Opinion statement
Patients with either primary or metastatic brain tumors quite often have cognitive impairment. Maintaining cognitive function is important to brain tumor patients and a decline in cognitive function is generally accompanied by a decline in functional independence and performance status. Cognitive decline can be a result of tumor progression, depression/anxiety, fatigue/sleep dysfunction, or the treatments they have received. It is our opinion that providers treating brain tumor patients should obtain pre-treatment and serial cognitive testing in their patients and offer mitigating and therapeutic interventions when appropriate. They should also support cognition-focused clinical trials.
Keywords: Cognition, Brain, Radiation, Neuropsychology, Memory, Attention
Introduction
Cognitive abilities in brain tumor patients can be affected by a myriad of factors: the tumor itself, depression and anxiety, fatigue, sleep dysfunction, pre-brain tumor cognitive baseline (premorbid functioning), pain, and brain tumor treatments themselves (surgery, chemotherapy, and radiation). Most often, attention, working memory, and information processing speed are affected but patients can present with a wide array of cognitive symptoms [1].
Radiation-induced cognitive decline (RICD) is considered a late effect of radiation therapy (RT) occurring in 30% or more of patients alive at 4 months after partial or whole brain irradiation. For those living over 6 months, that number may rise to 50% [2, 3]. Patients with RICD may be unable to continue working and in severe cases may not be able to live independently. Memantine, donepezil, methylphenidate, and Ginkgo biloba have all been utilized as mitigating pharmacologic strategies with modest levels of success [4••, 5••, 6•, 7]. Neurocognitive rehabilitation has been explored as a non-pharmacologic intervention [8•]. Preventative strategies include using radiosurgery (SRS) when appropriate for patients with brain metastases or whole-brain RT with hippocampal avoidance. Cytoprotective agents under investigation include ramipril, fenofibrate, tamoxifen, indomethacin, and pioglitazone [9–14]. Here, we review the pathogenesis, diagnosis/classification, and management of RICD and discuss strategies used to minimize its risk.
Pathogenesis
Microvascular damage, demyelination, direct damage to neurons and supportive brain parenchymal cells, stem-cell depletion, and changes in the brain microenvironment have all been reported as components of radiation-induced brain injury (RIBI), the histopathologic correlate of RICD [2, 15–38]. RIBI is characterized by multifocal white matter necrosis, neuro-inflammation with reactive microglia and astrocyte activation, and cerebrovascular injury. The cerebrovascular injury creates blood-brain-barrier disruption with perivascular edema, perivascular extracellular matrix accumulation, and haphazard microvascular proliferation. Recent evidence indicates chronic oxidative stress and persistent neuro-inflammation after RT plays a key role in RIBI [13, 39–51]. Inflammation-associated histologic findings include diffuse microglial activation, microglial aggregation at sites of necrosis, and mononuclear perivascular cuffing.
Initially, RICD was heavily attributed to depletion of neural stem cells in the subventricular zone of the hippocampus. More recently, there has been a growing appreciation for a neuroanatomical target theory which suggests that different regions or substructures within the brain hold unique significance relative to cognitive function and may have differing thresholds for radiation damage [52•].
Presentation and diagnosis
RICD is a clinical diagnosis and a diagnosis of exclusion. Disease progression, mental health comorbidity, severe fatigue, medication- or infection-induced delirium, and other forms of dementia such as Alzheimer’s disease and vascular dementia must be ruled out.
The early-delayed effects of radiation manifest between 1- and 6-month post-RT and may be reversible. They are thought to be a consequence of transient demyelination. Late RICD occurs 6 months or later after RT and is considered progressive and irreversible. It is often characterized by problems with working memory, attention, executive function, cognitive flexibility, and processing speed. Radiographic evidence of white matter damage can support the diagnosis of RICD [53–60]. Hallmark radiographic changes include periventricular hyperintensities on T2-weighted magnetic resonance imaging (MRI) [61], increases in diffusivity, and decreases in fractional anisotropy on diffusion tensor MRI [62–64], and periventricular and subcortical low-density lesions detectable on computed tomography [65]. The degree of radiologically detectable white matter injury is correlated with the extent of cognitive decline.
Neuropsychological assessment is often the key to diagnosing and managing RICD. The Mini-Mental Status Exam (MMSE) was used in some older clinical trials reporting cognitive outcomes in treated brain tumor patients but was subsequently shown to have inadequate sensitivity compared to comprehensive neuropsychological testing [66]. Physicians should refer their patients to board-certified (or board-eligible) neuropsychologists who have experience working with brain tumor patients.
The value of neuropsychological evaluation
Ideally, neuropsychological assessment occurs at the time of initial diagnosis and before any treatments (including surgery) have been initiated. Cognitive evaluation prior to resection can predict patient outcomes [1] and can help determine whether a preoperative functional MRI, intraoperative language mapping, or transcranial magnetic stimulation may be useful [67]. After treatment with surgery, radiation, chemotherapy, or a combination of all three, serial neuropsychological evaluations following treatment can track treatment-related cognitive decline and can be an early indicator of tumor recurrence (even prior to radiographic progression) [68].
The neuropsychologic evaluation of brain tumor patients should be comprehensive in order to allow for consideration of all factors that may contribute to cognitive functioning (mood, fatigue, pain, and premorbid functioning). The report can be beneficial to patients and their families in a variety of ways. It may prompt the patient’s physicians to offer them medications, cognitive rehabilitation, or home health [8•, 69]. A neuropsychologic assessment can also help determine patient capacity for decision-making, recommendations for driving, care needs, and provide documentation for any necessary disability paperwork [1].
Key neurocognitive tests and domains evaluated in a comprehensive evaluation
While most neurocognitive tests are marketed as assessing “language” or “memory,” multiple factors can contribute to a single score. For example, the Trail Making Test Part A (TMT-A) is commonly referred to as a test of attention but visual acuity, numeracy, and processing speed all contribute to performance on this test [70]. The Trail Making Test Part B (TMT-B) measures cognitive set- shifting (and is commonly appreciated as a measure of executive functioning) but also requires visual processing and motor speed.
The Digit Span (DS) test measures basic and complex attention (often referred to as working memory). This test is divided into three tasks: forward span, backward span, and numeric sequencing to provide a total score. To measure more complex working memory through the mental manipulation and sequencing of alpha-numeric information, the Letter-Number Sequencing (LNS) test can be used.
The Hopkins Verbal Learning Test-Revised (HVLT-R) is used to measure multiple aspects of learning and memory. Containing 12 words that span three semantic categories, this test allows for the measurement of what is referred to as effortful encoding, delayed (free) recall, and yes/no recognition. This allows for the elucidation between overt forgetting and retrieval-based deficit. There are six alternate forms of the HVLT-R which allows for repeated administration over short periods of time.
The Controlled Oral Word Association Test (COWAT) is a commonly appreciated measure of phonemic and semantic verbal fluency. Patients are asked to list words beginning with a specific letter of the alphabet and then those belonging to a specific category, which necessitates executive processing and linguistic fluency. The Boston Naming Test (BNT) is used to measure object naming and is exceptionally sensitive to language-dominant hemisphere disruption.
Other commonly used measures include those specifically designed to assess information processing speed such as the Coding and Symbol Search and those designed to assess verbal and visual abstract reasoning such as the Similarities and Matrix Reasoning which are both components of the Wechsler Adult Intelligence Scale, 4th edition (WAIS-IV).
A comparison of the patient’s raw and normalized scores over time can be completed using standard metrics that include a change of 1 standard deviation (SD) from baseline and/or normative data and reliable change indices (RCI) that allows for a correction of practice effects often seen in repeated examination.
Estimating premorbid functioning, depression, fatigue, and quality of life
Premorbid functioning is assessed through a combination of considerations that includes single-word reading, and educational, occupational, and social demographic predictions, otherwise known as the Barona estimate.
Emotional and behavioral characteristics are measured via self-report questionnaires like the Beck Depression Inventory (BDI –2) and the Beck Anxiety Inventory (BAI). Fatigue is similarly measured via the Fatigue Severity Scale (FSS) and functional activities of daily living can be measured by the Barthel ADL (basic ADL) or the Functional Activities Questionnaire (FAQ; instrumental activities). Cancer-specific questionnaires that assess disease-related quality of life and cancer type-specific symptoms are also available in the public domain and include the FACT-Cog and the FACT-Br.
Classification of patients with RICD
Although the cognitive effects from brain tumors and their treatment range from mild to severe, there is no accepted classification or phenotyping of cognitive impairment in brain tumor survivors. This stands in stark contrast to the literature on Alzheimer’s disease (AD). The concept of mild cognitive impairment (MCI) as a syndrome prodromal to AD and related dementias was introduced over 20 years ago [71]. The National Institute on Aging (NIA) and the Alzheimer’s Association (AA) have specified criteria for MCI, dementia, and AD. MCI is defined by the presence of a personal concern regarding a change in cognition and a measured deficit in at least one major cognitive domain with relative preservation of basic and instrumental functional abilities [72]. Additional criteria have been proposed for MCI subtypes that distinguish amnestic from non-amnestic cognitive deficits and single cognitive domain deficits from multi-domain deficits [73]. These criteria have become standard in research and clinical care and have undergone recent updates [74]. Standardized diagnostic criteria for MCI [72, 75] and AD [76–78] have helped to stimulate, organize, and focus research efforts on AD. Our group recently proposed applying slightly modified MCI NIA-AA criteria to cancer survivors with cognitive dysfunction [79–81]. We then retrospectively reviewed patients enrolled on our phase 3 randomized trial of donepezil versus placebo to estimate the prevalence of MCI in brain tumor survivors. We found that two-thirds of post-RT brain tumor survivors met NIA-AA criteria for MCI. Of patients meeting MCI criteria, the majority (58%) were subclassified as amnestic MCI-multiple domain which is the phenotype most commonly associated with AD. Applying this classification schema to patients with RICD does have limitations. In particular, it does not help discern between patients with transient/early decline and those with progressive/late decline. Classification of cognitive impairment in brain tumor patients could lead to improved treatments for RICD and thus should be a research priority for the field.
Treatment options
Treatment options for RICD can be partitioned into pharmacologic or non- pharmacologic treatments. Pharmacologic treatment options include donepezil, memantine, methylphenidate, armodafinil, and gingko biloba. Non-pharmacologic treatments include cognitive rehabilitation and exercise programs.
Donepezil
Donepezil is an acetyl-cholinesterase inhibitor indicated for mild to moderate Alzheimer’s Dementia. A randomized, phase III, open-label, placebo-controlled trial enrolled irradiated brain tumor survivors (at least 6 months after RT) and evaluated cognition before treatment with donepezil or placebo and then after 24 weeks of medication [5••]. This study did not meet its pre-specified primary endpoint (a difference in a cognitive composite score between the treatment group and the placebo group). However, patients randomized to donepezil performed better on measures of verbal and working memory (HVLT-DR and - IR) as well as a measure of motor speed and dexterity (Grooved Pegboard). There was also an interaction effect between pre-randomization (baseline) cognitive performance and treatment group—patients with poorer initial cognitive functioning benefited more from donepezil across multiple domains and in their cognitive composite scores. Similarly, the quality-of-life analysis of this study showed that patients with greater baseline concern about their cognitive function showed improved quality-of-life based on several scales (compared to placebo), whereas patients with better baseline quality-of-life had a slight decrease in their quality-of-life survey scores with donepezil (versus placebo) [82]. For this reason, donepezil continues to be used in the clinic for patients who have objective memory deficits (particularly if they have deficiencies on the HVLT) or have at least moderate impairment relative to normative data in multiple domains and feel that their memory concerns are compromising their quality-of-life.
The starting dose of donepezil is 5 mg and after 4–6 weeks, this can be increased to 10 mg daily. Common side effects include headache, urgent and frequent bowel movements, anorexia, weight loss, insomnia, and vivid dreams. Care should be taken when prescribing this to patients with bradycardia, a prolonged QT interval, or severe COPD because increased cholinergic activity can worsen these conditions. Drug interactions or incompatibilities include non- steroidal anti-inflammatory drugs (NSAIDS), cardiac medications like beta- blockers or calcium-channel blockers, or cholinergic agents like pilocarpine.
Methylphenidate
Methylphenidate is a stimulant approved for treatment of Attention-Deficit Hyperactivity Disorder (ADHD) and ADD. Some patients have greater difficulties in attention (versus memory) and in these patents, a trial of methylphenidate is a reasonable option but there is no data supporting its use as a prophylactic agent. There is data that methylphenidate can improve fatigue, attention, and cognition in children who received WBRT and in patients with chemotherapy-related fatigue [83, 84]. However, a randomized, open-label, placebo-controlled phase III trial of methylphenidate taken during brain RT and for 8 weeks after showed no difference in FACIT-F scores or cognitive measures [6•]. This study was hindered by a higher than expected drop-out rate which may have left it underpowered to detect a true difference.
The starting dose of immediate-release methylphenidate is 5 mg BID which can be increased by 5 to 10 mg increments per week for a maximum of 60 mg/day divided into two or three doses. Common side effects include increased heart rate, blood pressure, or palpitations; agitation, sweating, insomnia, emotionally liability, or psychosis; decreased appetite or weight loss; and decreased peripheral circulation.
Methylphenidate can lower seizure-thresholds so caution should be used in patients with a history of difficult-to-control seizures and is generally not prescribed for patients who are having break-through seizures on medications or. The use of methylphenidate is contraindicated for patients on Monoamine Oxidase Inhibitors (MAOIs). It should not be used in patients with glaucoma or a history of stroke, myocardial infarction, or tachycardia. Methylphenidate can cause anorexia and weight loss so do not prescribe it to patients who already have cancer-related anorexia.
Armodafinil and modafinil
Armodafinil and its predecessor modafinil are wakefulness-promoting drugs primarily used in the treatment of narcolepsy. In a randomized, placebo- controlled phase II trial, patients with primary brain tumors receiving at least 45 Gy were randomized to armodafinil or placebo during RT and for 1 month after RT. Fatigue, quality-of-life, and cognitive performance were all evaluated [85]. While there was not a significant difference in fatigue cores between groups at 4 weeks after RT, patients with greater baseline fatigue (below the group median) appeared to derive a benefit with improved fatigue levels and higher quality-of-life ratings in those receiving armodafinil compared to placebo.
The starting dose for armodafinil is 150 mg taken once daily. In narcolepsy, doses up to 250 mg daily can be used. However, the phase II trial used 150 mg daily without any dose-escalation. Common side effects include headache, nausea, weight loss, tremor, or dizziness among others. The starting dose of modafinil is 100 mg and can be increased every 3–7 days up to 400 mg daily. Caution should be used in patients with cardiac disease, hepatic impairment, or a history of Tourette’s syndrome.
Cognitive rehabilitation
Cognitive rehabilitation has been shown in a randomized trial to improve subjective and objective neurocognitive performance [8•]. In this study, low-grade glioma survivors with stable disease were randomized to a cognitive rehabilitation program or a wait list. The program consisted of 7 weeks of rehabilitation (which consisted of 6, 2-h sessions with a trained neuropsychologist and a computer-based retraining program) and a booster session 3 months after completion of the rehabilitation program (a telephone call discussing important aspects of retraining). The training focused on compensation mechanisms for impaired attention, memory, and executive function. Immediately after treatment, patients who participated in the rehabilitation program reported improved cognitive functioning on questionnaires but did not perform better on objective measures of cognitive performance. However, after 6 months, the patients who underwent rehabilitation performed objectively better on measures of verbal memory and attention compared to control patients and reported less mental fatigue. This group is currently in the process of validating a computer-based rehabilitation program [86].
Prevention
Radiosurgery
Currently, the most effect strategy to prevent RICD is to limit the integral dose of RT to the brain. Stereotactic radiosurgery (SRS) is highly focused radiation treatment given in a single dose and has supplanted WBRT in patients with a limited number of brain metastases because cognitive decline is less after SRS than after WBRT. In 2009, Chang et al. published the results of a randomized trial of SRS versus WBRT for patients with 1–3 brain metastases which showed less cognitive decline with SRS [87••]. At 4 months, only 24% of patients receiving SRS had declined in their HVLT-R score compared to 52% of patients in the WBRT arm. Overall survival was not different between the two arms. Subsequently, a N0574 (which randomized patients with 1–3 brain metastases to SRS alone or SRS + WBRT showed a much higher incidence of cognitive decline in the group receiving WBRT than those receiving SRS alone [88••]. Then NCGTGN107C/CEC 3 offered confirmation that cognitive-deterioration- free survival is longer in patients receiving SRS compared to WBRT. In this phase III trial, 194 patients who had undergone resection for a brain metastasis < 5 cm were randomized to postoperative SRS to the surgical cavity or WBRT [89••]. At 6 months, 52% of patients receiving SRS had cognitive decline compared with 85% of the patients receiving WBRT. Again, there was no difference seen in survival between the two groups. This was a landmark trial and will likely serve as a benchmark and historical control study for some years to come.
Hippocampal avoidance
The hippocampus has been recognized as a key anatomic location for memory for quite some time. After studies showed that metastases to the limbic circuit are rare, the concept of avoiding the hippocampus during WBRT to try to mitigate cognitive side effects evolved and was then tested in the multicenter phase II trial [14]. This trial (RTOG 0933) compared decline in HVLT-DR in patients treated with hippocampal avoidance to the decline in HVLT-DR in historical control patients. An initial report of a trial looking at WBRT plus memantine versus WBRT with hippocampal avoidance plus memantine (CC001) showed that time to cognitive decline was longer in the hippocampal avoidance arm [90••]. There are multiple additional clinical trials underway investigating the utility of hippocampal avoidance in patients with brain metastases from breast cancer, non-small-cell lung cancer, small-cell lung cancer, and glioblastoma.
Memantine
Memantine is an N-Methyl-D-Aspartate (NMDA) receptor antagonist approved for use in moderate to severe dementia. The NMDA receptor is a voltage-gated glutamate receptor that allow sodium and calcium influx into neurons when activated and holds special significance in synaptic plasticity. However, over-activation of the NMDA receptor has been linked to glucotoxicity (excitotoxic cell death).
A randomized, double-blind, placebo-controlled trial enrolled patients anticipated to receive WBRT for brain metastases. Patients received memantine or placebo during WBRT and after (for a total of 24 weeks) [4••]. The primary endpoint was patient performance on the HVLT-R-DR at 24 weeks. Similar to the donepezil trial, this study was a negative study (there was no statistical difference in HVLT-R-DR scores between memantine and placebo groups at 24 weeks). However, the study was underpowered as only 29% of patients enrolled completed the cognitive evaluation at 24 weeks. On secondary analysis, memantine did delay time to cognitive decline and was well-tolerated. Our institutional practice is to discuss the use of memantine with patients who require WBRT but who are anticipated to have relatively long survival (e.g., patients with EGFR or ALK-mutated lung cancer or breast cancer with innumerable metastases which preclude the use of SRS). We do not routinely offer it for patients with uncontrolled systemic disease or poor performance status. For patients who have already received RT and have memory deficits that have not improved with donepezil, memantine is also a reasonable option.
The starting dose for memantine is 5 mg. After 1 week, it can be titrated to 5 mg twice daily (BID), then after another week, 10 mg in the morning and 5 mg in the evening (15 mg total) and then after another week, 10 mg BID. Common side effects include headaches, dizziness, drowsiness, confusion, irritability, and constipation. Caution should be used in patients with liver or kidney dysfunction. An abnormal urine pH can lead to decreased drug excretion and higher than anticipated blood levels of memantine.
Investigational cytoprotective agents
Preclinical data suggests that angiotensin-converting enzyme (ACE) inhibitors such as a ramipril taken during RT may protect against RICD by decreasing radiation-induced inflammation [9, 10]. There is an ongoing cooperative group trial investigating its ability to reduce RICD in patients with glioblastoma receiving chemoradiation. Similarly, there is preclinical data that peroxisomal proliferation-activated receptor (PPAR) agonists such as pioglitazone can protect against RICD by via transcriptional inhibition effect on the NF-κB pathway which is a key mediator the pro-inflammatory cascade triggered by RT. There are emerging data that PPAR-γ agonists can be neuroprotective in stroke, Alzheimer’s disease and Parkinson’s disease [91–95]. An initial safety study of pioglitazone in brain tumor patients has been completed [96] and there are plans for a cooperative group trial evaluating the efficacy of pioglitazone in the prevention of RICD.
Developing a treatment plan for each patient
For patients with complaints of cognitive changes post-RT, it is imperative to elucidate from the patient and their caregivers whether depression or fatigue are significant issues. If depression and anxiety do not seem to be significant concerns, try to determine their cognitive concerns. Often, it is either short- term/working memory or attention. Review their medication list to see if there are any anti-cholinergic drugs (most often for urinary symptoms), sedating medications (especially things like opioids and gabapentin) which could be contributing to cognitive problems. If this is the case, evaluate how necessary these medications are for the patient and consider reducing or removing them. Progressive disease should be ruled out with imaging. Reversible causes of cognitive changes should be ruled out which generally involves checking B12/folate levels, TSH/free T4, morning cortisol, and testosterone levels (in men).
The next step is usually neuropsychological testing. For high-functioning patients who are working, we recommend comprehensive evaluation. For patients with a lower performance status, an abbreviated testing session with selected tests (see above) may be more appropriate and give you enough information to create a care plan.
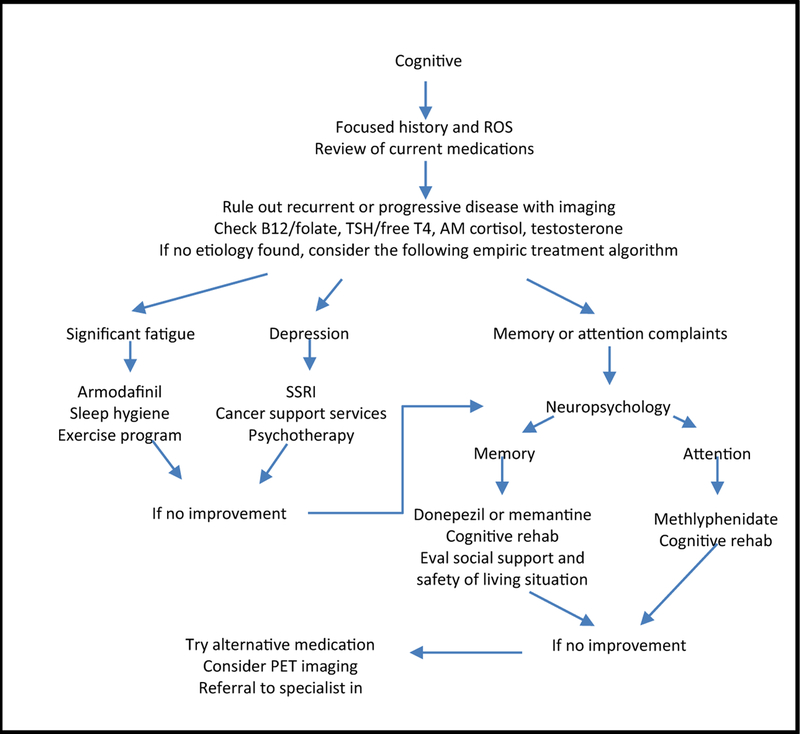
Our strategy has been to first address depression and fatigue (see Fig.1). If the patient acknowledges depression or severe fatigue and the neuropsychologist mentions these factors in their report, then consider starting your patient on anti-depressants. Usually, we start with a serotonin-uptake inhibitor (SSRI). In patients with both depression and severe fatigue, methylphenidate and an SSRI can be started synchronously with methylphenidate acting as a bridge since it can take 6 weeks for the clinical effect of SSRIs to become evident. Be cautious about using the dopamine-norepinephrine reuptake inhibitor bupropion in patients with a history of seizures. If fatigue (without depression) is a key factor, then consider armodafinil or modafinil. These medications should also be used cautiously if the patient has a history of seizures. For patients whose fatigue seems related to sleep dysfunction, it can be helpful to discuss sleep hygiene practices, rule out obstructive sleep apnea, and consider melatonin supplementation.
Fig. 1.
Treatment algorithm for brain tumor patients with cognitive decline.
If depression and fatigue do not appear to be significant factors then try to discern from the report whether measures of working memory (such as the HVLT), measures of attention (DS and TMT-A), or measures of executive function (COWA and TMT-B) are below normative data. For patients with complaints of short-term memory problems and corresponding low scores on HVLT-IR and HVLT-DR, a trial of donepezil or memantine may be appropriate. For patients who complain of attentional difficulties and with deficiencies on DS and TMT-A, a trial of methylphenidate may be appropriate. If you have not prescribed these medications previously, we would advise using a program that checks for drug interactions to confirm that none of the patients’ existing medications present a contraindication to the use of the medication you are prescribing.
Acknowledgments
Glenn J. Lesser has received clinical trial support from Vascular Biogenics, Incyte, NewLink Genetics, Novartis, Immunocellular Therapeutics, and Pfizer; has received compensation fromMonteris Medical and Insys Therapeutics for service as a consultant; and has received compensation from Stemline Therapeutics for serving as Data Safety and Monitoring Board Chair for a study.
Footnotes
This article is part of the Topical Collection on Neuro-oncology
Conflict of Interest
Christina K. Cramer has received speaker’s honoraria from Monteris Medical, and is also a member of teaching faculty for an on-site SBRT training course to which Elekta refers customers and for which Elekta reimburses the department.
Tiffany L. Cummings declares that she has no conflict of interest.
Rachel N. Andrews declares that she has no conflict of interest.
Michael Chan has received speaker’s honoraria from Monteris Medical, and is also a member of teaching faculty for an on-site SBRT training course to which Elekta refers customers and for which Elekta reimburses the department. Roy Strowd declares that he has no conflict of interest.
Stephen R. Rapp declares that he has no conflict of interest.
Edward G. Shaw declares that he has no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Johnson DR, Sawyer AM, Meyers CA, O’Neill BP, Wefel JS. Early measures of cognitive function predict survival in patients with newly diagnosed glioblastoma.Neuro- Oncology. 2012;14(6):808–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greene-Schloesser D, Robbins ME, Peiffer AM, Shaw EG, Wheeler KT, Chan MD. Radiation-induced brain injury: a review. Front Oncol. 2012;2:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meyers CA, Smith JA, Bezjak A, Mehta MP, Liebmann J, Illidge T, et al. Neurocognitive function and progression in patients with brain metastases treated with whole-brain radiation and motexafin gadolinium: results of a randomized phase III trial. J Clin Oncol. 2004;22(1):157–65. [DOI] [PubMed] [Google Scholar]
- 4.••.Brown PD, Pugh S, Laack NN, Wefel JS, Khuntia D, Meyers C, et al. Memantine for the prevention of cognitive dysfunction in patients receiving whole-brain radiotherapy: a randomized, double-blind, placebo-controlled trial. Neuro-Oncology. 2013;15(10):1429–37 [DOI] [PMC free article] [PubMed] [Google Scholar]; This randomized, placebo-controlled study did not meet its pre- specified primary endpoint but did show that time to cognitive decline is longer in patients who receive memantine during and after WBRT.
- 5.••.Rapp SR, Case LD, Peiffer A, Naughton MM, Chan MD, Stieber VW, et al. Donepezil for irradiated brain tumor survivors: a phase III randomized placebo-controlled clinical trial. J Clin Oncol. 2015;33(15):1653–9 [DOI] [PMC free article] [PubMed] [Google Scholar]; This was a seminal randomized, phase III, open-label, placebo- controlled trial enrolled irradiated brain tumor survivors (at least 6 months after RT) and evaluated cognition before treatment with donepezil or placebo and then after 24 weeks of medication. This study did not meet its pre-specified primary endpoint but patients randomized to donepezil performed better on measures of verbal and working memory (HVLT-DR and -IR). There was also an interaction effect between pre- randomization (baseline) cognitive performance and treatment group—patients with poorer initial cognitive functioning benefited more from donepezil. For this reason, donepezil continues to be used in the clinic.
- 6.•.Butler JM Jr, Case LD, Atkins J, Frizzell B, Sanders G, Griffin P, et al. A phase III, double-blind, placebocontrolled prospective randomized clinical trial of dthreo-methylphenidate HCl in brain tumor patients receiving radiation therapy. Int J Radiat Oncol Biol Phys. 2007;69(5):1496–501 [DOI] [PubMed] [Google Scholar]; This was a randomized, open-label, placebo-controlled phase III trial of methylphenidate taken during brain RT and for 8 weeks after, showed no difference fatigue or cognition between patients taking methylphenidate and those taking placebo. This study was hindered by a higher than expected dropout rate which may have left it underpowered to detect a true difference. There are other smaller studies to support the use of methylphenidate in treating cancer-related fatigue which is why methylphenidate is still used clinically.
- 7.Attia A, Rapp SR, Case LD, D’Agostino R, Lesser G, Naughton M, et al. Phase II study of Ginkgo biloba in irradiated brain tumor patients: effect on cognitive function, quality of life, and mood. J Neuro-Oncol. 2012;109(2):357–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.•.Gehring K, Sitskoorn MM, Gundy CM, Sikkes SA, Klein M, Postma TJ, et al. Cognitive rehabilitation in patients with gliomas: a randomized, controlled trial. J Clin Oncol. 2009;27(22):3712–22 [DOI] [PubMed] [Google Scholar]; In this study, low-grade glioma survivors with stable disease were randomized to a cognitive rehabilitation program or the wait list. The program consisted 7 weeks of rehabilitation. Immediately after treatment, patients who participated in the rehabilitation program reported improved cognitive functioning on questionnaires but did not perform better on objective measures of cognitive performance. However, after 6 months, the patients who underwent rehabilitation performed objectively better on measures of verbal memory and attention compared to control patients and reported less mental fatigue.
- 9.Jenrow KA, Brown SL, Liu J, Kolozsvary A, Lapanowski K, Kim JH. Ramipril mitigates radiation-induced impairment of neurogenesis in the rat dentate gyrus. Radiat Oncol. 2010;5:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee TC, Greene-Schloesser D, Payne V, Diz DI, Hsu FC, Kooshki M, et al. Chronic administration of the angiotensin-converting enzyme inhibitor, ramipril, prevents fractionated whole-brain irradiation-induced perirhinal cortex-dependent cognitive impairment. Radiat Res. 2012;178(1):46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramanan S, Kooshki M, Zhao W, Hsu FC, Riddle DR, Robbins ME. The PPARalpha agonist fenofibrate preserves hippocampal neurogenesis and inhibits microglial activation after whole-brain irradiation. Int J Radiat Oncol Biol Phys. 2009;75(3):870–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu JL, Tian DS, Li ZW, Qu WS, Zhan Y, Xie MJ, et al. Tamoxifen alleviates irradiation-induced brain injury by attenuating microglial inflammatory response in vitro and in vivo. Brain Res. 2010;1316:101–11. [DOI] [PubMed] [Google Scholar]
- 13.Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302(5651):1760–5. [DOI] [PubMed] [Google Scholar]
- 14.Gondi V, Pugh SL, Tome WA, Caine C, Corn B, Kanner A, et al. Preservation of memory with conformal avoidance of the hippocampal neural stem-cell compartment during whole-brain radiotherapy for brain metastases (RTOG 0933): a phase II multi-institutional trial. J Clin Oncol. 2014;32(34):3810–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Begolly S, Olschowka JA, Love T, Williams JP, O’Banion MK. Fractionation enhances acute oligodendrocyte progenitor cell radiation sensitivity and leads to long term depletion. Glia. 2018;66(4):846–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andrews RN, Metheny-Barlow LJ, Peiffer AM, Hanbury DB, Tooze JA, Bourland JD, et al. Cerebrovascular remodeling and neuroinflammation is a late effect of radiation-induced brain injury in non-human primates. Radiat Res. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hanbury DB, Robbins ME, Bourland JD, Wheeler KT, Peiffer AM, Mitchell EL, et al. Pathology of fractionated whole-brain irradiation in rhesus monkeys (Macaca mulatta). Radiat Res. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schultheiss TE, Kun LE, Ang KK, Stephens LC. Radiation response of the central nervous system. Int J Radiat Oncol Biol Phys. 1995;31(5):1093–112. [DOI] [PubMed] [Google Scholar]
- 19.Monje ML, Mizumatsu S, Fike JR, Palmer TD. Irradiation induces neural precursor-cell dysfunction. Nat Med. 2002;8(9):955–62. [DOI] [PubMed] [Google Scholar]
- 20.Ungvari Z, Podlutsky A, Sosnowska D, Tucsek Z, Toth P, Deak F, et al. Ionizing radiation promotes the acquisition of a senescence-associated secretory phenotype and impairs angiogenic capacity in cerebromicrovascular endothelial cells: role of increased DNA damage and decreased DNA repair capacity in microvascular radiosensitivity. J Gerontol A Biol Sci Med Sci. 2013;68(12):1443–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shirai K, Mizui T, Suzuki Y, Okamoto M, Hanamura K, Yoshida Y, et al. X irradiation changes dendritic spine morphology and density through reduction of cytoskeletal proteins in mature neurons. Radiat Res. 2013;179(6):630–6. [DOI] [PubMed] [Google Scholar]
- 22.Sanchez MC, Benitez A, Ortloff L, Green LM. Alterations in glutamate uptake in NT2-derived neurons and astrocytes after exposure to gamma radiation. Radiat Res. 2009;171(1):41–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rola R, Raber J, Rizk A, Otsuka S, VandenBerg SR, Morhardt DR, et al. Radiation-induced impairment of hippocampal neurogenesis is associated with cognitive deficits in young mice. Exp Neurol. 2004;188(2):316–30. [DOI] [PubMed] [Google Scholar]
- 24.Rogers LR. Cerebrovascular complications in cancer patients. Neurol Clin. 2003;21(1):167–92. [DOI] [PubMed] [Google Scholar]
- 25.Raber J, Rola R, LeFevour A, Morhardt D, Curley J, Mizumatsu S, et al. Radiation-induced cognitive impairments are associated with changes in indicators of hippocampal neurogenesis. Radiat Res. 2004;162(1):39–47. [DOI] [PubMed] [Google Scholar]
- 26.Puspitasari A, Koganezawa N, Ishizuka Y, Kojima N, Tanaka N, Nakano T, et al. X irradiation induces acute cognitive decline via transient synaptic dysfunction. Radiat Res. 2016;185(4):423–30. [DOI] [PubMed] [Google Scholar]
- 27.O’Connor MM, Mayberg MR. Effects of radiation on cerebral vasculature: a review. Neurosurgery. 2000;46(1):138–49 discussion 50–1. [DOI] [PubMed] [Google Scholar]
- 28.Monje ML, Palmer T. Radiation injury and neurogenesis. Curr Opin Neurol. 2003;16(2):129–34. [DOI] [PubMed] [Google Scholar]
- 29.Mizumatsu S, Monje ML, Morhardt DR, Rola R, Palmer TD, Fike JR. Extreme sensitivity of adult neurogenesis to low doses of X-irradiation. Cancer Res. 2003;63(14):4021–7. [PubMed] [Google Scholar]
- 30.Kurita H, Kawahara N, Asai A, Ueki K, Shin M, Kirino T. Radiation-induced apoptosis of oligodendrocytes in the adult rat brain. Neurol Res. 2001;23(8):869–74. [DOI] [PubMed] [Google Scholar]
- 31.Ji S, Tian Y, Sun R, Lu Y, Zhang L, Chen L, et al. Radiation-induced hippocampal neurogenesis impairment and cognitive deficits is associated with inhibition of BDNF-Trk-B signaling. Int J Radiat Oncol Biol Phys. 87(2):S628. [Google Scholar]
- 32.Irvine KA, Blakemore WF. A different regional response by mouse oligodendrocyte progenitor cells (OPCs) to high-dose X-irradiation has consequences for repopulating OPC-depleted normal tissue. Eur J Neurosci. 2007;25(2):417–24. [DOI] [PubMed] [Google Scholar]
- 33.Hsu YC, Wang LF, Lee KW, Ho KY, Huang CJ, Kuo WR. Cerebral radionecrosis in patients with nasopharyngeal carcinoma. Kaohsiung J Med Sci. 2005;21(10):452–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chari DM, Huang WL, Blakemore WF. Dysfunctional oligodendrocyte progenitor cell (OPC) populations may inhibit repopulation of OPC depleted tissue. J Neurosci Res. 2003;73(6):787–93. [DOI] [PubMed] [Google Scholar]
- 35.Chakraborti A, Allen A, Allen B, Rosi S, Fike JR. Cranial irradiation alters dendritic spine density and morphology in the hippocampus. PLoS One. 2012;7(7):e40844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brown WR, Thore CR, Moody DM, Robbins ME, Wheeler KT. Vascular damage after fractionated whole- brain irradiation in rats. Radiat Res. 2005;164(5):662–8. [DOI] [PubMed] [Google Scholar]
- 37.Brown WR, Blair RM, Moody DM, Thore CR, Ahmed S, Robbins ME, et al. Capillary loss precedes the cognitive impairment induced by fractionated whole-brain irradiation: a potential rat model of vascular dementia. J Neurol Sci. 2007;257(1–2):67–71. [DOI] [PubMed] [Google Scholar]
- 38.Atkinson SL, Li YQ, Wong CS. Apoptosis and proliferation of oligodendrocyte progenitor cells in the irradiated rodent spinal cord. Int J Radiat Oncol Biol Phys. 2005;62(2):535–44. [DOI] [PubMed] [Google Scholar]
- 39.Kyrkanides S, Moore AH, Olschowka JA, Daeschner JC, Williams JP, Hansen JT, et al. Cyclooxygenase-2 modulates brain inflammation-related gene expression in central nervous system radiation injury. Brain Res Mol Brain Res. 2002;104(2):159–69. [DOI] [PubMed] [Google Scholar]
- 40.Zhou K, Bostrom M, Ek CJ, Li T, Xie C, Xu Y, et al. Radiation induces progenitor cell death, microglia activation, and blood-brain barrier damage in the juvenile rat cerebellum. Sci Rep. 2017;7:46181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang J, Tong F, Cai Q, Chen LJ, Dong JH, Wu G, et al. Shenqi fuzheng injection attenuates irradiation- induced brain injury in mice via inhibition of the NF- kappaB signaling pathway and microglial activation. Acta Pharmacol Sin. 2015;36(11):1288–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yoritsune E, Furuse M, Kuwabara H, Miyata T, Nonoguchi N, Kawabata S, et al. Inflammation as well as angiogenesis may participate in the pathophysiology of brain radiation necrosis. J Radiat Res. 2014;55(4):803–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu P, Xu Y, Hu B, Wang J, Pan R, Murugan M, et al. Extracellular ATP enhances radiation-induced brain injury through microglial activation and paracrine signaling via P2X7 receptor. Brain Behav Immun. 2015;50:87–100. [DOI] [PubMed] [Google Scholar]
- 44.Schnegg CI, Kooshki M, Hsu FC, Sui G, Robbins ME. PPARdelta prevents radiation-induced proinflammatory responses in microglia via transrepression of NF- kappaB and inhibition of the PKCalpha/MEK1/2/ERK1/2/AP-1 pathway. Free Radic Biol Med. 2012;52(9):1734–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schindler MK, Forbes ME, Robbins ME, Riddle DR. Aging-dependent changes in the radiation response of the adult rat brain. Int J Radiat Oncol Biol Phys. 2008;70(3):826–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ramanan S, Kooshki M, Zhao W, Hsu F-C, Robbins ME. PPARα ligands inhibit radiation-induced microglial inflammatory responses by negatively regulating NF-κB and AP-1 pathways. Free Radic Biol Med. 2008;45(12):1695–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hwang SY, Jung JS, Kim TH, Lim SJ, Oh ES, Kim JY, et al. Ionizing radiation induces astrocyte gliosis through microglia activation. Neurobiol Dis. 2006;21(3):457–67. [DOI] [PubMed] [Google Scholar]
- 48.Acharya MM, Green KN, Allen BD, Najafi AR, Syage A, Minasyan H, et al. Elimination of microglia improves cognitive function following cranial irradiation. Sci Rep. 2016;6:31545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Robbins ME, Zhao W, Garcia-Espinosa MA, Diz DI. Renin-angiotensin system blockers and modulation of radiation-induced brain injury. Curr Drug Targets. 2010;11(11):1413–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Robbins ME, Payne V, Tommasi E, Diz DI, Hsu FC, Brown WR, et al. The AT1 receptor antagonist, L-158,809, prevents or ameliorates fractionated whole- brain irradiation-induced cognitive impairment. Int J Radiat Oncol Biol Phys. 2009;73(2):499–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Robbins ME, Zhao W. Chronic oxidative stress and radiation-induced late normal tissue injury: a review. Int J Radiat Biol. 2004;80(4):251–9. [DOI] [PubMed] [Google Scholar]
- 52.•.Peiffer AM, Leyrer CM, Greene-Schloesser DM, Shing E, Kearns WT, Hinson WH, et al. Neuroanatomical target theory as a predictive model for radiation-induced cognitive decline. Neurology. 2013;80(8):747–53 [DOI] [PMC free article] [PubMed] [Google Scholar]; This was an important analysis of several prospective studies which linked post-RT cognitive function to the dose distribution in the brain relative to specific anatomic regions-of-interest. This suggests that ultimatley, a dose-volume-histogram analysis of neural substructures could help predict (and reduce the risk) of RICD.
- 53.Yoshita M, Fletcher E, Harvey D, Ortega M, Martinez O, Mungas DM, et al. Extent and distribution of white matter hyperintensities in normal aging, MCI, and AD. Neurology. 2006;67(12):2192–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kester MI, Goos JD, Teunissen CE, Benedictus MR, Bouwman FH, Wattjes MP, et al. Associations between cerebral small-vessel disease and Alzheimer disease pathology as measured by cerebrospinal fluid biomarkers. JAMA Neurol. 2014;71(7):855–62. [DOI] [PubMed] [Google Scholar]
- 55.Capizzano A, Acion L, Bekinschtein T, Furman M, Gomila H, Martinez A, et al. White matter hyperintensities are significantly associated with cortical atrophy in Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2004;75(6):822–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brickman AM, Muraskin J, Zimmerman ME. Structural neuroimaging in Alzheimer’s disease: do white matter hyperintensities matter? Dialogues Clin Neurosci. 2009;11(2):181–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brickman AM, Honig LS, Scarmeas N, Tatarina O, Sanders L, Albert MS, et al. Measuring cerebral atrophy and white matter hyperintensity burden to predict the rate of cognitive decline in Alzheimer disease. Arch Neurol. 2008;65(9):1202–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brickman AM. Contemplating Alzheimer’s disease and the contribution of white matter hyperintensities. Curr Neurol Neurosci Rep. 2013;13(12):415 10.1007/s11910-013-0415-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Johannesen TB, Lien HH, Hole KH, Lote K. Radiological and clinical assessment of long-term brain tumour survivors after radiotherapy. Radiother Oncol. 2003;69(2):169–76. [DOI] [PubMed] [Google Scholar]
- 60.Constine LS, Konski A, Ekholm S, McDonald S, Rubin P. Adverse effects of brain irradiation correlated with MR and CT imaging. Int J Radiat Oncol Biol Phys. 1988;15(2):319–30. [DOI] [PubMed] [Google Scholar]
- 61.Tsuruda JS, Kortman KE, Bradley WG, Wheeler DC, Van Dalsem W, Bradley TP. Radiation effects on cerebral white matter: MR evaluation. AJR Am J Roentgenol. 1987;149(1):165–71. [DOI] [PubMed] [Google Scholar]
- 62.Chapman CH, Nagesh V, Sundgren PC, Buchtel H, Chenevert TL, Junck L, et al. Diffusion tensor imaging of normal-appearing white matter as biomarker for radiation-induced late delayed cognitive decline. Int J Radiat Oncol Biol Phys. 2012;82(5):2033–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chapman CH, Zhu T, Nazem-Zadeh M, Tao Y, Buchtel HA, Tsien CI, et al. Diffusion tensor imaging predicts cognitive function change following partial brain radiotherapy for low-grade and benign tumors. Radiother Oncol. 2016;120(2):234–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mabbott DJ, Noseworthy MD, Bouffet E, Rockel C, Laughlin S. Diffusion tensor imaging of white matter after cranial radiation in children for medulloblastoma: correlation with IQ. Neuro-Oncology. 2006;8(3):244–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Laukkanen E, Klonoff H, Allan B, Graeb D, Murray N. The role of prophylactic brain irradiation in limited stage small cell lung cancer: clinical, neuropsychologic, and CT sequelae. Int J Radiat Oncol Biol Phys. 1988;14(6):1109–17. [DOI] [PubMed] [Google Scholar]
- 66.Meyers CA, Wefel JS. The use of the mini-mental state examination to assess cognitive functioning in cancer trials: no ifs, ands, buts, or sensitivity. J Clin Oncol. 2003;21(19):3557–8. [DOI] [PubMed] [Google Scholar]
- 67.Noll KR, Bradshaw ME, Rexer J, Wefel JS. Neuropsychological practice in the oncology setting. Arch Clin Neuropsychol. 2018;33(3):344–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Meyers CA, Hess KR. Multifaceted end points in brain tumor clinical trials: cognitive deterioration precedes MRI progression. Neuro-Oncology. 2003;5(2):89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ferguson RJ, Ahles TA, Saykin AJ, McDonald BC, Furstenberg CT, Cole BF, et al. Cognitive-behavioral management of chemotherapy-related cognitive change. Psychooncology. 2007;16(8):772–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tombaugh TN. Trail Making Test A and B: normative data stratified by age and education. Arch Clin Neuropsychol. 2004;19(2):203–14. [DOI] [PubMed] [Google Scholar]
- 71.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56(3):303–8. [DOI] [PubMed] [Google Scholar]
- 72.Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):270–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Winblad B, Palmer K, Kivipelto M, Jelic V, Fratiglioni L, Wahlund LO, et al. Mild cognitive impairment– beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med. 2004;256(3):240–6. [DOI] [PubMed] [Google Scholar]
- 74.Jack CR Jr, Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, et al. NIA-AA Research Framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018;14(4):535–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256(3):183–94. [DOI] [PubMed] [Google Scholar]
- 76.McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr, Kawas CH, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):263–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.McKhann G, Drachman D, Folstein MF, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–44. [DOI] [PubMed] [Google Scholar]
- 78.American Psychiatric Association. American Psychiatric Association.Task Force on DSM-IV. Diagnostic and statistical manual of mental disorders: DSM-IV. 4th ed Washington, DC: American Psychiatric Association; 1994. xxvii, 886 p. p. [Google Scholar]
- 79.Gifford A, Lawrence J, Case D, Rapp S, Baker L, Craft S, et al. Mild cognitive impairment (MCI) in chemotherapy-treated breast cancer survivors. J Clin Oncol. 2015;33(15):9560. [Google Scholar]
- 80.Gifford AR, Lawrence JA, Baker LD, Balcueva EP, Case D, Craft S, et al. National Institute on Aging/Alzheimer’s Association criteria for Mild Cognitive Impairment applied to chemotherapy treated breast cancer survivors . J Oncol Res. 2017;1(1). [PMC free article] [PubMed] [Google Scholar]
- 81.Cramer CK, McKee N, Case LD, Chan MD, Cummings TL, Lesser GJ, et al. Mild cognitive impairment in longterm brain tumor survivors following brain irradiation. J Neurooncol. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Naughton MJ, Case LD, Peiffer A, Chan M, Stieber V, Moore D, et al. Quality of life of irradiated brain tumor survivors treated with donepezil or placebo: results of the WFU CCOP research base protocol 91105. Neurooncol Pract. 2018;5(2):114–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mulhern RK, Khan RB, Kaplan S, Helton S, Christensen R, Bonner M, et al. Short-term efficacy of methylphenidate: a randomized, double-blind, placebo- controlled trial among survivors of childhood cancer. J Clin Oncol. 2004;22(23):4795–803. [DOI] [PubMed] [Google Scholar]
- 84.Bruera E, Driver L, Barnes EA, Willey J, Shen L, Palmer JL, et al. Patient-controlled methylphenidate for the management of fatigue in patients with advanced cancer: a preliminary report. J Clin Oncol. 2003;21(23):4439–43. [DOI] [PubMed] [Google Scholar]
- 85.Page BR, Shaw EG, Lu L, Bryant D, Grisell D, Lesser GJ, et al. Phase II double-blind placebo-controlled randomized study of armodafinil for brain radiation- induced fatigue. Neuro-Oncology. 2015;17(10):1393–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.van der Linden SD, Sitskoorn MM, Rutten GM, Gehring K. Feasibility of the evidence-based cognitive telerehabilitation program Remind for patients with primary brain tumors. J Neuro-Oncol. 2018;137(3):523–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.••.Chang EL, Wefel JS, Hess KR, Allen PK, Lang FF, Kornguth DG, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol. 2009;10(11):1037–44 [DOI] [PubMed] [Google Scholar]; This randomized trial of SRS alone versus SRS + WBRT for patients with a limited number of brain metastases demonstrated that treating patients with SRS rather than WBRT can preserve their cognition without compromising overall survival. At 4 months, only 24% of patients receiving SRS had declined in their HVLT-R score compared to 52% of patients in the WBRT arm.
- 88.••.Brown PD, Jaeckle K, Ballman KV, Farace E, Cerhan JH, Anderson SK, et al. Effect of radiosurgery alone vs radiosurgery with whole brain radiation therapy on cognitive function in patients with 1 to 3 brain metastases: a randomized clinical trial. JAMA. 2016;316(4):401–9 [DOI] [PMC free article] [PubMed] [Google Scholar]; N0574 randomized patients with 1–3 brain metastases to SRS alone or SRS + WBRT and showed a much higher incidence of cognitive decline in the group receiving WBRT than those receiving SRS alone.
- 89.••.Brown PD, Ballman KV, Cerhan JH, Anderson SK, Carrero XW, Whitton AC, et al. Postoperative stereotactic radiosurgery compared with whole brain radiotherapy for resected metastatic brain disease (NCCTG N107C/CEC.3): a multicentre, randomised, controlled, phase 3 trial. Lancet Oncol. 2017;18(8):1049–60 [DOI] [PMC free article] [PubMed] [Google Scholar]; NCGTGN107C/CEC 3 offered confirmation that cognitive- deterioration-free survival is longer in patients receiving SRS compared to WBRT. In this phase III trial, 194 patients who had undergone resection for a brain metastasis G 5 cm were randomized to postoperative SRS to the surgical cavity or WBRT [90]. At 6 months, 52% of patients receiving SRS had cognitive decline compared with 85% of the patients receiving WBRT. Again, there was no difference seen in survival between the two groups. This was a landmark trial and will likely serve as a benchmark and historical control study for some years to come.
- 90.••.Gondi V, Deshmukh S, Brown PD, Wefel JS, Tome WA, Bruner DW, et al. Preservation of neurocognitive function (NCF) with conformal avoidance of the hippocampus during whole-brain radiotherapy (HA-WBRT) for brain metastases: preliminary results of phase III trial NRG Oncology CC001. Int J Radiat Oncol Biol Phys. 2018;102(5):1607 [Google Scholar]; This is an initial report of a trial looking at WBRT plus memantine versus WBRT with hippocampal avoidance plus memantine (CC001). The early results indicate that time to cognitive decline is longer in the hippocampal avoidance + memantine versus WBRT + memantine.
- 91.Breidert T, Callebert J, Heneka MT, Landreth G, Launay JM, Hirsch EC. Protective action of the peroxisome proliferator-activated receptor-gamma agonist pioglitazone in a mouse model of Parkinson’s disease. J Neurochem. 2002;82(3):615–24. [DOI] [PubMed] [Google Scholar]
- 92.Chang KL, Pee HN, Yang S, Ho PC. Influence of drug transporters and stereoselectivity on the brain penetration of pioglitazone as a potential medicine against Alzheimer’s disease. Sci Rep. 2015;5:9000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dehmer T, Heneka MT, Sastre M, Dichgans J, Schulz JB. Protection by pioglitazone in the MPTP model of Parkinson’s disease correlates with I kappa B alpha induction and block of NF kappa B and iNOS activation. J Neurochem. 2004;88(2):494–501. [DOI] [PubMed] [Google Scholar]
- 94.Sundararajan S, Gamboa JL, Victor NA, Wanderi EW, Lust WD, Landreth GE. Peroxisome proliferator- activated receptor-gamma ligands reduce inflammation and infarction size in transient focal ischemia. Neuroscience. 2005;130(3):685–96. [DOI] [PubMed] [Google Scholar]
- 95.Zhao Y, Patzer A, Gohlke P, Herdegen T, Culman J. The intracerebral application of the PPARgamma-ligand pioglitazone confers neuroprotection against focal is- chaemia in the rat brain. Eur J Neurosci. 2005;22(1):278–82. [DOI] [PubMed] [Google Scholar]
- 96.Cramer CK, Alphonse-Sullivan N, Isom S, Metheny- Barlow LJ, Cummings TL, Page BR, et al. Safety of pioglitazone during and after radiation therapy in patients with brain tumors: a phase I clinical trial . J Can- cer Res Clin Oncol. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]