Supplemental Digital Content is available in the text.
Keywords: antiplatelet agents, efficacy, myocardial infarction, secondary prevention, stroke
Abstract
Background and Purpose—
We assessed the efficacy and safety of antiplatelet agents after noncardioembolic stroke or transient ischemic attack and examined how these vary according to patients’ demographic and clinical characteristics.
Methods—
We did a network meta-analysis (NMA) of data from 6 randomized trials of the effects of commonly prescribed antiplatelet agents in the long-term (≥3 months) secondary prevention of noncardioembolic stroke or transient ischemic attack. Individual patient data from 43 112 patients were pooled and reanalyzed. Main outcomes were serious vascular events (nonfatal stroke, nonfatal myocardial infarction, or vascular death), major bleeding, and net clinical benefit (serious vascular event or major bleeding). Subgroup analyses were done according to age, sex, ethnicity, hypertension, qualifying diagnosis, type of vessel involved (large versus small vessel disease), and time from qualifying event to randomization.
Results—
Aspirin/dipyridamole combination (RRNMA-adj, 0.83; 95% CI, 0.74–0.94) significantly reduced the risk of vascular events compared with aspirin, as did clopidogrel (RRNMA-adj, 0.88; 95% CI, 0.78–0.98), and aspirin/clopidogrel combination (RRNMA-adj, 0.83; 95% CI, 0.71–0.96). Clopidogrel caused significantly less major bleeding and intracranial hemorrhage than aspirin, aspirin/dipyridamole combination, and aspirin/clopidogrel combination. Aspirin/clopidogrel combination caused significantly more major bleeding than aspirin, aspirin/dipyridamole combination, and clopidogrel. Net clinical benefit was similar for clopidogrel and aspirin/dipyridamole combination (RRNMA-adj, 0.99; 95% CI, 0.93–1.05). Subgroup analyses showed no heterogeneity of treatment effectiveness across prespecified subgroups. The excess risk of major bleeding associated with aspirin/clopidogrel combination compared with clopidogrel alone was higher in patients aged <65 years than it was in patients ≥65 years (RRNMA-adj, 3.9 versus 1.7).
Conclusions—
Results favor clopidogrel and aspirin/dipyridamole combination for long-term secondary prevention after noncardioembolic stroke or transient ischemic attack, regardless of patient characteristics. Aspirin/clopidogrel combination was associated with a significantly higher risk of major bleeding compared with other antiplatelet regimens.
Stroke survivors are at increased risk of recurrent ischemic events, including recurrent stroke and myocardial infarction (MI).1 Particularly in the first hours and days after a transient ischemic attack (TIA) or stroke, risk of recurrence is high.2,3 Recurrent strokes lead to dementia more often and have higher case fatality than first strokes.4 Antiplatelet therapy is a cornerstone in secondary prevention and successfully reduces the frequency of vascular events5; for patients with noncardioembolic stroke or TIA the relative risk reduction of aspirin was 13%.6
Guidelines vary, but most recommend aspirin, aspirin/dipyridamole combination or clopidogrel as first-line treatment in long-term secondary prevention after noncardioembolic stroke or TIA.7,8 Given the mixed evidence and important differences between various antiplatelet agents, it becomes challenging for clinicians to select an optimal agent for an individual patient.
A few network meta-analyses (NMA) have been performed to compare the long-term efficacy of antiplatelet therapies among patients with stroke or TIA9–13; however, these analyses were performed on aggregated data from randomized controlled trials that included patients with different underlying causes of ischemic stroke/TIA. As a result, these analyses could not adequately restrict their study population to patients with noncardioembolic stroke or TIA, while appropriate use of antiplatelet drugs after TIA or ischemic stroke depends on whether the underlying cause is cardioembolic or not. Furthermore, these analyses based on published, aggregate data could not deal with differences in reported outcome definitions (eg, vascular death including or excluding hemorrhagic deaths from any origin). Also, not all trials reported the results of intracranial hemorrhage or major bleeding, thus some comparisons between antiplatelet therapies for safety outcomes were lacking. In addition, individual trials are usually not powered for subgroup analyses, and meta-analyses using published aggregate data on subgroups have substantial limitations because of the inability to systematically adjust for potential confounders. A pooled individual participant data analytic approach is most suitable for assessing subgroup effects with sufficient power and adequate adjustment for potential confounders.14
Therefore, we performed an individual patient data network meta-analysis (IPD-NMA) to compare the efficacy and safety of antiplatelet therapies frequently used for long-term secondary stroke prevention in patients with noncardioembolic stroke or TIA and among patient subgroups.
Methods
Data Availability
Requests for access to data from the Cerebrovascular Antiplatelet Trialists’ database will be considered by the Cerebrovascular Antiplatelet Trialists’ Steering Committee.
Study Population
A detailed description of the design of the IPD-NMA has been described elsewhere.15 Briefly, we obtained data for patients from trials investigating the efficacy of antiplatelet therapy in long-term secondary prevention after a TIA or ischemic stroke. Trials were eligible if they randomized patients with TIA or ischemic stroke to antiplatelet regimens (monotherapy or dual therapy) for long-term secondary prevention after stroke. Because homogeneity and consistency assumptions underlie NMA,16 we did not include RCTs assessing aspirin versus placebo because such studies had a wide range of daily doses (75–1500 mg). Although the benefit of aspirin is quite consistent at low, medium, and high doses of aspirin,6 side effects appear to be dose-related. Another reason for excluding these RCTs is that the evaluation of antiplatelet therapy versus placebo has become less clinically important. We also excluded randomized studies of short-duration (<3 months), those that only assessed surrogate outcomes, or those that specifically focused on patients with lacunar infarcts. Studies that examined triflusal, cilostazol, terutroban, ticlopidine, or dipyridamole alone were also excluded, as our interest was to evaluate the efficacy and safety of commonly prescribed antiplatelet regimens in patients with noncardioembolic ischemic stroke or TIA. We used the methods described in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement.
Data Extraction
For all eligible trials, we sought to obtain individual patient data. Data were obtained on the following baseline variables: demographics (age, sex, and ethnicity), smoking, medical history (hypertension, hypercholesterolemia, diabetes mellitus, history of stroke or TIA, history of cardiovascular disease, and history of heart failure), clinical presentation (nature of qualifying event [TIA versus minor ischemic stroke], type of vessel involved [small vessel disease versus large vessel disease] time from event to randomization, and severity of stroke at entry), and randomized treatment allocation (aspirin, clopidogrel, aspirin/dipyridamole combination, and aspirin/clopidogrel combination). Data were also obtained on the nature and timing of the following outcome variables: any recurrent stroke, recurrent ischemic stroke, MI, major bleeding, intracranial bleeding, and cause of any deaths. All data were merged into a single composite database, the Cerebrovascular Antiplatelet Trialists’ database. Detailed consideration was given to the definitions of baseline variables used in the original trials. When definitions were identical, comparable data were merged. If possible, differences in definitions of baseline variables between studies were resolved by reconstruction of definitions to achieve comparability. We excluded patients with a possible cardioembolic origin of their TIA or stroke (those with a history of atrial fibrillation or Trial of ORG 10172 in Acute Stroke Treatment [TOAST] classification cardioembolic stroke).
Redefinition of Outcome Events
Detailed consideration was given to the outcome definitions used in the original trial reports.
We accepted the reported definitions of ischemic stroke, intracranial hemorrhage (including intracerebral hemorrhage, subarachnoid hemorrhage, and epidural and subdural hematomas), all-cause mortality, death from nonvascular causes, and MI as defined by the trial investigators and did not attempt to retrospectively reclassify events.15 Composite outcome definitions of stroke and vascular death vary across the trials.15 For the combined analysis, subdural and epidural hematomas were counted as intracranial hemorrhages, but not as strokes.15 Vascular death includes hemorrhagic deaths from any origin.15
The primary efficacy outcomes of interest were serious vascular events (defined as the composite of stroke, MI, or vascular death) and ischemic events (composite of ischemic stroke, MI, or vascular death [excluding hemorrhagic death]). Primary safety outcomes were major (including fatal) bleeding and primary intracranial hemorrhage. There were minor differences in definition of major bleeding between trials,15 but designations made in the original trials were not changed. Major bleedings were fatal, intracranial, required hospital admission, or led to significant disability. Secondary exploratory outcomes included net clinical benefit outcome (defined as the composite of stroke, MI, vascular death, or major bleeding) and ischemic stroke.
Statistical Analysis
All analyses were by intention to treat based on the randomized treatment allocation. For each outcome, we cross-checked individual data against previous publications (Table I in the online-only Data Supplement). Second, we calculated unadjusted and adjusted risk ratios for each outcome within each trial with Poisson regression with robust SEs. In the adjusted analyses, we account for the following prespecified covariates: age, sex, hypertension, diabetes mellitus, current smoking, qualifying diagnosis (stroke versus TIA). Pooled unadjusted and adjusted risk ratios (RR) were obtained by random-effects network meta-analyses with package netmeta in R. We estimated ranking probabilities for all antiplatelet regimens of being at each possible rank for each treatment. The treatment hierarchy was summarized and reported as Surface Under the Cumulative Ranking Curve, which measures the average probability that a treatment is better than the competing treatments.17 The network results were assessed for consistency by comparing them with the results from individual trials or pairwise meta-analyses. To investigate the consistency of the primary results, we also did an analysis of patients who used treatment (on-treatment analysis), in which we included only the outcome events that arose while study treatment was being taken or before the 28th day after the discontinuation of treatment.
Third, to determine whether the results were affected by patient characteristics, we did subgroup network meta-analyses for the main outcomes (serious vascular events and major bleeding) according to the following characteristics: sex, age (<65 versus ≥65 years), ethnicity (Asian versus non-Asian), hypertension (yes versus no), qualifying diagnosis (stroke versus TIA), type of vessel involved (large versus small vessel disease), and time from qualifying event to randomization (≤21 days versus >21 days). These variables were selected following a review of risk scores, clinical guidelines, trial subgroup analyses, and clinical advice.7,8,18–23 All subgroup analyses are reported as adjusted effects (adjusted for the same prespecified covariates as in the primary analyses). Fourth, we performed several sensitivity analyses in which we either omitted the MATCH trial, where only patients with ischemic stroke/TIA at high vascular risk were included, or omitted the CHARISMA trial, where patients with previous symptomatic cerebrovascular disease within the previous 5 years were included, or omitted the ESPRIT trial in which an open, nonblinded study design was used. We did analyses with IBM SPSS Statistics (version 23), Review Manager (version 5.3), and R (version 3.3.1).
Results
Six trials (CAPRIE, ESPS-2, MATCH, CHARISMA, ESPRIT, and PRoFESS19–24) met the inclusion criteria, including 48 023 patients with a TIA or ischemic stroke recruited between 1989 and 2006. Table II in the online-only Data Supplement presents the main characteristics of the 6 trials. After exclusion of patients randomized to placebo or dipyridamole alone (n=3303) and patients with a possible cardioembolic origin of their stroke (n=1608), 43 112 patients remained for the analyses. The antiplatelet treatment comparisons are visualized in a network (Figure 1). Detailed results of the individual trials and pairwise meta-analyses are given in Table I in the online-only Data Supplement.
Figure 1.

Network of randomized controlled trial evidence. Ellipses represent comparators. Arrows represent comparisons of interventions for which trial data were available. Patient numbers represent the total number of patients enrolled in each trial informing the comparison of interest. ASA indicates aspirin; ASACLO, aspirin/clopidogrel combination; ASADIP, aspirin/dipyridamole combination; and CLO, clopidogrel.
Patient characteristics stratified by trial arm are presented in Table 1. The median time to randomization was 21 days (range, 15–124) and patients were followed for a median of 2.0 years (1.5–3.5). Mean age was 65±10 years and 36% were female. Ninety percent had a stroke as qualifying event and small vessel disease was diagnosed in 50% of the patients. Patient characteristics were similar between treatment options, except for a greater proportion of patients with vascular risk factors in patients treated with clopidogrel monotherapy or the aspirin/clopidogrel combination, and a greater proportion of patients with large vessel disease in patients treated with aspirin monotherapy. In terms of study quality, all 6 trials were rated as low risk of bias studies (Figure I in the online-only Data Supplement).
Table 1.
Baseline Characteristics of Patients Included in the Trials

Serious Vascular Events
A total of 5424 (12.6%) serious vascular events and 5022 (11.6%) ischemic events occurred. The adjusted NMA treatment effects are reported in Table 2. The results are consistent with the unadjusted NMA results, the results from individual trials or pairwise meta-analyses and the on-treatment analyses (Tables I, III, and IV in the online-only Data Supplement). Aspirin/dipyridamole combination significantly reduced the risk of serious vascular events compared with aspirin (RRNMA-adj, 0.83; 95% CI, 0.74–0.94), as did clopidogrel (RRNMA-adj, 0.88; 95% CI, 0.78–0.98), and aspirin/clopidogrel combination (RRNMA-adj, 0.83; 95% CI, 0.71–0.96). There were no statistically significant differences with respect to the occurrence of serious vascular event risks between patients taking clopidogrel, aspirin/dipyridamole combination, or aspirin/clopidogrel combination. Similarly, clopidogrel, aspirin/dipyridamole combination, and aspirin/clopidogrel combination significantly reduced the risk of ischemic events compared with aspirin (RRs range, 0.83–0.91); aspirin/dipyridamole combination and aspirin/clopidogrel combination significantly reduced the risk of ischemic stroke compared with aspirin.
Table 2.
Adjusted Treatment Effect Estimates From Network Meta-Analysis for Efficacy and Safety

Major Bleeding Events
In terms of safety, 1530 (3.5%) major bleedings and 380 (0.9%) intracranial hemorrhages occurred. Clopidogrel caused significantly less major bleeding (RRNMA-adj, 0.76; 95% CI, 0.63–0.91) and intracranial hemorrhage (RRNMA-adj, 0.63; 95% CI, 0.43–0.91) than aspirin. Aspirin/dipyridamole combination caused significantly more major bleeding (RRNMA-adj, 1.14; 95% CI, 1.00–1.30) and intracranial hemorrhage (RRNMA-adj, 1.40; 95% CI, 1.08–1.82) than clopidogrel. Aspirin/clopidogrel combination caused significantly more major bleeding than aspirin, clopidogrel, and aspirin/dipyridamole combination (Table 2).
The net clinical benefit outcome (serious vascular events or major bleeding) was similar for clopidogrel and aspirin/dipyridamole combination (RRNMA-adj, 0.99; 95% CI, 0.93–1.05). Risk of this combined outcome was reduced by clopidogrel (RRNMA-adj, 0.89; 95% CI, 0.82–0.96) and aspirin/dipyridamole (RRNMA-adj, 0.87; 95% CI, 0.80–0.95) compared with aspirin.
Sensitivity Analysis and Ranking
All results were similar after exclusion of 7252 (17%) patients with TIA/ischemic stroke at high vascular risk in MATCH, after exclusion of 4240 (10%) patients in CHARISMA who had symptomatic cerebrovascular disease within the previous 5 years, or after exclusion of 2739 (6%) patients randomized in ESPRIT in which an open, nonblinded study design was used (Table V in the online-only Data Supplement). The ranking of treatments based on cumulative probability (Surface Under the Cumulative Ranking Curve) is presented in Table VI in the online-only Data Supplement. In terms of efficacy, the most effective treatments were aspirin/clopidogrel combination and aspirin/ dipyridamole combination. Both treatments have a probability around 75% of being superior to a competing treatment. Clopidogrel has the highest probability of being the best treatment modality in terms of safety (99%), followed by aspirin/dipyridamole combination (65%). Combining the rankings for efficacy and safety indicates that both clopidogrel and aspirin/dipyridamole combination seemed to be best choices, because both had a favorable balance between efficacy and safety (Figure 2; Table VI in the online-only Data Supplement).
Figure 2.
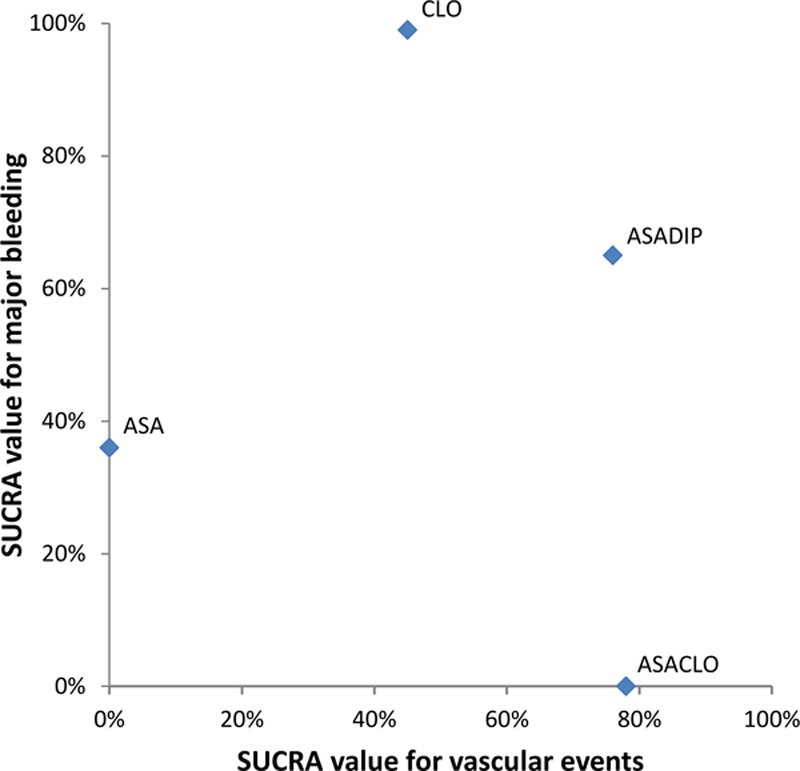
Clustered ranking plot for the outcomes serious vascular events and major bleeding. The probabilities of each treatment being ranked best in terms of efficacy (serious vascular events) and safety (major bleeding) outcomes are represented by their Surface Under the Cumulative Ranking curve (SUCRA) values. Treatments lying in the upper right corner are more effective in preventing serious vascular events, with lower propensity to cause major bleeding than the other treatments (highest net clinical benefit).
Subgroup Analyses
We also investigated whether the treatment effect differed between certain subgroups of patients (Table VII in the online-only Data Supplement). For serious vascular events, there was no evidence of heterogeneity of treatment effect across any of the prespecified subgroups. Excess risks of major bleeding were similar for most of the subgroups, apart from patient age. Aspirin/clopidogrel combination showed more major bleeding complications than clopidogrel, especially in younger patients. The adjusted excess risk for major bleeding varied from 1.7× higher (RRNMA-adj, 1.7; 95% CI, 1.3–2.2) in patients aged ≥65 years to a ≈4-fold excess risk (RRNMA-adj, 3.9; 95% CI, 2.5–6.0) in patients aged <65 years.
This subgroup effect was already apparent in the MATCH trial: patients older than 65 years assigned to aspirin/clopidogrel had a 1.6× increased risk of major bleeding (92 [3.0%/y] of 2169 patients versus 54 [1.8%/y] of 2097 assigned to clopidogrel; RRadj, 1.6; 95% CI, 1.2–2.3). Patients younger than 65 years assigned to aspirin/clopidogrel had a 4-fold increased risk of major bleeding (67 [3.2%/y] of 1466 patients versus 16 [0.7%/y] of 1520 assigned to clopidogrel; RRadj, 4·3; 95% CI, 2.5–7.5).
Discussion
Our collaborative IPD-NMA indicates that clopidogrel and aspirin/dipyridamole combination both showed a favorable balance between efficacy and safety. Benefits were seen across a wide range of subgroups.
Long-term combination of clopidogrel and aspirin resulted in significantly more major bleeding complications compared with aspirin or clopidogrel alone, doubling the number of events. Also, older age was positively associated with higher bleeding risks for all antiplatelet regimens. However, major bleeding risk did not further increase in older patients on the aspirin/clopidogrel combination compared with younger patients, indicating a risk ceiling effect of ≈3% per year. This effect is likely to be related to the fact that patients with high bleeding risks were not included in the trials, due to strict exclusion criteria or that their bleeding led to premature death. The unexpected effect of age on treatment effect observed by pooling these trials was present in the MATCH trial, but has not been reported. Our findings suggest that future trials of new antiplatelet regimens in long-term stroke prevention should examine risk of bleeding for younger and older patients separately. Also, coprescription of a proton-pump inhibitor could be considered in future studies, as has been suggested recently.25
To date, several network meta-analyses have been conducted to assess the effects of different antiplatelet regimens in the secondary stroke prevention.9–13 One NMA showed that the aspirin/dipyridamole combination was better than using clopidogrel or aspirin alone in the secondary prevention of serious vascular events after TIA or ischemic stroke10; this finding was not consistent with our analysis. We consider the main reason to be that results of the PRoFESS trial, which showed similar rates of recurrent stroke in patients receiving aspirin/dipyridamole combination and in patients receiving clopidogrel, were published after this review. In another NMA, Malloy et al11 reported that more bleeding events seemed to occur with the combination of aspirin and clopidogrel than with other treatments, and our results are in line with that finding. Two recent network analyses have shown that cilostazol had the best risk-benefit profile for long-term secondary prevention after stroke or TIA.12,13 We excluded trials that assessed cilostazol, since all trials that investigated the effect of cilostazol in the long-term secondary stroke prevention were performed in patients of Asian descent26–28; therefore the effect of cilostazol may not be generalizable to non-Asian populations. More randomized controlled trials in non-Asian patients are needed to determine whether the use of cilostazol is a good option for long-term secondary stroke prevention. Other conventional pairwise meta-analyses focused on the effect of short-term and long-term dual antiplatelet therapy compared with monotherapy.29,30 However, antiplatelet agents used in dual and single antiplatelet therapies varied across trials.
Analysis of individual patient data has advantages over meta-analysis of overall trial results. The availability of individual data for a large number of patients enabled us to make a more precise assessment of the relative treatment effects of antiplatelet agents than has been possible previously. One of the strengths of our study is the standardized definition of composite outcomes. Differences between the trials in the definition of composite outcomes made it previously impossible to combine reported aggregate results satisfactorily. Also, we could study safety outcomes such as major bleeding and intracranial hemorrhage in more detail and could restrict our study population to patients with noncardioembolic ischemic stroke or TIA. Furthermore, we were able to assess potential heterogeneous treatment effects among different subgroups. We did an IPD-NMA to combine the evidence from all relevant (direct and indirect) treatment comparisons into one single analysis, while fully preserving randomization. Although NMA has been criticized, results from conventional random-effects meta-analyses of direct within-trial comparisons were concordant with results from our IPD-NMA.
Our meta-analysis had some limitations. Although the sample size was large, the ability to provide adjusted treatment effect estimates for all subgroups analyzed was limited by the number of patients in each subgroup. Second, too few studies were available to be able to study between-trial heterogeneity. Third, we compared treatments for several relevant clinical outcomes and subgroups. Given the strong, predefined rationale (see published protocol15), we did not explicitly adjust for multiple comparisons. Fourth, trial populations were similar in many respects, but they varied in some entry criteria. These differences, however, allowed us to explore and confirm a consistent benefit across wide ranges of age, qualifying diagnoses, and additional patient characteristics. The consistency of results across all 6 trials suggests that our findings are generalizable to a broad range of patients with noncardioembolic ischemic stroke or TIA. Fifth, most patients in the secondary stroke prevention trials were already beyond the very early high-risk period after their initial TIA or stroke when recruited. We found no evidence for differences in treatment effects in patients randomized in the subacute and late phases, but acute effects might differ. The results of the POINT and CHANCE trial suggest that the aspirin/clopidogrel combination is beneficial over aspirin alone when initiated early after stroke and continued for about 3 weeks.31,32
Our findings raise questions about the mechanisms by which clopidogrel and aspirin/dipyridamole combination cause major bleeding. CYP2C19 genetic variants decrease the efficacy of clopidogrel, but no association between bleeding risk and carrier status is observed yet.31,33,34 It is therefore not clear if CYP2C19 genetic variants influence the risk of bleeding.
Hence, both clopidogrel and aspirin/dipyridamole combination can be used in the long-term secondary prevention of noncardioembolic stroke or TIA. The aspirin/clopidogrel combination significantly increases the risk of major bleeding compared with other antiplatelet regimens. Given the similar net clinical benefit outcome of clopidogrel and aspirin/dipyridamole combination, selection of antiplatelet therapy for the secondary prevention of stroke must be individualized according to patient needs, bleeding risks, and costs.
Acknowledgments
We thank Sanofi-Aventis and Bristol-Myers Squibb for giving access to the databases of CAPRIE, MATCH, and CHARISMA. We thank the ESPRIT Steering Committee for providing access to the ESPRIT data. Boehringer Ingelheim Pharmaceuticals, Inc supported this study by providing access to the clinical trial databases of ESPS-2 and PRoFESS.
Sources of Funding
This study was funded by grants from the Dutch Heart Foundation (grant 2013T128) and the Netherlands Organization for Health Research and Development (ZonMw; grant 916.11.129). The study was designed, conducted, analyzed, and interpreted by the investigators independent of all sponsors and pharmaceutical companies.
Disclosures
Dr Diener received honoraria for participation in clinical trials, contribution to advisory boards, or oral presentations from Abbott, Allergan, AstraZeneca, Bayer Vital, BMS, Boehringer Ingelheim, Daiichi-Sankyo, Johnson & Johnson, MSD, Medtronic, Novartis, Pfizer, Portola, Sanofi-Aventis, Servier, St. Jude, and WebMD. Dr Diener has no ownership interest and does not own stocks of any pharmaceutical company. Dr Diener received research grants from the German Research Council, German Ministry of Education and Research, European Union, National Institutes of Health, Bertelsmann Foundation and Heinz-Nixdorf Foundation. Drs Diener and Sacco received research support from Boehringer Ingelheim. Dr Bath has an ownership interest in Platelet Solutions Ltd and has received an advisory board fee from Sanofi and National Institute of Health Research funding. The other authors report no conflicts.
Supplementary Material
Footnotes
Guest Editor for this article was Jeffrey L. Saver, MD.
The online-only Data Supplement is available with this article at https://www.ahajournals.org/doi/suppl/10.1161/STROKEAHA.118.024497.
References
- 1.Touzé E, Varenne O, Chatellier G, Peyrard S, Rothwell PM, Mas JL. Risk of myocardial infarction and vascular death after transient ischemic attack and ischemic stroke: a systematic review and meta-analysis. Stroke. 2005;36:2748–2755. doi: 10.1161/01.STR.0000190118.02275.33. doi: 10.1161/01.STR.0000190118.02275.33. [DOI] [PubMed] [Google Scholar]
- 2.Rothwell PM, Warlow CP. Timing of TIAs preceding stroke: time window for prevention is very short. Neurology. 2005;64:817–820. doi: 10.1212/01.WNL.0000152985.32732.EE. doi: 10.1212/01.WNL.0000152985.32732.EE. [DOI] [PubMed] [Google Scholar]
- 3.Giles MF, Rothwell PM. Risk of stroke early after transient ischaemic attack: a systematic review and meta-analysis. Lancet Neurol. 2007;6:1063–1072. doi: 10.1016/S1474-4422(07)70274-0. doi: 10.1016/S1474-4422(07)70274-0. [DOI] [PubMed] [Google Scholar]
- 4.Pendlebury ST, Rothwell PM. Prevalence, incidence, and factors associated with pre-stroke and post-stroke dementia: a systematic review and meta-analysis. Lancet Neurol. 2009;8:1006–1018. doi: 10.1016/S1474-4422(09)70236-4. doi: 10.1016/S1474-4422(09)70236-4. [DOI] [PubMed] [Google Scholar]
- 5.Baigent C, Blackwell L, Collins R, Emberson J, Godwin J, Peto R, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet. 2009;373:1849–1860. doi: 10.1016/S0140-6736(09)60503-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Algra A, van Gijn J. Cumulative meta-analysis of aspirin efficacy after cerebral ischaemia of arterial origin. J Neurol Neurosurg Psychiatry. 1999;66:255. doi: 10.1136/jnnp.66.2.255. doi: 10.1136/jnnp.66.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.The European Stroke Organisation (ESO) Executive Committee and the ESO Writing Committee. Guidelines for management of ischaemic stroke and transient ischaemic attack 2008. Cerebrovasc Dis. 2008;25:457–507. doi: 10.1159/000131083. doi: 10.1159/000131083. [DOI] [PubMed] [Google Scholar]
- 8.Kernan WN, Ovbiagele B, Black HR, Bravata DM, Chimowitz MI, Ezekowitz MD, et al. American Heart Association Stroke Council, Council on Cardiovascular and Stroke Nursing, Council on Clinical Cardiology, and Council on Peripheral Vascular Disease. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:2160–2236. doi: 10.1161/STR.0000000000000024. doi: 10.1161/STR.0000000000000024. [DOI] [PubMed] [Google Scholar]
- 9.Kent DM, Thaler DE. Stroke prevention–insights from incoherence. N Engl J Med. 2008;359:1287–1289. doi: 10.1056/NEJMe0806806. doi: 10.1056/NEJMe0806806. [DOI] [PubMed] [Google Scholar]
- 10.Thijs V, Lemmens R, Fieuws S. Network meta-analysis: simultaneous meta-analysis of common antiplatelet regimens after transient ischaemic attack or stroke. Eur Heart J. 2008;29:1086–1092. doi: 10.1093/eurheartj/ehn106. doi: 10.1093/eurheartj/ehn106. [DOI] [PubMed] [Google Scholar]
- 11.Malloy RJ, Kanaan AO, Silva MA, Donovan JL. Evaluation of antiplatelet agents for secondary prevention of stroke using mixed treatment comparison meta-analysis. Clin Ther. 2013;35:1490.e7–1500.e7. doi: 10.1016/j.clinthera.2013.09.004. doi: 10.1016/j.clinthera.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 12.Xie W, Zheng F, Zhong B, Song X. Long-term antiplatelet mono- and dual therapies after ischemic stroke or transient ischemic attack: network meta-analysis. J Am Heart Assoc. 2015;4:e002259. doi: 10.1161/JAHA.115.002259. doi: 10.1161/JAHA.115.002259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Niu PP, Guo ZN, Jin H, Xing YQ, Yang Y. Antiplatelet regimens in the long-term secondary prevention of transient ischaemic attack and ischaemic stroke: an updated network meta-analysis. BMJ Open. 2016;6:e009013. doi: 10.1136/bmjopen-2015-009013. doi: 10.1136/bmjopen-2015-009013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koopman L, van der Heijden GJ, Hoes AW, Grobbee DE, Rovers MM. Empirical comparison of subgroup effects in conventional and individual patient data meta-analyses. Int J Technol Assess Health Care. 2008;24:358–361. doi: 10.1017/S0266462308080471. doi: 10.1017/S0266462308080471. [DOI] [PubMed] [Google Scholar]
- 15.Greving JP, Diener HC, Csiba L, Hacke W, Kappelle LJ, Koudstaal PJ, et al. Cerebrovascular Antiplatelet Trialists’ Collaborative Group. Individual patient data meta-analysis of antiplatelet regimens after noncardioembolic stroke or TIA: rationale and design. Int J Stroke. 2015;10(suppl A100):145–150. doi: 10.1111/ijs.12581. doi: 10.1111/ijs.12581. [DOI] [PubMed] [Google Scholar]
- 16.Caldwell DM, Gibb DM, Ades AE. Validity of indirect comparisons in meta-analysis. Lancet. 2007;369:270; author reply 271. doi: 10.1016/S0140-6736(07)60138-X. doi: 10.1016/S0140-6736(07)60138-X. [DOI] [PubMed] [Google Scholar]
- 17.Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol. 2011;64:163–171. doi: 10.1016/j.jclinepi.2010.03.016. doi: 10.1016/j.jclinepi.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 18.Wijnhoud AD, Maasland L, Lingsma HF, Steyerberg EW, Koudstaal PJ, Dippel DW. Prediction of major vascular events in patients with transient ischemic attack or ischemic stroke: a comparison of 7 models. Stroke. 2010;41:2178–2185. doi: 10.1161/STROKEAHA.110.580985. doi: 10.1161/STROKEAHA.110.580985. [DOI] [PubMed] [Google Scholar]
- 19.CAPRIE Steering Committee. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). Lancet. 1996;348:1329–1339. doi: 10.1016/s0140-6736(96)09457-3. doi: 10.1016/S0140-6736(96)09457-3. [DOI] [PubMed] [Google Scholar]
- 20.Diener HC, Bogousslavsky J, Brass LM, Cimminiello C, Csiba L, Kaste M, et al. MATCH Investigators. Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high-risk patients (MATCH): randomised, double-blind, placebo-controlled trial. Lancet. 2004;364:331–337. doi: 10.1016/S0140-6736(04)16721-4. doi: 10.1016/S0140-6736(04)16721-4. [DOI] [PubMed] [Google Scholar]
- 21.Bhatt DL, Fox KA, Hacke W, Berger PB, Black HR, Boden WE, et al. CHARISMA Investigators. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med. 2006;354:1706–1717. doi: 10.1056/NEJMoa060989. doi: 10.1056/NEJMoa060989. [DOI] [PubMed] [Google Scholar]
- 22.Halkes PH, van Gijn J, Kappelle LJ, Koudstaal PJ, Algra A The ESPRIT Study Group. Aspirin plus dipyridamole versus aspirin alone after cerebral ischaemia of arterial origin (ESPRIT): randomised controlled trial. Lancet. 2006;367:1665–1673. doi: 10.1016/S0140-6736(06)68734-5. doi: 10.1016/S0140-6736(06)68734-5. [DOI] [PubMed] [Google Scholar]
- 23.Sacco RL, Diener HC, Yusuf S, Cotton D, Ounpuu S, Lawton WA, et al. PRoFESS Study Group. Aspirin and extended-release dipyridamole versus clopidogrel for recurrent stroke. N Engl J Med. 2008;359:1238–1251. doi: 10.1056/NEJMoa0805002. doi: 10.1056/NEJMoa0805002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Diener HC, Cunha L, Forbes C, Sivenius J, Smets P, Lowenthal A. European Stroke Prevention Study. 2. Dipyridamole and acetylsalicylic acid in the secondary prevention of stroke. J Neurol Sci. 1996;143:1–13. doi: 10.1016/s0022-510x(96)00308-5. [DOI] [PubMed] [Google Scholar]
- 25.Li L, Geraghty OC, Mehta Z, Rothwell PM Oxford Vascular Study. Age-specific risks, severity, time course, and outcome of bleeding on long-term antiplatelet treatment after vascular events: a population-based cohort study. Lancet. 2017;390:490–499. doi: 10.1016/S0140-6736(17)30770-5. doi: 10.1016/S0140-6736(17)30770-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gotoh F, Tohgi H, Hirai S, Terashi A, Fukuuchi Y, Otomo E, et al. Cilostazol stroke prevention study: a placebo-controlled double-blind trial for secondary prevention of cerebral infarction. J Stroke Cerebrovasc Dis. 2000;9:147–157. doi: 10.1053/jscd.2000.7216. doi: 10.1053/jscd.2000.7216. [DOI] [PubMed] [Google Scholar]
- 27.Huang Y, Cheng Y, Wu J, Li Y, Xu E, Hong Z, et al. Cilostazol Versus Aspirin for Secondary Ischaemic Stroke Prevention Cooperation Investigators. Cilostazol as an alternative to aspirin after ischaemic stroke: a randomised, double-blind, pilot study. Lancet Neurol. 2008;7:494–499. doi: 10.1016/S1474-4422(08)70094-2. doi: 10.1016/S1474-4422(08)70094-2. [DOI] [PubMed] [Google Scholar]
- 28.Shinohara Y, Katayama Y, Uchiyama S, Yamaguchi T, Handa S, Matsuoka K, et al. CSPS 2 group. Cilostazol for prevention of secondary stroke (CSPS 2): an aspirin-controlled, double-blind, randomised non-inferiority trial. Lancet Neurol. 2010;9:959–968. doi: 10.1016/S1474-4422(10)70198-8. doi: 10.1016/S1474-4422(10)70198-8. [DOI] [PubMed] [Google Scholar]
- 29.Lee M, Saver JL, Hong KS, Rao NM, Wu YL, Ovbiagele B. Risk-benefit profile of long-term dual- versus single-antiplatelet therapy among patients with ischemic stroke: a systematic review and meta-analysis. Ann Intern Med. 2013;159:463–470. doi: 10.7326/0003-4819-159-7-201310010-00006. doi: 10.7326/0003-4819-159-7-201310010-00006. [DOI] [PubMed] [Google Scholar]
- 30.Wong KS, Wang Y, Leng X, Mao C, Tang J, Bath PM, et al. Early dual versus mono antiplatelet therapy for acute non-cardioembolic ischemic stroke or transient ischemic attack: an updated systematic review and meta-analysis. Circulation. 2013;128:1656–1666. doi: 10.1161/CIRCULATIONAHA.113.003187. doi: 10.1161/CIRCULATIONAHA.113.003187. [DOI] [PubMed] [Google Scholar]
- 31.Wang Y, Zhao X, Lin J, Li H, Johnston SC, Lin Y, et al. CHANCE Investigators. Association between CYP2C19 loss-of-function allele status and efficacy of clopidogrel for risk reduction among patients with minor stroke or transient ischemic attack. JAMA. 2016;316:70–78. doi: 10.1001/jama.2016.8662. doi: 10.1001/jama.2016.8662. [DOI] [PubMed] [Google Scholar]
- 32.Johnston SC, Easton JD, Farrant M, Barsan W, Conwit RA, Elm JJ, et al. Clinical Research Collaboration, Neurological Emergencies Treatment Trials Network, and the POINT Investigators. Clopidogrel and aspirin in acute ischemic stroke and high-risk TIA. N Engl J Med. 2018;379:215–225. doi: 10.1056/NEJMoa1800410. doi: 10.1056/NEJMoa1800410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McDonough CW, McClure LA, Mitchell BD, Gong Y, Horenstein RB, Lewis JP, et al. CYP2C19 metabolizer status and clopidogrel efficacy in the Secondary Prevention of Small Subcortical Strokes (SPS3) study. J Am Heart Assoc. 2015;4:e001652. doi: 10.1161/JAHA.114.001652. doi: 10.1161/JAHA.114.001652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang J, Zhao HD, Tan J, Ding YL, Gu ZQ, Zou JJ. CYP2C19 polymorphism and antiplatelet effects of clopidogrel in Chinese stroke patients. Pharmazie. 2013;68:183–186. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Requests for access to data from the Cerebrovascular Antiplatelet Trialists’ database will be considered by the Cerebrovascular Antiplatelet Trialists’ Steering Committee.


