Supplemental Digital Content is available in the text.
Keywords: copper, metals, molybdenum, rubidium, selenium, stroke, titanium
Abstract
Background and Purpose—
Circulating metals synchronously reflect multiple metal exposures from both natural and anthropogenic sources, which may be linked with the risk of stroke. However, there is a lack of prospective studies investigating the associations of multiple metal exposures with incident stroke.
Methods—
We performed a nested case-control study within the ongoing Dongfeng-Tongji cohort launched in 2008. A total of 1304 incident stroke cases (1035 ischemic strokes and 269 hemorrhagic strokes) were prospectively identified by December 31, 2016, and matched to incident identity sampled controls according to age (within 1 year), sex, and blood sampling date (within 1 month). We determined the concentrations of 24 plasma metals and assessed the associations of plasma multiple metal concentrations with incident stroke using conditional logistic regression and elastic net model.
Results—
The average follow-up was 6.1 years. After adjusting for established risk confounders, copper, molybdenum, and titanium were significantly associated with higher risk of ischemic stroke (odds ratios according to per interquartile range increase, 1.29 [95% CI, 1.13–1.46], 1.19 [95% CI, 1.05–1.35], and 1.30 [95% CI, 1.07–1.59]), whereas rubidium and selenium were associated with lower risk of hemorrhagic stroke (odds ratios according to per interquartile range increase, 0.66 [95% CI, 0.50–0.87] and 0.68 [95% CI, 0.51–0.91]). The predictive plasma metal scores based on multiple metal exposures were significantly associated with higher risk of ischemic and hemorrhagic stroke (adjusted odds ratios according to per interquartile range increase, 1.37 [95% CI, 1.20–1.56] and 1.53 [95% CI, 1.16–2.01]).
Conclusions—
Plasma copper, molybdenum, and titanium were associated with higher risk of ischemic stroke, whereas plasma rubidium and selenium were associated with lower risk of hemorrhagic stroke. These findings may have important public health implications given the ever-increasing burden of stroke worldwide.
Stroke is the second leading cause of death and has contributed to increasing disease burden globally.1 The situation is even worse in China, which had the highest estimated lifetime risk of stroke.2 The general populations are synchronously exposed to multiple metals existed in the natural environment. Consequently, these metals can disturb body’s metabolic functions and contribute to adverse health effects,3 including stroke. It is essential to explore the associations of multiple metal exposures with stroke. A recent meta-analysis of 37 studies with different design suggested that single metal exposures of arsenic, lead, and copper were associated with higher risk of cardiovascular disease (CVD).4 Several prospective studies have also assessed the associations between risk of CVD and single metal exposures such as arsenic,5 lead,6,7 and selenium8; however, no firm conclusion can be made about the associations of metal exposures with stroke risk. A meta-analysis based on 11 longitudinal studies observed drinking water arsenic across low-moderate to high levels were significantly associated with increased CVD incidence among 408 945 general populations.5 Nevertheless, this analysis found no significant association of arsenic exposure with incident stroke.5 A cohort study based on 13 946 US adults suggested baseline blood lead concentrations were prospectively associated with greater stroke mortality.6 However, a systematic review suggested the positive association of lead exposure with stroke was lack of sufficient evidence to deduce a causal relationship.7 According to a recent meta-analysis of 16 prospective studies, circulating selenium levels were associated with lower risk of incident CVD within the range of 55 to 145 μg/L.8 Currently, there is limited information about the association of copper exposure with risk of stroke. A prospective study conducted among 58 646 healthy Japanese revealed higher intake of copper from diet was associated with higher risk of stroke mortality (median copper intake of 1.21 mg/d for men and 1.10 mg/d for women).9
Metals’ mixture is one of the most prevalent pollution worldwide and coexisted in the environment.10 Effects of metals’ mixture cannot always be evaluated using simple additive assumptions; however, previous studies have only evaluated the relationship between single metal exposures and stroke risk. Prospective evidence on the associations of multiple metal exposures with incident stroke remains scarce. Therefore, we conducted a nested case-control study to prospectively estimate the associations of the concentrations of 24 plasma metals with incident stroke among Chinese adults.
Methods
The data, analytical methods of this study are available from the corresponding author upon reasonable request.
Population and Study Design
The design of DFTJ cohort (Dongfeng-Tongji) has been previously described.11 In brief, the DFTJ cohort is an ongoing prospective cohort study launched in Hubei Province of China. The cohort enrolled 27 009 retired employees of Dongfeng Motor Corporation during September 2008 and June 2010. In the first follow-up in 2013, the cohort newly enrolled 14 120 Dongfeng Motor Corporation retirees. Baseline information was collected through questionnaire survey and physical examination, and blood samples were drawn when the participants joined the cohort.
We conducted a nested case-control study based on the DFTJ cohort. Subjects without blood sample for metal detection at baseline (n=2517) and lost to follow-up (n=709) were excluded from the study. We further excluded participants with stroke (n=2500), coronary heart disease (n=5501), and cancer (n=2644) before the date of baseline blood sampling. After exclusion, a total of 29 763 participants were eligible for our analysis. All participants provided written informed consent, and the study was approved by the Ethics and Human Subject Committees of the Tongji Medical College.
Stroke Cases and Controls
Stroke was diagnosed based on the World Health Organization definition of stroke12 and imaging results consistent with stroke. Methods of determination of incident stroke are shown in the online-only Data Supplement. Stroke subtypes were carefully classified by physicians according to computerized tomography scan or magnetic resonance imaging diagnoses into 2 major categories: (1) ischemic stroke, including subtypes of large artery atherosclerosis infarction, lacunar infarction, cardioembolic infarction, other demonstrated cause of infarction, and undetermined cause of infarction according to the Trial of ORG 10172 in Acute Stroke Treatment classification;13 (2) hemorrhagic stroke, including subtypes of intracerebral hemorrhage and subarachnoid hemorrhage.
During an average of 6.1±2.3 years of follow-up, we identified 1304 incident stroke cases from the remaining 29 763 participants up to the date of December 31, 2016. These strokes included 1035 ischemic strokes (382 large artery atherosclerosis infarction, 206 cardioembolic infarction, 228 lacunar infarction, and 219 undetermined cause of infarction) and 269 hemorrhagic strokes (233 intracerebral hemorrhage and 36 subarachnoid hemorrhage). For each incident stroke case, we randomly selected a control from the remaining 29 763 eligible participants who were stroke-free at the time of the case event.14 Cases and controls were matched on age (within 1 year), sex, and blood sampling date (within 1 month).
Detection of Plasma Metals
The baseline blood samples were drawn in the morning after overnight fasting, collected in EDTA tubes, centrifuged, and stored at −80°C within 2 hours. We used Agilent 7700x inductively coupled plasma mass spectrometer to measure total concentrations of 24 plasma metals (aluminum, arsenic, barium, cobalt, copper, lead, manganese, mercury, molybdenum, nickel, rubidium, selenium, strontium, thallium, titanium, tungsten, vanadium, zinc) based on the previously described methods.15 We added gold into the diluent with the concentration of 100 μg/L to eliminate the memory effect of mercury.16
We included metals with detection rates above 80.0% in the analysis. Therefore, antimony, tin, and uranium with low detection rates (23.24%, 52.45%, and 21.74% of samples below the limits of detection, respectively, Table I in the online-only Data Supplement) were excluded from further analyses. According to previous metal correlation study and literature review,15 we considered plasma cadmium, chromium, and iron might not be reliable biomarkers of exposure and excluded them from further analyses. We also previously conducted metal variability study among 138 healthy participants within 5 years and calculated the intraclass correlation coefficients (ICCs) and Pearson correlation coefficients (rs) to assess the reproducibility of repeated measurements.15
Details of quality-control methods for metal measurement and definition of covariates are shown in the online-only Data Supplement.
Statistical Analyses
Baseline characteristics of cases and controls were compared using t tests or Mann-Whitney U tests for continuous variables and χ2 tests for categorical variables.
We applied conditional logistic regression models and restricted cubic splines to evaluate the associations of each plasma metal concentration with incident stroke. The final model was adjusted for potential stroke risk factors of body mass index, smoking status, drinking status, regular exercise, family history of stroke, hyperlipidemia, diabetes mellitus, and hypertension. Sensitivity analyses were conducted after excluding stroke events within 1 year after completion of their baseline survey (143 ischemic strokes and 42 hemorrhagic strokes). We further applied conditional logistic regression models to evaluate the effect of different metal exposures on subtypes of stroke. Moreover, we performed cross-sectional analyses to explore the associations of metal levels with stroke risk factors, included 1268 subjects without incident stroke.
Elastic net regression is a regularized regression combining the Lasso and Ridge penalties to avoid overfitting, reduce the occurrence of false positives, and improve prediction performance.17 We used elastic net regression models with 10-fold cross-validation to select stroke-associated metals. The predictive plasma metal scores were calculated as the weighted sum of selected multiple metals with weights equal to the regression coefficients according to elastic net model.
We evaluated the associations of predictive plasma metal scores with incident stroke by using conditional logistic regression with the adjustment of the same covariates. Stratified analyses were further conducted according to age, sex, body mass index, ever-smoking, hypertension, and hyperlipidemia. We further computed the area under the receiver operating characteristic curve, net reclassification improvement, and the integrated discrimination improvement to evaluate the improvement of predictive ability for plasma metal scores beyond traditional risk factors.
Analyses were performed using SAS version 9.3 (SAS Institute, Inc, Cary, NC) and R version 3.4.1.
Results
Characteristics of the Study Population
After 6.1±2.3 years of follow-up, a total of 1304 incident stroke cases were identified and classified into 1035 ischemic strokes and 269 hemorrhagic strokes. Among the population, mean age was 66.55±7.59 years, and 62.1% were men. Compared with the controls, we observed elevated body mass index, higher prevalence of diabetes mellitus, hypertension, and lower rate of stroke family history in the ischemic cases (Table II in the online-only Data Supplement). The incident hemorrhagic stroke cases were more likely to be hypertensive compared with their controls. Ischemic stroke cases also had higher concentrations of plasma copper and molybdenum and lower levels of plasma selenium at the baseline. Meanwhile, concentrations of molybdenum and strontium were higher in hemorrhagic stroke cases, whereas plasma mercury, rubidium, and selenium were lower.
Metal Exposures and Incident Stroke
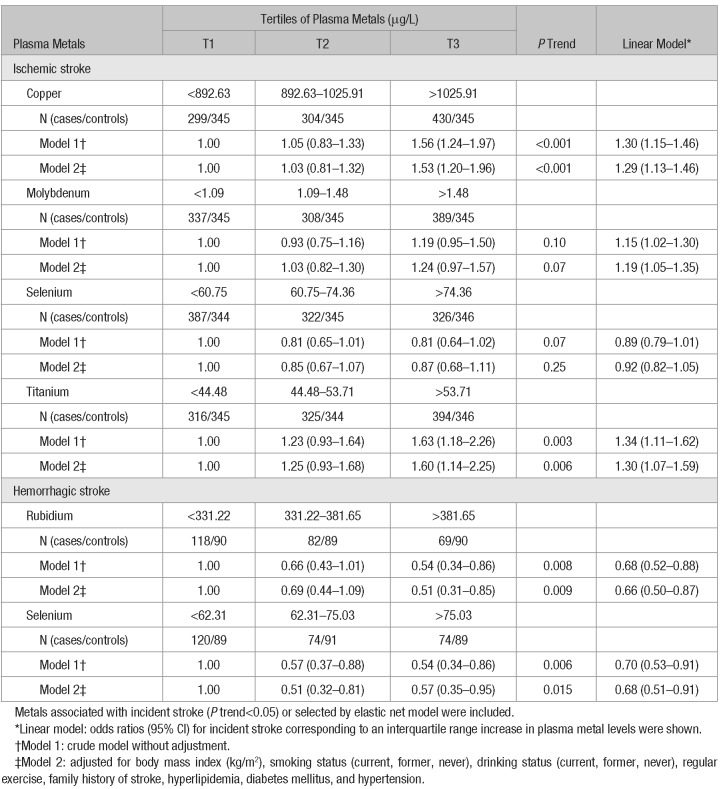
Table 1 presents the odds ratios (ORs; 95% CI) for incident stroke according to metals associated with incident stroke or selected by elastic net model. We observed the plasma concentrations of copper, molybdenum, and titanium were associated with higher risk of ischemic stroke. The multivariate-adjusted ORs (95% CI) for incident stroke per interquartile range increase were 1.29 (1.13–1.46; P trend<0.001) for copper, 1.19 (1.05–1.35; P trend=0.07) for molybdenum, and 1.30 (1.07–1.59; P trend=0.006) for titanium. In addition, the associations of plasma rubidium and selenium with lower risk of hemorrhagic stroke were identified. The multivariate-adjusted ORs (95% CI) according to per interquartile range increase were 0.66 (0.50–0.87; P trend=0.009) for rubidium and 0.68 (0.51–0.91, P trend=0.015) for selenium. The adjusted ORs (95% CI) for incident stroke according to 18 plasma metals were presented in Table III in the online-only Data Supplement.
Table 1.
Adjusted Odds Ratios (95% CI) for Incident Stroke According to Plasma Metals
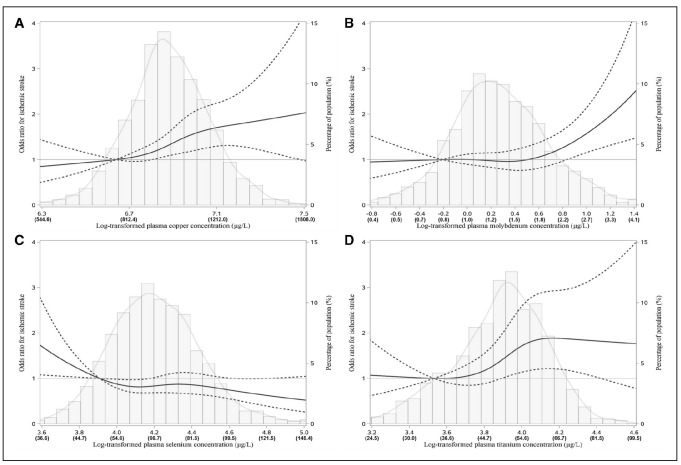
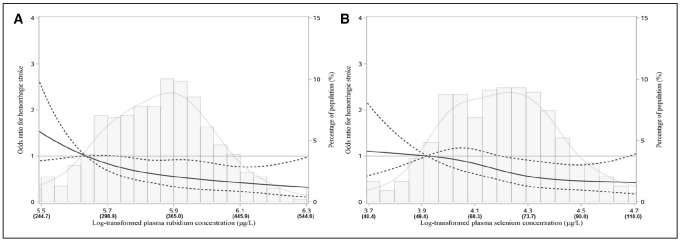
In the spline regression analysis, we observed significant positive linear relations between ischemic stroke and copper, titanium, and selenium (P<0.001, P=0.011, and P=0.043, respectively), and nonlinear association of molybdenum with ischemic stroke (P=0.006; Figure 1). In terms of hemorrhagic stroke, the inverse linear relations were identified for rubidium and selenium (P=0.001 and P=0.008, respectively; Figure 2). The associations did not materially change in the sensitivity analyses (Table IV, Figures I and II in the online-only Data Supplement).
Figure 1.
The restricted cubic spline for the associations of plasma metals with incident ischemic stroke. The restricted cubic spline for the associations of plasma copper (A), molybdenum (B), selenium (C), and titanium (D) with ischemic stroke. The bars represent histograms of plasma metal distribution among the total population. The lines represent adjusted odds ratios for the log-transformed levels of plasma metals in the conditional regression model. Knots were placed at the 20th, 40th, 60th, and 80th percentiles of the plasma metal distribution, and the reference value was set at the percentile of 10th. Models were adjusted for body mass index (kg/m2), smoking status (current, former, never), drinking status (current, former, never), regular exercise, family history of stroke, hyperlipidemia, diabetes mellitus, and hypertension.
Figure 2.
The restricted cubic spline for the associations of plasma metals with incident hemorrhagic stroke. The restricted cubic spline for the associations of plasma rubidium (A) and selenium (B) with hemorrhagic stroke. The bars represent histograms of plasma metal distribution among the total population. The lines represent adjusted odds ratios for the log-transformed levels of plasma metals in the conditional regression model. Knots were placed at the 20th, 40th, 60th, and 80th percentiles of the plasma metal distribution, and the reference value was set at the percentile of 10th. Models were adjusted for body mass index (kg/m2), smoking status (current, former, never), drinking status (current, former, never), regular exercise, family history of stroke, hyperlipidemia, diabetes mellitus, and hypertension.
In the analyses of effects of metal exposures on stroke subtypes, we observed plasma copper levels associated with higher risk of large artery atherosclerosis infarction (Table V in the online-only Data Supplement). We also found that plasma titanium concentrations were associated with higher risk of lacunar infarction, and plasma molybdenum levels were associated with higher risk of undetermined cause of infarction. As for hemorrhagic stroke, the associations of plasma rubidium and selenium with lower risk of intracerebral hemorrhagic were identified. However, there was no significant association between plasma metal levels and subarachnoid hemorrhage.
We further observed associations of plasma copper and titanium concentrations with higher prevalence of hyperlipidemia according to the cross-sectional analyses (Figure III in the online-only Data Supplement). Moreover, plasma molybdenum concentrations were associated with lower prevalence of overweight.
Multiple Metal Exposures and Incident Stroke
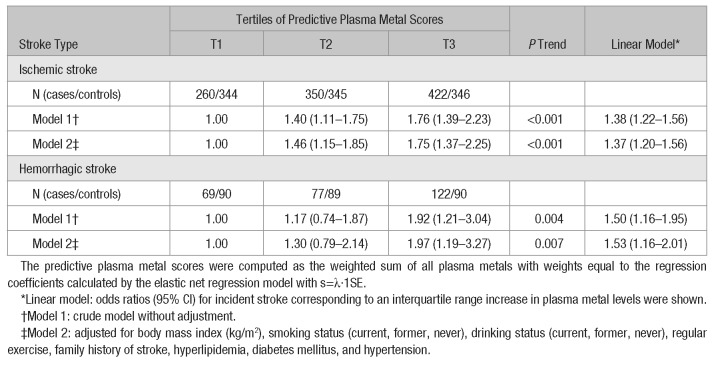
Elastic net model selected 3 metals (copper, molybdenum, and selenium) for ischemic stroke and 2 metals (rubidium and selenium) for hemorrhagic stroke to establish predictive plasma metal scores. The multivariate-adjusted ORs (95% CI) corresponding to an interquartile range increase in predictive plasma metal scores were 1.37 (1.20–1.56; P trend<0.001) for ischemic stroke and 1.53 (1.16–2.01; P trend=0.007) for hemorrhagic stroke (Table 2). According to the stratified analyses, associations of predictive plasma metal scores with hemorrhagic stroke were stronger among ever-smokers (P trend=0.006; Figure IV in the online-only Data Supplement).
Table 2.
Adjusted Odds Ratios (95% CI) for Incident Stroke According to Predictive Plasma Metal Scores
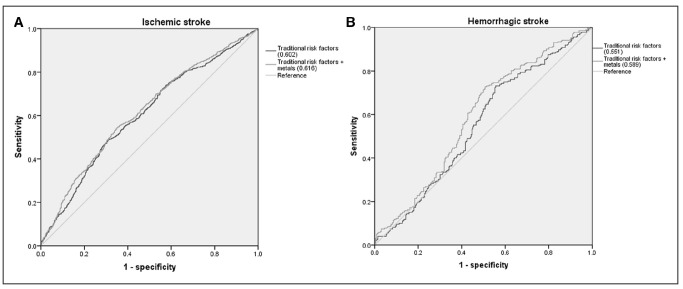
The addition of predictive plasma metal scores significantly improved area under the receiver operating characteristic curve beyond conventional model from 0.602 to 0.616 for ischemic stroke (P difference =0.025) and from 0.551 to 0.589 for hemorrhagic stroke (Pdifference =0.009, Figure 3). Compared with the conventional models, the estimated relative integrated discrimination improvements and net reclassification improvements were 24.53% (P<0.001) and 19.89% (P<0.001) for ischemic stroke, whereas 48.76% (P=0.010) and 17.23% (P=0.045) for hemorrhagic stroke.
Figure 3.
Receiver operator characteristic curves for prediction of incident stroke. Receiver operator characteristic curves for prediction of ischemic stroke (A) and hemorrhagic stroke (B). Blue curve represents conventional model including age, sex, body mass index (kg/m2), smoking status (current, former, never), drinking status (current, former, never), regular exercise, family history of stroke, hyperlipidemia, diabetes mellitus, and hypertension; green curve represents conventional model + metals selected by elastic net model with s=λ·1SE.
Discussion
This prospective study among Chinese population suggested plasma levels of copper, molybdenum, and titanium were significantly associated with higher risk of ischemic stroke, whereas rubidium and selenium were associated with lower risk of hemorrhagic stroke. Moreover, predictive plasma metal scores based on selected metals were significantly associated with higher risk of both ischemic and hemorrhagic stroke after adjusting for confounding factors. To our knowledge, this is the first prospective study in China to evaluate the associations of internal multiple metal exposures with incident stroke.
As for the metals identified associated with higher risk of ischemic stroke, copper is an essential, yet toxic, trace element for human body.18 Several prospective studies have provided evidence that copper exposure was associated with higher risk of CVD mortality.19,20 A cohort study comprising 4035 middle-aged French participants revealed that serum copper was associated with 30% elevation of CVD mortality by comparing the extreme quartiles (mean concentration of 952.5 μg/L).19 Another prospective investigation conducted among 3253 Germans also suggested the association between serum copper levels and greater risk of CVD mortality (median concentration of 1050 μg/L).20 Nevertheless, there is only one existing prospective study evaluating the association of copper exposure with stroke risk. It was conducted among 58 646 healthy Japanese, which suggested higher dietary intake of copper was associated with greater risk of stroke mortality.9 Copper was primarily distributed into plasma after absorption and transported to organs through peripheral circulation.21 Plasma copper levels, which kept a dynamic equilibrium with organs, are traditionally measured and used to reflect copper exposure status.18 The previous variability study indicated plasma copper was a reliable biomarker to reflect chronic exposure (ICC, 0.74; rs=0.72).15 Comparing with previous studies,19,20 the median concentration of 963.36 μg/L in our control group reflected a moderate level of copper exposure. Several investigations confirmed that copper would play a role in the development of carotid atherosclerosis,22,23 which is the main cause of ischemic stroke. Our analyses also suggested associations of plasma copper concentrations with increased prevalence of hyperlipidemia, which was a major carotid atherosclerosis factor.23 However, copper was also related to the impairment of endothelial function,24 promotion of reactive oxygen species production,25 and processes of inflammation,19,26 which may involve in carotid plagues and further contribute to cerebral ischemic injury.
The current study identified plasma selenium levels were associated with lower risk of hemorrhagic stroke. A meta-analysis of 16 prospective studies summarized a benefit of selenium within the range of 55 to 145 μg/L on CVD risk.8 Another meta-analysis (with 14 cohort studies) indicated a linearly inverse association of selenium with coronary heart disease risk throughout the selenium concentrations of 35 to 153 μg/L.27 Nevertheless, the association of selenium exposure with stroke risk was inconsistent. A cohort study of 13 887 US adults found no significant association of serum selenium (mean concentration of 125.6 μg/L) with stroke mortality.28 In a cohort study of 1103 Chinese subjects, no association was observed between stroke mortality and serum selenium (mean concentration of 73 μg/L).29 On the contrary, a cross-sectional study conducted among 2077 Canadian participants noted blood selenium to be associated with lower stroke prevalence.30 Plasma selenium was acknowledged as a reliable biomarker for chronic selenium exposure (ICC, 0.64; rs=0.73).15 Comparing with the reference values for trace elements (plasma selenium, 50–120 μg/L),31 the median concentration of 67.6 μg/L in our control group reflected a relatively low level of selenium exposure. The protective effect of selenium on stroke was primarily owing to its antioxidative properties and detoxification effects.32 Selenium is presented in antioxidant enzyme glutathione peroxidases as a cofactor, which would reduce lipids peroxidation and platelet aggregation.32 Selenium also prevent arteriosclerosis from metal-induced oxidative damage and formation of inactive complexes,33 thus to protect the cardiovascular system from cardiotoxic metals.34 Therefore, the association of plasma selenium with lower risk of stroke warrants further study to confirm the conclusion.
This study indicated a novel association of plasma molybdenum with higher ischemic stroke risk. Molybdenum is an essential element for humans, and molybdenum deficiency or excess may be associated with a series of diseases.35 A cross-sectional analysis beyond 20 293 US subjects recognized elevated urinary molybdenum (mean concentration of 69.10 µg/L) was associated with higher blood pressure.36 However, a cohort study demonstrated the simultaneous supplementation of molybdenum would reduce 8% of stroke mortality.37 As the molybdenum levels in blood, plasma, and urine are influenced by dietary intake, specific biomarker for molybdenum status has not been characterized.38 Compared with previous studies (ranged 0.3–1.1 µg/L),21 the median plasma molybdenum concentration of 1.27 µg/L in the current study was relatively high. Further investigations are warranted to explore the biomarkers for molybdenum status and to elucidate the underlying biological mechanisms of molybdenum on stroke.
Our analysis was also the first to reveal that plasma titanium concentrations were associated with higher risk of ischemic stroke. Titanium is widely used in metallic alloys, pharmaceuticals, and pigments in the forms of titanium compounds and is extensively exposed to the public.39 Nevertheless, there are scarce studies exploring its potential health effect on human health. We previously conducted a nested case-control study (1621 pairs) and observed a novel positive association of plasma titanium concentrations with incident coronary heart disease.15 Animal studies demonstrated that oral inhalation of TiO2 might be related to inflammatory response and lipid metabolism dysfunction, contributing to the cause of CVD.40 This study also showed plasma titanium levels were associated with higher prevalence of hyperlipidemia. According to previous study, plasma titanium shares good reproducibility over time (ICC, 0.56; rs=0.52).15 More studies are required to explore the metabolism of titanium and its health effect on stroke.
Our analysis firstly observed significant associations of plasma rubidium concentrations with lower hemorrhagic stroke risk. Although rubidium was reported to have anticancer efficacy,41 few studies have examined the association of rubidium with cardiovascular outcome. A recent cross-sectional study among 823 Chinese adults demonstrated urinary rubidium (median concentration of 1560 µg/g creatinine) was associated with lower risk of hypertension.42 On the contrary, an animal study found elevated rubidium concentrations in tissues among hypertensive rats.43 Although the reproducibility of plasma rubidium concentrations was satisfactory (ICC, 0.65; rs=0.51),15 a reliable biomarker for rubidium internal exposure needs to be confirmed.
Given the general populations were widely exposed to multiple metals, even a modest increment of stroke risk due to metal exposures could have substantial public health implications. Management of multiple metal exposures could potentially decrease the burden of stroke. Although the deficiency status of trace elements was linked to a series of diseases, the potential impact of excessive copper and molybdenum on human health was not viewed as a primary concern. This study implied copper and molybdenum exposures were potential risk factors of stroke, and therefore, it is necessary to establish their dietary reference values for stroke prevention. The association between selenium exposure and reduction of stroke risk indicated more clinical trials were needed to clarify the health effects of selenium supplementation. It is necessary to conduct more epidemiological and mechanism studies to explore the relation of titanium and rubidium with stroke. Moreover, because the general populations were simultaneously exposed to multiple metals in the real-world scenario, this study suggests a new approach for multiple metals risk assessment.
The strength of this study mostly lies on the prospective design and considerable sample size. Moreover, this study adopted appropriate analytical methods to select stroke-associated metals and systematically estimated the associations of multiple metal exposures with incident stroke. We also evaluated the associations of plasma metal concentrations with traditional stroke risk factors, which elucidated the potential biological mechanisms of metal exposures impact on stroke. Additionally, incident stroke events were carefully confirmed by the physicians following the standard criteria and further classified into subtypes; therefore, the potential undiagnosed and misdiagnosed cases were minimized.
This study also has potential limitations. First, plasma metals may not be appropriate biomarkers for various metal exposures. Therefore, we previously summarized plasma metals which were not suitable biomarkers of exposure15 and further excluded them from analyses. Second, there is no optimal approach to simultaneously reflect long-term exposure status for all metals, but reproducibility was fair to good for most plasma metals according to previous study.15 Finally, although we could not explore the sources of metals, circulating metals can be viewed as internal biomarkers integrating all sources of exposure.
Conclusions
We observed the plasma copper, molybdenum, and titanium were associated with higher risk of ischemic stroke, whereas plasma rubidium and selenium were associated with lower risk of hemorrhagic stroke. Our findings may have significant impact on public health, given the ever-increasing burden of stroke worldwide.
Acknowledgments
We thank all Dongfeng-Tongji cohort participants, staff, and investigators for their contribution to the study.
Sources of Funding
This work was supported by the Natural National Scientific Foundation of China (91643202 and 81390542); the Foundation of National Key Program of Research and Development of China (2016YFC0900800); the 111 Project and the Program for Changjiang Scholars and Innovative Research Team in University; Fundamental Research Funds for the Central Universities, Huazhong University of Science and Technology, China (2016YXZD045); Sanming Project of Medicine in Shenzhen (SZSM201511007); and the China Postdoctoral Science Foundation (2018M642858).
Disclosures
None.
Supplementary Material
Footnotes
The online-only Data Supplement is available with this article at https://www.ahajournals.org/doi/suppl/10.1161/STROKEAHA.119.025060.
References
- 1.Feigin VL, Norrving B, Mensah GA. Global burden of stroke. Circ Res. 2017;120:439–448. doi: 10.1161/CIRCRESAHA.116.308413. doi: 10.1161/CIRCRESAHA.116.308413. [DOI] [PubMed] [Google Scholar]
- 2.Feigin VL, Nguyen G, Cercy K, Johnson CO, Alam T, Parmar PG, et al. GBD 2016 Lifetime Risk of Stroke Collaborators. Global, regional, and country-specific lifetime risks of stroke, 1990 and 2016. N Engl J Med. 2018;379:2429–2437. doi: 10.1056/NEJMoa1804492. doi: 10.1056/NEJMoa1804492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rehman K, Fatima F, Waheed I, Akash MSH. Prevalence of exposure of heavy metals and their impact on health consequences. J Cell Biochem. 2018;119:157–184. doi: 10.1002/jcb.26234. doi: 10.1002/jcb.26234. [DOI] [PubMed] [Google Scholar]
- 4.Chowdhury R, Ramond A, O’Keeffe LM, Shahzad S, Kunutsor SK, Muka T, et al. Environmental toxic metal contaminants and risk of cardiovascular disease: systematic review and meta-analysis. BMJ. 2018;362:k3310. doi: 10.1136/bmj.k3310. doi: 10.1136/bmj.k3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moon KA, Oberoi S, Barchowsky A, Chen Y, Guallar E, Nachman KE, et al. A dose-response meta-analysis of chronic arsenic exposure and incident cardiovascular disease. Int J Epidemiol. 2017;46:1924–1939. doi: 10.1093/ije/dyx202. doi: 10.1093/ije/dyx202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Menke A, Muntner P, Batuman V, Silbergeld EK, Guallar E. Blood lead below 0.48 micromol/L (10 microg/dL) and mortality among US adults. Circulation. 2006;114:1388–1394. doi: 10.1161/CIRCULATIONAHA.106.628321. doi: 10.1161/CIRCULATIONAHA.106.628321. [DOI] [PubMed] [Google Scholar]
- 7.Navas-Acien A, Guallar E, Silbergeld EK, Rothenberg SJ. Lead exposure and cardiovascular disease–a systematic review. Environ Health Perspect. 2007;115:472–482. doi: 10.1289/ehp.9785. doi: 10.1289/ehp.9785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang X, Liu C, Guo J, Song Y. Selenium status and cardiovascular diseases: meta-analysis of prospective observational studies and randomized controlled trials. Eur J Clin Nutr. 2016;70:162–169. doi: 10.1038/ejcn.2015.78. doi: 10.1038/ejcn.2015.78. [DOI] [PubMed] [Google Scholar]
- 9.Eshak ES, Iso H, Yamagishi K, Maruyama K, Umesawa M, Tamakoshi A. Associations between copper and zinc intakes from diet and mortality from cardiovascular disease in a large population-based prospective cohort study. J Nutr Biochem. 2018;56:126–132. doi: 10.1016/j.jnutbio.2018.02.008. doi: 10.1016/j.jnutbio.2018.02.008. [DOI] [PubMed] [Google Scholar]
- 10.Kapraun DF, Wambaugh JF, Ring CL, Tornero-Velez R, Setzer RW. A method for identifying prevalent chemical combinations in the U.S. population. Environ Health Perspect. 2017;125:087017. doi: 10.1289/EHP1265. doi: 10.1289/EHP1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang F, Zhu J, Yao P, Li X, He M, Liu Y, et al. Cohort profile: the Dongfeng-Tongji cohort study of retired workers. Int J Epidemiol. 2013;42:731–740. doi: 10.1093/ije/dys053. doi: 10.1093/ije/dys053. [DOI] [PubMed] [Google Scholar]
- 12.Walker AE, Robins M, Weinfeld FD. The National Survey of Stroke. Clinical findings. Stroke. 1981;12(2 Pt 2)(suppl 1):I13–I44. [PubMed] [Google Scholar]
- 13.Adams HP, Jr, Biller J. Classification of subtypes of ischemic stroke: history of the trial of org 10172 in acute stroke treatment classification. Stroke. 2015;46:e114–e117. doi: 10.1161/STROKEAHA.114.007773. doi: 10.1161/STROKEAHA.114.007773. [DOI] [PubMed] [Google Scholar]
- 14.Lubin JH, Gail MH. Biased selection of controls for case-control analyses of cohort studies. Biometrics. 1984;40:63–75. [PubMed] [Google Scholar]
- 15.Yuan Y, Xiao Y, Feng W, Liu Y, Yu Y, Zhou L, et al. Plasma metal concentrations and incident coronary heart disease in Chinese adults: the Dongfeng-Tongji Cohort. Environ Health Perspect. 2017;125:107007. doi: 10.1289/EHP1521. doi: 10.1289/EHP1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Allibone J, Fatemian E, Walker PJ. Determination of mercury in potable water by ICP-MS using gold as a stabilising agent. J Anal At Spectrom. 1999;14:235–239. [Google Scholar]
- 17.Waldmann P, Mészáros G, Gredler B, Fuerst C, Sölkner J. Evaluation of the lasso and the elastic net in genome-wide association studies. Front Genet. 2013;4:270. doi: 10.3389/fgene.2013.00270. doi: 10.3389/fgene.2013.00270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bost M, Houdart S, Oberli M, Kalonji E, Huneau JF, Margaritis I. Dietary copper and human health: current evidence and unresolved issues. J Trace Elem Med Biol. 2016;35:107–115. doi: 10.1016/j.jtemb.2016.02.006. doi: 10.1016/j.jtemb.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 19.Leone N, Courbon D, Ducimetiere P, Zureik M. Zinc, copper, and magnesium and risks for all-cause, cancer, and cardiovascular mortality. Epidemiology. 2006;17:308–314. doi: 10.1097/01.ede.0000209454.41466.b7. doi: 10.1097/01.ede.0000209454.41466.b7. [DOI] [PubMed] [Google Scholar]
- 20.Grammer TB, Kleber ME, Silbernagel G, Pilz S, Scharnagl H, Lerchbaum E, et al. Copper, ceruloplasmin, and long-term cardiovascular and total mortality (the Ludwigshafen Risk and Cardiovascular Health Study). Free Radic Res. 2014;48:706–715. doi: 10.3109/10715762.2014.901510. doi: 10.3109/10715762.2014.901510. [DOI] [PubMed] [Google Scholar]
- 21.Ellingsen DG, et al. Molybdenum. In: Nordberg GF, Fowler BA, Nordberg M, editors. In: Handbook on the Toxicology of Metals. Burlington, VT: Academic Press; 2014. [Google Scholar]
- 22.Stadler N, Lindner RA, Davies MJ. Direct detection and quantification of transition metal ions in human atherosclerotic plaques: evidence for the presence of elevated levels of iron and copper. Arterioscler Thromb Vasc Biol. 2004;24:949–954. doi: 10.1161/01.ATV.0000124892.90999.cb. doi: 10.1161/01.ATV.0000124892.90999.cb. [DOI] [PubMed] [Google Scholar]
- 23.Ari E, Kaya Y, Demir H, Asicioglu E, Keskin S. The correlation of serum trace elements and heavy metals with carotid artery atherosclerosis in maintenance hemodialysis patients. Biol Trace Elem Res. 2011;144:351–359. doi: 10.1007/s12011-011-9103-0. doi: 10.1007/s12011-011-9103-0. [DOI] [PubMed] [Google Scholar]
- 24.Jiang Y, Wang LP, Dong XH, Cai J, Jiang GJ, Zhang C, et al. Trace amounts of copper in drinking water aggravate cerebral ischemic injury via impairing endothelial progenitor cells in mice. CNS Neurosci Ther. 2015;21:677–680. doi: 10.1111/cns.12427. doi: 10.1111/cns.12427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burkitt MJ. A critical overview of the chemistry of copper-dependent low density lipoprotein oxidation: roles of lipid hydroperoxides, alpha-tocopherol, thiols, and ceruloplasmin. Br J Pharmacol. 2001;394:117–135. doi: 10.1006/abbi.2001.2509. [DOI] [PubMed] [Google Scholar]
- 26.Ford ES. Serum copper concentration and coronary heart disease among US adults. Am J Epidemiol. 2000;151:1182–1188. doi: 10.1093/oxfordjournals.aje.a010168. doi: 10.1093/oxfordjournals.aje.a010168. [DOI] [PubMed] [Google Scholar]
- 27.Flores-Mateo G, Navas-Acien A, Pastor-Barriuso R, Guallar E. Selenium and coronary heart disease: a meta-analysis. Am J Clin Nutr. 2006;84:762–773. doi: 10.1093/ajcn/84.4.762. doi: 10.1093/ajcn/84.4.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bleys J, Navas-Acien A, Guallar E. Serum selenium levels and all-cause, cancer, and cardiovascular mortality among US adults. Arch Intern Med. 2008;168:404–410. doi: 10.1001/archinternmed.2007.74. doi: 10.1001/archinternmed.2007.74. [DOI] [PubMed] [Google Scholar]
- 29.Wei WQ, Abnet CC, Qiao YL, Dawsey SM, Dong ZW, Sun XD, et al. Prospective study of serum selenium concentrations and esophageal and gastric cardia cancer, heart disease, stroke, and total death. Am J Clin Nutr. 2004;79:80–85. doi: 10.1093/ajcn/79.1.80. doi: 10.1093/ajcn/79.1.80. [DOI] [PubMed] [Google Scholar]
- 30.Hu XF, Sharin T, Chan HM. Dietary and blood selenium are inversely associated with the prevalence of stroke among Inuit in Canada. J Trace Elem Med Biol. 2017;44:322–330. doi: 10.1016/j.jtemb.2017.09.007. doi: 10.1016/j.jtemb.2017.09.007. [DOI] [PubMed] [Google Scholar]
- 31.Wilhelm M, Ewers U, Schulz C. Revised and new reference values for some trace elements in blood and urine for human biomonitoring in environmental medicine. Int J Hyg Environ Health. 2004;207:69–73. doi: 10.1078/1438-4639-00260. doi: 10.1078/1438-4639-00260. [DOI] [PubMed] [Google Scholar]
- 32.Rayman MP. Selenium and human health. Lancet. 2012;379:1256–1268. doi: 10.1016/S0140-6736(11)61452-9. doi: 10.1016/S0140-6736(11)61452-9. [DOI] [PubMed] [Google Scholar]
- 33.Hu XF, Eccles KM, Chan HM. High selenium exposure lowers the odds ratios for hypertension, stroke, and myocardial infarction associated with mercury exposure among Inuit in Canada. Environ Int. 2017;102:200–206. doi: 10.1016/j.envint.2017.03.002. doi: 10.1016/j.envint.2017.03.002. [DOI] [PubMed] [Google Scholar]
- 34.Houston MC. Role of mercury toxicity in hypertension, cardiovascular disease, and stroke. J Clin Hypertens. 2011;13:621–627. doi: 10.1111/j.1751-7176.2011.00489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schwarz G, Belaidi AA. Molybdenum in human health and disease. Met Ions Life Sci. 2013;13:415–450. doi: 10.1007/978-94-007-7500-8_13. doi: 10.1007/978-94-007-7500-8_13. [DOI] [PubMed] [Google Scholar]
- 36.Shiue I, Hristova K. Higher urinary heavy metal, phthalate and arsenic concentrations accounted for 3-19% of the population attributable risk for high blood pressure: US NHANES, 2009-2012. Hypertens Res. 2014;37:1075–1081. doi: 10.1038/hr.2014.121. doi: 10.1038/hr.2014.121. [DOI] [PubMed] [Google Scholar]
- 37.Qiao YL, Dawsey SM, Kamangar F, Fan JH, Abnet CC, Sun XD, et al. Total and cancer mortality after supplementation with vitamins and minerals: follow-up of the Linxian General Population Nutrition Intervention Trial. J Natl Cancer Inst. 2009;101:507–518. doi: 10.1093/jnci/djp037. doi: 10.1093/jnci/djp037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Institute of Medicine (US) Panel on Micronutrients. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. Washington, DC: National Academy Press; 2001. [PubMed] [Google Scholar]
- 39.Shi H, Magaye R, Castranova V, Zhao J. Titanium dioxide nanoparticles: a review of current toxicological data. Part Fibre Toxicol. 2013;10:15. doi: 10.1186/1743-8977-10-15. doi: 10.1186/1743-8977-10-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen Z, Wang Y, Zhuo L, Chen S, Zhao L, Luan X, et al. Effect of titanium dioxide nanoparticles on the cardiovascular system after oral administration. Toxicol Lett. 2015;239:123–130. doi: 10.1016/j.toxlet.2015.09.013. doi: 10.1016/j.toxlet.2015.09.013. [DOI] [PubMed] [Google Scholar]
- 41.Su Y, Chen LJ, He JR, Yuan XJ, Cen YL, Su FX, et al. Urinary rubidium in breast cancers. Clin Chim Acta. 2011;412:2305–2309. doi: 10.1016/j.cca.2011.08.035. doi: 10.1016/j.cca.2011.08.035. [DOI] [PubMed] [Google Scholar]
- 42.Wu W, Jiang S, Zhao Q, Zhang K, Wei X, Zhou T, et al. Environmental exposure to metals and the risk of hypertension: a cross-sectional study in China. Environ Pollut. 2018;233:670–678. doi: 10.1016/j.envpol.2017.10.111. doi: 10.1016/j.envpol.2017.10.111. [DOI] [PubMed] [Google Scholar]
- 43.Gélinas Y, Schmit JP. Comparisons between the inorganic content of healthy and hypertensive rat tissues by inductively coupled plasma-mass spectrometry. Biometals. 1994;7:155–162. doi: 10.1007/BF00140486. [DOI] [PubMed] [Google Scholar]