Abstract
Megakaryocytes are hematopoietic cells which are responsible for the production of blood platelets. The traditional view of megakaryopoiesis describes the cellular journey from hematopoietic stem cells (HSCs), through a hierarchical series of progenitor cells, ultimately to a mature megakaryocyte. Once mature, the megakaryocyte then undergoes a terminal maturation process involving multiple rounds of endomitosis and cytoplasmic restructuring to allow platelet formation. However, recent studies have begun to redefine this hierarchy and shed new light on alternative routes by which HSCs are differentiated into megakaryocytes. In particular, the origin of megakaryocytes, including the existence and hierarchy of megakaryocyte progenitors, has been redefined, as new studies are suggesting that HSCs originate as megakaryocyte-primed and can bypass traditional lineage checkpoints. Overall, it is becoming evident that megakaryopoiesis does not only occur as a stepwise process, but is dynamic and adaptive to biological needs. In this review, we will re-examine the canonical dogmas of megakaryopoiesis and provide an updated framework for interpreting the roles of traditional pathways in the context of new megakaryocyte biology.
Keywords: Megakaryopoiesis, Hematopoiesis, Megakaryocyte, Platelet, Hematopoietic Stem Cell
Introduction
The role of platelets in both the initiation and propagation of cardiovascular disease has been firmly established over the past two decades1–3. The importance of platelets in cardiovascular disease pathology is further underscored by the ongoing successful use of antiplatelet agents in reducing myocardial infarction, stroke, and vascular-related death4. As such, a large body of work exists examining how platelet function can be modulated in both health (hemostasis) and disease (thrombosis). However, there is surprisingly little known about megakaryocytes, the cells that generate platelets. Megakaryocytes are large (50–100μm) cells that are quite rare - making up approximately 0.05% of the cell population in human bone marrow5. After megakaryocytes are terminally differentiated, they undergo a complex maturation process that is required for platelet production. As they mature, megakaryocytes undergo endomitosis, resulting in a polyploid nucleus that is 16N on average, but has been observed up to 128N. In addition, megakaryocytes create an invaginated membrane system that is continuous with the plasma membrane, permeates the cytoplasm, and provides the extra membrane necessary for platelet formation. Once mature, megakaryocytes extend long processes called proplatelets into the blood vessel lumen. This elaborate process is powered by the megakaryocyte cytoskeleton, and more specifically, the sliding and telescoping of microtubules over each other6. Once released into the lumen, the shear forces associated with flowing blood help facilitate fragmentation of proplatelets into 1–2μm platelets7.
Megakaryopoiesis is the process by which megakaryocytes are derived from hematopoietic stem cells (HSCs), primarily in the bone marrow, along the myeloid branch of hematopoiesis. HSCs in the bone marrow sit at the top of a hierarchical system and give rise to increasingly restricted progenitors which eventually produce, and continuously replenish, approximately one trillion mature blood cells per day8. During steady-state hematopoiesis, all blood lineages are produced through a series of committed progenitors, the megakaryocyte being derived through the multipotent progenitor (MPP), common myeloid progenitor (CMP), and megakaryocyte erythroid progenitor (MEP)9, 10. However, recent studies have begun to redefine this hierarchy and shed new light on alternative routes by which HSCs are differentiated into megakaryocytes. In this review, we will focus on new studies that both refine and challenge the field of megakaryopoiesis.
Hematopoiesis and Megakaryopoiesis
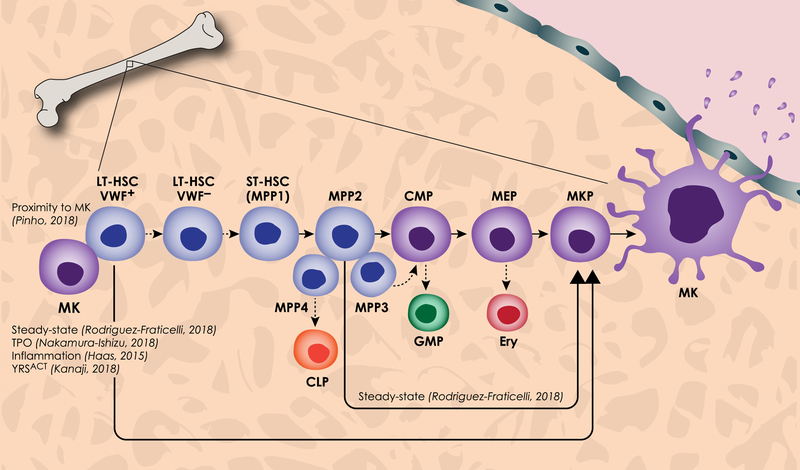
Hematopoiesis occurs in a succession of tissues during vertebrate embryonic development, beginning in the yolk sac around embryonic day 7.5 in mice and during the third week of human development8, 11, 12. Following this, HSCs are derived from hemogenic endothelial cells in the aorta-gonad-mesonephros and placenta and migrate to the fetal liver, thymus, spleen, and finally the bone marrow811. This spatially and temporally varied developmental process gives rise to independent waves of hematopoiesis. Early hematopoiesis in the yolk sac produces blood cells essential for survival, primarily oxygen-carrying erythrocytes and platelet producing megakaryocytes13. HSCs from the aorta-gonad-mesonephros and placenta are preferentially self-renewing, whereas HSCs in the fetal liver and bone marrow are primed to differentiate into all blood lineages14. Bone marrow long term HSCs (LT-HSCs) are largely quiescent – a state marked by G0 cell cycle and low mitochondrial activity – during steady-state hematopoiesis15. However, during times of biological demand such as hemorrhage, HSCs lose quiescence and become mobilized to differentiate. Canonical hematopoiesis occurs through LT-HSCs, short term HSCs (ST-HSCs), which are capable of reconstituting all mature lineages but have limited self-renewal capability, and multipotent progenitors (MPPs), a group of non-renewing lineage-biased progenitor cells (Figure 1). Finally, MPPs differentiate into more committed progenitors such as the common lymphoid progenitor (CLP) and the common myeloid progenitor (CMP), which further branches into the megakaryocyte-erythroid progenitor (MEP), a shared progenitor between megakaryocytes and erythroblasts, and the granulocyte-macrophage progenitor (GMP) (Figure 1). These committed progenitors give rise to terminally differentiated mature blood cells.
Figure 1. Canonical and new models of hematopoiesis and megakaryopoiesis.
Canonical megakaryopoiesis involves differentiation through the long term HSC (LT-HSC), short term HSC (ST-HSC) or MPP1, multipotent progenitor (primarily MPP2), common myeloid progenitor (CMP), megakaryocyte erythroid progenitor (MEP), and megakaryocyte progenitor (MKP). The megakaryocyte (MK) then undergoes a maturation process involving an increase in size, increase in DNA content, and extension of proplatelets through sinusoids in the blood vessel into circulation. Also in this canonical hematopoiesis pathway, deemphasized with dashed arrows, VWF+ LT-HSCs differentiate directly into VWF− LT-HSCs which give rise to all MPPs. MPP4 primarily differentiates into the common lymphoid progenitor (CLP), MPP3 to the granulocyte macrophage progenitor (GMP), MPP2 to the MEP and erythroblast (Ery). The megakaryocyte-biased pathway is highlighted here, including new findings which propose that VWF+ LT-HSCs are principally biased towards the megakaryocyte lineage through the MPP2 and the MEP. Furthermore, VWF+ LT-HSC and MPP2 populations differentiate directly to MKs, bypassing intermediate progenitors, a phenomenon seen during steady-state, TPO induction, inflammation, and expression of YRSACT. Additionally, VWF+ LT-HSCs reside in close proximity to, and are influenced by, megakaryocytes in the bone marrow.
In recent years, the dogma of hematopoietic hierarchy, which has traditionally been determined by surface markers via flow cytometry, has been challenged. In particular, the origin of megakaryocytes, including the existence and hierarchy of megakaryocyte progenitors, has been redefined. First, the MPP population has been further characterized to include subsets of lineage-biased MPPs16, 17. Additionally, several groups have shown through various technologies that traditionally defined, self-renewing LT-HSCs are capable of unilaterally differentiating into megakaryocytes18–24. Finally, the existence of the MEP population itself has been challenged. While a common progenitor capable of producing erythroblasts and megakaryocytes exists in ex vivo assays, newer technologies have shown that this bipotent progenitor is transient and difficult to detect in native hematopoiesis, and the classification of the MEP may require refinement. Both the canonical pathway and pathways recently discovered to impact megakaryopoiesis are outlined in Figure 1.
Identification of Megakaryocyte-biased MPPs and HSCs
As platelets are often the first line of defense not only during vascular injury, but also during states of inflammation, infection, and wound healing, it is not surprising that there would be a hematopoietic demand to quickly make megakaryocytes. Thus, rapid differentiation from an HSC to a megakaryocyte is necessary to replenish the platelet pool. More recently, MPPs have been defined as a set of unique multipotent, but lineage-biased, progenitors. Specifically, MPPs exist in multiple states, designated MPP 1–4, each having a unique signature and lineage bias16. MPP1 is classified as a ST-HSC, MPP3 is largely granulocyte/macrophage biased, and MPP4 is largely lymphoid biased17. MPP2 is biased towards the megakaryocyte/erythroid lineage, and it is the only MPP capable of replenishing platelets in a transplant model17. This megakaryocyte-biased MPP2 population has more recently been shown to directly differentiate into a megakaryocyte, bypassing the canonical CMP and MEP progenitors24. Furthermore, an expanding body of work has uncovered the persistence of a megakaryocyte-biased, self-renewing HSC that, while originally thought to be employed under times of hematopoietic stress, is increasingly recognized as a precursor to a large proportion of megakaryocytes during native hematopoiesis18–24.
The heterogeneity of HSCs and their self-renewal and repopulating abilities has historically been demonstrated with single-cell HSC transplantation; individual HSCs exhibit varying degrees of quiescence as well as myeloid or lymphoid bias25–28. A megakaryocyte-like subset of myeloid-biased HSCs (FLT3 negative), was first recognized due to its increased expression of megakaryocytic transcripts such as the transcription factor GATA1, the thrombopoietin receptor Mpl, and the platelet and endothelial aggregation factor von Willebrand Factor (VWF)29. Further examination of these megakaryocyte-like HSCs defined a subset of HSCs expressing the megakaryocyte-specific integrin CD4130. CD41-expressing HSCs have a myeloid-bias, increase with age, and are associated with the megakaryocyte specific transcription factor, GATA130, 31. These observations further led to the question of whether megakaryocyte-like, myeloid-biased HSCs were specifically megakaryocyte and platelet biased. A seminal study using a VWF-GFP reporter found that approximately 60% of HSCs in the bone marrow are VWF+ and that these HSCs are strongly biased towards reconstituting megakaryocytes and platelets in a transplant assay18. Furthermore, this study revealed that VWF+ HSCs are hierarchically above VWF− HSCs, suggesting that HSCs originate as megakaryocyte-primed and loss of megakaryocyte markers is necessary for differentiation into other lineages18. Further exploring the heterogeneity of HSCs, Yamamoto et al. showed that traditionally defined self-renewing HSCs are capable of differentiating directly into myeloid-biased, megakaryocyte-biased, or megakaryocyte/erythroid biased HSCs, all of which maintain self-renewal capability19. Two studies also identified high c-Kit receptor density on HSCs as a marker self-renewal capability and megakaryocyte bias20, 21. Complementing these studies, Carrelha et al. used sensitive analysis (detection as low as 0.01%) of peripheral blood cells after HSC transplantation to define VWF+, megakaryocyte-biased HSCs as unipotent – capable of repopulating platelets but no other blood lineages22. Megakaryocyte-biased HSCs have also been observed in human fetal liver, cord blood, and bone marrow cells23. Taken together, these data strongly support the existence of a repopulating HSC that is partially or completely biased towards differentiating into megakaryocytes and producing platelets.
One significant caveat to many HSC lineage tracing studies is that they rely on HSC transplant into lethally irradiated mice, which may not fully recapitulate native hematopoiesis. However, a recent paper by Rodriguez-Fraticelli et al. confirmed the existence of megakaryocyte-biased MPP2 and HSC populations during native hematopoiesis24. In this paper, megakaryocyte-biased HSCs were observed during native hematopoiesis by tracking inducible random transposon integration, thereby bypassing the need for lethal irradiation and transplant. This study reveals that LT-HSCs and MPP2 populations are both direct sources of megakaryocytes and replenish approximately 31% of the native pool. Interestingly, mature megakaryocytes are capable of differentiating directly from the LT-HSC and skipping the MPP2 intermediate. Furthermore, this study did not find megakaryocytes that had a shared lineage tracing with erythroblasts, failing to recognize the existence of a MEP. This study supports the idea that megakaryocyte-biased HSCs are not only strongly biased, but constitute a sub-population of HSCs primed to directly differentiate into megakaryocytes, bypassing other commitment steps. However, the specific megakaryocyte differentiation pathways taken during normal and pathologic hematopoiesis is a question that remains unanswered and will likely be the topic of important research in the coming years.
Redefining the Megakaryocyte-Erythroid Progenitor
The new dogma suggesting that megakaryocytes are capable of differentiating directly from a lineage-biased HSC or MPP, and may do so frequently, brings to attention the importance of the traditionally accepted progenitor, the MEP. Rodriguez-Fraticelli and colleagues found no evidence of common lineage between megakaryocytes and erythroblasts using a novel method of interrogating native hematopoiesis24. However, flow cytometry, CFU assays, and transplant studies have historically identified progenitors with bipotent megakaryocyte/erythroid potential32. Additionally, Yamamoto et al. showed in a reporter-based system the existence of repopulating megakaryocyte/erythroid-biased HSCs in addition to megakaryocyte-biased HSCs19. These data strongly support the existence of a bipotent progenitor. One possibility for this discrepancy is the inconsistent definition of the MEP. Xavier-Ferrucio et al. suggest that the classic flow cytometry gating scheme for mouse MEPs represents a population that is largely erythroid committed (CD34− Lin− c-Kit+ Sca− FcgR−)9, 32. Thus, phenotyping of the MEP may require further dissection, as a series of sub-progenitors have been defined using the CD150 and CD105 markers – Pre-MK/E (CD150+/CD105−), Pre-CFU-E (CD150+/CD105+), and CFU-E (CD150−/CD105+)33. A recent study aimed to further refine the MEP gating strategy to include additional markers (lin− CD34+ Flt3− CD45RA− CD38 mid MPL+ CD36− CD41−) which reconstitute all three megakaryocyte, erythroid, and megakaryocyte/erythroid colonies in a functional assay34. As such, it is possible that a progenitor with only megakaryocyte and erythroid potential exists, but may require refined phenotyping, may be too transient to detect in native hematopoiesis such as in the transposon study, or may exist higher up in hematopoiesis as a self-renewing, bipotent HSC19, 24. Regardless, the origins of embryonic hematopoiesis suggest a common progenitor, as erythrocytes and megakaryocytes are the first terminally differentiated blood cells to be produced during embryonic hematopoiesis. Furthermore, the existence of a common progenitor between the megakaryocyte and erythrocyte is supported by the similar genetic signature and reliance on common transcription factors, such as GATA135, 36. It is important to continue to define these various cell types in multiple ways, including more sensitive phenotyping and functional assays, to determine both their fate and potential. Overall, it is becoming evident that hematopoiesis does not only occur as a branched tree hierarchy, but is dynamic and adaptive to biological needs.
Signaling Pathways in Megakaryopoiesis
Megakaryocyte development is regulated at multiple levels by different cytokines, the most critical of which is thrombopoietin (TPO) (Figure 2). TPO, along with its receptor c-mpl, was discovered and cloned in 1994 where it was shown to promote development and maturation of megakaryocytes from their HSC precursors37–44. TPO predominately regulates megakaryocyte differentiation from the HSC, and thus all progenitor cells primed to become megakaryocytes, including HSCs, CMPs and MEPs, express c-mpl45. This regulation is the result of a feedback loop; TPO is constitutively produced by the liver and then circulates in plasma, where it is sequestered by circulating platelets in a c-mpl-dependent manner. As such, reduction in platelet counts leads to increased levels of circulating TPO which is free to exert its stimulatory effects on bone marrow HSCs, thus increasing megakaryocyte (and platelet) numbers46–48. TPO signaling results in internalization of the c-mpl-TPO receptor-ligand complex and initiation of multiple signal transduction pathways including JAK2, STAT3/STAT5, MAPK/ERK, and PI3K/AKT49–52. Specifically, TPO induces phosphorylation of JAK2 which phosphorylates downstream targets including activation of the transcription factors STAT3/STAT551. Signaling through these pathways causes downstream activation of megakaryocyte-specific transcription factors and regulation of expression of megakaryocyte specific genes. Studies in TPO and c-mpl KO mice show a global decrease in HSCs, with a particularly drastic reduction in megakaryocytes and platelets53. However, TPO and c-mpl KO mice are able to make a small (but sufficient) number of platelets. In addition, humans with a complete loss of functional c-Mpl have a median platelet count of 21 × 109/L or below, suggesting that patients without functional TPO signaling also retain a form of platelet production and that TPO-independent pathways of megakaryopoiesis in mammals exist54. Although TPO is a critical regulator of megakaryopoiesis, it does not have an essential role in the final stages of proplatelet formation and release.
Figure 2. Key cytokines and transcription factors involved in the regulation of megakaryocyte maturation.
Megakaryopoiesis is regulated at multiple stages via cytokines (blue). In the classical pathway, megakaryopoiesis begins with thrombopoietin signaling via it’s receptor c-mpl (red), which is expressed throughout every differentiation stage; from the hematopoietic stem cell (HSC) through to platelets produced from the mature megakaryocyte (MK). Various lineage fate decisions lead to the upregulation of transcription factors and consequent surface receptor expression, which are currently used to define a committed, mature MK. Expression of GATA1/FOG1 and FLI-1 lead to expression of CD41 (orange), CD42b (grey) and GPIX (grey), respectively. The expression of RUNX1 and NFE2 are crucial in terminal maturation processes, including polyploidization (upregulation of MYH10, downregulation of MYH10 and Notch4) and cytoskeletal rearrangement (expression of Tubulin B1 (green)). In addition to TPO, other cytokines have been found to affect megakaryopoiesis, both in a TPO-dependent (IL-1β, IL-3, IL-6, YRSACT) and independent fashion (IL-1α, CCL5 IGF-1).
TPO has also recently been shown to regulate HSC quiescence and mobilization by stimulation of HSC entry into the cell cycle55, 56. Considering the newly-recognized megakaryocyte primed HSCs, recent work has sought to reconcile the effect of TPO on HSCs and megakaryocyte bias. Nakamura-Ishizu and colleagues found that TPO stimulation biases HSCs towards megakaryocytes in vivo and may cause differentiation directly to megakaryocytes while bypassing other progenitors57. This report found that HSCs, which are normally quiescent and have low mitochondrial activity and number, are mobilized to differentiate into megakaryocytes after TPO stimulation. This process was marked by increased mitochondrial activity, increased cycling, decreased pro-apoptotic gene expression, and increased expression of the cell surface marker CD9. This gives a possible mechanism explaining HSC mobilization and megakaryocyte-biased differentiation through TPO signaling.
Alternative signaling effectors of megakaryopoiesis
Although residual platelet production persists in the absence of TPO signaling, TPO-independent regulators of megakaryopoiesis remain elusive. However, some alternative pathways have been shown to enhance this process. In fact, the interleukin family was found to influence megakaryopoiesis before the discovery of TPO; in early studies, injections of IL-1β and IL-6 increased platelet counts in vivo58, 59. However, these cytokines were ultimately found to be associated with increased plasma TPO, suggesting that interleukins upregulate TPO production, rather than directly stimulating megakaryopoiesis. IL-3 also increases megakaryocyte colony size and numbers in vitro, but does not affect megakaryocyte maturation60, 61. IL-1α is perhaps the only known interleukin to have a possible TPO-independent phenotype, as it acts on mature megakaryocytes to cause rupture, and thus shedding of proplatelets into the bone marrow62. More recently, other cytokines and chemokines not in the interleukin family have been identified as megakaryocyte-promoting factors. Insulin-like growth factor (IGF-1) promotes CD34+ differentiation towards the megakaryocyte lineage, a process mediated through AKT signaling with the assistance of steroid receptor coactivator 363. In vivo administration of IGF-1 increases platelet counts in both lethally irradiated mice and c-mpl knockout mice, suggesting a TPO-independent phenotype63. Another chemokine, CCL5 (RANTES), has also recently been shown to increase megakaryocyte ploidy, proplatelet production, and suppress apoptosis in vitro64. Seeing as these cytokines are often upregulated during inflammation, it remains to be seen if they also regulate megakaryopoiesis during normal hematopoiesis and/or in a TPO-independent manner. Additionally, in the context of recent studies, it is possible that these inflammatory cytokines also bias HSCs towards the megakaryocytic lineage.
Recent studies have identified the Notch pathway as a mediator of megakaryopoiesis. Notch signaling is required for murine megakaryocyte commitment from the HSC and increases CD41+ cells, CMPs, and MEPs, potentially via AKT signaling and consequent suppression of FOXO65, 66. However, in human CD34+ cells, Notch activation inhibits terminal megakaryocyte maturation67. Furthermore, characterization of mouse MEPs revealed that Notch signaling through recombinant DLL skewes towards the erythroid lineage and away from the megakaryocyte lineage68. Li and colleagues demonstrated that downregulation of receptor Notch4 by RUNX1 is required for normal megakaryocyte development in human cells69. Specifically, deletion of the RUNX1-binding site in intron 29 of NOTCH4 causes an increase in Notch4 expression and inhibition of megakaryocyte differentiation. Pharmacological inhibition of Notch signaling enhances human megakaryopoiesis in vitro69. Overall, megakaryopoiesis appears to be regulated by Notch signaling at different stages of development, potentially by requiring Notch signaling for development of earlier progenitors, but downregulating Notch for terminal megakaryocyte commitment.
Signaling affecting HSC bias
TPO signaling not only directs differentiation of HSCs along the megakaryocyte lineage through committed progenitors, but also skews HSCs towards a direct megakaryocyte bias, bypassing other progenitors22, 24. Seeing as the discovery of megakaryocyte-biased HSCs is relatively new, the TPO-independent effectors of HSC bias directly towards megakaryocytes are not known. However, a recent study identified a tyrosyl-tRNA synthetase variant (YRSACT), an activated version of an enzyme responsible for protein synthesis, as a potential TPO-independent regulator of megakaryocyte differentiation70. YRSACT administration increases platelet counts in acute models of thrombocytopenia as well as in mice deficient in TPO signaling. The TPO-independent effect was attributed to skewing of HSCs towards megakaryopoiesis, as was determined by an increased population of megakaryocytes positive for the Sca cell surface marker, a marker for HSCs. While a direct effect of YRSACT on HSC bias remains to be seen, these data indicate that TPO-independent mechanisms of megakaryocyte-biased HSC skewing may exist.
Transcription Factors in Megakaryopoiesis
Hematopoietic cell fate and megakaryocyte differentiation is largely coordinated by the temporal expression of various transcription factors. Just as HSCs and megakaryocytes share similar cell surface receptors, they also have similar expression of transcription factors. Transcription factors are involved in fetal HSC specification, adult HSC maintenance and renewal, and megakaryocyte differentiation. For example, SCL and RUNX, two transcription factors necessary for megakaryocyte differentiation, are also essential for fetal HSC determination, but are dispensable for adult HSC maintenance71, 72. Likewise, GATA2 is required for HSC maintenance as well as further downstream during early megakaryopoiesis73, 74. A conditional knockout mouse of ETV6, an ETS family transcription factor, also has impaired HSC viability and megakaryocyte differentiation75.
Transcription factors regulate differentiation along each lineage commitment decision point from HSC to megakaryocyte. The first step of myeloid commitment, the CMP juncture, requires a balance of expression of antagonistic transcription factors GATA1 and PU.1, which influence hematopoietic skewing towards the MEP and GMP, respectively76–78. MEP commitment to the megakaryocyte or erythrocyte is coordinated by expression of various transcription factors that must be expressed in a time- and dose-dependent manner. Of these, NF-E2, SCL, GFI1B, GATA1, GATA2, KLF1, and ETV6 are all expressed in the MEP, whereas transcription factors EKLF and c-Myb are exclusively expressed in the erythrocyte lineage and FLI1 and RUNX1 are exclusively expressed in the megakaryocyte lineage79–82 (Figure 2). GATA2 and GATA1, along with its cofactor FOG1, are expressed in an antagonistic manner in the MEP; GATA2 promotes megakaryopoiesis at the expense of erythropoiesis, while GATA1 promotes erythropoiesis83–86. However, these relationships are not exclusive; GATA1 is largely studied for its requirement in erythropoiesis and activation of globin gene expression but is also essential for megakaryopoiesis and transcription of the gene encoding megakaryocyte-specific receptor glycoprotein IIb84, 87, 88. Also functioning in the MEP to skew towards megakaryocyte fate, RUNX1 actively represses the erythroid-specific transcription factor KLF1, thus allowing a shift in the ratio of increased megakaryocyte-specific FLI1 to KLF1 and promoting megakaryocyte differentiation80, 82. During later stages of megakaryopoiesis, RUNX1 directly represses and activates the expression of myosins MYH10 and MYL9, respectively, the regulated expression of which are required for megakaryocyte maturation89, 90. Furthermore, megakaryocyte transcription factor FLI1 (which is negatively regulated by ETV6) regulates expression of megakaryocyte receptors GPIX and GPIba (Figure 2)91, 92. Finally, the transcription factor NFE2 is required during the terminal stage of megakaryocyte maturation, proplatelet production. Mice lacking NFE2 have normal proliferation of megakaryocytes, but defective platelet production (Figure 2)93, 94. This is largely due to transcriptional regulation of the megakaryocyte specific microtubule component β1-tubulin by NFE295, 96. Several of these transcription factors, including GATA1, RUNX1, FLI1, GFI1b, and ETV6, are mutated in inherited thrombocytopenias35, 97–105. In fact, most of these genes were originally discovered by sequencing individuals with congenital thrombocytopenia, underscoring their importance in maintaining megakaryocyte and platelet number.
Canonical transcription factor regulation of megakaryopoiesis has largely been explained by the previously mentioned transcription factors; however, new and more accurate, high-throughput technologies has revealed that these transcription factors are not the sole actors. Genome wide ChIP- sequencing of GATA1, GATA2, SCL, FLI1, and RUNX1-targeted promoters in human megakaryocytes found that these factors bind promoters simultaneously in multiple combinations106. Further complicating this process, many of these transcription factors act as both transcriptional activators or repressors, depending on their association with other factors91, 107. Thus, it is difficult to predict the effect of up- or down-regulating a single transcription factor. While many of the essential transcription factors are well known, recent work suggests that the list of transcription factors involved in megakaryopoiesis is much longer than previously thought. A recent large-scale screen of CRISPR/Cas9 in megakaryocytes uncovered several unrecognized transcription factors that influence megakaryopoiesis, including those downstream of the Notch pathway (HES7), in the NFAT family of transcription factors, as well as Histone Deacetylases108. Of particular interest, the prospect that histone modifiers affect megakaryopoiesis suggests a layer of epigenetic regulation, further complicating the process of transcription factor accessibility.
Megakaryocyte-biased HSCs, as defined by high CD41 expression, high c-Kit expression, or VWF expression, are also enriched for megakaryocytic transcription factors such as GATA1, GFI1b, GATA2, and FLI1, suggesting that HSC megakaryocyte bias may occur at the transcriptional level prior to differentiation18, 20, 30. This is further supported by clonal analysis and single-cell gene expression of megakaryocyte-primed HSCs, which have a similar transcriptional signature to terminally differentiated megakaryocytes24. Interestingly, genome-wide chromatin mapping in hematopoietic cells found that megakaryocytes and HSCs have strongly overlapping active chromatin signatures109. These shared open chromatin sites also correspond to megakaryocytic transcription factor binding sites, an overlap that is not seen between megakaryocytes and erythroid progenitors, suggesting that epigenetics controls unilateral differentiation from HSC to megakaryocyte109. These new data provide an updated framework in which to view transcriptional control regulating canonical hematopoiesis. In addition, it provides intriguing new ideas supporting the existence of megakaryocyte-biased HSC populations. In the future, it will be interesting to see if researchers will look to transcription factors or epigenetics to help further define the biases inherent in HSC subpopulations.
Interactions between HSCs, megakaryocytes, and bone marrow cells
The bone marrow microenvironment regulates HSC number and quiescence, but the exact mechanism is controversial. The interaction between HSCs and the bone marrow was originally thought to be mediated by expression of and signaling through HSC receptors such as Tie2 and c-mpl, the ligands of which (angiopoietin and thrombopoietin, respectively) are produced by osteoblasts56, 110, 111. However, subsequent studies found that HSCs are often in close proximity to perivascular cells adjacent to sinusoids in the bone marrow112. This relationship was attributed to production of the chemokine CXCL12/SDF1 by perivascular cells, signaling through which regulates HSC quiescence and maintenance113. Of note, megakaryocytes are also primarily localized to vascular sinusoids, as it is necessary for proplatelets to extend and release into the vascular space, a process guided by a sphingosine 1-phosphate gradient from the blood114. This interaction between the vascular sinusoids and megakaryocytes is also mediated by the chemokine CXCL12/SDF1 and interaction with the CXCR4 receptor as well as an interaction between podoplanin on perivascular cells and CLEC-2 on megakaryocytes115–118. Additionally, megakaryocyte maturation and platelet production is accelerated by IL9, a cytokine produced by bone marrow osteoblasts119. More widely and recently studied, however, is the fact that shared interactions with perivascular cells position HSCs and megakaryocytes in close proximity to each other. Whole mount imaging of bone marrow revealed a physical association of HSCs with megakaryocytes120. This physical interaction is thought to be functional due to the fact that megakaryocyte depletion results in proliferation of HSCs and an associated loss in quiescence, an interaction largely attributed to megakaryocyte release of CXCL4 (PF4)120. Additional studies have found that megakaryocyte-derived TPO and TGF-β also mediate HSC quiescence120–122. In addition to direct release of proteins, recent attention has also turned to the release of microparticles within the bone marrow space as a method of cell-to-cell communication. In fact, Jiang and colleagues revealed that megakaryocyte-derived microparticles bind to HSCs and induce them to differentiate into megakaryocytes, providing a possible mechanism of direct communication between the megakaryocyte and the HSC123.
Although evidence suggests that megakaryocytes are essential for HSC maintenance, in vivo imaging has often found that HSC distribution throughout the bone marrow is random, and cell depletion studies showing these effects are not representative of native hematopoiesis124. Recent work has sought to parse these data by examining HSC and megakaryocyte niches in the context of new studies showing the heterogeneity of HSCs. In particular, a study by Pinho et al. examined significant niche association in the native bone marrow of the two subsets of VWF+ and VWF− HSCs. This study found that almost all VWF+ (megakaryocyte-biased) HSCs exist within 5μm of megakaryocytes, but are not significantly associated with arterioles125. Of note, the converse was true for VWF− HSCs, indicating that spatial positioning of HSCs in relation to megakaryocytes and arterioles may contribute to or be associated with HSC heterogeneity. This study builds on previous findings that megakaryocyte depletion, resulting in loss of PF4, causes expansion of the HSC population, by adding that this expansion is largely specific to VWF+ HSCs120, 125. Similarly, PF4 injection selectively reduces the number of VWF+ HSCs. This study also sought to uncover the role of periarteriolar cells on lineage-biased HSCs by depleting NG2+ periarteriolar cells and tracking the independent effects on VWF+ and VWF− HSCs. Periarteriolar cell depletion selectively reduces the number and alters the localization of VWF− cells, but does not have an effect on VWF+ cells125. These data begin to unify the controversial literature about bone marrow niches and the relationship between HSCs and megakaryocytes by viewing HSCs as a heterogeneous population. Nonetheless, it is well-supported that the bone marrow microenvironment provides a space for cell-cell communication that is essential for functional hematopoiesis and megakaryopoiesis. Interestingly, a recent study revealed that the vascular architecture of the bone marrow is more complex than previously thought;126 a network of trans-cellular capillaries traverses perpendicularly through long bones, providing even more vascular area for HSC and megakaryocyte interaction. Thus, as technologies to map and define the bone marrow advance, it is likely that the relationships between HSCs, megakaryocytes, and their cellular neighbors will evolve as well.
Bone marrow structure/ 3D contacts
Although the exact niche distribution of megakaryocytes and HSCs is not completely defined, the three-dimensional architecture of the extracellular space in the bone marrow does affect megakaryocyte differentiation and maturation. The bone marrow extracellular environment is composed of fibrillar matrix proteins and glycosaminoglycans which create a low stiffness, elastic, 3D matrix127. This environment plays an important role in HSC maintenance and differentiation, and is also essential for megakaryocyte maturation and platelet production128, 129. In general, a less stiff matrix favors platelet production, while increased stiffness favors HSC and megakaryocyte maturation at the expense of proplatelet formation130. Recently, megakaryocytes were found to contribute to the bone marrow microenvironment, in that they can create their own niche that includes fibronectin, type IV collagen, and laminin131. Megakaryocytes synthesize these extracellular matrix components through TPO and TGF-beta signaling132. In addition, megakaryocytes synthesize the lysyl oxidase LOX1, which is largely responsible for crosslinking collagen I in the bone marrow133. LOX1 is expressed in immature megakaryocytes and downregulated as megakaryocytes mature, perhaps speaking to the different stiffness requirements necessary for megakaryocyte maturation versus proplatelet formation134. Indeed, this may be one mechanism by which megakaryocytes can alter their microenvironment as they mature, thereby inhibiting the production of proplatelets until the necessary maturation state is achieved. Another important component of the bone marrow extracellular matrix is a diverse number of glycosaminoglycans such as hyaluronic acid, which is depolymerized by the megakaryocyte to modulate its own maturation135. A knockout mouse of hyaluronidase-2, the depolymerizing enzyme of hyaluronic acid in megakaryocytes, causes megakaryocytes with a malformed demarcation membrane system and thrombocytopenia135.
All of these interactions depend on cellular receptors and subsequent signaling in the megakaryocyte, which are not only involved in creating and degrading the extracellular matrix, but are also essential for sensing the extracellular matrix stiffness. Megakaryocyte integrins such as α2β1 as well as a family of mechano-sensing ion channels, Piezo, are essential for environment sensing and proplatelet production136–138. These data exemplify the multi-dimensional relationship between megakaryocytes and the bone marrow, including a reciprocal relationship with both the extracellular environment and the residing HSCs. While researchers have often overlooked the impact of the microenvironment by using cells in culture, recent work has begun to address the importance of the three-dimensional microenvironment and the contribution of its tensegrity, stiffness, and molecular components on megakaryopoiesis. Investigators have replicated the stiffness and various components of the bone marrow in vitro by culturing megakaryocytes in hydrogels, scaffolds, and 3D silk matrices, all of which increase megakaryocyte ploidy and proplatelet production in vitro139–142. The success of these culture systems underscores the importance of the continued use of 3D culture systems in the study of megakaryocyte maturation in the future.
New concepts in megakaryocyte biology
Emergency megakaryopoiesis during inflammatory stress
In recent years, several landmark studies have revealed novel insights into the regulation of megakaryopoiesis under stress conditions. It has been well-documented that systemic inflammation can lead to acute thrombocytopenia or thrombocytosis143–145. It is therefore not surprising that mechanisms are in place to rapidly respond to inflammation and increase platelet counts. These mechanisms may lay dormant during homeostasis when TPO signaling dominates decisions about megakaryocyte production. Research into the situations in which ‘emergency hematopoiesis’ is employed have described novel pathways towards megakaryopoiesis and focused on HSC bias towards the megakaryocyte lineage. Such new insights have significant implications for the plasticity of the bone marrow to respond to stress and the particular importance of megakaryopoiesis.
Inflammatory signaling accentuates HSC bias towards the megakaryocytic lineage. It was originally observed that the megakaryocyte-biased MPP2 is preferred in regenerative states after bone marrow transplant17. Furthermore, in studies conducted by Haas and colleagues, mimicking viral, bacterial, and general acute infection upregulates expression of megakaryocyte proteins from preexisting dormant transcripts146. Specifically, megakaryocyte proteins were upregulated when mice were injected with type-1 interferon and LPS, which occurred via signaling through STAT1/mTOR and TLR4/MYD88/TRIF, respectively. Thrombocytopenia induced by other means, such as platelet-depleting antibodies, do not trigger the same increase in megakaryocyte protein expression. In conjunction with the known effects of other inflammatory cytokines on megakaryopoiesis, these findings may help to explain the thrombocytosis that is present in inflammatory states.
New insights into cell cycle regulation of megakaryopoiesis
Cell cycle regulation and cell fate decisions during hematopoiesis is an area of growing interest. Cell cycle activity is associated with loss of HSC quiescence, pushing progenitors out of dormancy and thus disrupting their self-renewal ability147. As previously mentioned, TPO signaling is a potent stimulator of HSC entry into the cell cycle and megakaryocyte-bias57. Interestingly, the cell cycle is also involved in cell fate determination of non-quiescent, committed progenitor cells. Work from Lu and colleagues shows that differences in cell cycle speed in the MEP may contribute to fate decisions between erythroid and megakaryocyte lineages148. This work reveals that MEPs with increased cell cycle speed are pushed towards the erythrocyte lineage, whereas those with decreased cell cycle speed skew towards megakaryocyte specification148. Furthermore, the differentiated megakaryocyte undergoes multiple rounds of endomitosis as a part of maturation, a process requiring both coordination and restriction of cell cycle components149–153. Ultimately, tight regulation of the cell cycle is required for all stages of megakaryopoiesis, but the overlap of these processes during endomitosis and lineage commitment, as well the extent of this regulation in HSC commitment is just beginning to be appreciated and understood.
Alternative functions of megakaryocytes
Megakaryocytes have other roles in hematopoiesis that are independent of their function in platelet production, including the ability to behave as immune and inflammatory cells. Megakaryocytes express several surface markers related to immune function, including members of the TLR family (TLR1, 2, 3, 4 and 6), FcyRIIA (humans only), and CD40L (the ligand of CD40 on immune cells)154, 155. Mature megakaryocytes also present antigens via expression of MHC I, which is transferred to platelets, thus increasing their ability to cross-present antigens156, 157. Megakaryocytes also contribute to inflammation by packaging cytokines and chemokines into their alpha granules and microparticles, which when released contribute to pathogenesis of proinflammatory conditions such as systemic lupus and inflammatory arthritis123, 158–160. Another way in which megakaryocytes contribute to immunity is through emperipolesis, a process by which other bone marrow cells, particularly neutrophils, are internalized by mature megakaryocytes whilst staying morphologically intact161. Although the functional significance of this is still unclear, it has been hypothesized that emperipolesis allows the exchange of membrane and other cellular material between the cells. In addition to the role of megakaryocytes in the bone marrow, extramedullary megakaryocytes reside in the lung and may be positioned to allow them to function more effectively as immune effector cells162. Combined with recent knowledge that inflammatory and infectious stimuli trigger megakaryocyte-primed HSCs to fast-track differentiation, these data suggest a relationship between cellular phenotype of megakaryocytes and response to inflammation and infection.
Concluding remarks
The changing dogma of megakaryopoiesis, specifically the differentiation of megakaryocytes directly from biased HSCs, opens new opportunities to investigate megakaryopoiesis more carefully. Firstly, the molecular processes involved in megakaryocyte-biased HSC differentiation have only been superficially investigated. It has been proposed that canonical signaling molecules and transcription factors are involved in this process, but further work is needed to tease apart the triggers of HSC commitment towards a direct megakaryocyte lineage. Furthermore, megakaryocytes should be studied not solely as platelet-producing cells, but also as cells involved in maintaining the bone marrow niche and cell-to-cell communication. It is necessary to determine if megakaryocytes themselves exist as a heterogeneous population with varied roles. Finally, megakaryocytes and megakaryopoiesis have been shown to be dynamic in the setting of inflammation and disease, suggesting that these processes are sensitive to their environment. The changes in megakaryocytes and platelets during disease may exacerbate inflammation by changing the bone marrow environment as well as the molecular signature of platelets. Understanding these changes may help to diagnose and treat inflammatory diseases more effectively. The biological drive for direct differentiation from HSC to megakaryocyte is intuitive due to the body’s need to replenish platelet pools during blood loss. However, it is also possible that, given the diverse roles of megakaryocytes, direct differentiation from the HSC is also essential for bone marrow maintenance and immune and inflammatory responses. To this extent, the question remains whether the route of differentiation (canonical or directly from the HSC) results in molecularly and functionally different megakaryocytes poised for different roles. Overall, this is an exciting time in the field of megakaryopoiesis; the changes in dogma are not simply rewriting what is known, but are also helping to clarify previously controversial topics and opening opportunities for new investigation.
Supplementary Material
Highlights:
New advances in interrogating hematopoiesis have revealed the presence of a megakaryocyte-biased HSC which may differentiate directly into a megakaryocyte.
Megakaryocyte-biased HSCs express megakaryocyte specific proteins such as VWF and CD41, can be triggered by extracellular signals such as TPO, and reside in close proximity to megakaryocytes in the bone marrow.
Megakaryocyte-biased HSC differentiation may occur under steady-state conditions, but is also upregulated during times of stress such as inflammation.
Acknowledgdements:
We sincerely thank Kristin Johnson for producing the figures in this paper.
Dr. Machlus is supported by the National Institute of Diabetes and Digestive and Kidney Diseases (K01DK111515) at the National Institutes of Health, and is an American Society of Hematology Scholar. Dr. Noetzli is supported by the Program in Blood Coagulation and Vascular Biology T32 through the National Heart, Lung, and Blood Institute at the National Institutes of Health (5T32HL007917-19).
The authors declare no relevant conflict of interest.
References
- 1.Massberg S, Brand K, Gruner S, Page S, Muller E, Muller I, Bergmeier W, Richter T, Lorenz M, Konrad I, Nieswandt B, Gawaz M. A critical role of platelet adhesion in the initiation of atherosclerotic lesion formation. J Exp Med. 2002;196:887–896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Farndale RW, Sixma JJ, Barnes MJ, de Groot PG. The role of collagen in thrombosis and hemostasis. J Thromb Haemost. 2004;2:561–573 [DOI] [PubMed] [Google Scholar]
- 3.Coppinger JA, Cagney G, Toomey S, Kislinger T, Belton O, McRedmond JP, Cahill DJ, Emili A, Fitzgerald DJ, Maguire PB. Characterization of the proteins released from activated platelets leads to localization of novel platelet proteins in human atherosclerotic lesions. Blood. 2004;103:2096–2104 [DOI] [PubMed] [Google Scholar]
- 4.Antithrombotic Trialists C. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002;324:71–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ebaugh FG Jr., Bird RM. The normal megakaryocyte concentration in aspirated human bone marrow. Blood. 1951;6:75–80 [PubMed] [Google Scholar]
- 6.Bender M, Thon JN, Ehrlicher AJ, Wu S, Mazutis L, Deschmann E, Sola-Visner M, Italiano JE, Hartwig JH. Microtubule sliding drives proplatelet elongation and is dependent on cytoplasmic dynein. Blood. 2015;125:860–868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thon JN, Italiano JE. Visualization and manipulation of the platelet and megakaryocyte cytoskeleton. Methods Mol Biol. 2012;788:109–125 [DOI] [PubMed] [Google Scholar]
- 8.Doulatov S, Notta F, Laurenti E, Dick JE. Hematopoiesis: A human perspective. Cell Stem Cell. 2012;10:120–136 [DOI] [PubMed] [Google Scholar]
- 9.Akashi K, Traver D, Miyamoto T, Weissman IL. A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature. 2000;404:193–197 [DOI] [PubMed] [Google Scholar]
- 10.Debili N, Coulombel L, Croisille L, Katz A, Guichard J, Breton-Gorius J, Vainchenker W. Characterization of a bipotent erythro-megakaryocytic progenitor in human bone marrow. Blood. 1996;88:1284–1296 [PubMed] [Google Scholar]
- 11.Orkin SH, Zon LI. Hematopoiesis: An evolving paradigm for stem cell biology. Cell. 2008;132:631–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tavian M, Hallais MF, Peault B. Emergence of intraembryonic hematopoietic precursors in the pre-liver human embryo. Development. 1999;126:793–803 [DOI] [PubMed] [Google Scholar]
- 13.Tober J, Koniski A, McGrath KE, Vemishetti R, Emerson R, de Mesy-Bentley KK, Waugh R, Palis J. The megakaryocyte lineage originates from hemangioblast precursors and is an integral component both of primitive and of definitive hematopoiesis. Blood. 2007;109:1433–1441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galloway JL, Zon LI. Ontogeny of hematopoiesis: Examining the emergence of hematopoietic cells in the vertebrate embryo. Curr Top Dev Biol. 2003;53:139–158 [DOI] [PubMed] [Google Scholar]
- 15.Hirao A, Arai F, Suda T. Regulation of cell cycle in hematopoietic stem cells by the niche. Cell Cycle. 2004;3:1481–1483 [DOI] [PubMed] [Google Scholar]
- 16.Cabezas-Wallscheid N, Klimmeck D, Hansson J, Lipka DB, Reyes A, Wang Q, Weichenhan D, Lier A, von Paleske L, Renders S, Wunsche P, Zeisberger P, Brocks D, Gu L, Herrmann C, Haas S, Essers MAG, Brors B, Eils R, Huber W, Milsom MD, Plass C, Krijgsveld J, Trumpp A. Identification of regulatory networks in hscs and their immediate progeny via integrated proteome, transcriptome, and DNA methylome analysis. Cell Stem Cell. 2014;15:507–522 [DOI] [PubMed] [Google Scholar]
- 17.Pietras EM, Reynaud D, Kang YA, Carlin D, Calero-Nieto FJ, Leavitt AD, Stuart JM, Gottgens B, Passegue E. Functionally distinct subsets of lineage-biased multipotent progenitors control blood production in normal and regenerative conditions. Cell Stem Cell. 2015;17:35–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sanjuan-Pla A, Macaulay IC, Jensen CT, Woll PS, Luis TC, Mead A, Moore S, Carella C, Matsuoka S, Bouriez Jones T, Chowdhury O, Stenson L, Lutteropp M, Green JC, Facchini R, Boukarabila H, Grover A, Gambardella A, Thongjuea S, Carrelha J, Tarrant P, Atkinson D, Clark SA, Nerlov C, Jacobsen SE. Platelet-biased stem cells reside at the apex of the haematopoietic stem-cell hierarchy. Nature. 2013;502:232–236 [DOI] [PubMed] [Google Scholar]
- 19.Yamamoto R, Morita Y, Ooehara J, Hamanaka S, Onodera M, Rudolph KL, Ema H, Nakauchi H. Clonal analysis unveils self-renewing lineage-restricted progenitors generated directly from hematopoietic stem cells. Cell. 2013;154:1112–1126 [DOI] [PubMed] [Google Scholar]
- 20.Shin JY, Hu W, Naramura M, Park CY. High c-kit expression identifies hematopoietic stem cells with impaired self-renewal and megakaryocytic bias. J Exp Med. 2014;211:217–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grinenko T, Arndt K, Portz M, Mende N, Gunther M, Cosgun KN, Alexopoulou D, Lakshmanaperumal N, Henry I, Dahl A, Waskow C. Clonal expansion capacity defines two consecutive developmental stages of long-term hematopoietic stem cells. J Exp Med. 2014;211:209–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carrelha J, Meng Y, Kettyle LM, Luis TC, Norfo R, Alcolea V, Boukarabila H, Grasso F, Gambardella A, Grover A, Hogstrand K, Lord AM, Sanjuan-Pla A, Woll PS, Nerlov C, Jacobsen SEW. Hierarchically related lineage-restricted fates of multipotent haematopoietic stem cells. Nature. 2018;554:106–111 [DOI] [PubMed] [Google Scholar]
- 23.Notta F, Zandi S, Takayama N, Dobson S, Gan OI, Wilson G, Kaufmann KB, McLeod J, Laurenti E, Dunant CF, McPherson JD, Stein LD, Dror Y, Dick JE. Distinct routes of lineage development reshape the human blood hierarchy across ontogeny. Science. 2016;351:aab2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rodriguez-Fraticelli AE, Wolock SL, Weinreb CS, Panero R, Patel SH, Jankovic M, Sun J, Calogero RA, Klein AM, Camargo FD. Clonal analysis of lineage fate in native haematopoiesis. Nature. 2018;553:212–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dykstra B, Kent D, Bowie M, McCaffrey L, Hamilton M, Lyons K, Lee SJ, Brinkman R, Eaves C. Long-term propagation of distinct hematopoietic differentiation programs in vivo. Cell Stem Cell. 2007;1:218–229 [DOI] [PubMed] [Google Scholar]
- 26.Muller-Sieburg CE, Cho RH, Thoman M, Adkins B, Sieburg HB. Deterministic regulation of hematopoietic stem cell self-renewal and differentiation. Blood. 2002;100:1302–1309 [PubMed] [Google Scholar]
- 27.Muller-Sieburg CE, Cho RH, Karlsson L, Huang JF, Sieburg HB. Myeloid-biased hematopoietic stem cells have extensive self-renewal capacity but generate diminished lymphoid progeny with impaired il-7 responsiveness. Blood. 2004;103:4111–4118 [DOI] [PubMed] [Google Scholar]
- 28.Spangrude GJ, Brooks DM, Tumas DB. Long-term repopulation of irradiated mice with limiting numbers of purified hematopoietic stem cells: In vivo expansion of stem cell phenotype but not function. Blood. 1995;85:1006–1016 [PubMed] [Google Scholar]
- 29.Mansson R, Hultquist A, Luc S, Yang L, Anderson K, Kharazi S, Al-Hashmi S, Liuba K, Thoren L, Adolfsson J, Buza-Vidas N, Qian H, Soneji S, Enver T, Sigvardsson M, Jacobsen SE. Molecular evidence for hierarchical transcriptional lineage priming in fetal and adult stem cells and multipotent progenitors. Immunity. 2007;26:407–419 [DOI] [PubMed] [Google Scholar]
- 30.Gekas C, Graf T. Cd41 expression marks myeloid-biased adult hematopoietic stem cells and increases with age. Blood. 2013;121:4463–4472 [DOI] [PubMed] [Google Scholar]
- 31.Miyawaki K, Arinobu Y, Iwasaki H, Kohno K, Tsuzuki H, Iino T, Shima T, Kikushige Y, Takenaka K, Miyamoto T, Akashi K. Cd41 marks the initial myelo-erythroid lineage specification in adult mouse hematopoiesis: Redefinition of murine common myeloid progenitor. Stem Cells. 2015;33:976–987 [DOI] [PubMed] [Google Scholar]
- 32.Xavier-Ferrucio J, Krause DS. Concise review: Bipotent megakaryocytic-erythroid progenitors: Concepts and controversies. Stem Cells. 2018;36:1138–1145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pronk CJ, Rossi DJ, Mansson R, Attema JL, Norddahl GL, Chan CK, Sigvardsson M, Weissman IL, Bryder D. Elucidation of the phenotypic, functional, and molecular topography of a myeloerythroid progenitor cell hierarchy. Cell Stem Cell. 2007;1:428–442 [DOI] [PubMed] [Google Scholar]
- 34.Sanada C, Xavier-Ferrucio J, Lu YC, Min E, Zhang PX, Zou S, Kang E, Zhang M, Zerafati G, Gallagher PG, Krause DS. Adult human megakaryocyte-erythroid progenitors are in the cd34+cd38mid fraction. Blood. 2016;128:923–933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nichols KE, Crispino JD, Poncz M, White JG, Orkin SH, Maris JM, Weiss MJ. Familial dyserythropoietic anaemia and thrombocytopenia due to an inherited mutation in gata1. Nat Genet. 2000;24:266–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stachura DL, Chou ST, Weiss MJ. Early block to erythromegakaryocytic development conferred by loss of transcription factor gata-1. Blood. 2006;107:87–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bartley TD, Bogenberger J, Hunt P, Li YS, Lu HS, Martin F, Chang MS, Samal B, Nichol JL, Swift S, et al. Identification and cloning of a megakaryocyte growth and development factor that is a ligand for the cytokine receptor mpl. Cell. 1994;77:1117–1124 [DOI] [PubMed] [Google Scholar]
- 38.de Sauvage FJ, Hass PE, Spencer SD, Malloy BE, Gurney AL, Spencer SA, Darbonne WC, Henzel WJ, Wong SC, Kuang WJ, et al. Stimulation of megakaryocytopoiesis and thrombopoiesis by the c-mpl ligand. Nature. 1994;369:533–538 [DOI] [PubMed] [Google Scholar]
- 39.Kaushansky K, Lok S, Holly RD, Broudy VC, Lin N, Bailey MC, Forstrom JW, Buddle MM, Oort PJ, Hagen FS, et al. Promotion of megakaryocyte progenitor expansion and differentiation by the c-mpl ligand thrombopoietin. Nature. 1994;369:568–571 [DOI] [PubMed] [Google Scholar]
- 40.Lok S, Kaushansky K, Holly RD, Kuijper JL, Lofton-Day CE, Oort PJ, Grant FJ, Heipel MD, Burkhead SK, Kramer JM, et al. Cloning and expression of murine thrombopoietin cdna and stimulation of platelet production in vivo. Nature. 1994;369:565–568 [DOI] [PubMed] [Google Scholar]
- 41.Kaushansky K The mpl ligand: Molecular and cellular biology of the critical regulator of megakaryocyte development. Stem Cells. 1994;12 Suppl 1:91–96; discussion 96–97 [PubMed] [Google Scholar]
- 42.Kuter DJ, Beeler DL, Rosenberg RD. The purification of megapoietin: A physiological regulator of megakaryocyte growth and platelet production. Proc Natl Acad Sci U S A. 1994;91:11104–11108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sohma Y, Akahori H, Seki N, Hori T, Ogami K, Kato T, Shimada Y, Kawamura K, Miyazaki H. Molecular cloning and chromosomal localization of the human thrombopoietin gene. FEBS Lett. 1994;353:57–61 [DOI] [PubMed] [Google Scholar]
- 44.Wendling F, Maraskovsky E, Debili N, Florindo C, Teepe M, Titeux M, Methia N, Breton-Gorius J, Cosman D, Vainchenker W. Cmpl ligand is a humoral regulator of megakaryocytopoiesis. Nature. 1994;369:571–574 [DOI] [PubMed] [Google Scholar]
- 45.Huang H, Cantor AB. Common features of megakaryocytes and hematopoietic stem cells: What’s the connection? J Cell Biochem. 2009;107:857–864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fielder PJ, Hass P, Nagel M, Stefanich E, Widmer R, Bennett GL, Keller GA, de Sauvage FJ, Eaton D. Human platelets as a model for the binding and degradation of thrombopoietin. Blood. 1997;89:2782–2788 [PubMed] [Google Scholar]
- 47.Kuter DJ, Rosenberg RD. The reciprocal relationship of thrombopoietin (c-mpl ligand) to changes in the platelet mass during busulfan-induced thrombocytopenia in the rabbit. Blood. 1995;85:2720–2730 [PubMed] [Google Scholar]
- 48.McCarty JM, Sprugel KH, Fox NE, Sabath DE, Kaushansky K. Murine thrombopoietin mrna levels are modulated by platelet count. Blood. 1995;86:3668–3675 [PubMed] [Google Scholar]
- 49.Miyakawa Y, Drachman JG, Gallis B, Kaushansky A, Kaushansky K. A structure-function analysis of serine/threonine phosphorylation of the thrombopoietin receptor, c-mpl. J Biol Chem. 2000;275:32214–32219 [DOI] [PubMed] [Google Scholar]
- 50.Miyakawa Y, Oda A, Druker BJ, Kato T, Miyazaki H, Handa M, Ikeda Y. Recombinant thrombopoietin induces rapid protein tyrosine phosphorylation of janus kinase 2 and shc in human blood platelets. Blood. 1995;86:23–27 [PubMed] [Google Scholar]
- 51.Miyakawa Y, Oda A, Druker BJ, Miyazaki H, Handa M, Ohashi H, Ikeda Y. Thrombopoietin induces tyrosine phosphorylation of stat3 and stat5 in human blood platelets. Blood. 1996;87:439–446 [PubMed] [Google Scholar]
- 52.Yamada M, Komatsu N, Okada K, Kato T, Miyazaki H, Miura Y. Thrombopoietin induces tyrosine phosphorylation and activation of mitogen-activated protein kinases in a human thrombopoietin-dependent cell line. Biochem Biophys Res Commun. 1995;217:230–237 [DOI] [PubMed] [Google Scholar]
- 53.Kimura S, Roberts AW, Metcalf D, Alexander WS. Hematopoietic stem cell deficiencies in mice lacking c-mpl, the receptor for thrombopoietin. Proc Natl Acad Sci U S A. 1998;95:1195–1200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van den Oudenrijn S, Bruin M, Folman CC, Peters M, Faulkner LB, de Haas M, von dem Borne AE. Mutations in the thrombopoietin receptor, mpl, in children with congenital amegakaryocytic thrombocytopenia. Br J Haematol. 2000;110:441–448 [DOI] [PubMed] [Google Scholar]
- 55.Qian H, Buza-Vidas N, Hyland CD, Jensen CT, Antonchuk J, Mansson R, Thoren LA, Ekblom M, Alexander WS, Jacobsen SE. Critical role of thrombopoietin in maintaining adult quiescent hematopoietic stem cells. Cell Stem Cell. 2007;1:671–684 [DOI] [PubMed] [Google Scholar]
- 56.Yoshihara H, Arai F, Hosokawa K, Hagiwara T, Takubo K, Nakamura Y, Gomei Y, Iwasaki H, Matsuoka S, Miyamoto K, Miyazaki H, Takahashi T, Suda T. Thrombopoietin/mpl signaling regulates hematopoietic stem cell quiescence and interaction with the osteoblastic niche. Cell Stem Cell. 2007;1:685–697 [DOI] [PubMed] [Google Scholar]
- 57.Nakamura-Ishizu A, Matsumura T, Stumpf PS, Umemoto T, Takizawa H, Takihara Y, O’Neil A, Majeed A, MacArthur BD, Suda T. Thrombopoietin metabolically primes hematopoietic stem cells to megakaryocyte-lineage differentiation. Cell Rep. 2018;25:1772–1785e1776 [DOI] [PubMed] [Google Scholar]
- 58.Kimura H, Ishibashi T, Shikama Y, Okano A, Akiyama Y, Uchida T, Maruyama Y. Interleukin-1 beta (il-1 beta) induces thrombocytosis in mice: Possible implication of il-6. Blood. 1990;76:2493–2500 [PubMed] [Google Scholar]
- 59.Kaser A, Brandacher G, Steurer W, Kaser S, Offner FA, Zoller H, Theurl I, Widder W, Molnar C, Ludwiczek O, Atkins MB, Mier JW, Tilg H. Interleukin-6 stimulates thrombopoiesis through thrombopoietin: Role in inflammatory thrombocytosis. Blood. 2001;98:2720–2725 [DOI] [PubMed] [Google Scholar]
- 60.Segal GM, Stueve T, Adamson JW. Analysis of murine megakaryocyte colony size and ploidy: Effects of interleukin-3. J Cell Physiol. 1988;137:537–544 [DOI] [PubMed] [Google Scholar]
- 61.Yang YC, Ciarletta AB, Temple PA, Chung MP, Kovacic S, Witek-Giannotti JS, Leary AC, Kriz R, Donahue RE, Wong GG, et al. Human il-3 (multi-csf): Identification by expression cloning of a novel hematopoietic growth factor related to murine il-3. Cell. 1986;47:3–10 [DOI] [PubMed] [Google Scholar]
- 62.Nishimura S, Nagasaki M, Kunishima S, Sawaguchi A, Sakata A, Sakaguchi H, Ohmori T, Manabe I, Italiano JE Jr., Ryu T, Takayama N, Komuro I, Kadowaki T, Eto K, Nagai R. Il-1alpha induces thrombopoiesis through megakaryocyte rupture in response to acute platelet needs. J Cell Biol. 2015;209:453–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen S, Hu M, Shen M, Wang S, Wang C, Chen F, Tang Y, Wang X, Zeng H, Chen M, Gao J, Wang F, Su Y, Xu Y, Wang J. Igf-1 facilitates thrombopoiesis primarily through akt activation. Blood. 2018;132:210–222 [DOI] [PubMed] [Google Scholar]
- 64.Machlus KR, Johnson KE, Kulenthirarajan R, Forward JA, Tippy MD, Soussou TS, El-Husayni SH, Wu SK, Wang S, Watnick RS, Italiano JE Jr., Battinelli EM. Ccl5 derived from platelets increases megakaryocyte proplatelet formation. Blood. 2016;127:921–926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mercher T, Cornejo MG, Sears C, Kindler T, Moore SA, Maillard I, Pear WS, Aster JC, Gilliland DG. Notch signaling specifies megakaryocyte development from hematopoietic stem cells. Cell Stem Cell. 2008;3:314–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cornejo MG, Mabialah V, Sykes SM, Khandan T, Lo Celso C, Lopez CK, Rivera-Munoz P, Rameau P, Tothova Z, Aster JC, DePinho RA, Scadden DT, Gilliland DG, Mercher T. Crosstalk between notch and akt signaling during murine megakaryocyte lineage specification. Blood. 2011;118:1264–1273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Poirault-Chassac S, Six E, Catelain C, Lavergne M, Villeval JL, Vainchenker W, Lauret E. Notch/delta4 signaling inhibits human megakaryocytic terminal differentiation. Blood. 2010;116:5670–5678 [DOI] [PubMed] [Google Scholar]
- 68.Weiss-Gayet M, Starck J, Chaabouni A, Chazaud B, Morle F. Notch stimulates both self-renewal and lineage plasticity in a subset of murine cd9high committed megakaryocytic progenitors. PLoS One. 2016;11:e0153860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li Y, Jin C, Bai H, Gao Y, Sun S, Chen L, Qin L, Liu PP, Cheng L, Wang QF. Human notch4 is a key target of runx1 in megakaryocytic differentiation. Blood. 2018;131:191–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kanaji T, Vo MN, Kanaji S, Zarpellon A, Shapiro R, Morodomi Y, Yuzuriha A, Eto K, Belani R, Do MH, Yang XL, Ruggeri ZM, Schimmel P. Tyrosyl-trna synthetase stimulates thrombopoietin-independent hematopoiesis accelerating recovery from thrombocytopenia. Proc Natl Acad Sci U S A. 2018;115:E8228–E8235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mikkola HK, Klintman J, Yang H, Hock H, Schlaeger TM, Fujiwara Y, Orkin SH. Haematopoietic stem cells retain long-term repopulating activity and multipotency in the absence of stem-cell leukaemia scl/tal-1 gene. Nature. 2003;421:547–551 [DOI] [PubMed] [Google Scholar]
- 72.Ichikawa M, Asai T, Saito T, Seo S, Yamazaki I, Yamagata T, Mitani K, Chiba S, Ogawa S, Kurokawa M, Hirai H. Aml-1 is required for megakaryocytic maturation and lymphocytic differentiation, but not for maintenance of hematopoietic stem cells in adult hematopoiesis. Nat Med. 2004;10:299–304 [DOI] [PubMed] [Google Scholar]
- 73.Tsai FY, Keller G, Kuo FC, Weiss M, Chen J, Rosenblatt M, Alt FW, Orkin SH. An early haematopoietic defect in mice lacking the transcription factor gata-2. Nature. 1994;371:221–226 [DOI] [PubMed] [Google Scholar]
- 74.Rodrigues NP, Tipping AJ, Wang Z, Enver T. Gata-2 mediated regulation of normal hematopoietic stem/progenitor cell function, myelodysplasia and myeloid leukemia. Int J Biochem Cell Biol. 2012;44:457–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hock H, Meade E, Medeiros S, Schindler JW, Valk PJ, Fujiwara Y, Orkin SH. Tel/etv6 is an essential and selective regulator of adult hematopoietic stem cell survival. Genes Dev. 2004;18:2336–2341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rekhtman N, Radparvar F, Evans T, Skoultchi AI. Direct interaction of hematopoietic transcription factors pu.1 and gata-1: Functional antagonism in erythroid cells. Genes Dev. 1999;13:1398–1411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Arinobu Y, Mizuno S, Chong Y, Shigematsu H, Iino T, Iwasaki H, Graf T, Mayfield R, Chan S, Kastner P, Akashi K. Reciprocal activation of gata-1 and pu.1 marks initial specification of hematopoietic stem cells into myeloerythroid and myelolymphoid lineages. Cell Stem Cell. 2007;1:416–427 [DOI] [PubMed] [Google Scholar]
- 78.Rhodes J, Hagen A, Hsu K, Deng M, Liu TX, Look AT, Kanki JP. Interplay of pu.1 and gata1 determines myelo-erythroid progenitor cell fate in zebrafish. Dev Cell. 2005;8:97–108 [DOI] [PubMed] [Google Scholar]
- 79.Athanasiou M, Mavrothalassitis G, Sun-Hoffman L, Blair DG. Fli-1 is a suppressor of erythroid differentiation in human hematopoietic cells. Leukemia. 2000;14:439–445 [DOI] [PubMed] [Google Scholar]
- 80.Bouilloux F, Juban G, Cohet N, Buet D, Guyot B, Vainchenker W, Louache F, Morle F. Eklf restricts megakaryocytic differentiation at the benefit of erythrocytic differentiation. Blood. 2008;112:576–584 [DOI] [PubMed] [Google Scholar]
- 81.Dore LC, Crispino JD. Transcription factor networks in erythroid cell and megakaryocyte development. Blood. 2011;118:231–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kuvardina ON, Herglotz J, Kolodziej S, Kohrs N, Herkt S, Wojcik B, Oellerich T, Corso J, Behrens K, Kumar A, Hussong H, Urlaub H, Koch J, Serve H, Bonig H, Stocking C, Rieger MA, Lausen J. Runx1 represses the erythroid gene expression program during megakaryocytic differentiation. Blood. 2015;125:3570–3579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cantor AB, Orkin SH. Transcriptional regulation of erythropoiesis: An affair involving multiple partners. Oncogene. 2002;21:3368–3376 [DOI] [PubMed] [Google Scholar]
- 84.Ikonomi P, Noguchi CT, Miller W, Kassahun H, Hardison R, Schechter AN. Levels of gata-1/gata-2 transcription factors modulate expression of embryonic and fetal hemoglobins. Gene. 2000;261:277–287 [DOI] [PubMed] [Google Scholar]
- 85.Ikonomi P, Rivera CE, Riordan M, Washington G, Schechter AN, Noguchi CT. Overexpression of gata-2 inhibits erythroid and promotes megakaryocyte differentiation. Exp Hematol. 2000;28:1423–1431 [DOI] [PubMed] [Google Scholar]
- 86.Galloway JL, Wingert RA, Thisse C, Thisse B, Zon LI. Loss of gata1 but not gata2 converts erythropoiesis to myelopoiesis in zebrafish embryos. Dev Cell. 2005;8:109–116 [DOI] [PubMed] [Google Scholar]
- 87.Martin F, Prandini MH, Thevenon D, Marguerie G, Uzan G. The transcription factor gata-1 regulates the promoter activity of the platelet glycoprotein iib gene. J Biol Chem. 1993;268:21606–21612 [PubMed] [Google Scholar]
- 88.Evans T, Reitman M, Felsenfeld G. An erythrocyte-specific DNA-binding factor recognizes a regulatory sequence common to all chicken globin genes. Proc Natl Acad Sci U S A. 1988;85:5976–5980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lordier L, Bluteau D, Jalil A, Legrand C, Pan J, Rameau P, Jouni D, Bluteau O, Mercher T, Leon C, Gachet C, Debili N, Vainchenker W, Raslova H, Chang Y. Runx1-induced silencing of non-muscle myosin heavy chain iib contributes to megakaryocyte polyploidization. Nat Commun. 2012;3:717. [DOI] [PubMed] [Google Scholar]
- 90.Jalagadugula G, Mao G, Kaur G, Goldfinger LE, Dhanasekaran DN, Rao AK. Regulation of platelet myosin light chain (myl9) by runx1: Implications for thrombocytopenia and platelet dysfunction in runx1 haplodeficiency. Blood. 2010;116:6037–6045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kwiatkowski BA, Bastian LS, Bauer TR Jr., Tsai S, Zielinska-Kwiatkowska AG, Hickstein DD. The ets family member tel binds to the fli-1 oncoprotein and inhibits its transcriptional activity. J Biol Chem. 1998;273:17525–17530 [DOI] [PubMed] [Google Scholar]
- 92.Kwiatkowski BA, Zielinska-Kwiatkowska AG, Bauer TR Jr., Hickstein DD. The ets family member tel antagonizes the fli-1 phenotype in hematopoietic cells. Blood Cells Mol Dis. 2000;26:84–90 [DOI] [PubMed] [Google Scholar]
- 93.Shivdasani RA, Rosenblatt MF, Zucker-Franklin D, Jackson CW, Hunt P, Saris CJ, Orkin SH. Transcription factor nf-e2 is required for platelet formation independent of the actions of thrombopoietin/mgdf in megakaryocyte development. Cell. 1995;81:695–704 [DOI] [PubMed] [Google Scholar]
- 94.Levin J, Peng JP, Baker GR, Villeval JL, Lecine P, Burstein SA, Shivdasani RA. Pathophysiology of thrombocytopenia and anemia in mice lacking transcription factor nf-e2. Blood. 1999;94:3037–3047 [PubMed] [Google Scholar]
- 95.Lecine P, Villeval JL, Vyas P, Swencki B, Xu Y, Shivdasani RA. Mice lacking transcription factor nf-e2 provide in vivo validation of the proplatelet model of thrombocytopoiesis and show a platelet production defect that is intrinsic to megakaryocytes. Blood. 1998;92:1608–1616 [PubMed] [Google Scholar]
- 96.Schwer HD, Lecine P, Tiwari S, Italiano JE Jr., Hartwig JH, Shivdasani RA. A lineage-restricted and divergent beta-tubulin isoform is essential for the biogenesis, structure and function of blood platelets. Curr Biol. 2001;11:579–586 [DOI] [PubMed] [Google Scholar]
- 97.Hart A, Melet F, Grossfeld P, Chien K, Jones C, Tunnacliffe A, Favier R, Bernstein A. Fli-1 is required for murine vascular and megakaryocytic development and is hemizygously deleted in patients with thrombocytopenia. Immunity. 2000;13:167–177 [DOI] [PubMed] [Google Scholar]
- 98.Monteferrario D, Bolar NA, Marneth AE, Hebeda KM, Bergevoet SM, Veenstra H, Laros-van Gorkom BA, MacKenzie MA, Khandanpour C, Botezatu L, Fransen E, Van Camp G, Duijnhouwer AL, Salemink S, Willemsen B, Huls G, Preijers F, Van Heerde W, Jansen JH, Kempers MJ, Loeys BL, Van Laer L, Van der Reijden BA. A dominant-negative gfi1b mutation in the gray platelet syndrome. N Engl J Med. 2014;370:245–253 [DOI] [PubMed] [Google Scholar]
- 99.Raslova H, Komura E, Le Couedic JP, Larbret F, Debili N, Feunteun J, Danos O, Albagli O, Vainchenker W, Favier R. Fli1 monoallelic expression combined with its hemizygous loss underlies paris-trousseau/jacobsen thrombopenia. J Clin Invest. 2004;114:77–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Song WJ, Sullivan MG, Legare RD, Hutchings S, Tan X, Kufrin D, Ratajczak J, Resende IC, Haworth C, Hock R, Loh M, Felix C, Roy DC, Busque L, Kurnit D, Willman C, Gewirtz AM, Speck NA, Bushweller JH, Li FP, Gardiner K, Poncz M, Maris JM, Gilliland DG. Haploinsufficiency of cbfa2 causes familial thrombocytopenia with propensity to develop acute myelogenous leukaemia. Nat Genet. 1999;23:166–175 [DOI] [PubMed] [Google Scholar]
- 101.Stockley J, Morgan NV, Bem D, Lowe GC, Lordkipanidze M, Dawood B, Simpson MA, Macfarlane K, Horner K, Leo VC, Talks K, Motwani J, Wilde JT, Collins PW, Makris M, Watson SP, Daly ME, Genotyping UK, Phenotyping of Platelets Study G. Enrichment of fli1 and runx1 mutations in families with excessive bleeding and platelet dense granule secretion defects. Blood. 2013;122:4090–4093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Topka S, Vijai J, Walsh MF, Jacobs L, Maria A, Villano D, Gaddam P, Wu G, McGee RB, Quinn E, Inaba H, Hartford C, Pui CH, Pappo A, Edmonson M, Zhang MY, Stepensky P, Steinherz P, Schrader K, Lincoln A, Bussel J, Lipkin SM, Goldgur Y, Harit M, Stadler ZK, Mullighan C, Weintraub M, Shimamura A, Zhang J, Downing JR, Nichols KE, Offit K. Germline etv6 mutations confer susceptibility to acute lymphoblastic leukemia and thrombocytopenia. PLoS Genet. 2015;11:e1005262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhang MY, Churpek JE, Keel SB, Walsh T, Lee MK, Loeb KR, Gulsuner S, Pritchard CC, Sanchez-Bonilla M, Delrow JJ, Basom RS, Forouhar M, Gyurkocza B, Schwartz BS, Neistadt B, Marquez R, Mariani CJ, Coats SA, Hofmann I, Lindsley RC, Williams DA, Abkowitz JL, Horwitz MS, King MC, Godley LA, Shimamura A. Germline etv6 mutations in familial thrombocytopenia and hematologic malignancy. Nat Genet. 2015;47:180–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Moriyama T, Metzger ML, Wu G, Nishii R, Qian M, Devidas M, Yang W, Cheng C, Cao X, Quinn E, Raimondi S, Gastier-Foster JM, Raetz E, Larsen E, Martin PL, Bowman WP, Winick N, Komada Y, Wang S, Edmonson M, Xu H, Mardis E, Fulton R, Pui CH, Mullighan C, Evans WE, Zhang J, Hunger SP, Relling MV, Nichols KE, Loh ML, Yang JJ. Germline genetic variation in etv6 and risk of childhood acute lymphoblastic leukaemia: A systematic genetic study. Lancet Oncol. 2015;16:1659–1666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Noetzli L, Lo RW, Lee-Sherick AB, Callaghan M, Noris P, Savoia A, Rajpurkar M, Jones K, Gowan K, Balduini CL, Pecci A, Gnan C, De Rocco D, Doubek M, Li L, Lu L, Leung R, Landolt-Marticorena C, Hunger S, Heller P, Gutierrez-Hartmann A, Xiayuan L, Pluthero FG, Rowley JW, Weyrich AS, Kahr WH, Porter CC, Di Paola J. Germline mutations in etv6 are associated with thrombocytopenia, red cell macrocytosis and predisposition to lymphoblastic leukemia. Nat Genet. 2015;47:535–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tijssen MR, Cvejic A, Joshi A, Hannah RL, Ferreira R, Forrai A, Bellissimo DC, Oram SH, Smethurst PA, Wilson NK, Wang X, Ottersbach K, Stemple DL, Green AR, Ouwehand WH, Gottgens B. Genome-wide analysis of simultaneous gata1/2, runx1, fli1, and scl binding in megakaryocytes identifies hematopoietic regulators. Dev Cell. 2011;20:597–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tripic T, Deng W, Cheng Y, Zhang Y, Vakoc CR, Gregory GD, Hardison RC, Blobel GA. Scl and associated proteins distinguish active from repressive gata transcription factor complexes. Blood. 2009;113:2191–2201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhu F, Feng M, Sinha R, Seita J, Mori Y, Weissman IL. Screening for genes that regulate the differentiation of human megakaryocytic lineage cells. Proc Natl Acad Sci U S A. 2018;115:E9308–E9316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Heuston EF, Keller CA, Lichtenberg J, Giardine B, Anderson SM, Center NIHIS, Hardison RC, Bodine DM. Establishment of regulatory elements during erythro-megakaryopoiesis identifies hematopoietic lineage-commitment points. Epigenetics Chromatin. 2018;11:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Arai F, Hirao A, Ohmura M, Sato H, Matsuoka S, Takubo K, Ito K, Koh GY, Suda T. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell. 2004;118:149–161 [DOI] [PubMed] [Google Scholar]
- 111.Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, Martin RP, Schipani E, Divieti P, Bringhurst FR, Milner LA, Kronenberg HM, Scadden DT. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425:841–846 [DOI] [PubMed] [Google Scholar]
- 112.Kiel MJ, Yilmaz OH, Iwashita T, Yilmaz OH, Terhorst C, Morrison SJ. Slam family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–1121 [DOI] [PubMed] [Google Scholar]
- 113.Sugiyama T, Kohara H, Noda M, Nagasawa T. Maintenance of the hematopoietic stem cell pool by cxcl12-cxcr4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006;25:977–988 [DOI] [PubMed] [Google Scholar]
- 114.Zhang L, Orban M, Lorenz M, Barocke V, Braun D, Urtz N, Schulz C, von Bruhl ML, Tirniceriu A, Gaertner F, Proia RL, Graf T, Bolz SS, Montanez E, Prinz M, Muller A, von Baumgarten L, Billich A, Sixt M, Fassler R, von Andrian UH, Junt T, Massberg S. A novel role of sphingosine 1-phosphate receptor s1pr1 in mouse thrombopoiesis. J Exp Med. 2012;209:2165–2181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang JF, Liu ZY, Groopman JE. The alpha-chemokine receptor cxcr4 is expressed on the megakaryocytic lineage from progenitor to platelets and modulates migration and adhesion. Blood. 1998;92:756–764 [PubMed] [Google Scholar]
- 116.Hamada T, Mohle R, Hesselgesser J, Hoxie J, Nachman RL, Moore MA, Rafii S. Transendothelial migration of megakaryocytes in response to stromal cell-derived factor 1 (sdf-1) enhances platelet formation. J Exp Med. 1998;188:539–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Avecilla ST, Hattori K, Heissig B, Tejada R, Liao F, Shido K, Jin DK, Dias S, Zhang F, Hartman TE, Hackett NR, Crystal RG, Witte L, Hicklin DJ, Bohlen P, Eaton D, Lyden D, de Sauvage F, Rafii S. Chemokine-mediated interaction of hematopoietic progenitors with the bone marrow vascular niche is required for thrombopoiesis. Nat Med. 2004;10:64–71 [DOI] [PubMed] [Google Scholar]
- 118.Tamura S, Suzuki-Inoue K, Tsukiji N, Shirai T, Sasaki T, Osada M, Satoh K, Ozaki Y. Podoplanin-positive periarteriolar stromal cells promote megakaryocyte growth and proplatelet formation in mice by clec-2. Blood. 2016;127:1701–1710 [DOI] [PubMed] [Google Scholar]
- 119.Xiao M, Wang Y, Tao C, Wang Z, Yang J, Chen Z, Zou Z, Li M, Liu A, Jia C, Huang B, Yan B, Lai P, Ding C, Cai D, Xiao G, Jiang Y, Bai X. Osteoblasts support megakaryopoiesis through production of interleukin-9. Blood. 2017;129:3196–3209 [DOI] [PubMed] [Google Scholar]
- 120.Bruns I, Lucas D, Pinho S, Ahmed J, Lambert MP, Kunisaki Y, Scheiermann C, Schiff L, Poncz M, Bergman A, Frenette PS. Megakaryocytes regulate hematopoietic stem cell quiescence through cxcl4 secretion. Nat Med. 2014;20:1315–1320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Nakamura-Ishizu A, Takubo K, Fujioka M, Suda T. Megakaryocytes are essential for hsc quiescence through the production of thrombopoietin. Biochem Biophys Res Commun. 2014;454:353–357 [DOI] [PubMed] [Google Scholar]
- 122.Zhao M, Perry JM, Marshall H, Venkatraman A, Qian P, He XC, Ahamed J, Li L. Megakaryocytes maintain homeostatic quiescence and promote post-injury regeneration of hematopoietic stem cells. Nat Med. 2014;20:1321–1326 [DOI] [PubMed] [Google Scholar]
- 123.Jiang J, Kao CY, Papoutsakis ET. How do megakaryocytic microparticles target and deliver cargo to alter the fate of hematopoietic stem cells? J Control Release. 2017;247:1–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Acar M, Kocherlakota KS, Murphy MM, Peyer JG, Oguro H, Inra CN, Jaiyeola C, Zhao Z, Luby-Phelps K, Morrison SJ. Deep imaging of bone marrow shows non-dividing stem cells are mainly perisinusoidal. Nature. 2015;526:126–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Pinho S, Marchand T, Yang E, Wei Q, Nerlov C, Frenette PS. Lineage-biased hematopoietic stem cells are regulated by distinct niches. Dev Cell. 2018;44:634–641e634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Grüneboom A, Ibrahim Hawwari DW, Culemann Stephan, Müller Sylvia, Henneberg Sophie, Brenzel Alexandra, Merz Simon, Bornemann Lea, Zec Kristina, Wuelling Manuela, Kling Lasse, Hasenberg Mike, Voortmann Sylvia, Lang Stefanie, Baum Wolfgang, Ohs Alexandra, Kraff Oliver, Quick Harald H., Jäger Marcus, Landgraeber Stefan, Dudda Marcel, Danuser Renzo, Stein Jens V., Rohde Manfred, Gelse Kolja, Garbe Annette I., Adamczyk Alexandra, Westendorf Astrid M., Hoffmann Daniel, Christiansen Silke, Engel Daniel Robert, Vortkamp Andrea, Krönke Gerhard, Herrmann Martin, Kamradt Thomas, Schett Georg, Hasenberg Anja & Gunzer Matthias A network of trans-cortical capillaries as mainstay for blood circulation in long bones. Nat Metabolism. 2019;1:236–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Long MW, Briddell R, Walter AW, Bruno E, Hoffman R. Human hematopoietic stem cell adherence to cytokines and matrix molecules. J Clin Invest. 1992;90:251–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Whetton AD, Graham GJ. Homing and mobilization in the stem cell niche. Trends Cell Biol. 1999;9:233–238 [DOI] [PubMed] [Google Scholar]
- 129.Chitteti BR, Kacena MA, Voytik-Harbin SL, Srour EF. Modulation of hematopoietic progenitor cell fate in vitro by varying collagen oligomer matrix stiffness in the presence or absence of osteoblasts. J Immunol Methods. 2015;425:108–113 [DOI] [PubMed] [Google Scholar]
- 130.Abbonante V, Di Buduo CA, Gruppi C, De Maria C, Spedden E, De Acutis A, Staii C, Raspanti M, Vozzi G, Kaplan DL, Moccia F, Ravid K, Balduini A. A new path to platelet production through matrix sensing. Haematologica. 2017;102:1150–1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Malara A, Currao M, Gruppi C, Celesti G, Viarengo G, Buracchi C, Laghi L, Kaplan DL, Balduini A. Megakaryocytes contribute to the bone marrow-matrix environment by expressing fibronectin, type iv collagen, and laminin. Stem Cells. 2014;32:926–937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Abbonante V, Di Buduo CA, Gruppi C, Malara A, Gianelli U, Celesti G, Anselmo A, Laghi L, Vercellino M, Visai L, Iurlo A, Moratti R, Barosi G, Rosti V, Balduini A. Thrombopoietin/tgf-beta1 loop regulates megakaryocyte extracellular matrix component synthesis. Stem Cells. 2016;34:1123–1133 [DOI] [PubMed] [Google Scholar]
- 133.Lucero HA, Kagan HM. Lysyl oxidase: An oxidative enzyme and effector of cell function. Cellular and molecular life sciences : CMLS. 2006;63:2304–2316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Eliades A, Papadantonakis N, Bhupatiraju A, Burridge KA, Johnston-Cox HA, Migliaccio AR, Crispino JD, Lucero HA, Trackman PC, Ravid K. Control of megakaryocyte expansion and bone marrow fibrosis by lysyl oxidase. J Biol Chem. 2011;286:27630–27638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Petrey AC, Obery DR, Kessler SP, Flamion B, de la Motte CA. Hyaluronan depolymerization by megakaryocyte hyaluronidase-2 is required for thrombopoiesis. Am J Pathol. 2016;186:2390–2403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Mossuz P, Schweitzer A, Molla A, Berthier R. Expression and function of receptors for extracellular matrix molecules in the differentiation of human megakaryocytes in vitro. Br J Haematol. 1997;98:819–827 [DOI] [PubMed] [Google Scholar]
- 137.Sabri S, Jandrot-Perrus M, Bertoglio J, Farndale RW, Mas VM, Debili N, Vainchenker W. Differential regulation of actin stress fiber assembly and proplatelet formation by alpha2beta1 integrin and gpvi in human megakaryocytes. Blood. 2004;104:3117–3125 [DOI] [PubMed] [Google Scholar]
- 138.Ilkan Z, Wright JR, Goodall AH, Gibbins JM, Jones CI, Mahaut-Smith MP. Evidence for shear-mediated ca(2+) entry through mechanosensitive cation channels in human platelets and a megakaryocytic cell line. J Biol Chem. 2017;292:9204–9217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Aguilar A, Pertuy F, Eckly A, Strassel C, Collin D, Gachet C, Lanza F, Leon C. Importance of environmental stiffness for megakaryocyte differentiation and proplatelet formation. Blood. 2016;128:2022–2032 [DOI] [PubMed] [Google Scholar]
- 140.Di Buduo CA, Wray LS, Tozzi L, Malara A, Chen Y, Ghezzi CE, Smoot D, Sfara C, Antonelli A, Spedden E, Bruni G, Staii C, De Marco L, Magnani M, Kaplan DL, Balduini A. Programmable 3d silk bone marrow niche for platelet generation ex vivo and modeling of megakaryopoiesis pathologies. Blood. 2015;125:2254–2264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Sullenbarger B, Bahng JH, Gruner R, Kotov N, Lasky LC. Prolonged continuous in vitro human platelet production using three-dimensional scaffolds. Exp Hematol. 2009;37:101–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Currao M, Malara A, Di Buduo CA, Abbonante V, Tozzi L, Balduini A. Hyaluronan based hydrogels provide an improved model to study megakaryocyte-matrix interactions. Exp Cell Res. 2016;346:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Levin J, Conley CL. Thrombocytosis associated with malignant disease. Arch Intern Med. 1964;114:497–500 [DOI] [PubMed] [Google Scholar]
- 144.Morowitz DA, Allen LW, Kirsner JB. Thrombocytosis in chronic inflammatory bowel disease. Ann Intern Med. 1968;68:1013–1021 [DOI] [PubMed] [Google Scholar]
- 145.Larkin CM, Santos-Martinez MJ, Ryan T, Radomski MW. Sepsis-associated thrombocytopenia. Thromb Res. 2016;141:11–16 [DOI] [PubMed] [Google Scholar]
- 146.Haas S, Hansson J, Klimmeck D, Loeffler D, Velten L, Uckelmann H, Wurzer S, Prendergast AM, Schnell A, Hexel K, Santarella-Mellwig R, Blaszkiewicz S, Kuck A, Geiger H, Milsom MD, Steinmetz LM, Schroeder T, Trumpp A, Krijgsveld J, Essers MA. Inflammation-induced emergency megakaryopoiesis driven by hematopoietic stem cell-like megakaryocyte progenitors. Cell Stem Cell. 2015;17:422–434 [DOI] [PubMed] [Google Scholar]
- 147.Orford KW, Scadden DT. Deconstructing stem cell self-renewal: Genetic insights into cell-cycle regulation. Nat Rev Genet. 2008;9:115–128 [DOI] [PubMed] [Google Scholar]
- 148.Lu YC, Sanada C, Xavier-Ferrucio J, Wang L, Zhang PX, Grimes HL, Venkatasubramanian M, Chetal K, Aronow B, Salomonis N, Krause DS. The molecular signature of megakaryocyte-erythroid progenitors reveals a role for the cell cycle in fate specification. Cell Rep. 2018;25:2083–2093e2084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Datta NS, Williams JL, Caldwell J, Curry AM, Ashcraft EK, Long MW. Novel alterations in cdk1/cyclin b1 kinase complex formation occur during the acquisition of a polyploid DNA content. Mol Biol Cell. 1996;7:209–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lordier L, Jalil A, Aurade F, Larbret F, Larghero J, Debili N, Vainchenker W, Chang Y. Megakaryocyte endomitosis is a failure of late cytokinesis related to defects in the contractile ring and rho/rock signaling. Blood. 2008;112:3164–3174 [DOI] [PubMed] [Google Scholar]
- 151.Trakala M, Partida D, Salazar-Roa M, Maroto M, Wachowicz P, de Carcer G, Malumbres M. Activation of the endomitotic spindle assembly checkpoint and thrombocytopenia in plk1-deficient mice. Blood. 2015;126:1707–1714 [DOI] [PubMed] [Google Scholar]
- 152.Trakala M, Rodriguez-Acebes S, Maroto M, Symonds CE, Santamaria D, Ortega S, Barbacid M, Mendez J, Malumbres M. Functional reprogramming of polyploidization in megakaryocytes. Dev Cell. 2015;32:155–167 [DOI] [PubMed] [Google Scholar]
- 153.Zhang Y, Wang Z, Ravid K. The cell cycle in polyploid megakaryocytes is associated with reduced activity of cyclin b1-dependent cdc2 kinase. J Biol Chem. 1996;271:4266–4272 [DOI] [PubMed] [Google Scholar]
- 154.Markovic B, Wu Z, Chesterman CN, Chong BH. Quantitation of soluble and membrane-bound fc gamma riia (cd32a) mrna in platelets and megakaryoblastic cell line (meg-01). Br J Haematol. 1995;91:37–42 [DOI] [PubMed] [Google Scholar]
- 155.Crist SA, Elzey BD, Ahmann MT, Ratliff TL. Early growth response-1 (egr-1) and nuclear factor of activated t cells (nfat) cooperate to mediate cd40l expression in megakaryocytes and platelets. J Biol Chem. 2013;288:33985–33996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zufferey A, Speck ER, Machlus KR, Aslam R, Guo L, McVey MJ, Kim M, Kapur R, Boilard E, Italiano JE Jr., Semple JW. Mature murine megakaryocytes present antigen-mhc class i molecules to t cells and transfer them to platelets. Blood Adv. 2017;1:1773–1785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Chapman LM, Aggrey AA, Field DJ, Srivastava K, Ture S, Yui K, Topham DJ, Baldwin WM 3rd, Morrell CN. Platelets present antigen in the context of mhc class i. J Immunol. 2012;189:916–923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Flaumenhaft R, Dilks JR, Richardson J, Alden E, Patel-Hett SR, Battinelli E, Klement GL, Sola-Visner M, Italiano JE Jr., Megakaryocyte-derived microparticles: Direct visualization and distinction from platelet-derived microparticles. Blood. 2009;113:1112–1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Boilard E, Nigrovic PA, Larabee K, Watts GF, Coblyn JS, Weinblatt ME, Massarotti EM, Remold-O’Donnell E, Farndale RW, Ware J, Lee DM. Platelets amplify inflammation in arthritis via collagen-dependent microparticle production. Science. 2010;327:580–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Linge P, Fortin PR, Lood C, Bengtsson AA, Boilard E. The non-haemostatic role of platelets in systemic lupus erythematosus. Nat Rev Rheumatol. 2018;14:195–213 [DOI] [PubMed] [Google Scholar]
- 161.Cunin P, Bouslama R, Machlus K, Martinez-Bonet M, Lee P, Nelson-Maney N, Morris A, Guo L, Weyrich A, Sola-Visner M, Boilard E, Italiano J, Nigrovic P. Megakaryocyte emperipolesis mediates membrane transfer from intracytoplasmic neutrophils to platelets. bioRxiv. 2018;10:504–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lefrancais E, Ortiz-Munoz G, Caudrillier A, Mallavia B, Liu F, Sayah DM, Thornton EE, Headley MB, David T, Coughlin SR, Krummel MF, Leavitt AD, Passegue E, Looney MR. The lung is a site of platelet biogenesis and a reservoir for haematopoietic progenitors. Nature. 2017;544:105–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.