp. among children residing in study compounds between 18 and 27 months of age (the projected age range for index children at the end of the study) to assess baseline prevalence of these pathogens. STHs were not measured at enrollment among these proxy children because it was not logistically feasible to deworm infected children at baseline. We also collected 100-ml samples from primary drinking water sources accessed by study households and household stored drinking water (if available). We transported the samples on ice to field labs and enumerated Escherichia coli in each sample by membrane filtration followed by culture on MI medium.
Randomization and blinding
A few weeks after enrollment, clusters were randomly assigned to intervention/control arms at the University of California, Berkeley, by an investigator independent of the field research team (BFA) using a random number generator. Groups of 9 geographically adjacent clusters were block-randomized into the 6 intervention arms, the double-sized active control arm, and the passive control arm (the passive control arm was not included in the parasite assessment). Participants and other community members were informed of their intervention/control group assignment after the baseline survey. Blinding (masking) of participants was not possible given the nature of the interventions. Data and stool sample collectors were not informed of cluster assignment, but could have inferred treatment status by observing intervention hardware. Lab technicians were blinded to intervention status. Two authors (AJP and JS) independently replicated the statistical analyses while blinded to intervention status.
Intervention delivery
Intervention delivery occurred within 3 months after enrollment. In the water intervention arms (water treatment, WSH, and WSHN), community health promoters encouraged drinking water treatment with chlorine (liquid sodium hypochlorite) using either manual dispensers installed at the point of collection (community water source) in study villages or bottled chlorine provided directly to households every 6 months. In the sanitation intervention arms (sanitation, WSH, and WSHN), households in study compounds received new latrines, or existing latrines were upgraded and improved by installing a plastic slab that included a lid. All households in sanitation arm study compounds were provided with a child potty for each child <3 years as well as a “sani-scoop” to remove animal and human feces from the compound. Households were encouraged to use latrines for defecation and for disposal of child and animal feces. In the handwashing intervention arms (handwashing, WSH, and WSHN), study compounds were provided with 2 handwashing stations—near the latrine for handwashing after defecation and near the cooking area for handwashing before preparing food. Stations included dual foot-pedal-operated jerry cans that could be tipped to dispense either soapy water or rinse water. Households were responsible for keeping the stations stocked with rinse water, and community health promoters refilled soap regularly. In the nutrition intervention arms (nutrition and WSHN), small-quantity LNSs were provided to children 6–24 months of age. Children received monthly rations of LNSs for addition to their other food twice per day. Nutrition messaging included promoting dietary diversity during pregnancy and lactation, early initiation of breastfeeding, exclusive breastfeeding at age 0–6 months, continued breastfeeding through age 24 months, timely introduction of complementary foods, dietary diversity for child feeding, and child feeding during illness. Intervention delivery was at the cluster level for the water intervention (all compounds in villages assigned to the cluster had access to the chlorine dispensers), at the compound level for sanitation and handwashing (non-study compounds in the cluster did not receive handwashing stations or improved toilets), and at the child level for the nutrition intervention (only index children and their siblings under 24 months received LNSs).
Community health promoters were nominated by mothers in the community and trained to provide intervention-specific behavior change activities and instructions on hardware use and provision of nutrition supplements. They were also trained to measure the mid-upper arm circumference of the index children to identify and provide referrals for potential cases of severe acute malnutrition. Each intervention consisted of a comprehensive behavior change package of key messages; visual aids in the form of flip charts, posters, and reminder cue cards; interactive activities with songs, games, or pledges to commit to practice target behaviors; and the distribution of arm-specific hardware, products, or supplements. Households in the active control group received visits from promoters to measure child mid-upper arm circumference and provide malnutrition referrals, but did not receive any intervention-related hardware or messaging. Promoters were instructed to visit households monthly. Key messages and promoter materials are available at https://osf.io/fs23x/.
Adherence to the interventions was measured during unannounced household visits after 1 year and 2 years of intervention exposure (S1 Text).
Measurement of parasite infections
Stool samples were collected from index children and older children in sterile containers and transported on ice to the closer of 2 central field labs located in Kakamega and Bungoma. Field staff revisited households up to 3 times to collect stool samples. A. lumbricoides, T. trichiura, and hookworm eggs were immediately enumerated (same day) by double-slide Kato—Katz microscopy with 41.7-mg templates. Both slides created from each stool sample were counted by a trained parasitologist, and 2 different parasitologists counted each slide from the same sample. A supervisor with expertise in STH egg identification reviewed 10% of all slides, and any discrepancies were corrected. STH egg counts were averaged for analysis if both slides from 1 stool sample were positive; if 1 slide was negative, the count for the positive slide was used for analysis. Two aliquots of stool (1 mixed with ethanol) were transported on dry ice to the Eastern and Southern Africa Centre of International Parasite Control laboratory at the Kenya Medical Research Institute in Nairobi, Kenya, for further analysis.
One aliquot was analyzed by monoclonal enzyme-linked immunosorbent assay (ELISA) (Giardia II, Alere International, Galway, Ireland) for the presence or absence of G. duodenalis cysts. Samples were measured by ELISA in duplicate; if there was a discrepancy between duplicates, the sample was rerun. DNA was extracted from the other aliquot (preserved in ethanol) for stool samples collected from children in the control, WSH, and WSHN groups. Four quantitative polymerase chain reaction (qPCR) assays were run in duplicate on each sample to detect the following targets: N. americanus, A. duodenale, T. trichiura, and A. lumbricoides (see S1 Text for further details) [30].
Outcomes
STH and Giardia infections were prespecified outcomes in the parent WASH Benefits trial prior to the start of data collection; see Fig 3 in Arnold et al. [28]. Parasite infections were measured after 2 years of intervention exposure. The main indicators of parasite infections were the prevalence of each individual STH infection, any STH infection, and Giardia infection among index and older children from the same compound. Additional indicators of parasite infections included intensity of Ascaris, Trichuris, and hookworm measured in eggs per gram (epg) of feces; intensity binary category of Ascaris infection, measured as low intensity (1–5,000 epg) or moderate/high intensity (>5,000 epg), following World Health Organization (WHO) cutoffs; prevalence of coinfection with 2 or 3 STHs; and prevalence of coinfection with Giardia and any STH. The trial’s original protocol included E. histolytica and Cryptosporidium spp. as additional protozoan endpoints. At enrollment, Giardia prevalence was 40% among 535 children 18–27 months old in study compounds, while Cryptosporidium spp. prevalence was 1% and E. histolytica prevalence was 0%. We determined that the extremely low baseline prevalence of E. histolytica and Cryptosporidium spp. made these trial endpoints futile due to limited statistical power, and since each required a separate assay on the ELISA platform, the study’s steering committee decided to not test for them at follow-up.
Sample size calculations
All households in all clusters enrolled into the main trial were invited to participate in the measurement of parasite infections. The main trial was powered for a minimum detectable effect of 0.15 in length-for-age Z score and a relative risk of diarrhea of 0.7 or smaller for a comparison of any intervention with the double-sized control group, assuming a type I error (α) of 0.05 and power (1 − β) of 0.8, 10% loss to follow-up, and a 1-sided test for a 2-sample comparison of means (the main trial statistical analysis plan was later changed to employ 2-sided tests). This led to a planned design of 100 clusters per arm and 10 index children per cluster. Given this design and a single post-intervention measure, we estimated that the trial’s sample size would be sufficient at 80% power with a 2-sided α of 0.05 to detect a relative reduction of 18% in infection prevalence of any parasite (2-sided tests were planned due to a lack of evidence that all interventions would have a protective effect). Our minimum detectable effect calculations assumed 50% prevalence in the control arm, a village intraclass correlation (ICC) of 0.14, 2 children measured per enrolled household (index child plus an older sibling), and 70% successful stool collection and analysis. For perspective, this minimum detectable effect is much smaller than typical effect sizes reported in meta-analyses of the association between improved water, sanitation, and handwashing and helminth/protozoan infections (e.g., odds ratios between 0.46 and 0.58 for sanitation facilities and helminth infections) [2,31].
Statistical analysis
All statistical analyses and comparisons between arms (water treatment, sanitation, handwashing, WSH, nutrition, and WSHN compared to active control) were prespecified prior to unblinding of investigators, and the analysis plan was published with a time stamp on the Open Science Framework (https://osf.io/k2s47/). Replication scripts and data are also provided at the same link. Our alternative hypothesis for all comparisons was that group means were not equal (2-sided tests). We estimated unadjusted and adjusted intention-to-treat effect differences between study arms using targeted maximum likelihood estimation with influence-curve-based standard errors that treated clusters as independent units and allowed for outcome correlation within clusters [32,33]. Our parameters of interest for dichotomous outcomes were prevalence ratios (PRs) (prevalence in the intervention group divided by the prevalence in the control group). Our parameter of interest for helminth intensity was the relative fecal egg count reduction. We calculated the relative reduction using both geometric and arithmetic means. We did not perform statistical adjustments for multiple outcomes to preserve interpretation of effects and because many of our outcomes were correlated [34]. We estimated adjusted parameters by including variables that were associated with the outcome, to potentially improve the precision of our estimates. We prescreened covariates (S1 Text) to assess whether they were associated (p-value < 0.2) with each outcome prior to including them in adjusted statistical models [35]. We conducted subgroup analyses to explore effect modification on Ascaris and Giardia infection presence by the following factors: index child status (LNSs were given only to index children and siblings under 24 months), consumed deworming medicine in past 6 months (Ascaris only), consumed soil in past week (index children only), >8 people in compound, and time since defecation before stool collection. Statistical analyses were conducted using R version 3.3.2 (https://www.r-project.org).
Results
Enrollment
Pregnant women were enrolled into the cluster-randomized controlled trial from Kakamega, Bungoma, and Vihiga counties in Kenya’s western region. Enrollment occurred between November 27, 2012, and May 21, 2014; 8,246 pregnant women were enrolled. Clusters with an average of 12 eligible pregnant women each were randomized by geographic proximal blocks into 1 of 8 study arms: water treatment (chlorine treatment of drinking water); improved sanitation (provision of toilets with plastic slabs and hardware to manage child feces); handwashing with soap; combined WSH; improved nutrition (infant and young child feeding counseling plus small-quantity LNSs); combined WSHN; a double-sized active control; and a passive control. Children in the passive control arm were purposively excluded from parasitology measurement: Only the active control group is considered hereafter (Fig 1). Parasite infections were measured among children born to enrolled pregnant women (index children) as well as their older siblings or an older child in the same compound.
Enrollment characteristics of the study population were similar between arms (Table 1). Most households accessed springs or wells as their primary drinking water source. In the control group, 24% of households accessed unprotected water sources, such as springs, dug wells, and surface water. The microbial quality of drinking water was very poor, as has been reported previously for this study area [36]; 96% (n = 1,829) of source water samples and 94% (n = 5959) of stored drinking water samples contained E. coli contamination. Most (82%) households owned a latrine, but only 15% had access to a latrine with a slab or ventilation pipe (Table 1). Soap and water availability for handwashing at a designated handwashing location was low (<10%).
Table 1. Baseline characteristics by treatment assignment.
| Characteristic | Control (n = 1,916) |
Water (n = 903) |
Sanitation (n = 886) |
Handwashing (n = 911) |
WSH (n = 909) |
Nutrition (n = 840) |
Nutrition + WSH (n = 920) |
|---|---|---|---|---|---|---|---|
| Maternal | |||||||
| Age (years) | 26 | 26 | 26 | 26 | 26 | 26 | 26 |
| Completed primary school | 47.9 | 49.4 | 48.4 | 43.8 | 47.1 | 48.6 | 47.7 |
| Paternal | |||||||
| Completed primary school | 62.4 | 63.9 | 58.7 | 59.0 | 61.5 | 63.5 | 62.5 |
| Works in agriculture | 41.1 | 44.2 | 42.6 | 42.1 | 43.2 | 43.6 | 42.8 |
| Household | |||||||
| Number of persons | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| Has electricity | 6.4 | 6.7 | 8.1 | 7.2 | 7.0 | 6.9 | 7.3 |
| Has a cement floor | 5.6 | 8.1 | 5.5 | 4.5 | 5.4 | 5.7 | 6.1 |
| Drinking water | |||||||
| Primary source protected | 75.7 | 75.3 | 75.9 | 77.5 | 68.6 | 71.5 | 76.1 |
| Stored water observed at home | 81.5 | 81.3 | 82.0 | 82.8 | 79.5 | 81.0 | 81.0 |
| Reported treating currently stored water | 12.6 | 11.1 | 12.8 | 12.6 | 13.2 | 11.6 | 14.3 |
| Sanitation | |||||||
| Daily defecating in the open | |||||||
| Children: 3 to <8 years | 11.7 | 12.6 | 12.7 | 13.8 | 12.8 | 14.4 | 12.3 |
| Children: 0 to <3 years | 77.5 | 80.2 | 74.8 | 76.2 | 76.4 | 78.5 | 78.0 |
| Latrine | |||||||
| Owned by compound | 81.7 | 83.2 | 81.2 | 82.8 | 82.7 | 83.1 | 83.5 |
| Has slab or ventilation pipe | 17.3 | 17.7 | 15.7 | 18.4 | 17.5 | 15.0 | 16.4 |
| Visible feces on slab or floor | 47.6 | 78.3 | 76.3 | 50.1 | 52.5 | 50.4 | 50.2 |
| Has a child potty | 2.3 | 2.7 | 2.0 | 3.1 | 2.6 | 1.7 | 2.2 |
| Human feces observed in the compound | 8.6 | 7.3 | 8.0 | 9.3 | 8.1 | 8.7 | 9.5 |
| Handwashing | |||||||
| Handwashing station has water and soap | 5.0 | 6.2 | 4.7 | 5.6 | 6.9 | 6.7 | 5.7 |
Data are mean or percent. Protected water sources include piped water, borewells, protected springs, protected dug wells, and rainwater collection.
WSH, water treatment, sanitation, and handwashing.
Indicators of intervention uptake
After 1 year of intervention, 89%–90% of households that received the sanitation intervention had access to an improved latrine with a slab or a ventilation pipe (compared to 18% in the control arm), and 79%–82% of these had access to an improved latrine after 2 years of intervention. In the water intervention arms, 40%–44% of households had a detectable chlorine residual in their stored drinking water at the 1-year follow-up (compared to 3% of control households), and 19%–23% had chlorine detected after 2 years. In the handwashing intervention arms, 76%–78% of households had soap and water available at a handwashing station (compared to 12% in the control arm) after 1 year, and this decreased to 19%–23% at 2 years. Consumption of LNS sachets by children in the nutrition intervention arms was 95%–96% of the expected 2 sachets per day at the 1-year follow-up, and 114%–116% of expected at the 2-year follow-up (>100% is possible because additional LNS sachets were delivered in case of future delivery delays) (S1 and S2 Tables).
Infection prevalence
STH and Giardia infections were measured after 2 years of exposure to the interventions. We collected stool specimens from 9,077 children aged 2–15 years at the 2-year survey during January 2015–July 2016, including 4,928 index children (median age in years: 2.0; IQR 1.9, 2.1) and 4,149 older children (median age in years: 5.0; IQR 4.2, 6.4) residing in an index child’s compound (Fig 1). A total of 2,346 children in 158 control clusters, 1,117 children in 77 water clusters, 1,160 children in 77 sanitation clusters, 1,141 children in 77 handwashing clusters, 1,064 children in 76 WSH clusters, 1,072 children in 78 nutrition clusters, and 1,177 children in 79 WSHN clusters provided stool specimens. Stool specimens were successfully collected from 95% (4,928 of 5,202) of available index children and from 93% (4,149 of 4,484) of available older children 2 years after intervention delivery (Fig 1 shows number of children not available due to there being no live birth, death, refusal, or absence; S3 and S4 Tables show characteristics of children lost to follow-up, by treatment status). In the control group, 22.6% of children were infected with Ascaris (ICC: 0.10), 2.2% with hookworm (ICC: 0.04), 1.2% with Trichuris (ICC: 0.07) (measured by Kato–Katz microscopy), and 39% with Giardia (measured by ELISA) (S5 Table). Ascaris infection prevalence was similar for index children (22.8%) and older children (22.3%) in the control group. Caregivers reported that 39% of index children and 10% of older children had consumed soil in the past 7 days.
Effect of interventions on parasite infection prevalence
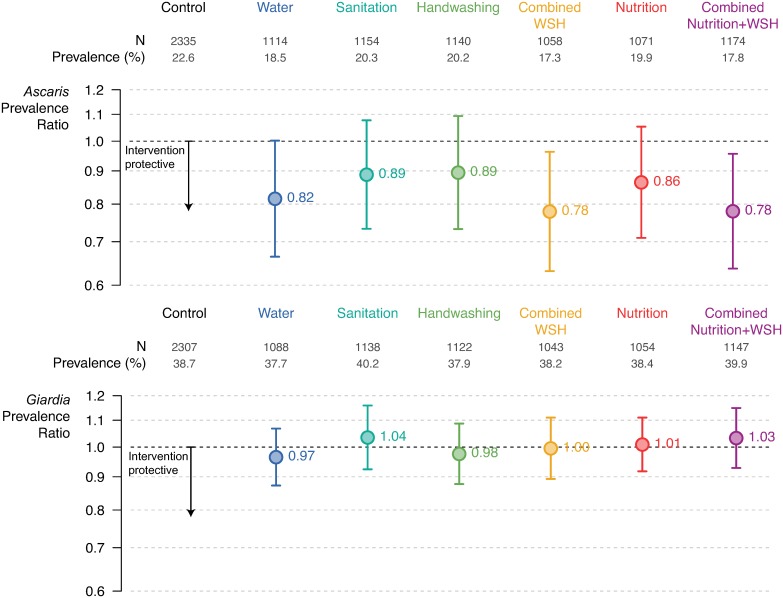
Infection prevalence of each STH, any STH, and Giardia was compared between each intervention group (water treatment, sanitation, handwashing, WSH, nutrition, and WSHN) and the double-sized active control group; see Methods for further details of the analysis. Compared to the control group, Ascaris infection prevalence was 18% lower in the water arm (PR: 0.82 [95% CI 0.67, 1.00]), 22% lower in the combined WSH arm (PR: 0.78 [95% CI 0.63, 0.96]), and 22% lower in the WSHN arm (PR: 0.78 [95% CI 0.64, 0.96]) (Fig 2; S5 Table). We did not observe that the individual interventions sanitation (PR: 0.89 [95% CI 0.73, 1.08]), handwashing (PR: 0.89 [95% CI 0.73, 1.09]), or nutrition (PR: 86 [95% CI 0.71, 1.05] reduced Ascaris infection on their own (Fig 2). The combined WSH intervention reduced infection with any STH by 23% (PR: 0.77 [95% CI 0.63, 0.95]), and the combined WSHN intervention reduced infection with any STH by 19% (PR: 0.81 [95% CI 0.66, 0.98]) (S5 Table). No interventions significantly reduced the prevalence of hookworm and Trichuris, though the low prevalence in the control arm meant that any reduction due to intervention would be difficult to detect in the trial (S5 Table). No interventions reduced Giardia prevalence (Fig 2).
Fig 2. Effect of the interventions on infection with Ascaris and Giardia: Data includes all index children and older siblings combined.
Prevalence ratios estimated by targeted maximum likelihood estimation. Error bars show 95% confidence intervals for the prevalence ratios. WSH, water treatment, sanitation, and handwashing.
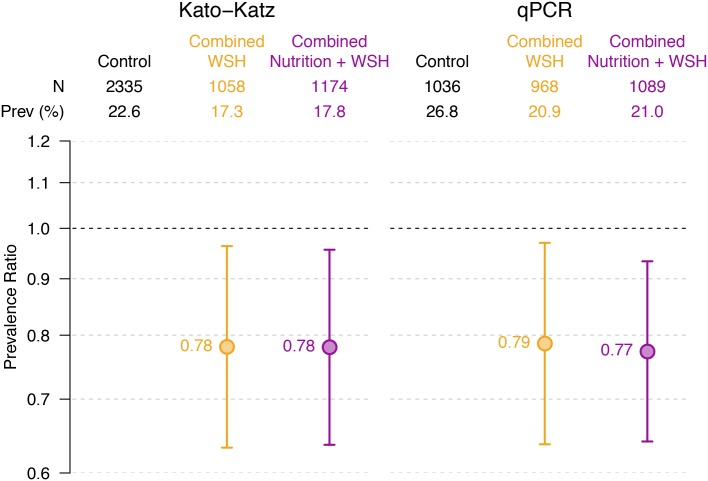
We reanalyzed all stool samples collected from children enrolled in the control, WSH, and WSHN arms by qPCR to validate our estimates based on microscopy measurements. These 3 arms were selected for the qPCR subset analysis prior to unblinding of investigators to results and were chosen based on the hypothesis that these arms would be the most likely to have low-intensity STH infections if any of the interventions were effective. qPCR analyses resulted in almost identical intervention effect estimates to those based on microscopy (Fig 3; S6 Table). Compared to the control group, Ascaris infection prevalence was 21% lower (PR: 0.79 [95% CI 0.64, 0.97]) in the WSH group and 23% lower (PR: 0.77 [95% CI 0.64, 0.93]) in the WSHN group. We also did not detect any significant effects of the interventions on Trichuris or hookworm infections using qPCR data (S6 Table).
Fig 3. Effect of the combined interventions on infection with Ascaris estimated with Kato–Katz microscopy (left) and by qPCR (right).
Prevalence ratios estimated by targeted maximum likelihood estimation. Error bars show 95% confidence intervals for the prevalence ratios. qPCR, quantitative PCR; WSH, water treatment, sanitation, and handwashing.
Effect of interventions on infection intensity
Ascaris infection intensity was lower in children in the water arm (fecal egg count reduction [FECR] with geometric means: −16% [95% CI −32%, −1%]), the WSH arm (FECR: −19% [95% CI −33%, −5%]), and the WSHN arm (FECR: −18% [95% CI −32%, −4%]) compared to the control arm; FECR with arithmetic means showed similar results (Table 2). The prevalence of heavy/moderate intensity Ascaris infections was 10.0% in the water arm, 10.9% in WSH, and 10.3% in WSHN compared to 12.7% in the control arm; these differences were not statistically significant at the 95% confidence level (S5 Table).
Table 2. Effect of the interventions on infection intensity, measured by FECR with arithmetic and geometric means.
| Outcome and arm | N | Geometric mean | Arithmetic mean | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Log10 mean*, epg | FECR | 95% CI | p-Value | Arithmetic mean, epg | FECR | 95% CI | p-Value | ||
| Ascaris FECR | |||||||||
| Control | 2,335 | 0.60 | 3,641 | ||||||
| Water | 1,114 | 0.40 | −0.16 | −0.32, −0.01 | 0.04 | 2,682 | −0.26 | −0.52, −0.01 | 0.04 |
| Sanitation | 1,154 | 0.50 | −0.09 | −0.25, 0.07 | 0.27 | 3,443 | −0.04 | −0.32, 0.23 | 0.75 |
| Handwashing | 1,140 | 0.50 | −0.08 | −0.25, 0.08 | 0.31 | 3,386 | −0.03 | −0.34, 0.28 | 0.85 |
| WSH | 1,058 | 0.40 | −0.19 | −0.33, −0.05 | 0.01 | 2,571 | −0.27 | −0.52, −0.02 | 0.03 |
| Nutrition | 1,071 | 0.50 | −0.10 | −0.25, 0.04 | 0.16 | 3,303 | −0.11 | −0.34, 0.12 | 0.35 |
| Nutrition + WSH | 1,174 | 0.40 | −0.18 | −0.32, −0.04 | 0.01 | 2,927 | −0.21 | −0.46, 0.03 | 0.09 |
| Hookworm FECR | |||||||||
| Control | 2,335 | −0.25 | 12 | ||||||
| Water | 1,114 | −0.23 | 0.02 | −0.02, 0.05 | 0.37 | 10 | −0.20 | −0.84, 0.44 | 0.54 |
| Sanitation | 1,154 | −0.24 | 0.01 | −0.02, 0.04 | 0.42 | 10 | −0.16 | −0.90, 0.57 | 0.67 |
| Handwashing | 1,140 | −0.21 | 0.03 | 0.00, 0.07 | 0.08 | 23 | 0.93 | −1.39, 3.25 | 0.43 |
| WSH | 1,058 | −0.26 | −0.02 | −0.04, 0.01 | 0.18 | 3 | −0.74 | −0.91, −0.58 | 0.00 |
| Nutrition | 1,071 | −0.23 | 0.03 | −0.01, 0.06 | 0.14 | 12 | 0.16 | −1.17, 1.50 | 0.81 |
| Nutrition + WSH | 1,174 | −0.23 | 0.02 | −0.01, 0.06 | 0.22 | 24 | 1.02 | −1.87, 3.91 | 0.49 |
| Trichuris FECR | |||||||||
| Control | 2,335 | −0.27 | 6 | ||||||
| Water | 1,114 | −0.27 | 0.00 | −0.03, 0.03 | 0.92 | 6 | 0.04 | −1.91, 1.98 | 0.97 |
| Sanitation | 1,154 | −0.27 | 0.00 | −0.03, 0.02 | 0.77 | 4 | −0.19 | −1.49, 1.11 | 0.78 |
| Handwashing | 1,140 | −0.26 | 0.01 | −0.02, 0.04 | 0.46 | 6 | 0.03 | −1.40, 1.46 | 0.97 |
| WSH | 1,058 | −0.29 | −0.02 | −0.04, 0.00 | 0.06 | 0 | −0.91 | −1.07, −0.75 | 0.00 |
| Nutrition | 1,071 | −0.28 | −0.02 | −0.04, 0.01 | 0.18 | 2 | −0.64 | −1.22, −0.05 | 0.03 |
| Nutrition + WSH | 1,174 | −0.29 | −0.02 | −0.05, 0.00 | 0.10 | 1 | −0.81 | −1.15, −0.47 | 0.00 |
FECR estimated by targeted maximum likelihood estimation. FECRs are expressed as proportions (percentage change/100).
*Value of 0.5 epg substituted for samples below the detection limit, to calculate log-transformed mean.
epg, eggs per gram; FECR, fecal egg count reduction; WSH, water treatment, sanitation, and handwashing.
The FECR with arithmetic means indicated that children in the WSH arm had lower intensity infections with hookworm (3 epg versus 11 epg in control arm) (Table 2). In addition, the FECR with arithmetic means indicated lower Trichuris infection intensity in the WSH (0 epg versus 6 epg in control arm), nutrition (2 epg), and WSHN (1 epg) arms. Children who received the WSHN intervention had 27% lower prevalence of coinfection with STH and Giardia compared to the control group (PR: 0.73 [95% CI 0.56, 0.97]) (S4 Table). STH coinfection was rare: <2% in the control arm and at similarly low levels in intervention arms (S5 Table).
Adjusted models and subgroup analyses
Adjusted effect estimates were similar to unadjusted effects (S5 Table). Subgroup analyses of intervention effects stratified by index children versus older children, reported soil consumption (index children only), number of people living in the compound, deworming (Ascaris only), and time since defecation did not show any strong effect modification (S8 Table).
Discussion
Our findings demonstrate that an integrated water, sanitation, and handwashing intervention targeting the household environment in rural Kenya reduced Ascaris infection prevalence by 22%, while a water treatment intervention reduced Ascaris infection by 18%. Almost identical effect estimates generated by analyzing stool samples with microscopy and qPCR in a subset of arms lent additional credibility to the overall results (Fig 3). In addition, we found that improved nutrition did not enhance the effectiveness of the WSH intervention. Trichuris and hookworm prevalence were too low to precisely assess intervention impact in this setting, and Giardia was unaffected by the interventions. Although the integrated WSH intervention did not succeed in improving child growth or reducing symptomatic diarrhea in this trial [29], our findings confirm that WSH can effectively reduce helminth infection prevalence.
A limited number of RCTs have previously analyzed the effect of WSH interventions on STH infection. Several school-based RCTs combining deworming with handwashing promotion have reported significant reductions in Ascaris reinfection prevalence in China, Ethiopia, and Peru [37–39]. A school-based integrated WSH intervention combined with deworming in rural Kenya also reduced the odds of Ascaris reinfection [40]. While previous RCTs demonstrate the success of school-based deworming combined with hygiene promotion, our results contribute new evidence from a large cluster-randomized trial that improving WSH in the household environment can reduce Ascaris infections in a rural, low-income setting.
We did not detect an effect of the sanitation intervention alone on STH infection prevalence. One potential explanation for the lack of impact may be that transitioning households from using traditional pit latrines to pit latrines with slabs may not have a measurable impact on STH transmission. A shift from households practicing open defecation to using latrines might be more likely to reduce STH transmission, with little additional benefit from improving latrine quality. A recent trial in Côte d’Ivoire reported greater reduction in hookworm infection prevalence among communities that received a community-led total sanitation intervention (designed to reduce open defecation levels) integrated with community-wide MDA compared to community-wide MDA alone, although it should be noted that the trial was not randomized and had limited statistical power [41]. A second explanation for the lack of effect of the sanitation intervention may be that sanitation interventions are more effective at interrupting environmental transmission of pathogens when they are implemented at the community level [42], whereas our intervention only improved sanitation access in compounds with enrolled pregnant women. However, a recent cluster-randomized trial of a community-wide sanitation intervention integrated with deworming in Timor-Leste found that the intervention did not reduce helminth infection prevalence more than deworming alone [22]. A third explanation is that it could require >2 years of improved sanitation access to substantially reduce levels of helminth eggs in soil (Ascaris eggs can survive in soil for several years).
The reductions in Ascaris prevalence in the combined arms could have resulted from improved water quality alone; Ascaris prevalence was 18% lower in the water treatment arm than the control arm, a similar magnitude to the 22% reduction in the integrated intervention arms. Near identical reductions in Ascaris infection across all 3 water intervention arms suggests that water could have been an important transmission pathway in this population, which was interrupted by chlorine treatment. STH transmission through water is consistent with a recently published substudy among the control, sanitation, and WSH arms in our trial that found no effect of the sanitation intervention on STH egg prevalence in soil collected from the household entrance [43]. However, we cannot completely rule out contribution to reductions from other interventions in the combined arms; Ascaris prevalence was lower (20%) in the single intervention arms sanitation, handwashing, and nutrition, compared to 23% prevalence in the control arm. Chlorine is not known to inactivate Ascaris eggs, but 1 experimental study did find that chlorine can delay egg development and infectivity [44]; it’s possible that delayed egg infectivity could reduce the risk of consuming an infective egg through drinking water. The proportion of households using jerry cans (a plastic water container with a narrow capped opening) to safely store drinking water was slightly higher in the water intervention arms than the other arms (S1 and S2 Tables). Our findings indicate that drinking water is an understudied transmission pathway for Ascaris. We believe drinking water treatment should be further investigated as an STH control strategy, and that chlorine should be further explored as a method for inhibiting Ascaris egg development in drinking water supplies. While improved sanitation and handwashing have been suggested as control strategies for Ascaris, water treatment has not been previously recommended as an Ascaris control strategy, yet it appeared to be the most effective environmental intervention that we tested in this trial. Our results have the potential to shape future guidance for STH control programs to emphasize water treatment for Ascaris control.
The combined WSHN intervention was similarly effective to WSH in reducing Ascaris prevalence, and improved nutrition did not reduce STH or Giardia infection on its own. Together, these results suggest that the improved nutrition intervention did not reduce parasite infection in this population. Trials investigating the impact of micronutrient supplementation on STH infection or reinfection have reported mixed results [25]. Our results are consistent with a Kenyan trial that found no effect of school-based micronutrient supplementation on reinfection with Ascaris [45]. Considering that interventions in this trial did not include treatment with antiparasitic drugs, further research would be valuable to understand if LNSs could prevent parasite infections after drug treatment.
Our findings suggest that combined interventions may not achieve additive or multiplicative effects on Ascaris infection. Similar reductions in Ascaris infection prevalence were observed in the water and combined WSH arms, and in the WSH and WSHN arms. Given limited resources, combining the interventions implemented in this trial may not be a cost-effective strategy to reduce helminth infections as drinking water interventions alone may yield similar benefits.
Giardia prevalence was unaffected by any of the interventions in this trial. Our results stand in contrast to results from the parallel WASH Benefits trial conducted in Bangladesh [46], which detected reductions in Giardia infection prevalence in the handwashing, sanitation, combined WSH, and combined WSHN arms [47]. One potential explanation for the lack of intervention effects in this trial is that water could be the primary transmission pathway for Giardia in this study setting, and Giardia is highly resistant to chlorination. The majority of households in the WASH Benefits Bangladesh trial accessed protected tubewells, providing water with lower levels of fecal contamination compared to the springs and shallow wells accessed by households in this trial [36,48]. Another potential explanation is that handwashing rates with soap were not high enough at the time of measurement to interrupt Giardia transmission; presence of soap and water at a handwashing station decreased from 78% at 1 year to 19% at 2 years among households in the WSH arm (S1 and S2 Tables). Giardia is also zoonotic [4]; exposure to avian and ruminant fecal contamination in the household environment could mitigate the effect of improved sanitation on transmission. Animal feces management was not a targeted behavior of the intervention packages.
This trial had some limitations. Chlorination does not inactivate protozoa, but was selected as the most appropriate water treatment intervention for the study context considering previous local acceptability, affordability, and effectiveness against bacterial and viral enteric pathogens. We measured parasite infections 2 years after intervention delivery; measurement among the study population at 1 year could have produced different results because of higher intervention adherence at that time (S1 Table) and different child age-related exposures (e.g., younger children may be more likely to consume soil). We were unable to blind study participants due to the nature of the interventions; however, our outcomes were objective indicators of infection analyzed by blinded laboratory technicians, and blinded analysts replicated the data analysis.
During our trial, Kenya implemented a national school-based targeted MDA program to reduce STH prevalence [49], and 43% of study children were reported to have consumed deworming medication in the past 6 months (S8 Table). Reported consumption of deworming medicine was similar across study arms, suggesting no systematic differences in program coverage or intensity between arms (S9 Table). We observed similar Ascaris prevalence among study index children (23%, median age 2 years) and older children (22%, median age 5 years), suggesting that school-based MDA could be missing a key reservoir of infection among young, preschool-aged children. Moreover, an environmental survey conducted during the national deworming program in our study region reported common detection of STH eggs in soil collected from the entrance to homes, with Ascaris eggs detected in soil in 19% of households [50]. Taken together, these findings suggest that additional control strategies beyond school-based deworming might be necessary to fully interrupt environmental STH transmission.
In contrast to most previous trials evaluating the effect of WSH or nutrition on STH infection, administering deworming medication was not included with our intervention. Our findings represent the potential impact of WSH and nutrition interventions in the context of exposure to a deworming program implemented at the national scale. Although the magnitude of Ascaris prevalence reduction observed in the WSH and water arms may be lower than what could be achieved by drug treatment in the short term, reduced STH infection after 2 years of intervention exposure indicates sustained impact. Our results support the proposal that improved WSH could complement chemotherapy in the global effort to eliminate STH transmission.
Supporting information
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
We thank Charles Arnold for data management assistance, Michael Kremer for input on the study design, Araka Sylvie Biyaki and Samara Loewenstein for contributing to sample analysis, the WASH Benefits staff at Innovations for Poverty Action, and our study participants.
The contents are the responsibility of the authors and do not necessarily reflect the views of the US Agency for International Development or the US Government.
Abbreviations
- ELISA
enzyme-linked immunosorbent assay
- epg
eggs per gram
- FECR
fecal egg count reduction
- ICC
intraclass correlation coeffcient
- LNS
lipid-based nutrient supplement
- MDA
mass drug administration
- PR
prevalence ratio
- qPCR
quantitative polymerase chain reaction
- RCT
randomized controlled trial
- STH
soil-transmitted helminth
- WSH
water treatment, sanitation, and handwashing
- WSHN
water treatment, sanitation, handwashing, and nutrition
Data Availability
All replication scripts and data are available on Open Science Framework at the following link: https://osf.io/k2s47/.
Funding Statement
This research was financially supported in part by Global Development grant OPPGD759 from the Bill & Melinda Gates Foundation to the University of California, Berkeley (CN, CPS, JMC, AJP, SN, AL, BFA, RM, JK, KB, GR, TM, PC, MP, LS received funds from this grant). This manuscript was also made possible by the generous support of the American people through the United States Agency for International Development (USAID) (grant AID-OAA-F-13-00040 to Innovations for Poverty Action) (AJP and CN received funds through this grant). Additional funding was provided by a grant from the Task Force for Global Health to Innovations for Poverty Action (NTDSC 088G). Funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Pullan RL, Smith JL, Jasrasaria R, Brooker SJ. Global numbers of infection and disease burden of soil transmitted helminth infections in 2010. Parasit Vectors. 2014;7:37 10.1186/1756-3305-7-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Speich B, Croll D, Fürst T, Utzinger J, Keiser J. Effect of sanitation and water treatment on intestinal protozoa infection: a systematic review and meta-analysis. Lancet Infect Dis. 2016;16:87–99. 10.1016/S1473-3099(15)00349-7 [DOI] [PubMed] [Google Scholar]
- 3.Bethony J, Brooker S, Albonico M, Geiger SM, Loukas A, Diemert D, et al. Soil-transmitted helminth infections: ascariasis, trichuriasis, and hookworm. Lancet. 2006;367:1521–32. 10.1016/S0140-6736(06)68653-4 [DOI] [PubMed] [Google Scholar]
- 4.Certad G, Viscogliosi E, Chabé M, Cacciò SM. Pathogenic mechanisms of Cryptosporidium and Giardia. Trends Parasitol. 2017;33:561–6. 10.1016/j.pt.2017.02.006 [DOI] [PubMed] [Google Scholar]
- 5.Dickson R, Awasthi S, Williamson P, Demellweek C, Garner P. Effects of treatment for intestinal helminth infection on growth and cognitive performance in children: systematic review of randomised trials. BMJ. 2000;320:1697–701. 10.1136/bmj.320.7251.1697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taylor-Robinson DC, Maayan N, Soares-Weiser K, Donegan S, Garner P. Deworming drugs for soil-transmitted intestinal worms in children: effects on nutritional indicators, haemoglobin and school performance. Cochrane Database Syst Rev. 2015;7:CD000371 10.1002/14651858.CD000371.pub6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Donowitz JR, Alam M, Kabir M, Ma JZ, Nazib F, Platts-Mills JA, et al. A Prospective longitudinal cohort to investigate the effects of early life giardiasis on growth and all cause diarrhea. Clin Infect Dis. 2016;63:792–7. 10.1093/cid/ciw391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simsek Z, Zeyrek FY, Kurcer MA. Effect of Giardia infection on growth and psychomotor development of children aged 0–5 years. J Trop Pediatr. 2004;50:90–3. 10.1093/tropej/50.2.90 [DOI] [PubMed] [Google Scholar]
- 9.Prado MS, Cairncross S, Strina A, Barreto ML, Oliveira-Assis AM, Rego S. Asymptomatic giardiasis and growth in young children; a longitudinal study in Salvador, Brazil. Parasitology. 2005;131:51–6. 10.1017/S0031182005007353 [DOI] [PubMed] [Google Scholar]
- 10.Berkman DS, Lescano AG, Gilman RH, Lopez SL, Black MM. Effects of stunting, diarrhoeal disease, and parasitic infection during infancy on cognition in late childhood: a follow-up study. Lancet. 2002;359:564–71. 10.1016/S0140-6736(02)07744-9 [DOI] [PubMed] [Google Scholar]
- 11.Owada K, Nielsen M, Lau CL, Clements ACA, Yakob L, Soares Magalhaes RJ. Measuring the effect of soil-transmitted helminth infections on cognitive function in children: systematic review and critical appraisal of evidence. Adv Parasitol. 2017;98:1–37. 10.1016/bs.apar.2017.05.002 [DOI] [PubMed] [Google Scholar]
- 12.Stoltzfus RJ, Kvalsvig JD, Chwaya HM, Montresor A, Albonico M, Tielsch JM, et al. Effects of iron supplementation and anthelmintic treatment on motor and language development of preschool children in Zanzibar: double blind, placebo controlled study. BMJ. 2001;323:1389 10.1136/bmj.323.7326.1389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Joseph SA, Casapía M, Montresor A, Rahme E, Ward BJ, Marquis GS, et al. The effect of deworming on growth in one-year-old children living in a soil-transmitted helminth-endemic area of Peru: a randomized controlled trial. PLoS Negl Trop Dis. 2015;9(10):e0004020 10.1371/journal.pntd.0004020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Campbell SJ, Nery SV, Doi SA, Gray DJ, Magalhães RJS, McCarthy JS, et al. Complexities and perplexities: a critical appraisal of the evidence for soil-transmitted helminth infection-related morbidity. PLoS Negl Trop Dis. 2016;10(5):e0004566 10.1371/journal.pntd.0004566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clarke NE, Clements ACA, Doi SA, Wang D, Campbell SJ, Gray D, et al. Differential effect of mass deworming and targeted deworming for soil-transmitted helminth control in children: a systematic review and meta-analysis. Lancet. 2017;389:287–97. 10.1016/S0140-6736(16)32123-7 [DOI] [PubMed] [Google Scholar]
- 16.Jia T-W, Melville S, Utzinger J, King CH, Zhou X-N. Soil-transmitted helminth reinfection after drug treatment: a systematic review and meta-analysis. PLoS Negl Trop Dis. 2012;6(5):e1621 10.1371/journal.pntd.0001621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Campbell SJ, Nery SV, McCarthy JS, Gray DJ, Magalhães RJS, Clements ACA. A critical appraisal of control strategies for soil-transmitted helminths. Trends Parasitol. 2016;32:97–107. 10.1016/j.pt.2015.10.006 [DOI] [PubMed] [Google Scholar]
- 18.Savioli L, Smith H, Thompson A. Giardia and Cryptosporidium join the “Neglected Diseases Initiative”. Trends Parasitol. 2006;22:203–8. 10.1016/j.pt.2006.02.015 [DOI] [PubMed] [Google Scholar]
- 19.Strunz EC, Addiss DG, Stocks ME, Ogden S, Utzinger J, Freeman MC. Water, sanitation, hygiene, and soil-transmitted helminth infection: a systematic review and meta-analysis. PLoS Med. 2014;11(3):e1001620 10.1371/journal.pmed.1001620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patil SR, Arnold BF, Salvatore AL, Briceno B, Ganguly S, Colford JM, et al. The effect of India’s Total Sanitation Campaign on defecation behaviors and child health in rural Madhya Pradesh: a cluster randomized controlled trial. PLoS Med. 2014;11(8):e1001709 10.1371/journal.pmed.1001709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clasen T, Boisson S, Routray P, Torondel B, Bell M, Cumming O, et al. Effectiveness of a rural sanitation programme on diarrhoea, soil-transmitted helminth infection, and child malnutrition in Odisha, India: a cluster-randomised trial. Lancet Glob Health. 2014;2:e645–53. 10.1016/S2214-109X(14)70307-9 [DOI] [PubMed] [Google Scholar]
- 22.Vaz Nery S, Traub RJ, McCarthy JS, Clarke NE, Amaral S, Llewellyn S, et al. WASH for WORMS: a cluster-randomized controlled trial of the impact of a community integrated water, sanitation, and hygiene and deworming intervention on soil-transmitted helminth infections. Am J Trop Med Hyg. 2019;100:750–61. 10.4269/ajtmh.18-0705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crump JA, Mendoza CE, Priest JW, Glass RI, Monroe SS, Dauphin LA, et al. Comparing serologic response against enteric pathogens with reported diarrhea to assess the impact of improved household drinking water quality. Am J Trop Med Hyg. 2007;77:136–41. [PubMed] [Google Scholar]
- 24.Zambrano LD, Priest JW, Ivan E, Rusine J, Nagel C, Kirby M, et al. Use of serologic responses against enteropathogens to assess the impact of a point-of-use water filter: a randomized controlled trial in Western Province, Rwanda. Am J Trop Med Hyg. 2017;97:876–87. 10.4269/ajtmh.16-1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yap P, Utzinger J, Hattendorf J, Steinmann P. Influence of nutrition on infection and re-infection with soil-transmitted helminths: a systematic review. Parasit Vectors. 2014;7:229 10.1186/1756-3305-7-229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2015;505:559–63. 10.1038/nature12820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Glendinning L, Nausch N, Free A, Taylor DW, Mutapi F. The microbiota and helminths: sharing the same niche in the human host. Parasitology. 2014;141:1255–71. 10.1017/S0031182014000699 [DOI] [PubMed] [Google Scholar]
- 28.Arnold BF, Null C, Luby SP, Unicomb L, Stewart CP, Dewey KG, et al. Cluster-randomised controlled trials of individual and combined water, sanitation, hygiene and nutritional interventions in rural Bangladesh and Kenya: the WASH Benefits study design and rationale. BMJ Open. 2013;3:e003476 10.1136/bmjopen-2013-003476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Null C, Stewart CP, Pickering AJ, Dentz HN, Arnold BF, Arnold CD, et al. Effects of water quality, sanitation, handwashing, and nutritional interventions on diarrhoea and child growth in rural Kenya: a cluster-randomised controlled trial. Lancet Glob Health. 2018;6:e316–29. 10.1016/S2214-109X(18)30005-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pilotte N, Papaiakovou M, Grant JR, Bierwert LA, Llewellyn S, McCarthy JS, et al. Improved PCR-based detection of soil transmitted helminth infections using a next-generation sequencing approach to assay design. PLoS Negl Trop Dis. 2016;10(3):e0004578 10.1371/journal.pntd.0004578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ziegelbauer K, Speich B, Mäusezahl D, Bos R, Keiser J, Utzinger J. Effect of sanitation on soil-transmitted helminth infection: systematic review and meta-analysis. PLoS Med. 2012;9(1):e1001162 10.1371/journal.pmed.1001162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gruber S, van der Laan MJ. tmle: an R package for targeted maximum likelihood estimation. J Stat Softw. 2012;51:1–35. 10.18637/jss.v051.i1323504300 [DOI] [Google Scholar]
- 33.Balzer LB, van der Laan MJ, Petersen ML. Adaptive pre‐specification in randomized trials with and without pair‐matching. Stat Med. 2016;35:4528–45. 10.1002/sim.7023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schulz KF, Grimes DA. Multiplicity in randomised trials I: endpoints and treatments. Lancet. 2005;365:1591–5. 10.1016/S0140-6736(05)66461-6 [DOI] [PubMed] [Google Scholar]
- 35.Pocock SJ, Assmann SE, Enos LE, Kasten LE. Subgroup analysis, covariate adjustment and baseline comparisons in clinical trial reporting: current practice and problems. Stat Med. 2002;21:2917–30. 10.1002/sim.1296 [DOI] [PubMed] [Google Scholar]
- 36.Pickering AJ, Arnold BF, Dentz HN, Colford JM, Null C. Climate and health co-benefits in low-income countries: a case study of carbon financed water filters in Kenya and a call for independent monitoring. Environ Health Perspect. 2017;125:278–83. 10.1289/EHP342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bieri FA, Gray DJ, Williams GM, Raso G, Li Y-S, Yuan L, et al. Health-education package to prevent worm infections in Chinese schoolchildren. N Engl J Med. 2013;368:1603–12. 10.1056/NEJMoa1204885 [DOI] [PubMed] [Google Scholar]
- 38.Mahmud MA, Spigt M, Bezabih AM, Pavon IL, Dinant G-J, Velasco RB. Efficacy of handwashing with soap and nail clipping on intestinal parasitic infections in school-aged children: a factorial cluster randomized controlled trial. PLoS Med. 2015;12(6):e1001837 10.1371/journal.pmed.1001837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gyorkos TW, Maheu-Giroux M, Blouin B, Casapía M. Impact of health education on soil-transmitted helminth infections in schoolchildren of the Peruvian Amazon: a cluster-randomized controlled trial. PLoS Negl Trop Dis. 2013;7(9):e2397 10.1371/journal.pntd.0002397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Freeman MC, Clasen T, Brooker SJ, Akoko DO, Rheingans R. The impact of a school-based hygiene, water quality and sanitation intervention on soil-transmitted helminth reinfection: a cluster-randomized trial. Am J Trop Med Hyg. 2013;89:875–83. 10.4269/ajtmh.13-0237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hürlimann E, Silué KD, Zouzou F, Ouattara M, Schmidlin T, Yapi RB, et al. Effect of an integrated intervention package of preventive chemotherapy, community-led total sanitation and health education on the prevalence of helminth and intestinal protozoa infections in Côte d’Ivoire. Parasit Vectors. 2018;11:115 10.1186/s13071-018-2642-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Harris M, Alzua ML, Osbert N, Pickering A. Community-level sanitation coverage more strongly associated with child growth and household drinking water quality than access to a private toilet in rural Mali. Environ Sci Technol 2017;51:7219–27. 10.1021/acs.est.7b00178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Steinbaum L, Mboya J, Mahoney R, Njenga SM, Null C, Pickering AJ. Effect of a sanitation intervention on soil-transmitted helminth prevalence and concentration in household soil: a cluster-randomized controlled trial and risk factor analysis. PLoS Negl Trop Dis. 2019;13(2):e0007180 10.1371/journal.pntd.0007180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oh K-S, Kim G-T, Ahn K-S, Shin S-S. Effects of disinfectants on larval development of Ascaris suum eggs. Korean J Parasitol. 2016;54:103–7. 10.3347/kjp.2016.54.1.103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Olsen A, Thiong’o FW, Ouma JH, Mwaniki D, Magnussen P, Fleischer Michaelsen K, et al. Effects of multimicronutrient supplementation on helminth reinfection: a randomized, controlled trial in Kenyan schoolchildren. Trans R Soc Trop Med Hyg. 2003;97:109–14. 10.1016/s0035-9203(03)90042-3 [DOI] [PubMed] [Google Scholar]
- 46.Luby SP, Rahman M, Arnold BF, Unicomb L, Ashraf S, Winch PJ, et al. Effects of water quality, sanitation, handwashing, and nutritional interventions on diarrhoea and child growth in rural Bangladesh: a cluster randomised controlled trial. Lancet Glob Health. 2018;6:e302–15. 10.1016/S2214-109X(17)30490-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lin A, Ercumen A, Benjamin-Chung J, Arnold BF, Das S, Haque R, et al. Effects of water, sanitation, handwashing, and nutritional interventions on child enteric protozoan infections in rural Bangladesh: a cluster-randomized controlled trial. Clin Infect Dis. 2018;16:87 10.1093/cid/ciy320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pickering AJ, Ercumen A, Arnold BF, Kwong LH, Parvez SM, Alam M, et al. Fecal indicator bacteria along multiple environmental transmission pathways (water, hands, food, soil, flies) and subsequent child diarrhea in rural Bangladesh. Environ Sc Technol. 2018;52:7928–36. 10.1021/acs.est.8b00928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Okoyo C, Nikolay B, Kihara J, Simiyu E, Garn JV, Freeman MC, et al. Monitoring the impact of a national school based deworming programme on soil-transmitted helminths in Kenya: the first three years, 2012–2014. Parasit Vectors. 2016;9:408 10.1186/s13071-016-1679-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Steinbaum L, Njenga SM, Kihara J, Boehm AB, Davis J, Null C, et al. Soil-transmitted helminth eggs are present in soil at multiple locations within households in rural Kenya. PLoS ONE. 2016;11(6):e0157780 10.1371/journal.pone.0157780 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All replication scripts and data are available on Open Science Framework at the following link: https://osf.io/k2s47/.