Abstract
Background:
Sarcoidosis is a systemic granulomatous disease of unknown etiology that affects the lungs in 90% of patients, but has a wide range of disease manifestations and outcomes including chronic and progressive courses. Noninvasive biomarkers are needed to assess these outcomes and guide decisions for long term monitoring and treatment. Interferon-gamma (IFN-γ)-inducible chemotactic cytokines (chemokines), CXCL9, CXCL10 and CXCL11, show promise in this regard because they have been implicated in the pathogenesis of and reflect the burden of granulomatous inflammation. CXCL11 has been reported to have unique functional properties in modulating adaptive immunity in model systems so our goal was to examine serum levels of CXCL11 in relation to clinical outcomes in a heterogeneous cohort of sarcoidosis subjects.
Methods:
CXCL19, CXCL10, and CXCL11 serum levels were measured in sarcoidosis and healthy subjects using ELISA assay. We determined relationships between CXCL11 and standard clinical inflammatory markers, expression of IFN-γ-related genes in whole blood, organ involvement, dyspnea scores, and measures of pulmonary function.
Results:
In a cross-sectional analysis of 104 sarcoidosis subjects, serum CXCL11 was significantly elevated compared to 49 healthy controls (p < 0.001). CXCL11 was positively correlated with CXCL9 and CXCL10 (p < 0.001), sedimentation rate (p < 0.01), and mean expression of three IFN-γ-related genes in whole blood (GBP1, STAT1, and STAT2) (p < 0.001). CXCL11 was inversely correlated with FVC %predicted (%pred) and FEV1 %pred and higher levels were associated with higher patient-reported dyspnea scores. We found positive correlations between CXCL11 and number of organs involved. Using survival analyses, we found that CXCL11 levels were predictive of future pulmonary function test (PFT) decline (log rank <0.001 and HR of log10(CXCL11) = 5.1, 95% CI 1.2 – 21, p = 0.026).
Conclusions:
The pattern of expression of serum CXCL11 in sarcoidosis patients suggests that this blood measure could be helpful in identifying patients that need longer-term monitoring for progressive thoracic and extra-thoracic sarcoidosis.
Keywords: Sarcoidosis, CXCL11, CXCL9, CXCL10, Interferon-gamma, Chemokine
1. Introduction
Sarcoidosis is a systemic inflammatory disease characterized by non-necrotizing granulomatous inflammation that predominantly affects the lungs, but can affect almost any organ system [1]. As in other granulomatous diseases, CD4+ T cells that produce interferon-gamma (IFN-γ) play a vital role in sarcoidosis-associated inflammation, although the specific antigen for these T cells is unknown [2–7]. These T helper cells traffic to affected areas due, in part, to their expression of the chemokine receptor CXCR3 [8]. This receptor binds to several different ligands, including the chemotactic cytokines (chemokines), CXCL9, CXCL10, and CXCL11, which are produced by epithelial, interstitial, and immune cells including endothelial cells, fibroblasts, and macrophages [9]. All three of these chemokines are induced by interferon-gamma (IFN-γ) as well as Toll-like receptor ligands and TNF-α [9–11].
Several studies have shown that protein and gene expression levels of CXCL9, CXCL10, and CXCL11 are increased in lung lavage or lung tissue in sarcoidosis subjects, suggesting a pathogenic role in the disease [12–15]. We have been interested in the potential for these chemokines to provide insights into sarcoidosis pathogenesis and to serve as informative biologic markers. Our prior work has focused on analysis of peripheral blood, from which we and others have found a robust signal of interferon inflammation, suggesting that these chemokines could be helpful as predictive disease markers [16–18].
Although CXCL9, CXCL10, and CXCL11 share common functions with respect to T cell homing [15], CXCL11 has been shown to bind to different splice variants of CXCR3 as well as other receptors (e.g. CXCR7), which may allow it to serve different functions [9]. CXCL11 has also been shown to have differential effects on T cells in mice including mediating polarization of T “effector” cells to a more regulatory phenotype, although the mechanism by which this could potentially occur in humans is unclear [19]. CXCL9 and CXCL10 have been shown to be increased in the serum of sarcoidosis patients [17], but little is known about the role(s) of serum CXCL11 in disease manifestations or clinical course. Given that CXCL11 is induced by IFN-γ, we hypothesized that CXCL11 would 1) be increased in sarcoidosis subjects compared to health and 2) be correlated with objective measures of disease severity. In the current study, we sought to 1) assess the serum levels of CXCL11 in relation to CXCL9, CXCL10, and other blood markers of inflammation, 2) analyze the relationship of CXCL11 to clinical features such as organ involvement and pulmonary function tests (PFTs), and 3) examine the predictive value of CXCL11 for longitudinal decline in pulmonary function.
2. Methods
2.1. Study Population and Measurements
Sarcoidosis subjects who met established ATS diagnostic criteria [20] were enrolled at any point in their disease course as part of the University of California, San Francisco (UCSF) sarcoidosis cohort as previously described [21]. Healthy control subjects were recruited from the Bay Area community. Blood samples and clinical data that were collected at study visits and used in these analyses included demographics, date of tissue biopsy, organ involvement assessed by physician review of medical records [21], UCSD “Shortness of Breath” (dyspnea) questionnaire [22–24], chest X-ray imaging, clinical laboratory tests, and pulmonary function tests which include forced expiratory volume in 1 second (FEV1) percent predicted (%pred), forced vital capacity (FVC %pred), FEV1/FVC ratio, diffusing capacity for carbon monoxide (DLCO %pred), and total lung capacity (TLC %pred). Immunosuppression use was recorded at each study visit and categorized as a binary variable of use of none or any of the following: oral corticosteroids, methotrexate, azathioprine, colchicine, hydroxycholoroquine, or anti-TNF-α therapy. Subjects had follow-up study visits every 6 to 12 months for up to five years with collection of all the same clinical data except for chest imaging. Gene expression levels of IFN-γ-related genes were measured from whole blood RNA from the same subject samples used for this analysis (results have been previously reported [18]).
2.2. Protein Assay
Levels of CXCL9, CXCL10, and CXCL11 in serum were measured using Quantikine® Colorimetric Sandwich ELISA kits purchased from R&D Systems (Minneapolis, MN, USA). The assays have been optimized for detection of these proteins within human serum. Samples were thawed, aliquoted into sample wells in duplicate, and processed in one batch. Duplicates were averaged for each sample. Seven standard control samples were run on each plate along with a reagent blank to generate standard curves to calculate protein concentrations. Per manufacturer’s instructions, the lowest detectable levels for CXCL9, CXCL10, and CXCL11 were 31.2 pg/mL, 7.8 pg/mL, and 62.5 pg/mL respectively; the upper limits of detection were 2000 pg/mL, 500 pg/mL, and 4000 pg/mL, respectively.
2.3. Statistical Analysis
Data were analyzed for normality using a Shapiro-Wilk test. Chemokine levels were normalized using log10 transformation given their skewed distributions. The Chi-squared or Fisher-exact tests were used for categorical variables. ANOVA analysis was used for more than two group comparison analyses, unless variables were non-normally distributed in which case, a Kruskal-Wallis test was used along with a Dunn’s test of multiple pairwise comparisons. The t-test or Wilcoxon rank-sum test were used for two group comparisons of continuously distributed parametric or non-parametric data, respectively. Spearman rank correlation tests were used to determine the correlation coefficients between CXCL11 and variables of interest. The variable termed, “Interferon Factor” (IFN Factor) consisted of a composite score of centered and scaled means for three genes as described previously [18]. Where indicated, analyses were adjusted for several confounders including presence of immunosuppression use, sex, age, race, and prior smoking using ordered logistic regression (as opposed to linear regression since 50.4% of values were below the lower limit of detection for the CXCL11 measurement, which does not allow for normalization after log10 transformation). To adjust for immunosuppression use, sex, age, and race in our analysis of CXCL11 and organ number, we performed a non-parametric Kernel regression with bootstrap testing using 1000 replicates to determine statistical significance given the highly skewed distribution of CXCL11 values [25, 26]; we also used linear and ordered logistic regression models to confirm our findings. We performed time to event analyses for PFT decline using the log-rank test and Cox proportional hazards modeling. Analyses were done using Stata/SE 15.1 software (StataCorp LLC, College Station, TX) and GraphPad Prism 6 software (GraphPad Software, Inc., La Jolla, CA).
3. Results
3.1. Characteristics of sarcoidosis subjects & healthy controls
The sample sizes for our sample for cross-sectional analyses consisted of 104 sarcoidosis subjects and 49 healthy controls who had paired blood measurements and clinical data available (N = 96 from enrollment visit and N = 8 from visit 2 or 3). The demographic characteristics are summarized in Table 1. On average, sarcoidosis subjects were older than healthy controls. Gender, race, and prior tobacco smoking history were similar between the two groups. Table 2 summarizes Scadding stage, immunosuppression history, extra-thoracic organ involvement and pulmonary physiology testing results for sarcoidosis subjects [21]. Two subjects with Scadding stage 0 had prior lung involvement that had radiographically resolved at enrollment, five had abnormal pulmonary function tests, and two had isolated neurologic disease. Fifty-four percent of subjects were taking systemic immunosuppressive therapy at time of their blood draw visit. The majority of subjects had extra-thoracic involvement defined by physician assessment of medical records.
Table 1.
Demographics
| Healthy Control | Sarcoidosis | p-Value | |
|---|---|---|---|
| N = 49 (%) | N = 104 (%) | ||
| Female | 31 (63) | 65 (63) | 0.93 |
| Age (years) | 45 ± 1.9 | 51 ± 0.92 | <0.001 |
| Race | 0.15 | ||
| African American | 5(10) | 15(14) | |
| White | 30(61) | 73 (70) | |
| Hispanic | 9(18) | 7 (6.7) | |
| Other Ethnicity | 5(10) | 9 (8.7) | |
| Ever Smokers | 15(31) | 47 (45) | 0.087 |
Table 2.
Sarcoidosis Subject Characteristics
| Imaging: Scadding Stage | N (%) |
|---|---|
| 0 | 9 (8.7) |
| 1 | 13 (13) |
| 2 | 49 (47) |
| 3 | 10 (10) |
| 4 | 23 (22) |
| Immunosuppression use | 56 (54) |
| Extra-thoracic involvement | 71 (69) |
| Pulmonary Function Tests | |||
|---|---|---|---|
| N | Mean (SD) | Range | |
| FVC %predicted | 96 | 94 (15) | 59 – 129 |
| FEV1 %predicted | 96 | 89 (17) | 28 – 133 |
| FEV1/FVC | 96 | 0.75 (0.092) | 0.34 – 0.99 |
| DLCO %predicted | 67 | 72 (15) | 39 – 108 |
| TLC %predicted | 63 | 94 (15) | 59 – 124 |
DLCO = Diffusing Capacity of the Lungs for Carbon Monoxide, FEV1 = Forced Expiratory Volume in 1 Second, FEV1/FVC = Forced Expiratory Volume in 1 Second to Forced Vital Capacity ratio, FVC = Forced Vital Capacity, TLC = Total Lung Capacity.
3.2. Chemokine levels were increased in sarcoidosis relative to healthy controls
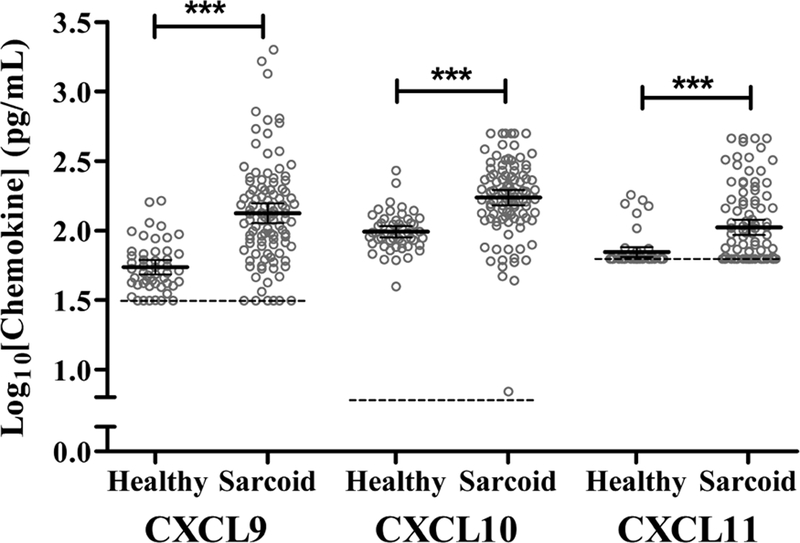
There were no statistically significant correlations between CXCL9, CXCL10, or CXCL11 levels and age, gender, or prior smoking status in either healthy controls or sarcoidosis subjects (p >0.05) in analyses that compared disease and health separately. While CXCL9 and CXCL10 levels did not vary by race, African American subjects, regardless of case status, had significantly higher CXCL11 levels than other races (p < 0.05). The levels of all three chemokines were increased in sarcoidosis subjects relative to healthy controls (p < 0.001) (Figure 1). Although sarcoidosis subjects were older on average than healthy controls, all chemokine levels were statistically significantly higher in sarcoidosis after adjusting for age, race, sex, and prior smoking status using ordered logistic regression. Amongst sarcoidosis subjects, there was no statistically significant difference in CXCL11 levels between those on or off immunosuppression treatment (p = 0.30).
Fig. 1.
Serum chemokine levels in sarcoidosis and healthy control subjects.
Levels of the CXCL9, CXL10, and CXCL11 were measured using Quanitikine® ELISA kits from 104 sarcoidosis subjects and 49 healthy control subjects. Measured values are displayed as log10 transformations of chemokines with bars designating mean values with 95% confidence intervals (*** = p < 0.001). The lower limits of assay detection are denoted by the dashed lines and grey open circles represent individual subject measurements. Abbreviations: “Healthy” = healthy control measurements; “Sarcoid” = sarcoidosis measurements.
3.3. Relationship of CXCL11 to inflammatory blood markers
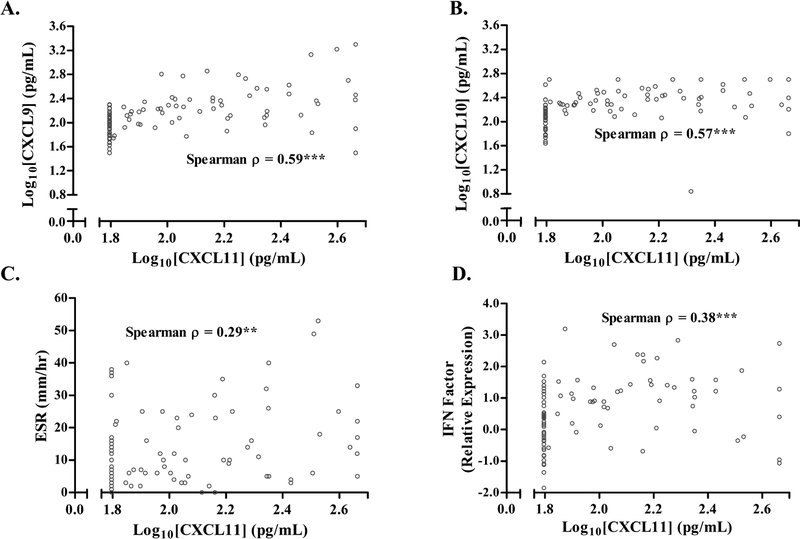
Among sarcoidosis subjects, we assessed the correlation of CXCL11 with inflammatory markers and genes related to IFN-γ signaling. CXCL11 levels had a statistically significant positive correlation with levels of CXCL9 and CXCL10 (Spearman ρ = 0.59 and 0.57, respectively) (Figure 2A–B), although CXCL9 and CXCL10 had a stronger correlation with each other (Spearman ρ = 0.718). In healthy controls, CXCL9 and CXCL10 were not statistically significantly correlated with CXCL11 and had a lower inter-correlation with each other (Spearman ρ = 0.32) (Supplemental Figure S1 A&B). CXCL11 was also positively correlated with erythrocyte sedimentation rate (ESR) (Spearman ρ = 0.38) (Figure 2C) while correlation with serum angiotensin-converting enzyme (ACE) level was weak (ρ=0.19, 0.062). We previously reported significant increases in gene transcript levels related to IFN-γ pathways from whole blood in sarcoidosis subjects relative to healthy controls [18]. Given that CXCL11 is known to be induced by IFN-γ, we evaluated its association with IFN-γ-related genes we previously measured. We found a statistically significant correlation between CXCL11 and STAT1, STAT2, and GBP1 separately or using the mean value of the three genes (previously referred to as the “IFN Factor”) (Figure 2D and Supplementary Table S1) [18]. CXCL11 was also positively correlated with several other IFN-γ related genes, including ICAM1, IRF1, IRF7, IRF9, and TAP1, with the greatest correlation occurring for TAP1 (a surface transporter involved in HLA-1 processing) and ICAM1 (a transmembrane protein involved in leukocyte trafficking), which have both have been implicated in sarcoidosis (ρ = 0.39 and 0.37, respectively, p < 0.01) (Supplementary Table S1) [27–30]. These correlations remained statistically significant after adjusting for age, sex, race, immunosuppression use, and prior smoking status using ordered logistic regression. These same associations were not seen between these genes and other markers such CRP, ESR, or ACE (p > 0.05), except for GBP1 and ACE (ρ = 0.21, p = 0.044). Sedimentation rate and the IFN Factor were not statistically significantly correlated with CXCL11 in healthy controls (Supplemental Figure S1 C&D). These results indicate that CXCL11 levels may be more specific for sarcoidosis-related IFN-γ-type inflammation compared to clinically available protein inflammatory markers (e.g. CRP and ESR).
Fig. 2.
Correlations of serum CXCL11 with other blood measurements in sarcoidosis subjects.
In sarcoidosis subjects, CXCL11 levels were positively correlated with A) CXCL9 and B) CXCL10, C) ESR and D) a gene expression composite score reflecting IFN-γ-related signaling (genes, GBP1, STAT1, and STAT2). Blood samples were processed and analyzed as described in Methods. Data are displayed as log10 transformation of individual CXCL11 levels (represented by open gray circles) with Spearman correlations (**p <0.01 and ***p<0.001). Abbreviations: ESR = Erythrocyte Sedimentation Rate, IFN Factor = Interferon Factor, IFN-γ = Interferon-gamma.
3.4. Relationship of CXCL11 to pulmonary function and organ involvement
CXCL11 levels were statistically significantly increased in subjects with abnormal (<80% predicted) FVC %pred, FEV1 %pred, and DLCO %pred (Figure 3A). We found that percent FVC %pred was negatively correlated with CXCL11 in analyses with or without adjusting for immunosuppression use and prior smoking using ordered logistic regression (p = 0.014) (Spearman ρ = −0.22, p = 0.031) (Figure 3B). There was a negative correlation with FEV1 %pred (ρ = −0.21, p = 0.058), which was significant after adjusting for immunosuppression use and prior smoking (p = 0.022) (Figure 4C). There was also a trend towards a negative correlation with DLCO (p = 0.095) in adjusted analyses. Comparison of CXCL11 levels with patient reported outcome scores for dyspnea where increasing score indicates more dyspnea showed a positive correlation using both adjusted and unadjusted analyses (Figure 4D) [22–24]. In contrast, there were no correlations between dyspnea scores and CXCL9 or CXCL10. Collectively these results show that CXCL11 correlates with objective and subjective and subjective measures of pulmonary measurements.
Fig. 3.
Relationships between serum CXCL11 levels and pulmonary function measurements and dyspnea scores.
A) CXCL11 levels were compared between sarcoidosis subjects dichotomized into two groups based on normal or abnormal spirometry and diffusing capacity measurements using a threshold of 80% predicted. B&C) Correlations between CXCL11 and both FVC %pred and FEV1 %pred. D) Correlations between CXCL11 and dyspnea scores where increasing score reflects more dyspnea. Data are displayed as A) log10 transformation of serum CXCL11 (individual levels denoted by grey open circles), bars representing mean values with 95% confidence intervals, and the lower limits of assay detection denoted by the dashed line; statistical significance was determined using the Wilcoxon rank-sum test (***p< 0.001 *p <0.05). For B-D), Spearman correlations of log10 transformation of CXCL11 levels (and Dyspnea scores) where indicated (*p <0.05, †p = 0.058).
Abbreviations: FEV1 %pred = Forced Expiratory Volume in 1 Second percent predicted, FVC %pred = Forced Vital Capacity percent predicted, DLCO %pred = Diffusing Capacity for Carbon Monoxide percent predicted.
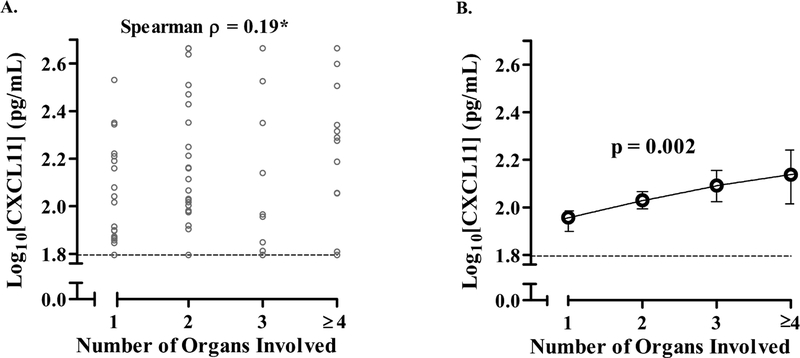
Fig. 4.
Relationship between serum CXCL11 levels and the number of organs involved with sarcoidosis.
A) Correlation of CXCL11 levels and number of organs involved in sarcoidosis subjects. Organ assessments were performed by study physician review of the patient’s records. Thoracic lymphadenopathy and/or parenchymal involvement was counted as one organ system (thoracic) and subjects with four or greater organs were counted as four or greater organs. B) Relationship between CXCL11 levels and organ involvement after adjusting for age, race, gender, and immunosuppression use. In A), data are displayed as the log10 transformation of individual CXCL11 levels (individual values denoted by open gray circles) with the lower limits of assay detection denoted by the dashed line and the Spearman correlation coefficient (*p < 0.05). In B), predicted values for log10CXCL11 at each organ number are plotted (open circles) with 95% confidence intervals based on a non-parametric regression model adjusting for age, race, gender, and immunosuppression use; statistical significance was calculated by bootstrap analysis with 1000 replicates (p = 0.002). Sample size for each number of organs involved 1) N = 36; 2) N = 35; 3) N = 16; > 4) N = 17.
We tested whether CXCL11 had an association with extra-thoracic organ involvement. First, using a binary grouping, we found higher CXCL11 levels in subjects who had more than one organ involved with disease as compared to only one organ involved (mean CXCL11 150 vs. 96 pg/mL, SD = 61 vs. 120, p = 0.032, N = 36 vs. 68). Next, to determine if there was a correlation between CXCLl1 and greater number of organs involved, we categorized subjects into groups with 1, 2, 3, or 4 or more organs and found a positive correlation (Spearman ρ = 0.19, p = 0.044). To determine if this positive correlation was present while adjusting for age, race, gender, and immunosuppression use, we performed a non-parametric Kernel regression [25, 26] and found that CXCL11 positively correlated with number of organs involved (p = 0.002) (Figure 4B). Similar results were found using both an adjusted linear and ordered logistic regression models (p = 0.047 and p = 0.038).
3.4. CXCL11 levels and longitudinal pulmonary function decline
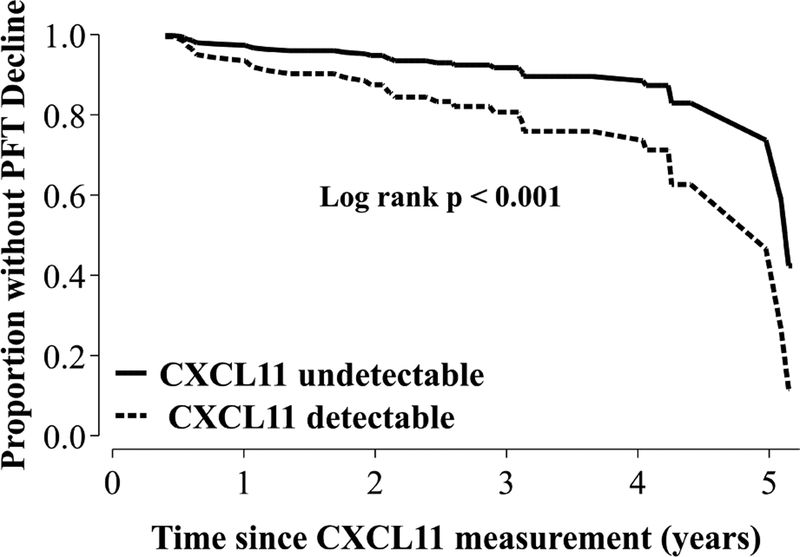
To determine the predictive value of CXCL11, we used longitudinal pulmonary function measures to identify subjects who had a decline in absolute FVC and DLCO values of ≥10% or ≥15%, respectively over the course of their follow-up (up to five years) (N = 33) [31]. We then performed a time to event analysis to determine if serum CXCL11 level measured at enrollment was predictive for risk of eventual PFT decline. To calculate total time at risk, we included all sarcoidosis subjects who had at least one follow-up visit and censored subjects at the time of their last follow-up study visit, which amounted to 226 person-years. We determined a log-rank statistic and Cox proportional hazard while adjusting for age, sex, race, and immunosuppression use, as well as initial CXR Scadding stage to account for initial radiographic burden of pulmonary involvement [32]. The adjusted log-rank test p-value for the effect of increasing CXCL11 on cumulative incidence of absolute FVC or DLCO decline was <0.001. We found a positive hazard ratio (HR) for CXCL11 for eventual PFT decline (HR of log10(CXCL11) = 5.1, 95% CI 1.2 – 21, p = 0.026). A relationship between CXCL11 and decline in lung function was also found if subjects were dichotomized as either having CXCL11 levels that were undetectable (62.5 pg/mL; N = 37) or detectable (>62.5 pg/mL; N = 48) (adjusted log-rank test p <0.001 and adjusted HR (2.5, 95% CI 1.1 – 5.7, p = 0.027) (Figure 5). These data show that CXCL11 is informative not only in cross-sectional assessment of disease severity, but also in longitudinal assessment of physiologic decline.
Fig. 5.
Relationship between serum CXCL11 measurement and longitudinal decline in lung function.
Thirty-three subjects were identified who experienced a decline in absolute FVC or DLCO of 10% or 15%, respectively, over the study period (5 years). Total time at risk for all subjects analyzed was 226 person-years. We used a Cox proportional hazard modeling that adjusted for age, race, sex, immunosuppression use, and initial radiographic burden of lung involvement as assessed by chest x-ray “Scadding Stage” to determine a hazard ratio for CXCL11. We also dichotomized subjects based on whether they had a CXCL11 level that was undetectable (<62.5 pg/mL, N = 37) or detectable (>62.5 pg/mL, N = 48). Using this dichotomous designation, the adjusted log rank p-value was <0.001 and the HR was also statically significant (hazard ratio 2.5, 95% CI 1.1 – 5.7 p = 0.027). Data are displayed as adjusted survival curves for detectable versus undetectable CXCL11 levels. Abbreviations: DLCO = Diffusing Capacity for Carbon Monoxide, FVC = Forced Vital Capacity, HR = hazard ratio.
4. Discussion
Sarcoidosis is a systemic disease involving granulomatous inflammation and a common inflammatory signature characterized by IFN-γ [5, 6, 16, 33]. In this study, we performed cross-sectional and longitudinal analyses to examine the pattern of induction of the chemokine, CXCL11, in relation to other IFN-induced chemokines and manifestations of sarcoidosis [17]. We found that CXCL11 was not only increased in sarcoidosis subjects relative to healthy controls, but also correlated with inflammatory blood markers as well as clinical measures of disease severity including pulmonary function tests, organ involvement, and patient-reported dyspnea. One of the major challenges in managing patients with sarcoidosis is determining their disease activity and overall prognosis given the lack of a gold standard test for granulomatous inflammation, aside from tissue biopsy, which is invasive and impractical to do longitudinally. Because of this, the heterogeneous nature of our cohort prevented us from establishing the relationship between CXCL11 and definitive presence of on-going granulomatous inflammation. However, we did find that CXCL11 had useful prognostic value given that subjects with higher CXCL11 levels had a shorter time to PFT decline, suggesting that those with higher CXCL11 levels could have worse outcomes. We believe that one of the reasons CXCL11 shows promise as a serum marker is because it reflects IFN-γ-mediated inflammation in the tissue.
CXCL11 recruits immune cells, including CD4+ T helper cells, to sites of inflammation in response to local production of IFN-γ [8, 34]. The potential relevance of this mechanism to sarcoidosis-associated granulomatous inflammation in the local environment has been suggested by previous studies showing that relative to healthy controls, sarcoidosis subjects have increased CXCL11 in bronchoalveolar lavage (BAL) fluid, alveolar macrophages, and lung tissue [12–14]. Our study takes this concept further by not only demonstrating that levels in the serum are also increased in sarcoidosis relative to health, but these serum levels also correlate with pulmonary disease manifestations. Furthermore, CXCL11 levels correlated with systemic manifestations, in addition to measures of pulmonary involvement, as evidenced by the positive correlation with total number of organs involved.
While other inflammatory markers have been shown to be increased in the peripheral blood of sarcoidosis subjects, including CRP and ESR, the potential value of CXCL11 as a biologic marker is that it is more specific for IFN-γ-mediated inflammation [35, 36]. In our analyses, neither of these traditional markers were correlated with the IFN-γ-related genes STAT1, STAT2, GBP1, TAP1, or ICAM1. Of note, ACE level was not associated with any of the measures of disease severity we analyzed, although it was increased in sarcoidosis subjects relative to healthy controls (data not shown) and weakly correlated with GBP1. Aside from sarcoidosis, the level of CXCL11 in the serum has shown promise as a predictive marker in other granulomatous diseases such as tuberculosis (TB). Kim et al., showed that whole blood Cxcl11 mRNA expression was increased in subjects with active pulmonary TB compared to healthy controls [37]. Other groups have also shown that CXCL11 levels (as well as CXCL9 and CXCL10) are increased in in the serum of subjects with confirmed active TB as compared to suspected TB cases and healthy controls [38, 39].
Although CXCL9 and CXCL10 are similar to CXCL11 with respect to their relationship to IFN-γ and the CXCR3 receptor, our specific interest in CXCL11 is driven by its unique properties. Mechanistically, CXCL9, CXCL10, and CXCL11 all bind to CXCR3, which has three splice variants (CXCR3A, CXCR3B, and CXCR3alt) [9]. All three chemokines bind to CXCR3A and CXCR3B, but CXCL11 is the only one that binds to the CXCR3alt spice variant, as well as the CXCR7 receptor [40, 41]. While some aspects of CXCL11 function are unclear, such as the fact that it has been shown to polarize T cells to a regulatory phenotype in mice, other properties, such as its unique affinity for CXCR7, suggest that it may serve specific roles in trafficking of leucocytes to areas of granulomatous inflammation [19]. For example, in a zebrafish model of mycobacterial infection, abrogation of expression of the orthologues of CXCL11 and CXCR3 resulted in decreased granulomatous inflammation [42]. In humans, although CXCR7 has not been found to be expressed by blood leucocytes from healthy human donors, it has been found to be expressed in atherosclerosis-related macrophages and upregulated during the transition of monocytes to tissue-infiltrating macrophages [43]. CXCL11 may thus play a role in both the trafficking of monocytes to areas of inflammation and in their differentiation into macrophages associated with granulomas.
This study has several important limitations. First, the sensitivity of the CXCL11 ELISA assay that we used had a lower limit of detection of 62.5 pg/mL. Future studies should try to use assays that have lower levels of detection to allow for better interpretation of values before this lower limit, which may even strengthen the associations we have reported. Second, because our cohort includes subjects who were not enrolled at the time of their diagnosis, our ability to extrapolate our data to newly-diagnosed subjects is limited and we cannot generalize about the prognostic information of CXCL11 levels at initial diagnosis. However, despite the lack of a gold standard to determine if subjects in our cohort had clinically active or resolved disease, the relationship between CXCL11 and both cross-sectional and longitudinal measures of disease severity suggest that has it value even in a heterogeneous population, which is important to clinicians treating sarcoidosis patients at all phases of their disease. Third, we also cannot address whether CXCL11 is associated with progressive fibrotic lung disease as our cohort sample size was too small to test this question. Lastly, we are cautious about interpreting CXCL11 in non-white populations given our sample size and recommend performing chemokine measurements in larger cohorts that include regions with higher prevalence of non-white populations, such as the GRADS cohort [44].
5. Conclusions and Future Directions
In summary, we provide evidence that CXCL11 is increased in the serum of sarcoidosis subjects relative to healthy controls. Moreover, serum CXCL11 appeared to correlate with measures of sarcoidosis disease burden, specifically organ involvement, respiratory symptom severity, and pulmonary function abnormalities. CXCL11 may also be useful in identifying those at risk for more rapid pulmonary function decline. Additional human-based and model system studies will be necessary to measure the expression of CXCL11 in patients with newly diagnosed sarcoidosis and to understand how CXCL11 and other interferon-related chemokines are modulated, either directly or indirectly, by corticosteroids. Determining the relationship of serum CXCL11 levels with circulating immune cells, such as monocytes and their expression of chemokine receptors would further the mechanistic understanding of this chemokine in the disease.
Supplementary Material
Highlights.
Serum CXCL11 was elevated in sarcoidosis subjects vs. healthy controls (p<0.001).
Serum CXCL11 positively correlated with CXCL9, CXCL10, ESR and IFN-γ-related genes.
Serum CXCL11 negatively correlated with pulmonary function measures (FEV1 & FVC).
Serum CXCL11 positively correlated with organs involvement in sarcoidosis subjects.
Subjects with increased CXCL11 levels suffered declines in PFTs more quickly.
Acknowledgements
The authors thank the following individuals for their specific contributions: Michael M. Li, B.S., Eli P.B. Darnell, B.S., and Suresh Garudadri, B.S., for assistance with sample acquisition, analysis, and management of the database; and Owen Solberg, Ph.D., for database programming. We would also like to thank all of the participants who volunteered their time for this study.
Funding This work was supported by the National Institutes of Health (R56IO87652 and T32HL007185).
Abbreviations:
- ATS
American Thoracic Society
- ACE
Angiotensin Converting Enzyme
- ANOVA
Analysis of Variance
- BAL
Bronchoalveolar lavage
- CRP
C-Reactive Protein
- ESR
Erythrocyte Sedimentation Rate
- DLCO
Diffusing Capacity of the Lungs for Carbon Monoxide
- ELISA
Enzyme-Linked Immunosorbent Assay
- FEV1
Forced Expiratory Volume in 1 Second
- FVC
Forced Vital Capacity
- FEV1FVC
Forced Expiratory Volume in 1 Second to Forced Vital Capacity ratio
- IFN Factor
Interferon Factor
- IFN-γ
Interferon-gamma
- %pred
Percent predicted
- PFT
Pulmonary Function Test
- TLC
Total Lung Capacity
- TB
Tuberculosis
- TNF-α
Tumor Necrosis Factor alpha
- UCSF
University of San Francisco
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest All the other authors declare that they have no conflict of interest.
Ethical Approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent Informed consent was obtained from all individual participants included in the study.
Research Involving Animal Studies This article does not contain any studies with animals performed by any of the authors.
References
- [1].Chappell AG, Cheung WY, Hutchings HA. Sarcoidosis: a long-term follow up study. Sarcoidosis, vasculitis, and diffuse lung diseases : official journal of WASOG. 2000;17(2):167–73. [PubMed] [Google Scholar]
- [2].Robinson BW, McLemore TL, Crystal RG. Gamma interferon is spontaneously released by alveolar macrophages and lung T lymphocytes in patients with pulmonary sarcoidosis. J Clin Invest. 1985;75(5):1488–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Prasse A, Georges CG, Biller H, Hamm H, Matthys H, Luttmann W, Virchow JC. Th1 cytokine pattern in sarcoidosis is expressed by bronchoalveolar CD4(+) and CD8(+) T cells. Clinical and experimental immunology. 2000;122(2):241–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Inui N, Chida K, Suda T, Nakamura H. TH1/TH2 and TC1/TC2 profiles in peripheral blood and bronchoalveolar lavage fluid cells in pulmonary sarcoidosis. The Journal of allergy and clinical immunology. 2001;107(2):337–44. [DOI] [PubMed] [Google Scholar]
- [5].Mollers M, Aries SP, Dromann D, Mascher B, Braun J, Dalhoff K. Intracellular cytokine repertoire in different T cell subsets from patients with sarcoidosis. Thorax. 2001;56(6):487–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Wahlstrom J, Katchar K, Wigzell H, Olerup O, Eklund A, Grunewald J. Analysis of intracellular cytokines in CD4+ and CD8+ lung and blood T cells in sarcoidosis. American journal of respiratory and critical care medicine. 2001;163(1):115–21. [DOI] [PubMed] [Google Scholar]
- [7].Kriegova E, Fillerova R, Tomankova T, Hutyrova B, Mrazek F, Tichy T, Kolek V, du Bois RM, Petrek M. T-helper cell type-1 transcription factor T-bet is upregulated in pulmonary sarcoidosis. European Respiratory Journal. 2011;38(5):1136–44. [DOI] [PubMed] [Google Scholar]
- [8].Groom JR, Luster AD. CXCR3 ligands: redundant, collaborative and antagonistic functions. Immunology and cell biology. 2011;89(2):207–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Van Raemdonck K, Van den Steen PE, Liekens S, Van Damme J, Struyf S. CXCR3 ligands in disease and therapy. Cytokine Growth Factor Rev. 2015;26(3):311–27. [DOI] [PubMed] [Google Scholar]
- [10].Proost P, Verpoest S, Van de Borne K, Schutyser E, Struyf S, Put W, Ronsse I, Grillet B, Opdenakker G, Van Damme J. Synergistic induction of CXCL9 and CXCL11 by Toll-like receptor ligands and interferon-gamma in fibroblasts correlates with elevated levels of CXCR3 ligands in septic arthritis synovial fluids. Journal of leukocyte biology. 2004;75(5):777–84. [DOI] [PubMed] [Google Scholar]
- [11].Loos T, Dekeyzer L, Struyf S, Schutyser E, Gijsbers K, Gouwy M, Fraeyman A, Put W, Ronsse I, Grillet B, Opdenakker G, Van Damme J, Proost P. TLR ligands and cytokines induce CXCR3 ligands in endothelial cells: enhanced CXCL9 in autoimmune arthritis. Laboratory investigation; a journal of technical methods and pathology. 2006;86(9):902–16. [DOI] [PubMed] [Google Scholar]
- [12].Piotrowski WJ, Mlynarski W, Fendler W, Wyka K, Marczak J, Gorski P, Antczak A. Chemokine receptor CXCR3 ligands in bronchoalveolar lavage fluid: associations with radiological pattern, clinical course, and prognosis in sarcoidosis. Polskie Archiwum Medycyny Wewnetrznej. 2014;124(7–8):395–402. [DOI] [PubMed] [Google Scholar]
- [13].Schnerch J, Prasse A, Vlachakis D, Schuchardt KL, Pechkovsky DV, Goldmann T, Gaede KI, Muller-Quernheim J, Zissel G. Functional Toll-Like Receptor 9 Expression and CXCR3 Ligand Release in Pulmonary Sarcoidosis. American journal of respiratory cell and molecular biology. 2016;55(5):749–57. [DOI] [PubMed] [Google Scholar]
- [14].Li H, Zhao X, Wang J, Zong M, Yang H. Bioinformatics analysis of gene expression profile data to screen key genes involved in pulmonary sarcoidosis. Gene. 2017;596:98–104. [DOI] [PubMed] [Google Scholar]
- [15].Nishioka Y, Manabe K, Kishi J, Wang W, Inayama M, Azuma M, Sone S. CXCL9 and 11 in patients with pulmonary sarcoidosis: a role of alveolar macrophages. Clinical and experimental immunology. 2007;149(2):317–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Koth LL, Solberg OD, Peng JC, Bhakta NR, Nguyen CP, Woodruff PG. Sarcoidosis blood transcriptome reflects lung inflammation and overlaps with tuberculosis. American journal of respiratory and critical care medicine. 2011;184(10):1153–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Su R, Nguyen ML, Agarwal MR, Kirby C, Nguyen CP, Ramstein J, Darnell EP, Gomez AD, Ho M, Woodruff PG, Koth LL. Interferon-inducible chemokines reflect severity and progression in sarcoidosis. Respir Res. 2013;14:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Su R, Li MM, Bhakta NR, Solberg OD, Darnell EP, Ramstein J, Garudadri S, Ho M, Woodruff PG, Koth LL. Longitudinal analysis of sarcoidosis blood transcriptomic signatures and disease outcomes. The European respiratory journal. 2014;44(4):985–93. [DOI] [PubMed] [Google Scholar]
- [19].Zohar Y, Wildbaum G, Novak R, Salzman AL, Thelen M, Alon R, Barsheshet Y, Karp CL, Karin N. CXCL11-dependent induction of FOXP3-negative regulatory T cells suppresses autoimmune encephalomyelitis. J Clin Invest. 2014;124(5):2009–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Hunninghake GW, Costabel U, Ando M, Baughman R, Cordier JF, du Bois R, Eklund A, Kitaichi M, Lynch J, Rizzato G, Rose C, Selroos O, Semenzato G, Sharma OP. ATS/ERS/WASOG statement on sarcoidosis. American Thoracic Society/European Respiratory Society/World Association of Sarcoidosis and other Granulomatous Disorders. Sarcoidosis, vasculitis, and diffuse lung diseases : official journal of WASOG. 1999;16(2):149–73. [PubMed] [Google Scholar]
- [21].Benn BS, Lehman Z, Kidd SA, Ho M, Sun S, Ramstein J, Arger NK, Nguyen CP, Su R, Gomez A, Gelfand JM, Koth LL. Clinical and Biological Insights from the University of California San Francisco Prospective and Longitudinal Cohort. Lung. 2017. [DOI] [PubMed] [Google Scholar]
- [22].Eakin EG, Resnikoff PM, Prewitt LM, Ries AL, Kaplan RM. Validation of a new dyspnea measure: the UCSD Shortness of Breath Questionnaire. University of California, San Diego. Chest. 1998;113(3):619–24. [DOI] [PubMed] [Google Scholar]
- [23].Swigris JJ, Yorke J, Sprunger DB, Swearingen C, Pincus T, du Bois RM, Brown KK, Fischer A. Assessing dyspnea and its impact on patients with connective tissue disease-related interstitial lung disease. Respiratory medicine. 2010;104(9):1350–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Swigris JJ, Han M, Vij R, Noth I, Eisenstein EL, Anstrom KJ, Brown KK, Fairclough D. The UCSD shortness of breath questionnaire has longitudinal construct validity in idiopathic pulmonary fibrosis. Respiratory medicine. 2012;106(10):1447–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Linton O, Nielsen JP. A Kernel-Method of Estimating Structured Nonparametric Regression-Based on Marginal Integration. Biometrika. 1995;82(1):93–100. [Google Scholar]
- [26].Delaigle A, Fan J, Carroll RJ. A Design-Adaptive Local Polynomial Estimator for the Errors-in-Variables Problem. Journal of the American Statistical Association. 2009;104(485):348–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kim DS, Paik SH, Lim CM, Lee SD, Koh Y, Kim WS, Kim WD. Value of ICAM-1 expression and soluble ICAM-1 level as a marker of activity in sarcoidosis. Chest. 1999;115(4):1059–65. [DOI] [PubMed] [Google Scholar]
- [28].Ma W, Lehner PJ, Cresswell P, Pober JS, Johnson DR. Interferon-gamma rapidly increases peptide transporter (TAP) subunit expression and peptide transport capacity in endothelial cells. The Journal of biological chemistry. 1997;272(26):16585–90. [DOI] [PubMed] [Google Scholar]
- [29].Foley PJ, Lympany PA, Puscinska E, Zielinski J, Welsh KI, du Bois RM. Analysis of MHC encoded antigen-processing genes TAP1 and TAP2 polymorphisms in sarcoidosis. American journal of respiratory and critical care medicine. 1999;160(3):1009–14. [DOI] [PubMed] [Google Scholar]
- [30].Schiffer R, Baron J, Dagtekin G, Jahnen-Dechent W, Zwadlo-Klarwasser G. Differential regulation of the expression of transporters associated with antigen processing, TAP1 and TAP2, by cytokines and lipopolysaccharide in primary human macrophages. Inflammation research : official journal of the European Histamine Research Society [et al. ]. 2002;51(8):403–8. [DOI] [PubMed] [Google Scholar]
- [31].Baughman RP, Teirstein AS, Judson MA, Rossman MD, Yeager H Jr, Bresnitz EA, DePalo L, Hunninghake G, Iannuzzi MC, Johns CJ, McLennan G, Moller DR, Newman LS, Rabin DL, Rose C, Rybicki B, Weinberger SE, Terrin ML, Knatterud GL, Cherniak R. Clinical characteristics of patients in a case control study of sarcoidosis. Am J Respir Crit Care Med. 2001;164(10 Pt 1):1885–9. [DOI] [PubMed] [Google Scholar]
- [32].Keir G, Wells AU. Assessing pulmonary disease and response to therapy: which test? Seminars in respiratory and critical care medicine. 2010;31(4):409–18. [DOI] [PubMed] [Google Scholar]
- [33].Prior C, Haslam PL. Increased levels of serum interferon-gamma in pulmonary sarcoidosis and relationship with response to corticosteroid therapy. The American review of respiratory disease. 1991;143(1):53–60. [DOI] [PubMed] [Google Scholar]
- [34].Groom JR, Luster AD. CXCR3 in T cell function. Exp Cell Res. 2011;317(5):620–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].McDonnell MJ, Saleem MI, Wall D, Gilmartin JJ, Rutherford RM, O’Regan A. Predictive value of C-reactive protein and clinically relevant baseline variables in sarcoidosis. Sarcoidosis, vasculitis, and diffuse lung diseases : official journal of WASOG. 2016;33(4):331–40. [PubMed] [Google Scholar]
- [36].Kamphuis LS, Bonte-Mineur F, van Laar JA, van Hagen PM, van Daele PL. Calcium and vitamin D in sarcoidosis: is supplementation safe? Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2014;29(11):2498–503. [DOI] [PubMed] [Google Scholar]
- [37].Kim S, Lee H, Kim H, Kim Y, Cho JE, Jin H, Kim DY, Ha SJ, Kang YA, Cho SN, Lee H. Diagnostic performance of a cytokine and IFN-gamma-induced chemokine mRNA assay after Mycobacterium tuberculosis-specific antigen stimulation in whole blood from infected individuals. The Journal of molecular diagnostics : JMD. 2015;17(1):90–9. [DOI] [PubMed] [Google Scholar]
- [38].Lee K, Chung W, Jung Y, Kim Y, Park J, Sheen S, Park K. CXCR3 ligands as clinical markers for pulmonary tuberculosis. The international journal of tuberculosis and lung disease : the official journal of the International Union against Tuberculosis and Lung Disease. 2015;19(2):191–9. [DOI] [PubMed] [Google Scholar]
- [39].Chung W, Lee K, Jung Y, Kim Y, Park J, Sheen S, Lee J, Kang D, Park K. Serum CXCR3 ligands as biomarkers for the diagnosis and treatment monitoring of tuberculosis. The international journal of tuberculosis and lung disease : the official journal of the International Union against Tuberculosis and Lung Disease. 2015;19(12):1476–84. [DOI] [PubMed] [Google Scholar]
- [40].Korniejewska A, McKnight AJ, Johnson Z, Watson ML, Ward SG. Expression and agonist responsiveness of CXCR3 variants in human T lymphocytes. Immunology. 2011;132(4):503–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Burns JM, Summers BC, Wang Y, Melikian A, Berahovich R, Miao Z, Penfold ME, Sunshine MJ, Littman DR, Kuo CJ, Wei K, McMaster BE, Wright K, Howard MC, Schall TJ. A novel chemokine receptor for SDF-1 and I-TAC involved in cell survival, cell adhesion, and tumor development. The Journal of experimental medicine. 2006;203(9):2201–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Torraca V, Cui C, Boland R, Bebelman JP, van der Sar AM, Smit MJ, Siderius M, Spaink HP, Meijer AH. The CXCR3-CXCL11 signaling axis mediates macrophage recruitment and dissemination of mycobacterial infection. Disease models & mechanisms. 2015;8(3):253–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Ma W, Liu Y, Ellison N, Shen J. Induction of C-X-C chemokine receptor type 7 (CXCR7) switches stromal cell-derived factor-1 (SDF-1) signaling and phagocytic activity in macrophages linked to atherosclerosis. The Journal of biological chemistry. 2013;288(22):15481–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Moller DR, Koth LL, Maier LA, Morris A, Drake W, Rossman M, Leader JK, Collman RG, Hamzeh N, Sweiss NJ, Zhang Y, O’Neal S, Senior RM, Becich M, Hochheiser HS, Kaminski N, Wisniewski SR, Gibson KF. Rationale and Design of the Genomic Research in Alpha-1 Antitrypsin Deficiency and Sarcoidosis (GRADS) Study. Sarcoidosis Protocol. Ann Am Thorac Soc. 2015;12(10):1561–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.