Abstract
Introduction:
Hepatocellular carcinoma (HCC) is one of the most common and lethal cancers. Progress has been made in treatment of HCC; however, improved outcomes are much needed. The increased metabolic needs of cancer cells underscore the importance of metabolic pathways in cancer cell survival. Lipid metabolism has a role in HCC development; aberrant overexpression of several key enzymes is seen in many solid human tumors.
Areas covered:
We discuss aberrant lipid metabolism and the promise of multiple targets, in particular related to HCC treatment. We searched PubMed and clinicaltrials.gov for published and unpublished studies from 2000 to 2019. These terms were used: lipids, fatty acid metabolism, lipid metabolism, liver cancer, HCC, de novo fatty acid synthesis, ATP citrate lyase, stearoyl CoA denaturase, fatty acid synthase, acetyl coenzyme A carboxylase, CD147, KLF4, monoglyceride lipase, AMP activated protein kinase.
Expert opinion:
The importance of dysregulation of fatty acid synthesis in cancer is a growing area of research. HCC demonstrates significant alteration in lipid metabolism, representing great potential as a target for novel therapeutics. Various agents have demonstrated promising anti-neoplastic activity. This strategy deserves further development for improved outcomes.
Keywords: Fatty acid, hepatocellular carcinoma, liver cancer, lipids, lipid metabolism, stearoyl co-A desaturase, SCD1
1. Introduction
Liver cancer is the sixth most common form of cancer worldwide [1]. Hepatocellular carcinoma (HCC) is the most common cause of primary liver cancer. Due to the global prevalence and high mortality associated with HCC, research has been focused on identifying risk factors for the development of HCC and also potential new therapies for more effective treatment of this devastating disease. Cirrhosis is one of the most well described risk factors for the development of HCC [2]. Cirrhosis can be the result of viral hepatitis, chronic alcohol use, non-alcoholic steatohepatitis (NASH), or certain genetic disorders, such as Wilson’s Disease, Alpha-1 anti-trypsin deficiency, and hemochromatosis. The development of HCC has also been described in patients without documented cirrhosis who have hepatitis B infection, hepatitis C infection, diabetes mellitus, non-alcoholic fatty liver disease (NAFLD), a history of tobacco use or obesity [2].
HCC is often diagnosed late and therefore carries a poor prognosis. In fact, worldwide, liver cancer is the third leading cause of cancer-related death. Globally, liver cancer incidence is estimated to represent 4.7% of all new cancer cases, and 8.2% of all cancer-related deaths. In the United States, for example, the average 1-year survival rate is <50% and average 5-year survival rate is <10% for liver cancer [3].
Curative therapy options are not available, and though much progress has been made in recent years, therapeutic options and outcomes for patients with HCC are still limited. Sorafenib arose as the singular standard in 2007 and remained the sole standard for many years. Since 2017, we have witnessed a dynamically changing treatment landscape for patients with advanced HCC, including the emergence of a number of inhibitors of tyrosine kinase signaling and angiogenic molecules and immune checkpoint inhibitors. Currently, there are several approved drugs for systemic treatment of HCC – sorafenib, lenvatinib, regorafenib, cabozantinib, and nivolumab [4–7]. Sorafenib and lenvatinib are multikinase inhibitors used as first-line treatment in HCC. Sorafenib targets Raf serine/threonine kinases, VEGF receptors 1–3, PDGFR- β, FLT3, KIT, and RET, while lenvatinib targets VEGF receptors 1–3, FGF receptors 1–4, PDGF receptor α, RET, and KIT. Regorafenib and nivolumab are approved as second-line treatment. Regorafenib, like sorafenib and lenvatinib, targets VEGF receptors 1–3, PDGFR, FGFR, KIT, RET, RAF-1, and BRAF, while nivolumab is a programmed cell death protein-1 (PD–1) immune checkpoint inhibitor that has also demonstrated positive results in HCC patients. None of these therapies address the altered metabolic functions of HCC cells, including lipid metabolism.
Several studies have sought to elucidate the underlying mechanisms enabling the generation and survival of HCC cells as these processes may serve as therapeutic targets. One such potential target is lipid metabolism. Altered lipid metabolism has demonstrated a role in the development of HCC; however, the mechanism by which lipid metabolism is altered during the natural progression of HCC is poorly understood. Herein, we review the current literature to provide insight into lipid metabolism as a source of potential novel therapeutic targets for the treatment of HCC. Our review focuses on targets such as Stearoyl-Coenzyme A desaturase-1 (SCD1), fatty acid synthase (FASN), acetyl CoA carboxylase (ACC), Kruppel-like Factor 4 (KLF4), Monoglyceride Lipase (MGLL), and basigin (CD147), which all play roles in lipid metabolism.
2. Normal de novo fatty acid synthesis
De novo fatty acid (FA) synthesis occurs in high energy or fed states. During FA synthesis, glucose is taken up in the liver where it is then converted to FAs for storage in the form of triacylglycerols (TAGs) [8]. The initial step in FA synthesis is glycolysis. Glycolysis results in the production of pyruvate from glucose [8]. This reaction takes place in the cytosol of the hepatocyte. After pyruvate has been produced, pyruvate enters into the mitochondria and is converted to citrate via the citric acid (TCA) cycle [9]. Once citrate has been formed, citrate is expelled out of the mitochondria via the citrate shuttle. Citrate is converted into oxaloacetate and acetyl CoA via ATP-citrate lyase (ACL) [9]. The oxaloacetate is further broken down into pyruvate and NADPH. The acetyl CoA and NADPH are then used for FA synthesis [9]. Acetyl CoA is converted to Malonyl CoA via ACC [10]. Malonyl CoA and Acetyl CoA are combined using FASN to help form saturated fatty acids (SFA) (palmitoyl-CoA and stearoyl-CoA) [10]. Critically, these are then converted to monounsaturated fatty acids (MUFA) palmitoleoyl-CoA and oleoyl-CoA by SCD [11]. MUFAs are critical as building blocks for membrane synthesis, prostaglandin synthesis, and as the source for TAGs. They are important to cancer cell survival via their role in the induction of autophagy, enhancement of cell membrane turnover, effecting intracellular signaling and gene transcription, and increasing energy production.
Once these FAs have been produced, they are prepared for storage. In the liver, glucose is converted into glyceraldehyde 3-phosphate (G3P) via glycolysis [12]. G3P is bound with activated FAs via acyltransferases (AT) to form lysophosphatidic acid. An additional activated FA is added via AT to form phosphatidic acid. This is further converted to diacylglycerol (DAG) via phosphatase. Diacylglycerol acyltransferase (DGAT) then converts DAG to TAG [12]. The liver packages TAG with other substrates to form very low density lipoproteins (VLDL). These VLDLs enter the blood stream for transport of TAGs to other cells within the body. TAGs are transferred in the blood via chylomicrons from enterocytes. Endogenously produced TAGs are carried via VLDL and TAGs absorbed from the diet in enterocytes are carried in the blood via chylomicrons [10]. These TAGs are a main source of energy for normal metabolic cellular function [13].
3. Fatty acid synthesis in HCC
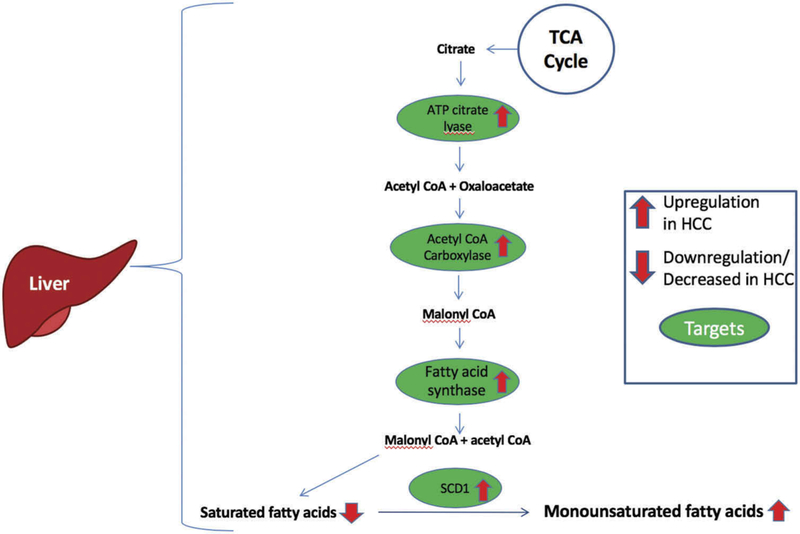
Dysregulation of FA synthesis has become a growing area of research as multiple studies have revealed altered lipid metabolism as a culprit in the pathogenesis of cancer [13–17]. The increased metabolic needs of cancer cells in a setting of reduced nutrient availability underscore the importance of reprogramming of metabolic pathways in cancer cell survival. These include increased de novo lipogenesis. Recent studies have implicated lipid biosynthesis and desaturation as a requirement for HCC tumorigenesis, survival and progression [18–20]. Though a dedicated, comprehensive evaluation of FA biosynthesis machinery in HCC is lacking, several studies have described aberrant overexpression of enzymes in this process, such as FASN, ACL, ACC, and Stearoyl CoA desaturase-1 (SCD1), in a wide variety of solid human tumors including HCC (Figure 1). Blocking the FA biosynthesis pathway has been shown to suppress cancer cell growth and several potential targets in these pathways may serve as effective drug targets as part of a therapeutic strategy for HCC.
Figure 1.
Fatty acid synthesis in HCC. Numerous role players in fatty acid biosynthesis are upregulated in HCC. Pyruvate is converted to citrate via the TCA cycle. ATP citrate lvase upregulated in HCC, catalyzes the conversion of citrate to cytosolic acetyl CoA and oxaloacetate. Acetyl CoA is converted to malonyl CoA via acetyl CoA carboxylase. FASN then combines malonyl CoA and acetyl CoA to form SFA. These SFA are converted to monosaturated fatty acids by SCDl.
3.1. ATP citrate lyase
ATP Citrate Lyase (ACLY) is the cytosolic enzyme that catalyzes the Mg-ATP-dependent synthesis of oxaloacetate and acetylcoenzyme A (acetyl-CoA) from citrate and co-enzyme A [21]. Human ACLY is a polypeptide characterized by an N-terminal citryl-CoA synthetase module that consists of CCSβ and CCSα regions, as well as a C-terminal citryl-CoA lyase (CCL) domain [22]. In turn, acetyl-coA is a versatile molecule involved in several important biosynthetic pathways. It is necessary for the production of malonyl-CoA as the first step in FA synthesis, and is required for the creation of acetoacetyl-CoA in the mevalonate pathway of cholesterol systhesis. It is also required for acetylation reactions that modify proteins, such as histone acetylation [23]. Studies of the ligand-dependent conformational changes in the ACLY tetramer and their biologic, protumorigenic effects have identified a number of potential molecular targets within the ACLY protein [22,24]. With its position in the midst of intracellular metabolic activity, ACLY is a strategic enzyme linking both the glycolytic and lipidic metabolism so important to tumors. Given this critical location at the juncture of two major metabolic processes, and also the important role acetyl-coA plays in lipid and cellular metabolism, ACLY is an important molecule in cancer cell physiology and survival and thus is a significant potential therapeutic target.
ACLY expression and activity have proven to be aberrantly expressed in many tumor types, including HCC [25–30]. Ference et al. demonstrated that genetic variants that resulting in either ACLY inhibition or HMGCR inhibition did not result in an increased risk of cancer [31]. Further, experimental pharmacological or genetic inhibition have demonstrated significant inhibition of cancer cell proliferation and induction of apoptosis. Interestingly, in these studies a differential sensitivity to ACLY inhibition based upon the cellular rate of glucose utilization and lactate production has been seen. Highly glycolytic cells are more dependent on ACLY activity for their survival, and thus more sensitive to inhibitory pressure. This preferential sensitivity may make ACLY targeted therapeutics safer since cells with a normal metabolism and a growth rate would be much less influenced by its inhibition [9,32–34]. Also of note is that ACLY knockdown or inhibition has demonstrated an important impact upon cancer stem cells, particularly those that are located in hypoxic regions of tumors and more strongly rely on glycolysis [35].
ACLY inhibitors have reported positive results in human clinical trials as cholesterol-lowering drugs [36–42]. Several natural and synthetic inhibitors of ACLY exist and many, both by themselves and in combination with other agents including chemotherapy have demonstrated anti-proliferative effects on several cancer cell lines in vitro and in vivo [32,43–48]. There have, however, not yet been any human clinical trials dedicated to evaluating ACLY inhibitors as anti-neoplastic therapeutics. While additional investigations of existing ACLY inhibitors are still necessary to explore their potential as antineoplastic agents, the development of more potent and selective ACLY inhibitors is an unexplored field and, given the importance of this enzyme as a potential topic, a significant unmet need.
3.2. Stearoyl-CoA desaturase-1
SCD is an enzyme located on the endoplasmic reticulum that helps catalyze double bond formation at the cis-delta-9 position of FAs [49]. In particular, it converts palmitoyl-CoA to palmitoleoyl-CoA and stearoyl-Coa to oleoyl-CoA [49]. SCD is immediately downstream of FASN in this enzymatic cascade and, functionally, is the rate limiting enzyme in this process, converting SFA into MUFA through insertion of a cis-double bond at the Δ9 position of the carbon chain [50]. Five isoforms of SCD have been described, of which two are expressed in humans (SCD1 and SCD5) [17,51]. Each of these isoforms demonstrates differential tissue distribution and expression patterns, but preserved enzymatic function [52,53].
SCD1 has been noted to be upregulated in a number of cancers, including HCC [49]. By interrogating large cohorts of clinically available HCC tissue samples, SCD1 has been found to be overexpressed. This overexpression was correlated with tumor differentiation and with shorter disease-free survival [54]. Additionally, studies have demonstrated upregulation of SCD1 in HCC cells in response to some chemotherapeutics and also to sorafenib [54,55]. SCD1 upregulation is thought to contribute to increased cell proliferation and evasion of apoptosis by cancer cells. In normal cells, as SFA levels increase, ACC is inhibited through a negative feedback mechanism. Rising SFA levels play a role in signaling apoptosis via their role in signaling oxidative and endoplasmic reticulum stress. However, with increased levels of SCD1, SFA levels remain low as much of the available SFA is converted to MUFA [49]. Increased SCD1 resulting in decreased SFA levels is thought to aid cancer cells in evasion of programmed cell death.
Early in vivo studies in mice with targeted disruption in the SCD1 gene showed reduced body adiposity, increased insulin sensitivity and resistance to diet-induced obesity, thus SCD plays a central role in FA metabolism and metabolic disorders [56,57]. Modulation of SCD represented a promising therapeutic strategy for the treatment of obesity and diabetes. Researchers have utilized various methods to develop novel inhibitors of SCD1 as possible therapeutics. One such molecule, MK-8245 (Merck & Co), is a SCD inhibitor with moderate bioavailability across multiple animal species. A phase 1 study of the compound in human subjects for the treatment of diabetes noted general safety and tolerability [58]. To overcome SCD1’s deleterious activity of on target toxicity in sensitive tissues, such as the eyes and skin, CV Therapeutics, Merck Research Laboratories and Xenon/Novartis and others produced compounds that preferentially distributed to the liver [59–61]. In another clever strategy, a high throughput drug screen identified two chemical series, oxalamides and benzothiazoles, that were selectively toxic at low nanomolar concentrations in human lung cancer cell lines expressing cytochrome P450 (CYP) 4F11, which metabolized the compounds into irreversible inhibitors of SCD [62].
In more recent years, SCD1 inhibitors were discovered to also be anticancer agents and shown to inhibit tumor growth in numerous murine in vivo studies that include prostate, breast, liver, renal, and lung cancers [17,52,53,63–65]. An example of recently discovered SCD1 inhibitors, von Roemeling et al. developed and tested a novel computational-based drug discovery method aimed at development of a novel SCD1 inhibitor. The group combined Quantitative Structure–Activity Relationship (QSAR) modeling with cell-based and enzymatic assay testing to analyze compound activity and specificity. Results rendered from two known SCD1 inhibitors, A939572 and ChemBL375265, compared with those of computational matches ultimately led to the discovery of SSI-1, SSI-2, SSI-3, and SSI-4 as novel SCD inhibitors. In vitro administration of these novel inhibitors to breast, colon, liver, ovarian, endometrial, thyroid, prostate, and lung cancer, as well as melanoma cell lines resulted in attenuated cell proliferation while having no effect upon normal primary cells in culture (i.e. renal, ovarian, and breast) [63]. Additionally, the lead compound for development, SSI-4 demonstrated excellent bioavailability and tumor suppression in patient-derived xenograft models of clear cell renal cell carcinoma [63]. Ma et al. also demonstrated that pharmacologic SCD1 inhibition with this novel compound, SSI-4, in a previously developed sorafenib resistant HCC cell line, effectively mitigated sorafenib resistance by induction of ER stress and apoptotic cell death [54]. Furthermore, in an in vivo HCC model combined treatment with sorafenib and SSI-4 showed maximal anti-tumor efficacy [54].
To date, essentially no SCD1 inhibitors have been tested clinically in humans as cancer therapeutics, thus limiting not only knowledge regarding their safety and efficacy, but also the understanding of mechanisms by which SCD1 levels are altered in HCC pathophysiology.
3.3. Fatty acid synthase
Researchers have yet to fully elucidate the roles FASN plays in liver tumorigenesis and HCC metabolism, in particular. Similar to SCD1, FASN has been identified as a potential target in HCC due to its integral role in catalyzing endogenous FA synthesis within cells [66,67]. Decades of investigation has resulted in the identification of numerous FASN inhibitors demonstrating preclinical antitumor activity, such as C75, C93, orlistat, GSK2194069, GSK837149A, and TVB-2640 [68–70]. Unfortunately, many have shown unfavorable toxicity profiles, preventing them from moving into the clinic as viable treatment options for patients.
Recent in vitro studies of Fasnall, a thiophenopyrimidine, which targets FASN, demonstrated anti-proliferative and apoptotic activity [71]. When administered to HER2+ breast cancer cell lines (SKBR3 and BT474), Fasnall significantly increased apoptotic activity, as evidenced by the increase incaspase-3 and caspase-9, known markers of apoptosis. These apoptotic affects were further demonstrated in Her2 positive breast cancer mice (MMTV-Neu) administered Fasnall alone and in combination with carboplatin. Both cohorts demonstrated a significantly reduced tumor volume and a doubled median survival time was seen in the Fasnall only cohort.
Another small molecule reversible inhibitor of FASN, TVB-2640 (3-V Biosciences, Menlo Park, CA), is being studied for the treatment of NASH, as well as multiple cancer types [72]. To date, TVB-2640 is the only FASN inhibitor to have moved into a phase 1 human clinical trial. This study was initiated to investigate safety and to determine the recommended phase II dose of TVB-2640 as monotherapy and in combination with paclitaxel or docetaxel in patients with advanced or metastatic solid tumor cancers [73]. The trial enrolled 17 patients with non-small cell lung cancer on the monotherapy arm. Although no objective responses were observed, an interesting trend of longer median time to progression was observed among patients with KRAS-mutant non-small cell lung cancer compared to wild-type. This is consistent with known negative effects of FASN inhibition on RAS pathway signaling. Fourteen patients with non-small cell lung cancer were treated in the combination arm with paclitaxel resulting in one partial response and five patients with stable disease lasting longer than 6 months. Again, there was a notable trend toward longer median time to progression in KRAS-mutant patients compared to patients with KRAS wild-type disease (28 weeks vs. 14 weeks). A total of 15 patients with breast cancer, all heavily pre-treated with an average of 7 prior systemic regimens, were treated with the combination of TVB-2640 and paclitaxel. Interestingly, the majority of these patients (13 of 15) had taxane resistance. Treatment in this group demonstrated three confirmed partial responses and three patients with stable disease lasting longer than 6 months. Among patients with HER2-positive disease, responses were achieved in two of the three patients. This subset of breast cancer is known to demonstrate the highest expression of FASN, suggesting a possible role for both Her2 positivity and FASN expression as biomarkers of response. A phase 2 trial of TVB-2640 in combination with paclitaxel and the Her2 targeting agent trastuzumab is set to open soon for patients with Her2 positive breast cancer ( NCT03179904) [74]. In the cohort of patients with ovarian cancer, two confirmed partial responses and two patients with stable disease lasting longer than 6 months were observed. Dose-limiting side effects included palmar-plantar erythrodysesthesia, as well as eye toxicity including iritis, corneal edema, and keratitis. These toxicities were considered on-target effects of inhibiting lipogenesis and were reversible. Trials investigating this compound in other malignancies, such as high-grade astrocytoma and colon cancer, are also underway ( NCT02980029, NCT03032484) [75,76]. The promising results from this study demonstrate the strong potential value in targeting lipid metabolism as an anti-cancer therapeutic strategy, including in patients with HCC.
3.4. Acetyl CoA carboxylase
ACC has been identified as an integral enzyme in canonical lipid metabolism in animals and humans [77,78]. During normal de novo FA synthesis, ACC is a rate-limiting enzyme that converts acetyl CoA to malonyl CoA. In mammals, two isoforms of ACC are expressed, ACC1 and ACC2. While ACC1 is highly expressed in lipid rich tissues (liver, adipose, and lactating mammary gland), ACC2 is highly expressed in oxidative tissues (heart and muscle) [79]. Given ACC’s role as rate-limiting enzymes in de novo FA synthesis, it is of special interest to investigators focused on establishing ACC as a therapeutic target in multiple cancer types, including HCC [15,80].
Biochemists recently designed and synthesized a series of ACC inhibitors. One of these novel spiropentacylamide derivatives, Compound 6o, demonstrated potent anti-proliferation activity against multiple human cancer cell lines- A549 (lung carcinoma), H1975 (small cell lung carcinoma), HCT116 (color-ectal carcinoma), SW620 (colon adenocarcinoma), and Cac0–2 (colon adenocarcinoma) [81]. Similarly, in vitro studies of human glioblastoma cells (U87 EGFRvIII) treated with a dual ACC inhibitor demonstrated an effect of reducing de novo lipogenesis, increasing cellular metabolic rate, though decreasing mitochondrial ATP production efficiency, and increasing apoptotic activity [82]. Svensson et al. showed anti-tumor synergy, tumor regression, and survival benefit using a novel ACC inhibitor combined with carboplatin in non-small cell lung cancer animal models [83].
While several studies have profiled ACC inhibition in preclinical analysis of various cancer types, little evidence exists on potential targets in HCC models exclusively [84–86]. A study by Wei et al. explored the efficacy of ND-654, a hepatoselective (~3000:1 liver to muscle exposure), allosteric inhibitor of ACC1 and ACC2, in rat models of liver cirrhosis and HCC. After establishment of cirrhosis and when HCCs were first developing, rats were treated daily by oral gavage with either (1) vehicle control, (2) ND-654, (3) sorafenib, or (4) ND-654 + sorafenib. Interestingly, dual ACC inhibition of ACC1 and ACC2 significantly reduced HCC incidence by 41% (p < 0.05), which was comparable to results with sorafenib alone (57% reduction, p < 0.01). The combination of ND-654 and sorafenib significantly reduced HCC incidence by 81% (p < 0.001) [87].
To further investigate the effects of ND-654 on ACC-mediated activity in HCC, Lally et al. conducted multiple in vivo studies in rats. They had demonstrated that mice with targeted loss-of-function point mutations of the AMP- activated protein kinase (AMPK) phosphorylation sites on ACC1 (ACC1 Ser79Ala) and ACC2 (ACC2 Ser212Ala) resulted in increased liver de novo lipogenesis and liver lesions. The same mutation in ACC1 increased de novo lipogenesis and proliferation in human liver cancer cells. The liver-specific ACC inhibitor (ND-654) mimics the effects of the above mutation and inhibits hepatic de novo lipogenesis and the development of HCC. In one experiment, rats were treated daily by oral gavage at the start of week 14 with either (1) vehicle control, (2) 10 mg/kg ND-654, or (3) 30 mg/kg ND-654. At day 115, survival rates were 40% and 100% for vehicle-treated and ND654-treated rats, respectively. In another experiment rats received (1) vehicle control, (2) ND-654, (3) sorafenib, or (4) ND-654 + sorafenib. ND-654 alone and sorafenib alone demonstrated a 41% (p < 0.05) and 57% (p < 0.05), respectively, decrease in HCC incidence, while the combination of ND-654 and sorafenib reduced HCC incidence by 81% (p < 0.05) [88].
Although multiple animal and human studies have demonstrated benefits of ACC inhibition in cancer treatment, in vitro and in vivo experiments by Rios Garcia et al. reported an increase in breast cancer metastasis and tumor recurrence following ACC1 knockdown via phosphorylation [89]. Data from these studies are by no means definitive in concluding that ACC inhibition itself leads to or promotes metastasis, however caution is necessary and further testing is warranted to determine the role and effects of ACC inhibition in various cancer types. Specifically, HCC animal models should be employed to obtain a deeper understanding of the role ACC inhibition plays in HCC disease progression and metastasis.
4. Other fatty acid metabolism targets
4.1. CD147 (basigin or emmprin)
CD147 is a transmembrane glycoprotein, greatly expressed on the surface of malignant cells including HCC [90]. It has been shown to regulate many bodily functions like immunologic differentiation and development, spermatogenesis, and also has an effect on sensory and nervous systems especially the function of photoreceptors [90]. Contradicting results exist in a limited set of studies regarding the prognostic relevance of CD147 in HCC [91].
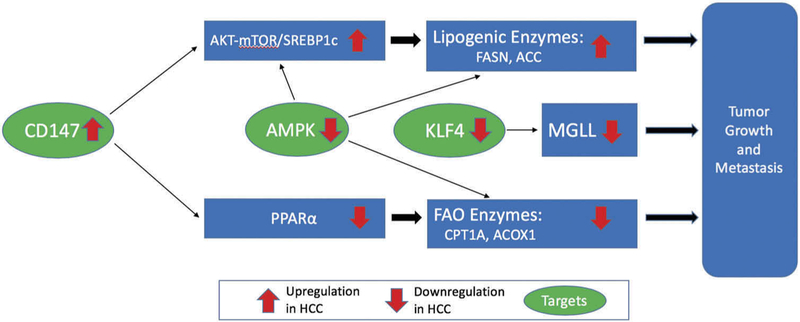
Particular to lipid metabolism, CD147 has been shown to increase lipogenesis and decrease FA oxidation, which makes it an important factor to be studied in cancer, including HCC (Figure 2). In fact, increased expression of CD147 is associated with significant tumor growth, metastasis, and angiogenesis [90]. CD147 increases lipogenesis by increasing levels of ACC1 and FASN, two of the main enzymes involved in lipogenesis, by activating the Akt/mTOR signaling pathway, increasing the expression of sterol regulatory element binding protein 1c (SREBP1c), a transcription factor involved in lipid synthesis which in turn directly activates the transcription of these lipogenic genes, thus promoting de novo lipogenesis.
Figure 2.
Other fatty acid metabolism targets. Upregulation of CD147 activates the AKT-mTOR signaling pathway, leading to increased expression of SREBPlc. Increased SREBPlc levels increases FASN and ACC expression, thus causing tumor growth and metastasis. Upregulation of CD147 also decreases PPAR α, which downregulates CPTlA and ACOXl. Tumor growth and metastasis results from downregulation of CPTlA and ACOXl. Decreased AMPK activity leads to tumor growth and metastasis by directly upregulating SREBPlc, FASN, and/or ACC. Decreased AMPK activity also downregulates CPTlA and ACOXl, thus leading to tumor growth and metastasis. Decreased KLF4 1evels leads to decreased MGLL activity, thus causing tumor growth and metastasis.
Regarding its role in decreasing FA oxidation (FAO), CD147 has been shown to downregulate both CPT1 and ACOX1, key enzymes in FAO, via downregulation of peroxisome proliferator-activated receptor alpha (PPARα). PPARα serves as a transcription factor specifically regulating the key enzymes in this process of FAO, including CPT1 and ACOX1. A study done exploring CD147’s effects on FAO in HCC specifically demonstrates that levels of PPARα are significantly increased upon CD147 knockout. In vitro and in vivo analysis demonstrated that CD147-mediated reprogramming of FA metabolism played a critical role in HCC proliferation and metastasis [92].
There has been at least one study in humans testing a novel therapeutic antibody targeting CD147, conjugated to an 131I radioisotope (I113-Metuximab) in HCC. In a rabbit model of HCC, combined treatment with the I113-Metuximab antibody led to significantly longer survival. Metastasis and tumor growth in the I113-Metuximab treatment group were also inhibited [93]. In a subsequent human clinical trial, patients received either radiofrequency ablation (RFA) followed by [(131)I] metuximab or RFA alone. The survival rate at 1-year and 2-year intervals in CD147 positive population were greater in combination therapy group when compared to RFA group alone. There was a greater benefit upon survival and recurrence rates in those patients treated with the combination therapy. The one- and two-year recurrence rates in the combination group were 31.8% and 58.5%, whereas those in the RFA group were 56.3% and 70.9%, respectively. The median time to overall tumor recurrence was 17 months in the combination group and 10 months in the RFA group (P = .03) [94].
4.2. KLF4 and monoglyceride lipase (MGLL)
MGLL plays a role in lipid metabolism by hydrolyzing monoacylglycerides to free FAs (FFAs) and glycerol. MGLL has been reported to be downregulated in HCC, and Yang et al. investigated the clinical significance in HCC patients (Figure 2). The study demonstrated that MGLL was frequently downregulated in HCC samples, especially in metastatic tumor tissues. Patients with low MGLL expression showed significantly lower 5 year-overall survival [95]. Functionally, they found that MGLL played an important role in HCC cell migration. Overexpression of MGLL suppressed cell migration and depletion of MGLL by shRNA promoted cell migration. KLF4, a zinc finger transcription factor, was noted to be directly bound to the promoter of MGLL and accelerated MGLL expression, which had an inhibitory effect on HCC cell migration. Expression levels of KLF4 correlated with MGLL expression in HCC tissues, all suggesting that KLF4 is a key regulator of MGLL. They effectively demonstrated the key role played by the KLF4-MGLL in suppressing HCC cell migration [95].
Interestingly, KLF4 has another role in lipid metabolism via hepatocyte nuclear factor 6 (HNF6). Both KLF4 and HNF6 expression levels have been linked to differentiation of HCC cells. Reduced KLF4 or HNF6 expression correlated with high grade, poorly differentiated HCC in one study [96]. HNF6 represses a subset of genes which are linked to lipid biosynthesis, FA biosynthesis, and lipid storage, while upregulating genes linked to FAO. KLF4 directly binds to the promoter site of HNF6 and activates HNF-6 expression. Restored HNF-6 expression upregulates expression of differentiation-associated markers and inhibits HCC cell migration and invasion, while HNF-6 knockdown did the opposite, as demonstrated in one study [96].
Given this data, KLF4 and HNF6 are two targets warranting further exploration as a therapeutic strategy. A potential target for investigation is FBOX22, which regulates and promotes the turnover of KLF4. FBOX22 interacts with and destabilizes KLF4 via polyubiquitination, promoting degradation of KLF4. Accordingly, FBXO22 expression has been shown to be markedly increased in human HCC tissues, which correlated with down-regulation of KLF4. FBXO22 demonstrated that it could promote HCC cell proliferation both in vitroand in vivo in one study [97]. Another target of possible interest for drug development is FOAX2 which regulates HNF6. A study was conducted in colorectal liver metastasis patients demonstrating that inhibition of FOAX2 caused upregulation of HNF6 leading to cell cycle arrest [98].
Though data is limited, there have also been studies investigating inhibitors of monoacylglycerol (MAGL) production, demonstrating effects on different types of cancers by inhibiting cell migration and invasiveness. MAGL inhibitors have been shown to have anti-cancer properties through modulating of FA release for the synthesis of protumorigenic signaling lipids [99–102].
Some have also shown that MAGL blockade in aggressive breast, ovarian, and melanoma cancer cells impairs cell migration, invasiveness, and tumorigenicity through lowering free-FAs and protumorigenic signaling lipids [103]. Other studies have shown that MAGL inhibitors impair colorectal cancer tumorigenesis [101].
4.3. AMP-activated protein kinase (AMPK)
AMPK is a multifaceted player in cellular metabolic functions, in part via its central role in lipid metabolism in its actions controlling the concentration of circulating FFAs by activating FAO, and by inhibiting lipolysis and lipogenesis. Within FA metabolism, AMPK inhibits de novo synthesis of FAs, cholesterol, and triglycerides (TG), encourages FA uptake, and activates FAO [104] (Figure 2). AMPK exerts an inhibitory effect upon FA synthesis via induction of the inhibitory phosphor-ylation of two critical players in this process: ACC and sterol regulatory element-binding protein 1 (SREBP1c) [105,106]. Additionally, AMPK inhibits cholesterol synthesis by inducing inhibitory phosphorylation of HMG-CoA reductase, the rate-limiting enzyme in cholesterol synthesis [107]. Its impact upon TG synthesis is accomplished by AMPK’s inhibitory action upon glycerol-3-phosphate acyltransferase, the enzyme catalyzing the first committed step of TG synthesis [108]. AMPK increases FA uptake by controlling, via unclear mechanisms, the translocation of transporter CD36 to the plasma membrane. Once inside, FAs are transported to the mitochondria for beta-oxidation by carnityl palmitoyltransferase-1 (CPT-1). AMPK increases FAO by increasing CPT-1 activity by inducing the inhibitory phosphorylation of ACC2. ACC2 is an enzyme which lies adjacent to CPT-1 in the outer membrane of the mitochondria where it inhibits production of malonyl-CoA, a very potent allosteric inhibitor of CPT-1 [104,106].
In patient samples of HCC, lower expression of phosphorylated AMPK has been found, compared with precancerous liver tissues. The expression of phosphorylated AMPK also negatively correlated with the level of Ki-67 (marker of cell proliferation), tumor grade, and tumor size in HCC, thus indicating that AMPK might have a suppressive effect on HCC [109].
AMPK as a therapeutic target has been studied in HCC specifically with AMPK activators, for example metformin and simvastatin, showing significant inhibitory effects on the proliferation of HCC and induction of cell cycle arrest [110,111]. Metformin, the common hypoglycemic agent used in diabetes, is also an AMPK activator and has demonstrated its ability to induce apoptosis in HCC cells. Phosphorylation of AMPK and expression of p53 were increased upon metformin treatment in one study. Further, this induction of the AMPK/p53 signaling axis was noted to induce micro-RNA-23a expression, which in turn stimulated activity of the transcription factor FOXA1 [109,112].
Contrary to its potential as a therapeutic target, AMPK has also been shown to have pro-oncogenic effect. Its expression may be beneficial in the earlier stages of carcinogenesis by enabling cells to facilitate both cell growth and proliferation by activating anabolic pathways, and by introducing genetic mutations through an increase in oxidative stress and a proinflammatory response, largely through a dysregulation of FA metabolism [104]. In fact, one particular study demonstrates that deficiency of liver kinase B1 (LKB1), a negative regulator of AMPK, promotes neutrophil recruitment and proinflammatory cytokine production in the lung tumor microenvironment [113]. It has been postulated that the different complexes of AMPK subunits have different functions depending on their combination. Thus, AMPK may indeed be a potential target for drug development, but one has to be mindful about the potential oncogenic effects and further study is critical.
5. Conclusion
Given the global burden of HCC, the rising incidence, and the modest outcomes with current therapeutics, there is a critical need for novel, innovative therapeutic options. Strategies targeting altered metabolic pathways that are critical to cancer cell survival deserve increased attention and development. Multiple viable targets exist and a number of agents are poised for potential advancement into the clinic with continued support.
6. Expert opinion
HCC represents a globally significant cause of cancer related death, and is rising in incidence. The paucity of effective therapeutic agents for HCC for decades has recently been met with a some modest success in drug development and drug approvals for treatment of advanced disease. However, the disease remains a significant global burden, and while systemic therapies involving tyrosine kinase inhibition of certain aspects of cell signaling, inhibition of angiogenesis, and single target immune checkpoint inhibition have achieved modest outcomes, there remains a critical need for more effective therapeutic strategies. This requires strategies targeting cancer processes heretofore unchallenged by novel therapeutics in this disease.
The increased metabolic needs of cancer cells, accomplished by alterations in both glucose and lipid metabolism pathways, in a setting of reduced nutrient availability underscore the importance of these pathways and is well recognized. Despite the recognition of metabolic adaptations made by cancer cells, HCC included, to respond to increased metabolic needs, therapeutic strategies targeting such adaptations are lacking. Lipid biosynthesis is one particular pathway in which adaptations are made, and recent studies implicate lipid biosynthesis and desaturation as essential for HCC survival [18–20]. The increase in lipid metabolism and imbalance in lipid physiology in HCC is further suggestive of a reliance on increased lipogenesis in this devastating malignancy. As we discuss, herein, emerging work has elucidated a number of attractive potential targets for novel therapeutics.
Given the tumor-specific overexpression of a number of important lipid metabolism proteins in HCC, and the rigorous regulation of this pathway in normal tissues, it is logical to believe that targeted inhibitors would be predicted to produce discriminative therapeutic responses in HCC tumors. While inhibitors of lipid metabolism should be individually investigated for efficacy in HCC, combinatorial therapeutics including these agents with complementary regimens including radiation, chemotherapy, other targeted agents and immunotherapies will need to be explored and will likely improve efficacy and outcomes.
Thus far, there has been little advancement of therapeutics targeting lipogenesis. There has indeed been much work by our group related to SCD1’s role as a potential target in a number of cancers including HCC, and a novel inhibitor of the enzyme, SSI-4, has demonstrated promising efficacy both singly and in combination with other drugs, including demonstration of synergitic activity with sorafenib in HCC models and with immune checkpoint inhibitors in breast cancer [17,52,54,114]. Inhibitors against a variety of other enzymes involved in de novo lipogenesis have been produced, some currently under further investigation for anti-tumorigenic efficacy including: ACC, ACL, carnitine palmitoyltransferase 1 (CPT-1), FASN, MAGL lipase, HMGCR [70,115–117]. Agents targeting ACC and FASN are readily available for clinical development in HCC, and have already demonstrated safety and activity in other cancers. HMGCR is of particular interest given it is the target of statins, a well known and widely used class of drugs employed for their cholesterol lowering effects. Indeed, statins might also exert antineoplastic properties. These agents have demonstrated antiproliferative effects in HCC, reduction in risk of HCC in patients at high risk for it, and those after initial liver resection for the disease. They have also demonstrated the ability to reverse resistance to sorafenib therapy [118–121].
While there is tremendous potential of and an unmet need for continued study of lipid metabolism as a therapeutic target for cancer, there are indeed fundamental challenges to the advancement of this research, including the use of rodents as preclinical models. While these models are seen as integral and suitable tools to evaluate many aspects of cancer biology and the efficacy of novel therapeutics, there are significant discrepancies in genetic variation, tissue distribution, expression, and regulatory mechanisms governing lipid metabolism that may limit their translational relevance in humans [122]. For example, mouse models express four isoforms of SCD, only one being homologous to human SCD [51]. Therefore, therapeutic efficacy and adverse events observed in rodent models in response to SCD inhibitors may not be predictive of toxicity profiles in humans. Alternative preclinical models that more accurately reflect lipid metabolism observed in humans may be needed to improve the predictive value of targeting this pathway in cancer.
Article Highlights.
HCC is one of the leading causes of cancer deaths worldwide and has limited therapeutic options.
Strategies targeting altered metabolic pathways that are so critical to cancer cell survival deserve increased attention and development.
Recent studies implicate lipid biosynthesis and desaturation as a requirement for HCC survival.
Multiple viable targets exist and a number of agents are poised for potential advancement into the clinic with continued support.
There are fundamental challenges to the advancement of research in this area, including the use of rodents as preclinical models. There are significant discrepancies in genetic variation, tissue distribution, expression, and regulatory mechanisms governing lipid metabolism that may limit their translational relevance in humans
There is a critical need for more effective therapeutic strategies.
This box summarizes key points contained in the article.
Funding
This research was funded in part by the following grants: Mayo Clinic Liver SPORE (NCI/NIH P50 CA210964; KM, JAC), NCI STTR R42 grant (CA195946; JAC) and Florida Department of Health (8BC01; JAC).
Declaration of interest
K Mody has received research support from Agios, Senwha Biosciences, Taiho, ArQule, Astra Zeneca, Genentech, Incyte, Tracon Pharmaceuticals, Medimmune, Puma Biotechnology and consulting fees from Astra Zeneca, Bayer, Celgene, Eisai, Exelixis, Merrimack and Vicus
JA Copland has filed a patent application for novel composition of matter for SCD1 inhibitors.
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Footnotes
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Ananthakrishnan A, Gogineni V, Saeian K. Epidemiology of primary and secondary liver cancers. Semin Intervent Radiol. 2006;23(1):47–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balogh J, Victor D, Asham EH, et al. Hepatocellular carcinoma: a review. J Hepatocell Carcinoma. 2016. October 05;3:41–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McGlynn KA, London WT. The global epidemiology of hepatocellular carcinoma: present and future. Clin Liver Dis. 2011. May;15(2):223–43, vii-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abou-Alfa GK, Meyer T, Cheng A-L, et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Engl J Med. 2018. July 05;379(1):54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017. June 24;389(10088):2492–2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepato-cellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017. January 7;389(10064):56–66. [DOI] [PubMed] [Google Scholar]
- 7.Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391(10126):1163–1173. [DOI] [PubMed] [Google Scholar]
- 8.Bechmann LP, Hannivoort RA, Gerken G, et al. The interaction of hepatic lipid and glucose metabolism in liver diseases. J Hepatol. 2012. April 01;56(4):952–964. [DOI] [PubMed] [Google Scholar]
- 9.Bauer DE, Hatzivassiliou G, Zhao F, et al. ATP citrate lyase is an important component of cell growth and transformation. Oncogene. 2005. September 15;24(41):6314–6322. [DOI] [PubMed] [Google Scholar]
- 10.Litwack G. Chapter 9 - lipids In: Litwack G, editor. Human biochemistry. Boston: Academic Press; 2018. p. 199–255. [Google Scholar]
- 11.Miyazaki M, Kim HJ, Man WC, et al. Oleoyl-CoA is the major de novo product of stearoyl-CoA desaturase 1 gene isoform and substrate for the biosynthesis of the Harderian gland 1-alkyl-2,3-diacylglycerol. J Biol Chem. 2001. October 19;276(42):39455–39461. [DOI] [PubMed] [Google Scholar]
- 12.Semenkovich CF, Goldberg AC, Goldberg IJ. Chapter 37 - disorders of lipid metabolism In: Melmed S, Polonsky KS, Larsen PR, et al. , editors. Williams textbook of endocrinology. 13 ed Philadelphia: Content Repository Only!; 2016. p. 1660–1700. [Google Scholar]
- 13.Santos CR, Schulze A. Lipid metabolism in cancer. Febs J. 2012. August;279(15):2610–2623. [DOI] [PubMed] [Google Scholar]
- 14.Menendez JA, Lupu R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat Rev Cancer. 2007. October 01 online;7:763. [DOI] [PubMed] [Google Scholar]
- 15.Abramson HN. The lipogenesis pathway as a cancer target. J Med Chem. 2011. August 25;54(16):5615–5638. [DOI] [PubMed] [Google Scholar]
- 16.Ackerman D, Simon MC. Hypoxia, lipids, and cancer: surviving the harsh tumor microenvironment. Trends Cell Biol. 2014. August;24(8):472–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.von Roemeling CA, Marlow LA, Wei JJ, et al. Stearoyl-CoA desaturase 1 is a novel molecular therapeutic target for clear cell renal cell carcinoma. Clin Cancer Res. 2013. May 1;19(9):2368–2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muir K, Hazim A, He Y, et al. Proteomic and lipidomic signatures of lipid metabolism in NASH-associated hepatocellular carcinoma. Cancer Res. 2013. August 1;73(15):4722–4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nelson GM, Ahlborn GJ, Allen JW, et al. Transcriptional changes associated with reduced spontaneous liver tumor incidence in mice chronically exposed to high dose arsenic. Toxicology. 2009. December 21;266(1–3):6–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Falvella FS, Pascale RM, Gariboldi M, et al. Stearoyl-CoA desaturase 1 (Scd1) gene overexpression is associated with genetic predisposition to hepatocarcinogenesis in mice and rats. Carcinogenesis. 2002. November;23(11):1933–1936. [DOI] [PubMed] [Google Scholar]
- 21.Watson JA, Fang M, Lowenstein JM. Tricarballylate and hydroxycitrate: substrate and inhibitor of ATP: citrate oxaloacetate lyase. Arch Biochem Biophys. 1969. December;135(1):209–217. [DOI] [PubMed] [Google Scholar]
- 22.Verschueren KHG, Blanchet C, Felix J, et al. Structure of ATP citrate lyase and the origin of citrate synthase in the Krebs cycle. Nature. 2019. April;568(7753):571–575. [DOI] [PubMed] [Google Scholar]
- 23.Zaidi N, Swinnen JV, Smans K. ATP-citrate lyase: a key player in cancer metabolism. Cancer Res. 2012. August 1;72(15):3709–3714. [DOI] [PubMed] [Google Scholar]
- 24.Wei J, Leit S, Kuai J, et al. An allosteric mechanism for potent inhibition of human ATP-citrate lyase. Nature. 2019. April;568 (7753):566–570. [DOI] [PubMed] [Google Scholar]
- 25.Yahagi N, Shimano H, Hasegawa K, et al. Co-ordinate activation of lipogenic enzymes in hepatocellular carcinoma. Eur J Cancer. 2005. June;41(9):1316–1322. [DOI] [PubMed] [Google Scholar]
- 26.Migita T, Narita T, Nomura K, et al. ATP citrate lyase: activation and therapeutic implications in non-small cell lung cancer. Cancer Res. 2008. October 15;68(20):8547–8554. [DOI] [PubMed] [Google Scholar]
- 27.Zhou Y, Bollu LR, Tozzi F, et al. ATP citrate lyase mediates resistance of colorectal cancer cells to SN38. Mol Cancer Ther. 2013. December;12(12):2782–2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Teng L, Chen Y, Cao Y, et al. Overexpression of ATP citrate lyase in renal cell carcinoma tissues and its effect on the human renal carcinoma cells in vitro. Oncol Lett. 2018. May;15(5):6967–6974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beckner ME, Fellows-Mayle W, Zhang Z, et al. Identification of ATP citrate lyase as a positive regulator of glycolytic function in glioblastomas. Int J Cancer. 2010. May 15;126(10):2282–2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carrer A, Trefely S, Zhao S, et al. Acetyl-CoA metabolism supports multistep pancreatic tumorigenesis. Cancer Discov. 2019. March;9(3):416–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ference BA, Ray KK, Catapano AL, et al. Mendelian randomization study of ACLY and cardiovascular disease. N Engl J Med. 2019. March 14;380(11):1033–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hatzivassiliou G, Zhao F, Bauer DE, et al. ATP citrate lyase inhibition can suppress tumor cell growth. Cancer Cell. 2005. October;8(4):311–321. [DOI] [PubMed] [Google Scholar]
- 33.Zaidi N, Royaux I, Swinnen JV, et al. ATP citrate lyase knockdown induces growth arrest and apoptosis through different cell- and environment-dependent mechanisms. Mol Cancer Ther. 2012. September;11(9):1925–1935. [DOI] [PubMed] [Google Scholar]
- 34.Wellen KE, Hatzivassiliou G, Sachdeva UM, et al. ATP-citrate lyase links cellular metabolism to histone acetylation. Science (New York, NY). 2009. May 22;324(5930):1076–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hanai JI, Doro N, Seth P, et al. ATP citrate lyase knockdown impacts cancer stem cells in vitro. Cell Death Dis. 2013. June;27(4):e696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Filippov S, Pinkosky SL, Newton RS. LDL-cholesterol reduction in patients with hypercholesterolemia by modulation of adenosine triphosphate-citrate lyase and adenosine monophosphate-activated protein kinase. Curr Opin Lipidol. 2014. August;25(4):309–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pinkosky SL, Filippov S, Srivastava RA, et al. AMP-activated protein kinase and ATP-citrate lyase are two distinct molecular targets for ETC-1002, a novel small molecule regulator of lipid and carbohydrate metabolism. J Lipid Res. 2013. January;54(1):134–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rose-Kahn G, Bar-Tana J. Inhibition of rat liver acetyl-CoA carboxylase by beta, beta’-tetramethyl-substituted hexadecanedioic acid (MEDICA 16). Biochim Biophys Acta. 1990. February 6;1042(2):259–264. [DOI] [PubMed] [Google Scholar]
- 39.Gutierrez MJ, Rosenberg NL, Macdougall DE, et al. Efficacy and safety of ETC-1002, a novel investigational low-density lipoprotein-cholesterol-lowering therapy for the treatment of patients with hypercholesterolemia and type 2 diabetes mellitus. Arterioscler Thromb Vasc Biol. 2014. March;34(3):676–683. [DOI] [PubMed] [Google Scholar]
- 40.Ballantyne CM, Hoogeveen RC, Raya JL, et al. Efficacy, safety and effect on biomarkers related to cholesterol and lipoprotein metabolism of rosuvastatin 10 or 20 mg plus ezetimibe 10 mg vs. simvastatin 40 or 80 mg plus ezetimibe 10 mg in high-risk patients: results of the GRAVITY randomized study. Atherosclerosis. 2014. January;232(1):86–93. [DOI] [PubMed] [Google Scholar]
- 41.Lemus HN, Mendivil CO. Adenosine triphosphate citrate lyase: emerging target in the treatment of dyslipidemia. J Clin Lipidol. 2015. May-Jun;9(3): 384–389. [DOI] [PubMed] [Google Scholar]
- 42.Ray KK, Bays HE, Catapano AL, et al. Safety and efficacy of bempedoic acid to reduce LDL cholesterol. N Engl J Med. 2019. March 14;380(11):1022–1032. [DOI] [PubMed] [Google Scholar]
- 43.Berkhout TA, Havekes LM, Pearce NJ, et al. The effect of (−)-hydroxycitrate on the activity of the low-density-lipoprotein receptor and 3-hydroxy-3-methylglutaryl-CoA reductase levels in the human hepatoma cell line Hep G2. Biochem J. 1990. November 15;272(1):181–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schwartz L, Abolhassani M, Guais A, et al. A combination of alpha lipoic acid and calcium hydroxycitrate is efficient against mouse cancer models: preliminary results. Oncol Rep. 2010. May;23(5):1407–1416. [DOI] [PubMed] [Google Scholar]
- 45.Guais A, Baronzio G, Sanders E, et al. Adding a combination of hydroxycitrate and lipoic acid (METABLOC) to chemotherapy improves effectiveness against tumor development: experimental results and case report. Invest New Drugs. 2012. February;30(1):200–211. [DOI] [PubMed] [Google Scholar]
- 46.Gao Y, Islam MS, Tian J, et al. Inactivation of ATP citrate lyase by Cucurbitacin B: A bioactive compound from cucumber, inhibits prostate cancer growth. Cancer Lett. 2014. July 10;349(1):15–25. [DOI] [PubMed] [Google Scholar]
- 47.Jernigan FE, Hanai JI, Sukhatme VP, et al. Discovery of furan carboxylate derivatives as novel inhibitors of ATP-citrate lyase via virtual high-throughput screening. Bioorg Med Chem Lett. 2017. February 15;27(4):929–935. [DOI] [PubMed] [Google Scholar]
- 48.Koerner SK, Hanai JI, Bai S, et al. Design and synthesis of emodin derivatives as novel inhibitors of ATP-citrate lyase. Eur J Med Chem. 2017. January 27;126:920–928. [DOI] [PubMed] [Google Scholar]
- 49.Igal RA. Stearoyl-CoA desaturase-1: a novel key player in the mechanisms of cell proliferation, programmed cell death and transformation to cancer. Carcinogenesis. 2010;31(9):1509–1515. [DOI] [PubMed] [Google Scholar]; •• Describe the integral role of SCD-1 in fatty acid metabolism and systematically detail how SCD-1 upregulation aids in cancer cell growth and apoptosis evasion.
- 50.Ntambi JM. Regulation of stearoyl-CoA desaturase by polyunsatu-rated fatty acids and cholesterol. J Lipid Res. 1999. September;40(9):1549–1558. [PubMed] [Google Scholar]
- 51.Liu X, Strable MS, Ntambi JM. Stearoyl CoA desaturase 1: role in cellular inflammation and stress. Adv Nutr. 2011. January;2(1):15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.von Roemeling CA, Marlow LA, Pinkerton AB, et al. Aberrant lipid metabolism in anaplastic thyroid carcinoma reveals stearoyl CoA desaturase 1 as a novel therapeutic target. J Clin Endocrinol Metab. 2015. May;100(5):E697–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.von Roemeling CA, Copland JA. Targeting lipid metabolism for the treatment of anaplastic thyroid carcinoma. Expert Opin Ther Targets. 2016. February 01;20(2):159–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ma MKF, Lau EYT, Leung DHW, et al. Stearoyl-CoA desaturase regulates sorafenib resistance via modulation of ER stress-induced differentiation. J Hepatol. 2017. November;67(5):979–990. [DOI] [PubMed] [Google Scholar]; •• This study established that SCD overexpression is correlated with tumor cell differentiation and shorter disease-free survival in HCC cells.
- 55.Bansal S, Berk M, Alkhouri N, et al. Stearoyl-CoA desaturase plays an important role in proliferation and chemoresistance in human hepatocellular carcinoma. J Surg Res. 2014. January;186(1):29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Miyazaki M, Flowers MT, Sampath H, et al. Hepatic stearoyl-CoA desaturase-1 deficiency protects mice from carbohydrate-induced adiposity and hepatic steatosis. Cell Metab. 2007. December;6(6):484–496. [DOI] [PubMed] [Google Scholar]
- 57.Ntambi JM, Miyazaki M. Recent insights into stearoyl-CoA desaturase-1. Curr Opin Lipidol. 2003. June;14(3):255–261. [DOI] [PubMed] [Google Scholar]
- 58.Pharmacokinetics and pharmacodynamics of MK-8245 in participants with type 2 diabetes (MK-8245–012). [Cited 2019 Apr 9]. Available from: https://clinicaltrials.gov/ct2/show/results/NCT00972322
- 59.Zhang Z, Dales NA, Winther MD. Opportunities and challenges in developing stearoyl-coenzyme A desaturase-1 inhibitors as novel therapeutics for human disease. J Med Chem. 2014. June 26;57(12):5039–5056. [DOI] [PubMed] [Google Scholar]
- 60.Koltun DO, Parkhill EQ, Vasilevich NI, et al. Novel, potent, selective, and metabolically stable stearoyl-CoA desaturase (SCD) inhibitors. Bioorg Med Chem Lett. 2009. April 1;19(7):2048–2052. [DOI] [PubMed] [Google Scholar]
- 61.Sun S, Zhang Z, Raina V, et al. Discovery of thiazolylpyridinone SCD1 inhibitors with preferential liver distribution and reduced mechanism-based adverse effects. Bioorg Med Chem Lett. 2014. January 15;24(2):526–531. [DOI] [PubMed] [Google Scholar]
- 62.Theodoropoulos PC, Gonzales SS, Winterton SE, et al. Discovery of tumor-specific irreversible inhibitors of stearoyl CoA desaturase. Nat Chem Biol. 2016. February 01 online;12:218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.von Roemeling CA, Caulfield TR, Marlow L, et al. Accelerated bottom-up drug design platform enables the discovery of novel stearoyl-CoA desaturase 1 inhibitors for cancer therapy. Oncotarget. 2018. January 2;9(1):3–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fritz V, Benfodda Z, Rodier G, et al. Abrogation of de novo lipogenesis by stearoyl-CoA desaturase 1 inhibition interferes with oncogenic signaling and blocks prostate cancer progression in mice. Mol Cancer Ther. 2010. June;9(6):1740–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li W, Bai H, Liu S, et al. Targeting stearoyl-CoA desaturase 1 to repress endometrial cancer progression. Oncotarget. 2018. February 23;9(15):12064–12078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lee S, Zhang C, Liu Z, et al. Network analyses identify liver-specific targets for treating liver diseases. Mol Syst Biol. 2017. August 21;13(8):938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Buckley D, Duke G, Heuer TS, et al. Fatty acid synthase - Modern tumor cell biology insights into a classical oncology target. Pharmacol Ther. 2017;177:23–31. [DOI] [PubMed] [Google Scholar]
- 68.Zhou W, Simpson PJ, McFadden JM, et al. Fatty acid synthase inhibition triggers apoptosis during S phase in human cancer cells. Cancer Res. 2003. November 1;63(21):7330–7337. [PubMed] [Google Scholar]
- 69.Hardwicke MA, Rendina AR, Williams SP, et al. A human fatty acid synthase inhibitor binds beta-ketoacyl reductase in the keto-substrate site. Nat Chem Biol. 2014. September;10(9):774–779. [DOI] [PubMed] [Google Scholar]
- 70.Flavin R, Peluso S, Nguyen PL, et al. Fatty acid synthase as a potential therapeutic target in cancer. Future Oncol. 2010. April;6(4):551–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Alwarawrah Y, Hughes P, Loiselle D, et al. Fasnall, a selective FASN inhibitor, shows potent anti-tumor activity in the MMTV-neu model of HER2(+) breast cancer. Cell Chem Biol. 2016. June 23;23(6):678–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Duke G, Wagman AS, Buckley D, et al. LBP-515 - Establishing the foundation for a novel, first-in-class, fatty acid synthase inhibitor, TVB-2640, for the treatment of NASH. J Hepatol. 2017. January 01;66(1, Supplement):S99–S100. [Google Scholar]
- 73.Falchook G, Patel M, Infante J, et al. Abstract CT153: first in human study of the first-in-class fatty acid synthase (FASN) inhibitor TVB-2640. Cancer Res. 2017;77(13Supplement):CT153. [Google Scholar]; •• As the only FASN inhibitor that has moved to human trials, TVB-2640 performed favorably both as monotherapy and in combination with chemotherapeutic agents in advanced/meta-static solid tumors.
- 74.FASN inhibitor TVB-2640, paclitaxel, and trastuzumab in treating patients with HER2 positive advanced breast cancer. [Cited 2019 Apr 9]. Available from: https://ClinicalTrials.gov/show/NCT03179904
- 75.TVB 2640 for resectable colon cancer other resectable cancers; a window trial. [Cited 2019 Apr 9]. Available from: https://ClinicalTrials.gov/show/NCT02980029
- 76.TVB- 2640 in combination with bevacizumab in patients with first relapse of high grade astrocytoma. [Cited 2019 Apr 9]. Available from: https://ClinicalTrials.gov/show/NCT03032484
- 77.Wakil SJ, Abu-Elheiga LA. Fatty acid metabolism: target for metabolic syndrome. J Lipid Res. 2009. April;50(Suppl):S138–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chow JD, Lawrence RT, Healy ME, et al. Genetic inhibition of hepatic acetyl-CoA carboxylase activity increases liver fat and alters global protein acetylation. Mol Metab. 2014. July;3(4):419–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Abu-Elheiga L, Almarza-Ortega DB, Baldini A, et al. Human acetyl-CoA carboxylase 2. Molecular cloning, characterization, chromosomal mapping, and evidence for two isoforms. J Biol Chem. 1997. April 18;272(16):10669–10677. [DOI] [PubMed] [Google Scholar]
- 80.Wang C, Rajput S, Watabe K, et al. Acetyl-CoA carboxylase-a as a novel target for cancer therapy. Front Biosci (Schol Ed). 2010. January 1;2:515–526. [DOI] [PubMed] [Google Scholar]
- 81.Wei Q, Mei L, Yang Y, et al. Design, synthesis and biological evaluation of novel spiro-pentacylamides as acetyl-CoA carboxylase inhibitors. Bioorg Med Chem. 2018. August 7;26(14):3866–3874. [DOI] [PubMed] [Google Scholar]
- 82.Jones JEC, Esler WP, Patel R, et al. Inhibition of Acetyl-CoA Carboxylase 1 (ACC1) and 2 (ACC2) reduces proliferation and de novo lipogenesis of EGFRvIII human glioblastoma cells. PloS One. 2017;12(1):e0169566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Svensson RU, Parker SJ, Eichner LJ, et al. Inhibition of acetyl-CoA carboxylase suppresses fatty acid synthesis and tumor growth of non-small cell lung cancer in preclinical models. Nat Med. 2016. September 19;22(10):1108–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sugimoto Y, Naniwa Y, Nakamura T, et al. A novel acetyl-CoA carboxylase inhibitor reduces de novo fatty acid synthesis in HepG2 cells and rat primary hepatocytes. Arch Biochem Biophys. 2007. December 1;468(1):44–48. [DOI] [PubMed] [Google Scholar]
- 85.Calvisi DF, Wang C, Ho C, et al. Increased lipogenesis, induced by AKT-mTORC1-RPS6 signaling, promotes development of human hepatocellular carcinoma. Gastroenterology. 2011. March;140(3):1071–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang MD, Wu H, Huang S, et al. HBx regulates fatty acid oxidation to promote hepatocellular carcinoma survival during metabolic stress. Oncotarget. 2016. February 9;7(6):6711–6726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wei L, Harriman G, Ghoshal S, et al. Abstract 3781: combination therapy with a liver selective acetyl-CoA carboxylase inhibitor ND-654 and sorafenib improves efficacy in the treatment of cirrhotic rats with hepatocellular carcinoma. Cancer Res. 2016;76(14 Supplement):3781. [Google Scholar]
- 88.Lally JSV, Ghoshal S, DePeralta DK, et al. Inhibition of Acetyl-CoA carboxylase by phosphorylation or the inhibitor ND-654 suppresses lipogenesis and hepatocellular carcinoma. Cell Metab. 2019. January 8;29(1):174–82.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• In pre-clinical studies, ACC inhibition with ND-654 alone demonstrated a 41% decrease in HCC incidence and when combined with sorafenib (a first line HCC therapy), HCC incidence is decreased by 81%.
- 89.Rios Garcia M, Steinbauer B, Srivastava K, et al. Acetyl-CoA carboxylase 1-dependent protein acetylation controls breast cancer metastasis and recurrence. Cell Metab. 2017. December 5;26(6):842–55.e5. [DOI] [PubMed] [Google Scholar]
- 90.Muramatsu T Basigin (CD147), a multifunctional transmembrane glycoprotein with various binding partners. J Biochem. 2016. May;159(5):481–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lee A, Rode A, Nicoll A, et al. Circulating CD147 predicts mortality in advanced hepatocellular carcinoma. J Gastroenterol Hepatol. 2016;31(2):459–466. [DOI] [PubMed] [Google Scholar]
- 92.Li J, Huang Q, Long X, et al. CD147 reprograms fatty acid metabolism in hepatocellular carcinoma cells through Akt/mTOR/SREBP1c and P38/PPARalpha pathways. J Hepatol. 2015. December;63(6):1378–1389. [DOI] [PubMed] [Google Scholar]
- 93.Niu H, Wang R, Cheng J, et al. Treatment of (131)I-labeled anti-CD147 monoclonal antibody in VX2 carcinoma-induced liver tumors. Oncol Rep. 2013. July;30(1):246–252. [DOI] [PubMed] [Google Scholar]
- 94.Bian H, Zheng JS, Nan G, et al. Randomized trial of [131I] metuximab in treatment of hepatocellular carcinoma after percutaneous radiofrequency ablation. J Natl Cancer Inst. 2014. September;106(9). [DOI] [PubMed] [Google Scholar]; •• When combined with radiofrequency ablation in a human clinical trial, Metuximab (antibody targeting CD147) with I131 radioisotope showed a recurrence-free survival of 17 months in HCC patients.
- 95.Yang X, Zhang D, Liu S, et al. KLF4 suppresses the migration of hepatocellular carcinoma by transcriptionally upregulating monoglyceride lipase. Am J Cancer Res. 2018;8(6):1019–1029. [PMC free article] [PubMed] [Google Scholar]
- 96.Sun H, Tang H, Xie D, et al. Krüppel-like factor 4 blocks hepatocellular carcinoma dedifferentiation and progression through activation of hepatocyte nuclear factor-6. Clin Cancer Res off J Am Assoc Cancer Res. 2016. September 02;22(2):502–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tian X, Dai S, Sun J, et al. F-box protein FBXO22 mediates polyubiquitination and degradation of KLF4 to promote hepatocellular carcinoma progression. Oncotarget. 2015. May 28;6(26):22767–22775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lehner F, Kulik U, Klempnauer J, et al. Inhibition of the liver enriched protein FOXA2 recovers HNF6 activity in human colon carcinoma and liver hepatoma cells. PloS One. 2010. October 13;5(10):e13344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fowler CJ. Monoacylglycerol lipase – a target for drug development? Br J Pharmacol. 2012. November 30;166(5):1568–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mulvihill MM, Nomura DK. Therapeutic potential of monoacylglycerol lipase inhibitors. Life Sci. 2013. November 08;92(8–9):492–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ye L, Zhang B, Seviour EG, et al. Monoacylglycerol lipase (MAGL) knockdown inhibits tumor cells growth in colorectal cancer. Cancer Lett. 2011. August 1;307(1):6–17. [DOI] [PubMed] [Google Scholar]
- 102.Nomura DK, Lombardi DP, Chang JW, et al. Monoacylglycerol lipase exerts dual control over endocannabinoid and fatty acid pathways to support prostate cancer. Chem Biol. 2011. July 29;18(7):846–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kopp F, Komatsu T, Nomura DK, et al. The glycerophospho meta-bolome and its influence on amino acid homeostasis revealed by brain metabolomics of GDE1(−/−) mice. Chem Biol. 2010. August 27;17(8):831–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jeon S-M. Regulation and function of AMPK in physiology and diseases. Exp Mol Med. 2016. July 15;48(7):e245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Li Y, Xu S, Mihaylova MM, et al. AMPK phosphorylates and inhibitsSREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metab. 2011. April 6;13(4):376–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hardie DG, Pan DA. Regulation of fatty acid synthesis and oxidation by the AMP-activated protein kinase. Biochem Soc Trans. 2002. November;30(Pt 6):1064–1070. [DOI] [PubMed] [Google Scholar]
- 107.Carling D, Clarke PR, Zammit VA, et al. Purification and characterization of the AMP-activated protein kinase. Copurification of acetyl-CoA carboxylase kinase and 3-hydroxy-3-methylglutaryl-CoA reductase kinase activities. Eur J Biochem. 1989. December 8;186(1–2):129–136. [DOI] [PubMed] [Google Scholar]
- 108.Muoio DM, Seefeld K, Witters LA, et al. AMP-activated kinase reciprocally regulates triacylglycerol synthesis and fatty acid oxidation in liver and muscle: evidence that sn-glycerol-3-phosphate acyltransferase is a novel target. Biochem J. 1999. March 15;338(Pt3):783–791. [PMC free article] [PubMed] [Google Scholar]
- 109.Cheng J, Huang T, Li Y, et al. AMP-activated protein kinase suppresses the In Vitro and In Vivo proliferation of hepatocellular carcinoma. PloS One. 2014. April 07;9(4):e93256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hsieh FS, Chen YL, Hung MH, et al. Palbociclib induces activation of AMPK and inhibits hepatocellular carcinoma in a CDK4/6--independent manner. Mol Oncol. 2017. August;11(8):1035–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wang ST, Ho HJ, Lin JT, et al. Simvastatin-induced cell cycle arrest through inhibition of STAT3/SKP2 axis and activation of AMPK to promote p27 and p21 accumulation in hepatocellular carcinoma cells. Cell Death Dis. 2017. February 23;8(2):e2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sun Y, Tao C, Huang X, et al. Metformin induces apoptosis of human hepatocellular carcinoma HepG2 cells by activating an AMPK/p53/miR-23a/FOXA1 pathway. Onco Targets Ther. 2016;9:2845–2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Koyama S, Akbay EA, Li YY, et al. STK11/LKB1 Deficiency promotes neutrophil recruitment and proinflammatory cytokine production to suppress T-cell activity in the lung tumor microenvironment. Cancer Res. 2016. March 1;76(5):999–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Roemeling CAEV, Caulfield, Qie Y, et al. Abstract LB-189: blockade of stearoyl CoA desaturase 1 promotes immunogenic clearance of tumors. Cancer Res. 2017;77(13 Supplement):LB–189. [Google Scholar]
- 115.Zhang F, Du G. Dysregulated lipid metabolism in cancer. World J Biol Chem. 2012. August 26;3(8):167–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhao Y, Butler EB, Tan M. Targeting cellular metabolism to improve cancer therapeutics. Cell Death Dis. 2013. March 7;4:e532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Beckers A, Organe S, Timmermans L, et al. Chemical inhibition of acetyl-CoA carboxylase induces growth arrest and cytotoxicity selectively in cancer cells. Cancer Res. 2007. September 1;67(17):8180–8187. [DOI] [PubMed] [Google Scholar]
- 118.Higashi T, Hayashi H, Kitano Y, et al. Statin attenuates cell proliferative ability via TAZ (WWTR1) in hepatocellular carcinoma. Med Oncol. 2016. November;33(11):123. [DOI] [PubMed] [Google Scholar]
- 119.Kawaguchi Y, Sakamoto Y, Ito D, et al. Statin use is associated with a reduced risk of hepatocellular carcinoma recurrence after initial liver resection. Biosci Trends. 2017. November 20;11(5):574–580. [DOI] [PubMed] [Google Scholar]
- 120.Zhou T-Y, Zhuang L-H, Hu Y, et al. Inactivation of hypoxia-induced YAP by statins overcomes hypoxic resistance tosorafenib in hepatocellular carcinoma cells. Sci Rep. 2016. August 01 online;6:30483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhou YY, Zhu GQ, Wang Y, et al. Systematic review with network meta-analysis: statins and risk of hepatocellular carcinoma. Oncotarget. 2016. April 19;7(16):21753–21762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bergen WG, Mersmann HJ. Comparative aspects of lipid metabolism: impact on contemporary research and use of animal models. J Nutr. 2005. November;135(11):2499–2502. [DOI] [PubMed] [Google Scholar]