Abstract
Introduction:
Traditional histopathological allograft biopsy evaluation provides, within hours, diagnoses, prognostic information, and mechanistic insights into disease processes. However, proponents of an array of alternative monitoring platforms, broadly classified as “invasive” or “noninvasive” depending on whether allograft tissue is needed, question the value proposition of tissue histopathology. The authors explore the pros and cons of current analytical methods relative to the value of traditional and next generation histopathological evaluation of tissue biopsies.
Objectives:
Illustrate the continuing value of traditional histopathological tissue assessment and “next generation pathology (NGP)”, broadly defined as staining/labeling techniques coupled with digital imaging and automated image analysis.
Summary:
Noninvasive imaging and fluid (blood, urine) analyses promote low-risk, global organ assessment, and “molecular” data output, respectively; invasive alternatives promote objective, “mechanistic” insights by creating gene lists with variably increased/decreased expression compared to steady-state/baseline. Proponents of alternative approaches contrast their preferred methods with traditional histopathology that: 1) fail to cite the main value of traditional and NGP - retention of spatial and inferred temporal context available for innumerable objective analyses; and 2) belie an unfamiliarity with the impact of advances in imaging and software-guided analytics on emerging histopathology practices. Illustrative NGP examples demonstrate the value of multi-dimensional data that preserves tissue-based spatial and temporal contexts.
Conclusion:
We outline a path forward for clinical NGP implementation where “software-assisted sign-out” will enable pathologists to conduct objective analyses that can be incorporated into their final reports and improve patient care.
INTRODUCTION
Strengths of Traditional Histopathology and Modern Alternatives
Traditional histopathology strengths and value derive primarily from its visual nature and retention of spatial and inferred temporal context at a submicron resolution level. Up to 80% of environmental stimuli are processed via visual pathways1 and ~30%–50% of the cerebral cortex is devoted to processing visual signals.2 When pathologists view organs at submicron resolution levels, hardwired visual dominance pathways immediately integrate inputs into legacy networks of pathophysiological insights. Pathologists can rapidly, reliably, and accurately: 1) identify rare (eg, isolated intimal arteritis, viral inclusions) and/or focal (eg, scars, lymphoid nodules, etc) events; 2) appreciate social relationships among cells (eg, lymphocytic tubulitis, capillaritis); 3) “age” tissue alterations into acute (hours to days), subacute (days to weeks), and chronic (months to years); and 4) recognize and rank order multiple insults.
Rapid conceptualization by humans/pathologists, comes at the expense of more precise quantitative abilities3 leading to important criticisms of histopathology: conventional tissue staining combined with human-only visual analysis is semiquantitative, thus, somewhat subjective, non-mechanistic and poorly reproducible.4–8 Humans are intrinsically built for estimation and not precise counting.3 Other shortcomings of traditional histopathology may include: sampling errors, biopsies are invasive, risky, and an unpleasant patient experience; and granular morphological or contextual information might not be required in some circumstances.
Considerations regarding the value of liver, kidney, or heart allograft biopsy acquisition for histopathological examination are evolving. The English Oxford Dictionary defines “value” as “the regard that something is held to deserve; the importance, worth, or usefulness of something.”9 Under noncomplex clinical circumstances (eg, slowly evolving presumed single insult), the value of traditional histopathological biopsy analysis is questioned allowing newer noninvasive imaging tools and high throughput tissue, blood, and bodily fluid -omics platforms combined with software-assisted analytics to enter the diagnostic tool kit.
Advocates of newer noninvasive, imaging (reviewed in references 10-14) and fluid (eg, urine or blood6,15–19) analytical techniques promote safety and global organ assessment, mitigating biopsy shortcomings of riskiness, sampling errors, and undesirability of use for serial measurements.5,8,10,13,20,21 However, noninvasive radiographic approaches: 1) produce low spatial resolution output; 2) often rely on indirect measurements (eg, liver stiffness as a surrogate of fibrosis) interfered with by non-fibrosis-related processes (eg, intraorgan congestion, inflammation, and patient obesity); and/or 3) introduce operator-dependent measurement variations (reviewed in references 10-14). Blood and fluid analyses provide no spatial context regarding intra-allograft events and only indirectly monitor allografts.
Advocates of high-throughput alternative tissue methodologies (eg, -omics platforms) promote value over traditional histopathology with respect to objectivity, data output, and “mechanistic” insights. Again, valuable spatial and inferred temporal contexts are destroyed while risks, patient avoidances, and sampling issues are the same as traditional biopsy analyses. Moreover, these methods use traditional histopathology for validation and often yield a narrow range of simplified single-insult diagnoses,4,7,17,18,22–25 that may overlap (eg, BK nephropathy and TCMR).7,17,23–25
Complex data outputs are visually displayed for human consumption (eg, scatter plots, heatmaps, volcano plots) or reported as “gene lists” without rank ordering insults when more than one insult is present. Gene lists reported can vary by organ, circumstance, or lab.17 Sample preparation and storage, interlaboratory assay reproducibility, and interpretive expertise distribution needs standardization, resulting in calls for centralization.7,26 Large transplant centers will evaluate the cost-benefit ratio of waiting several days (mRNA expression arrays) vs. the relatively short (hours) turnaround time of biopsy evaluation and the ability to confidently act on results for patient management.
Nevertheless, “gene lists” derived from -omics platforms represent an advancement and provide important details that can be layered on legacy histopathology mechanistic insights.27–41 For example: 1) transcripts during TCMR show increased leukocyte populations (eg, T, B, and NK cells, macrophages) and monocyte-macrophage-derived chemokines17,23,33,42; 2) endothelial cell mRNA profiles are perturbed in AMR25,28,31,42–44; and 3) chronic inflammation, mast cell, and immunoglobulin transcripts increase over time in kidney allografts.7,45 How effectively non-histopathology-based platforms will adapt to clinical care complexities remains forthcoming. Will “grind and bind” -omics platforms be able to: 1) rank multiple insults, 2) recognize focal defects and rare events, 3) adapt to variations in tissue age and sex, 4) time post-transplant, 5) inform drug regimens, 6) provide location data regarding donor MHC antigen expression and 7) provide spatial interactions among various cell types?
Interstitial inflammation scoring in kidney allografts encompasses peculiarities not encountered with liver allografts. If renal interstitial inflammation is located in areas of scarring or occupies <10% of non-scarred renal cortex, a Banff “i” (inflammation) score of “i0” (no inflammation) is registered.46 Any liver inflammation is registered as abnormal. “Non-scored” (i0) kidney lymphocytic infiltrates are undoubtedly contributing to the mRNA profile, which questions the value of correlations between kidney histopathology and mRNA expression profiles.
Roles of Traditional Histopathology
Traditional histopathological liver biopsy evaluation (within hours) provides47,48: 1) diagnosis(es); 2) pathophysiological processes involved in tissue injury (eg, TCMR, AMR, vascular issues); 3) a rank ordering of multiple simultaneous insults; 4) structural/architectural integrity assessment, including potentially irreversible damage; 5) guidance for therapeutic intervention; and 6) potentially excess tissue for translational research purposes. These traditional roles are based on hundreds of thousands (if not millions) of assays, validated over decades, and are widely available because of distributed expertise, and used as endpoints in clinical trials,49–53 and included in USFDA guidelines.54
Ranking multiple insults and recognizing mechanisms of tissue injury are increasingly important as noninvasive radiology tests decrease liver biopsy numbers conducted in noncomplex settings where one insult is imagined. For example, various radiographical approaches10–14 might be adequate for serial monitoring of recurrent or de novo nonalcoholic fatty liver disease in an otherwise stable liver allograft recipient with negative DSA and autoimmune and viral hepatitis serologies. Patients with complex simultaneous insults (eg, suboptimal biliary tract drainage coexistent and TCMR with central perivenulitis) will likely be missed using radiographic monitoring alone and treatment (eg, stent placement versus augmented IS) greatly differs.
To maximize the value proposition of traditional histopathology, pathologists need to comprehensively communicate results and diagnoses for incorporation into patient management algorithms51,55 (see Appendix S1). This approach is superior to issuance of “descriptive” reports. Granular templated, Banff-based, semiquantitative scoring of microscopic findings51,55 (eg, portal inflammation and portal, subsinusoidal, or perivenular fibrosis scored: none: 0; minimal: 1; mild: 2; moderate: 3; and severe) standardizes EMR databases (Figure 1). Templated scoring facilitates translational research and enables “digital” queries of archival material, which can be melded with other data (eg, demographics, serology, mRNA expression arrays, urine proteomics) and subjected to analyses.56–61 Scoring standardization via digital WSI archives facilitates proficiency training and consensus criteria development.62 This is an important step toward NGP implementation when objective software-assisted scoring will replace semiquantitative scoring and provide data for DIA algorithm validation. Subsequent NGP “hybrid” approaches will combine human conceptualization skills and “savant-like” DIA capabilites3,63 to produce “software-assisted sign-out”.
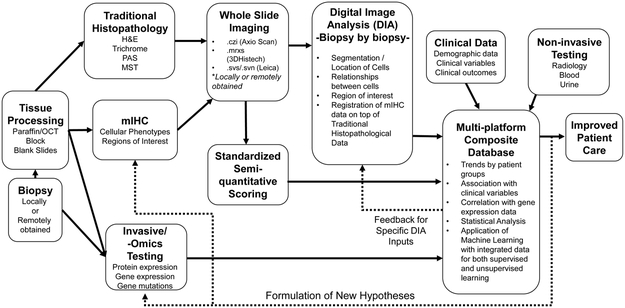
Figure 1.
Overall workflow and NGP integration with other data sources. Locally- or remotely obtained tissue biopsies can be triaged for traditional and NGP and tissue shared with other “invasive” non-histopathology platforms either before or after FFPE processing. WSI can be created at the core laboratory or electronically transferred from remote sites thus saving repeat traditional histochemical staining and preserving tissue for other NGP assays. NGP-generated data can then be integrated with clinical and serological data, noninvasive imaging and fluid -omics and invasive tissue-based -omics platforms for analysis. Dashed lines indicate iterative processes where feedback from a later stage is incorporated to optimize a result.
Continued Value of Traditional Histopathology
Reasons repeatedly cited by advocates for indication and protocol liver allograft biopsies64,65 and native liver biopsies in the context of hepatocellular carcinoma,66 NASH,52,67 chronic viral,68 and autoimmune hepatitis69 include: 1) detecting subclinical pathology56,57,65,70–72 (reviewed in references 60, 64, 73, and 74); 2) discerning and analyzing pathological events at a micron resolution scale guiding pathophysiological insights52,55,67; 3) sampling organ-restricted events (inflammation, swelling, etc); 4) serving as an endpoint in management algorithms and clinical trials49–53; 5) providing detailed meaningful results within hours; and 6) providing tissue samples for other platforms.56,57,65,68,70–72 Despite protestations that histopathology is “non-mechanistic,” it discovers, documents, and provides initial pathophysiological insights into many disease processes.28,36–38,41,56–58,75–78
Recently recognized examples where liver allograft biopsy’s value helped advance the field includes recognition and monitoring of: 1) acute58,59,77,79 and indolent chronic antibody-mediated injury57,80–83; 2) processes facilitating IS minimization49,60,61,84,85 (similar to those used to monitor AIH)69,86; and 3) regenerative medicine attempts to create and/or rehabilitate donor organs (reviewed in reference 87). Liver allograft recipient management complacency based on relatively normal liver injury tests in long-term survivors ignored decades-long concerning histopathological data of subclinical inflammation and indolently progressive fibrosis (reviewed in references 55, 73, 74, and 82-84). Serum DSA monitoring combined with traditional histopathological analysis showed a robust association among inflammation, fibrosis, and DSA positivity.56,57,83 Uninflamed biopsies more often are indicative of successful IS minimization.49,55,61,72,84,85
Introduction to Next Generation Pathology
Alternative monitoring approaches should be measured against NGP, which can be broadly defined as the use of multiplex tissue labeling techniques coupled with digital imaging and viewing, automated DIA, and creation and utilization of archival systems (reviewed in references 88-92). NGP enhances the value equation for “histopathology” and can be aggregated with data from other monitoring platforms (Figure 1). Tissue staining can range from standard histochemical stains (eg, H&E, trichrome) to mIHC labeling of multiple (usually two to six) proteins, mRNA, and/or DNA analytes/targets. A “panel” refers to a complete set of stains on one slide, which can be analyzed for spatial relationships among analytes.
NGP increases the value equation for biopsy evaluation by: 1) replacing semiquantitative scoring with objective quantitative data extraction; 2) creating digital archives to feed ML platforms (see below); and 3) leveraging hypothesis-driven translational research results to develop robust, clinically-applicable panels for clinical utilization by a preexisting network of expert pathologists who are familiar with automated immunostaining, standardization, and quality control platforms (eg, CLIA, CAP).
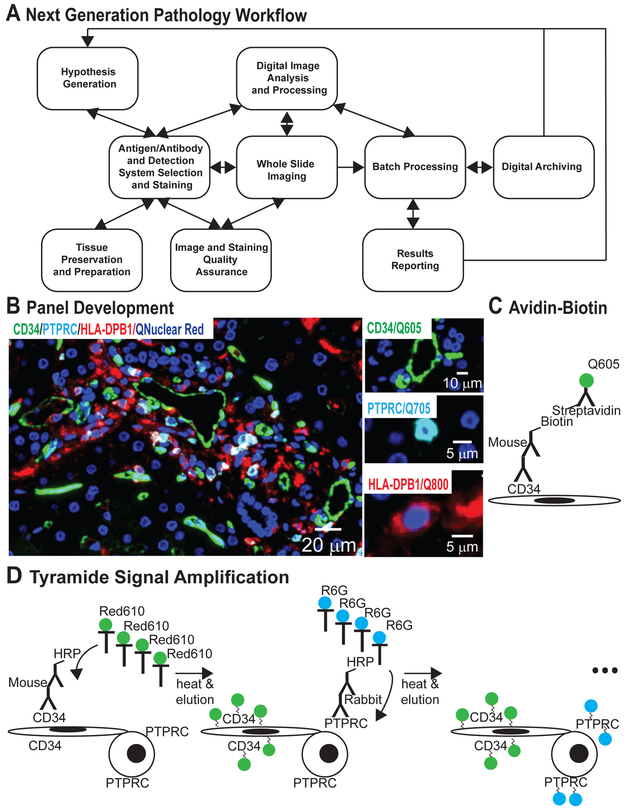
NGP follows the general workflow (Figure 2, Table S1): 1) hypotheses formulation and antigen/primary antibody selection; 2) tissue preparation; 3) slide staining; 4) digital slide scanning; 5) image/stain quality assurance; 6) DIA and biostatistical summary; and 7) digital archiving.
Figure 2.
Next generation pathology workflow and initial panel development. (A) NGP requires hypothesis generation, potential antigenic targets, available antibodies, and preferred detection systems. Tissue preservation and preparation should facilitate staining. Analytics occur simultaneously and iteratively with panel development, scanning optimization, and QA; each step providing feedback to others. Samples are then batch processed using the same protocol/metrics for each sample without human intervention culminating in objective data reporting and archiving. (B) The CD34/PTPRC/HLA-DPB1 multiplex IHC panel: CD34 (green) labels elongated endothelial cells lining the portal microvasculature. PTPRChigh (cyan) mostly labels lymphocytes245 resulting in circular, cytoplasmic “donut”. HLA-DPB1 (red) can appear on any antigen presenting cell (ie, oddly shaped macrophages, round PTPRC positive B cells, and dendritic and endothelial cells). The larger image shows an overlay of all labeled antigens with nuclei shown in blue. See Table S1 for sample workflow details. (C) Avidin-biotin amplification systems are used for sequential detection of proteins via primary antibodies from the same host species (eg, detection of CD34). (D) Tyramide signal amplification systems are used for sequential detection of primary antibodies from the same or different host species. The catalyst horseradish peroxidase activates tyramide-conjugated fluorophores causing them to covalently bind to protein tyrosine residues near the target antigen. Covalent binding enables heat deactivation, denaturation, and elution of primary and secondary antibodies leaving the fluorophore covalently bound to the target antigen and facilitating subsequent staining steps.
Biopsy Procurement and Tissue Preservation
Biopsy value is only as good as the tissue obtained; best practices will ensure that biopsy value can be maximized. At most institutions, interventional radiologists conduct biopsies under ultrasound guidance using biopsy guns. After local anesthesia, the needles are advanced to the organ surface then Tru-Cut needles are triggered to automatically obtain a tissue core (recommended 16-gauge) of a specified depth/chamber depth (22 mm).
When our institution drifted from AASLD standard practice guidelines47 of two 2.0 cm 16-gauge (16G) needle cores toward one 1.0–1.5 cm 18-gauge (18G) core the rate inadequate 18G core biopsies increased (approaching 30%), which in turn, prompted a formal study. Thinner gauge needles were originally intended to sample liver malignancies where a greater risk of bleeding exists.93–95 Our prospective randomized study (n: 150), comparing 16G and 18G cores96 showed: 1) no difference in pain or complications rates; 2) shorter 16G core lengths (1.7 cm vs 1.9 cm; P <0.05); and 3) more portal tracts in 16G cores (14 vs. 13; p=0.1). Of 18G biopsies, 29% were inadequate compared to 19% of 16G biopsies (p=0.17), despite 16G cores being significantly shorter.97 Two recent larger studies (n: 427; n: 194)98,99 showed significantly higher inadequacy rates with 18G needles, but Iqbal et al98 showed higher pain rates with 16G needles. Myers et al (n: 4275; gauge unreported) showed that 0.75% of patients experienced significant complications: 0.51% pain requiring hospital admission, 0.35% bleeding; and deaths (0.14%) occurred exclusively in cancer patients.100 Similar results were reported in three other studies,94,101,95 where terminal complications were restricted to cirrhotic and/or cancer patients.94,101
Needle gauge significantly affects tissue volume. Nicholson et al102 compared 16G (0.7 mm) versus 18G (0.5 mm) kidney tissue core diameters. For a 2.0 cm-long biopsy, the cylinder volume formula (πr2h) shows that 16G cores yield double the tissue volume. FFPE tissue mRNA expression profiling can require up to three consecutive 20 µm curls103 compared to a single 2–3 μm section for panel staining (see Appendix S2). To maximize approaches available for tissue monitoring, 16G cores should be the standard, and two cores are preferable. Quality metrics for all biopsy-obtaining specialists should address the consequences of: 1) inadequate biopsies; 2) institutional review board approval for a second core; and 3) restricted ability to conduct studies on excess tissue.
Tissue preservation CAP guidelines exist to ensure maintenance of target antigenicity for IHC-based clinical assays104 (eg, HER2,105,106 ER and PgR107). Immediate tissue snap freezing is ideal for antigen preservation but is generally outside of routine clinical workflow and improper cryopreservation leads to inferior morphology preservation for DIA (see Appendix S3). Clinical standard FFPE tissue preparation results in less quality variation and preserves morphology for DIA. Since formalin preparations (10% NBF vs. 4% paraformaldehyde) can produce analytical variability on an antigen-specific basis, a standard should be chosen.108,109 Room temperature fixation in 10–20x tissue volume of 10% NBF for one hour/mm tissue thickness (generally 6 to 48 hours) is standard for most antigens.110 Preservation-sensitive antigens (ex. p-AKT, p-ERK1/2) require immediate fixation; slicing large specimens facilitates proper fixative penetration111,112 (See Appendix S2). While no official CAP guidance is available regarding specimen fixation for “molecular pathology” assays, guidelines exist for assay validation from a variety of inputs (ie, unfixed vs. fixed vs. FFPE).113,114
Formulation of Hypotheses and Antigen/Primary Antibody Selection
Hypothesis testing often leads to innumerable marker queries and panel combinations that can be simplified into key processes. Examples include characterization of immune infiltrates (eg, T, B, and NK cells, and monocyte/macrophage panels)60,115; relationships of cell types to tissue structures (eg, leukocytes to bile ducts or hepatocytes or capillaries via TBX21/GZMB/CK19/CAM5.2 or PTPRC/CD34/HLA-DPB1, respectively)115–121; spatial relationships among immune infiltrates (virtual immunological synapses60); cancer signaling pathways118,120,121; stereotypic responses to injury/stress (eg, endothelial and myofibroblast proliferation/activation via CD34/ACTA2/MKI67); and tolerogenic vs proinflammatory liver APCs (eg, IRF4/IRF8/LILRB2/HLA-DPB1/ITGAX). Cancer studies primarily focus on the tumor immune microenvironment – a mirror image of transplant immunology122–126. Since perturbed genes “travel in herds7,45“ and are network-linked,127 hypothesis-driven or -omics-derived “gene lists” can inform more selective, and potentially more enlightening, NGP targets for study because it enables analyses of spatial relationships. New technologies combine spatial resolving capabilities of digital imaging with tissue-targeted mRNA expression analysis.128
Primary antibody or ISH probe selection is key to panel development success. Ideally, primary antibody candidates should have a robust publication use record in human tissues, be easily implemented clinically, and show compatible antigen retrieval characteristics with panel coanalytes. Novel antibodies may not meet this standard, but time is wasted trying to facilitate “poor-performing” antibodies are compounded during attempted panel inclusion. In our experience, ~70% of antibodies show robust FFPE tissue staining; the Human Protein Atlas is a generally reliable reference.129–131 RTU or IVD antibodies should be considered as the starting point of clinically-targeted panel development92 (See Table S1).
Multiplex Staining Approaches
Traditional mIHC approaches include the following (or combinations thereof): (1) simultaneous protein detection via primary antibodies from different host species; (2) sequential protein detection via primary antibodies from the same host species with interspersed blocking steps (Figure 2C); (3) sequential detection of proteins via primary antibodies from the same/different host species with antibody denaturation/elution between detection steps (Figure 2D). Targets can be localized with chromogens (eg, 3,3’-diaminobenzidine [DAB], 3-amino-9-ethylcarbazole [AEC], and alkaline phosphatase substrates132 and/or fluorescent probes [ie, organic fluorophores, Opal Dyes, and Qdots]).
Antigen retrieval is usually required for FFPE tissues and impacts panel co-analyte selection because of potential incompatibilities (reviewed in references 133 and 134). Amplification systems (eg, biotin-avidin, tyramide, hapten-based, rolling circle, polymer-based, silver89,135–137) help to detect low expressing antigens or deterioration-sensitive targets. Newer methodologies, like multiplex ion beam imaging,138,139 can detect ~100 analytes in FFPE tissues with: (1) improved signal-to-noise ratio and assay linearity; (2) elimination of spectral overlap; (3) analyte signal stability; (4) stable analyte registry; and (5) improved resolution compared to other mass-based reporter systems.138 CODEX utilizes oligonucleotide-labelled antibodies, iteratively queried by labelled dNTPs, and visualized by standard fluorescent microscopy.140 CODEX revealed 66 targets in frozen tissue sections and touts lack of antigen degradation during repeated deactivation.140 Although new techniques circumvent the need for primary antibodies from unique species, we find it helpful (see Appendix S4). Protocols should be validated in a variety of tissue-specific specimens (ie, test stain targeted tissue; see Appendixes S5–S7).
Implementing mIHC fluorescence panels requires adjustments for possible spectral overlap and/or tissue autofluorescence (see Appendixes S5–S7). Complex mIHC panels may require splitting across several thin serial sections and digitally aligning serial sections. Algorithms can align WSI at regional (eg, portal tracts) and cell-based levels.141 The latter requires more consideration, validation, and manual input to override error. Lopez et al achieved an automated registration accuracy of <50 μm142 advocating thinner (3–4 μm) section use (see Appendix S2).
Our initial mIHC panel development using Qdots5,20 was based on indirect detection of primary antibodies using biotinylated secondary antibodies and streptavidin-linked Qdots (Figure 2, B and C; reviewed in reference 143). Initial Qdot advantages included: brightness, photostability, and a common excitation wavelength (acquisition speed facilitated by efficient metal halide excitation sources). Qdot shortcomings became apparent over time, including: lot number staining variation; signal quenching by a variety of commonly used IHC reagents; and background staining (especially in liver, see Appendix S7), which led to unreliable staining for some panels.144,145 The availability of enhanced camera sensitivity led to a transition to fluorophore-conjugated TSA reagents (Figure 2D, for caveats see Appendix S8).122,123,146,147 Heat deactivation and elution remove primary and secondary antibodies from the antigen binding site (to below detection levels) at each staining step. Fluorophores remain covalently bound to tyrosine residues near the antigen site via peroxidase activation.123,136,148
Automation of Multiplex Immunohistochemistry
Automation is essential to mIHC clinical implementation because it reduces human variation and errors while producing more predictable results and shorter turnaround times: week versus day(s) and is available in most clinical laboratories. We have experience with two platforms – LabVision Autostainer 360–2D (ThermoScientific) and Discovery ULTRA (Ventana Medical Systems/Roche). The LabVision is suited to avidin-biotin based detection systems but is limited compared to newer platforms (eg, Discovery ULTRA, Leica Bond III) by inabilities to: 1) deparaffinize; 2) enable individual protocolized slide heating for antigen retrieval or incubations (as needed for TSA and polymer-based detection reagents and ISH), which may shorten primary antibody incubation time; 3) prevent evaporation during lengthy incubations (>2–3 hours); and 4); enable more than one staining protocol per run. Newer platforms enable completion of mIHC four to five analyte panels in “overnight runs (8–15 hours)”. “Open” platforms allow investigators to customize their reagents in conjunction with vendor-specific reagents.
WSI Considerations
Clinical NGP imaging considerations include: 1) overall system costs (ie, money and time)149; 2) workflow integration; 3) WSI resolution; 4) scan time for transmitted light microscopy applications; 5) fluorescence scanning capabilities; 6) digital storage space; 7) trained personnel; 8) LIMS integration; 9) telepathology; and 10) regulatory hurdles.150–152 Comprehensive reviews cover selection, implementation, and practical use of digital pathology systems in clinical practice or research settings.153–155 Industry advocate groups (eg, CAP, Royal College of Pathologists) and software workflow improvements drive standardization and validation guidelines with respect to device and viewing platform calibration, maintenance, analytics, and validation cohort size,156–158 In 2017, the USFDA granted Philips’ IntelliSite scanner approval for primary diagnostic use.159 “Subscription-based” companies/services offer economical alternatives to a large upfront capital or personnel investments.160,161
Retrospective studies confirm diagnostic concordance between WSI and glass slides using the industry clinical standard of 20x WSI magnification (0.5 microns/pixel resolution).162,163 Higher 40X magnification scanning (<0.25 micron/pixel resolution) is: 1) required for transplant and “inflammatory” pathology (eg, recognition of leukocyte phenotypes and glomerular basement membrane abnormalities)164,165; 2) supported by nearly all WSI vendors; 3) alleviates pathologist eye strain compared to 20X; and 4) yields over 95% diagnostic concordance compared to conventional light microscopy.166
Fluorescence WSI processes require upfront mIHC experimental design considerations such as fluorophore/imaging filter pairs with available excitation sources to reduce imaging artifacts such as photobleaching and poor signal-to-noise. Web accessible resources can assist with optimal component selection.167 Improvements enabling routine use of fluorescence WSI, include: camera technologies,168 fast excitation sources, wavelength separation,169 improved axial resolution (Z-dimension) techniques (ie, confocal WSI scanners), and mechanical speed.170 Emerging high-speed focus systems can leverage new camera technologies to shorten capture times,171 and correlative microscopy approaches will enable comprehensive multi-modal imaging studies of tissue events.172
Fluorescence WSI scanners enable comprehensive software-based mIHC quantification (eg, NearCYTE173; Figure 3) and social relationships among cell types60,119,174 (Figures 3 and 4). Graphical mIHC data displays can mimic flow cytometry while preserving spatial context and “tether” index populations back to tissue locations119 (Figure 5). Brown et al demonstrated equivalence between mIHC and traditional flow cytometry for assessing lymphocyte populations.158 A mIHC WSI enables systematic “reanalysis” of tissues as insights are gained from analytic datasets. The potential for combining fluorescence and brightfield imaging to predict tissue labeling “in silico” will extend spectral sensitivity.175 Emergence of immune checkpoint inhibitors in cancer therapy will trigger widespread adaptation of digital imaging capabilities.
Figure 3.
An example of objective quantitative analysis of replacing semiquantitative scoring. (A) An H&E image of a biopsy with obvious portal lymphocytic inflammation (top right) would be scored semi-quantitatively as mild to moderate portal inflammation. The lymphocyte phenotypes and quantitative analysis of each cell type are undiscernible from this traditionally stained and scoring approach. However, human only analysis can be improved by mIHC staining and automated DIA. (B) For example, CD3(green)/MAC387(red)/CD163(cyan) panel can be used to detect and quantify total T cells (CD3) and recent monocyte/macrophage immigrants (MAC387) signaling some type of liver injury,246,247 and resident Kupffer cells (CD163). The red box identifies the high-power field shown in D. (C) A combination of pixel-based and QH can identify individual cells and their phenotypic characteristics based on analyte expression, which can be used to objectively report the total number of each cell type per mm2 of biopsy area or in certain tissue areas (eg, portal tracts). Here we localize nuclear (blue) or cytoplasmic (green) analytes in possible identified cytoplasmic regions (grey) per segmented nucleus. (D) The number of cells matching the criteria defined in C are then indicated by Nearcyte, a hand-crafted” tissue-tethered cytometric software and can be quantified. (E) An unsupervised clustering algorithm can be used to identify and highlight abnormal “clustering” of CD3+ T cells using objective computational algorithms, such as mixed gaussian models (yellow ovals). The proximity to and type of APC present in these clusters, like MAC387+ or CD163+ cells, can be analyzed to determine whether recent immigrants or liver-based, possibly more tolerogenic, cells, respectively, are interacting with tissue-based CD3+ cells. MAC387+ cells are indicated with green dots.
Figure 4.
Digital image analysis. (A) and (B) are from two patients at time 0 (before attempted IS weaning). Overall the severity of the negligible portal and lobular inflammation is similar based on analysis of the H&E-stained sections. However, when the same biopsies (C) and (D), respectively, are mIHC stained for CD34 (green), PTPRC (cyan), HLA-DPB1 (red), and QNuclear Deep Red stain (blue) the stark difference between the two is easily recognizable. (C) DIA to identify pairings (virtual immunological synapses) between APC and PTPRC+ cells (yellow circles) highlights a vast difference between the two biopsies that is not recognizable on the H&E stains. Note the noticeable difference between (C) and (D) that is not noticeable on the H&E stains. (E) DIA is also able to recognize portal tract regions defined as a 100 μm radius zone centered on 5 or more HLA-DPB1+ (red) cells within a 25 μm area (large thin yellow circles). (F) DIA is able to identify “virtual immunological synapses” formed between two cells that may not be apparent to the human eye, in this case a CD34−/PTPRC−/HLA-DPB1+ (red) cell within 5 μm of a CD34−/PTPRC+/HLA-DPB1+ (leukocyte) cell (small thick yellow circles). Quantitation of these features can be queried and combined with spatial information to infer relationships about antigen presenting cells in portal tracts.
Figure 5.
Multidimensional morphometric data generation and tissue-tethered cytometry. DIA can dissect tissue cell populations and produce data outputs mimicking flow cytometry, while maintaining the spatial context of mIHC panels. Automated detection of tissue area, nuclear segmentation, and analyte expression/cell are performed via NearCYTE. This mIHC panel included CD4 (green), CD8 (red), FOXP3 (magenta), TBX21 (cyan), and nuclei (blue) to help characterize different T cell phenotypes. (A–F) Cellular subpopulations can be graphically represented on the mIHC-stained tissue sections: (A; CD4+/FOXP3+ cells; yellow circles) and (B; CD4+/TBX21+ cells; red circles). (C) Overlay of all mIHC panel components with DIA can be used to quantify cell types and inform relationships among cell types and tissue structures. Morphometric data, maintaining spatial relationships between cell types, can be translated into a traditional flow plot with the color intensity representative of the percentage of cells (green less cell density to red more cell density). (D) Cell plot representing CD4+ versus CD8+ cells from all cells that had at least one mIHC panel label. (E) CD8+ cells were queried for subpopulations expressing TBX21. (F) CD4+ cells were queried for subpopulations into putative Tregs expressing FOXP3 or Th1 cells expressing TBX21.
WSI quality assurance for DIA is a time-consuming, but essential, two-component process needed to eliminate: 1) scanning errors (eg, focus problems, tissue folds, etc)176,177 and 2) inadequate staining. Scanning errors are being minimized by algorithmically incorporating color and focusing “metrics” to automatically detect problematic areas.178,179 Staining quality assessment requires domain knowledge of expected staining patterns, which has been more difficult to automate.
Digital Imaging in Transplantation and Analytical Approaches
Digital imaging and DIA have a 25-year history in clinical translational transplantation research and enables a worldwide network of expert pathologists. Digital images have been used to evaluate the reproducibility of diagnoses and/or lesion scoring in kidney180–182 and heart183 allografts and C4d deposition in liver184 and kidney183 allografts. DIA approaches included: 1) pixel-based; 2) QH; 3) combinations of pixel-based, spatial and QH (ie, hand-crafted); and 4) ML. When compared with estimation techniques (eg, stereology), WSI-based analyses have provided results with acceptable correlation to human-generated assessment.185,186
Pixel-based “software recognition” of specified color(s) differences has been used to quantify: 1) fibrosis in liver,61,187–191 kidney,192–196 and heart173,197 allografts with stains that enhance fibrous and non-fibrous tissue contrast (eg, Masson’s and light-green trichrome, Picrosirius Red with/out polarization, collagen III immunostaining); 2) severity and phenotype of inflammatory infiltrates in kidney115,198–200 and heart201 allografts; 3) severity of steatosis in donor livers202–205; and 4) scoring of chronic allograft damage index206 parameters. Results show that human semiquantitative scoring correlates with software-based DIA, but the latter is generally more reproducible - a key criticism of human-only traditional evaluations. Donor liver202–205 steatosis severity evaluation, augmented by Oil Red O lipid staining205, might benefit from DIA standardization because humans tend to overestimate the severity of steatosis compared to software-derived values.203 Since pixel-based analysis is not computationally taxing, readily available devices such as smartphone cameras can be leveraged to assess semi-supervised steatosis quantification in donor liver biopsies.207 Once digital fibrosis quantification tools are standardized, they will erode the subjective and poorly reproducible criticisms of histopathology.
Although pixel-based approaches are useful and simple, human intervention can be required, generated data can be limited, and spatial contexts are generally ignored. For example, pixel-based assessment of liver187–190 and kidney192,194,195,206 allograft fibrosis and kidney115,198–200 and heart201 inflammatory infiltrates often rely on humans either capturing and/or excluding ROI (eg, glomeruli, blood vessels, etc) or segmenting/selecting cortical tissue on WSI, which introduces subjectivity. Biopsies from architecturally normal or minimally fibrotic livers can yield a wide range of quantitative fibrosis expression because of sampling of variably-sized portal tracts; therefore, an assessment of architectural integrity is needed.191,208,209
QH groups similarly colored pixels into biologically relevant structures (eg, nuclei) that satisfy relevant predefined criteria (eg, shape and/or size). Preserved spatial data (eg, XY coordinates, morphological characteristics of discrete structures) requires high sensitivity and specificity detection algorithms, limiting adaptability due to routine error sources (ie, tissue artifacts or stain variation210). QH has been used for glomerular parameterization,211,212 fibrosis assessment213 and/or determining total infiltrative cell types and/or characteristics of particular cell types (Figures 5 and 6) within tissues.60,115,118,119,121,214,215 Quantitative DIA evaluation of nephropathology protocol biopsies for interstitial fibrosis and tubular atrophy lesions has shown prognostic significance.216 Assessment of assay reproducibility is essential for standardization and determining usefulness.
Figure 6.
Vendor-neutral web-based viewing solution. This vendor-neutral viewing platform enables direct side-by-side comparisons of biopsies from the same patient at two different time points. The “Weaning/Enrollment Biopsy” (left) and “Biopsy at 16 months post-weaning (right) show representative portal tracts from H&E-stained (top row) and CD34/HLA-DPB1+/PTPRChigh stained mIHC panel (bottom row). Quantitative queries, analyses, and mechanistic insights can be carried out with respect to treatment changes between the two timepoints. This platform also enables receipt of WSI created elsewhere thus eliminating the need to repeat common stains (H&E, PAS, trichrome, etc) preserving tissue for other analyses.
Complex DIA combines pixel-based and QH analyses into higher order relationships using rule sets to define tissue structures (eg, algorithmic detection of “virtual immunological synapses” in pediatric liver allografts distinguished among patient clusters enrolled in IS minimization trials; Figures 3 and 4).60 Madabhushi et al discuss the concept of “hand-crafted” DIA features, or “features which can be connected to specific measurable image attributes and have some degree of interpretability.”217 They report interest in “graph-based” approaches (ie, using tessellations and other human interpretable spatial relationship metrics) to characterize image feature arrangement (eg, arrangement of nuclei).217,218 Recognition of specific pathology patterns (eg, lymphocyte clustering; Figure 3) can correlate with downstream effects (eg, rejection).219 Standardization of thresholding, deconvolution, and automated ROI selection can reduce variability in quantification compared to “human-powered” reproducibility.220,221
We20,60,61,116,118–121,214 and others90,122,222–225 utilized a combination of pixel-based and QH based techniques to analyze: 1) graft-infiltrative cells and relationships between and among them (Figure 5); 2) tumor immune microenvironments; and 3) side-by-side comparison of biopsies from the same recipient over time (Figure 6). Automated detection of tissue area, nuclear segmentation, and analyte expression/cell are performed via NearCYTE, a “hand-crafted” tissue-tethered cytometric software (Figure 5).173 Multidimensional individual cell-based morphometric data (eg, size, shape, analyte expression pattern, XY coordinates, etc) can identify specific cell populations (Figure 5A-C), and subpopulations (Figure 5B-E) while maintaining spatial relationship with other cell types (ie, tissue-tethered cytometry119). Specific cell populations and interactions can be interrogated within a spatial context while retaining architectural features (eg, virtual immune synapses in portal tracts60). This approach can run “asynchronous” to pathology review and provide supporting diagnostic material (eg, density specific cell populations per mm2 of area).
DIA creates a wealth of digital data including XY coordinates, color intensity, and size, and can be used to determine spatial relationships among cells (eg, Treg, NKT cells) and/or structures (eg, portal tracts, glomeruli) not readily appreciated and/or quantifiable by humans. Humans are “handicapped” when attempting to utilize multidimensional “big” data resources, because the data inputs are not readily amenable to routine representation methods for pattern detection. A subset of Artificial Intelligence: Machine Learning (ML) enables algorithms to “learn” by observation of input variables that are tied to particular outcomes. ML can then “predict” outcomes on fresh data sets,226,227 which has the potential to eliminate human imposed constraints and biases.
ML methods are classified by algorithmic “learning” approaches to input data: supervised and unsupervised.226,227 Supervised learning, the most common, “learns” by algorithmic mapping of training data variables to a known outcome (eg, variables that create a “formula” to give a known output).226,227 A practical biopsy-centric application of supervised ML includes Content Based Histopathological Image Retrieval, where WSI archives are searched for image patterns (via color, texture or other measured attributes) of a chosen pathology (ie, interface hepatitis lesions or FSGS lesions in kidneys).228,229 Modern ML can “blend” both supervised and unsupervised techniques to optimize performance.226,227
“Deep Learning” (DL; deep convolutional network) is a supervised ML method226,227 that is employed successfully in the healthcare arena (eg, the IBM Watson Oncology tool; https://www.ibm.com/us-en/marketplace/ibm-watson-for-oncology). DL creates data information relationships at multiple levels, similar to humans (reviewed in reference 230), can incorporate absolute data values, and combine them with “abstractions” such as how quickly those values are changing. Benefits include higher training efficiency and leveraging of cloud-based parallel computing resources for performance (eg, nuclear classification of colon cancers to predict outcome).231,232
ML techniques utilizing unsupervised learning do not rely on any predetermined labeling of training or outcome data, but instead depend on assumptions to discover patterns.226,227 Unsupervised learning-based ML challenges include: 1) methods that are typically computationally expensive; 2) subtle pathology patterns (eg, “subclinical” rejection) are not well characterized making training and validation challenging, especially considering biopsy size and architectural variance; and 3) influence of data artifacts, such as histology or intra-laboratory specimen preparation variance.
Unsupervised approaches operate bias-free, and hold promise to unlock biological/pathological signatures (Figure 3E). Engagement and inclusion of pathologist-oriented insights in unsupervised learning processes provide unmatched value to minimize caveats233: 1) reducing non-specificity from “savant” DIA data algorithms (eg, training data selection to avoid histopathology artifact contribution), and 2) predictions grounded in observable and understandable facts.234
Regardless of the ML approach, pathology practices will inevitably evolve toward ML incorporation into the routine workflow to maximize specimen value. Practical applications will enable “probing” biological questions by incorporating causal modeling, distinguishing which variables influence the prediction process. Routine screening tasks (eg, fibrosis-based architectural distortion quantification) will be automated, promoting efficiency and decreasing prognostic variations for subtle changes. Pathology classifications will evolve to incorporate these subtleties, invisible to the human eye, to resolve diagnostic dilemmas (eg, invasive versus in situ carcinomas).
Digital Archiving
WSI scanners create “pixel” characteristic data from stained slides, leading to a requirement for scalable and cost-effective data storage (eg, our digital archives occupy 80+ TB). WSI data contains or is linked to, metadata (eg, semi-quantitatively scored parameters, demographics, serological laboratory data, -omics platform data, etc), including associative details undiscernible to humans, but informative to ML (Figure 1). Linking metadata and intra-WSI pixel data is critical to full NGP utilization and integration into the clinical workflow. Archival systems should be user friendly and support multiple data sources. Commercial scanner vendors offer image management systems, but they: 1) are often rigid and relatively closed, making it difficult to integrate with existing laboratory information systems; and 2) have considerable upfront and ongoing maintenance costs.
A WSI archive of longitudinally-obtained biopsies and associated metadata yields several benefits because it enables: 1) immediate availability of prior patient biopsies regardless of pathologist location; 2) side-by-side biopsy comparisons, special stains, and fluorescence microscopy (Figure 6); 3) lesion grading accuracy by comparing to consensus archival reference standards; 4) remote access for expert consultation; 5) immediate harvesting of electronically-defined groupings for mechanistic, translational research, and quality assurance initiatives; and 6) batch processing DIA comparisons between or among groups to test various hypotheses and for ML algorithms. For example, simply by changing selection criteria for group inclusion (eg, DSA+ versus DSA-; male versus female, etc) various WSI parameters (eg, portal inflammation) can be batch processed for comparison without having to rearrange and link associated metadata – even for parameters not previously evaluated.
Industry advocate groups (eg, Digital Imaging and Communications in Medicine) promote image storage formats and data exchange235 standards that facilitate WSI exchange and interoperability.236 Open source WSI software, image format interpretation, data collection and analysis frameworks enable high throughput algorithm execution and downstream organization.237 The Open Microscopy Environment238 and Digital Slide Archive239 groups developed storage models and database systems that can represent and manage multi-dimensional WSI data. Cloud-based computing platforms are being leveraged for solutions that integrate search, storage, and DIA processing in a secure environment that can be cost-effectively deployed.240,241
NGP Translation Into a Clinical Transplant Pathology Workflow
Clinical translation of NGP platforms is needed to exploit its capabilities by distributing capabilities amongst a preexisting worldwide network of expert pathologists. Our core laboratory for multicenter and corporate-sponsored clinical trials transitioned more than a decade ago to an entirely digital workflow, including: complete barcode accessioning and tissue and slide tracking, WSI creation, automated WSI analyses, and archiving. This prototypical clinical setup has been successful primarily because of: 1) the relatively small daily case load (about 20 biopsy slides/day including standard and mIHC stains); 2) the relatively small size for the vast majority of specimens (liver, kidney, and heart biopsies), which translates into a “controllable” scanning real estate; 3) parallel Zeiss Axio Scan.Z1 WSI scanners; and 4) a team of domain experts devoted to each aspect of the NGP workflow.
Histochemically-stained biopsies are scanned quickly (~11 mm2 at 7 min/WSI at 40x, 0.11 μm/pixel resolution) whereas a four-antigen mIHC WSI biopsy might require 20–30 min/WSI. Larger specimens (eg, sections from failed allografts) require a lengthy scan time (179.74 mm2 at 66 min/WSI). In our experience, mIHC IF scanning may be reliably accomplished with a 20x apochromatic objective; yielding optimal balance between scan time and image quality (signal-to-noise), with a wider field providing greater context for focus determination. The value equation of creating WSI for all specimens in a routine clinical workflow is questionable at this time. All WSIs are subjected to intense, time consuming scrutiny for semiquantitative scoring, which will be replaced by objective “analytical modules” in the viewing software.
Problematic areas for clinical implementation include: 1) lack of a best-of-breed technology to address all considerations for an optimal WSI system and workflow; 2) a paucity of competent trained personnel; 3) LIMS integration issues242, and 4) vendor-supplied WSI viewer incompatibility. Our development of a vendor-neutral web-based viewer complete with annotation and analytical capabilities addressed the last issue and eliminated user interface inefficiencies (Figure 6). Template development with WSI libraries specific to pathology scoring and studies will assist in LIMS system interface and improve standardization and expertise distribution.
NGP penetration into routine clinical patient care has been limited and requires commitment from anatomic pathology groups and clinical staff they support (eg, surgeons, hepatologists, nephrologists). Transplant biopsies require STAT results with “hours” turnaround time for diagnostic rendering. The necessary infrastructure for obtaining and properly triaging needle biopsies already exists and includes core(s) triaged for FFPE (1.5 – 2.0 cm needle cores) and a smaller needle core segment (3–5 mm) triaged for frozen section C4d staining. Although actual pathologist slide review time via WSI is slightly increased compared to glass slides for routine biopsy stains (personal observations and references 180 and 182), the time cost of implementing digital imaging for routine semiquantitative evaluation would currently not exceed two hours. Overnight mIHC staining and quantitative DIA would necessitate that NGP-derived data would require one to two days for more detailed objective data output (eg, pathogenic mechanisms, detailed assessments of the microvasculature.173 fibrosis, other architectural distortions).
Developmental arms of clinical immunostaining laboratories will be responsible for panel development, similar to the current workflow. Once validated, panel staining automation and QA by trained laboratory personnel can be implemented with overnight turnaround times for as many as four to five protein analyte panels. Staining platform vendors currently encourage chromogenic mIHC panels for clinical application because of limited imaging and DIA platform availability in clinical labs and pathologist familiarity with chromogenic labeling. Advantages of fluorescence labeling include: 1) precise signal quantification; 2) increased sensitivity; 3) spectral channel separation; 4) standardization; 5) increased number of color options, and 6) facilitation of DIA.243 Continued cost reductions in equipment and reagents will entice clinical labs to upgrade imaging and analytical capabilities. Care must be taken to ensure that mIHC panels are applied in a medically necessary way and subjected to standards and proficiency testing to be cognizant of patient costs.244
Conclusions
Our view of platforms “competing” with traditional and NGP histopathology is a welcoming one, especially during discovery and developmental phases because radiological, and -omics platforms are complementary. Computer hardware and software advances are driving a revolution in histopathology, which we have overviewed and illustrated its value. In our opinion, although fewer biopsies will likely be obtained, biopsies will be subjected to more intense scrutiny using NGP, which: 1) preserves spatial and inferred temporal contexts; 2) can rely on a worldwide distributed network of expert transplant pathophysiologists who, instead of relying on semiquantitative data rendering on biopsies, will have new toolkits that standardize objective quantitative output that limits or eliminates subjectivity and enhances reproducibility; and 3) engage various non-medical experts (eg, engineers, mathematicians, statisticians, and other biologists) into the realm of tissue analysis.
Integration of multiple data sources filtered through a variety of domain experts will provide a conceptual framework for scientific and patient care advancements. “Iterative loops” comparing morphometric, clinical, and -omics platforms data are informative and creative. Reliance on human input for morphology will continue, but software tools and ML will contribute to final biopsy interpretations once large WSI libraries are created and queried. Retention of spatial and inferred temporal context is essential because: 1) humans use visual inputs to conceptualize their environment; 2) it engages a worldwide network of expert mechanistic pathophysiologists who can interpret and develop in parallel -accelerating the rate of advancement; and 3) it retains software-based DIA and ML biopsy analyses. Until “grind and bind” or radiographic approaches discover methods to reconstruct the original spatial configuration from expression data or achieve the submicron spatial context resolution, respectively, both traditional histopathology and NGP are needed and will continue to be valued.
Supplementary Material
Acknowledgements
The authors thank the patients who agreed to participate in the clinical trials for which we serve as a core laboratory. The authors would also like to thank the following funding sources: Immune Tolerance Network (1UM1AI109565), Clinical Trials in Organ Transplantation in Children (1U01AI104336, 4U01AI104347), NIH/NAIAD (1U19AI131453, 4U01AI104347, U01AI100807), NIH/NIDDK (1R01DK114180), TransMedics, UPMC, and the Enduring Hearts Foundation.
ABBREVIATIONS:
- AASLD
American Association for the Study of Liver Diseases
- AMR
antibody-mediated rejection
- APCs
antigen presenting cells
- CAP
College of American Pathologists
- CLIA
Clinical Laboratory Improvement Amendments
- DIA
digital image analysis
- CODEX
Co-detection by InDEXing
- DSA
donor specific antigen
- EMR
electronic medical record
- FFPE
formalin-fixed, paraffin-embedded
- H&E
hematoxylin and eosin
- IHC
immunohistochemistry
- IS
immunosuppression
- ISH
in situ hybridization
- IPEX
chromogenic immunohistochemistry
- IVD
in vitro diagnostic
- mIHC
multiplex immunohistochemistry
- ML
machine learning
- LIMS
laboratory information management system
- mRNA
messenger ribonucleic acid
- NASH
nonalcoholic steatohepatitis
- NBF
neutral buffered formalin
- NGP
next generation pathology
- NK cells
natural killer cells
- PAS
periodic acid-Schiff
- Qdots
quantum dots
- QH
quantitative histomorphometry
- RTU
ready to use
- ROI
region of interest
- TCMR
T cell-mediated rejection
- TSA
tyramide signal amplification
- TTC
tissue-tethered cytometry
- USFDA
United States Food and Drug Administration
- WSI
whole slide images
Footnotes
Conflicts of interest: Authors have no conflicts to disclose.
Statement of Authorship: Contributions: MWT, AL, and AJD all contributed to writing of the manuscript, supplement, and production of figures
References
- 1.Jerath R, Crawford MW, Barnes VA. A unified 3D default space consciousness model combining neurological and physiological processes that underlie conscious experience. Front Psychol. 2015;6:1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Romih T Humans Are Visual Creatures. https://www.seyens.com/humans-are-visual-creatures/. Accessed October 12, 2016. [Google Scholar]
- 3.Snyder A Explaining and inducing savant skills: privileged access to lower level, less-processed information. Philos Trans R Soc Lond B Biol Sci. 2009;364(1522):1399–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khatri P, Sarwal MM. Using gene arrays in diagnosis of rejection. Curr Opin Organ Transplant. 2009;14(1):34–39. [DOI] [PubMed] [Google Scholar]
- 5.Isse K, Lesniak A, Grama K, Roysam B, Minervini MI, Demetris AJ. Digital transplantation pathology: combining whole slide imaging, multiplex staining and automated image analysis. Am J Transplant. 2012;12(1):27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kransdorf EP, Kobashigawa JA. Novel molecular approaches to the detection of heart transplant rejection. Per Med. 2017;14(4):293–297. [DOI] [PubMed] [Google Scholar]
- 7.Halloran PF, Famulski KS, Reeve J. Molecular assessment of disease states in kidney transplant biopsy samples. Nat Rev Nephrol. 2016;12(9):534–548. [DOI] [PubMed] [Google Scholar]
- 8.Caussy C, Reeder SB, Sirlin CB, Loomba R. Noninvasive, quantitative assessment of liver fat by MRI-PDFF as an endpoint in NASH trials. Hepatology. 2018;68(2):763–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Value English Oxford Living Dictionary. https://en.oxforddictionaries.com/definition/value. Oxford University Press; 2019. Accessed January 3, 2019. [Google Scholar]
- 10.Kennedy P, Wagner M, Castéra L, et al. Quantitative elastography methods in liver disease: current evidence and future directions. Radiology. 2018;286(3):738–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiang H, Zheng T, Duan T, Chen J, Song B. Non-invasive in vivo imaging grading of liver fibrosis. J Clin Transl Hepatol. 2018;6(2):198–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wong GL. Non-invasive assessments for liver fibrosis: the crystal ball we long for. J Gastroenterol Hepatol. 2018;33(5):1009–1015. [DOI] [PubMed] [Google Scholar]
- 13.Tapper EB, Loomba R. Noninvasive imaging biomarker assessment of liver fibrosis by elastography in NAFLD. Nat Rev Gastroenterol Hepatol. 2018;15(5):274–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mancini M, Summers P, Faita F, et al. Digital liver biopsy: bio-imaging of fatty liver for translational and clinical research. World J Hepatol. 2018;10(2):231–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Toby TK, Abecassis M, Kim K, et al. Proteoforms in peripheral blood mononuclear cells as novel rejection biomarkers in liver transplant recipients. Am J Transplant. 2017;17(9):2458–2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bonaccorsi-Riani E, Pennycuick A, Londono MC, et al. Molecular characterization of acute cellular rejection occurring during intentional immunosuppression withdrawal in liver transplantation. Am J Transplant. 2016;16(2):484–496. [DOI] [PubMed] [Google Scholar]
- 17.Khatri P, Roedder S, Kimura N, et al. A common rejection module (CRM) for acute rejection across multiple organs identifies novel therapeutics for organ transplantation. J Exp Med. 2013;210(11):2205–2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sigdel TK, Gao Y, He J, et al. Mining the human urine proteome for monitoring renal transplant injury. Kidney Int. 2016;89(6):1244–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deng MC, Eisen HJ, Mehra MR, et al. Noninvasive discrimination of rejection in cardiac allograft recipients using gene expression profiling. Am J Transplant. 2006;6(1):150–160. [DOI] [PubMed] [Google Scholar]
- 20.Isse K, Grama K, Abbott IM, et al. Adding value to liver (and allograft) biopsy evaluation using a combination of multiplex quantum dot immunostaining, high-resolution whole-slide digital imaging, and automated image analysis. Clin Liver Dis. 2010;14(4):669–685. [DOI] [PubMed] [Google Scholar]
- 21.Patel K, Bedossa P, Castera L. Diagnosis of liver fibrosis: present and future. Semin Liver Dis. 2015;35(2):166–183. [DOI] [PubMed] [Google Scholar]
- 22.Vitalone MJ, Sigdel TK, Salomonis N, Sarwal RD, Hsieh SC, Sarwal MM. Transcriptional perturbations in graft rejection. Transplantation. 2015;99(9):1882–1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Halloran PF, Venner JM, Madill-Thomsen KS, et al. Review: the transcripts associated with organ allograft rejection. Am J Transplant. 2018;18(4):785–795. [DOI] [PubMed] [Google Scholar]
- 24.Halloran PF, Potena L, Van Huyen JD, et al. Building a tissue-based molecular diagnostic system in heart transplant rejection: the heart Molecular Microscope Diagnostic (MMDx) System. J Heart Lung Transplant. 2017;36(11):1192–1200. [DOI] [PubMed] [Google Scholar]
- 25.Loupy A, Duong Van Huyen JP, Hidalgo L, et al. Gene expression profiling for the identification and classification of antibody-mediated heart rejection. Circulation. 2017;135(10):917–935. [DOI] [PubMed] [Google Scholar]
- 26.KASHI Clinical Laboratories. Molecular Microscope Diagnostic System (MMDx). https://www.molecular-microscope.com/clinicians. Accessed January 29, 2019.
- 27.Starzl TE, Marchioro TL, Terasaki PI, et al. Chronic survival after human renal homotransplantation. Lymphocyte-antigen matching, pathology and influence of thymectomy. Ann Surg. 1965;162(4):749–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kissmeyer-Nielsen F, Olsen S, Petersen VP, Fjeldborg O. Hyperacute rejection of kidney allografts, associated with pre-existing humoral antibodies against donor cells. Lancet. 1966;2(7465):662–665. [DOI] [PubMed] [Google Scholar]
- 29.Ogden DA, Porter KA, Terasaki PI, Marchioro TL, Holmes JH, Starzl TE. Chronic renal homograft function: correlation with histology and lymphocyte antigen matching. Am J Med. 1967;43(6):837–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Porter KA, Dossetor JB, Marchioro TL, et al. Human renal transplants. I. Glomerular changes. Lab Invest. 1967;16(1):153–181. [PubMed] [Google Scholar]
- 31.Porter KA. The effects of antibodies on human renal allografts. Transplant Proc. 1976;8(2):189–197. [PubMed] [Google Scholar]
- 32.Weil R 3rd, Clarke DR, Iwaki Y, et al. Hyperacute rejection of a transplanted human heart. Transplantation. 1981;32(1):71–72. [PMC free article] [PubMed] [Google Scholar]
- 33.Hancock WW. Analysis of intragraft effector mechanisms associated with human renal allograft rejection: immunohistological studies with monoclonal antibodies. Immunol Rev. 1984;77:61–84. [DOI] [PubMed] [Google Scholar]
- 34.Demetris AJ, Markus BH. Immunopathology of liver transplantation. Crit Rev Immunol. 1989;9(2):67–92. [PubMed] [Google Scholar]
- 35.Hammond EH, Yowell RL, Nunoda S, et al. Vascular (humoral) rejection in heart transplantation: pathologic observations and clinical implications. J Heart Transplant. 1989;8(6):430–443. [PubMed] [Google Scholar]
- 36.Halloran PF, Wadgymar A, Ritchie S, Falk J, Solez K, Srinivasa NS. The significance of the anti-class I antibody response. I. Clinical and pathologic features of anti-class I-mediated rejection. Transplantation. 1990;49(1):85–91. [DOI] [PubMed] [Google Scholar]
- 37.Halloran PF, Schlaut J, Solez K, Srinivasa NS. The significance of the anti-class I response. II. Clinical and pathologic features of renal transplants with anti-class I-like antibody. Transplantation. 1992;53(3):550–555. [PubMed] [Google Scholar]
- 38.Demetris AJ, Murase N, Nakamura K, et al. Immunopathology of antibodies as effectors of orthotopic liver allograft rejection. [Review]. Semin Liver Dis. 1992;12(1):51–59. [DOI] [PubMed] [Google Scholar]
- 39.Lajoie G, Nadasdy T, Laszik Z, Blick KE, Silva FG. Mast cells in acute cellular rejection of human renal allografts. Mod Pathol. 1996;9(12):1118–1125. [PubMed] [Google Scholar]
- 40.Racusen LC, Haas M. Antibody-mediated rejection in renal allografts: lessons from pathology. Clin J Am Soc Nephrol. 2006;1(3):415–420. [DOI] [PubMed] [Google Scholar]
- 41.Porter KA. Renal Transplantation In: Heptinstall RH, ed. Pathology of the Kidney. Vol III 4th ed. Boston/Toronto/London: Little, Brown and Company; 1992:1799–1934. [Google Scholar]
- 42.Halloran PF, de Freitas DG, Einecke G, et al. The molecular phenotype of kidney transplants. Am J Transplant. 2010;10(10):2215–2222. [DOI] [PubMed] [Google Scholar]
- 43.Drachenberg CB, Papadimitriou JC. Endothelial injury in renal antibody-mediated allograft rejection: a schematic view based on pathogenesis. Transplantation. 2013;95(9):1073–1083. [DOI] [PubMed] [Google Scholar]
- 44.Karlsson C, Karlsson MG. Effects of long-term storage on the detection of proteins, DNA, and mRNA in tissue microarray slides. J Histochem Cytochem. 2011;59(12):1113–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reeve J, Chang J, Salazar ID, Lopez MM, Halloran PF. Using molecular phenotyping to guide improvements in the histologic diagnosis of T cell-mediated rejection. Am J Transplant. 2016;16(4):1183–1192. [DOI] [PubMed] [Google Scholar]
- 46.Roufosse C, Simmonds N, Groningen MC, et al. A 2018 reference guide to the banff classification of renal allograft pathology. Transplantation. 2018;102(11):1795–1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rockey DC, Caldwell SH, Goodman ZD, Nelson RC, Smith AD. Liver biopsy. Hepatology. 2009;49(3):1017–1044. [DOI] [PubMed] [Google Scholar]
- 48.Pandey N, John S. Liver Biopsy [January 2018 ed]. In: StatPearls.Treasure Island, FL: StatPearls Publishing; 2018. [Google Scholar]
- 49.Feng S, Ekong UD, Lobritto SJ, et al. Complete immunosuppression withdrawal and subsequent allograft function among pediatric recipients of parental living donor liver transplants. JAMA. 2012;307(3):283–293. [DOI] [PubMed] [Google Scholar]
- 50.Zheng J, Song W. Alemtuzumab versus antithymocyte globulin induction therapies in kidney transplantation patients: a systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore). 2017;96(28):e7151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Haas M, Loupy A, Lefaucheur C, et al. The Banff 2017 Kidney Meeting Report: revised diagnostic criteria for chronic active T cell-mediated rejection, antibody-mediated rejection, and prospects for integrative endpoints for next-generation clinical trials. Am J Transplant. 2018;18(2):293–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bedossa P Diagnosis of non-alcoholic fatty liver disease/non-alcoholic steatohepatitis: why liver biopsy is essential. Liver Int. 2018;38 Suppl 1:64–66. [DOI] [PubMed] [Google Scholar]
- 53.Rodríguez-Perálvarez M, Rico-Juri JM, Tsochatzis E, Burra P, De la Mata M, Lerut J. Biopsy-proven acute cellular rejection as an efficacy endpoint of randomized trials in liver transplantation: a systematic review and critical appraisal. Transpl Int. 2016;29(9):961–973. [DOI] [PubMed] [Google Scholar]
- 54.Williams J FDA regulatory considerations for NASH clinical trial endpoints Global NASH Congress; 2018; London, UK. [Google Scholar]
- 55.Demetris AJ, Bellamy C, Hubscher SG, et al. 2016 comprehensive update of the Banff Working Group on liver allograft pathology: introduction of antibody-mediated rejection. Am J Transplant. 2016;16(10):2816–2835. [DOI] [PubMed] [Google Scholar]
- 56.O’Leary JG, Smith C, Cai J, et al. Chronic AMR in liver transplant: validation of the 1-year cAMR score’s ability to determine long-term outcome. Transplantation. 2017;101(9):2062–2070. [DOI] [PubMed] [Google Scholar]
- 57.O’Leary JG, Cai J, Freeman R, et al. Proposed diagnostic criteria for chronic antibody-mediated rejection in liver allografts. Am J Transplant. 2016;16(2):603–614. [DOI] [PubMed] [Google Scholar]
- 58.O’Leary JG, Kaneku H, Demetris AJ, et al. Antibody-mediated rejection as a contributor to previously unexplained early liver allograft loss. Liver Transpl. 2014;20(2):218–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.O’Leary JG, Michelle Shiller S, Bellamy C, et al. Acute liver allograft antibody-mediated rejection: an inter-institutional study of significant histopathological features. Liver Transpl. 2014;20(10):1244–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Feng S, Bucuvalas JC, Demetris AJ, et al. Evidence of chronic allograft injury in liver biopsies from long-term pediatric recipients of liver transplants. Gastroenterology. 2018;155(6):1838–1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Feng S, Demetris AJ, Spain KM, et al. Five-year histological and serological follow-up of operationally tolerant pediatric liver transplant recipients enrolled in WISP-R. Hepatology. 2017;65(2):647–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Barisoni L, Troost JP, Nast C, et al. Reproducibility of the NEPTUNE descriptor-based scoring system on whole-slide images and histologic and ultrastructural digital images. Mod Pathol. 2016;29(7):671–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Snyder A, Bahramali H, Hawker T, Mitchell DJ. Savant-like numerosity skills revealed in normal people by magnetic pulses. Perception. 2006;35(6):837–845. [DOI] [PubMed] [Google Scholar]
- 64.Mells G, Neuberger J. Protocol liver allograft biopsies. Transplantation. 2008;85(12):1686–1692. [DOI] [PubMed] [Google Scholar]
- 65.Mells G, Mann C, Hubscher S, Neuberger J. Late protocol liver biopsies in the liver allograft: a neglected investigation? Liver Transpl. 2009;15(8):931–938. [DOI] [PubMed] [Google Scholar]
- 66.Russo FP, Imondi A, Lynch EN, Farinati F. When and how should we perform a biopsy for HCC in patients with liver cirrhosis in 2018? A review. Dig Liver Dis. 2018;50(7):640–646. [DOI] [PubMed] [Google Scholar]
- 67.Brunt EM. Nonalcoholic fatty liver disease and the ongoing role of liver biopsy evaluation. Hepatol Commun. 2017;1(5):370–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gill US, Pallett LJ, Kennedy PTF, Maini MK. Liver sampling: a vital window into HBV pathogenesis on the path to functional cure. Gut. 2018;67(4):767–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mieli-Vergani G, Vergani D, Czaja AJ, et al. Autoimmune hepatitis. Nat Rev Dis Primers. 2018;4:18017. [DOI] [PubMed] [Google Scholar]
- 70.Londoño MC, Souza LN, Lozano JJ, et al. Molecular profiling of subclinical inflammatory lesions in long-term surviving adult liver transplant recipients. J Hepatol. 2018;69(3):626–634. [DOI] [PubMed] [Google Scholar]
- 71.Baumann AK, Schlue J, Noyan F, et al. Preferential accumulation of T helper cells but not cytotoxic T cells characterizes benign subclinical rejection of human liver allografts. Liver Transpl. 2016;22(7):943–955. [DOI] [PubMed] [Google Scholar]
- 72.Demetris AJ, Isse K. Tissue biopsy monitoring of operational tolerance in liver allograft recipients. Curr Opin Organ Transplant. 2013;18(3):345–353. [DOI] [PubMed] [Google Scholar]
- 73.Hubscher SG. What is the long-term outcome of the liver allograft? Journal of Hepatology. 2011;55(3):702–717. [DOI] [PubMed] [Google Scholar]
- 74.Hubscher S What does the long-term liver allograft look like for the pediatric recipient? Liver Transpl. 2009;15 Suppl 2:S19–S24. [DOI] [PubMed] [Google Scholar]
- 75.Demetris AJ, Jaffe R, Sheahan DG, et al. Recurrent hepatitis B in liver allograft recipients. Differentiation between viral hepatitis B and rejection. Am J Pathol. 1986;125(1):161–172. [PMC free article] [PubMed] [Google Scholar]
- 76.Demetris AJ, Jaffe R, Tzakis A, et al. Antibody-mediated rejection of human orthotopic liver allografts. A study of liver transplantation across ABO blood group barriers. Am J Pathol. 1988;132(3):489–502. [PMC free article] [PubMed] [Google Scholar]
- 77.Demetris AJ, Nakamura K, Yagihashi A, et al. A clinicopathological study of human liver allograft recipients harboring preformed IgG lymphocytotoxic antibodies. Hepatology. 1992;16(3):671–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Demetris AJ, Eghtesad B, Marcos A, et al. Recurrent hepatitis C in liver allografts: prospective assessment of diagnostic accuracy, identification of pitfalls, and observations about pathogenesis. Am J Surg Pathol. 2004;28(5):658–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.O’Leary JG, Demetris AJ, Friedman LS, et al. The role of donor-specific HLA alloantibodies in liver transplantation. Am J Transplant. 2014;14(4):779–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.OʼLeary JG, Demetris AJ, Philippe A, et al. Non-HLA antibodies impact on C4d staining, stellate cell activation and fibrosis in liver allografts. Transplantation. 2017;101(10):2399–2409. [DOI] [PubMed] [Google Scholar]
- 81.Varma S, Ambroise J, Komuta M, et al. Progressive fibrosis is driven by genetic predisposition, allo-immunity, and inflammation in pediatric liver transplant recipients. EBioMedicine. 2016;9:346–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Demetris AJ, Zeevi A, O’Leary JG. ABO-compatible liver allograft antibody-mediated rejection: an update. Curr Opin Organ Transplant. 2015;20(3):314–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Miyagawa-Hayashino A, Yoshizawa A, Uchida Y, et al. Progressive graft fibrosis and donor-specific human leukocyte antigen antibodies in pediatric late liver allografts. Liver Transpl. 2012;18(11):1333–1342. [DOI] [PubMed] [Google Scholar]
- 84.Banff Working Group on Liver Allograft Pathology. Importance of liver biopsy findings in immunosuppression management: biopsy monitoring and working criteria for patients with operational tolerance. Liver Transpl. 2012;18(10):1154–1170. [DOI] [PubMed] [Google Scholar]
- 85.Wong T, Nouri-Aria KT, Devlin J, Portmann B, Williams R. Tolerance and latent cellular rejection in long-term liver transplant recipients. Hepatology. 1998;28(2):443–449. [DOI] [PubMed] [Google Scholar]
- 86.Kerkar N, Yanni G. ‘De novo’ and ‘recurrent’ autoimmune hepatitis after liver transplantation: A comprehensive review. J Autoimmun. 2016;66:17–24. [DOI] [PubMed] [Google Scholar]
- 87.Solez K, Fung KC, Saliba KA, et al. The bridge between transplantation and regenerative medicine: beginning a new Banff classification of tissue engineering pathology. Am J Transplant. 2018;18(2):321–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Stack EC, Wang C, Roman KA, Hoyt CC. Multiplexed immunohistochemistry, imaging, and quantitation: a review, with an assessment of Tyramide signal amplification, multispectral imaging and multiplex analysis. Methods. 2014;70(1):46–58. [DOI] [PubMed] [Google Scholar]
- 89.Dixon AR, Bathany C, Tsuei M, White J, Barald KF, Takayama S. Recent developments in multiplexing techniques for immunohistochemistry. Expert Rev Mol Diagn. 2015;15(9):1171–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mansfield JR. Phenotyping multiple subsets of immune cells in situ in FFPE tissue sections: an overview of methodologies. Methods Mol Biol. 2017;1546:75–99. [DOI] [PubMed] [Google Scholar]
- 91.Kim SW, Roh J, Park CS. Immunohistochemistry for pathologists: protocols, pitfalls, and tips. J Pathol Transl Med. 2016;50(6):411–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Taylor CR. Immunohistochemistry in surgical pathology: principles and practice. Methods Mol Biol. 2014;1180:81–109. [DOI] [PubMed] [Google Scholar]
- 93.Demetris AJ, Ruppert K. Pathologist’s perspective on liver needle biopsy size? J Hepatol. 2003;39(2):275–277. [DOI] [PubMed] [Google Scholar]
- 94.Piccinino F, Sagnelli E, Pasquale G, Giusti G. Complications following percutaneous liver biopsy. A multicentre retrospective study on 68,276 biopsies. J Hepatol. 1986;2(2):165–173. [DOI] [PubMed] [Google Scholar]
- 95.Atwell TD, Smith RL, Hesley GK, et al. Incidence of bleeding after 15,181 percutaneous biopsies and the role of aspirin. AJR Am J Roentgenol. 2010;194(3):784–789. [DOI] [PubMed] [Google Scholar]
- 96.Tublin ME, Blair R, Martin J, Malik S, Ruppert K, Demetris A. Prospective study of the impact of liver biopsy core size on specimen adequacy and procedural complications. AJR Am J Roentgenol. 2018;210(1):183–188. [DOI] [PubMed] [Google Scholar]
- 97.Tublin ME, Blair R, Martin JA, Ruppert K, Demetris A. RC614–03 Ultrasound Guided Random Liver Biopsy: Impact of Biopsy Core Size on Specimen Adequacy and Procedural Complications Paper presented at: Radiological Society of North America 2015. Scientific Assembly and Annual Meeting; Chicago, IL. [Google Scholar]
- 98.Iqbal J, Sattar A, Al Qamari N, Hussain M, Rashid S. Compare the efficacy and complications of 16 gauge vs 18 gauge core biopsy needle in ultrasound guided percutaneous liver biopsies. J Dow Uni Health Sci. 2017;11(2):37–40. [Google Scholar]
- 99.Hall TC, Deakin C, Atwal GS, Singh RK. Adequacy of percutaneous non-targeted liver biopsy under real-time ultrasound guidance when comparing the Biopince™ and Achieve™ biopsy needle. Br J Radiol. 2017;90(1080):20170397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Myers RP, Fong A, Shaheen AA. Utilization rates, complications and costs of percutaneous liver biopsy: a population-based study including 4275 biopsies. Liver Int. 2008;28(5):705–712. [DOI] [PubMed] [Google Scholar]
- 101.Filingeri V, Sforza D, Tisone G. Complications and risk factors of a large series of percutaneous liver biopsies in patients with liver transplantation or liver disease. Eur Rev Med Pharmacol Sci. 2015;19(9):1621–1629. [PubMed] [Google Scholar]
- 102.Nicholson ML, Wheatley TJ, Doughman TM, et al. A prospective randomized trial of three different sizes of core-cutting needle for renal transplant biopsy. Kidney Int. 2000;58(1):390–395. [DOI] [PubMed] [Google Scholar]
- 103.Smith RN, Adam BA, Rosales IA, et al. RNA expression profiling of renal allografts in a nonhuman primate identifies variation in NK and endothelial gene expression. Am J Transplant. 2018;18(6):1340–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fitzgibbons PL, Bradley LA, Fatheree LA, et al. Principles of analytic validation of immunohistochemical assays: Guideline from the College of American Pathologists Pathology and Laboratory Quality Center. Arch Pathol Lab Med. 2014;138(11):1432–1443. [DOI] [PubMed] [Google Scholar]
- 105.Wolff AC, Hammond ME, Hicks DG, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013;31(31):3997–4013. [DOI] [PubMed] [Google Scholar]
- 106.Wolff AC, Hammond MEH, Allison KH, et al. Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice guideline focused update. J Clin Oncol. 2018;36(20):2105–2122. [DOI] [PubMed] [Google Scholar]
- 107.Hammond ME, Hayes DF, Dowsett M, et al. American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol. 2010;28(16):2784–2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Economou M, Schöni L, Hammer C, Galván JA, Mueller DE, Zlobec I. Proper paraffin slide storage is crucial for translational research projects involving immunohistochemistry stains. Clin Transl Med. 2014;3(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bass BP, Engel KB, Greytak SR, Moore HM. A review of preanalytical factors affecting molecular, protein, and morphological analysis of formalin-fixed, paraffin-embedded (FFPE) tissue: how well do you know your FFPE specimen? Arch Pathol Lab Med. 2014;138(11):1520–1530. [DOI] [PubMed] [Google Scholar]
- 110.Lott R, Tunnicliffe J, Sheppard E, et al. Practical Guide to Specimen Handling in Surgical Pathology. 6th ed: College of American Pathologists; November 2015. [Google Scholar]
- 111.Pinhel IF, Macneill FA, Hills MJ, et al. Extreme loss of immunoreactive p-Akt and p-Erk1/2 during routine fixation of primary breast cancer. Breast Cancer Res. 2010;12(5):R76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Neumeister VM, Anagnostou V, Siddiqui S, et al. Quantitative assessment of effect of preanalytic cold ischemic time on protein expression in breast cancer tissues. J Natl Cancer Inst. 2012;104(23):1815–1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Aziz N, Zhao Q, Bry L, et al. College of American Pathologists’ laboratory standards for next-generation sequencing clinical tests. Arch Pathol Lab Med. 2015;139(4):481–493. [DOI] [PubMed] [Google Scholar]
- 114.Endrullat C, Glökler J, Franke P, Frohme M. Standardization and quality management in next-generation sequencing. Appl Transl Genom. 2016;10:2–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Macedo C, Walters JT, Orkis EA, et al. Long-term effects of alemtuzumab on regulatory and memory T-cell subsets in kidney transplantation. Transplantation. 2012;93(8):813–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Corbitt N, Kimura S, Isse K, et al. Gut bacteria drive Kupffer cell expansion via MAMP-mediated ICAM-1 induction on sinusoidal endothelium and influence preservation-reperfusion injury after orthotopic liver transplantation. Am J Pathol. 2013;182(1):180–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Feng S, Demetris AJ, Ekong U, et al. Serum and Tissue DSA Subclass, Stellate and Endothelial Phenotype Monitoring in ITN029ST Tolerance Pediatric Liver Transplant Recipients Over 5+ Years of Follow-up. Joint International Conference of ILTS, ELITA & LICAGE; June 4–7, 2014; London, UK. [Google Scholar]
- 118.Isse K, Specht SM, Lunz JG 3rd, Kang LI, Mizuguchi Y, Demetris AJ. Estrogen stimulates female biliary epithelial cell interleukin-6 expression in mice and humans. Hepatology. 2010;51(3):869–880. [DOI] [PubMed] [Google Scholar]
- 119.Isse K, Lesniak A, Grama K, et al. Preexisting epithelial diversity in normal human livers: a tissue-tethered cytometric analysis in portal/periportal epithelial cells. Hepatology. 2013;57(4):1632–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mizuguchi Y, Isse K, Specht S, et al. Small proline rich protein 2a in benign and malignant liver disease. Hepatology. 2014;59(3):1130–1143. [DOI] [PubMed] [Google Scholar]
- 121.Mizuguchi Y, Specht S, Isse K, et al. Breast tumor kinase/protein tyrosine kinase 6 (Brk/PTK6) activity in normal and neoplastic biliary epithelia. J Hepatol. 2015;63(2):399–407. [DOI] [PubMed] [Google Scholar]
- 122.Feng Z, Puri S, Moudgil T, et al. Multispectral imaging of formalin-fixed tissue predicts ability to generate tumor-infiltrating lymphocytes from melanoma. J Immunother Cancer. 2015;3:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhang W, Hubbard A, Jones T, et al. Fully automated 5-plex fluorescent immunohistochemistry with tyramide signal amplification and same species antibodies. Lab Invest. 2017;97(7):873–885. [DOI] [PubMed] [Google Scholar]
- 124.Mezheyeuski A, Bergsland CH, Backman M, et al. Multispectral imaging for quantitative and compartment-specific immune infiltrates reveals distinct immune profiles that classify lung cancer patients. J Pathol. 2018;244(4):421–431. [DOI] [PubMed] [Google Scholar]
- 125.Ng SB, Fan S, Choo SN, et al. Quantitative analysis of a multiplexed immunofluorescence panel in T-cell lymphoma. SLAS Technol. 2018;23(3):252–258. [DOI] [PubMed] [Google Scholar]
- 126.Hu Z, Qian G, Müller S, et al. Biomarker quantification by multiplexed quantum dot technology for predicting lymph node metastasis and prognosis in head and neck cancer. Oncotarget. 2016;7(28):44676–44685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kauffman SA. The Origins of Order: Self Organization and Selection in Evolution. New York: Oxford University Press; 1993. [Google Scholar]
- 128.NanoString Technologies Inc. Digital Spatial Profiling (DSP) Technology. https://www.nanostring.com/scientific-content/technology-overview/digital-spatial-profiling-technology. Accessed June 15, 2018.
- 129.Thul PJ, Åkesson L, Wiking M, et al. A subcellular map of the human proteome. Science. 2017;356(6340):eaal3321. [DOI] [PubMed] [Google Scholar]
- 130.Uhlén M, Fagerberg L, Hallström BM, et al. Proteomics. Tissue-based map of the human proteome. Science. 2015;347(6220):1260419. [DOI] [PubMed] [Google Scholar]
- 131.Uhlen M, Zhang C, Lee S, et al. A pathology atlas of the human cancer transcriptome. Science. 2017;357(6352):eaan2507. [DOI] [PubMed] [Google Scholar]
- 132.Abcam plc. Substrates and chromogens for IHC. https://www.abcam.com/kits/substrates-and-chromogens-for-ihc. Accessed July 2, 2018.
- 133.Shi SR, Shi Y, Taylor CR. Antigen retrieval immunohistochemistry: review and future prospects in research and diagnosis over two decades. J Histochem Cytochem. 2011;59(1):13–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Shi SR, Liu C, Taylor CR. Standardization of immunohistochemistry for formalin-fixed, paraffin-embedded tissue sections based on the antigen-retrieval technique: from experiments to hypothesis. J Histochem Cytochem. 2007;55(2):105–109. [DOI] [PubMed] [Google Scholar]
- 135.Hunyady B, Krempels K, Harta G, Mezey E. Immunohistochemical signal amplification by catalyzed reporter deposition and its application in double immunostaining. J Histochem Cytochem. 1996;44(12):1353–1362. [DOI] [PubMed] [Google Scholar]
- 136.Tóth ZE, Mezey E. Simultaneous visualization of multiple antigens with tyramide signal amplification using antibodies from the same species. J Histochem Cytochem. 2007;55(6):545–554. [DOI] [PubMed] [Google Scholar]
- 137.Hagen J, Schwartz D, Kalyuzhny AE. Hapten-anti-hapten technique for two-color IHC detection of phosphorylated EGFR and H2AX using primary antibodies raised in the same host species. Methods Mol Biol. 2017;1554:155–160. [DOI] [PubMed] [Google Scholar]
- 138.Angelo M, Bendall SC, Finck R, et al. Multiplexed ion beam imaging of human breast tumors. Nat Med. 2014;20(4):436–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Rost S, Giltnane J, Bordeaux JM, Hitzman C, Koeppen H, Liu SD. Multiplexed ion beam imaging analysis for quantitation of protein expression in cancer tissue sections. Lab Invest. 2017;97(8):992–1003. [DOI] [PubMed] [Google Scholar]
- 140.Goltsev Y, Samusik N, Kennedy-Darling J, et al. Deep profiling of mouse splenic architecture with CODEX multiplexed imaging. Cell. 2018;174(4):968–981.e915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Trahearn N, Epstein D, Cree I, Snead D, Rajpoot N. Hyper-stain inspector: a framework for robust registration and localised co-expression analysis of multiple whole-slide images of serial histology sections. Sci Rep. 2017;7(1):5641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lopez XM, Barbot P, Van Eycke YR, et al. Registration of whole immunohistochemical slide images: an efficient way to characterize biomarker colocalization. J Am Med Inform Assoc. 2015;22(1):86–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Vorob’ev IA, Rafalovskaia-Orlovskaia EP, Gladkikh AA, Potashnikova DM, Barteneva NS. [Applications of fluorescent semiconductor nanocrystals in microscopy and cytometry]. Tsitologiia. 2011;53(5):392–403. [PubMed] [Google Scholar]
- 144.Prost S, Kishen RE, Kluth DC, Bellamy CO. Working with commercially available quantum dots for immunofluorescence on tissue sections. PLoS One. 2016;11(9):e0163856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zhang W, Hubbard A, Pang L, et al. Protecting quantum dot fluorescence from quenching to achieve a reliable automated multiplex fluorescence in situ hybridization assay. J Biomed Nanotechnol. 2015;11(9):1583–1596. [DOI] [PubMed] [Google Scholar]
- 146.Day WA, Lefever MR, Ochs RL, et al. Covalently deposited dyes: a new chromogen paradigm that facilitates analysis of multiple biomarkers in situ. Lab Invest. 2017;97(1):104–113. [DOI] [PubMed] [Google Scholar]
- 147.van Gijlswijk RP, Zijlmans HJ, Wiegant J, et al. Fluorochrome-labeled tyramides: use in immunocytochemistry and fluorescence in situ hybridization. J Histochem Cytochem. 1997;45(3):375–382. [DOI] [PubMed] [Google Scholar]
- 148.Pirici D, Mogoanta L, Kumar-Singh S, et al. Antibody elution method for multiple immunohistochemistry on primary antibodies raised in the same species and of the same subtype. J Histochem Cytochem. 2009;57(6):567–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Boyce BF. An update on the validation of whole slide imaging systems following FDA approval of a system for a routine pathology diagnostic service in the United States. Biotech Histochem. 2017;92(6):381–389. [DOI] [PubMed] [Google Scholar]
- 150.Parwani AV, Hassell L, Glassy E, Pantanowitz L. Regulatory barriers surrounding the use of whole slide imaging in the United States of America. J Pathol Inform. 2014;5(1):38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Cornish TC, Swapp RE, Kaplan KJ. Whole-slide imaging: routine pathologic diagnosis. Adv Anat Pathol. 2012;19(3):152–159. [DOI] [PubMed] [Google Scholar]
- 152.Hartman DJ, Pantanowitz L, McHugh JS, Piccoli AL, OLeary MJ, Lauro GR. Enterprise implementation of digital pathology: feasibility, challenges, and opportunities. J Digit Imaging. 2017;30(5):555–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Evans AJ, Salama ME, Henricks WH, Pantanowitz L. Implementation of whole slide imaging for clinical purposes: issues to consider from the perspective of early adopters. Arch Pathol Lab Med. 2017;141(7):944–959. [DOI] [PubMed] [Google Scholar]
- 154.Pantanowitz L Digital images and the future of digital pathology. J Pathol Inform. 2010;1:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Pantanowitz L, Valenstein PN, Evans AJ, et al. Review of the current state of whole slide imaging in pathology. J Pathol Inform. 2011;2:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Pantanowitz L, Sinard JH, Henricks WH, et al. Validating whole slide imaging for diagnostic purposes in pathology: guideline from the College of American Pathologists Pathology and Laboratory Quality Center. Arch Pathol Lab Med. 2013;137(12):1710–1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Cross S, Furness P, Igali L, Snead D, Treanor D. Best practice recommendations for implementing digital pathology. The Royal College of Pathologists. https://www.rcpath.org/uploads/assets/uploaded/d6b14330-a8b9-4f5e-bbe443f0d56de24a.pdf. Accessed January 29, 2019. [Google Scholar]
- 158.Brown JR, Wimberly H, Lannin DR, Nixon C, Rimm DL, Bossuyt V. Multiplexed quantitative analysis of CD3, CD8, and CD20 predicts response to neoadjuvant chemotherapy in breast cancer. Clin Cancer Res. 2014;20(23):5995–6005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Caccomo S FDA allows marketing of first whole slide imaging system for digital pathology. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm552742.htm. Accessed July 15, 2018. [Google Scholar]
- 160.Fasciano D, Pavlidakey P. 298 Digital pathology cost effective – fact or fiction? Am J Clin Pathol. 2018;149(Suppl 1):S126–S127. [Google Scholar]
- 161.Langbein W Smaller Labs Eyeing Digital Pathology as Options Grow. https://www.360dx.com/clinical-lab-management/smaller-labs-eyeing-digital-pathology-options-grow. Accessed April 23, 2018. [Google Scholar]
- 162.Shaw EC, Hanby AM, Wheeler K, et al. Observer agreement comparing the use of virtual slides with glass slides in the pathology review component of the POSH breast cancer cohort study. J Clin Pathol. 2012;65(5):403–408. [DOI] [PubMed] [Google Scholar]
- 163.Mukhopadhyay S, Feldman MD, Abels E, et al. Whole slide imaging versus microscopy for primary diagnosis in surgical pathology: a multicenter blinded randomized noninferiority study of 1992 cases (pivotal study). Am J Surg Pathol. 2018;42(1):39–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Bauer TW, Schoenfield L, Slaw RJ, Yerian L, Sun Z, Henricks WH. Validation of whole slide imaging for primary diagnosis in surgical pathology. Arch Pathol Lab Med. 2013;137(4):518–524. [DOI] [PubMed] [Google Scholar]
- 165.Słodkowska J, Pankowski J, Siemiatkowska K, Chyczewski L. Use of the virtual slide and the dynamic real-time telepathology systems for a consultation and the frozen section intra-operative diagnosis in thoracic/pulmonary pathology. Folia Histochem Cytobiol. 2009;47(4):679–684. [DOI] [PubMed] [Google Scholar]
- 166.Saco A, Diaz A, Hernandez M, et al. Validation of whole-slide imaging in the primary diagnosis of liver biopsies in a University Hospital. Dig Liver Dis. 2017;49(11):1240–1246. [DOI] [PubMed] [Google Scholar]
- 167.ThermoFisher Scientific. Molecular Probes™ Handbook: A Guide to Fluorescent Probes and Labeling Technologies. https://www.thermofisher.com/us/en/home/references/molecular-probes-the-handbook/introduction-to-fluorescence-techniques.html. Accessed July 8, 2018.
- 168.Beier HT, Ibey BL. Experimental comparison of the high-speed imaging performance of an EM-CCD and sCMOS camera in a dynamic live-cell imaging test case. PLoS One. 2014;9(1):e84614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Park KS, Kim DU, Lee J, Kim GH, Chang KS. Simultaneous multicolor imaging of wide-field epi-fluorescence microscopy with four-bucket detection. Biomed Opt Express. 2016;7(6):2285–2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Fu X, Lennerz JK, Onozato M, Iafrate A, Yagi Y. Evaluation of a confocal WSI scanner for FISH slide imaging and image analysis. Diagn Pathol. 2017;3:249. [Google Scholar]
- 171.Liao J, Bian L, Bian Z, et al. Single-frame rapid autofocusing for brightfield and fluorescence whole slide imaging. Biomed Opt Express. 2016;7(11):4763–4768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Loussert Fonta C, Humbel BM. Correlative microscopy. Arch Biochem Biophys. 2015;581:98–110. [DOI] [PubMed] [Google Scholar]
- 173.Feingold B, Picarsic J, Lesniak A, Popp BA, Wood-Trageser MA, Demetris AJ. Late graft dysfunction after pediatric heart transplantation is associated with fibrosis and microvasculopathy by automated, digital whole-slide analysis. J Heart Lung Transplant. 2017;36(12):1336–1343. [DOI] [PubMed] [Google Scholar]
- 174.Blom S, Paavolainen L, Bychkov D, et al. Systems pathology by multiplexed immunohistochemistry and whole-slide digital image analysis. Sci Rep. 2017;7(1):15580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Christiansen EM, Yang SJ, Ando DM, et al. In silico labeling: predicting fluorescent labels in unlabeled images. Cell. 2018;173(3):792–803.e719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Bautista PA, Yagi Y. Improving the visualization and detection of tissue folds in whole slide images through color enhancement. J Pathol Inform. 2010;1:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Kothari S, Phan JH, Wang MD. Eliminating tissue-fold artifacts in histopathological whole-slide images for improved image-based prediction of cancer grade. J Pathol Inform. 2013;4:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Lopez XM, D’Andrea E, Barbot P, et al. An automated blur detection method for histological whole slide imaging. PLoS One. 2013;8(12):e82710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Campanella G, Rajanna AR, Corsale L, Schüffler PJ, Yagi Y, Fuchs TJ. Towards machine learned quality control: a benchmark for sharpness quantification in digital pathology. Comput Med Imaging Graph. 2018;65:142–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Jen KY, Olson JL, Brodsky S, Zhou XJ, Nadasdy T, Laszik ZG. Reliability of whole slide images as a diagnostic modality for renal allograft biopsies. Hum Pathol. 2013;44(5):888–894. [DOI] [PubMed] [Google Scholar]
- 181.Ozluk Y, Blanco PL, Mengel M, Solez K, Halloran PF, Sis B. Superiority of virtual microscopy versus light microscopy in transplantation pathology. Clin Transplant. 2012;26(2):336–344. [DOI] [PubMed] [Google Scholar]
- 182.Al-Janabi S, Huisman A, Jonges GN, Ten Kate FJ, Goldschmeding R, van Diest PJ. Whole slide images for primary diagnostics of urinary system pathology: a feasibility study. J Renal Inj Prev. 2014;3(4):91–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Brazdziute E, Laurinavicius A. Digital pathology evaluation of complement C4d component deposition in the kidney allograft biopsies is a useful tool to improve reproducibility of the scoring. Diagn Pathol. 2011;6 Suppl 1: S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Neil DAH, Bellamy CO, Smith M, et al. Global quality assessment of liver allograft C4d staining during acute antibody-mediated rejection in formalin-fixed, paraffin-embedded tissue. Hum Pathol. 2018;73:144–155. [DOI] [PubMed] [Google Scholar]
- 185.Daunoravicius D, Besusparis J, Zurauskas E, et al. Quantification of myocardial fibrosis by digital image analysis and interactive stereology. Diagn Pathol. 2014;9:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Fu R, Ma X, Bian Z, Ma J. Digital separation of diaminobenzidine-stained tissues via an automatic color-filtering for immunohistochemical quantification. Biomed Opt Express. 2015;6(2):544–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Isgro G, Calvaruso V, Andreana L, et al. The relationship between transient elastography and histological collagen proportionate area for assessing fibrosis in chronic viral hepatitis. J Gastroenterol. 2013;48(8):921–929. [DOI] [PubMed] [Google Scholar]
- 188.Manousou P, Burroughs AK, Tsochatzis E, et al. Digital image analysis of collagen assessment of progression of fibrosis in recurrent HCV after liver transplantation. J Hepatol. 2013;58(5):962–968. [DOI] [PubMed] [Google Scholar]
- 189.Calvaruso V, Dhillon AP, Tsochatzis E, et al. Liver collagen proportionate area predicts decompensation in patients with recurrent hepatitis C virus cirrhosis after liver transplantation. J Gastroenterol Hepatol. 2012;27(7):1227–1232. [DOI] [PubMed] [Google Scholar]
- 190.Manousou P, Dhillon AP, Isgro G, et al. Digital image analysis of liver collagen predicts clinical outcome of recurrent hepatitis C virus 1 year after liver transplantation. Liver Transpl. 2011;17(2):178–188. [DOI] [PubMed] [Google Scholar]
- 191.Calvaruso V, Burroughs AK, Standish R, et al. Computer-assisted image analysis of liver collagen: relationship to Ishak scoring and hepatic venous pressure gradient. Hepatology. 2009;49(4):1236–1244. [DOI] [PubMed] [Google Scholar]
- 192.Diaz Encarnacion MM, Griffin MD, Slezak JM, et al. Correlation of quantitative digital image analysis with the glomerular filtration rate in chronic allograft nephropathy. Am J Transplant. 2004;4(2):248–256. [DOI] [PubMed] [Google Scholar]
- 193.Servais A, Meas-Yedid V, Buchler M, et al. Quantification of interstitial fibrosis by image analysis on routine renal biopsy in patients receiving cyclosporine. Transplantation. 2007;84(12):1595–1601. [DOI] [PubMed] [Google Scholar]
- 194.Meas-Yedid V, Servais A, Noël LH, et al. New computerized color image analysis for the quantification of interstitial fibrosis in renal transplantation. Transplantation. 2011;92(8):890–899. [DOI] [PubMed] [Google Scholar]
- 195.Farris AB, Adams CD, Brousaides N, et al. Morphometric and visual evaluation of fibrosis in renal biopsies. J Am Soc Nephrol. 2011;22(1):176–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Farris AB, Chan S, Climenhaga J, et al. Banff fibrosis study: multicenter visual assessment and computerized analysis of interstitial fibrosis in kidney biopsies. Am J Transplant. 2014;14(4):897–907. [DOI] [PubMed] [Google Scholar]
- 197.Pickering JG, Boughner DR. Fibrosis in the transplanted heart and its relation to donor ischemic time. Assessment with polarized light microscopy and digital image analysis. Circulation. 1990;81(3):949–958. [DOI] [PubMed] [Google Scholar]
- 198.Lerut E, Kuypers DR, Verbeken E, et al. Acute rejection in non-compliant renal allograft recipients: a distinct morphology. Clin Transplant. 2007;21(3):344–351. [DOI] [PubMed] [Google Scholar]
- 199.Bräsen JH, Khalifa A, Schmitz J, et al. Macrophage density in early surveillance biopsies predicts future renal transplant function. Kidney Int. 2017;92(2):479–489. [DOI] [PubMed] [Google Scholar]
- 200.Moon A, Smith GH, Kong J, Rogers TE, Ellis CL, Farris ABB. Development of CD3 cell quantitation algorithms for renal allograft biopsy rejection assessment utilizing open source image analysis software. Virchows Arch. 2018;472(2):259–269. [DOI] [PubMed] [Google Scholar]
- 201.Sorrentino C, Scarinci A, D’Antuono T, et al. Endomyocardial infiltration by B and NK cells foreshadows the recurrence of cardiac allograft rejection. J Pathol. 2006;209(3):400–410. [DOI] [PubMed] [Google Scholar]
- 202.El-Badry AM, Breitenstein S, Jochum W, et al. Assessment of hepatic steatosis by expert pathologists: the end of a gold standard. Ann Surg. 2009;250(5):691–697. [DOI] [PubMed] [Google Scholar]
- 203.Hall AR, Dhillon AP, Green AC, et al. Hepatic steatosis estimated microscopically versus digital image analysis. Liver Int. 2013;33(6): 926–935. [DOI] [PubMed] [Google Scholar]
- 204.Vertemati M, Petrella D, Gambacorta M, Bonacina E, Vizzotto L. Predictive value of computerized morphometric analysis of steatosis in donor livers. Anal Quant Cytopathol Histpathol. 2014;36(3): 137–146. [PubMed] [Google Scholar]
- 205.Reis H, Peterek PT, Wohlschlaeger J, et al. Oil Red O-assessed macrosteatosis in liver transplant donor biopsies predicts ischemia-reperfusion injury and clinical outcome. Virchows Arch. 2014;464(2):165–174. [DOI] [PubMed] [Google Scholar]
- 206.Lauronen J, Häyry P, Paavonen T. An image analysis-based method for quantification of chronic allograft damage index parameters. APMIS. 2006;114(6):440–448. [DOI] [PubMed] [Google Scholar]
- 207.Moccia S, Mattos LS, Patrini I, et al. Computer-assisted liver graft steatosis assessment via learning-based texture analysis. Int J Comput Assist Radiol Surg. 2018;13(9):1357–1367. [DOI] [PubMed] [Google Scholar]
- 208.Goodman ZD, Becker RL Jr., Pockros PJ, Afdhal NH. Progression of fibrosis in advanced chronic hepatitis C: evaluation by morphometric image analysis. Hepatology. 2007;45(4):886–894. [DOI] [PubMed] [Google Scholar]
- 209.Huang Y, de Boer WB, Adams LA, MacQuillan G, Bulsara MK, Jeffrey GP. Image analysis of liver biopsy samples measures fibrosis and predicts clinical outcome. J Hepatol. 2014;61(1):22–27. [DOI] [PubMed] [Google Scholar]
- 210.Irshad H, Veillard A, Roux L, Racoceanu D. Methods for nuclei detection, segmentation, and classification in digital histopathology: a review-current status and future potential. IEEE Rev Biomed Eng. 2014;7:97–114. [DOI] [PubMed] [Google Scholar]
- 211.Denic A, Alexander MP, Kaushik V, et al. Detection and clinical patterns of nephron hypertrophy and nephrosclerosis among apparently healthy adults. Am J Kidney Dis. 2016;68(1):58–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Klapczynski M, Gagne GD, Morgan SJ, et al. Computer-assisted imaging algorithms facilitate histomorphometric quantification of kidney damage in rodent renal failure models. J Pathol Inform. 2012;3:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Abe T, Hashiguchi A, Yamazaki K, et al. Quantification of collagen and elastic fibers using whole-slide images of liver biopsy specimens. Pathol Int. 2013;63(6):305–310. [DOI] [PubMed] [Google Scholar]
- 214.Castillo-Rama M, Sebagh M, Sasatomi E, et al. “Plasma Cell Hepatitis” in Liver Allografts: Identification and Characterization of an IgG4-Rich Cohort. Am J Transplant. 2013;13(11):2966–2977. [DOI] [PubMed] [Google Scholar]
- 215.Mizuguchi Y, Specht S, Lunz JG 3rd, et al. SPRR2a enhances p53 deacetylation through HDAC1 and down regulates p21 promoter activity. BMC Mol Biol. 2012;13:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Serón D, Moreso F. Protocol biopsies in renal transplantation: prognostic value of structural monitoring. Kidney Int. 2007;72(6):690–697. [DOI] [PubMed] [Google Scholar]
- 217.Madabhushi A, Lee G. Image analysis and machine learning in digital pathology: Challenges and opportunities. Med Image Anal. 2016;33:170–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Sharma H, Zerbe N, Lohmann S, Kayser K, Hellwich O, Hufnagl P. A review of graph-based methods for image analysis in digital histopathology. Diagn Pathol. 2015;1(2015):61. [Google Scholar]
- 219.Bhatia AK, Phan JH, Kothari S, Shehata B, Wang M. Automated analysis of histopathological whole slide images to diagnose pediatric heart transplant rejection. J Heart Lung Transplant. 2015;34(4):S327. [Google Scholar]
- 220.Kayser K, Görtler J, Borkenfeld S, Kayser G. How to measure diagnosis-associated information in virtual slides. Diagn Pathol. 2011;6 Suppl 1:S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Riber-Hansen R, Vainer B, Steiniche T. Digital image analysis: a review of reproducibility, stability and basic requirements for optimal results. APMIS. 2012;120(4):276–289. [DOI] [PubMed] [Google Scholar]
- 222.Wan S, Zhao E, Kryczek I, et al. Tumor-associated macrophages produce interleukin 6 and signal via STAT3 to promote expansion of human hepatocellular carcinoma stem cells. Gastroenterology. 2014;147(6):1393–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Yoshida N, Kinugasa T, Miyoshi H, et al. A high RORγT/CD3 ratio is a strong prognostic factor for postoperative survival in advanced colorectal cancer: analysis of helper T cell lymphocytes (Th1, Th2, Th17 and regulatory T cells). Ann Surg Oncol. 2016;23(3):919–927. [DOI] [PubMed] [Google Scholar]
- 224.Abadjian MZ, Edwards WB, Anderson CJ. Imaging the tumor microenvironment. Adv Exp Med Biol. 2017;1036:229–257. [DOI] [PubMed] [Google Scholar]
- 225.Saltz J, Gupta R, Hou L, et al. Spatial organization and molecular correlation of tumor-infiltrating lymphocytes using deep learning on pathology images. Cell Rep. 2018;23(1):181–193.e187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Djuric U, Zadeh G, Aldape K, Diamandis P. Precision histology: how deep learning is poised to revitalize histomorphology for personalized cancer care. NPJ Precis Oncol. 2017;1(1):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Jordan MI, Mitchell TM. Machine learning: trends, perspectives, and prospects. Science. 2015;349(6245):255–260. [DOI] [PubMed] [Google Scholar]
- 228.Li Z, Zhang X, Müller H, Zhang S. Large-scale retrieval for medical image analytics: a comprehensive review. Med Image Anal. 2018;43:66–84. [DOI] [PubMed] [Google Scholar]
- 229.Zheng Y, Jiang Z, Zhang H, et al. Histopathological whole slide image analysis using context-based CBIR. IEEE Trans Med Imaging. 2018;37(7):1641–1652. [DOI] [PubMed] [Google Scholar]
- 230.Janowczyk A, Madabhushi A. Deep learning for digital pathology image analysis: a comprehensive tutorial with selected use cases. J Pathol Inform. 2016;7:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Sirinukunwattana K, Ahmed Raza SE, Tsang Yee-Wah, Snead DR, Cree IA, Rajpoot NM. Locality sensitive deep learning for detection and classification of nuclei in routine colon cancer histology images. IEEE Trans Med Imaging. 2016;35(5):1196–1206. [DOI] [PubMed] [Google Scholar]
- 232.Mobadersany P, Yousefi S, Amgad M, et al. Predicting cancer outcomes from histology and genomics using convolutional networks. Proc Natl Acad Sci USA. 2018;115(13):E2970–E2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Holzinger A Interactive machine learning for health informatics: when do we need the human-in-the-loop? Brain Inform. 2016;3(2):119–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Samek W, Binder A, Montavon G, Lapuschkin S, Muller KR. Evaluating the Visualization of What a Deep Neural Network Has Learned. IEEE Trans Neural Netw Learn Syst. 2017;28(11):2660–2673. [DOI] [PubMed] [Google Scholar]
- 235.DICOM Standards Committee Working Group 26 Pathology. Digital Imaging and Communications in Medicine (DICOM) Supplement 145: Whole Slide Microscopic Image IOD and SOP Classes. ftp://medical.nema.org/medical/dicom/final/sup145_ft.pdf Accessed July 2, 2018.
- 236.Goode A, Gilbert B, Harkes J, Jukic D, Satyanarayanan M. OpenSlide: a vendor-neutral software foundation for digital pathology. J Pathol Inform. 2013;4:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Bankhead P, Loughrey MB, Fernández JA, et al. QuPath: open source software for digital pathology image analysis. Sci Rep. 2017;7(1):16878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Williams E, Moore J, Li SW, et al. The Image Data Resource: a bioimage data integration and publication platform. Nat Methods. 2017;14(8):775–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Gutman DA, Khalilia M, Lee S, et al. The Digital Slide Archive: a software platform for management, integration, and analysis of histology for cancer research. Cancer Res. 2017;77(21):e75–e78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Wang F, Oh TW, Vergara-Niedermayr C, Kurc T, Saltz J. Managing and querying whole slide images. Proc SPIE Int Soc Opt Eng. 2012;8319:pii 83190J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Wang F, Kong J, Cooper L, et al. A data model and database for high-resolution pathology analytical image informatics. J Pathol Inform. 2011;2:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Fraggetta F, Garozzo S, Zannoni GF, Pantanowitz L, Rossi ED. Routine digital pathology workflow: the Catania experience. J Pathol Inform. 2017;8:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Gaigalas AK, Li L, Henderson O, et al. The development of fluorescence intensity standards. J Res Natl Inst Stand Technol. 2001;106(2):381–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Yaziji H, Eisen R, Wick M, et al. Immunohistochemistry cocktails are here to stay: Center for Medicare and Medicaid Services should revise its new reimbursement policy. Am J Clin Pathol. 2012;138(1):10–11. [DOI] [PubMed] [Google Scholar]
- 245.Im M, Chae H, Kim T, et al. Comparative quantitative analysis of cluster of differentiation 45 antigen expression on lymphocyte subsets. Korean J Lab Med. 2011;31(3):148–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.McGuinness PH, Painter D, Davies S, McCaughan GW. Increases in intrahepatic CD68 positive cells, MAC387 positive cells, and proinflammatory cytokines (particularly interleukin 18) in chronic hepatitis C infection. Gut. 2000;46(2):260–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Huang R, Wu H, Liu Y, et al. Increase of infiltrating monocytes in the livers of patients with chronic liver diseases. Discov Med. 2016;21(113):25–33. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.