Abstract
Background:
Balance challenges are associated with not only the aging process but also a wide variety of psychiatric and neurological disorders. However, relatively little is known regarding the neural basis of balance and the effects of balance interventions on the brain.
Research Question:
This review synthesizes the existing literature to answer the question: What are the key brain structures associated with balance?
Methods:
This review examined 37 studies that assessed brain structures in relation to balance assessment or intervention. These studies provided 234 findings implicating 71 brain structures. The frequency of implication for each structure was examined based upon specific methodological parameters, including study design (assessment/intervention), type of balance measured (static/dynamic), population (clinical/non-clinical), and imaging analysis technique (region of interest [ROI]/voxel-based morphometry [VBM]).
Results:
Although a number of structures were associated with balance across the brain, the most frequently implicated structures included the cerebellum, basal ganglia, thalamus, hippocampus, inferior parietal cortex, and frontal lobe regions. Findings in the cerebellum and brainstem were most common in studies with clinical populations, studies that used an ROI approach, and studies that measured dynamic balance. Findings in the frontal, occipital, and parietal regions were also more common in studies that measured dynamic compared to static balance.
Significance:
While balance appears to be a whole-brain phenomenon, a subset of structures appear to play a key role in balance and are likely implicated in balance disorders. Some of these structures (i.e., the cerebellum, basal ganglia and thalamus) have a well-appreciated role in balance, whereas other regions (i.e., hippocampus and inferior parietal cortex) are not commonly thought to be associated with balance and therefore may provide alternative explanations for the neural basis of balance. Key avenues for future research include understanding the roles of all regions involved in balance across the lifespan and in different clinical populations.
Keywords: balance, postural control, magnetic resonance imaging, MRI, diffusion tensor imaging
Introduction
An estimated 17-30% of individuals will experience a balance disorder during their lifetime [1]. Balance disorders are associated with increased risk of falling which substantially impacts morbidity and mortality, especially in older adults [2]. However, balance challenges can occur at any phase of life and have been observed across the life span. Balance challenges commonly co-occur with both acquired and developmentally based neurological disorders, such as traumatic brain injury (TBI) [3], cerebral palsy [4], Down syndrome [5], and autism spectrum disorder [6]. Such balance challenges have previously been associated with individual differences in spatial reasoning skills [7], core autism symptoms [8,9], attentional control [10], and neuroplasticity [11,12]. However, despite the prevalence and impact of balance challenges, little is known about which brain structures underpin balance in human beings. The purpose of the present review was to summarize the existing human MRI literature in order to isolate key brain structures that underlie postural balance in clinical and non-clinical populations. In doing so, we expect to identify possible biomarkers to facilitate early detection of balance challenges and to lay the groundwork for efficacious interventions.
A previous review of the effects of balance training on spinal and supraspinal excitability [13] suggested that balance training reduces spinal reflex excitability and cortical involvement during balance, thereby theoretically increasing the reliance on subcortical structures. However, this review concluded that advanced electrophysiological and neuroimaging techniques would need to be implemented before we could fully test this theoretical model. Advantageously, such advanced techniques have been utilized in a number of studies in the ten years since publication of this review and have led to a better understanding of the brain correlates of balance and balance training. Specifically, magnetic resonance imaging (MRI) has examined balance in relation to both volume and microstructure of brain regions using structural and diffusion tensor imaging (DTI), respectively. Structural imaging has allowed for comparison of balance to volumetric measures of gray and white matter, while DTI-derived microstructural properties quantify white matter integrity using measures of fractional anisotropy (FA), median diffusivity (MD), axial diffusivity (AD), and radial diffusivity (RD). Together, these techniques promote a complementary understanding of the circuits that may play a role in balance. Structural and DTI brain imaging used in combination with clinical and laboratory based balance assessment, provide a clearer picture of the brain regions that are most critically implicated in postural balance and are most affected by balance training.
Structural neuroimaging provides two general mechanisms by which we can approach research questions about neuroanatomical regions. The first option allows our prior assumptions and previous findings from the literature to drive the imaging question that is asked. In these studies, predetermined regions of interest (ROIs) are established and observations are limited to within the fiducial lines of those region. The second option does not limit the anatomical exploration to a given location or region, but rather allows for examination of the entire brain through voxel based analysis. Each method has its strengths and limitations. An ROI based analysis may result in the collection and analysis of data that has the potential to demonstrate a significant volumetric relationship between the amygdala (for example) and performance on a standardized clinical balance assessment, but if the amygdala is not included as an ROI, the relationship may never be observed. That said, ROI studies allow for the examination of vast sums of imaging data based on a priori hypothesis that serve to limit the number of necessary multiple comparison corrections and increase the power of the study. In contrast, whole brain voxel based studies allow for the examination of the entire brain but at the cost of numerous comparisons and the required multiple comparison corrections, which may limit the power of the study. Looking across both ROI- and voxel-based studies is likely to provide a more complete understanding of which neural structures are associated with balance.
Previous work in rodents suggests a complicated pattern of cortical and subcortical changes associated with changes in balance [14]. Specifically, in response to an 8-day rotarod training the volume of some brain areas increased (i.e., the insular, piriform, and orbito-frontal cortices, hippocampal layer 7, anterior thalamus, amygdala, and the cerebellum), whereas the volume of other areas decreased (i.e., corpus callosum near the somatosensory cortex, corticospinal tract, ventral spinocerebellar tract, central lobule of the cerebellum, medial/superior nuclei, and retrosplenial cortex). In addition, better performance (i.e., better balance) was correlated with larger volume of the primary and secondary motor cortices, olfactory bulb, medulla, and frontal association cortex, but smaller volume of the thalamus and lobule III of the cerebellum. Better rotarod performance was also associated with increased FA of the hippocampus, hypothalamus, thalamus, medial longitudinal fasciculus, lobule 8 of the cerebellum, the striatum, and olfactory bulbs. In all, the results of this study suggest that the effects of balance training are widespread but particularly prominent in the cerebellum, the primary and secondary motor regions, frontal regions, and the hippocampus. Such research in rodents may provide preliminary evidence for brain structures involved in human postural stability.
Nevertheless, the pattern of results in rodents does not cleanly align with pre-existing suggestions that balance training reduces cortical involvement but increases subcortical involvement, as mice that demonstrated improved balance had smaller subcortical volume. This discrepancy raises the questions of whether humans demonstrate the same brain basis of balance as mice and which regions of the brain are most implicated in both balance performance and balance training, which are key gaps in the literature. Therefore, the aim of the present study was to characterize brain structures that subserve postural balance in humans, taking into account different clinical populations, structural imaging methodologies, data analysis strategies, and study designs. We approach this aim by examining evidence for brain metrics related to balance in populations with typical, exceptional, or impaired balance, and by examining changes to the brain that coincide with intensive balance training. Further, we use descriptive statistics to examine whether brain structures associated with balance varied as a function of balance assessment versus intervention, static versus dynamic balance, clinical versus non-clinical populations, or ROI versus voxel-based analysis.
Methods
The present review was conducted to identify key brain structures involved in postural balance and balance training. While there are studies that examine balance in relation to functional magnetic resonance imaging (fMRI) measures, we only included structural imaging findings in order to better reflect the lasting structural changes associated with balance performance and balance training. To locate studies that used MRI analysis to implicate specific brain regions with balance, we performed a database search during September 2017 using a PubMed search with key terms: postur[All Fields] OR postura[All Fields] OR postural[All Fields] OR postural'[All Fields] AND (("Balance"[Journal] OR "balance"[All Fields]) AND ("brain"[MeSH Terms] OR "brain"[All Fields]) AND ("magnetic resonance imaging"[MeSH Terms] OR ("magnetic"[All Fields] AND "resonance"[All Fields] AND "imaging"[All Fields]) OR “magnetic resonance imaging”[All Fields] OR "mri"[All Fields])) NOT("J Mol Catal A Chem"[Journal] OR "chemical"[All Fields]). Search terms were developed to capture relevant articles and to ensure that the terms relevant to the specific thesaurus of the database were included. The search yielded a total of 285 articles, which were then screened for inclusion.
Predetermined exclusionary criteria for studies included the sole use of non-human subjects, functional imaging, the use of an MRI scanner with strength below 1.5T (due to reduced resolution), case studies involving fewer than eight participants, or studies that did not involve the use of both structural brain and behavioral balance assessments or training. Articles were screened by a single person and then vetted for inclusion by a second individual. Ultimately a total of 37 studies were included for review. Imaging, participant summaries, and study design parameters for these 37 studies are depicted in Table 1. Details of the studies can be found in Supplementary Table 1. Studies varied in sample size (M = 113.6, range: 14-1387), average subject age, MRI scanner strength, and imaging parameters, but all studies assessed structural measures of the brain in relation to balance. As can be seen in Table 1, the majority of the studies included participants over the age of 40 years, used a 3T scanner, and assessed gray matter volume. The majority also implemented a balance assessment, looked at static balance, used an ROI analysis approach, and investigated a clinical population.
Table 1.
Imaging technique and participant summaries across the 37 studies reviewed.
| Study Parameter | Frequency |
|---|---|
| Participant Age (years) | |
| >40 | 25 |
| <40 | 12 |
| Scanner Magnetic Field | |
| Strength | |
| 3 T | 23 |
| 1.5 T | 14 |
| Imaging Technique | |
| Gray Matter Volume | 18 |
| Diffusion Tensor Imaging | 9 |
| Lesion Volume | 6 |
| White Matter Hyperintensity | 3 |
| Cortical Thickness | 2 |
| White Matter Volume | 1 |
| Ventricular Volume | 1 |
| Study Design | |
| Assessment | 27 |
| Intervention | 10 |
| Analysis Method | |
| ROI | 24 |
| VBM | 13 |
| Balance Metric | |
| Dynamic | 14 |
| Static | 20 |
| Dynamic and Static | 2 |
| Neither (professional vestibulo-visually trained subjects) | |
| Population | |
| Clinical | 24 |
| Non-Clinical | 7 |
| Clinical and Non-Clinical | 6 |
Brain structures were distinguished based on the naming conventions used in each study. In order to summarize key results across studies, findings were grouped into nine general anatomical regions: brainstem/cerebellar regions, frontal regions, subcortical regions, temporal regions, occipital regions, parietal regions, insular regions, ventricles/paraventricular regions, and corpus callosum. The hippocampus was included with temporal regions based on the developmental nature of the hippocampus, which ultimately is constructed from an infolding of the temporal cortical regions [15]. The insular regions were singled out due to their anatomical location and versatile function. White matter tracts that crossed multiple regions of the brain were grouped into the regions where their associated studies found the most change. The exception to this was the corpus callosum, which remained its own group due to its expansive and extensive connections throughout the brain.
Frequency of regional involvement in balance was defined as the number of times each region was implicated in balance across the papers reviewed. One concern we had with raw frequency was the bias inherent to combining findings across ROI studies (that can only detect findings in pre-specified areas of the brain) and VBM studies (that are able to detect findings across the brain). To try to account for this bias, we calculated a frequency-of-findings-per-paper proportion, dividing the number of times each region was implicated in balance by the number of papers that were designed to potentially detect a finding within that region. Papers were deemed to be able to detect a finding within a region if the study used an ROI defined within that region or if a study used a VBM approach in general. A very similar pattern of results occurred across both the raw frequency (Figure 1) and proportion (Supplementary Figure 1) measures, which is why raw frequency is reported here.
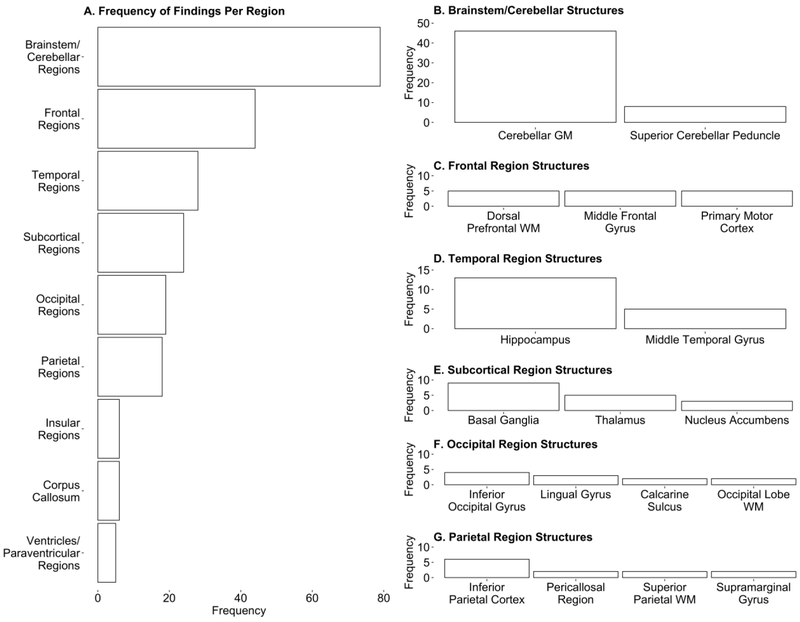
Figure 1.
Structural involvement in balance as indexed by total number of findings per region. A) Frequency of findings implicating each brain region in balance. B-G) Frequency of findings per structure within each region that had 15 or more total findings. Structures that contributed to 10% or more of that region’s total number of findings are reported. Remaining structures that contributed to less than 10% of the region’s total number of findings are not listed but can be found in Supplementary Table 1. GM, Gray Matter; WM, White Matter
Findings were further analyzed on the basis of experimental design (Balance Assessment/Intervention), balance paradigm (Static/Dynamic), population (Clinical/Non-clinical), and regional analysis method (ROI/VBM). Findings that investigated structural brain properties in relation to balance ability at a single time point were classified as “Assessment,” while findings that investigated the change in neural structure over the course of balance training intervention were classified as “Intervention.” Findings that quantified balance based on ability to remain still were considered to have used “Static” measures whereas those that required the participant to balance while in motion were considered to have used “Dynamic” measures of balance. Findings based on the specific populations with impaired balance were designated as “Clinical,” while those that were based on typically developing populations or populations with enhanced balance were designated as “Non-clinical.” Findings were also classified based on the regional analysis method used (i.e., ROI or VBM analysis approaches). All studies that used VBM included a family-wise error correction in the statistical assessment of their findings. Findings per major brain region were classified on an independent level as well as on a study level and showed very similar results (Supplementary Figure 2).
Results
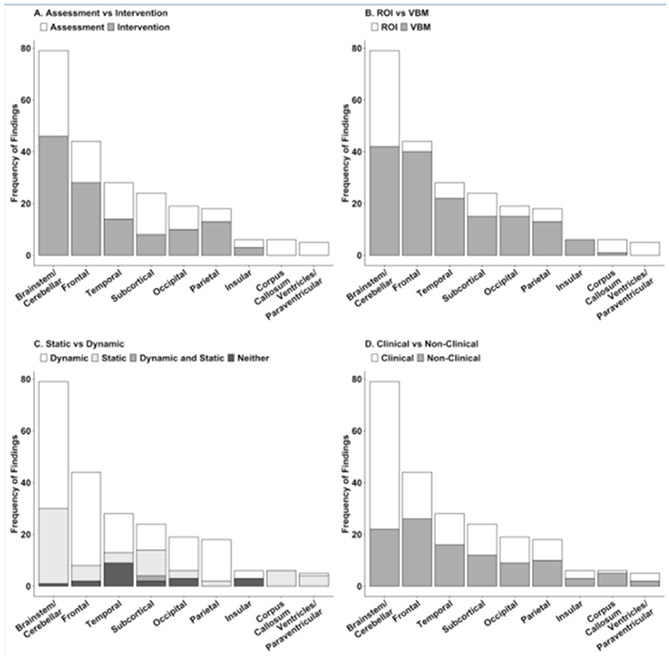
Across the 37 studies, a total of 234 findings in 71 brain structures were evaluated. Figure 1A shows that the brainstem/cerebellar region played the largest role in balance across both assessment and intervention paradigms, with 79 significant findings in the region coming from 22 studies (59% of studies; Figure 2). Within this region, findings in the cerebellar gray matter accounted for over half of the total brainstem/cerebellar findings (58%; Figure 1B). Findings were relatively evenly distributed across the cerebellar lobes (when the lobes were specified by the study), however the directionalities of the cerebellar findings were mixed (see Supplementary Table 1). For example, balance assessment studies generally showed that larger cerebellar gray matter volume was associated with better balance across clinical populations. However, individuals with ADHD showed an opposite pattern, as cerebellar gray matter correlated negatively with better balance. The cerebellum was also heavily implicated in balance training studies, although directionality of the volume change varied according to the population of study (see Supplementary Table 1). The superior cerebellar peduncle (SCP) accounted for 10.1% of the brainstem/cerebellar findings (Figure 1B) with higher white matter integrity (as indexed by DTI measures of higher FA and lower MD) being associated with better balance in assessment studies [16,17]. Intervention studies showed a similar pattern of increased FA being associated with better balance in balance-impaired populations [11,19]. Figure 2 depicts the degree to which these findings were associated with specific study parameters. Overall, findings in the brainstem and cerebellar region, were more common in intervention studies (46 findings; 58% of findings), clinical populations (57 findings; 72% of findings), and measurements of dynamic balance (49 findings; 62% of findings). Interestingly, 46% of findings (37 findings) in the brainstem and cerebellar region were based on ROI analysis (Figure 2B). This is proportion of ROI based findings in a single region was atypically high compared to other brain structures. Additionally, 34 of the 37 ROI based findings involved clinical populations.
Figure 2.
Frequency of specific parameters used to produce regional findings. A) Frequency of findings per region that used assessment versus intervention study designs. B) Frequency of findings per region that used ROI versus VBM analysis techniques. C) Frequency of findings per region that used static versus dynamic measures of balance. Studies that assessed balance using both static and dynamic metrics or a composite score of both static and dynamic balance are noted. D) Frequency of findings per region that looked at balance in clinical versus non-clinical populations. Studies that presented findings in both clinical and non-clinical populations are noted.
Following the brainstem/cerebellar region, the frontal region had the most findings with a total of 44 findings from 13 studies (35% of studies; Figure 1C). The inferior orbitofrontal cortex, primary motor cortex, superior frontal gyrus, and supplementary motor areas each accounted for 11.9% of the findings in the frontal region. Structural changes in these brain areas were highly variable across populations and not consistent across studies (see Supplementary Table 1). Figure 2 shows that findings in the frontal region were disproportionately from VBM analysis techniques (40 findings; 90% of findings) and involved more dynamic balance measurements than static balance (36 findings; 81% of findings).
Temporal regions were implicated in over half of the balance studies with 28 findings from 11 studies (33% of papers; Figure 1D). This was largely due to the hippocampus, which accounted for 46.4% of the temporal region findings. Increased hippocampal gray matter volume was associated with better balance in the case of people with expert balance [19] but poorer balance in the case of individuals 40 years or older [20,21]. Gray matter volume in the hippocampus fluctuated during balance training studies and directionality of these changes was inconsistent across populations and time. The middle temporal gyrus accounted for an additional 17.8% of the findings in the temporal lobe. Gray matter volume in the middle temporal gyrus varied with population and ability. Individuals with expert balance showed increased gray matter volume in the middle temporal gyrus, while typically developing individuals showed gray matter volume decreases during balance training that correlated positively with balance improvement. Changes in gray matter volume over the course of balance training were shown to correlate with balance improvement; however the directionality of such change was inconsistent across studies. While several of the findings in the temporal region were from a single study that did not measure balance directly, findings also came from studies that implemented both static and dynamic measures (Figure 2).
Subcortical regions were implicated in 9 studies included in this review (24% of studies; 1E) with 24 findings (Figure 1E). The basal ganglia and thalamus each accounted for over 20% of the subcortical findings and the nucleus accumbens each accounted for 12.5%. The majority of findings in the basal ganglia came from balance assessment studies (Figure 2). These findings were also in populations over 40 years old, all concluding that reduced size and the presence of white matter hyperintensities in the basal ganglia were associated with poorer balance ability. Interestingly, the gray matter volume in the basal ganglia, specifically the putamen, was seen to decrease over the course of balance training in typically developing individuals [12]. However, a separate study reported an increase in putamen size following balance training [22]. Lower gray matter volume in either the putamen or the caudate was also associated with worse balance in individuals with multiple sclerosis [16] and Alzheimer’s Disease [23], respectively. Findings in the thalamus accounted for 20.8% of the total findings in the subcortical regions and all stemmed from balance assessment studies. Gray matter volume in the thalamus was increased in individuals with exceptional balance and conversely decreased in individuals with balance impairments. None of the studies that implemented balance intervention noted significant changes in the thalamus. The distribution of findings among the study parameters in the subcortical regions was relatively consistent with what was to be expected (Figure 2).
Nineteen findings across nine studies (24% of studies) were attributed to structures within the occipital region (Figure 1F). Like that of the frontal regions, there were no structures within the occipital region that showed significant and distinct contributions to balance ability or improvement. Several structures within the occipital regions, including the inferior occipital gyrus and the lingual gyrus may play a role in balance but have not been implicated across studies frequently enough to draw conclusions about their distinct roles in balance. The majority of findings in the occipital region were implicated in dynamic balance (13 findings; 68% of findings) rather than static balance measures (Figure 2C).
The parietal regions had only 18 findings in 7 studies (18% of studies). However the inferior parietal cortex accounted for 33.3% of the findings in the parietal regions, potentially making it a critical region for understanding the neural mechanisms associated with balance. Typically developing individuals show decreased white matter integrity (increased MD and decreased FA) in this region over the course of balance training. Further, gray matter volume in the inferior parietal cortex in people with Parkinson disease was found to change over the course of balance training with an initial increase and subsequent decrease in volume over time. These changes, regardless of directionality, were associated with improved balance at all time points. The majority of findings in the parietal regions involved dynamic measures of balance (16 findings; 88% of findings) and VBM analysis methods (13 findings; 17% of findings; Figure 2).
The corpus callosum, insular regions and ventricles/paraventricular regions were all implicated in balance less than six times and in fewer than five studies indicating that while they may play a supportive role in balance, they likely are not the most critical underlying components of balance in the brain. Findings in the ventricles/paraventricular regions were exclusively from studies involving balance assessment (5 findings) and ROI analysis (5 findings), and findings in the corpus callosum were exclusively from studies involving static balance assessment (6 findings; Figure 2).
Discussion
The purpose of the present review was to better understand structures of the brain that are most highly associated with balance and balance improvement across a variety of methodological approaches. The present findings suggest that balance is a whole-brain phenomenon that is not isolated to a few specific regions but rather can be influenced and impacted by nearly every region of the brain. Nevertheless, certain structures, such as the cerebellum, the basal ganglia, the thalamus, the hippocampus, the inferior parietal cortex, and frontal lobe (broadly defined) may be particularly central to balance skills, regardless of whether balance is being trained or assessed.
Cerebellum
While the present results suggest that nearly every region of the brain is involved in balance, cerebellar gray and white matter had the highest count of findings, suggesting the key role of the cerebellum in balance acquisition and ability. Although it is unsurprising given the widely accepted role of the cerebellum in motor coordination and planning, the degree to which the cerebellum was implicated in balance above all other structures clearly demonstrates its importance in balance. In most cases, lower gray matter volume or lesions in the cerebellar lobes were associated with poorer balance [16,17,26-31], and higher volume was associated with better balance [19,22,32]. However, in the case of ADHD, higher gray matter volume in the cerebellum was associated with poorer balance [33]. This unique pattern found in the ADHD population may be due to the developmental aspect of ADHD, which is not present in typically developing or other populations that encounter balance deficits later in life after the brain has developed (i.e. MS, TBI, alcoholism, stroke, spinal cord injury). Given the developmental course of balance, it is possible that brain-behavior relations may be distinct in youth with developmental disorders compared to other populations, which should be considered in future examinations of balance and the brain.
The study population also seems to play a significant role in cerebellar gray matter adaptation to balance training. Specifically, typically developing individuals tended to show volume decreases across cerebellar lobules as balance improved over time [12,34], whereas individuals with balance impairment showed increases in cerebellar lobule volume as balance improved over time [27,34]. Interestingly, people with expert balance also showed increased gray matter volume in the cerebellum compared to individuals with average balance [19,32], thus paralleling the effects of balance training in balance impaired populations and contradicting the effects of balance training in the typically developing population. One potential explanation is that shorter-term training (i.e., a matter of weeks) may be related to reductions in cerebellar volume, whereas longer-term training (i.e., years of activities like professional dancing, figure skating, or slack-lining) may be indicative of increases in cerebellar volume. Speculatively, the pre-training volume of the cerebellum may also be a key factor, such that balance training may be able to normalize the cerebellum’s volume (i.e., cerebellar atrophy in individuals from clinical populations may be corrected with balance training). However, future research is needed to examine these possibilities.
The present results also suggest that findings within the cerebellum (and brainstem) were overrepresented in studies that used ROI methodologies and studies that used clinical populations. It is possible that the cerebellum and brainstem regions may be more implicated in clinical population than in non-clinical populations. However, this finding may also be an artifact stemming from the overrepresentation of studies with clinical populations using ROI methods (presumably due to smaller sample sizes or greater a priori evidence making it more likely for scientists to selectively examine the cerebellum in clinical populations). Future research is needed to determine whether the cerebellum is indeed more commonly implicated in clinical populations with balance challenges or if the overrepresentation of the cerebellum in balance studies is an artifact of narrowing the search space to the cerebellum in ROI studies.
The relatively high implication of the superior cerebellar peduncle (SCP) is also unsurprising, as it is known to transmit motor coordination information from the cerebellum to cortical areas. Therefore, it is logical that reduced white matter integrity, in the form of low FA and high MD, was consistently associated with poorer balance in individuals with balance disorders [16,35,36]. While not many studies have found changes in the SCP following balance intervention, a study conducted with participants with MS showed a sustained increase of FA in the SCP with balance training [18]. This finding may indicate that white matter changes in the SCP could correspond to gray matter changes in the cerebellum, although this theory this will need to be addressed in future research.
Hippocampus
The temporal regional findings were largely driven by the hippocampal region (i.e., the hippocampus and parahippocampal gyri), which accounted for 46.4% of the temporal lobe findings. Unlike regions such as the cerebellum, the hippocampus is not often thought to be at the forefront of balance or motor functions. However, the present results suggest that the hippocampus may play a key role in balance. Importantly, there was no difference in the representation of temporal lobe structures in studies that assessed balance versus studies that sought to improve balance skills. Therefore, it is unlikely that the hippocampus is only involved in the learning of new balance skills. Instead, the hippocampus and parahippocampus may be involved in the encoding or retrieval of spatial information, likely needed for successful balance (for a review see [37]). Speculatively, the role of the hippocampus in spatial and configural reasoning skills may be related to its role in balance ability [7,29].
While the findings in the hippocampal and parahippocampal structures within the temporal region may suggest that the temporal gray matter is more critical to balance than white matter, they also may point to a lack of exploration of temporal white matter in neuroimaging studies involving balance. It is possible that tracts leading toward and away from the hippocampal regions are also involved in balance and have yet to be explored or reported. Regardless, the present results suggest that the hippocampus may be a key area involved in both balance and balance training, although future research is needed to better understand the specific role that the hippocampus plays in balance over the course of the lifespan.
Basal Ganglia & Thalamus
Findings in the subcortical regions were mainly driven by the basal ganglia and thalamus thus highlighting their importance in balance. The basal ganglia are another known hub of motor function and therefore are highly implicated in balance disorders. Although there was no specific trend of changes within the sub-structures of the basal ganglia, gray matter volume reduction in any part of the basal ganglia structure commonly had negative effects on balance, and volume increases were commonly associated with improved balance [12,18,22,23,25,38].
Similar to the basal ganglia, the thalamus is thought to play a key role in balance as well as several other sensory-motor functions. Its role in motor ability has been documented through lesion studies of thalamic nuclei and correspondence with movement deficits [36]. Deficits specific to balance were also associated with atypical thalamic presentation such as white matter hyperintensities [38], impaired white matter integrity [18,24], and reduced gray matter volume [19]. The critical nature of the thalamus in balance is corroborated by the findings that individuals with expert balance had increased thalamic gray matter volume [29].
Inferior parietal cortex
Findings in the parietal region were driven heavily by the inferior parietal cortex, which made up almost half of the findings in the parietal region and had more findings than the basal ganglia or thalamus. Unlike many of the other regions implicated in balance, the inferior parietal cortex was most commonly implicated in balance intervention studies. In individuals with typical development, decreased white matter integrity (higher MD and lower FA) occurred over the course of a six-week dynamic balance intervention [39]. Gray matter volume in the inferior parietal cortex also changed with balance training in individuals with Parkinson disease in that gray matter volume fluctuations were positively associated with balance improvement [34]. However, these fluctuations were not always in the same direction but likely reflect a growth and subsequent pruning of the region occurring over the course of balance training. Specifically, gray matter volume of the inferior parietal cortex increased over two balance training sessions but then decreased after three weeks, while behavioral assessments showed only improvement of balance over time [34].
As the inferior parietal cortex is involved in a wide range of functions including perception, planning, and interpretation of sensory information [40,41], its role in balance is likely one of higher order motor integration and planning rather than motor execution [42]. This theory may help explain the general trend of decreasing gray matter and white matter integrity as balance improves. Because the brain works to automate tasks over time, refined perception, integration, and planning is needed to execute a novel or difficult task as compared to that which is needed to execute a familiar task [43]. Therefore, the pattern of inferior parietal cortex gray matter increase followed by decrease over the course of short-term balance training may reflect a pattern of learning and automatization of the balance task.
Frontal Lobe
Unlike the majority of other regions, the findings in the frontal regions were not driven by a particular structure but rather comprised of relatively few findings in several different structures within the frontal region. This variability in frontal structures led us to conclude that while the frontal regions may play a part in balance, their specific role in balance is not as well defined or might vary according to the type of balance being trained or assessed. This speculation is in part supported by overrepresentation of frontal areas in dynamic compared to static balance. Gaining a more nuanced understanding of frontal contributions to balance will be a key avenue of inquiry for future research. However, due to the lack of information in the current literature implicating specific structures within the frontal regions in balance, conclusions regarding their unique roles in balance cannot be drawn at this time.
Conclusions and Future Directions
Across clinical and non-clinical populations and across different structural imaging modalities, the results of this review suggest that the most heavily implicated brain structures in studies of balance are the cerebellar gray matter, superior cerebellar peduncle, basal ganglia, thalamus, hippocampus, and inferior parietal cortex. The convergence of such diverse studies on these particular regions and demonstration that this pattern of results was similar across different populations, imaging modalities/techniques, and assessment/manipulation of balance further adds to their significance as being involved in balance. These results suggest that when balance impairments are present, it is probable that at least one of these areas of the brain is involved. These results corroborate rodent data [14] that have indicated structural changes in cerebellar, subcortical, and hippocampal regions as a function of training. This corroboration gives validity to the rodent model of balance and may provide reason for its continued use to track brain plasticity as it pertains to balance. These rodent models also may be used to explore new methods of balance intervention targeted toward plasticity in specific regions.
Despite the high implication of the cerebellar, subcortical, hippocampal, and inferior parietal regions in balance, the results of this review suggest that balance is a whole-brain phenomenon that is not isolated to a handful of regions. Although the specific regions commonly appear to be associated with balance across populations and may provide insight into the underlying neurobiology of balance impairments at the global scale, these regions are unlikely to be the only ones that can affect or be affected by balance ability and training. As such, a VBM approach in future balance studies may be advisable to most accurately represent the complex pattern of structures involved in balance across the brain. Further, across different populations there is likely variability in the regions associated with balance impairments. Indeed, a limitation of this review is that the current literature is not robust enough for a reliable examination of brain-balance relationships unique to specific clinical populations. More research is needed to determine which brain regions may be uniquely associated with the specific balance challenges of different populations.
Another limitation of the present review is the inconsistency in the directionality of the effects for the brain regions associated with balance. Future research into the effects of balance on the brain may consider employing longitudinal designs with multiple imaging time points to examine the time-sensitive effects of balance training. Indeed, studies that have employed such a design during motor training (i.e. [39,44]) have found complex patterns of larger and smaller volumes at different stages of motor learning. Similarly, in studies of balance impairments, longitudinal designs would enable a better understanding of which brain structures are involved in the early stages of balance challenges and how those might change over time and contribute to more severely impacted balance at later stages.
Although the scope of the present review did not include studies that focused on functional imaging, it is also likely that function of these specific regions changes with time. Future studies may consider multimodal imaging techniques that include structural as well as functional imaging components (i.e., functional magnetic resonance imaging [fMRI], positron emission tomography [PET], functional near-infrared spectroscopy [fNIRS]). Such studies may illuminate alterations in function either with or without the presence of structural plasticity. Convergence of functional neuroimaging findings with the structural findings in the present review may expose a pattern of changes in the brain that can be attributed to balance.
In all, the present review provides evidence of the important role of the cerebellum, basal ganglia, thalamus, hippocampus, and inferior parietal cortex in balance and balance training. While more research is needed to disentangle how these areas change over time and impact balance across different clinical populations, these regions may serve as important biomarkers that can be used to isolate the cause of the balance impairment and monitor treatment progress. Further, the role of these regions in balance training may begin to shed light on differential patterns of balance-training-induced neuroplasticity across different structures of the brain. A number of individuals are impacted by balance challenges [37,42], and the present review may help clinicians and researchers prioritize which brain regions to examine when balance challenges are present.
Supplementary Material
Highlights.
Thirty-seven studies examined brain structures involved in balance using MRI
Balance and balance training related to 234 brain structures
Cerebellum, basal ganglia, thalamus, and hippocampus were highly implicated
Subcortical areas associated with balance but underrepresented in balance training
Cerebellum implicated more in clinical populations than non-clinical populations
Acknowledgements
This work was supported by the Brain and Behavior Research Foundation’s NARSAD Young Investigator Award [to BGT]; the Hartwell Foundation Individual Biomedical Award [to BGT]; the Eunice Kennedy Shriver National Institute of Child Health and Human Development [P30 HD003352 and U54 HD090256 to the Waisman Center and R01 HD094715 to BGT]; and the Neuroscience Training Program Training Grant [T32GM007507 to OJS]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Child Health & Development or the National Institutes of Health. We thank Scott Anderson for his comments on an earlier version of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests: None.
Citations
- [1].Murdin L, Schilder AGM, Epidemiology of balance symptoms and disorders in the community: a systematic review, Otol. Neurotol. Off. Publ. Am. Otol. Soc. Am. Neurotol. Soc. Eur. Acad. Otol. Neurotol 36 (2015) 387–392. doi: 10.1097/MAO.0000000000000691. [DOI] [PubMed] [Google Scholar]
- [2].Alamgir H, Muazzam S, Nasrullah M, Unintentional falls mortality among elderly in the United States: time for action, Injury. 43 (2012) 2065–2071. doi: 10.1016/j.injury.2011.12.001. [DOI] [PubMed] [Google Scholar]
- [3].Greenwald BD, Cifu DX, Marwitz JH, Enders LJ, Brown AW, Englander JS, Zafonte RD, Factors associated with balance deficits on admission to rehabilitation after traumatic brain injury: a multicenter analysis, J. Head Trauma Rehabil 16 (2001) 238–252. [DOI] [PubMed] [Google Scholar]
- [4].Rose J, Wolff DR, Jones VK, Bloch DA, Oehlert JW, Gamble JG, Postural balance in children with cerebral palsy, Dev. Med. Child Neurol 44 (2002) 58–63. [DOI] [PubMed] [Google Scholar]
- [5].Shumway-Cook A, Woollacott MH, Dynamics of postural control in the child with Down syndrome, Phys. Ther 65 (1985) 1315–1322. [DOI] [PubMed] [Google Scholar]
- [6].Lim YH, Partridge K, Girdler S, Morris SL, Standing Postural Control in Individuals with Autism Spectrum Disorder: Systematic Review and Meta-analysis, J. Autism Dev. Disord 47 (2017) 2238–2253. doi: 10.1007/s10803-017-3144-y. [DOI] [PubMed] [Google Scholar]
- [7].Riley MA, Mitra S, Saunders N, Kiefer AW, Wallot S, The interplay between posture control and memory for spatial locations, Exp. Brain Res 217 (2012) 43–52. doi: 10.1007/s00221-011-2970-y. [DOI] [PubMed] [Google Scholar]
- [8].Radonovich KJ, Fournier KA, Hass CJ, Relationship between postural control and restricted, repetitive behaviors in autism spectrum disorders, Front. Integr. Neurosci 7 (2013)28. doi: 10.3389/fnint.2013.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Travers BG, Powell PS, Klinger LG, Klinger MR, Motor difficulties in autism spectrum disorder: linking symptom severity and postural stability, J. Autism Dev. Disord 43 (2013) 1568–1583. doi: 10.1007/s10803-012-1702-x. [DOI] [PubMed] [Google Scholar]
- [10].Borel L, Alescio-Lautier B, Posture and cognition in the elderly: interaction and contribution to the rehabilitation strategies, Neurophysiol. Clin. Clin. Neurophysiol 44 (2014) 95–107. doi: 10.1016/j.neucli.2013.10.129. [DOI] [PubMed] [Google Scholar]
- [11].Drijkoningen D, Caeyenberghs K, Leunissen I, Vander Linden C, Leemans A, Sunaert S, Duysens J, Swinnen SP, Training-induced improvements in postural control are accompanied by alterations in cerebellar white matter in brain injured patients, Neuroimage Clin. 7(2015)240–251. doi: 10.1016/j.nicl.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Taubert M, Draganski B, Anwander A, Müller K, Horstmann A, Villringer A, Ragert P, Dynamic properties of human brain structure: learning-related changes in cortical areas and associated fiber connections, J. Neurosci. Off. J. Soc. Neurosci 30 (2010) 11670–11677. doi: 10.1523/JNEUROSCI.2567-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Taube W, Gruber M, Gollhofer A, Spinal and supraspinal adaptations associated with balance training and their functional relevance, Acta Physiol. Oxf. Engl 193 (2008) 101–116. doi: 10.1111/j.1748-1716.2008.01850.x. [DOI] [PubMed] [Google Scholar]
- [14].Scholz J, Niibori Y, W Frankland P, P Lerch J, Rotarod training in mice is associated with changes in brain structure observable with multimodal MRI, Neuroimage. 107 (2015) 182–189. doi: 10.1016/j.neuroimage.2014.12.003. [DOI] [PubMed] [Google Scholar]
- [15].Duvernoy HM, Cattin F, Risold P-Y, Vannson JL, Gaudron M, The human hippocampus: functional anatomy, vascularization and serial sections with MRI, Fourth edition, Springer, Heidelberg; ; New York, 2013. [Google Scholar]
- [16].Prosperini L, Sbardella E, Raz E, Cercignani M, Tona F, Bozzali M, Petsas N, Pozzilli C, Pantano P, Multiple sclerosis: white and gray matter damage associated with balance deficit detected at static posturography, Radiology. 268 (2013) 181–189. doi: 10.1148/radiol.13121695. [DOI] [PubMed] [Google Scholar]
- [17].Prosperini L, Petsas N, Raz E, Sbardella E, Tona F, Mancinelli CR, Pozzilli C, Pantano P, Balance deficit with opened or closed eyes reveals involvement of different structures of the central nervous system in multiple sclerosis, Mult. Scler. Houndmills Basingstoke Engl 20 (2014) 81–90. doi: 10.1177/1352458513490546. [DOI] [PubMed] [Google Scholar]
- [18].Prosperini L, Fanelli F, Petsas N, Sbardella E, Tona F, Raz E, Fortuna D, De Angelis F, Pozzilli C, Pantano P, Multiple sclerosis: changes in microarchitecture of white matter tracts after training with a video game balance board, Radiology. 273 (2014) 529–538. doi: 10.1148/radiol.14140168. [DOI] [PubMed] [Google Scholar]
- [19].Hüfner K, Binetti C, Hamilton DA, Stephan T, Flanagin VL, Linn J, Labudda K, Markowitsch H, Glasauer S, Jahn K, Strupp M, Brandt T, Structural and functional plasticity of the hippocampal formation in professional dancers and slackliners, Hippocampus. 21 (2011) 855–865. doi: 10.1002/hipo.20801. [DOI] [PubMed] [Google Scholar]
- [20].Beauchet O, Barden J, Liu-Ambrose T, Chester VL, Szturm T, Allah G, The relationship between hippocampal volume and static postural sway: results from the GAIT study, Age Dordr. Neth 38 (2016) 19. doi: 10.1007/s11357-016-9883-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Nyberg EM, Tanabe J, Honce JM, Krmpotich T, Shelton E, Hedeman J, Berman BD, Morphologic changes in the mesolimbic pathway in Parkinson’s disease motor subtypes, Parkinsonism Relat. Disord 21 (2015) 536–540. doi: 10.1016/j.parkreldis.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Burciu RG, Fritsche N, Granert O, Schmitz L, Spönemann N, Konczak J, Theysohn N, Gerwig M, van Eimeren T, Timmann D, Brain changes associated with postural training in patients with cerebellar degeneration: a voxel-based morphometry study, J. Neurosci. Off. J. Soc. Neurosci 33 (2013) 4594–4604. doi: 10.1523/JNEUROSCI.3381-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Lee Y-W, Lee H, Chung I-S, Yi H-A, Relationship between postural instability and subcortical volume loss in Alzheimer’s disease, Medicine (Baltimore). 96 (2017) e7286. doi: 10.1097/MD.0000000000007286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Wang H-C, Hsu J-L, Leemans A, Diffusion tensor imaging of vascular parkinsonism: structural changes in cerebral white matter and the association with clinical severity, Arch. Neurol 69 (2012) 1340–1348. doi: 10.1001/archneurol.2012.633. [DOI] [PubMed] [Google Scholar]
- [25].Macfarlane MD, Looi JCL, Walterfang M, Spulber G, Velakoulis D, Styner M, Crisby M, Orndahl E, Erkinjuntti T, Waldemar G, Garde E, Hennerici MG, Bäzner H, Blahak C, Wallin A, Wahlund L-O, LADIS Study Group, Shape abnormalities of the caudate nucleus correlate with poorer gait and balance: results from a subset of the LADIS study, Am. J. Geriatr. Psychiatry Off. J. Am. Assoc. Geriatr. Psychiatry 23 (2015) 59–71.e1. doi: 10.1016/j.jagp.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Bultmann U, Pierscianek D, Gizewski ER, Schoch B, Fritsche N, Timmann D, Maschke M, Frings M, Functional recovery and rehabilitation of postural impairment and gait ataxia in patients with acute cerebellar stroke, Gait Posture. 39 (2014) 563–569. doi: 10.1016/j.gaitpost.2013.09.011. [DOI] [PubMed] [Google Scholar]
- [27].Villiger M, Grabher P, Hepp-Reymond M-C, Kiper D, Curt A, Bolliger M, Hotz-Boendermaker S, Kollias S, Eng K, Freund P, Relationship between structural brainstem and brain plasticity and lower-limb training in spinal cord injury: a longitudinal pilot study, Front. Hum. Neurosci 9 (2015) 254. doi: 10.3389/fnhum.2015.00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Sullivan EV, Rose J, Pfefferbaum A, Effect of vision, touch and stance on cerebellar vermian-related sway and tremor: a quantitative physiological and MRI study, Cereb. Cortex N. Y. N 1991 16 (2006) 1077–1086. doi: 10.1093/cercor/bhj048. [DOI] [PubMed] [Google Scholar]
- [29].Sullivan EV, Rose J, Pfefferbaum A, Physiological and focal cerebellar substrates of abnormal postural sway and tremor in alcoholic women, Biol. Psychiatry 67 (2010) 44–51. doi: 10.1016/j.biopsych.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Buderath P, Gärtner K, Frings M, Christiansen H, Schoch B, Konczak J, Gizewski ER, Hebebrand J, Timmann D, Postural and gait performance in children with attention deficit/hyperactivity disorder, Gait Posture. 29 (2009) 249–254. doi: 10.1016/j.gaitpost.2008.08.016. [DOI] [PubMed] [Google Scholar]
- [31].Hocking DR, Birch RC, Bui QM, Menant JC, Lord SR, Georgiou-Karistianis N, Godler DE, Wen W, Hackett A, Rogers C, Trollor JN, Cerebellar volume mediates the relationship between FMR1 mRNA levels and voluntary step initiation in males with the premutation, Neurobiol. Aging 50 (2017) 5–12. doi: 10.1016/j.neurobiolaging.2016.10.017. [DOI] [PubMed] [Google Scholar]
- [32].Park IS, Yoon JH, Kim N, Rhyu IJ, Regional cerebellar volume reflects static balance in elite female short-track speed skaters, Int. J. Sports Med 34 (2013) 465–470. doi: 10.1055/S-0032-1327649. [DOI] [PubMed] [Google Scholar]
- [33].Hove MJ, Zeffiro TA, Biederman J, Li Z, Schmahmann J, Valera EM, Postural sway and regional cerebellar volume in adults with attention-deficit/hyperactivity disorder, NeuroImage Clin 8 (2015) 422–428. doi: 10.1016/jnicl.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Sehm B, Taubert M, Conde V, Weise D, Classen J, Dukart J, Draganski B, Villringer A, Ragert P, Structural brain plasticity in Parkinson’s disease induced by balance training, Neurobiol. Aging 35 (2014) 232–239. doi: 10.1016/j.neurobiolaging.2013.06.021. [DOI] [PubMed] [Google Scholar]
- [35].Drijkoningen D, Leunissen I, Caeyenberghs K, Hoogkamer W, Sunaert S, Duysens J, Swinnen SP, Regional volumes in brain stem and cerebellum are associated with postural impairments in young brain-injured patients, Hum. Brain Mapp 36 (2015) 4897–4909. doi: 10.1002/hbm.22958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Drijkoningen D, Caeyenberghs K, Leunissen F, Vander Linden C, Leemans A, Sunaert S, Duysens J, Swinnen SP, Training-induced improvements in postural control are accompanied by alterations in cerebellar white matter in brain injured patients, NeuroImage Clin. 7(2015)240–251. doi: 10.1016/j.nicl.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].King JA, Burgess N, Hartley T, Vargha-Khadem F, O’Keefe J, Human hippocampus and viewpoint dependence in spatial memory, Hippocampus. 12 (2002) 811–820. doi: 10.1002/hipo.10070. [DOI] [PubMed] [Google Scholar]
- [38].Ogama N, Sakurai T, Shimizu A, Toba K, Regional white matter lesions predict falls in patients with amnestic mild cognitive impairment and Alzheimer’s disease, J. Am. Med. Dir. Assoc 15 (2014)36–41. doi: 10.1016/j.jamda.2013.11.004. [DOI] [PubMed] [Google Scholar]
- [39].Taubert M, Draganski B, Anwander A, Müller K, Horstmann A, Villringer A, Ragert P, Dynamic properties of human brain structure: learning-related changes in cortical areas and associated fiber connections, J. Neurosci. Off. J. Soc. Neurosci 30 (2010) 11670–11677. doi: 10.1523/JNEUROSCI.2567-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Andersen RA, Essick GK, Siegel RM, Neurons of area 7 activated by both visual stimuli and oculomotor behavior, Exp. Brain Res 67 (1987) 316–322. [DOI] [PubMed] [Google Scholar]
- [41].Battaglia-Mayer A, Caminiti R, Lacquaniti F, Zago M, Multiple levels of representation of reaching in the parieto-frontal network, Cereb. Cortex N. Y. N 1991 13 (2003) 1009–1022. [DOI] [PubMed] [Google Scholar]
- [42].Battaglia-Mayer A, Archambault PS, Caminiti R, The cortical network for eye-hand coordination and its relevance to understanding motor disorders of parietal patients, Neuropsychologia. 44 (2006) 2607–2620. doi: 10.1016/j.neuropsychologia.2005.11.021. [DOI] [PubMed] [Google Scholar]
- [43].Doyon J, Benali H, Reorganization and plasticity in the adult brain during learning of motor skills, Curr. Opin. Neurobiol 15 (2005) 161–167. doi: 10.1016/j.conb.2005.03.004. [DOI] [PubMed] [Google Scholar]
- [44].Driemeyer J, Boyke J, Gaser C, Büchel C, May A, Changes in gray matter induced by learning--revisited, PloS One. 3 (2008) e2669. doi: 10.1371/journal.pone.0002669. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.