Abstract
The appropriate treatment for patients with coexistent chronic obstructive pulmonary disease (COPD) and heart failure (HF) remains unclear. Data from the Taiwan National Health Insurance Research Database was used for this retrospective cohort study. Patients diagnosed with both diseases between 1997 and 2012 were enrolled as the COPD-heart failure overlap cohort. Patients were categorized as non-users and users of specific COPD and HF medications. Medication prescriptions in each 3-month and 1-year period served as time-dependent covariates. The primary endpoint was cumulative survival. The validation study confirmed the accuracy of definitions of COPD (94.0% sensitivity) and HF (96.3% sensitivity).
The study included 275,436 patients with COPD-heart failure overlap, with a mean follow-up period of 9.32 years. The COPD-heart failure overlap cohort had more medical service use and higher mortality than did the COPD alone cohort. Use of inhaled corticosteroid (ICS)/long-acting β2 agonist (LABA) combinations, long-acting muscarinic antagonist (LAMA), angiotensin receptor blockers (ARBs), β blockers, aldosterone antagonists, and statins reduced mortality risk compared with non-use. Sensitivity and subgroup analyses confirmed the consistency and robustness of results.
ICS/LABA combinations, LAMA, ARBs, β blockers, aldosterone antagonists, and statins use was associated with a lower mortality risk in patients with COPD-heart failure overlap.
Keywords: chronic obstructive pulmonary disease, heart failure, COPD-heart failure overlap, long-acting β2 agonist, long-acting muscarinic antagonist
INTRODUCTION
Chronic obstructive pulmonary disease (COPD) and heart failure (HF) are major global health issues characterized by high mortality and morbidity [1, 2]. The COPD-heart failure overlap leads to important therapeutic challenges. The cornerstones of COPD therapy are long-acting inhaled bronchodilators (anticholinergics and β2 agonists) with or without inhaled corticosteroids (ICSs) [1]. Improvements in medical treatment have shifted the treatment of HF from the use of positive inotropic agents to efforts to limit and slow its progression [2]. Angiotensin-converting enzyme inhibitors (ACEIs), angiotensin receptor blockers (ARBs), β blockers, and aldosterone antagonists reduce hospitalization and mortality in patients with HF.
Although β blockers are established therapy for HF, this class of drugs is often underused in patients with COPD-HF overlap [3]. Furthermore, patients receiving ARBs were less likely to have cough compared with those receiving ACEIs [4]. ACEI-related cough could lead to bronchial hyperresponsiveness [5]. Moreover, the presence of bronchial hyperresponsiveness in COPD is associated with increased mortality [6]. More evidence of the survival benefit of this therapy is needed. Therefore, the aim of this study was to investigate the survival effects of medications recommended in guidelines for patients with COPD-heart failure overlap using a population-based nationwide dataset.
RESULTS
Clinical characteristics of the study population
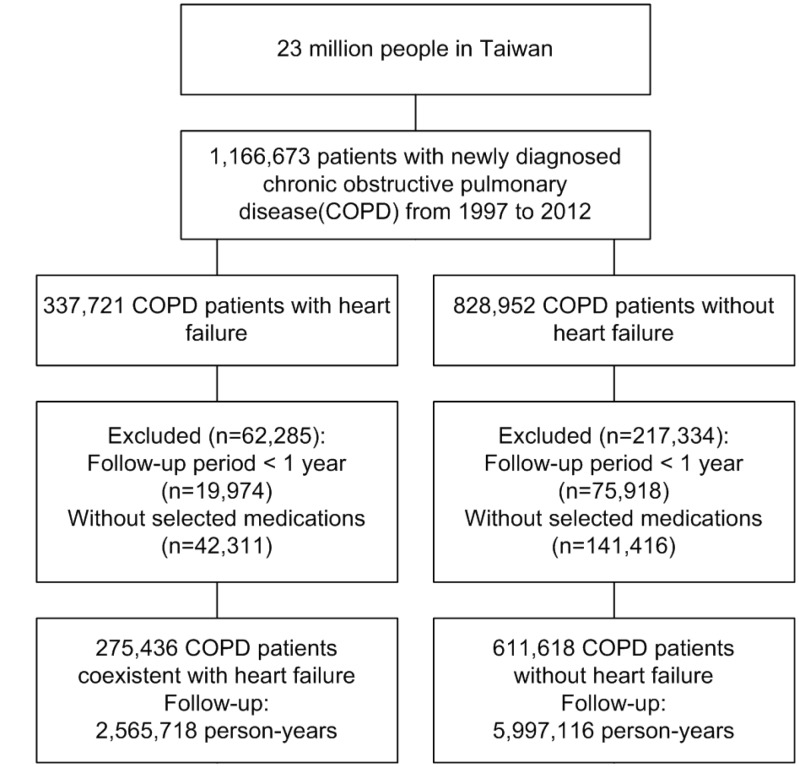
After the application of exclusion criteria, the study cohort consisted of 611,618 patients with COPD alone and 275,436 patients with COPD-heart failure overlap (Figure 1). The mean follow-up periods for the COPD-heart failure overlap and COPD alone cohorts were 9.32 and 9.81 years, respectively. Table 1 shows the baseline characteristics of the cohorts. The percentage of COPD-heart failure overlap patients increased significantly with age at the time of COPD diagnosis; the greatest proportion of these patients was aged ≥80 years (total, 45.90%; male, 40.56%; female, 53.66%; Figure 2A–2C). Compared with the COPD alone cohort, the COPD-heart failure overlap cohort was older (mean age, 64.38 vs. 70.63 years; p < 0.0001) and contained fewer men (64.02% vs. 59.14%; p < 0.0001). Compared with the COPD alone cohort, the COPD-heart failure overlap cohort had more outpatient visits (mean, 2.44 vs. 2.79), hospitalizations for COPD or HF (mean, 0.15 vs. 0.46; all p < 0.0001) per year.
Figure 1.
Flow diagram summarizing the process of enrollment.
Table 1. Characteristics of the COPD-heart failure overlap cohort and COPD alone cohort.
| Characteristics | COPD-heart failure overlap cohort | COPD alone cohort | p value | ||
| n | % | n | % | ||
| N | 275436 | 611618 | |||
| Age, years (mean ± SD) | 70.63±9.87 | 64.38±11.83 | <.0001 | ||
| Age | <.0001 | ||||
| <65 | 65199 | 23.67 | 287901 | 47.07 | |
| ≥65 | 210237 | 76.33 | 323717 | 52.93 | |
| Follow-up, years(mean ± SD) | 9.32±4.26 | 9.81±4.26 | <.0001 | ||
| Sex | <.0001 | ||||
| Female | 112546 | 40.86 | 220045 | 35.98 | |
| Male | 162890 | 59.14 | 391573 | 64.02 | |
| Comorbidities | |||||
| Diabetes mellitus | 101903 | 37.00 | 180785 | 29.56 | <.0001 |
| Hypertension | 224020 | 81.33 | 359818 | 58.83 | <.0001 |
| Cerebrovascular disease | 76127 | 27.64 | 121677 | 19.89 | <.0001 |
| Ischemia heart disease | 143048 | 51.94 | 189921 | 31.05 | <.0001 |
| Malignancy | 39000 | 14.16 | 105500 | 17.25 | <.0001 |
| Cirrhosis | 68138 | 24.74 | 171537 | 28.05 | <.0001 |
| Chronic kidney disease | 14457 | 5.25 | 8962 | 1.47 | <.0001 |
| Charlson Comorbidity Index | |||||
| 0–1 | 87422 | 31.74 | 294082 | 48.08 | <.0001 |
| >1 | 188014 | 68.26 | 317536 | 51.92 | <.0001 |
| Urbanization | |||||
| I | 58921 | 21.39 | 157047 | 25.68 | <.0001 |
| II | 113363 | 41.16 | 267800 | 43.78 | <.0001 |
| III | 64095 | 23.27 | 115765 | 18.93 | <.0001 |
| IV | 39057 | 14.18 | 71006 | 11.61 | <.0001 |
| Income level | |||||
| 0 | 64631 | 23.47 | 111657 | 18.26 | <.0001 |
| 1–15840 | 61442 | 22.31 | 117733 | 19.25 | <.0001 |
| 15841–25000 | 129684 | 47.08 | 286852 | 46.90 | <.0001 |
| ≧25000 | 19679 | 7.14 | 95376 | 15.59 | <.0001 |
| OPD visit/year(first year) | 2.79±4.96 | 2.44±4.26 | <.0001 | ||
| Exacerbation frequency of COPD (AE/year) | |||||
| 0 AE/year | 134269 | 48.75 | 449378 | 73.47 | <.0001 |
| >0, <1 AE/year | 117834 | 42.78 | 138584 | 22.66 | <.0001 |
| ≧1, <2 AE/year | 15114 | 5.49 | 15384 | 2.52 | <.0001 |
| ≧2, <3 AE/year | 4586 | 1.66 | 4582 | 0.75 | <.0001 |
| ≧ 3AE/year | 3633 | 1.32 | 3690 | 0.60 | <.0001 |
| Exacerbation frequency of HF (AE/year) | |||||
| 0 AE/year | 116224 | 42.20 | |||
| >0, <1 AE/year | 143611 | 52.14 | |||
| ≧1, <2 AE/year | 11197 | 4.07 | |||
| ≧2, <3 AE/year | 2792 | 1.01 | |||
| ≧ 3AE/year | 1612 | 0.58 | |||
| Hospitalization for COPD and HF/year (mean ± SD) | 0.46±0.75 | 0.15±0.49 | <.0001 | ||
| COPD Medications | |||||
| SABDs | 271527 | 98.58 | 592993 | 96.95 | <.0001 |
| LABAs alone | 69496 | 25.23 | 201216 | 32.90 | <.0001 |
| ICSs alone | 53268 | 19.34 | 112995 | 18.47 | <.0001 |
| ICS/LABA combinations | 60670 | 22.03 | 137063 | 22.41 | <.0001 |
| LAMA | 23948 | 8.69 | 54519 | 8.91 | <.0001 |
| HF Medications | |||||
| ACEIs | 178634 | 64.85 | 251327 | 41.09 | <.0001 |
| ARBs | 166788 | 60.55 | 262085 | 42.85 | <.0001 |
| Cardioselective β-blockers | 77381 | 28.09 | 124213 | 20.31 | <.0001 |
| Non-selective β-blockers | 64739 | 23.50 | 60261 | 9.85 | <.0001 |
| Loop Diuretics | 190593 | 69.20 | 265535 | 43.42 | <.0001 |
| Aldosterone antagonists | 81199 | 29.48 | 77585 | 12.69 | <.0001 |
| Digoxins | 105209 | 38.20 | 61995 | 10.14 | <.0001 |
| Statins | 88952 | 32.29 | 186115 | 30.43 | <.0001 |
Abbreviations: COPD, chronic obstructive pulmonary disease; HF, heart failure; SD, standard deviation; OPD, outpatient department; AE, acute exacerbation; SABDs, short-acting bronchodilators; LABAs, long-acting beta-agonists; LAMA, long-acting muscarinic antagonist; ICSs, inhaled corticosteroids; ACEIs, angiotensin-converting-enzyme inhibitors; ARBs, angiotensin receptor blockers.
Figure 2.
(A) Percentage of patients with COPD-heart failure overlap (COPD+HF) in different age groups. (B) Percentage of patients with COPD-heart failure overlap (COPD+HF) in different age groups in male sex. (C) Percentage of patients with COPD-heart failure overlap (COPD+HF) in different age groups in female sex.
Risk factors for mortality in patients with COPD-heart failure overlap
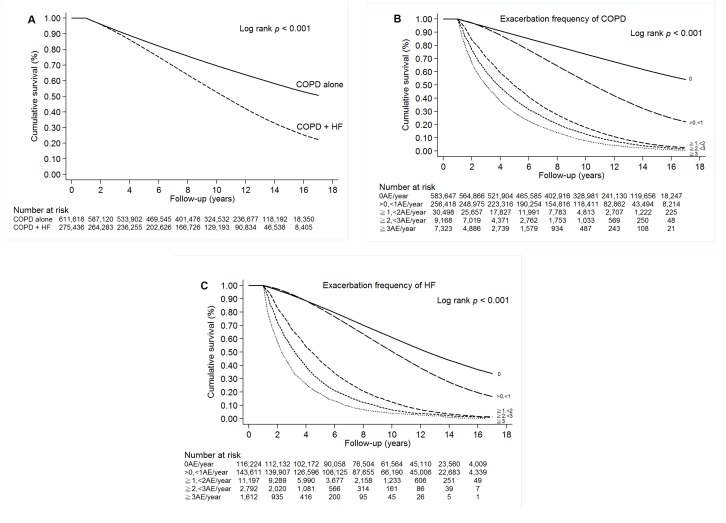
The cumulative survival hazard was significantly lower in the COPD-heart failure overlap cohort than in the COPD alone cohort in Kaplan–Meier analysis (log-rank test, p < 0.001; Figure 3A). Survival decreased significantly with increasing exacerbation frequency of COPD or HF in Kaplan–Meier analysis (Figure 3B and 3C); this result was sustained after adjustment for other factors (Supplementary Table 1). We identified the following independent risk factors for mortality in the COPD-heart failure overlap cohort: age ≥ 65 years (HR, 1.59; 95% CI, 1.59–1.60), male sex (HR, 1.11; 95% CI, 1.11–1.12), diabetes mellitus (HR, 1.07; 95% CI, 1.07–1.07), hypertension (HR, 1.05; 95% CI, 1.04–1.05), cerebrovascular disease (HR, 1.06; 95% CI, 1.06–1.06), malignancy (HR, 1.24; 95% CI, 1.24–1.24), chronic kidney disease (HR, 1.03; 95% CI, 1.03–1.03), and Charlson Comorbidity Index > 1 (HR, 1.03; 95% CI, 1.03–1.03; all p < 0.0001; Supplementary Table 1).
Figure 3.
(A) Survival of patients with COPD alone or COPD-heart failure overlap (COPD + HF). (B) Survival of patients with COPD. (C) Survival of patients with HF.
The effect of COPD medication use on mortality
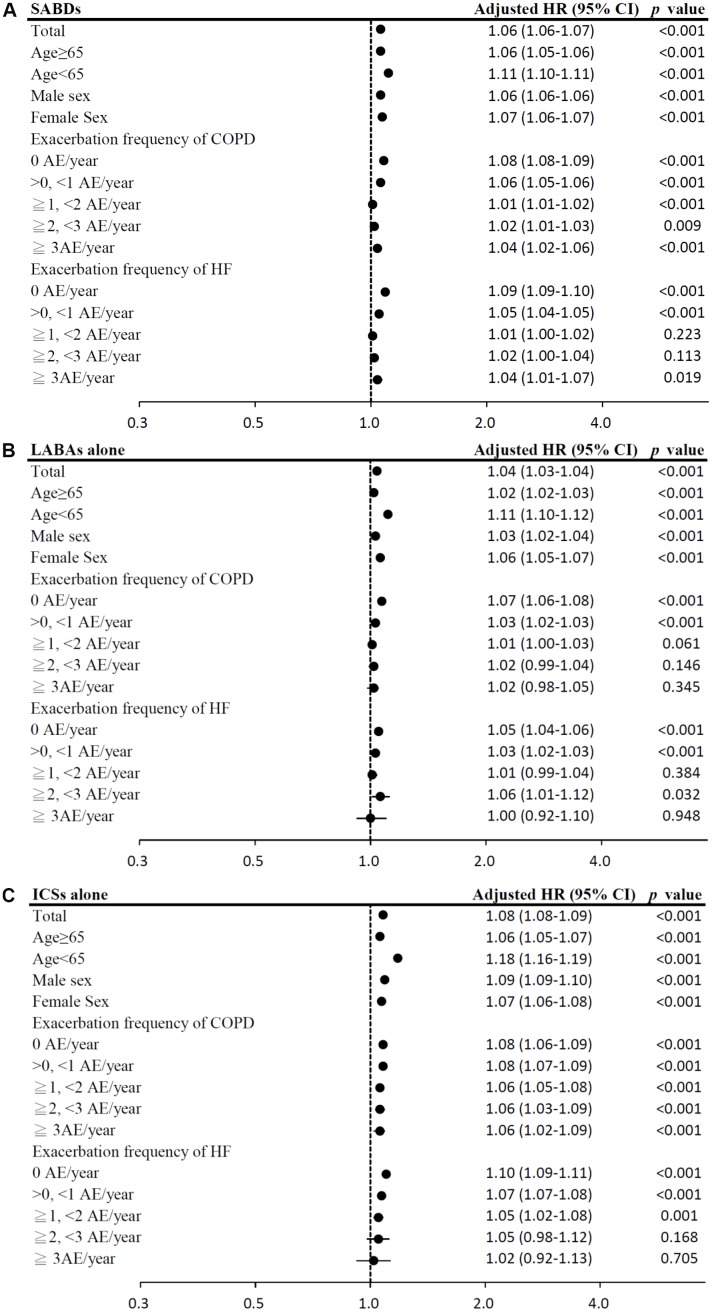
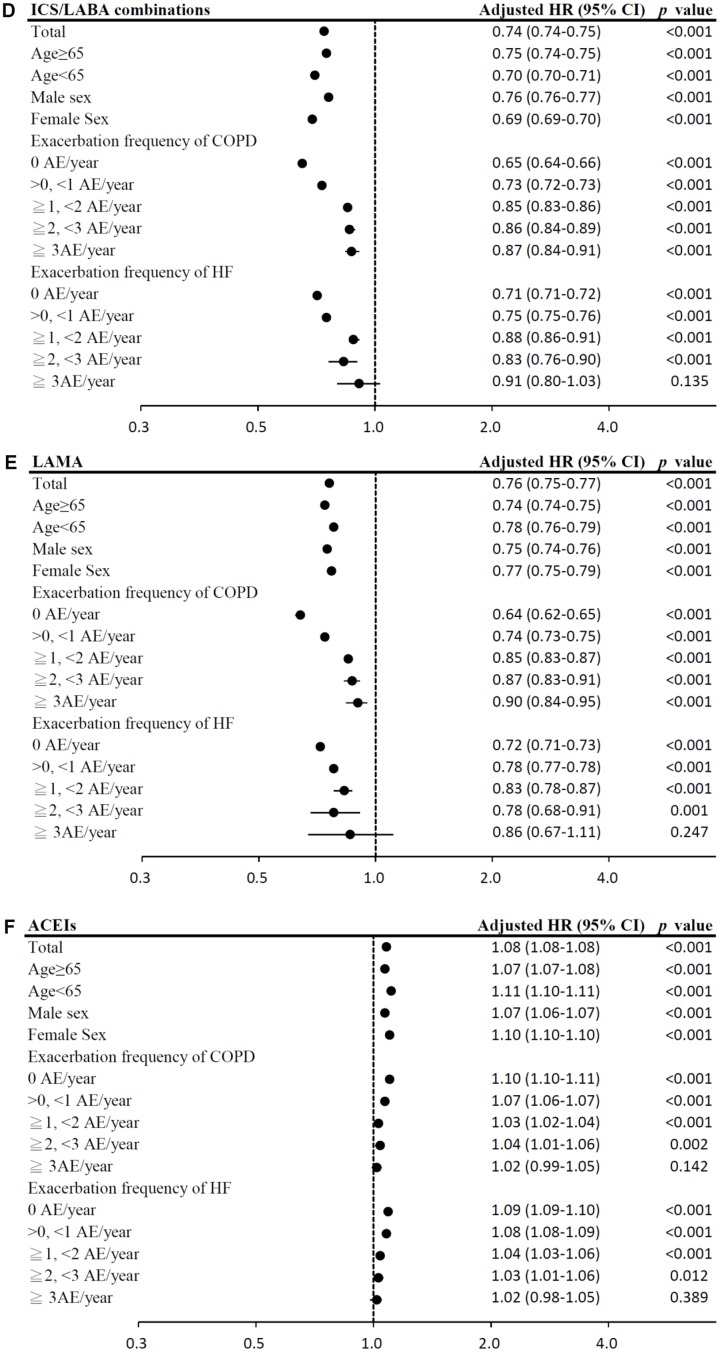
In the time-dependent Cox proportional-hazards model, the use of LAMA, and ICS/LABA combinations was associated with significantly lower mortality risks (model 1: LAMA: HR = 0.76, 95% CI = 0.75–0.77; ICS/LABA combinations: HR = 0.74, 95% CI = 0.74–0.75; model 2: LAMA: HR = 0.80, 95% CI = 0.79–0.81; ICS/LABA combinations: HR = 0.77, 95% CI = 0.77–0.78; all p < 0.0001; Table 2). In contrast, use of ICSs alone was associated with significantly higher mortality risks. The results of subgroup analyses are presented in Figure 4A–4E.
Table 2. Sensitivity analyses for medication effects on mortality in COPD-heart failure overlap patients.
| Variables | Time-dependent model | |||
| Model 1# | Model 2## | |||
| HR (95% CI) | p | HR (95% CI) | p | |
| COPD Medication | ||||
| Non-users of SABDs | Reference | Reference | ||
| SABDs | 1.06 (1.06–1.07) | <.0001 | 1.06 (1.06–1.06) | <.0001 |
| Non-users of LABAs alone | Reference | Reference | ||
| LABAs alone | 1.04 (1.03–1.04) | <.0001 | 1.05 (1.05–1.06) | <.0001 |
| Non-users of ICSs alone | Reference | Reference | ||
| ICSs alone | 1.08 (1.08–1.09) | <.0001 | 1.09 (1.08–1.10) | <.0001 |
| Non-users of ICS/LABA combinations | Reference | Reference | ||
| ICS/LABA combinations | 0.74 (0.74–0.75) | <.0001 | 0.77 (0.77–0.78) | <.0001 |
| Non-users of LAMA | Reference | Reference | ||
| LAMA | 0.76 (0.75–0.77) | <.0001 | 0.80 (0.79–0.81) | <.0001 |
| HF Medication | ||||
| Non-users of ACEIs | Reference | Reference | ||
| ACEIs | 1.08 (1.08–1.08) | <.0001 | 1.11 (1.11–1.12) | <.0001 |
| Non-users of ARBs | Reference | Reference | ||
| ARBs | 0.76 (0.76–0.76) | <.0001 | 0.80 (0.79–0.80) | <.0001 |
| Non-users of Cardioselective β-blockers | Reference | Reference | ||
| Cardioselective β-blockers | 0.72 (0.71–0.72) | <.0001 | 0.76 (0.76–0.77) | <.0001 |
| Non-users of Non-selective β-blockers | Reference | Reference | ||
| Non-selective β-blockers | 0.92 (0.92–0.93) | <.0001 | 0.96 (0.95–0.96) | <.0001 |
| Non-users of Loop diuretics | Reference | Reference | ||
| Loop diuretics | 1.07 (1.07–1.07) | <.0001 | 1.09 (1.08–1.09) | <.0001 |
| Non-users of Aldosterone antagonists | Reference | Reference | ||
| Aldosterone antagonists | 0.96 (0.96–0.96) | <.0001 | 0.99 (0.98–0.99) | 0.0002 |
| Non-users of Digoxins | Reference | Reference | ||
| Digoxins | 1.13 (1.12–1.13) | <.0001 | 1.12 (1.12–1.13) | <.0001 |
| Non-users of Statins | Reference | Reference | ||
| Statins | 0.75 (0.74–0.75) | <.0001 | 0.76 (0.76–0.77) | <.0001 |
Abbreviations: COPD, chronic obstructive pulmonary disease; HF, heart failure; HR, hazard ratio; CI, confidence interval; AE, acute exacerbation; SABDs, short-acting bronchodilators; LABAs, long-acting beta-agonists; LAMA, long-acting muscarinic antagonist; ICSs, inhaled corticosteroids; ACEIs, angiotensin-converting-enzyme inhibitors; ARBs, angiotensin receptor blockers.
HRs were adjusted for age, sex, income level, comorbidities, exacerbation frequency of COPD, exacerbation frequency of HF, Charlson Comorbidity Index, urbanization level and medications. All factors with p < 0.1 in univariate analyses were included in the Cox multivariate analysis.
#Medications were analyzed as time-dependent covariates (time period: 3 months).
##Medications were analyzed as time-dependent covariates (time period: 1 year)
Figure 4.
(A) Subgroup analysis of SABDs. (HRs were adjusted for age, sex, income level, comorbidities, exacerbation frequency of COPD, exacerbation frequency of HF, Charlson Comorbidity Index, urbanization level and medications; medications were analyzed as time-dependent covariates, time period: 3 months). (B) Subgroup analysis of LABAs alone. (C) Subgroup analysis of ICSs alone.
Figure 4.
(D) Subgroup analysis of ICS/LABA combinations. (E) Subgroup analysis of LAMA. (F) Subgroup analysis of ACEIs.
Figure 4.
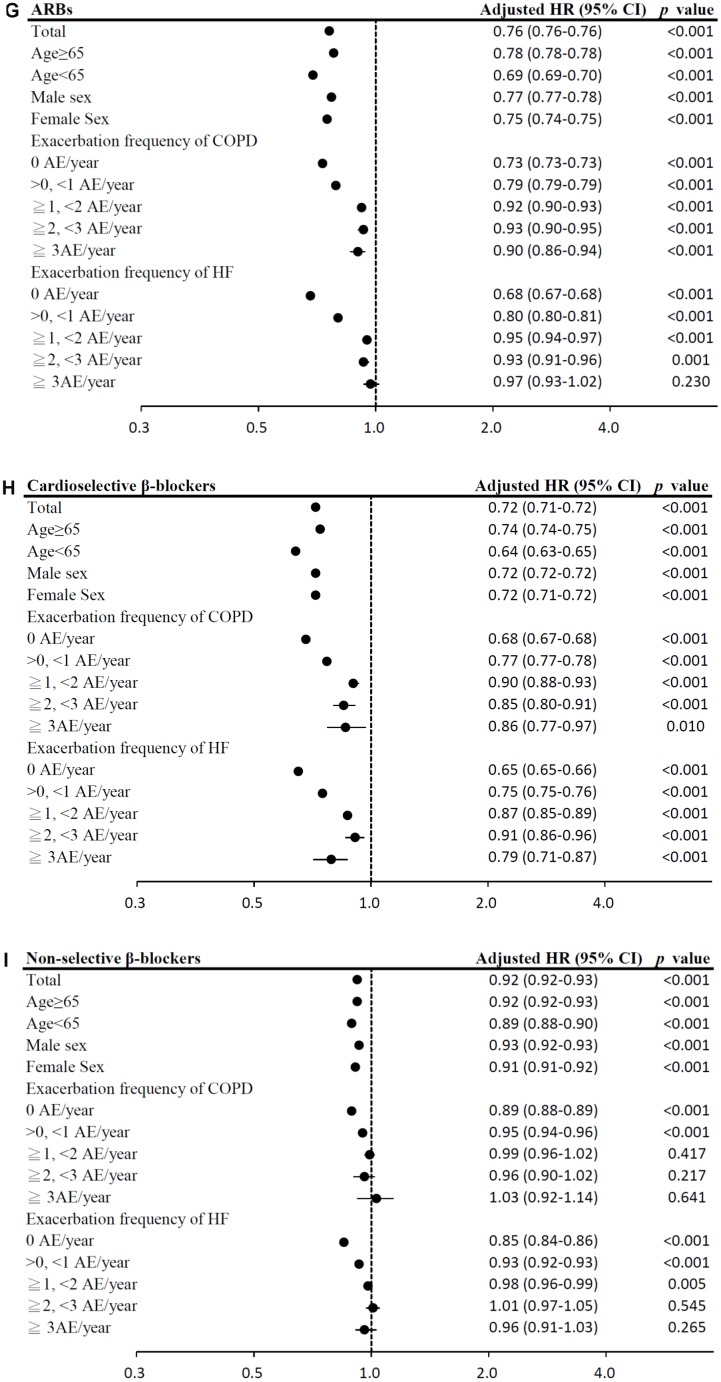
(G) Subgroup analysis of ARBs. (H) Subgroup analysis of cardioselective β blockers. (I) Subgroup analysis of non-selective β blockers.
Figure 4.
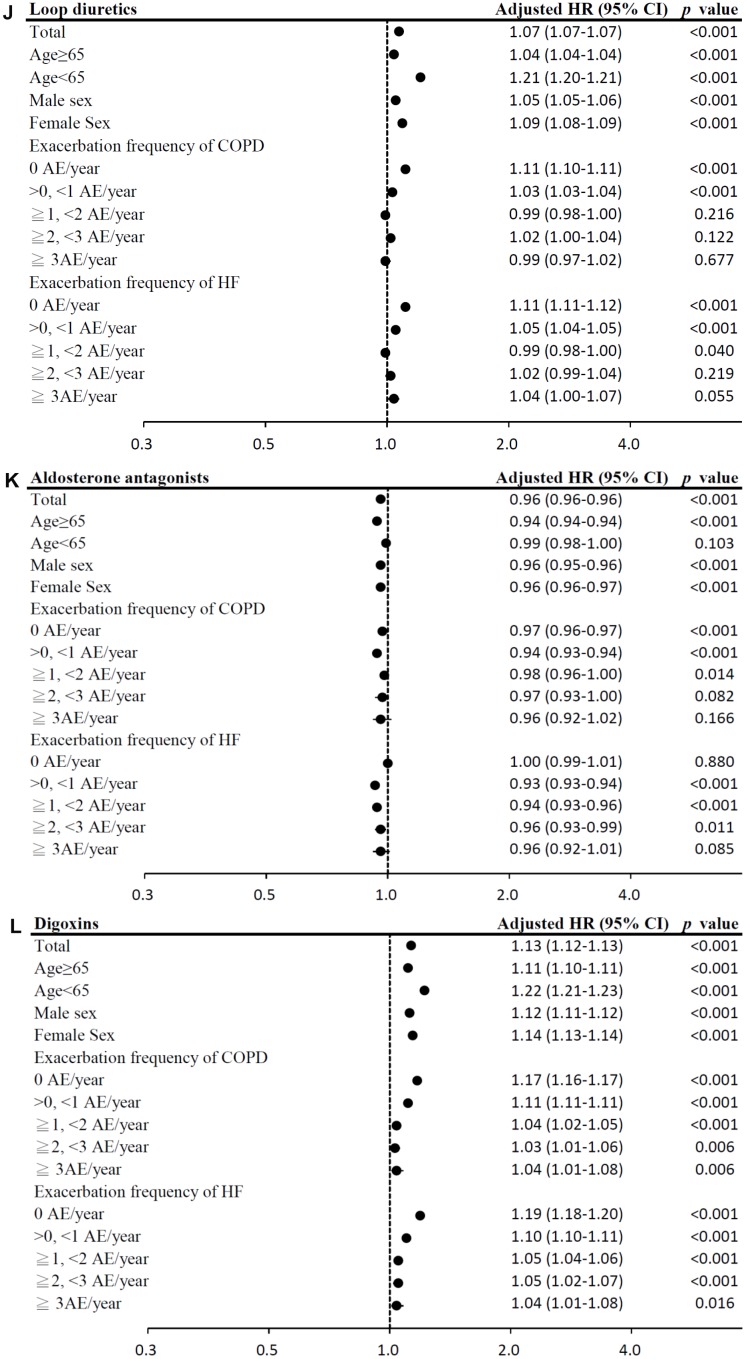
(J) Subgroup analysis of loop diuretics. (K) Subgroup analysis of aldosterone antagonists. (L) Subgroup analysis of digoxins.
The effect of HF medication use on mortality
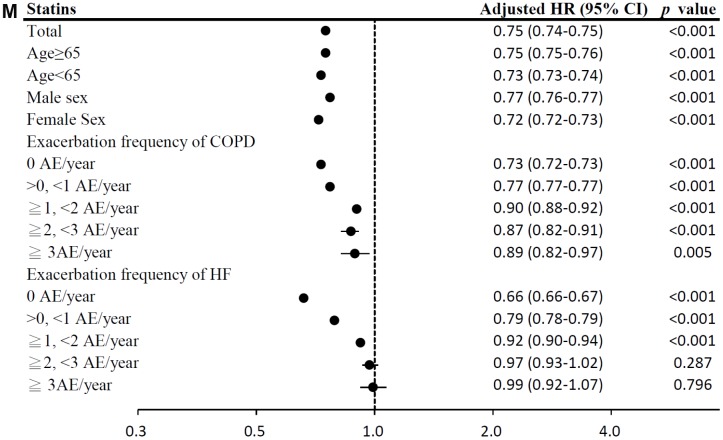
Among HF medication, the use of ARBs, cardioselective β blockers, non-selective β blockers, aldosterone antagonists, and statins was associated with significantly lower mortality risks (model 1: ARBs: HR = 0.76, 95% CI = 0.76–0.76; cardioselective β blockers: HR = 0.72, 95% CI = 0.71–0.72; non-selective β blockers: HR = 0.92, 95% CI = 0.92–0.93; aldosterone antagonists: HR = 0.96, 95% CI = 0.96–0.96; statins: HR = 0.75, 95% CI = 0.74–0.75; model 2: ARBs: HR = 0.80, 95% CI = 0.79–0.80; cardioselective β blockers: HR = 0.76, 95% CI = 0.76–0.77; non-selective β blockers: HR = 0.96, 95% CI = 0.95–0.96; aldosterone antagonists: HR = 0.99, 95% CI = 0.98–0.99; statins: HR = 0.76, 95% CI = 0.76–0.77; all p < 0.001; Table 2). However, digoxins use was associated with the highest mortality risk. The results of subgroup analyses are presented in Figure 4F–4M.
Figure 4.
(M) Subgroup analysis of statin.
Validation of COPD and HF definitions
2,325 patients with COPD and 3,402 patients with HF were identified from the claims database of Taipei Veterans General Hospital. Then, 300 patients from each group were randomly selected for validation of diagnoses. Of them, 276 patients with COPD underwent pulmonary function test and 288 patients with HF underwent echocardiogram. Diagnoses of COPD were confirmed by pulmonologists in 282/300 subjects (94.0% sensitivity) and by pulmonary function test in 238/276 subjects (86.2% sensitivity). Diagnoses of HF were confirmed by cardiologists in 289/300 subjects (96.3% sensitivity) and by echocardiography in 263/288 subjects (91.3% sensitivity).
DISCUSSION
This study revealed a greater medical burden in patients with COPD-heart failure overlap than in those with COPD alone. Our study demonstrated significant reductions in mortality associated with the use of LAMA, ICS/LABA combinations, ARBs, β blockers, aldosterone antagonists, and statins in patients with COPD-heart failure overlap. Results of this study, which involved large COPD-heart failure overlap and COPD cohorts – to our knowledge, the largest COPD-heart failure overlap cohort examined to date – are more valuable than those of clinical trials because they more closely reflect real-world practice. Furthermore, the definitions of COPD and HF were validated in this study, which indicated that the accuracy was excellent.
Despite the Heart Failure Society of America’s recommendation of β blocker use for HF [2], we previously documented the suboptimal use of β blockers in patients with COPD-heart failure overlap due to concern about their detrimental effects on COPD [7]. A population-based survey conducted by Lipworth et al. in UK enrolled 24,237 patients with HF alone and 10,853 patients with COPD-heart failure overlap, and showed that the use of β blockers in combination with ACEI/ARB was much lower in patients with COPD-heart failure overlap than in patients with HF alone (22.3% vs. 41.3%, p < 0.001) [3]. In turn, despite the Global Initiative for Chronic Obstructive Lung Disease’s recommendation of bronchodilator use in patients with COPD and COPD-heart failure overlap [1], concern about detrimental effects on HF has led to suboptimal use of these drugs [8].
This study demonstrated that LAMA, ICS/LABA combinations, ARBs, β blockers, aldosterone antagonists, and statins reduced mortality in patients with COPD-heart failure overlap. We also showed that cardioselective β blockers (bisoprolol and metoprolol) yield better survival outcomes than do non-selective β blockers in patients with COPD-heart failure overlap. Pulmonary effects appear to differ among β blockers; non-selective, but not cardioselective, β blockers have been found to reduce forced expiratory volume in 1 second (FEV1) and to increase airway hypersensitiveness in patients with COPD [9]. In a randomized cross-over study [10], bisoprolol is better tolerated than carvedilol on lung function in patients with moderate to severe COPD.
Paradoxically, as evidence for the efficacy of β blockers in COPD accumulates, studies have shown adverse cardiovascular effects of β agonists. The cardiac safety of LABA treatment, reported in clinical trials, is a result of administration by inhalation, which limits the transition of active drugs into the systemic circulation. However, inhaled β agonists still reach the systemic circulation through pulmonary circulation or absorption via the gastrointestinal tract after the swallowing of residual particles [11]. β agonists are associated with increased mortality and HF-related hospitalization in patients with existing HF [12]. A meta-analysis of randomized placebo-controlled trials showed that β-agonist treatment significantly increased the risk of a cardiovascular event in patients with obstructive airway disease compared with placebo (relative risk [RR] = 2.54; 95% CI, 1.59–4.05) [13]. Not surprisingly, SABDs and LABAs alone use was associated with increased risk of mortality in this study. Tiotropium, an inhaled LAMA approved for COPD maintenance treatment that was examined in this study, was associated with lower mortality than placebo in clinical trial [14]. In clinical trial [15], use of ICS/LABA combination over three years showed a decrease in mortality that almost reached statistical significance (HR=0.825, p = 0.052). A meta-analysis of randomized controlled trials showed that ICS/LABA combinations use was associated with a lower risk of death compared with use of ICSs alone [16]. According to international guideline for COPD [1], ICS monotherapy is not recommended for the management of COPD. Among older patients with COPD, ICS/LABA combinations use was associated with a lower risk of death compared with use of LABAs alone [17]. Sensitivity and subgroup analyses demonstrated that the benefits of LAMA and ICS/LABA combinations in patients with COPD and HF were consistent.
The most common side effect of ACEI therapy is cough, the prevalence of which approaches 50% in Chinese populations [18]. The incidence of ACEI-related cough has been reported to be 2.7 folds higher in Chinese subjects compared with Caucasians [19]. ACEI-induced cough may represent bronchial hyperresponsiveness in some patients [5]. ARBs do not appear to induce cough or bronchial hyperreactivity in patients with symptomatic asthma [20]. Indeed, bronchial hyperresponsiveness is an independent predictor of mortality in COPD patients [6]. In this study, almost all enrollees were Taiwanese. Our study showed that ARBs use is associated with better survival outcomes than ACEIs use. Similarly, use of statins (RR = 0.53; 95% CI, 0.43–0.65) and ARBs (RR = 0.62; 95% CI, 0.44–0.87), but not ACEIs (RR = 1.04; 95% CI, 0.86–1.25), was associated with survival benefit in 22,784 patients with COPD and high cardiovascular risk in a time-matched nested case-control study [21]. In a double-blind, placebo-controlled, randomized controlled trial [22], 80 patients with at least moderate COPD were randomized to either 10 weeks of therapy with an ACEI (10 mg enalapril) or placebo. Use of the ACEI significantly reduced the peak power response to exercise training in COPD patients. Recently, a population-based cohort study [23] also showed that statins use was associated with a lower mortality risk in patients with COPD.
In this study, use of SABDs, loop diuretics, and digoxins increased mortality risk in patients with COPD-heart failure overlap. Possible explanations for the higher mortality risk associated with SABD use are related to various cardiac side effects. In addition, excess SABD, loop diuretic, and digoxin use has been associated with poor control of COPD and HF, which may itself be the cause of this increased risk. Regardless of whether the association demonstrated is causal, however, chronic use of these drugs is a very powerful marker of high mortality risk in patients with COPD-heart failure overlap. Such patients warrant special attention.
This study was strengthened by using a nationwide population-based data to conduct a real-world study, which enrolled nearly all cases of COPD and HF in Taiwan with minimal selection and referral biases, as the NHI coverage rate is >99% and all cardiopulmonary-related healthcare is covered. To minimize immortal time bias, medication use was analyzed using time-dependent covariates in the Cox proportional-hazards model. Nevertheless, this study has some limitations. First, the claim-based dataset omitted some personal information, such as data on smoking and obesity. Although these factors appear to have no influence on medication selection and we made every effort to correct for confounding factors. Second, we could not assess drug adherence directly. However, this bias is toward the null hypothesis and would lead to underestimation of the actual effects of medications. Finally, the external validity of the results of this study should be of concern because all enrollees were Taiwanese. The generalizability of our findings to non-Asian populations must be further verified.
METHODS
Data Source
This cohort study used the Taiwan National Health Insurance Research Database (NHIRD), which is derived from the claims data of the Taiwan’s National Health Insurance (NHI) program, initiated in 1995, from more than 99% of Taiwan’s 23 million residents [24]. The NHIRD, provided for research purposes by Taiwan’s National Health Research Institute, contains healthcare data from NHI participants, including information on demographic characteristics; medical diagnoses, procedure, expenditure, and all detailed prescriptions. The accuracy of diagnoses recorded in the NHIRD, such as sleep apnea [24], pneumonia [24], asthma [25], COPD [25], tuberculosis contact [26], and tuberculosis [27] has been validated in our previous work. The study was exempted from full review by the Institutional Review Board of Taipei City Hospital because the datasets consisted of de-identified secondary data.
Study design and population
In this nationwide cohort study, we retrospectively analyzed medication effects on mortality in patients with COPD-heart failure overlap. All patients with COPD diagnoses recorded in the NHIRD between 1 January 1997 and 31 December 2012 were divided into the COPD alone and COPD-heart failure overlap cohorts. COPD and HF were identified using diagnostic codes from the International Classification of Diseases, 9th Revision, Clinical Modification (HF: 425.4, 425.9, 402.01, 402.11, 402.91, 404.01, 404.03, 404.11, 404.13, 404.91, 404.93, and 428.xx; COPD: 491.xx, 492.xx, and 496.xx). Patients with at least six recorded outpatient visits or one hospitalization for either disease were enrolled and followed until the date of death, withdrawal from insurance, or 31 December 2013. Information on the use of medications recommended in guidelines for patients with COPD or HF were extracted [1, 28]. Patients with follow-up periods < 1 year, those aged < 40 years, and those who were not prescribed medications for COPD or HF were excluded.
Potential confounders and severity classification
In the investigation of the effects of medications on mortality, age, sex, urbanization level, income level, Charlson Comorbidity Index, exacerbation frequency of COPD, exacerbation frequency of HF, and comorbidities were considered to be potential confounders. Because the most important factor in classifying COPD severity appears to be a previous history of exacerbations [29], exacerbation frequency of COPD was determined using the annual frequency of hospitalizations and emergency department visits for acute exacerbation during the follow-up period. Exacerbation frequency of HF was classified in the same way and the negative correlation between COPD/HF severity and cumulative survival has been validated in our previous study [7]. Based on the number of acute exacerbations (AE) per year, patients were assigned to the 0 AE/year, >0 and <1 AE/year, ≥1 and <2 AE/year, ≥2 and <3 AE/year, and ≥3 AE/year groups.
Exposure to COPD and HF medications
Patients who took ≥28 days of each medication recommended in guidelines for patients with COPD or HF during the follow-up period, were assigned to respective medication groups. Prescriptions of medications for COPD or HF were assessed during each time period (3 months or 1 year) for each individual patient [30]. A 3-month period was chosen, because medications are usually prescribed for 3 months at a time. A 1-year period was used to recheck the robustness of the results of 3-month period. COPD medication groups were: short-acting bronchodilators (SABDs), including short-acting β2 agonists (albuterol, fenoterol, terbutaline, procaterol) and short-acting muscarinic antagonists (ipratropium); long-acting β2 agonists (LABAs; salmeterol, formoterol, bambuterol, indacaterol); long-acting muscarinic antagonist (LAMA; tiotropium); ICSs (budesonide, fluticasone, beclomethasone) and ICS/LABA combinations (fluticasone/salmeterol, budesonide/formoterol, beclomethasone/formoterol). HF medication groups were: ACEIs (benazepril, captopril, enalapril, fosinopril, lisinopril, perindopril, perindopril, quinapril, ramipril), ARBs (losartan, candesartan, valsartan, irbesartan, telmisartan, eprosartan, olmesartan, azilsartan), cardioselective β blockers (bisoprolol, metoprolol), non-selective β blockers (carvedilol), loop diuretics (furosemide, bumetanide), aldosterone antagonists (spironolactone, eplerenone); digoxins (digoxin, digitoxin), and statins (atorvastatin, fluvastatin, lovastatin, pitavastatin, pravastatin, rosuvastatin, simvastatin).
Validation
The claims database of Taipei Veterans General Hospital (a 3,035-bed tertiary referral hospital in Taiwan) was used to validate the accuracy of the diagnoses of COPD and HF in this study. The contents of this database are used for reimbursement and are similar to those of the NHIRD [24–27]. The diagnoses of COPD and HF were validated by analysis of selected samples from this database using the same criteria. COPD was defined as COPD diagnosed by a pulmonologist, or fixed airflow limitation determined by pulmonary function test [1]. HF was defined as HF diagnosed by a cardiologist, or inadequate cardiac output determined by echocardiography [2]. Random sampling of claims data for validation was performed using SPSS version 20.
Statistical analyses
Data entry and analysis were performed using SAS statistical software (version 9.4; SAS Institute, Cary, NC, USA). Descriptive analysis was used to compare the COPD alone group and COPD-heart failure overlap group in terms of demographic data and the prescription medications during the study period. All the data were presented as mean ± standard deviation (SD) or percentage (%). Comparison between two groups was made by independent Student’s t-test for continuous variables or Pearson’s chi-square test for categorical variables as appropriate. Kaplan-Meier survival analysis and the log-rank test were used to examine the difference between the cohorts. We used the Cox proportional-hazards regression models to determine the survival effect of medication use during the follow-up period. Medication prescriptions in each 3-month and 1-year period served as time-dependent covariates. Patients who were prescribed ≥28 days of each medication within each interval were assigned to the respective medication groups and all others were defined as non-users [25]. Other baseline variables were analyzed as non–time-dependent covariates. All factors with p values < 0.1 in univariate analyses were included in the Cox multivariate analysis. The hazard ratio (HR) and 95% confidence intervals (CIs) were calculated after adjustment for possible confounding factors. Two-tailed p values < 0.05 were considered statistically significant. Subgroup analyses were performed according to age, sex, exacerbation frequency of COPD and HF.
CONCLUSIONS
This study demonstrated that patients with COPD-heart failure overlap have more outpatient visits per year, more hospitalization, and a shorter time to mortality than do patients with COPD alone. LAMA, ICS/LABA combination, ARB, β blocker, aldosterone antagonist, and statin use decreased the mortality risk.
Supplementary Material
ACKNOWLEDGEMENTS
We would like to thank Health Information and Epidemiology Laboratory of Chang Gung Memorial Hospital, Chia-yi Branch, for the comments and assistance in data analysis.
Footnotes
AUTHOR CONTRIBUTIONS: V-Y Su, Y-H Yang and K-Y Yang had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: V-Y Su, Y-H Yang, Y-H Tsai, P-C Chen, K-T Chou, D-W Perng, K-C Su, W-J Su, K-Y Yang. Acquisition of data: V-Y Su, Y-H Yang, Y-H Tsai, K-Y Yang. Analysis and interpretation of data: V-Y Su, Y-H Yang, Drafting of the manuscript: V-Y Su, Y-H Yang, Y-H Tsai, P-C Chen, K-T Chou, D-W Perng, W-J Su, K-C Su, K-Y Yang. Statistical analysis: V-Y Su, Y-H Yang. Study supervision: Y-H Tsai, P-C Chen, K-T Chou, D-W Perng, W-J Su, K-C Su, K-Y Yang.
CONFLICTS OF INTEREST: The authors declare that they have no conflicts of interest.
FUNDING: This study was supported in part by grants from Taipei City Hospital (10701-62-063) and the Ministry of Science and Technology Research Projects (MOST 103-2314-B-075-049-MY2 and MOST 105-2314-B-010-041-MY3) and Taipei Veterans General Hospital (V107C-077 and V108C-057) and the Featured Areas Research Center Program, Higher Education Sprout Project, Ministry of Education (MOE) in Taiwan and Chang Gung Memorial Hospital, Chia-yi Branch (CLRPG6G0041).
REFERENCES
- 1.Global initiative for chronic obstructive lung disease: Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. 2019. report http://goldcopd.Org/gold-reports/.
- 2.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM, Hollenberg SM, Lindenfeld J, Masoudi FA, et al. 2017 acc/aha/hfsa focused update of the 2013 accf/aha guideline for the management of heart failure: A report of the American college of cardiology/American heart association task force on clinical practice guidelines and the heart failure society of America. J Am Coll Cardiol. 2017; 70:776–803. 10.1016/j.jacc.2017.04.025 [DOI] [PubMed] [Google Scholar]
- 3.Lipworth B, Skinner D, Devereux G, Thomas V, Ling Zhi Jie J, Martin J, Carter V, Price DB. Underuse of β-blockers in heart failure and chronic obstructive pulmonary disease. Heart. 2016; 102:1909–14. 10.1136/heartjnl-2016-309458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caldeira D, David C, Sampaio C. Tolerability of angiotensin-receptor blockers in patients with intolerance to angiotensin-converting enzyme inhibitors: A systematic review and meta-analysis. Am J Cardiovasc Drugs. 2012; 12:263–77. 10.2165/11599990-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 5.Bucknall CE, Neilly JB, Carter R, Stevenson RD, Semple PF. Bronchial hyperreactivity in patients who cough after receiving angiotensin converting enzyme inhibitors. Br Med J (Clin Res Ed). 1988; 296:86–88. 10.1136/bmj.296.6615.86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vestbo J, Hansen EF. Airway hyperresponsiveness and COPD mortality. Thorax. 2001. (Suppl 2); 56:ii11–14. [PMC free article] [PubMed] [Google Scholar]
- 7.Su VY, Chang YS, Hu YW, Hung MH, Ou SM, Lee FY, Chou KT, Yang KY, Perng DW, Chen TJ, Liu CJ. Carvedilol, bisoprolol, and metoprolol use in patients with coexistent heart failure and chronic obstructive pulmonary disease. Medicine (Baltimore). 2016; 95:e2427. 10.1097/MD.0000000000002427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Le Jemtel TH, Padeletti M, Jelic S. Diagnostic and therapeutic challenges in patients with coexistent chronic obstructive pulmonary disease and chronic heart failure. J Am Coll Cardiol. 2007; 49:171–80. 10.1016/j.jacc.2006.08.046 [DOI] [PubMed] [Google Scholar]
- 9.van der Woude HJ, Zaagsma J, Postma DS, Winter TH, van Hulst M, Aalbers R. Detrimental effects of beta-blockers in COPD: a concern for nonselective beta-blockers. Chest. 2005; 127:818–24. 10.1378/chest.127.3.818 [DOI] [PubMed] [Google Scholar]
- 10.Jabbal S, Anderson W, Short P, Morrison A, Manoharan A, Lipworth BJ. Cardiopulmonary interactions with beta-blockers and inhaled therapy in COPD. QJM. 2017; 110:785–92. 10.1093/qjmed/hcx155 [DOI] [PubMed] [Google Scholar]
- 11.Cazzola M, Page CP, Rogliani P, Matera MG. β2-agonist therapy in lung disease. Am J Respir Crit Care Med. 2013; 187:690–96. 10.1164/rccm.201209-1739PP [DOI] [PubMed] [Google Scholar]
- 12.Au DH, Udris EM, Fan VS, Curtis JR, McDonell MB, Fihn SD. Risk of mortality and heart failure exacerbations associated with inhaled beta-adrenoceptor agonists among patients with known left ventricular systolic dysfunction. Chest. 2003; 123:1964–69. 10.1378/chest.123.6.1964 [DOI] [PubMed] [Google Scholar]
- 13.Salpeter SR, Ormiston TM, Salpeter EE. Cardiovascular effects of beta-agonists in patients with asthma and COPD: a meta-analysis. Chest. 2004; 125:2309–21. 10.1378/chest.125.6.2309 [DOI] [PubMed] [Google Scholar]
- 14.Celli B, Decramer M, Kesten S, Liu D, Mehra S, Tashkin DP, Investigators US, and UPLIFT Study Investigators. Mortality in the 4-year trial of tiotropium (UPLIFT) in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2009; 180:948–55. 10.1164/rccm.200906-0876OC [DOI] [PubMed] [Google Scholar]
- 15.Calverley PM, Anderson JA, Celli B, Ferguson GT, Jenkins C, Jones PW, Yates JC, Vestbo J, and TORCH investigators. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007; 356:775–89. 10.1056/NEJMoa063070 [DOI] [PubMed] [Google Scholar]
- 16.Nannini LJ, Poole P, Milan SJ, Kesterton A. Combined corticosteroid and long-acting beta(2)-agonist in one inhaler versus inhaled corticosteroids alone for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2013. August 30; CD006826. 10.1002/14651858.CD006826.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gershon AS, Campitelli MA, Croxford R, Stanbrook MB, To T, Upshur R, Stephenson AL, Stukel TA. Combination long-acting β-agonists and inhaled corticosteroids compared with long-acting β-agonists alone in older adults with chronic obstructive pulmonary disease. JAMA. 2014; 312:1114–21. 10.1001/jama.2014.11432 [DOI] [PubMed] [Google Scholar]
- 18.Woo KS, Nicholls MG. High prevalence of persistent cough with angiotensin converting enzyme inhibitors in Chinese. Br J Clin Pharmacol. 1995; 40:141–44. [PMC free article] [PubMed] [Google Scholar]
- 19.McDowell SE, Coleman JJ, Ferner RE. Systematic review and meta-analysis of ethnic differences in risks of adverse reactions to drugs used in cardiovascular medicine. BMJ. 2006; 332:1177–81. 10.1136/bmj.38803.528113.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tanaka H, Teramoto S, Oashi K, Saikai T, Tanaka S, Suzuki K, Hashimoto M, Abe S. Effects of candesartan on cough and bronchial hyperresponsiveness in mildly to moderately hypertensive patients with symptomatic asthma. Circulation. 2001; 104:281–85. 10.1161/01.CIR.104.3.281 [DOI] [PubMed] [Google Scholar]
- 21.Mancini GB, Etminan M, Zhang B, Levesque LE, FitzGerald JM, Brophy JM. Reduction of morbidity and mortality by statins, angiotensin-converting enzyme inhibitors, and angiotensin receptor blockers in patients with chronic obstructive pulmonary disease. J Am Coll Cardiol. 2006; 47:2554–60. 10.1016/j.jacc.2006.04.039 [DOI] [PubMed] [Google Scholar]
- 22.Curtis KJ, Meyrick VM, Mehta B, Haji GS, Li K, Montgomery H, Man WD, Polkey MI, Hopkinson NS. Angiotensin-converting enzyme inhibition as an adjunct to pulmonary rehabilitation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2016; 194:1349–57. 10.1164/rccm.201601-0094OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raymakers AJ, Sadatsafavi M, Sin DD, De Vera MA, Lynd LD. The impact of statin drug use on all-cause mortality in patients with COPD: A population-based cohort study. Chest. 2017; 152:486–93. 10.1016/j.chest.2017.02.002 [DOI] [PubMed] [Google Scholar]
- 24.Su VY, Liu CJ, Wang HK, Wu LA, Chang SC, Perng DW, Su WJ, Chen YM, Lin EY, Chen TJ, Chou KT. Sleep apnea and risk of pneumonia: A nationwide population-based study. CMAJ. 2014; 186:415–21. 10.1503/cmaj.131547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Su VY, Yang KY, Yang YH, Tsai YH, Perng DW, Su WJ, Chou KT, Su KC, Yen YF, Chen PC. Use of ics/laba combinations or lama is associated with a lower risk of acute exacerbation in patients with coexistent copd and asthma. J Allergy Clin Immunol Pract. 2018; 6:1927–1935.e3. 10.1016/j.jaip.2018.01.035 [DOI] [PubMed] [Google Scholar]
- 26.Su VY, Yen YF, Pan SW, Chuang PH, Feng JY, Chou KT, Chen YM, Chen TJ, Su WJ. Latent tuberculosis infection and the risk of subsequent cancer. Medicine (Baltimore). 2016; 95:e2352. 10.1097/MD.0000000000002352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Su VY, Su WJ, Yen YF, Pan SW, Chuang PH, Feng JY, Chou KT, Yang KY, Lee YC, Chen TJ. Statin use is associated with a lower risk of tb. Chest. 2017; 152:598–606. 10.1016/j.chest.2017.04.170 [DOI] [PubMed] [Google Scholar]
- 28.Lindenfeld J, Albert NM, Boehmer JP, Collins SP, Ezekowitz JA, Givertz MM, Katz SD, Klapholz M, Moser DK, Rogers JG, Starling RC, Stevenson WG, Tang WH, et al. , and Heart Failure Society of America. Hfsa 2010 comprehensive heart failure practice guideline. J Card Fail. 2010; 16:e1–194. 10.1016/j.cardfail.2010.04.004 [DOI] [PubMed] [Google Scholar]
- 29.Vestbo J, Hurd SS, Agustí AG, Jones PW, Vogelmeier C, Anzueto A, Barnes PJ, Fabbri LM, Martinez FJ, Nishimura M, Stockley RA, Sin DD, Rodriguez-Roisin R. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013; 187:347–65. 10.1164/rccm.201204-0596PP [DOI] [PubMed] [Google Scholar]
- 30.Ekström MP, Hermansson AB, Ström KE. Effects of cardiovascular drugs on mortality in severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013; 187:715–20. 10.1164/rccm.201208-1565OC [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.