Abstract
The adaptability of cassava to low fertile and marginal soils facilitates its production in subsistent agriculture. As a result, smallholder farmers rarely apply fertilizers. The current yield gap is therefore very large, calling for application of fertilizers and soil amendments to improve its productivity. Field experiments were carried out to assess the potential of partially substituting Phosphorus (P) fertilizers by in vitro-produced arbuscular mycorrhizal fungal (AMF) inoculants in cassava production in two agro-ecologies of Nigeria: Northern Guinea Savanna (Samaru) and Sudan Savanna (Minjibir). The experiments were laid out in a split plot design with P levels (0, 17.5, 35 and 52.5 kg P2O5 ha-1) as main plot and AMF inoculants (Control, Glomygel, Glomygel carrier, Mycodrip, Mycodrip carrier) as subplots. The results in Samaru showed that there was significant interaction between AMF and P in root fresh weight, total biomass and root to shoot ratio. The root fresh weights of the inoculated cassava increased proportionally with application of P. However, highest root fresh weight of cassava inoculated with Glomygel was observed at 35 kg P2O5 ha-1 recording 25% yield increase compared to 52.5 kg P2O5 ha-1 application. Interestingly, Cassava inoculated with Glomygel at 17.5 kg P2O5 ha-1 gave root fresh yield statistically similar to where 35 kg P2O5 ha-1 was applied. This represented a 50% reduction in P fertilizer use. Also, cassava inoculated with Glomygel increased leaf nutrient concentrations, which strongly correlated with the root fresh yield. However, no effects of inoculant carriers were observed in yield and nutrient concentrations. Contrarily, there was no significant treatment effect in Minjibir for nearly all the measured parameters. Cassava yield was however, higher in Minjibir than Samaru probably due to soil fertility and structural differences, which resulted in few observable effects of AMF and P treatments at Minjibir. We conclude that under low P conditions inoculation with in vitro produced AMF inoculants could be employed to reduce P fertilizer requirements for cassava and improve yields, but the variability of the responses as a result of soil heterogeneity and the identity of the fungal strain in the inoculant require further investigations before recommending the practice.
Introduction
Phosphorus (P) is one of the most important determinants of plant growth and in most soils it exists in forms that are largely unavailable for plant uptake [1]. Such is the case with Nigerian soils, which are mostly Oxisols, Ultisols and Alfisols [2]. Phosphorus can be as low as 2 mg kg-1 in the savanna soils of Nigeria [3] thus, making P one of the most limiting nutrients in those soils. Kamara et al. [4] reported that P levels were lower than critical values of 7 mg kg-1 (Mehlich-3 extractable P) in 92% and 93% of the fields surveyed in the Northern Guinea savanna and the Sudan savanna of Nigeria, respectively. Overcoming P deficiency in soils of savanna zones of Nigeria is one of the major challenges that smallholder farmers are facing. Therefore, improved P acquisition is essential in order to improve crop yields in extremely low-P conditions [5]. However, inorganic P fertilizers are not easily affordable for resource-constrained farmers who represent the majority of the farming community in sub-Saharan Africa. As a consequence, most farmers often apply rates of P that are too low to obtain a significant yield response. Hence, there is a need to provide cost-effective alternatives in order to increase the adoption of high quality agricultural inputs by reducing the cost of production, while enhancing crop productivity.
Some soil microbial inoculants could serve as possible alternatives as they offer the potential to increase agricultural yields and productivity in low-input systems [6]. Research is revealing the various mechanisms by which soil microbes can stimulate plant productivity [7]. In particular, symbionts of plant roots, namely arbuscular mycorrhizal fungi (AMF), have received considerable attention as a potential low-input solution to increasing the nutrient uptake efficiency of crops. The majority of plant species, including most agricultural crops, enter into symbioses with AMF, exchanging plant sugars for fungal-derived nutrients, such as phosphorus and nitrogen [8]. They increase plant growth and nutrient uptake and can improve plant tolerance to root pathogens and drought [9,10,11]. It has been demonstrated that plants can receive up to 100% of their P via the mycorrhizal pathway, and from 4 to 20% of plant carbon can be transferred to the fungi [12].
Cassava (manihot esculenta Crantz) is reported to be highly tolerant to soils with low levels of P [13]. This is because it can tolerate highly leached soils low in pH, high in exchangeable Al [14]. On the other hand, cassava forms a mutually beneficial association with AMF [15], which allows cassava to absorb sufficient P for healthy growth. Inoculation with AMF can, therefore, lead to growth increases in cassava [16]. According to report by [17], AMF inoculation of cassava can increase fresh root weight by up to 5 t ha-1. There are also several studies in Nigeria that have shown that cassava roots establish associations with AMF [18], while others demonstrated the impact of AMF on cassava growth [19,20]. These studies evidently showed that AMF could play an important role in increasing the sustainability of cassava cropping in Nigeria. However, the inoculants used in these studies were produced in large amounts, requiring much labor, time and space [18,21]. In most cases (e.g. [22]) the inoculum was soil-based, where a suitable trap crop is used to generate AMF propagules, which are produced in the soil. The soil is then used as inoculant. Such inoculants may be low in propagule number and contaminated with other potentially undesirable microorganisms. Another disadvantage of such inoculants is that they may be difficult to transport because of the large volume of the substrate required.
A biotechnological system of AMF production through in vitro cultivation offers unique advantage in producing AMF in sterile medium. Some small enterprises have developed efficient production systems allowing the concentration of very large numbers of AMF propagules in a small volume of sterile medium. This makes the product easy to transport, free of unwanted microorganisms and potentially economically viable for large-scale application [23]. Other advantages of in vitro AMF production technique include more precise quality control such as non-destructive microscopic observations of cultures, uncontaminated molecular analyses and mass production at an industrial scale. An example of in vitro produced AMF is Rhizophagus irregularis, which can be produced at a large scale in a sterile medium. In a field experiment, Ceballos et al. [24] reported that in vitro produced R. irregularis significantly increased cassava yields in Colombian soils by approximately 20% compared to the non-inoculated control. Hence, it is important to examine the potential of this in vitro produced R. irregularis to increase cassava production in Nigerian savanna soils.
While attention was focused more on improvement of cassava yield through inoculation with efficient AMF species, one of the prime factors important for AMF inoculants to be effective is the carrier substrate selected for its mass multiplication and production. As a result, a group of researchers tried different carrier substrates with nutrient solutions. According to Azcon-Aguilar and Barea [25], a careful selection of functionally compatible host/fungus/substrate combination is critical for success. For this reason, we hypothesized that carrier substrates of inoculants used in this study could have influence on the cassava yield. On the other hand, high availability of P could greatly affect the response of cassava to AMF inoculation. While some reports showed that high soil P levels reduce AMF benefit by decreasing their association with plants (e.g. [26]), others indicated that high soil P does not always have a negative effect on AMF [27]. According to Howeler [28], inoculation of cassava with AMF increased plant growth and dry matter production only when P was applied. To this end, there is need for proper diagnosis of soil available P status and judicious combination of P and AMF inoculants in low fertility soils. This is particularly important in regions like sub-Saharan Africa where crop inoculation with AMF inoculants is not common. In this study we, therefore, assessed the potential of combining P fertilization and application of in vitro-produced AMF inoculants to improve cassava productivity in soils of the two regions: Northern Guinea savanna and Sudan savanna of Nigeria. In addition, we tested the effect of inoculant carriers of cassava productivity in the two regions.
Materials and methods
Experimental site description
The experiments were conducted at two sites of the Institute for Agricultural Research (IAR) stations i.e. Samaru and Minjibir. Samaru station is located in the Northern Guinea Savanna of Nigeria at N11°10´31.3´´, E007˚36´38.9´ and 704 m above sea level (masl). The soil of the experimental site belongs to an Alfisol classified as Typic Haplustalf overlying basement complex rocks [29]. The rainy season in Samaru is mono modal with average annual rainfall of 1011 ± 161 mm. The season starts in April and lasts until October, while the dry season is between November and March with mean maximum and minimum temperatures of 32.2°C and 18.9°C respectively. The site had been fallowed for several years and was covered with a variety of weeds and shrubs that are predominantly black doka (Isoberlinia doka). Minjibir station is located in the Sudan Savanna of Nigeria at N12° 08´31. 5´´, E008°40’15.5´´ and 436 masl. The soil belongs to an Alfisol classified as Aeric Halaquept [30]. The rainy season in Minjibir is also mono modal with average annual rainfall ranging between 800 mm and 900 mm. The site has been under cultivation and the previous crop was cowpea (Vigna unguiculata) which was removed after cultivation.
Soil sampling and analysis
Soil samples for pre-planting analyses were taken at three depths: 0-15cm, 15-30cm and 30-45cm. Soil from the field was sampled by randomly selecting points in the field from which soil cores were taken. About 15 to 20 points were considered and soil cores were taken and bulked to make a pooled sample. The pooled soil sample was air dried, crushed and sieved through 2 mm mesh and prepared for laboratory analysis. Also, two profile pits were dug at each site and samples taken from each horizon for field characterization. The physical and chemical analyses were determined using standard methods thus: pH by the potentiometric method as described by [31]; organic carbon by chromic acid digestion [24]; cation exchange capacity (CEC) by saturation with 1 N NH4OAC and extraction of NH4OAC with 2 M KCl [32]; exchangeable acidity by the titration method after extraction with 1 N KCl [32]; ECEC by summation of exchangeable cations (Ca, Mg, K and Na) and exchangeable acidity; particle size analysis by the hydrometer method [33]; soil total nitrogen by digestion through wet oxidation based on Kjeldahl method [34]; and soil available P using Bray-1P [31]. These are reported in S1 Table. Also, approximate soil chemical characteristics based on nutritional requirement of cassava is presented in S2 Table.
Field preparation and layout
The fields were cleared, ploughed, harrowed and ridged, with 75cm spacing, using a tractor to create a fine tilth. The fields were then divided according to the experimental treatments using pegs. There were 160 plots in total (i.e. 2 site, 20 treatments replicated 4 times) and each plot size was 9 m x 8 m. Planting was carried out on 1st August 2014, during the rainy season. The cassava variety planted was TME 419; a high yielding variety released by the International Institute of Tropical Agriculture (IITA) and recommended for both regions. Cassava stem cuttings, approximately 25 cm long, were planted at a 1m x 0.75 m spacing giving a planting density of 13,333 plants per ha. The experiment was arranged in split plot design with phosphorus fertilizer rates as main plot and AMF inoculants as subplot. Treatments included four phosphorus fertilization levels (0, 17.5, 35 and 52.5 kg P2O5 ha-1) and five levels representing AMF inoculants or their carrier minus the fungus in the subplots (Glomygel, Glomygel carrier, Mycodrip, Mycodrip Carrier, and Control) in a full factorial experiment.
Treatment application
The application of fertilizer was based on the recommendation made by the National Root Crop Research Institute (NCRI) of Nigeria, where optimal levels are considered as 90 kg N ha-1, 35 kg P2O5 ha-1, and 75 kg K2O ha-1. Sources for the fertilizers applied during the trials were urea (46% N), Single Super Phosphate (18% P2O5) and Muriate of Potash (60% K2O). While N and K were applied uniformly as recommended, P was applied according to the treatments. The side dressing method for fertilizer application was adopted to ensure adequate fertilization, i.e. the fertilizers were put beside the plant at approximately 5 cm distance from the stem.
Two AMF inoculants, each containing a different isolates of the AMF R. irregularis were produced in vitro, and their carrier substrates were obtained in concentrated form (approximately 1000 spores per plant) and diluted before application. The product names of these inoculants were Glomygel (Mycovitro S.L., Spain) and Mycodrip (Symbiom s.r.o., Czech Republic). Glomygel and its carrier were diluted in water in the ratio inoculant or carrier: water = 1: 3 while the Mycodrip inoculants and its blend carrier were diluted with sterile sand in the ratio inoculant or carrier: sand = 1: 9 (Producer’s recommendations). Inoculation was done at 20 days after planting when the stem cuttings had developed fine roots. This was done by carefully lifting the cassava stem cuttings and applying the inoculants beneath the stem cuttings. Two milliliters of Glomygel suspension, or its carrier, were applied. Two grams of the Mycodrip inoculant, or its carrier, were applied per each cassava stake.
The infective propagule numbers in the experimental soils were determined before inoculation (result is available in S1 Table).
Harvesting
Harvesting was carried out at exactly 12 months after planting when the plants had attained maturity. This was done by hand pulling. A net plot of 4 m x 3 m consisting of 12 plants was marked out in each plot and the plant samples were taken therein. Both fresh weights of the roots, stem and leaves were taken in situ using a field scale. The subsamples of the leaves from each treatment were taken and oven dried at 70°C until constant weight.
Plant tissue analysis
The dried leaf samples were ground, sieved and analyzed for selected nutrient concentrations. The concentrations of total nitrogen and phosphorus in plant tissue were measured in a digest following the methods outlined in [35]. Potassium concentration was measured by complete oxidation of the samples using the Kjeldahl procedure followed by spectrophotometric analysis [35]. Micronutrient concentration (Mn, Cu, Zn and Fe) was measured on dried leaf samples using an atomic absorption spectrophotometer, after being digested as outlined in the procedure by [35].
Percentage root length colonization
Fine root samples of cassava were collected from each replicate of each treatment at harvesting and were washed in running tap water and stored in glass vials containing 50% ethanol for later analysis. The roots were then rinsed with water, at least four times, to remove the ethanol. The percent mycorrhizal colonization in cassava roots was determined according to the method outlined by [36]. The percentage root length colonized by AMF (% RLC) was determined by scoring the presence or absence of AMF crossing a graticule axis. Fifty intersections per slide were observed, counted and recorded. The % RLC was calculated using the following formula (1)
| (1) |
Mycorrhizal response ratio (MR)
The mycorrhizal response ratio (MR) was determined to evaluate the effects of AMF inoculation on harvestable root yield, total plant biomass and leaf nutrient P concentration, using Eqs 2–4, respectively. An MR value > 1 implies an effective inoculation response [6].
| (2) |
| (3) |
| (4) |
Statistical analysis
All data collected were fit into general linear model (GLM) and subjected to analysis of variance using SAS software version 9.4. Where significant F values were observed at a significance level of p < 0.05, a Tukey honest significant difference (HSD) test was used to separate the means. Also, Pearson correlation coefficient was used to examine the relationship between measured parameters. To compare the effects of two locations, the data from two locations were pooled and analyzed (S3 Table)
Result
Soil properties
The fertility trait of the soils was mostly similar except for higher organic matter in the Samaru than Minjibir and the higher available P in Minjibir (S1 Table). Plant available P was higher in Minjibir and was also higher than critical level of 7 mg P kg-1 soil (Bray 1 extract) for cassava production (S2 Table). The soils were moderately acidic, which was in agreement with the general characteristics of an Alfisol soil order of the tropics but within the suitable range for cassava production, as defined by [14]. The total N and organic carbon of both sites were very low (S1 Table). The exchangeable Ca was high in both soils while K fell within the medium classification for cassava production. Only Mg concentration was in the lowest class (S2 Table).
The physical characteristics of the soils showed great variability. The surface soil of Samaru was silt loam in nature and had a high percentage of silt and clay in the subsurface (S1 Table). This indicated that the soil is higher in pore spaces with lower pore volume space and poor drainage, having high clay content and, thus, shallow in nature. These characteristics are conducive to surface run off and waterlogging, and consequently, formation of a hardpan layer, that would restrict cassava root formation. The soil of Minjibir was loamy sand and had a good proportion of soil particles. The soil was deep, friable and well aerated which allows drainage. These attributes are favorable to good cassava root formation.
Yield and growth of cassava
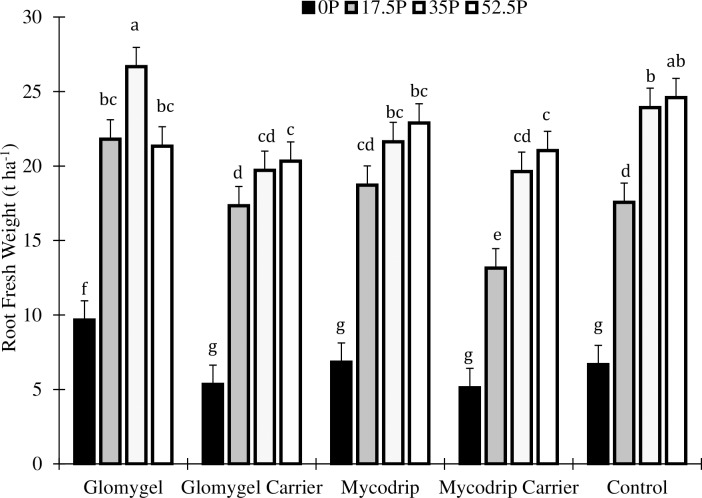
Analysis of the root fresh weight of cassava in Samaru revealed a highly significant interaction between phosphorus (P) and Inoculants (I) on root fresh yield (Table 1). Root fresh yield per hectare was affected by both P fertilization and inoculation with AMF at Samaru (Fig 1). However, the effect of AMF inoculation was not the same in all P fertilization treatments as shown by a significant P x I interaction. For example, inoculation with Glomygel increased cassava yield at all P levels except 52.5 kg P2O5 ha-1 compared to the control and the carrier. At 0 kg P2O5 ha-1, inoculation with Glomygel significantly increased cassava fresh yield over inoculation with all the other inoculants including the control (Fig 1). Here, inoculation with Glomygel increased cassava fresh yield by 81%, 41%, 89% and 45% over the Glomygel carrier, Mycodrip, Mycodrip carrier and the control respectively. Mycodrip carrier appeared to have a negative effect on plant growth compared to Mycodrip or the control.
Table 1. Summary table indicating the main effects of P fertilizers and AMF inoculants on the measured parameter F–values (p–values).
| Samaru | Minjibir | |||||
|---|---|---|---|---|---|---|
| Parameters | Phosphorus (P) | Inoculants (I) | Interaction P x I | Phosphorus (P) | Inoculants (I) | Interaction P x I |
| Root | 315.00 (<0.0001) | 19.66 (<0.0001) | 3.28 (0.0016) | 0.36 (0.78) | 0.13 (0.97) | 1.51 (0.15) |
| Shoot | 32.67 (<0.0010) | 5.82 (0.0007) | 1.20 (0.3119) | 1.51 (0.22) | 0.54 (0.71) | 1.86 (0.06) |
| Root/Shoot | 136.46 (<0.0001) | 3.22 (0.0202) | 2.07 (0.0382) | 0.83 (0.48) | 0.59 (0.67) | 1.57 (0.13) |
| Total Plant | 195.86 (<0.0001) | 16.07 (<0.0001) | 2.35 (0.0182) | 0.56 (0.64) | 0.24 (0.92) | 1.63 (0.11) |
| N concentration | 74.87 (<0.0001) | 5.90 (0.0006) | 1.12 (0.3698) | 0.61 (0.63) | 0.13 (0.97) | 0.49 (0.91) |
| P concentration | 128.75 (<0.0001) | 3.25 (0.0195) | 1.56 (0.1354) | 0.40 (0.75) | 0.28 (0.89) | 0.53 (0.88) |
| K concentration | 6.40 (<0.0001) | 2.65 (0.0447) | 1.56 (0.1368) | 0.66 (0.60) | 0.52 (0.72) | 0.59 (0.84) |
| Zn concentration | 51.27 (<0.0001) | 4.93 (0.0021) | 0.78 (0.6714) | 3.54 (0.0214) | 1.11 (0.36) | 0.81 (0.64) |
| Mn concentration | 80.47 (<0.0001) | 2.05 (0.102) | 1.15 (0.3473) | 1.02 (0.39) | 1.21 (0.32) | 0.62 (0.81) |
| Cu concentration | 15.63 (<0.0001) | 2.17 (0.087) | 1.32 (0.2368) | 2.16 (0.11) | 1.97 (0.11) | 1.20 (0.31) |
| Fe concentration | 48.61 (<0.0001) | 2.05 (0.1026) | 1.61 (0.1206) | 3.96 (0.0133) | 0.35 (0.84) | 1.36 (0.22) |
| PRC | 10.10 (<0.0001) | 5.87 (0.0006) | 1.04 (0.43) | 0.44 (0.72) | 0.24 (0.91) | 0.08 (1.00) |
| MR root | 2.88 (0.0457) | 14.49 (<0.0001) | 2.62 (0.0089) | 0.25 (0.86) | 0.21 (0.93) | 1.60 (0.13) |
| MR biomass | 5.62 (0.0022) | 11.64 (<0.0001) | 1.67 (0.10) | 0.65 (0.59) | 0.25 (0.91) | 1.56 (0.14) |
| MR P concentration | 11.4 (<0.0001) | 0.85 (0.4998) | 1.70 (0.097) | 0.16 (0.93) | 0.13 (0.97) | 0.02 (1.00) |
Sources of variation: P, I, and P × I.; for each measured parameter, the values in front of the brackets are F test-values, while the values between the brackets are p-values
Fig 1. Effects of different levels of P fertilizer application and inoculation with AMF on cassava root fresh weight in Samaru.
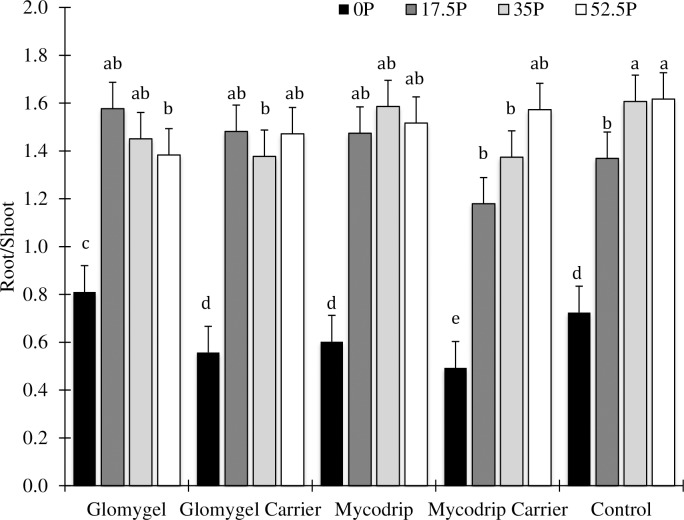
Significant differences in root/shoot ratio were also observed as a result of P fertilization and AMF inoculation in Samaru (Table 1). The highest ratio was obtained when 52.5 kg P2O5 ha-1 was applied (Fig 2). The combinations of 17.5 kg P2O5 ha-1 + Glomygel and 17.5 kg P2O5 ha-1 + Mycodrip were statistically similar in apportioning of biomass compared to when 52.5 kg P2O5 ha-1 was applied (Fig 2). Inoculation with Glomygel at 0 kg P2O5 ha-1 significantly increased the root/shoot ratio over other treatments. Here also the inoculation with Mycodrip carrier recorded lowest yield.
Fig 2. Effects of different levels of P fertilizer application and inoculation with AMF on cassava root: Shoot ratio in Samaru.
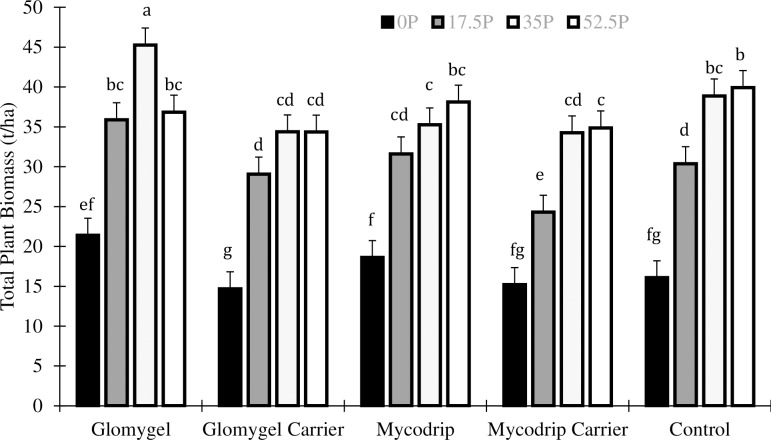
There was an interaction between P and I on total plant biomass which showed that highest total plant biomass yield was obtained with the application of 35 kg P2O5 ha-1 + Glomygel (as in root fresh yield) and this was statistically higher than all other treatments. Application of 17.5 kg P2O5 ha-1 + Glomygel gave a total plant biomass that was statistically similar to 35 kg P2O5 ha-1 (Fig 3). Additionally, the application of 17.5 kg P2O5 ha-1 + Glomygel increased the total plant biomass significantly over application of 17.5 kg P2O5 ha-1 in the control (Fig 3).
Fig 3. Effects of different levels of P fertilizer application and inoculation with AMF on total cassava biomass in Samaru.
Analysis of main and sub effects and the interaction of the treatments in Minjibir were not significant in all the yield components of cassava.
Nutrient concentration
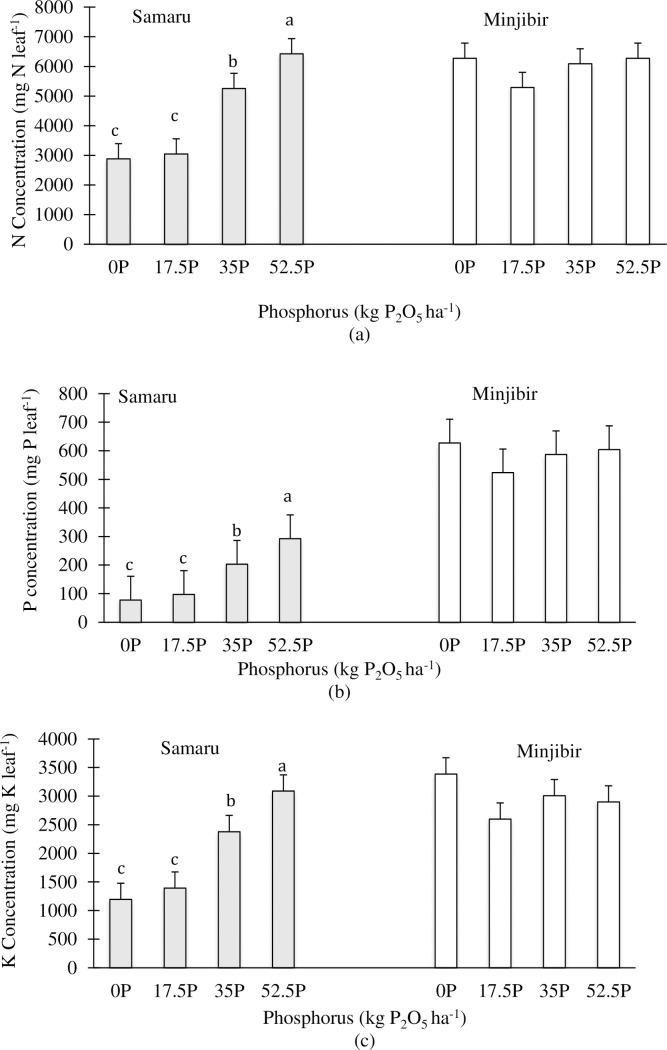
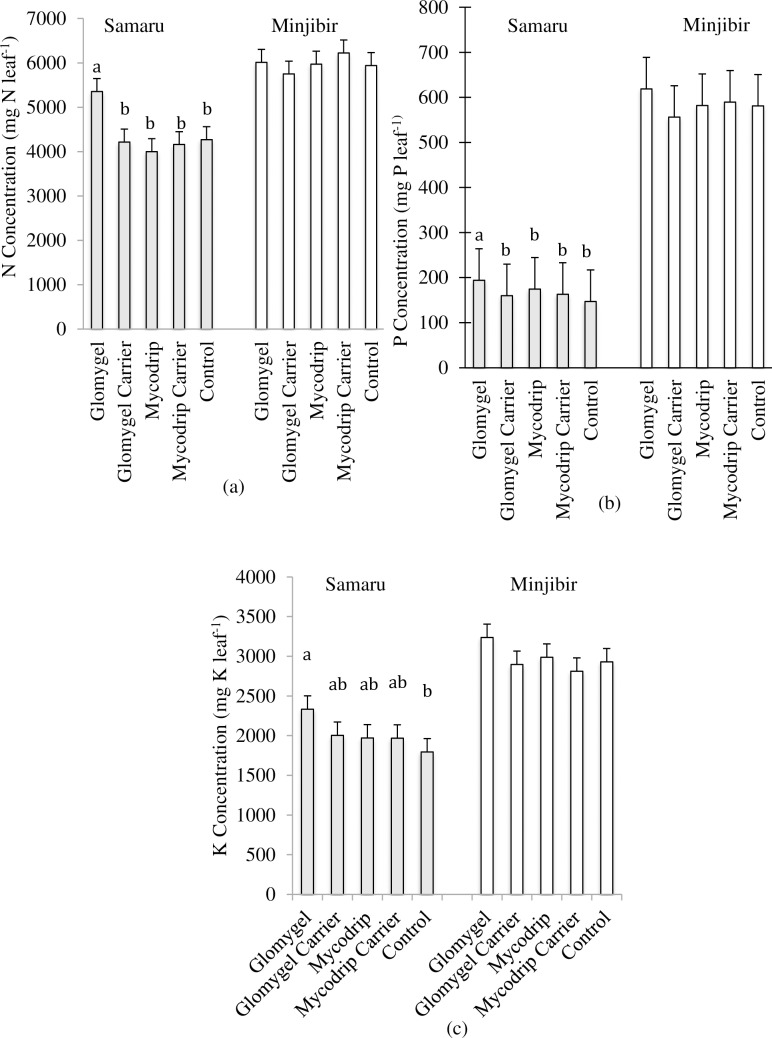
There were no significant P x I interactions in terms of leaf macronutrient concentrations at both sites (Table 1). However, in Samaru, analysis of the main and sub effects showed variation in the leaf macronutrient concentrations (Table 1). Application of P increased the leaf macronutrient concentrations proportional to P application i.e. an increase in P application improved the macronutrient concentration (Fig 4A, Fig 4B, Fig 4C). Increasing P application resulted in increased N leaf concentrations by 6%, 83% and 123% (Fig 4A); P concentration by 25%, 161% and 273% (Fig 4B); and K concentration by 17%, 99% and 159% (Fig 4C) for 17.5 kg P2O5 ha-1, 35 kg P2O5 ha-1 and 52.5 kg P2O5 ha-1 respectively.
Fig 4. Main P treatment effects on leaf macronutrient concentrations in cassava.
(a) Mean leaf N concentration (b) Mean leaf P concentration and (c) Mean leaf K concentration at both trial sites (Samaru and Minjibir) averaged over all the inoculation levels for the four different P fertilizer levels.
Leaf macronutrient concentrations also differed significantly among cassava treated with different inoculants (Table 1). Cassava inoculated with Glomygel resulted in significantly higher N concentration compared with other treatments. The magnitude of difference in N concentration between cassava inoculated with Glomygel and other treatments was 27% more than the Glomygel carrier, 34% more than Mycodrip, 29% more than the Mycodrip carrier and 25% more than control (Fig 5A). A similar trend was observed in both P and K concentrations although Glomygel was not statistically different from Mycodrip (Fig 5B and Fig 5C). Application of P at 52.5 kg P2O5 ha-1 resulted in significantly higher macronutrient leaf concentration when compared with application of 35 kg P2O5 ha-1 and 17.5 kg P2O5 ha-1. Also, application of 35 kg P2O5 ha-1 resulted in significantly different micronutrient concentrations to plants with 17.5 kg P2O5 ha-1; but there was no difference between application of 17.5 kg P2O5 ha-1 and 0 kg P2O5 ha-1. On the other hand, there was a significantly different leaf N and K concentrations of cassava inoculated with Glomygel compared to plants inoculated with Mycodrip. There were no significant effects on the leaf macronutrient concentrations as a result of P applications and AMF inoculation in Minjibir (Table 1).
Fig 5. Main inoculation treatment effects on leaf macronutrient concentrations in cassava.
(a) Mean leaf N concentration (b) Mean leaf P concentration and (c) Mean leaf K concentration at both trial sites ((Samaru and Minjibir) averaged over all P treatment- levels for the five inoculation levels.
There were no significant P x I interactions in micronutrient concentration at both locations (Table 1). However, P application increased micronutrient concentration at Samaru. Significant variation was also found in the leaf micronutrient concentrations as a result of AMF inoculation. Inoculation with Glomygel resulted in a significantly higher Zn concentrations compared to inoculation with Mycodrip and the carrier materials (Table 1). In Minjibir, analysis of Zn and Fe concentrations recorded a significant difference between the levels of P where application at 17.5 kg P2O5 ha-1 recorded lower concentration compared to other treatments (Table 1).
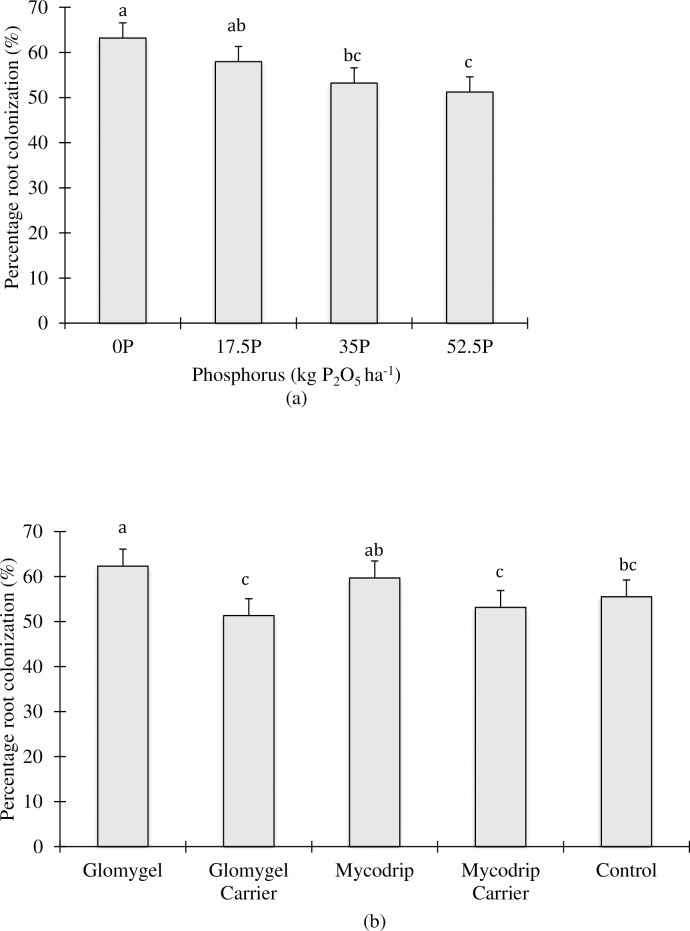
Percentage root colonization (PRC)
Analysis of PRC showed that there was no significant P x I interaction in both locations. However, in Samaru, main- and sub- treatment effects showed that there were variations among the treatments (Table 1; Fig 6A). There were also variations in PRC as a result of AMF inoculations (Table1; Fig 6B). Inoculation with Glomygel recorded a significantly higher PRC over its carrier and control while inoculation with Mycodrip recorded a significantly higher PRC than its carrier though not statistically different from control (Fig 6B). There was no significant PRC recorded in Minjibir at both main and sub treatment effects (Table 1).
Fig 6. Percentage of root length colonized by AMF in cassava at the Samaru trial site.
(a) Main P treatment effect; (b) main inoculation treatment effect.
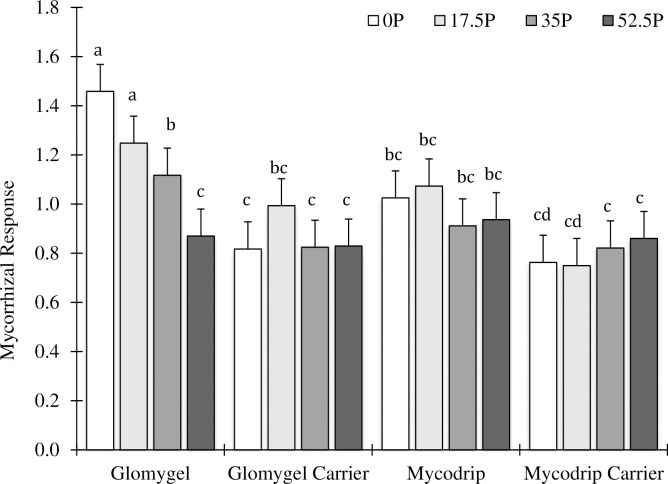
Mycorrhizal response (MR) ratio
Analysis of MR of root fresh yield in Samaru showed that there was significant P x I interaction (Table 1; Fig 7). Here, application of 0 kg P2O5 ha-1 + Glomygel recorded highest MR. Also, combination of 17.5 kg P2O5 ha-1 + Glomygel and 35 kg P2O5 ha-1 + Glomygel recorded MR that is above a unity. Similar trend was observed in Mycodrip in which the combination 17.5 Kg P2O5 ha-1 + Mycodrip was found to be greater than a unity (Fig 7). There were no P x I interactions in both biomass and P concentration in Samaru (Table 1). There were no P x I interactions recorded in MR ratios (root yield, biomass and P concentration) in Minjibir (Table 1). Analysis of the main and sub-treatment effects in Samaru however, showed that there was significant difference in MR in the total plant fresh biomass where application of 0 and 17.5 Kg P2O5 ha-1 showed higher MR when compared with application of 35 Kg P2O5 ha-1 and 52.5 Kg P2O5 ha-1. No main and sub effects were recorded in Minjibir for MR.
Fig 7. Mean mycorrhizal response of cassava for the different inoculation levels at Samaru across the different P levels.
Correlation between the measured parameters
Correlation analysis was carried out to relate the measured variables with one another (S4 Table). In Samaru, high correlations between yield components of cassava (root fresh weights, shoot fresh weights and root/shoot ratio) and leaf nutrient concentrations were highly significant (S4 Table). Moreover, PRC significantly correlated with the leaf nutrient concentrations showing higher nutrient uptakes with more PRC. Also, significant (p<0.05) correlation between PRC and MR root fresh yield (r = 0.216*) was recorded which shows there was increased harvestable root as a result of increased PRC.
Discussion
Site effect of co-applied P fertilizer and in-vitro AMF on cassava yield
The response of cassava to co-application of P and AMF inoculants was large in Samaru. However, in Minjibir there were almost no effects of P fertilization or AMF inoculation for almost all of the variables. Yield was much higher in Minjibir irrespective of treatment application, indicating that soil fertility at this site is much better than Samaru for cassava cultivation. The disparity in effects of the treatments between the two locations is likely to be the result of inherent differences in the properties of the soils such as texture, structure or compaction (S1 Table). The higher yield obtained in Minjibir was likely due to the fact that the soil was light textured, deep and better drained than Samaru. The soil was moderately suitable due to its medium level of soil chemical characteristics in comparison with the nutrient requirement for cassava production, as reported by [14]. The variability of the biological, physical and chemical properties of soil is a major reason for the variability of cassava production [37,38]. In comparison, the Samaru soil was less suitable as it was shallow in nature, having higher silt and clay contents (S1 Table) that would result in high susceptibility to compaction. Soil physical characteristics are likely to affect the development of cassava variety TME 419, which will produce tuberous roots to 1 m deep under favorable conditions such as those found at the site in Minjibir.
Effect of co-applying P fertilizer and in vitro AMF inoculants on cassava yield
The application of P fertilizer and the AMF inoculants in Samaru showed that the maximum cassava yield could be obtained by applying 66% of the full recommended P fertilization and one of the in vitro AMF inoculants (Glomygel). This yield was statistically similar to the situation where full P fertilizer was applied with no AMF inoculant (Fig 1). This shows that the recommended P fertilizer rate can be reduced (representing 33.3% P fertilizer reduction) in combination with one of the in vitro AMF inoculants in some soils. This is an important result, which indicates that farmers in the region can potentially use these inoculants to reduce P fertilizer usage. This finding is in line with a report by [23] who found that in vitro produced AMF could be used as an addition in order to reduce P fertilizer application.
The results showed that cassava yield in Samaru was increased by Glomygel at all levels of P fertilization, except at the recommended dose of P. Cassava that was not inoculated actually had a similar yield at the full recommended level of P application compared to the 35 kg P2O5 ha-1 level. This indicates that at this location, 66.6% of the recommended application of P fertilizer represents a more realistic optimum for growing cassava and that within this range of P fertilization, the AMF inoculant Glomygel, enhanced yields. This means that if the farmer inoculates the crop with AMF but does not apply any P fertilizer, or only a small amount of P fertilizer, then it is possible to get a significant growth response.
The mycorrhizal responsiveness of cassava to inoculation with the fungus differed according to the P fertilization treatment. When cassava was inoculated with Glomygel, the mycorrhizal responsiveness (essentially a measure of the efficacy of inoculation) was proportionally greater at no or low P fertilization than at higher P fertilization levels (Fig 7), even though overall yield of cassava at low P application was lower than at high P application. This would be expected given that there are many reports in literature that higher soil P levels reduce the mycorrhizal benefit by decreasing the AMF association with plants [26, 39, 40]. It was reported by [41] that formation and growth of AMF might only be negatively affected at high P supply levels when P is applied homogenously to the soil in pot experiments under controlled conditions, but this experiment indicates that such an effect is observable in field conditions. In this experiment, indeed, colonization of the cassava roots by AMF decreased with increasing P application in field conditions.
The two inoculants both contained the same species of fungus, but were different strains. The Mycodrip inoculant gave significantly higher cassava yields at some P fertilization levels than the Mycodrip carrier. However, the experiments showed a clear negative effect of the carrier alone (without AMF) on the yield of cassava. Thus, it may be that the fungal strain in Mycodrip also positively affects cassava growth but that the effect of the fungus was masked by the negative effect of the carrier.
It is impossible in these experiments to know the mechanism by which inoculation with Glomygel lead to an increase in cassava yield under the field conditions at Samaru. Irrespective of inoculation, cassava is naturally colonized by the local community of AMF. Thus, the effects of inoculation could either be due to a direct effect of the inoculated fungal strain on plant growth and P acquisition or through an indirect effect of the inoculated strain on the local AMF community or the other components of the soil microbiome [16]. Inoculation with Glomygel led to significantly higher levels of AMF colonization in cassava roots compared to the control or the carrier alone but we cannot say whether this was an increase in the colonization of cassava roots by the inoculant fungus or other members of the local AMF community. At present, molecular markers do not exist to track specific AMF individuals of this fungus in the roots, although with the generation of population genomic data on genetic variation in this fungal species this may be possible in the future [42].
Effects of co-applying P fertilizer and in vitro AMF inoculants on cassava nutrient concentration
One notable feature of the results was that cassava root yield and overall biomass accumulation at Samaru was affected by both P application and in vitro AMF inoculants, but that these two factors interacted with each other. Nutrient concentration was also affected by these two factors but strikingly these two factors did not interact. Increasing P application resulted in the concentration of increasingly more nutrients irrespective of the inoculation treatments. Inoculation with AMF only resulted in a significantly higher uptake of nutrients in the treatment with Glomygel, irrespective of P treatment.
Conclusion
Co-application of P fertilizer and in vitro AMF inoculants in the Northern Guinea Savanna (Samaru) and Sudan Savanna (Minjibir) of Nigeria showed a strong site effect demonstrating the need of local adaptation of the practices. Such a strong site effect also called for further investigation to identify AMF strains that could either perform at scale or specific to various locations. In the Samaru site, co-application of P and in vitro produced AMF led to improved yield components of cassava and at 66% of the current recommended P rate for cassava in the region in the presence of Glomygel. The yield increase offered by Glomygel at Samaru with the same level of P fertilization was up to 12% yield increase, which represents a significant yield increase for the farmer. Given that the inoculant is very easy to apply at planting, inoculation requires little extra labor cost for the farmer or special management throughout the crop cycle. Validation of this finding across the Northern Guinea Savanna of Nigeria and beyond and local availability of AMF inoculants would be important, as it would represent a significant cost reduction on P fertilizer for the resource-constrained farmers who represent the majority of the farming community in the region. This finding is crucial to establish potential local formulation of AMF inoculants using such strains, which could improve local availability of the products. It would be important to assess the reproducibility of the findings at a larger scale and in time using for instance various AMF strains to better inform the incorporation of effective AMF inoculants in integrated soil fertility management to improve cassava production in sub-Saharan Africa in general, and in Nigeria in particular.
Supporting information
A. Phosphurus levels: P0 = 0 kg P2O5 ha-1; P50 = 17.5 kg P2O5 ha-1; P100 = 35 kg P2O5 ha-1; P150 = 52.5 kg P2O5 ha-1. B. Arbuscular Mycorrhical Fungi levels: AMF + 1 = Glomygel Inoculant; AMF– 1 = Glomygel Carrier; AMF + 2 = Mycodrip Inoculants; AMF– 2 = Mycodrip Carrier; Control = absence of inoculant and carrier.
(TIF)
The infective propagule was determined at soil depth of 0–5 cm.
(DOCX)
Adapted from [43]
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
The authors would like to express their sincere gratitude to the International Institute of Tropical Agriculture (IITA) for sponsoring this work under the auspices of COMPRO II project. We thank the University of Lausanne for buying and providing the required inoculum.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This work was supported by the Bill and Melinda Gates Foundation (OPPGD1398).
References
- 1.Zafar M, Abbasil MK, Rahim N, Khaliq A, Shaheen A, Jamil M et al. (2011) Influence of integrated phosphorus supply and plant growth promoting rhizobacteria on growth, nodulation, yield and nutrient uptake in Phaseolus vulgaris. Afr J Biotech 10(74): 16793–16807. [Google Scholar]
- 2.Tsado PA, Osunde OA, Igwe CA, Adeboye MKA, Lawal BA (2012) Phosphorus sorption characteristics of some selected soil of the Nigerian Guinea Savanna. Int J Agri Sci 2(7): 613–618. [Google Scholar]
- 3.Nwoke OC, Vanlauwe B, Diels J, Sanginga N, Osunbi O (2004) The distribution of phosphorus fractions and desorption characteristics of some soils in the moist savanna zone of West Africa. Nut Cycl Agroecosyst 69: 127–141. [Google Scholar]
- 4.Kamara AY, Kwari J, Ekeleme F, Omoigui L, Abaidoo RC (2008) Effect of phosphorus application and soybean cultivar on grain and dry matter yield of subsequent maize in the tropical savannas of north-eastern Nigeria. Afr J Biotech 7(15): 2593–2599. [Google Scholar]
- 5.Xiurong W, Qiang P, Fengxian C, Xiaolong Y, Hong L (2011) Effects of co-inoculation with arbuscular mycorrhizal fungi and rhizobia on soybean growth as related to root architecture and availability of N and P. Mycorrhiza 21: 173–181. 10.1007/s00572-010-0319-1 [DOI] [PubMed] [Google Scholar]
- 6.Lekberg Y, Koide RT (2005) Is plant performance limited by abundance of arbuscular mycorrhizal fungi? A meta-analysis of studies published between 1988 and 2003. New Phytol. 168: 189–204. 10.1111/j.1469-8137.2005.01490.x [DOI] [PubMed] [Google Scholar]
- 7.Van der Heijden MGA, Bardgett RD, Van Straalen NM (2008) The unseen majority: soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol Lett 11: 296–310. 10.1111/j.1461-0248.2007.01139.x [DOI] [PubMed] [Google Scholar]
- 8.Verbruggen E, Van der Heijden MGA, Rillig MC, Kiers ET (2012) Mycorrhizal fungal establishment in agricultural soils: factors determining inoculation success: Min Rev New Phytol pp 1–6. [DOI] [PubMed] [Google Scholar]
- 9.Arumugam R, Rajasekaran S, Nagarajan SM (2010) Response of Arbuscular mycorrhizal fungi and Rhizobium inoculation on growth and chlorophyll content of Vigna unguiculata (L) Walp. J Appl Sci Environ Man 14(4): 113–115. [Google Scholar]
- 10.Auge RM (2001) Water relations, drought and vesicular-arbuscular mycorrhizal symbiosis. Mycorrhizae 11: 3–42. [Google Scholar]
- 11.Smith SE, Read DJ (2008). Mycorrhizal Symbiosis, 3rd Edn London: Academic. [Google Scholar]
- 12.Cavagnaro T.R. (2008). The role of arbuscular mycorrhzas in improving plant zinc nutrition under low soil zinc concentrations: a review. Plant Soil. 304, 315–325. [Google Scholar]
- 13.FAO (2013). Cassava: A guide to sustainable production intensification In Howeler R.H., Lutaladio N. and Thomas G. (eds). Food and Agricultural organization of United Nations. Rome; pp 15–19. [Google Scholar]
- 14.Howeler RH (2002) Cassava mineral nutrition and fertilization In: Hillocks RJ, Thresh MJ, Bellotti AC (Eds). Cassava: Biology, Production and Utilization. CABI Publishing Wallingford, Oxon, UK: pp 115–147. [Google Scholar]
- 15.Howeler RH (2012) Importance of mycorrhiza for phosphorus absorption by cassava In: Howeler RH (Ed). The Cassava Handbook–A reference manual based on the Asian regional cassava training course, held in Thailand. Cali, Colombia, CIAT; pp 497–523. [Google Scholar]
- 16.Rodriguez A, Sanders IR (2015) The role of community and population ecology in applying mycorrhizal fungi for improved food security. Int Soc Microbiol Ecol 9: 1053–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sieverding E (1991) Vesicular-arbuscular mycorrhiza management in tropical agrosystems. Eschborn, Germany: Deutche Gesellschaft für Technische Zusammenarbeit (GTZ). 371 p. [Google Scholar]
- 18.Oyetunji OJ, Ekanayake IJ, Osonubi O (2007) Chlorophyll Fluorescence Analysis for Assessing Water Deficit and Arbuscular Mycorrhizal Fungi (AMF) Inoculation in Cassava (Manihot esculenta Crantz). Adv Biol Res 1(3–4): 108–117. [Google Scholar]
- 19.Liasu MO, Atayese MO, Osonubi O (2006) Effect of mycorrhiza and pruning regimes on seasonality of hedgerow tree mulch contribution to alley-cropped cassava in Ibadan, Nigeria. Afr J Biotech 5: 1341–1349. [Google Scholar]
- 20.Oyetunji OJ, Osonubi O, Ekanayake IJ (2003) Contribution of an alley cropping system and arbuscular mycorrhizal fungi to maize productivity under cassava intercrop in the derived savannah zone. J Agric Sci 140: 1–6. [Google Scholar]
- 21.Okon IE, Solomon MG, Osonubi O (2010) The Effects of Arbuscular Mycorrhizal Fungal Inoculation and Mulch of Contrasting Chemical Composition on the Yield of Cassava under Humid Tropical Conditions. Sci World J 10: 505–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dare MO, Abaidoo R, Fagbola O, Asiedu R (2013) Diversity of arbuscular mycorrhizal fungi in soils of yam (Dioscorea spp.) cropping systems in four agroecologies of Nigeria. Arch Agron Soil Sci 59(4): 521–531. [Google Scholar]
- 23.Ceballos I, Ruiz M, Fernandez C, Pena R, Rodriguez A, Sanders IR (2013) The in vitro mass-prodcued model mycorrhizal fungus, Rhizophagus irregularis, significantly increases yields of the globally important food security crop cassava. PLOS One 8(8): 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heanes DL (1984). Determination of total organic carbon in soils by an improved chromic acid digestion and spectrophotometric procedure. Commun Soil Sci Plant Anal 15: 1191–1213. [Google Scholar]
- 25.Azcon-Aguilar C, Barea JM (1997) Applying mycorrhiza biotechnology to horticulture: significance and potentials. Sci Hort 68: 1–24. [Google Scholar]
- 26.Liu W, Zhang Y, Jiang S, Deng Y, Christie P, Murray PJ et al. (2016) Arbuscular mycorrhizal fungi in soil and roots respond differently to phosphorus inputs in an intensively managed calcareous agricultural soil. Sci. Rep 6, 24902; 10.1038/srep24902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gosling P, Andrew M, Maude P, Hammond JP, Bending GD (2013) Contrasting arbuscular mycorrhizal communities colonizing different host plants show a similar response to a soil phosphorus concentration gradient. New Phytol 198: 546–556. 10.1111/nph.12169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Howeler RH, Edwards DG, Asher CJ (1988) The Effect of Soil Sterilization and Mycorrhizal Inoculation on the Growth, Nutrient Uptake and Critical Phosphorus Concentration of Cassava. Intern symp trop root tuber crops. pp 519–537. [Google Scholar]
- 29.Ogunwole JO, Babalola OA, Oyinlola EY, Raji BA (2001) A pedological characterization of soils in the Samaru area of Nigeria. Sam J Agric Res 17: 71–77. [Google Scholar]
- 30.Malgwi WB (2001) Characterization of salt affected soils in some selected locations in the North Western zone of Nigeria. Unpublished PhD Thesis Ahmadu Bello University Zaria, 230pp.
- 31.Anderson JM, Ingram, JSI (1993) A handbook of Method: Tropical soil Biology and Fertility. 2nd edition. pp 82–87.
- 32.TSBF. (1993) A handbook of methods. pp 221.
- 33.Bouyoucos GH (1951) A recalibration of the hydrometer for making mechanical analysis of soils. Agron J 43: 434–438. [Google Scholar]
- 34.Bremner JM, Mulvaney CS (1982) Nitrogen total. In Page AL, Milland RH, Keeney DR (Eds.). Methods of soil analysis. Part 2, Agron J 9: 595–624. [Google Scholar]
- 35.Okalebo JR, Gathua KW, Woomer PL (2002) Laboratory methods of soil and plant analyses: A working manual TSBF UNESCO-ROSTA. pp 22–25.
- 36.Brundrett M, Piche Y, Peterson L (1994) A new method for obverting the morphology of VAM. Can J Bot 62: 2128–2134. [Google Scholar]
- 37.Anikwe MAN, Obi ME (1999) Evaluation of an index model for quantifying the productivity of soils in southeastern Nigeria using maize as a test crop. J Agric Environ 1: 80–89. [Google Scholar]
- 38.Rodríguez J, González AM, Leiva FR, Guerrero L (2008) Fertilización por sitio específico en un cultivo de maíz (Zea mays L.) en la Sabana de Bogotá. Agron Colomb 26(2): 308–321. [Google Scholar]
- 39.Liu A, Hamel C, Hamilton R, Smith D (2000) Mycorrhizae formation and nutrient uptake of new corn (Zea mays L.) hybrids with extreme canopy and leaf architecture as influenced by soil N and P levels. Plant Soil 221: 157–166. 10.1023/A:1004777821422 [DOI] [Google Scholar]
- 40.Vierheiling H (2004) Regulatory mechanisms during the plant- arbuscular mycorrhizal fungus interaction. Can J Bot 82(8): 1166–1176. [Google Scholar]
- 41.Olsson PA, Bååth E, Jakobsen I (1997) Phosphorus effects on the mycelium and storage structures of an arbuscular mycorrhizal fungus as studied in the soil and roots by analysis of fatty acid signatures. Appl Environ Microbiol 63: 3531–3538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Savary R, Masclaux FG, Wyss T, Droh G, Cruz Corella J, Machado AP et al. (2018) A population genomics approach shows widespread geographical distribution of cryptic genomic forms of the symbiotic fungus Rhizophagus irregularis. ISME J 12: 17–30. 10.1038/ismej.2017.153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ande OT (2011) Soil suitability evaluation and management for cassava production in the derived savanna area of south western Nigeria. Int J Soil Sci 6(2): 142–149. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A. Phosphurus levels: P0 = 0 kg P2O5 ha-1; P50 = 17.5 kg P2O5 ha-1; P100 = 35 kg P2O5 ha-1; P150 = 52.5 kg P2O5 ha-1. B. Arbuscular Mycorrhical Fungi levels: AMF + 1 = Glomygel Inoculant; AMF– 1 = Glomygel Carrier; AMF + 2 = Mycodrip Inoculants; AMF– 2 = Mycodrip Carrier; Control = absence of inoculant and carrier.
(TIF)
The infective propagule was determined at soil depth of 0–5 cm.
(DOCX)
Adapted from [43]
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.