Abstract
Cardiac disease is a major cause of morbidity and mortality for adult gorillas. Previous research indicates a sex-based difference with predominantly males demonstrating evidence of left ventricular hypertrophy. To evaluate these findings, we analyzed serum markers with cardiac measures in a large sample of gorillas. The study sample included 44 male and 25 female gorillas housed at American Association of Zoo and Aquariums (AZA)-accredited zoos. Serum samples were collected from fasted gorillas during routine veterinary health exams and analyzed to measure leptin, adiponectin, IGF-1, insulin, ferritin, glucose, triglycerides, and cholesterol. Cardiac ultrasonography via transthoracic echocardiogram was performed simultaneously. Three echocardiographic parameters were chosen to assess cardiac disease according to parameters established for captive lowland gorillas: left ventricular internal diameter, inter-ventricular septum thickness, and left ventricular posterior wall thickness. Our data revealed that high leptin, low adiponectin, and lowered cholesterol were significantly and positively correlated with measures of heart thickness and age in males but not in females. Lowered cholesterol in this population would be categorized as elevated in humans. High leptin and low adiponectin are indicative of increased adiposity and suggests a potential parallel with human obesity and cardiovascular disease in males. Interestingly, while females exhibited increased adiposity with age, they did not progress to cardiac disease.
Introduction
Cardiac disease is a major cause of death for gorillas in zoological settings [1, 2]. This is a male-biased disease, characterized by left ventricular hypertrophy and cardiomyopathy, but with an absence of atherosclerosis [1, 3]. In humans, left ventricular dysfunction and clinical heart failure are associated with obesity and insulin resistance [4, 5]. Previous studies demonstrated that both obesity and insulin resistance occur in captive gorillas [6], raising the possibility that these factors are associated with gorilla heart disease. Understanding the epidemiology and risk factors of heart disease in gorillas is critical for managing their health in zoo settings, as their status in the wild in critically endangered [7].
Materials and methods
The study sample included 44 male (ages 4–50 years, mean 21.4 ± 10.99) and 25 female (ages 5 to 51 years, mean 21.3 ± 7.35) gorillas housed at AZA-accredited zoos. This research was reviewed and approved by Cleveland Metroparks Zoo Scientific Review Committee. Serum samples were collected from fasted gorillas during routine veterinary health exams by or under the supervision of a veterinarian. Serum samples were analyzed using enzyme immunoassay using commercially available kits for leptin (Mercodia 10-1199-01), adiponectin (B-Bridge K-1002-1), IGF-1 (IDS AC-27), insulin (Mercodia 10-1132-01), and ferritin (Calbiotech FR065T). Each of these assays was validated for use in gorillas by the Cleveland Metroparks Zoo Endocrinology Laboratory. For each assay, a pooled sample dilution curve was parallel to the standard curve with no difference between slopes (adiponectin t(10) = 1.03, p > 0.05; leptin t(9) = 0.1, p > 0.05; insulin t(9) = 1.43, p > 0.05; IGF-1 t(15) = 0.37, p > 0.05; ferritin t(10) = 0.58, p > 0.05). Recovery for high and low concentrations was > 90% for each concentration and assay. Glucose, triglycerides, and cholesterol were measured using an IDEXX Vettest 8008 Chemistry Autoanalyzer.
Cardiac ultrasonography via transthoracic echocardiogram was performed at the same time using standard protocols [1]. Three echocardiographic parameters were chosen to assess cardiac disease according to parameters established for captive lowland gorillas [1]. These measures were chosen because progressive left ventricular hypertrophy is the predominant finding in heart disease of male gorillas. These were left ventricular internal diameter (LVID), inter-ventricular septum thickness (IVS), and the left ventricular posterior wall thickness (LVPW).
Statistical analyses
For the male and female samples separately, Spearman’s partial correlation coefficients, i.e., controlling for age, were calculated among the six hematology markers as well as the glucose-to-insulin ratio. Using Spearman’s partial correlation coefficients normalized the outliers that were present within the sample. Principal components analyses with varimax rotation were then performed on each Spearman matrix to correct for general skewness and outliers. The major principal components were then analyzed for relationships with echocardiograph measures indicative of heart disease in make gorillas using Pearson’s r correlation analyses.
Results
Levels of blood markers and heart thicknesses, by sex
Males
Summary statistics for all measures are provided in Table 1 and raw data are provided in S1 Table. Principal component (PC) 1 explained 26% of the variation (Table 2). High values of PC1 corresponded to high values of leptin, and low values of both adiponectin and cholesterol. High leptin with low adiponectin is associated with increased adiposity in captive gorillas [8]. Within this sample, mean cholesterol was higher than 200 mg/dl, which is not unusual for this species but higher than what is typically observed in humans. A mean of 284 mg/dl was previously reported for captive gorillas, which was 1.5 times higher than for free-ranging gorillas [9]. Taken together, PC1 is most indicative of elevated adiposity and cholesterol, although decreased relative to the present sample, would still be elevated compared with wild gorilla or normal human populations.
Table 1. Summary data for serum biomarkers and heart measures.
| Males (N = 44) | Females (N = 25) | |
|---|---|---|
| Age (years) | 21.4 ± 10.99 | 21.3 ± 7.35 |
| Serum biomarkers | ||
| Leptin (ng/mL) | 8.51 ± 10.07 | 13.25 ± 9.03 |
| Adiponectin (ng/mL) | 0.89 ± 0.90 | 0.82 ± 0.79 |
| IGF-1 (μg/L) | 369.66 ± 165.59 | 202.02 ± 99.03 |
| Insulin (mU/L) | 8.78 ± 10.29 | 8.13 ± 16.33 |
| Glucose (mg/dL) | 89.16 ± 15.39 | 101.32 ± 33.17 |
| Glucose:Insulin ratio | 43.46 ± 53.91 | 52.87 ± 56.61 |
| Ferritin (ng/mL) | 431.56 ± 208.85 | 667.09 ± 976.74 |
| Cholesterol (mg/dL) | 255.45 ± 63.64 | 217.88 ± 66.03 |
| Triglycerides (mg/dL) | 127.05 ± 64.01 | 162.08 ± 84.31 |
| Heart measurements | ||
| LVID (mm) | 5.89 ± 1.06 | 4.34 ± 0.66 |
| IVS (mm) | 1.47 ± 0.43 | 1.12 ± 0.30 |
| LVPW (mm) | 1.48 ± 0.40 | 1.16 ± 0.31 |
Table 2. Principal component factor loadings (varimax normalized).
| Factor 1 | Factor 2 | Factor 3 | ||||
|---|---|---|---|---|---|---|
| Males | Females | Males | Females | Males | Females | |
| Leptin | 0.67 | 0.79 | 0.08 | 0.19 | 0.57 | 0.15 |
| Cholesterol | -0.80 | -0.75 | -0.09 | 0.47 | -0.02 | -0.11 |
| Adiponectin | -0.68 | -0.67 | 0.02 | -0.43 | -0.06 | 0.28 |
| IGF-1 | 0.16 | 0.03 | 0.86 | 0.83 | -0.20 | 0.33 |
| Ferritin | 0.14 | 0.13 | -0.62 | -0.21 | -0.53 | -0.89 |
| Triglycerides | 0.47 | 0.21 | -0.05 | -0.16 | 0.71 | 0.72 |
| Glucose:Insulin | 0.01 | -0.10 | 0.07 | -0.70 | -0.79 | 0.15 |
| % Variation Explained | 26% | 25% | 16% | 24% | 25% | 22% |
PC2 explained 16% of total blood marker variation, with high values of PC2 reflecting high IGF1 (see Table 2). PC3 explained 25% of the variation. High values of PC3 reflect high triglycerides and a low glucose-to-insulin ratio (See Table 2). Taken together, both PC2 and PC3 are suggestive of dysregulated glucose metabolism as a major component explaining the variation within serum biomarkers in the males.
Principal components were then compared with the echocardiogram measures (Table 3, Figs 1 and 2). In males, PC1 increased with the two heart measures of IVS (Pearson’s r = 0.39, p < 0.01) and LVPW (Pearson’s r = 0.40, p < 0.01) and with age (Pearson’s r = 0.42, p < 0.01; see Table 3, Figs 1 and 2). PC2 correlated positively with age (Pearson’s r = 0.69, p < 0.01), but not with any of the heart measures (all p’s > 0.05). PC3 did not correlate with age or any heart measure (all p’s > 0.05).
Table 3. Pearson correlations of principal components factor loadings with heart measures and age.
The first number is the Pearson correlation coefficient, the second is the significance level (2-tailed).
| LVID | IVS | LVPW | Age | |
|---|---|---|---|---|
| Males Factor 1 | 0.01, p = 0.94 | 0.39, p < 0.01 | 0.40, p < 0.01 | 0.42, p < 0.01 |
| Males Factor 2 | 0.14, p = 0.36 | 0.06, p = 0.70 | 0.20, p = 0.21 | 0.69, p < 0.01 |
| Males Factor 3 | 0.22, p = 0.16 | 0.03, p = 0.87 | 0.04, p = 0.79 | -0.14, p = 0.38 |
| Females Factor 1 | 0.20, p = 0.33 | 0.33, p = 0.11 | 0.28, p = 0.18 | 0.20, p = 0.35 |
| Females Factor 2 | 0.14, p = 0.50 | 0.14, p = 0.51 | 0.11, p = 0.60 | 0.18, p = 0.35 |
| Females Factor 3 | -0.07, p = 0.74 | 0.15, p = 0.48 | 0.17, p = 0.41 | -0.03, p = 0.88 |
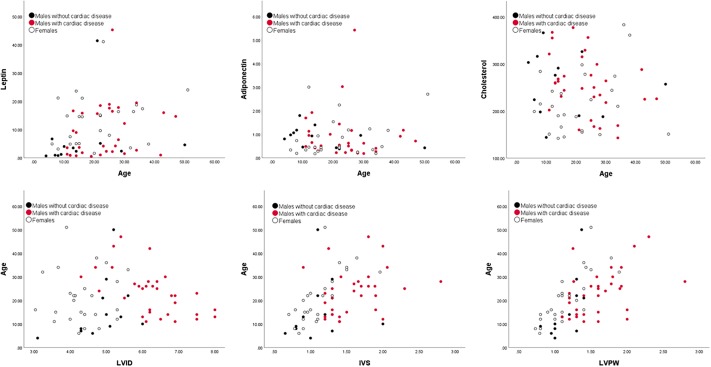
Fig 1. The significant variables for principal component 1 plotted against heart measures.
Leptin, adiponectin and cholesterol are each plotted against heart measures LVID, IVS, and LVPW. For males, principal component 1 was significantly correlated with heart measures IVS and LVPW. Males that were previously diagnosed with heart disease are indicated by red circles, healthy males are depicted by black circles and females by white circles.
Fig 2. The significant variables for principal component 1 plotted against age and age plotted against heart measures.
Leptin, adiponectin and cholesterol are each plotted against age. For males, principal component 1 was significantly correlated with age. Age is also plotted against each heart measure. Males that were previously diagnosed with heart disease are indicated by red circles, healthy males are depicted by black circles and females by white circles.
This multivariate approach appears to identify the leptin/adiponectin ratio coupled with the inverse of cholesterol as a major axis of variation in the blood marker system as well as a primary correlate of heart thickness in male gorillas.
Females
For the sample of 25 adult female gorillas the same multivariate axes were examined. While the sample of females was smaller, the eigen-solutions for the male and female samples were remarkably consistent, which implies that the blood marker inter-correlation structures were similar. Summary statistics can be found in Table 1, raw data are provided in S1 Table.
PC1 explained 25% of the female gorilla blood marker variation. As in males, the high values of PC1 reflected high leptin, low adiponectin, and lowered cholesterol (Table 2). The PC1 pairs of numerical loadings were virtually the same, i.e., the first eigenvectors for males and females represent equivalent principal components. PC2 represented 24% of the variation, and was strongly positively correlated with high IGF1, as was the case of the males. The difference in the correlation structure comparing males and females was that in PC2 the only other large loading for females was the glucose-to-insulin ratio vs. ferritin in males. PC3 loaded the same as males for triglycerides. The only other difference between the sexes was the second highest loading for PC3 was low glucose-to-insulin ratio in males versus low ferritin for females (Table 2, Figs 1 and 2).
While the bivariate and multivariate correlation and principal component structures were very similar in both sexes, there was no significant correlation between the measures of heart disease or age in females (Table 3). Although the age distributions of both males and females were very similar, the heart thicknesses were significantly smaller in females, (Figs 1 and 2); therefore, females simply have not progressed as far as males out on the first component, despite equivalent ages. That is, the primary dimensions of risk of heart disease are similar in both sexes, however for all practical purposes the older females appear to have the cardiac thicknesses of younger males.
Discussion
The present results indicate a strong relationship between adiposity and heart disease in captive male gorillas. Interestingly, females show a similar relationship between age and obesity, yet no association between adiposity and heart disease. In terms of body composition, adiposity refers to the amount of fat an organism possesses [10]. Obesity, it turn, is defined as having excess adipose tissue relative to lean body mass [11]. Accurate measures of body condition account for both body weight and lean mass (i.e., body mass index, BMI), but these measures may not always be easily transferred to nonhuman species. As hindgut fermenters, gorillas possess an extensive colon for processing fiber, making typical measures, such as waist circumference, less useful. Our previous work showed that, for male gorillas, absolute weight was the best predictor of the serum biomarkers of leptin and adiponectin [6, 8]. In females, leptin was positively correlated with hip width, shoulder width, and widest point [8]. Taken together, leptin is the best predictor of body condition in captive gorillas.
In humans, high leptin is associated with cardiac hypertrophy and obesity [12]. Adiponectin protects cardiomyocytes from hypertrophy [13]. We found similar patterns in male gorillas, with high leptin and low adiponectin associated with indicators of left ventricular hypertrophy. While the same patterns of biomarkers were present in female gorillas, there was no association with left ventricular hypertrophy. With left ventricular hypertrophy in humans, independent of blood pressure and other cardiovascular risk factors, women are less prone to LVH than men until women enter menopause [14]. Estrogens attenuate cardiac hypertrophy in mouse model by downregulating prohypertrophic signaling pathways that lead to cardiac hypertrophy [15]. Estrogens may serve to prevent cardiac hypertrophy in female gorillas exhibiting similar conditions to those of males. Testosterone does not provide a cardioprotective mechanism, rather, has been demonstrated to induce cardiac myocyte hypertrophy [16]. In human males, obesity and aging are associated with lower serum testosterone levels [17]. This suggests that high testosterone levels may not be a factor in left ventricular hypertrophy in male gorillas, as it is associated with age and obesity.
The finding of an association between heart disease and lowered serum cholesterol in males is surprising and seemingly in contrast to what is known regarding heart disease in humans. Studies in human health identify hyperlipidemia as a risk factor in cardiovascular disease [18]. Other studies have examined the influence of the gut microbiota on cholesterol metabolism [19]. The gut microbiota are also shown to have significant effect on cardiovascular health [20]. We previously demonstrated that the fecal microbiome of male gorillas with cardiac disease differs from that of healthy males [21]. A future avenue of investigation will focus on the role of the gorilla gut microbiome in cholesterol metabolism in association with heart disease.
Supporting information
All data used for the present analyses are included here. For the column labeled sex, F = female, M = male. Age is provide in years. Units for serum markers and heart measures are provided in Table 1 of the main text.
(XLSX)
Acknowledgments
The authors would like to acknowledge the help and assistance of the Great Ape Heart Project (GAHP) based at Zoo Atlanta and Dr. Marietta Danforth. The GAHP cardiac database was made possible in part by the Institute of Museum and Library Services Grant #LG-26-12-0526-12.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
The Great Ape Heart Project was made possible in part by funding from the Institute of Museum and Library Services Grant #LG-26-12-0526-12 to HM and PMD (https://www.imls.gov). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Murphy HW, Dennis P, Devlin W, Meehan T, Kutinsky I. Echocardiographic parameters of captive western lowland gorillas (Gorilla gorilla gorilla). J Zoo Wildlife Med. 2011;42:572–9. [DOI] [PubMed] [Google Scholar]
- 2.Strong V, Baiker K, Brennan ML, Redrobe S, Rietkerk F, Cobb M, et al. A retrospective review of western lowland gorilla (Gorilla gorilla gorilla) mortality in European zoologic collections between 2004 and 2014. Zoo Wildl Med. 2017;48:277–86. [DOI] [PubMed] [Google Scholar]
- 3.Lowenstine LJ, McManamon R, Terio KA. Comparative pathology of aging great apes: bonobos, chimpanzees, gorillas, and orangutans. Vet Pathol. 2016;53:250–76. 10.1177/0300985815612154 [DOI] [PubMed] [Google Scholar]
- 4.Banerjee S, Peterson LR. Myocardial metabolism and cardiac performance in obesity and insulin resistance. Curr Cardiol Rep. 2007;9:143–9. [DOI] [PubMed] [Google Scholar]
- 5.Tong LJ, Flach EJ, Sheppard MN, Pocknell A, Banerjee AA, Boswood A, et al. Fatal arrythmogenic right ventricular cardiomyopathy in 2 related subadult chimpanzees (Pan troglodytes). Vet Pathol. 2014;51:858–67. 10.1177/0300985813501333 [DOI] [PubMed] [Google Scholar]
- 6.Less EH, Lukas KE, Bergl R, Ball R, Kuhar CW, Lavin SR, et al. Implementing a low-starch biscuit-free diet in zoo gorillas: the impact on health. Zoo Biol. 2014;33:74–80. 10.1002/zoo.21115 [DOI] [PubMed] [Google Scholar]
- 7.Strindberg S, Maisels F, Williamson EA, Blake S, Stokes EJ, Aba’a R, et al. Guns, germs, and trees determine density and distribution of gorillas and chimpanzees in Western Equatorial Africa. Science advances. 2018;4(4):eaar2964 10.1126/sciadv.aar2964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Less EH. Adiposity in captive gorillas (Gorilla gorilla gorilla): the effects of diet and behavior [dissertation]. Case Western Reserve University2012.
- 9.Baitchman EJ, Calle PP, Clippinger TL, Deem SL, James SB, Raphael BL, et al. Preliminary evaluation of bloood lipid profiles in captive western lowland gorillas (Gorilla gorilla gorilla). Zoo Wildl Med. 2006;37:126–9. [DOI] [PubMed] [Google Scholar]
- 10.Frankenfield DC, Rowe WA, Cooney RN, Smith JS, Becker D. Limits of body mass index to detect obesity and predict body composition. Nutrition. 2001;17:26–30. [DOI] [PubMed] [Google Scholar]
- 11.Richardson J, VBeerman K, Heiss C, Shultz J. Comparison of body weight and body fat classifications of competitive school-age club swimmers. J Am Diet Assoc. 2000;100:237–40. 10.1016/S0002-8223(00)00072-9 [DOI] [PubMed] [Google Scholar]
- 12.Hall ME, Harmancey R, Stec DE. Lean heart: Role of leptin in cardiac hypertrophy and metabolism. World Journal of Cardiology. 2015;7:511–24. 10.4330/wjc.v7.i9.511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amin RH, Mathews ST, Alli A, Leff T. Endogenously produced adiponectin protects cardiomyocytes from hypertrophy by a PPARƴ-dependent autocrine mechanism. Am J Physiol Heart Circ Physiol. 2010;299(H690–H698). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Agabiti-Rosei E, Muiesan ML. Left ventricular hypertrophy and heart failure in women. J Hypertens Suppl. 2002;20:S34–S8. [PubMed] [Google Scholar]
- 15.Haines CD, Harvey PA, Leinwand LA. Estrogens mediate cardiac hypertrophy in a stimulus-dependent manner. Endocrinology. 2012;153:4480–90. 10.1210/en.2012-1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duran J, Oyarce C, Pavez M, Valladares D, Basualto-Alarcon C, Lagos D, et al. GSK-3β/NFAT Signaling Is Involved in Testosterone-Induced Cardiac Myocyte Hypertrophy. PloS one. 2016;11:e0168255 10.1371/journal.pone.0168255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fui MNT, Dupuis P, Grossman M. Lowered testosterone in male obesity: mechanisms, morbidity, and management. Asian J Androl. 2014;16:223–31. 10.4103/1008-682X.122365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nelson RH. Hyperlipidemia as a risk factor for cardiovascular disease. Prim Care. 2013;40:195–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kriaa A, Bourgin M, Poiron A, Mkaouar H, Jablaoui A, Gerard P, et al. Microbial impact on cholesterol and bile acid metabolism: current status and future prospects. J Lipid Res. 2019;60:323–32. 10.1194/jlr.R088989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tang WHW, Kitai T, Hazen SL. Gut microbiota in cardiovascular health and disease. Circ Res. 2017;120:1183–96. 10.1161/CIRCRESAHA.117.309715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krynak KL, Burke DJ, Martin RA, Dennis PM. Gut microbiome composition is associated with cardiac disease in zoo-housed western lowland gorillas (Gorilla gorilla gorilla). FEMS Microbiol Lett. 2017;364: 10.1093/femsle/fnx149 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
All data used for the present analyses are included here. For the column labeled sex, F = female, M = male. Age is provide in years. Units for serum markers and heart measures are provided in Table 1 of the main text.
(XLSX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.