Summary
Wrist actigraphy (ACT) may overestimate sleep and underestimate wake, and the agreement may be lower in people with chronic conditions who often have poor sleep and low activity levels. The purpose of this systematic review is to compare the agreement between ACT and polysomnographic (PSG) measures of sleep in adults without chronic conditions and sleep complaints (healthy) and with chronic conditions. We conducted a systematic review and metaanalysis using PRISMA guidelines. We searched PubMed, OVIDEMBASE, OVIDMEDLINE, OVIDPsycINFO, CENTRAL, CINAHL, ClinicalTrials.gov, International Clinical Trials Registry, and Open Grey. We included 96 studies with a total of 4,134 participants, of whom 762 (18.4) were healthy adults and 724 (17.5%) were adults with chronic conditions. Among adults with chronic conditions, ACT overestimated TST, compared to PSG [M = 22.42 minutes (CI 95%: 11.92, 32.91 minutes)] and SE [M = 5.21% (CI 95%: 1.41% to 9.00%)]. ACT underestimated SOL [M = −7.70 minutes (CI 95%: −15.22, −0.18 minutes)], and WASO [M = −10.90 minutes (CI 95%: −26.01, 4.22 minutes)]. These differences were consistently larger between ACT and PSG sleep measures compared to healthy adults. Research is needed to better understand factors that influence the agreement between ACT and PSG among people with chronic conditions.
Keywords: actigraphy, polysomnography, systematic review, meta-analysis, adults, chronic conditions, healthy adults, total sleep time, sleep onset latency, wake after sleep onset, sleep efficiency
INTRODUCTION
Researchers have used actigraphs, accelerometers that estimate sleep characteristics from motor activity, since the early 1980’s using computerized algorithms calibrated against polysomnography (PSG), the “gold standard” of sleep measurement. ACT, compared with PSG, is less intrusive and expensive, more ecologically valid, and more practical for continuous data collection over days or weeks (1,2). These advantages contribute to the feasibility of ACT to estimate sleep characteristics and evaluate the effects of sleep interventions in clinical and community settings (e.g., home, nursing home, acute care hospital) and across the spectrum of health and illness (1–3). Narrative reviews indicate various levels of agreement between ACT and PSG in single studies of several populations (1). The American Academy of Sleep Medicine published guidelines for the use of wrist actigraphy for the diagnoses of sleep disorders in 2007 (4) and updated the systematic review and clinical guidelines for the use of actigraphy in people with sleep disorders in 2018 (5,6). The authors concluded that ACT is useful in estimating total sleep time (TST) and sleep onset latency (SOL) among adults with insomnia compared to PSG (5). However, this review did not compare ACT and PSG in people with medical or psychiatric disorders. Despite the use of ACT in many studies of people with chronic conditions who also frequently have poor sleep, the extent to which ACT measurements are concordant with PSG measures in these groups is not known.
Unlike PSG that relies on electro-encephalography (EEG), electro-oculography (EOG), and electromyography (EMG) to quantify sleep characteristics, ACT estimates of sleep are derived from motor activity. ACT is generally a reliable and valid measure of sleep among people without chronic conditions and with good quality sleep, yielding good agreement rates with PSG for total sleep time (3). However, because ACT measures activity (7), it may overestimate sleep and under-estimate wake with lower levels of agreement for sleep outcomes that depend upon correct identification of wake, such as SOL, SE, and WASO (3). These measures may be biased by increased or decreased motor activity, the actigraph technology (e.g., microelectromechanical system [MEMs] vs. solid-state piezoelectric), software or settings used. For example, agreement may be lower among people who lie still while awake without electrophysiologic sleep, such as those with insomnia (8,9), and those whose daily activity may be limited due to illness, treatment (10,11) or other factors that may limit motor activity (e.g., cardiac or pulmonary conditions). Although ACT is widely used to measure sleep in a variety of clinical settings and among adults with chronic conditions, there have been notably few attempts to validate its use in these patients or to synthesize existing data to assess rates of agreement with PSG. Therefore, we conducted a systematic review of the literature to (1) evaluate the agreement between wrist actigraph measured sleep characteristics (TST, SE, SOL, WASO) and PSG measured sleep characteristics and (2) to compare the degree of agreement between studies of adults without chronic conditions and sleep complaints (healthy) and adults with chronic comorbid conditions.
METHODS
Review Design
We conducted a systematic review and meta-analysis using the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines (12).
Inclusion and Exclusion Criteria
We included both observational studies and randomized control trials if they reported sleep characteristics for both ACT and PSG at baseline. Prior to beginning this review, we searched PROSPERO for ongoing or recently completed systematic reviews.
Inclusion and exclusion criteria are shown in Supplementary Table 1. We limited the studies to those with participants who were ≥ 18 years of age because of developmental differences in sleep characteristics and sleep disturbance between adults and children (13). We included studies with participants expected to have normal sleep, as well as those with suspected sleep disturbance for a variety of reasons, such as sleep disorders, advanced age, menopause, comorbid medical conditions (e.g., cancer, cardiovascular disease, neurological disorders, diabetes, obesity), psychiatric conditions (e.g., depression, schizophrenia, bipolar disorder, substance abuse), disability (e.g., motor disabilities, total blindness), or work conditions (e.g., rotating or shift work or jet lag associated with travel across time zones).
We included only studies that used research accelerometers and excluded studies that reported only activity-tracking devices directly available to consumers because the software, technical specifications, and validity data are often proprietary and not publicly available. In addition, the U.S. Food and Drug Administration has not approved most of these devices for clinical sleep research and treatment (14). We also excluded studies that studied physiological phenomena during zero gravity conditions.
Literature Search
We searched multiple databases, including PubMed, OVIDEMBASE, OVIDMEDLINE, OVIDPsycINFO, CENTRAL, and CINAHL. A health sciences librarian with expertise in systematic reviews created the search strategies, conducted the searches on June 21, 2017, and then searched for updated results on March 14, 2019. The final OVIDMEDLINE search strategy is shown in Supplementary Table 2. We adapted the syntax and subject headings from this search for use in other databases. We also searched the International Clinical Trials Registry Platform Search Portal and ClinicalTrials.gov to identify ongoing or recently completed trials. We searched grey literature from the Grey Literature Report, OpenDOAR, and OpenGrey. As relevant studies were identified, reviewers checked for additional relevant cited articles.
Identification and Selection of Studies
We imported the records into Covidence, an electronic systematic review management system, to screen the reports based on the inclusion and exclusion criteria. Three independent reviewers screened all abstracts and, if necessary, discussed disagreements until consensus was reached. Full-texts were screened by two reviewers and disagreements were reviewed and solved by the third reviewer (Supplementary Table 1).
Data Evaluation
We evaluated the quality of studies for risk of bias with Quality Assessment in Diagnostic Accuracy Studies (QUADAS-2), a tool for assessment of diagnostic test accuracy (15). While not all of the included studies were diagnostic test accuracy studies, the four domains used for risk and applicability assessment in QUADAS-2 are relevant to our purpose: 1) patient selection, 2) index text (actigraphy), 3) reference standard (PSG/EEG), and 4) flow and timing (e.g., the extent to which all participants received the same reference standard, inclusion of all participants in the analyses, and the simultaneous recording of ACT and PSG).
Data Management and Analysis
One reviewer extracted the data into an Excel spreadsheet, and a second reviewer checked it for accuracy. Data extracted included study authors, year of publication, sample characteristics, study setting, PSG settings, actigraph device, scoring method, ACT and PSG means and standard deviations for TST, SOL, WASO, and SE, and epoch by epoch sensitivity, specificity, and accuracy. For the meta-analysis, we used Review Manager 5.3 from Cochrane Reviews (16) to create forest plots with random effects (due to the heterogeneous nature of the studies). We also computed the I2, a meta-analysis statistic that indicates the heterogeneity of the data, with values greater than 75% indicating substantial heterogeneity (17). Data were also reported in narrative form when insufficient for meta-analysis.
RESULTS
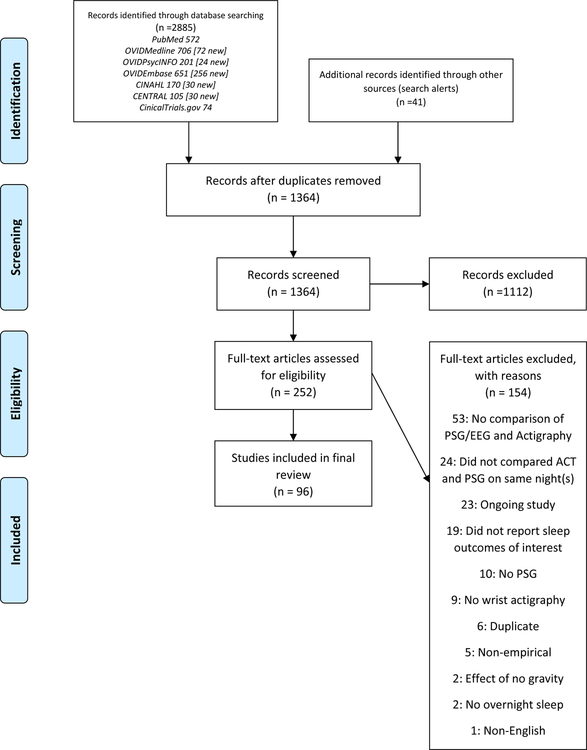
Our search resulted in 1364 records after the removal of duplicates. Screening of titles and abstracts resulted in the exclusion of 1112 records. We screened 252 full-text records and included 96 studies reported in 98 articles. See Figure 1 for a flow of the records through the screening process.
Figure 1.
PRISMA diagram: Flow of studies through inclusion and exclusion
Study Populations
A summary of each study sample description, mean or median age, and gender distribution is provided in Supplementary Table 3. There were 4,134 participants included in all the studies [age M = 43.4 (SD 16.7) years, 45.1% female]. The studies in this review included participants without chronic conditions or sleep disorders, those with sleep disorders, and others with a variety of chronic medical and mental health conditions including low back pain (18), dementia (10), bipolar disorder (19,34), schizophrenia (19), atopic dermatitis (20), depression (22,64), chronic fatigue (23), type 1 diabetes (25), heart failure (31), chronic obstructive pulmonary disease (33), Creutzfeldt-Jakob disease (39), Parkinson’s disease (43,48), Huntington’s disease gene carriers (45), depression and insomnia (47), and fibromyalgia (51) (see Table 1 for study descriptions of those with and without chronic conditions).
Table 1.
Characteristics of Studies comparing Actigraphy and Polysomnographic Measures of Sleep in Healthy Participants and with Chronic Conditions
| Authors, reference number | Sample | Age M (SD) | Gender N (%) | Setting | Actigraph Characteristics (type, scoring threshold) | |||
|---|---|---|---|---|---|---|---|---|
| Alsaadi et al. 2014 (18) | N = 50 Low back pain | 42.7 (15.15) | 24 (48%) female | 1-night research sleep lab | Actiwatch-2 (Phillips Respironics), medium threshold | |||
| Ancoli-Israel et al. 1997 (10) | N = 10 Nursing home, wheel chair bound, severe dementia | 86.4 (6.0) 74–96 years | (82%) female | 1-night in own bed in nursing home | Actillume recorder (Ambulatory Monitoring, Inc., Ardsley, NY) | |||
| Baandrup et al. 2015 (19) | N = 42 Schizophrenia and bipolar disorder | 86.4 (9.5) | 17 (40.5%) female | 1-night at home unattended | Actiwatch Spectrum (Philips Respironics, Murrysville, PA, USA), medium threshold | |||
| Bender et al. 2008 (20) | N = 20 Mild to moderate atopic dermatitis | 18/20 < 50 years | 13 (65%) female | 2-nights sleep lab | Actigraph Motionlogger (Ambulatory Monitoring Inc, Ardsley, NY) | |||
| Chakar et al. 2017 (21) | N = 38 Healthy | 23.5 (15) | Not reported | 1-night in sleep lab | Actiwatch-2 (Philips Respironics, Murrysville, PA, USA), default setting, | |||
| Cook et al. 2017 (22) | N = 21 Major depressive disorder | 26.5 (4.6) | 17 (90%) female | 1-night in lab | Actiwatch-2 | |||
| Creti et al. 2010 (23) | N = 49 Chronic fatigue | 42.78 (1173), range 16–73 | 41 (83.67%) female | 1-night in lab | Actitrac (IM Systems Co, Baltimore, MD, USA) | |||
| de Souza et al. 2003 (24) | N = 21 Healthy | Range: 18–33 years | 14 (66.67%) female | 1-night after adaption night in sleep lab | Basic 32 C Mini Motionlogger Actigraph - (Ambulatory Monitoring, Inc., Ardsley, USA), zero crossing mode | |||
| Farabi et al. 2017 (25) | N = 27 18 to 30-year old with Type 1 Diabetes | 23.8 (4.1) | 16 (59.26%) female | 1-night in sleep lab | Actiwatch-2 (Philips Respironics), medium threshold | |||
| Fonseca et al .2017 (26) | N = 35 Healthy | 52.0 (6.9) | 20 (57.1%) | 2-nights in hotel | Actiwatch Spectrum (Philips Respironics Inc., Murrysville, PA, USA), medium threshold | |||
| Fuller et al. 2017 (27) | N = 21 Male elite athletes | 22.5 (2.7) | 21 (100%) male | 4-nights in home | Actical® Z series activity monitors (Philips Respironics, Oregon, USA), medium threshold | |||
| Jean-Louis et al. 2001 (28) | N = 46 Healthy postmenopausal women and healthy young adults | 55.28 (6.98) Range: 19–77 |
42 (91.3%) female | 24-hours in home | The Actillume manufacturer (Ambulatory Monitoring, Ardsley, NY) | |||
| Jean-Louis et al. 2001a (29) | N = 5 Healthy young adults | 25 (6) | Not reported | 5-nights in Circadian Pacemaker Laboratory at UCSD | The Mini Motionlogger (Ambulatory Monitoring, Inc. Ardsley, NY), Zero-Crossing Mode | |||
| Jean-Louis et al. 1996 (30) | N = 20 Healthy | 29.95 (8.98) Range 21 to 53 years | 9 (45%) female | 1-night in lab | Gaehwiler Electronic (no. CH-8634; Hombrechtikon, Switzerland), analyzed using the Actigraph Data Analysis Software. | |||
| Jeon et al. 2019 (31) | N = 155 Heart failure | 60.5 (16.1) | 54 (34.8%) female | 1-night at home | Actiwatch-64 (Philips Respironics), medium threshold | |||
| Jumabhoy et al. 2017 (32) | N=22 Healthy | 29.3 (114) | Not reported | 1-night in laboratory | Actiwatch Spectrum Pro | |||
| Kapella et al. 2017 (33) | N=50 Chronic Obstructive Pulmonary Disease | 63.2 (8.4) | 15 (30.0%) | 1-night in lab | Actiwatch 2 | |||
| Kaplan et al. 2012 (34) | N = 54 Bipolar and healthy controls | 35.6 (11.65) | 69 (77.8%) female | 2-nights in lab | Actiwatch-64 (Mini Mitter, Philips Respironics Inc., Bend, OR, USA), medium threshold | |||
| Kogure et al. 2011 (35) | N = 6 Healthy participants | 32.2 (3.9) Range: 30–40 | 4 (66.6%) female | 2-nights in home | Micro-Mini Actigraph (Ambulatory Monitoring Inc.) Cole-Kripke algorithm | |||
| Kosmadopoulos et al .2014 (36) | N = 22 Young adults | 23.9 (3.8) | 4 (18.8%) female | 2-night in sleep lab | Actiwatch-64 (Mini-Mitter Philips Respironics, Bend, OR), medium threshold | |||
| Kripke et al. 1978 (37) | N = 5 Normal subjects | Not reported | Not reported | Number of nights not reported conducted at home | Researchers made own device | |||
| Kuo et al 2017 (38) | N=81 Healthy | 28.4 (5.8) | 34 (42.0%) female | 1-night in lab | Device developed by the authors | |||
| Landolt et al. 2006 (39) | N = 7 Creutzfeldt-Jakob disease | 64.2 (9.25) | 2 (28.6%) female | 1-night in lab | Actiwatch (Cambridge Technology, UK) | |||
| Latshang et al. 2016 (40) | N = 51 Healthy men | Median age 24 (quartiles 20–28 years) | 51 (100%) male | 1-night in lab or hostel | MSR2005; Electronics GmbH, Henggart, Switzerland | |||
| Leproult et al. 2015 (41) | N = 16 Healthy non-obese | Median 25 (IOR 23, 27.8) | 13 (81.3%) female | 1-night at home | Actiwatch-2 (Medys, Kappellen, Belgium) | |||
| Mack et al. 2009 (42) | N = 20 Healthy adults | 33.6 (12.6) | 10 (50%) females | 1-night in sleep lab | Not reported | |||
| Maglione et al. 2013 (43) | N = 61 Mild to moderate Parkinson’s disease | 67.74 (8.88) | 22 (36.7%) female | 1-night in sleep lab | Actiwatch-L (Philips Respironics Andover, Ma) | |||
| Mantua et al. 2016 (44) | N = 40 Healthy young adults | 22.37 (4.92) Range: 18–30 |
19 (47.5%) female | 1-night at home | Actiwatch Spectrum (Philips Respironics, Bend, OR, USA) | |||
| Maskevich et al. 2017 (45) | N = 7 Huntington’s Disease Gene Carriers (late presymptomatic or early disease) | 54.1 (6.4) | 6 (85.7%) female | 1-night at lab | Actiwatch Spectrum Pro | |||
| Markwald et al. 2016 (46) | N = 29 Healthy adults | 24.0 (5.3) | 8 (27.6%) females | 1-night in sleep lab | The Actiwatch-64 actigraph (Philips Respironics, Bend, OR, USA), medium threshold | |||
| McCall & McCall 2012 (47) | N = 60 People with depression and insomnia | 41.5 (12.5) | 40 (66.7) female | 1-night in lab | Actiwatch-64 (MiniMitter) medium threshold | |||
| Memon et al. 2017 (48) | N=25 Parkinson Disease | 63.6 (8.4) | 6 (24%) female | 1 to 3 nights | Micro Motionlogger Sleepwatch; Ambulatory Monitoring Inc. NY USA) | |||
| Mikkelsen et al. 2018 (49) | N=15 Healthy | 35.0 (14.3) | 9 (60%) female | 1-night in lab | Actiwatch MW8 | |||
| Montgomery-Downs et al. 2012 (50) | N = 24 Healthy adults | 26.1 Range: 19–41 | 9 (40%) female | 1-night in sleep lab | Actiwatch-64 (Philips Respironics), medium threshold | |||
| Mundt et al. 2016 (51) | N = 113 Insomnia and fibromyalgia | 52.68 (10.91) | 110 (97.3%) female | 1-night in home | Actiwatch-2 (Phillips Respironics, Bend, OR), high threshold | |||
| Nussbaumer-Ochsner et al. 2011 (52) | N = 16 Healthy mountaineers who had experienced high altitude pulmonary edema | Median 47 (quartile range 40 to 49) | 2 (12.5%) female | 1-night location not specified | Actigraph (MSR, Henggart, Switzerland), | |||
| Paquet et al 2007 (54) | N = 15 Healthy | 39.3 (15.1) Range: 20–60 | 8 (53.33%) female | 3-nights in lab | Actiwatch-L (Mini Mitter, Respironics Inc, Bend, OR), medium threshold | |||
| Peterson et al. 2012 (55) | N = 11 Healthy | Range: 24–55 | 7 (63.64%) female | 1-night in lab | Actiwatch Spectrum (Philips Home Health Care Solutions, Bend, OR, USA) | |||
| Pigeon et al. 2018 (56) | N = 27 Healthy | 28.4 (116) | 11 (40.7%) female | 1-night in lab | Actiwatch-2 | |||
| Pollak et al. 2001 (57) | N = 14 Healthy young adults and older adults | Range: 21–72 | 7 (50%) female | 7 days and nights Chronobiology Laboratory | CSA (Model 7164 Activity Monitor, Computer Science and Applications, Inc., Shalimar, Florida) and the IM (ActiTrac, IM Systems, Inc., Baltimore, Maryland), Simple threshold | |||
| Sargent et al. 2016 (59) | N = 16 Endurance cyclists | 19.3 (15) | 16 (100%) male | 9-nights in private bedroom at training camp | Actigraph not reported, medium threshold | |||
| Shambroom et al. 2016 (60) | N = 26 Healthy adults | 38 (13) Range 19 to 60 | 13 (50%) female | 2-nights in lab | Actiwatch-64 (Mini Mitter Philips Respironics, Bend, OR, USA), Medium threshold | |||
| Slater et al. 2015 (61) | N = 108 Health adults | 22.7 (0.2) | 51 (47.22%) female | 1-night in lab | GT3X+ activity monitors (Actigraph, FL, USA) | |||
| Tonetti et al. 2008 (62) | N = 12 Healthy | 22.97 (2.62) | 6 (50%) female | 7-nights in lab | Actiwatchw (AW; Cambridge Neurotechnology Ltd, Cambridge, UK; Mini Mitter, Respironics Inc., Bend, Oregon, USA), Medium threshold | |||
| Wichniak et al. 2018 (63) | N = 30 Healthy | 24.2 (4.9) | 15 (50%) female | 3-nights in laboratory | Motionwatch 8 (CamNTech) | |||
Sleep Study Settings
See Supplementary Table 3 for a summary of the study settings. Most of the sleep measurements occurred in a sleep laboratory (n = 61 studies); 18 studies took place in participants’ homes; 2 studies were conducted in an intensive care unit; and 2 in hotels. Five reports did not specify the setting. Five studies had mixed settings (i.e., home and laboratory, laboratory and hostel, and home and hospital). One study was conducted in a hospital ward, one in a nursing home, and one in a private room at a training camp. The majority were conducted on a single night [n = 67 (69.8%)].
Actigraph Types and Settings
Researchers used several types of actigraphs. The most commonly used included Actiwatch-2 (Phillips Respironics, Inc.) (n = 12 studies), Actiwatch-64 (Phillips Respironics, Inc.) (n = 12 studies), the Actiwatch Spectrum (Phillips Respironics) (n =7 studies), Actiwatch-L (Philips Respironics, Andover, MA) (n = 6), Motionlogger (Ambulatory Monitoring Inc., Ardsley, NY) (n = 4 studies), Actiwatch (Cambridge Neurotechnology) (n = 4 studies), and Actillume (Ambulatory Monitoring Inc, Ardsley, NY) (n = 4 studies). In 4 studies the researchers developed their own actigraphs. Ten studies did not report the type of actigraph used. Forty-one studies used 45 other actigraph models. See Supplementary Table 3 for a full list of the actigraphs and scoring thresholds used in each study.
Risk of Bias of Included Studies
Overall the risk of bias was low. The largest potential risk of bias was in the criteria for participant selection and was explained by limitations in reporting methods of recruiting and identifying participants. Twenty-eight studies had a low risk of bias in patient selection; 60 had unclear risk of bias; and 10 had high risk of bias. The next most common area for potential bias was flow and timing, for which 70 studies had low risk of bias; 17 had unclear risk for bias; and six had high risk of bias (Supplementary Table 4)
Total Sample
Sixty-four studies included mean and standard deviations for one or more of the sleep variables (TST, SOL, SE, or WASO) (See Supplementary Figure 1.1 to 1.4). These studies included adults without chronic conditions and sleep complaints and adults who had a variety of sleep and chronic conditions (e.g., insomnia, sleep disordered breathing, periodic limb movements, type 1 diabetes, depression). ACT overestimated TST by M = 17.88 minutes [(95% CI 7.90, 27.85) n = 3437 participants] and overestimated SE by M = 3.77% [(95% CI 1.87, 5.59) n = 2905 participants], compared to PSG. ACT underestimated SOL by M = −6.94 minutes [(95% CI – 9.63, - 4.24) n = 2534 participants] and underestimated WASO by M = −12.87 minutes [(CI 95% − 19.30, − 6.43) n = 2537 participants.]
Forty-nine studies reported sensitivity, specificity or accuracy using an epoch by epoch method (n = 1,582 participants). Sensitivity is the percentage of epochs scored as sleep by ACT compared to the epochs scored as sleep by PSG. Specificity is the percentage of epochs scored as wake compared to the epochs scored as wake by PSG. Accuracy is the percentage of epochs scores as sleep or wake compared to the epochs scored as sleep or wake by PSG (see Supplementary Table 4). The mean sensitivity for all studies was M = .83 (SD .120). The mean specificity was M = .51 (SD = .19), and mean accuracy was M =.82 (SD .07).
Sixty-four studies reported correlations (interclass correlations, Person’s correlations, or Spearman’s correlations) between ACT and PSG variables. The range of the correlations varied considerably for TST (.115 to .98), SOL (−07 to .90), SE (−.18 to .90) and WASO (.00 to .85). See Supplementary Table 3 for the studies that reported correlations.
Healthy Adults
Twenty-nine studies (n = 762; 18.4% of the sample) included healthy adults which was defined as adults without chronic conditions or sleep complaints and excluded people with medical, mental health conditions, and sleep complaints. The mean age was 28.0 (SD 8.0) years (range 18 to 77 years; 40.0% female). Compared to PSG, there was a non-statistically significant trend for ACT to overestimate TST [M = 11.23 minutes (CI 95%: −3.98, 26.44 minutes) n = 404]; and SE [M = 1.90% higher (CI 95%: −0.65%, 4.44%) n = 328 participants]; and underestimate WASO [M = −3.11 minutes lower (CI 95%: −10.88 minutes, 4.65 minutes) n = 297 participants] . ACT significantly underestimated SOL [M = −8.09 minutes (CI 95%: −12.46, −3.71 minutes) n = 419 participants]. See Supplementary Figures 2.1 to 2.4 for the forest plots for the data obtained from this group. These studies were very heterogeneous, as indicated by the I2.ranging from .80–87.
Eighteen studies that healthy adults reported sensitivity, specificity or accuracy. The sensitivity M = .89 (SD .216), n = 461. The specificity M = .53 (SD .20), n = 461); and the accuracy M = .88 (SD .04), n = 437. See Table 2 for sensitivity, specificity, and accuracy for healthy adults and chronic conditions.
Table 2.
Sensitivity, Specificity, and Accuracy Actigraphy and Polysomnographic Measures of Sleep in Healthy Participants and with Chronic Conditions
| Authors, reference number | Sample size | Sample Description | Sensitivity | Specificity | Accuracy |
|---|---|---|---|---|---|
| Healthy | |||||
| Chakar et al. 2017 (21) | 38 | Healthy | .96 | .48 | |
| De Sousa et al. 2003 (24) | 21 | Healthy | .991 .972 |
.341 .442 |
|
| Fonseca et al. 2017 (26) | 49 | Healthy | .455 | .971 | .918 |
| Jean-Louis et al. 2001 (28) | 31 | Healthy | .948 | .313 | .921 |
| Jean-Louis et al. 2001a (29) | 5 | Healthy | .987 | . 277 | .95 |
| Jumabhoy et al. 2017 (32) | 22 | Healthy | .942–.992 among the different devices | .188–.356 among the different devices | |
| Kogure et al. 2011 (35) | 6 | Healthy | .985 | .342 | .931 |
| Kosmadopoulos et al. 2014 (36) | 22 | Young adults | .958 | .377 | .877 |
| Kuo et al. 2017 (38) | 59 | Healthy | .952 | .713 | .921 |
| Markwald et al. 2016 (46) | 26 | Healthy | .967 | .370 | .893 |
| Montgomery-Downs et al. 2012 (50) | 24 | Healthy | .957 | .389 | |
| O’Hare et al. 2015 (53) | 20 | No sleep- disordered breathing or primary insomnia | .973 | .339 | .855 |
| Paquet et al. 2007(54) | 15 | Healthy | .953 | .543 | .907 |
| Pigeon et al. 2018 (56) | 20 | Healthy | .887 | ||
| Sargent et al. 2016(59) | 16 | Endurance cyclists | .875 | .771 | .809 |
| Shambroom et al. 2012 (60) | 26 | Healthy | .876 | ||
| Slater et al. 2015 (61) | 108 | Healthy | .897 | .456 | .841 |
| Webster et al. 1982 (68) | 14 | Healthy | .930 | ||
| Chronic Conditions | |||||
| Alsaadi et al. 2014 (18) | 33 | Low back pain | .90 | .67 | |
| Ancoli-Israel et al. 1997 (10) | 10 | Dementia | .87 | .90 | |
| Farabi et al 2017 (25) | 27 | 18 to 30-year old with Type 1 Diabetes | .81 | ||
| Maskevich et al. 2017 (45) | 7 | Huntington’s Disease Gene Carriers | .97 | .31 | .80 |
Sensitivity: percentage of epochs scored as sleep by actigraph
Specificity: percentage of epochs scored as wake by actigraph
Accuracy: percentage of epochs scored as sleep or wake
1: Cole & Kripke algorithm; 2: Sadeh’s algorithm
Twenty studies of healthy adults reported correlations between sleep variables measured with ACT compared with PSG (see Table 3 for correlations for healthy and chronic conditions samples). The correlations for the TST measures ranged from .19 to .98; SOL correlations varied from −.07 to .80, while the range of correlations for SE was −.18 to .89, and the range for WASO was −.69 to .82.
Table 3.
Correlations between Actigraphy and Polysomnographic Measures of Sleep in Healthy Participants and with Chronic Conditions
| Authors, reference number | Sample | TST | SOL | SE | WASO |
|---|---|---|---|---|---|
| Healthy | |||||
| Chakar et al. 2017 (21) | Healthy | .88** | .01** | .77* | |
| de Souza et al. 2003 (24) | Healthy | .69a .64b |
.39a .41b |
||
| Fuller et al. 2017 (27) | Male elite athletes | 83*** | .24** | .35** | .33** |
| Jean-Louis et al. 2001 (28) | Healthy | .98 | |||
| Jean-Louis et al. 2001a (29) | Healthy | .81 | .55 | ||
| Jean-Louis et al. 1996 (30) | Healthy | 97*** | |||
| Kaplan et al. 2012 (34) | Healthy | .91** | .41** | 51*** | .35** |
| Kogure et al. 2011 (35) | Healthy | 95*** | .69*** | .89*** | 82*** |
| Kosmadopoulos et al. 2014 (36) | Young adults | −. 69** | −.69** | ||
| Kripke et al. 1978 (37) | Normal subjects | .95** | |||
| Kuo et al. 2017 (38) | Healthy | .93 | .53 | .84 | .75 |
| Latshang et al. 2016 (40) | Healthy | .19 | .16 | .27* | |
| Leproult et al. 2015 (41) | Healthy | .95*** | |||
| Mantua et al. 2016 (44) | Healthy | 94** | .35** | ||
| Mikkelsen et al. 2019 (49) | Healthy | .88 | .79 | .52 | .57 |
| Nussbaumer-Ochsner et al. 2011 (52) | Healthy | 92** | .13* | .82 | |
| Sargent et al. 2016 (59) | Endurance cyclists | .251** | .306*** | .258** | |
| Shambroom et al. 2016 (60) | Healthy | .60 | −.07 | .36 | .21 |
| Slater et al. 2015 (61) | Healthy | .51 | .32 | .59 | .68 |
| Chronic Conditions | |||||
| Alsaadi et al. 2014 (18) | Low back pain | .80 | .33 | .55 | .52 |
| Ancoli-Israel et al. 1997 (10) | Dementia | 0.91*** | |||
| Baandrup et al. 2015 (19) | Schizophrenia and bipolar | .78 | .00 | .00 | .00 |
| Bender et al. 2008 (20) | Atopic dermatitis | .606** | .442* | ||
| Creti et al. 2010 (23) | Chronic fatigue | 792*** | −.036 | .673*** | .586*** |
| Farabi et al. 2017 (25) | Type 1 diabetes | .66 | .38 | .57 | .48 |
| Jeon et al. 2019 (31) | Heart failure | .62 | .13 | .34 | .28 |
| Kaplan et al. 2012 (34) | Bipolar | .92** | .33** | .49** | .59*** |
| Maglione et al 2013 (43) | Parkinson’s disease | .496*** | .383** | .400** | |
| Mundt et al. 2016 (56) | Insomnia and fibromyalgia | .60** | .08* | .46** | .49** |
Note. CID=chronic insomnia disorder, SDB=sleep-disordered breathing, SE=sleep efficiency, SOL=sleep onset latency, TST=total sleep time, WASO=wake after sleep onset
: Cole’s algorithm,
: Sadeh’s algorithm
p ≥. 05.
p < .05.
p < .001, if no * study did not report p-value
Chronic Conditions
Fourteen studies included a total of 724 (17.5%) participants [M age = 50.9 (SD 19.5), 62.6% female] who had chronic conditions, including low back pain (18), dementia (10), bipolar disorder (19,34), schizophrenia (19), atopic dermatitis (20), depression (22,64), chronic fatigue (23), type 1 diabetes (25), heart failure (31), chronic obstructive pulmonary disease (33), Creutzfeldt-Jakob disease (39), Parkinson’s disease (43,48), Huntington’s disease gene carriers (45), depression and insomnia (47), and fibromyalgia (51). Among these participants, ACT overestimated TST, compared to PSG [M = 22.42 minutes (CI 95%: 11.92, 32.91 minutes) n = 648 participants] and SE [M = 5.21% (CI 95%: 1.41% to 9.00%) n = 622 participants], while ACT underestimated SOL [M = −7.70 minutes (CI 95%: −15.22, −0.18 minutes) n = 484 participants], and these were statistically significant. There was a non-significant trend that suggested a difference in WASO [M = −10.90 minutes (CI 95%: −26.01, 4.22 minutes) n = 560 participants]. (See Supplementary Figure 3.1 to 3.4 for the forest plots for the studies that included participants with chronic conditions.) The reports of total sleep time were less heterogeneous (I2 = 34%), while reports of the other sleep characteristics were highly heterogeneous (I2 ranged from 83% to 90%).
Only four studies of people with chronic conditions reported sensitivity [M = .90 (SD .05], n = 50] and specificity [M = .67 (SD .15), n = 50] with epoch by epoch agreement of ACT and PSG. Only two studies reported accuracy (.80 and .81) ,(n = 34) (25,45) (Table 2). Ten studies that included participants with chronic conditions reported correlations between ACT and PSG variables for TST (.496 –.92), SOL (-.036–.38), SE (.00–67) and WASO (.00–.59) (Table 3).
DISCUSSION
This systematic review advances methodological research comparing ACT and PSG by comparing the level of agreement between ACT and PSG among people with chronic conditions. Our findings demonstrate that ACT consistently over-estimates sleep, as indicated by TST and SE, and under-estimates wake, as indicated by SOL and WASO among people with chronic conditions, compared to these measures in healthy adults. Although past studies suggest that ACT overestimates TST and SE and underestimates SOL and WASO in adults (5), these differences were much larger and statistically significant in people with chronic conditions. While the differences for participants with chronic conditions were generally within the range of clinically acceptable difference noted in the recent American Academy of Sleep Medicine (AASM) Review (5), the ranges from the AASM review were estimated for specific sleep disorders. The “clinical” importance of variations in sleep characteristics may vary depending on the sleep-related outcome of interest in chronic conditions (e.g., metabolic control, cardiovascular outcomes, sleep-related symptoms).
Chronic medical and psychiatric conditions are frequently associated with sleep disturbance (i.e., short or long sleep, fragmented sleep, altered sleep timing and perceptions of poor sleep/insomnia), although these characteristics may or may not represent primary sleep disorders. Causes of sleep disturbance among people with medical and psychiatric chronic conditions are multi-factorial and may include primary sleep disorders (e.g., insomnia, sleep-disordered breathing, or restless legs syndrome), as well as treatments (e.g., medications, devices), comorbidity, emotional distress (e.g., anxiety, depression), and the clinical environment, among others. Some medical or psychiatric conditions may also be associated with changes in motor activity that may influence activity counts detected by the actigraph and thereby contribute to agreement or discrepancy in obtained sleep measurements (31). For example, patients with cardiac or pulmonary conditions may have limited mobility, and people with depression may have psychomotor slowing. Jeon and colleagues (27) found smaller discrepancies between ACT and PSG among heart failure patients under the age of 60 than older patients, and smaller discrepancies were associated with more advanced age, higher levels of daytime motor activity and more severe insomnia and self-reported sleep disturbance in older but not younger participants. Aside from this study, few studies have considered the potential contributions of clinical and demographic characteristics or motor activity levels to ACT measures. It may be helpful to adapt electronic sleep scoring algorithms to address these factors. However, given the large number of populations and studies of adults with chronic conditions, this may be a costly and time-consuming process that could benefit from automated algorithms.
An important consideration when studying sleep in people with chronic conditions is the trajectory of the chronic conditions and related changes in treatment and environment that may influence sleep and may thereby also influence the agreement, disagreement, or accuracy of ACT compared to PSG. For example, sleep may be more fragmented during physiological exacerbations of chronic conditions than during more stable phases. Changes in motor activity secondary to changes in health may influence the consistency of the agreement between ACT and PSG over time, but these factors have seldom been evaluated. Studies that include only single nights of recording may also lead to greater bias associated with the first night effect due to the intrusive nature of PSG measurement (47). Given theseobservations and the proliferation of studies using actigraphs, the limited number of studies comparing ACT and PSG measures among people with chronic conditions is somewhat surprising. For example, ACT has been used extensively in oncology research (66), but we found no studies comparing ACT and PSG in oncology patients, and it is unclear that our findings can be generalized to groups of participants with varied conditions or treatments.
Heterogeneity of study results was very high as illustrated by the I2 statistic, which indicates a lack of lack of consistency across studies with respect to over- or under-estimation of sleep. There was no discernible trend with respect to population tested, device used, or data collection method employed that could explain this lack of consistency. However, these variations should be considered in future work.
Consistent with previous reviews, studies in this review generally suggest that ACT was very sensitive to sleep but was not as specific in determining wake. This characteristic may limit the usefulness of wrist actigraphy as a measure of sleep or napping during the day, and daytime napping may be more common among people with chronic disorders. However, we did address sleep during the day, and the small number of studies that reported sensitivity and specificity, especially among people with chronic conditions, is a limitation.
We found a wide range of correlations reported in both the healthy and chronic conditions subgroup. Correlations between sleep variables that were at or near zero suggest that the relationship between ACT and PSG may suggest that there are non-linear relationships between ACT and PSG, thus looking only at correlations in a validation study may be insufficient.
Strengths and Limitations
This study has several strengths, including the use of a standardized meta-analysis and systematic review approach; a systematic search strategy designed by a trained university research librarian, and the involvement of multiple reviewers. We were able to combine the results of many, but not all studies using quantitative methods. Limitations of the review include the possibility that we did not identify all relevant literature and restriction to English-language articles. Although studies reviewed were generally of high quality, the largest risk of bias was due to incomplete reporting of recruitment of the study sample and study sample characteristics.
Although this review was among the first to evaluate the use of ACT among people with chronic conditions, there was wide variability in the nature of the chronic medical and psychiatric conditions, the ages and settings of the study participants, and the types of actigraphs and scoring algorithms used. This variability likely influenced the finding of statistical heterogeneity. Future studies are needed for specific populations with chronic medical and psychiatric conditions to more fully evaluate the levels of agreement or disagreement between ACT and PSG.
Although consumer wearable devices are increasingly used to estimate sleep, our findings are limited to research actigraphs and are not generalizable to these devices that vary widely in physical properties and scoring methods (which are proprietary and may change without the user being alerted). Mantua et al. (2016) (44) conducted a validation study of 4 different consumer wearable devices and found that TST was valid in 3 of the devices. A recent review about consumer wearable devices for the measurement of sleep found that the majority of validation studies were conducted in healthy adults, and similar to our findings, consumer wearables tend to overestimate sleep duration and that they perform more poorly in those with sleep disorders. (67) However, validation research is limited in adults with chronic conditions. As more detailed information becomes available for adults with chronic conditions, these devices should be a focus of a future systematic review.
Conclusions
Based on the results of this study, the direction of the agreements or discrepancy between ACT and PSG is similar, but the magnitude of the difference is somewhat larger in adults with chronic conditions compared to healthy adults. Future research is needed to better understand these differences in more diverse chronic conditions and to address specific factors (e.g., demographic and clinical factors) that may impact these differences.
Supplementary Material
Practice Points.
ACT tends to overestimate total sleep time and sleep efficiency and to underestimate sleep onset latency and wake after sleep onset in adults;
These differences were consistently larger in people with chronic conditions;
The differences identified in this review were within the specified “clinically” acceptable range for adult sleep disorders identified in the recent American Academy of Sleep Medicine review.
Research Agenda.
There were limited studies comparing ACT and PSG in adults with chronic conditions;
Causes of the agreement or discordance between ACT and PSG in people with chronic conditions remain unknown and are an area for future research;
Age, chronic sleep disorders, comorbidity, symptoms, decreased motor activity, disability, and other concerns related to chronic conditions may bias ACT vs. PSG measures and should be considered in future studies.
Acknowledgments:
This research was funded by grants T32NR008346 PI Margaret Grey (Samantha Conley), T32AG019134 PI Thomas Gill (Brienne Miner) and P30AG021342 PI Thomas Gill (Brienne Miner), and R01NR016191 (Nancy S. Redeker)
Glossary:
- ACT
actigraphy
- PSG
polysomnography
- TST
total sleep time
- SOL
sleep onset latency
- SE
sleep efficiency
- WASO
wake after sleep onset
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: None
Contributor Information
Samantha Conley, Yale School of Nursing.
Andrea Knies, Yale School of Medicine.
Janene Batten, Yale University Reference Librarian.
Garrett Ash, Yale School of Nursing.
Brienne Miner, Yale School of Medicine.
Youri Hwang, Yale School of Nursing.
Sangchoon Jeon, Yale School of Nursing.
Nancy S. Redeker, Yale School of Nursing.
References
- (1).Martin JL, Hakim AD. Wrist actigraphy. Chest 2011. June;139(6):1514–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Sadeh A The role and validity of actigraphy in sleep medicine: an update. Sleep Med Rev 2011. August;15(4):259–267 [DOI] [PubMed] [Google Scholar]
- (3).Van de Water AT, Holmes A, Hurley DA. Objective measurements of sleep for non-laboratory settings as alternatives to polysomnography--a systematic review. J Sleep Res 2011. March;20(1 Pt 2):183–200. [DOI] [PubMed] [Google Scholar]
- (4).Morgenthaler T, Alessi C, Friedman L, Owens J, Kapur V, Boehlecke B, et al. Practice parameters for the use of actigraphy in the assessment of sleep and sleep disorders: an update for 2007. Sleep 2007. April;30(4):519–529. [DOI] [PubMed] [Google Scholar]
- (5).Smith MT, McCrae CS, Cheung J, Martin JL, Harrod CG, Heald JL, et al. Use of Actigraphy for the Evaluation of Sleep Disorders and Circadian Rhythm Sleep-Wake Disorders: An American Academy of Sleep Medicine Systematic Review, Meta-Analysis, and GRADE Assessment. J Clin Sleep Med 2018. July 15;14(7):1209–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Smith MT, McCrae CS, Cheung J, Martin JL, Harrod CG, Heald JL, et al. Use of Actigraphy for the Evaluation of Sleep Disorders and Circadian Rhythm Sleep-Wake Disorders: An American Academy of Sleep Medicine Clinical Practice Guideline. J Clin Sleep Med 2018. July 15;14(7):1231–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Tryon WW. Issues of validity in actigraphic sleep assessment. Sleep 2004. February 1;27(1):158–165 [DOI] [PubMed] [Google Scholar]
- (8).Lichstein KL, Stone KC, Donaldson J, Nau SD, Soeffing JP, Murray D, et al. Actigraphy validation with insomnia. Sleep 2006;29(2):232–9 [PubMed] [Google Scholar]
- (9).Taibi DM, Landis CA, Vitiello MV. Concordance of polysomnographic and actigraphic measurement of sleep and wake in older women with insomnia. Journal of Clinical Sleep Medicine 2013;9(3):217–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Ancoli-Israel S, Clopton P, Klauber MR, Fell R, Mason W. Use of wrist activity for monitoring sleep/wake in demented nursing-home patients. Sleep 1997;20(1):24–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Beecroft JM, Ward M, Younes M, Crombach S, Smith O, Hanly PJ. Sleep monitoring in the intensive care unit: comparison of nurse assessment, actigraphy and polysomnography. Intensive Care Med 2008;34(11):2076–83 [DOI] [PubMed] [Google Scholar]
- (12).Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 2009. October;62(10):1006–1012 [DOI] [PubMed] [Google Scholar]
- (13).Robinson PD, Waters K. Are children just small adults? The differences between paediatric and adult sleep medicine. Intern Med J 2008. September;38(9):719–731. [DOI] [PubMed] [Google Scholar]
- (14).Evenson KR, Goto MM, Furberg RD. Systematic review of the validity and reliability of consumer-wearable activity trackers. Int J Behav Nutr Phys Act 2015. December 18;12:159–015- 0314–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool fosr the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011. October 18;155(8):529–536. [DOI] [PubMed] [Google Scholar]
- (16).Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager. 2014;5.3. [Google Scholar]
- (17).Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003. September 6;327(7414):557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *(18).Alsaadi SM, McAuley JH, Hush JM, Bartlett DJ, McKeough ZM, Grunstein RR, et al. Assessing sleep disturbance in low back pain: the validity of portable instruments. PLoS ONE Electronic Resource] 2014;9(4):e95824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Baandrup L, Jennum PJ. A validation of wrist actigraphy against polysomnography in patients with schizophrenia or bipolar disorder. Neuropsychiatric Disease and Treatment Vol 11 2015, ArtID 2271–2277 2015;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Bender BG, Ballard R, Canono B, Murphy JR, Leung DYM. Disease severity, scratching, and sleep quality in patients with atopic dermatitis. J Am Acad Dermatol 2008;58(3):415–20. [DOI] [PubMed] [Google Scholar]
- (21).Chakar B, Senny F, Poirrier AL, Cambron L, Fanielle J, Poirrier R. Validation of midsagittal jaw movements to measure sleep in healthy adults by comparison with actigraphy and polysomnography. 2017;10(3):122–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *(22).Cook JD, Prairie ML, Plante DT. Utility of the Fitbit Flex to evaluate sleep in major depressive disorder: A comparison against polysomnography and wrist-worn actigraphy. J Affect Disord 2017;217:299–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *(23).Creti L, Libman E, Baltzan M, Rizzo D, Bailes S, Fichten CS. Impaired sleep in chronic fatigue syndrome: how is it best measured? Journal of Health Psychology 2010;15(4):596–607. [DOI] [PubMed] [Google Scholar]
- (24).de Souza L, Benedito-Silva A, Pires MLN, Poyares D, Tufik S, Calil HM. Further validation of actigraphy for sleep studies. Sleep 2003;26(1):81–5. [DOI] [PubMed] [Google Scholar]
- (25).Farabi SS., Quinn L, Carley DW. Validity of Actigraphy in Measurement of Sleep in Young Adults with Type 1 Diabetes. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Fonseca P, Weysen T, Goelema MS, Most EIS, Radha M, Lunsingh Scheurleer C, et al. Validation of Photoplethysmography-Based Sleep Staging Compared With Polysomnography in Healthy Middle-Aged Adults. 2017;40(7):01. [DOI] [PubMed] [Google Scholar]
- (27).Fuller KL, Juliff L, Gore CJ, Peiffer JJ, Halson SL. Software thresholds alter the bias of actigraphy for monitoring sleep in team-sport athletes. J Sci Med Sport 2017 [DOI] [PubMed] [Google Scholar]
- (28).Jean-Louis G, Kripke DF, Cole RJ, Assmus JD, Langer RD. Sleep detection with an accelerometer actigraph: comparisons with polysomnography. Physiol Behav 2001;72(1–2):21–8. [DOI] [PubMed] [Google Scholar]
- (29).Jean-Louis G, Kripke DF, Mason WJ, Elliott JA, Youngstedt SD. Sleep estimation from wrist movement quantified by different actigraphic modalities. J Neurosci Methods 2001;105(2):185–91 [DOI] [PubMed] [Google Scholar]
- (30).Jean-Louis G, von Gizycki H, Zizi F, Fookson J, Spielman A, Nunes J, et al. Determination of sleep and wakefulness with the actigraph data analysis software (ADAS). Sleep 1996;19(9):739–43. [PubMed] [Google Scholar]
- *(31).Jeon S, Conley S, Redeker NS. Discrepancy between wrist-actigraph and polysomnographic measures of sleep in patients with stable heart failure and a novel approach to evaluating discrepancy 2019;28 (2) (no pagination). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Jumabhoy R, Dao PD, Maskevich S, Stout JC, Drummond SP. Wrist-worn activity monitoring devices overestimate sleep duration and efficiency in healthy adults. 2017;40 (Supplement 1):A288–A289. [Google Scholar]
- (33).Kapella MC, Vispute S, Zhu B, Herdegen JJ. Actigraphy scoring for sleep outcome measures in chronic obstructive pulmonary disease. 2017;37:124–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *(34).Kaplan KA, Talbot LS, Gruber J, Harvey AG. Evaluating sleep in bipolar disorder: comparison between actigraphy, polysomnography, and sleep diary. Bipolar Disord 2012;14(8):870–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Kogure T, Shirakawa S, Shimokawa M, Hosokawa Y. Automatic sleep/wake scoring from body motion in bed: validation of a newly developed sensor placed under a mattress. Journal of Physiological Anthropology 2011;30(3):103–9. [DOI] [PubMed] [Google Scholar]
- (36).Kosmadopoulos A, Sargent C, Darwent D, Zhou X, Roach GD. Alternatives to polysomnography (PSG): a validation of wrist actigraphy and a partial-PSG system. Behavior Research Methods 2014;46(4):1032–41. [DOI] [PubMed] [Google Scholar]
- (37).Kripke DF, Mullaney DJ, Messin S, Wyborney VG. Wrist actigraphic measures of sleep and rhythms. Electroencephalogr Clin Neurophysiol 1978;44(5):674–676. [DOI] [PubMed] [Google Scholar]
- (38).Kuo C, Liu Y, Chang D, Young C, Shaw F, Liang S. Development and Evaluation of a Wearable Device for Sleep Quality Assessment. 2017;64(7):1547–1557. [DOI] [PubMed] [Google Scholar]
- (39).Landolt HP, Glatzel M, Blattler T, Achermann P, Roth C, Mathis J, et al. Sleep-wake disturbances in sporadic Creutzfeldt-Jakob disease. Neurology 2006;66(9):1418–24. [DOI] [PubMed] [Google Scholar]
- (40).Latshang TD, Mueller DJ, Lo Cascio CM, Stowhas AC, Stadelmann K, Tesler N, et al. Actigraphy of wrist and ankle for measuring sleep duration in altitude travelers. High Altitude Medicine and Biology 2016;17(3):194–202. [DOI] [PubMed] [Google Scholar]
- (41).Leproult R, Deliens G, Gilson M, Peigneux P. Beneficial impact of sleep extension on fasting insulin sensitivity in adults with habitual sleep restriction. Sleep 2015;38(5):707–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Mack DC, Patrie JT, Felder RA, Suratt PM, Alwan M. Sleep assessment using a passive ballistocardiography-based system: preliminary validation. Conference Proceedings: Annual International Conference of the IEEE Engineering in Medicine & Biology Society 2009;2009:4319–222. [DOI] [PubMed] [Google Scholar]
- (43).Maglione JE, Liu L, Neikrug AB, Poon T, Natarajan L, Calderon J, et al. Actigraphy for the assessment of sleep measures in Parkinson’s disease. Sleep 2013;36(8):1209–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Mantua J, Gravel N, Spencer RMC. Reliability of sleep measures from four personal health monitoring devices compared to research-based actigraphy and polysomnography. Sensors (Switzerland) 2016;16(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Maskevich S, Jumabhoy R, Dao PDM, Stout JC, Drummond SPA. Pilot Validation of Ambulatory Activity Monitors for Sleep Measurement in Huntington’s Disease Gene Carriers. 2017;6(3):249–253. [DOI] [PubMed] [Google Scholar]
- (46).Markwald RR, Bessman SC, Reini SA, Drummond SP. Performance of a Portable Sleep Monitoring Device in Individuals with High Versus Low Sleep Efficiency. Journal of Clinical Sleep Medicine 2016;12(1):95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *(47).McCall C, McCall WV. Objective vs. subjective measurements of sleep in depressed insomniacs: first night effect or reverse first night effect? Journal of Clinical Sleep Medicine 2012;8(1):59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *(48).Memon R, Memon A, Joop A, Pilkington J, Wood K, Amara A. Comparison of actigraphy with polysomnographic studies in patients with Parkinson’s disease. 2017;32. [Google Scholar]
- (49).Mikkelsen KB, Ebajemito JK, Bonmati-Carrion M, Santhi N, Revell VL, Atzori G, et al. Machine-learning-derived sleep-wake staging from around-the-ear electroencephalogram outperforms manual scoring and actigraphy. 2018;(no pagination). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Montgomery-Downs H, Insana SP, Bond JA. Movement toward a novel activity monitoring device. Sleep & Breathing 2012;16(3):913–7. [DOI] [PubMed] [Google Scholar]
- *(51).Mundt JM, Crew EC, Krietsch K, Roth AJ, Vatthauer K, Robinson ME, et al. Measuring treatment outcomes in comorbid insomnia and fibromyalgia: concordance of subjective and objective assessments. Journal of Clinical Sleep Medicine 2016;12(2):215–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Nussbaumer-Ochsner Y, Schuepfer N, Siebenmann C, Maggiorini M, Bloch KE. High altitude sleep disturbances monitored by actigraphy and polysomnography. High Alt Med Biol 2011;12(3):229–36. [DOI] [PubMed] [Google Scholar]
- (53).O’Hare E, Flanagan D, Penzel T, Garcia C, Frohberg D, Heneghan C. A comparison of radio-frequency biomotion sensors and actigraphy versus polysomnography for the assessment of sleep in normal subjects. Sleep & Breathing 2015;19(1):91–8 [DOI] [PubMed] [Google Scholar]
- (54).Paquet J, Kawinska A, Carrier J. Wake detection capacity of actigraphy during sleep. Sleep 2007;30(10):1362–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Peterson BT, Chiao P, Pickering E, Freeman J, Zammit GK, Ding Y, et al. Comparison of actigraphy and polysomnography to assess effects of zolpidem in a clinical research unit. Sleep Med 2012;13(4):419–24 [DOI] [PubMed] [Google Scholar]
- (56).Pigeon WR, Taylor M, Bui A, Oleynk C, Walsh P, Bishop TM. Validation of the sleep-wake scoring of a new wrist-worn sleep monitoring device. 2018;14(6):1057–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Pollak CP, Tryon WW, Nagaraja H, Dzwonczyk R. How accurately does wrist actigraphy identify the states of sleep and wakefulness? Sleep 2001;24(8):957–65. [DOI] [PubMed] [Google Scholar]
- (58).Sanchez-Ortuno M, Edinger JD, Means MK, Almirall D. Home is where sleep is: an ecological approach to test the validity of actigraphy for the assessment of insomnia. Journal of Clinical Sleep Medicine 2010;6(1):21–9. [PMC free article] [PubMed] [Google Scholar]
- (59).Sargent C, Lastella M, Halson SL, Roach GD. The validity of activity monitors for measuring sleep in elite athletes. J Sci Med Sport 2016;19(10):848–53. [DOI] [PubMed] [Google Scholar]
- (60).Shambroom JR, Fabregas SE, Johnstone J. Validation of an automated wireless system to monitor sleep in healthy adults. J Sleep Res 2012;21(2):221–30 [DOI] [PubMed] [Google Scholar]
- (61).Slater JA, Botsis T, Walsh J, King S, Straker LM, Eastwood PR. Assessing sleep using hip and wrist actigraphy. Sleep and Biological Rhythms 2015;13(2):172–180. [Google Scholar]
- (62).Tonetti L, Pasquini F, Fabbri M, Belluzzi M, Natale V. Comparison of two different actigraphs with polysomnography in healthy young subjects. Chronobiol Int 2008;25(1):145–53. [DOI] [PubMed] [Google Scholar]
- (63).Wichniak A, Wierzbicka A, Gaczkowska A, Rostek K, Jernajczyk W. Validation of the sleep assessment algorithm in the medical application Nightly and comparing it to polysomnography in 30 healthy individuals. 2018;27 (Supplement 1):384. [Google Scholar]
- (64).Jean-Louis G, Mendlowicz MV, Gillin JC, Rapaport MH, Kelsoe JR, Zizi F, et al. Sleep estimation from wrist activity in patients with major depression. Physiol Behav 2000;70(1–2):49–53. [DOI] [PubMed] [Google Scholar]
- (66).Madsen MT, Huang C, Gogenur I. Actigraphy for measurements of sleep in relation to oncological treatment of patients with cancer: a systematic review. Sleep Med Rev 2015. April;20:73–83 [DOI] [PubMed] [Google Scholar]
- (67).Baron KG, Duffecy J, Berendsen MA, Cheung Mason I, Lattie EG, Manalo NC. Feeling validated yet? A scoping review of the use of consumer-targeted wearable and mobile technology to measure and improve sleep. Sleep Med Rev 2018. August;40:151–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (68).Webster JB, Kripke DF, Messin S, Mullaney DJ, Wyborney G. An activity-based sleep monitor system for ambulatory use. Sleep 1982;5(4):389–399. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.