Abstract
Psychiatric symptoms that coincide with reproductive transitions are related to changes in sex steroids, but studies show that this relationship is governed by individual women’s vulnerability to change rather than by differences in level. There is growing interest in the role of allopregnanolone (ALLO), a 3-α reduced metabolite of progesterone and a strong allosteric modulator of the GABAA receptor, in such symptoms, with enough evidence now across various times of reproductive transition to offer an overview of the role of this hormone in reproductive psychiatry. This review offers a brief overview, focusing on literature of the last three years, of the relationship between allopregnanolone and mood at menarche; in the menstrual cycle; in the peripartum; and in the menopausal transition. ALLO dysregulation is identified in all of these transitions and found to be associated with mood symptoms, though evidence of its exact role; its relationship to other systems; and directionality is not consistent.
Keywords: allopregnanolone, pregnancy, postpartum, PMDD, mood, anxiety, perimenopause
Introduction
Mood and anxiety disorders that occur in conjunction with reproductive transitions (menarche, the menstrual cycle, the perinatal period, and perimenopause) represent a unique opportunity in biological psychiatry – for these transitions are one of the few points in the lifespan, of men or women, for which we can identify a unique biological trigger and focus our research on biological elements that may be related to any increase or difference in psychiatric symptoms. The obvious biological trigger at these points is the change in levels of sex steroids, but the literature attempting to connect changes in estradiol and progesterone (P4) to symptoms has yielded few studies that can directly connect levels of these hormones to psychopathology. Instead, what has emerged is an understanding that certain women are vulnerable to sex steroid transitions (Bloch et al., 2000), and the search for the mechanism of that vulnerability is ongoing.
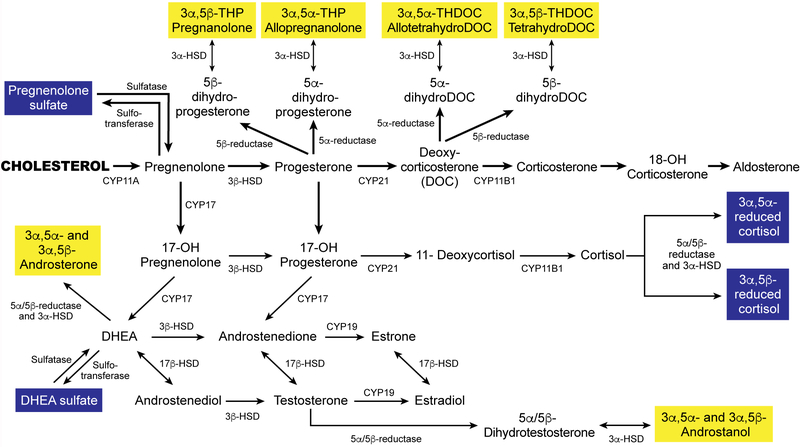
There is, however, increasing interest in the role of one class of sex steroids in reproductive psychiatry – the neuroactive steroids (NASs). This broad term encompasses both neurosteroids (derivatives of cholesterol synthesized de novo within the brain) as well as steroids synthesized in the adrenal glands that cross over the blood-brain barrier to act within the brain (McEvoy, Payne, & Osborne, 2018). Some limited literature exists for a role in mood and anxiety related to reproductive transitions for many of these NASs, including pregnanolone, allotetrahydroDOC, DHEA, DHEA-S, and testosterone (as reviewed in (McEvoy et al., 2018)), but the bulk of the evidence concerns allopregnanolone (ALLO), a 3-α reduced metabolite of progesterone. Figure 1 shows the synthesis of ALLO and related molecules. Each of these neuroactive steroids is formed by a reduction of its immediate precursor. Those highlighted in yellow, including ALLO, are inhibitory, and are potent allosteric modulators of the GABAA receptor. When they are present in low concentrations, they act by enhancing GABA action at the receptor (through altering both frequency and duration of chloride channel opening); when they are present in higher concentrations they can active the receptor directly (Carver & Reddy, 2013; Morrow, 2007; Rupprecht, 2003). The result is increased inhibition, including anxiolytic and sedative effects. In addition, ALLO has been shown to potentiate dopamine release, possibly enhancing feelings of pleasure and reward (Bristot et al., 2014; Rouge-Pont et al., 2002). Considerable animal and human research outside of reproductive periods supports the anxiolytic properties of ALLO (Schule, Nothdurfter, & Rupprecht, 2014), its connection with depression, bipolar disorder, negative symptoms of schizophrenia, and various anxiety disorders (Backstrom et al., 2014; Le Melledo & Baker, 2004; Schule et al., 2014; Uzunova, Ceci, Kohler, Uzunov, & Wrynn, 2003), and the reversal of ALLO dsyregulation with administration of antidepressants (Bristot et al., 2014; Uzunova et al., 2004).
Figure 1.
Synthesis of allopregnanolone from its precursors, cholesterol and progesterone.
In this paper, we hope to offer a brief overview of the evidence concerning allopregnanolone and psychiatric symptoms in the four major areas of reproductive transition mentioned above. We have made no attempt to be completely comprehensive, but have rather used the literature to lay a foundation of knowledge from older studies and then focus on new evidence from the last three years. Evidence to support this overview was found by conducting several related literature searches in PubMed, using the terms “allopregnanolone,” “mood,” “depression,” and “anxiety” in conjunction with “menarche,” “puberty,” “menstrual cycle,” “premenstrual dysphoric disorder,” “perinatal depression,” “postpartum depression,” “perimenopause,” and “menopause.” Additional articles were added by reviewing the references uncovered in these searches. Some of these areas have been the subject of review papers in the past; the contribution of this paper is to synthesize all of these areas together in a brief, user-friendly version designed for the general psychiatric reader.
Allopregnanolone and Menarche
Early research on allopregnanolone established that puberty is a time of gradually increasing allopregnanolone levels for both boys and girls at all Tanner stages; this was contrasted to early childhood, when allopregnanolone levels did not change at all (though levels of the related molecules progesterone and cortisol DID change in early childhood) (Fadalti et al., 1999). These results indicated a possible unique role for allopregnanolone in the changing neuroendocrine mechanisms involved in puberty for both sexes. Animal research has indicated a role for GABA agonists in synaptic pruning in female adolescent mice, which may affect spatial learning and could have implications for mood and anxiety symptoms (though these were not directly measured) (Afroz, Shen, & Smith, 2017) as well as a role for ALLO in neurosecretory functions related to the biology of reproduction (Giuliani et al., 2013). Overweight girls at puberty have been found to have elevated levels of ALLO, leading some to speculate that emotional and behavioral issues for overweight adolescent girls may be related to paradoxical reaction to this hypersecretion, though direct evidence for that is scant (Predieri et al., 2007). Corroborating evidence for this theory was offered over ten years ago, in a study that administered ALLO to pubertal female mice, causing increased anxiety and leading to a conclusion that the adolescent response to ALLO may be different that the adult response (Shen et al., 2007). There has been limited subsequent work, so the question of the relationship between ALLO and mood at menarche (and its directionality) is still largely unexamined.
Allopregnanolone and the Menstrual Cycle
In contrast to the dearth of evidence about menarche, the body of literature concerning variations in ALLO across the menstrual cycle, and in particular the role ALLO may play in symptoms of premenstrual dysphoric disorder (PMDD), is substantial. In 2014, Backstrom and colleagues offered a comprehensive overview of the theoretical mechanisms of ALLO’s role in mood across the menstrual cycle, and in particular its role in symptoms of PMDD. They cited well-established evidence pointing to the rise of ALLO (in tandem with progesterone) across the luteal phase for all women (as well as flat levels of ALLO across all phases in anovulatory cycles) (Backstrom et al., 2014). Moreover, numerous studies have found that the administration of exogenous progesterone induces negative mood states in women vulnerable to mood changes in the premenstrual period (Schmidt, Nieman, Danaceau, Adams, & Rubinow, 1998), and that blocking ALLO may improve symptoms (Nyberg, Backstrom, Zingmark, Purdy, & Poromaa, 2007). Stress reactivity has also been found to be related to the size of the increase in ALLO in the luteal phase (Ossewaarde et al., 2010). There is some evidence that the induction of negative mood states is not acting through the standard progesterone receptor, thus indicating that a metabolite, rather than progesterone itself, is a more likely mechanism (Chan, Mortola, Wood, & Yen, 1994). One early study found increased levels of ALLO and an increased ALLO/P4 ratio in women with PMDD compared to healthy controls, but also found that levels were lower in more symptomatic than less symptomatic women (in those with PMDD only), and that PMDD women failed to exhibit expected stress-related increases in ALLO (Girdler, Straneva, Light, Pedersen, & Morrow, 2001), indicating dysregulation but yielding uncertain conclusions about directionality. This dyregulation may also differ according to prior history of depression, and may be related to the metabolic pathways that govern conversion of P4 to ALLO (Klatzkin, Morrow, Light, Pedersen, & Girdler, 2006).
The common finding of induction of negative mood effects by rising allopregnanolone in the luteal phase is surprising, for it stands in contrast to the anxiolytic effects of the hormone cited in other contexts. Backstrom and colleagues explain this in terms of a paradoxical effect in certain individuals (similar to the paradoxical actions that can be seen with benzodiazepines at the same receptor), and provide evidence of an inverted U-shaped pattern of reactivity, with both low and high doses being anxiolytic and doses consistent with physiological doses typical of the luteal phase being anxiogenic (Backstrom, Bixo, & Stromberg, 2015; Backstrom et al., 2011). Others have concluded that it may not be absolute levels themselves, whether low or high, but the state of change that triggers symptoms (Schiller, Schmidt, & Rubinow, 2014).
Several additional studies in the last few years have added supporting evidence for these theories. One group blocked the action of 5-α reductase, the enzyme responsible for the conversion of P4 to ALLO, in healthy women and those with PMDD; women with PMDD had a significant reduction in symptoms when the luteal phase rise in ALLO was thus prevented (Martinez et al., 2016). The same group explored whether PMDD women have altered production of P4-derived neurosteroids, and found no difference from healthy women, but some evidence that differences in sulfotransferase activity may underlie enhanced sensitivity in PMDD (Nguyen et al., 2017). Timby and colleagues reported on direct brain effects of ALLO in women with PMDD sensitive to this paradoxical effect. They measured saccadic eye movement velocity (a common measure of sedation) in healthy women and those with PMDD after ALLO administration, and found that the healthy women displayed expected sedation while the PMDD women had an altered sensitivity that was greater in the luteal than the follicular phase (opposite to the pattern in healthy women) (Bixo, Johansson, Timby, Michalski, & Backstrom, 2018; Timby et al., 2016). This group also reviewed fMRI research, with the most consistent finding being increased activity in the amygdala in women with PMDD, a finding that is exacerbated by exogenous progesterone administration. They also point to animal research showing that this paradoxical sensitivity to ALLO in women with PMDD may be driven by GABAA receptor plasticity; a mouse model has shown increased anxiety-like behavior after ALLO administration when the α4βδ subunits of the receptor were upregulated (Bixo et al., 2018). Finally, some studies have used functional MRI to report on connections between endogenous levels of ALLO and other sex steroids and alterations in resting state functional connectivity in the brain, with more connections found in the late luteal phase than at other times in healthy women (and no data yet on women with PMDD) (Syan et al., 2017).
The evidence on PMDD thus points to a definitive role for ALLO in the symptoms of PMDD, with numerous studies finding that ALLO levels typical of the luteal phase (at the top of an inverted U-shaped curve) induce negative symptoms in women with PMDD, and others finding that it may be changing or fluctuating levels that cause symptoms rather than the absolute levels. It is clear, however, that this unique sensitivity either to levels or to fluctuations occurs in vulnerable women only (i.e., it is not present in women without PMDD) – and we do not yet know what the source of that vulnerability is.
Allopregnanolone and the Peripartum
As progesterone changes dramatically across pregnancy and after childbirth, so does allopregnanolone. ALLO levels rise steadily through pregnancy (produced primarily by the placenta) until shortly before delivery, when the ratio of ALLO to its precursor progesterone begins to drop. Both then drop precipitously immediately after childbirth. These rising levels of ALLO across pregnancy may lead to a down-regulation of receptors, which then fail to recover appropriately in women who develop symptoms in the postpartum (Gilbert Evans, Ross, Sellers, Purdy, & Romach, 2005; Maguire & Mody, 2008; Osborne et al., 2017). Knockout mice who were unable to downregulate the delta subunit of the GABAA receptor during pregnancy or to upregulate it postpartum developed depressive and anxiety-like behaviors in the postpartum. These symptoms were specific to the postpartum and did not occur when the mice were given an inhibitor that replaced the activity of the knocked out gene (Maguire & Mody, 2008). Similarly, an inability to recover receptor concentration postpartum has been associated with PPD (Gilbert Evans et al., 2005). Mood symptoms may therefore be arising either as a direct effect of changing hormone levels; as an indirect effect due to changes in receptor concentration or configuration; or as a result of mediating factors such as immune system or HPA axis changes.
Evidence of a relationship between perinatal mood and anxiety symptoms and absolute levels of ALLO has been mixed. Low ALLO has been correlated with higher third trimester depression scores (Hellgren, Akerud, Skalkidou, Backstrom, & Sundstrom-Poromaa, 2014), but not with those in the second trimester (Hellgren, Comasco, Skalkidou, & Sundstrom-Poromaa, 2017). Second trimester ALLO was correlated with negative emotional symptoms in another study, but it was measured in combination with pregnanolone and it is unclear if that relationship would have held for ALLO (Crowley et al., 2016). Mean ALLO levels measured across the peripartum have been positively correlated with anxiety scores (as measured by the STAI-S (Spielberger State-Trait Anxiety Inventory-State) (Deligiannidis et al., 2016). And mean ALLO levels at 6 weeks postpartum in over 1500 women have shown no difference between healthy postpartum women and those with PPD (Guintivano et al., 2018).
Low second trimester ALLO has also been found to predict subsequent PPD in women with pre-existing mood disorders, with each additional ng/mL f ALLO leading to a 63% reduction in the risk for PPD (Osborne et al., 2017); this work has now been replicated in a broader population containing women with mood disorders and healthy controls (Osborne et al., unpublished data). This group reported no effect when examining third trimester levels, and another small study also failed to find an association between third trimester ALLO and subsequent PPD (Deligiannidis et al., 2013), possibly because the extremely high levels of the third trimester may mean this is not the most sensitive time to detect an association. Those studies that have found an association not with concurrent but with subsequent symptoms indicate that interactions with other systems or other longer acting changes (receptor plasticity, gene transcription or expression, changes in rate-limiting enzymes (Agis-Balboa, Guidotti, & Pinna, 2014) may be important to understanding how ALLO interacts with perinatal symptoms.
Recent treatment trials of a synthetic formulation of ALLO, brexanolone, in PPD have shown promising results. A phase 2 randomized placebo-controlled trial resulted in a 70% remission rate in severe PPD in the treatment arm (n = 10) that was sustained after 30 days, while only one of the 11 women in the placebo group achieved remission (Kanes et al., 2017). Phase 3 results have not shown as dramatic a difference, but continue to show a statistically significant difference in remission rates between placebo and two different doses of brexanolone (Meltzer-Brody et al., 2018). The drug has been submitted for FDA approval and, if obtained, it will be the first ever drug approved specifically for PPD.
A very interesting recent review provides a possible link between these peripartum studies, where low ALLO seems to be linked to symptomatology (either concurrently or subsequently), and those in PMDD, where high ALLO is found to exacerbate symptoms. Burke and colleagues considered data on late postpartum depression in the context of breastfeeding cessation, and postulated that resumption of menses may alter GABAA receptors in such a way as to induce the paradoxical reactions found in PMDD studies (Burke, Susser, & Hermann, 2018).
Allopregnanolone and the Menopausal Transition
Evidence for the role of ALLO in the menopausal transition is not as abundant as in fertile women – and has been particularly sparse in recent years. Some have found that ALLO levels do not decline significantly in women across the menopausal transition (though they do in men), with levels in postmenopausal women similar to levels in the follicular phase of cycling women (Genazzani et al., 1998); others have found that levels are decreased compared to cycling women (Barbaccia et al., 2000; Bernardi et al., 2003). Depressed postmenopausal women have lower levels of ALLO than do healthy postmenopausal controls, and these levels are not altered by treatment with either an SSRI or estrogen (Morgan et al., 2010). This relationship may be different for women with premature ovarian failure than for women who undergo menopause at the normal age; one study found that ALLO levels were higher in women with premature ovarian failure when compared to standard postmenopausal women (Bernardi et al., 1998). One interesting area of exploration in the perimenopause has been alterations of the hormonal milieu during hormone replacement therapy (HRT). ALLO levels increase with all forms of HRT that contain progestins (Bernardi et al., 2003). Women with climacteric symptoms using estradiol 2 mg/day were randomized to placebo or progesterone suppositories in two doses and levels of progesterone and several metabolites were measured; the women were then sorted into low, medium, and high groups on the basis of their allopregnanolone concentrations. Significantly more negative mood symptoms were found in the medium ALLO group (corresponding to luteal phase levels) during progesterone administration, both between and within women (Andreen et al., 2005). This effect may be driven by dose, for another group using much lower doses of progesterone (100 mg vs. 400 and 800 in the prior study) found only mild sluggishness in response to increased ALLO (de Wit, Schmitt, Purdy, & Hauger, 2001). Exogenous ALLO itself has been tried as a vaginal supplement to estradiol therapy, but only for tolerability, and effects on mood are unknown.
Most recently, the relationship between ALLO levels and depressed mood was examined in women in the late menopausal transition and early postmenopause. ALLO levels were lower in women who had passed the menopausal transition and were correlated with time since last menses. Specific depressive symptoms were related to lower levels of ALLO, but this differed according to phase, with shallow sleep and digestive symptoms in late menopause transition and feelings of guilt, sleep disorders, and general somatic symptoms in early post-menopause (Slopien et al., 2018). Surprisingly, this study appears to be the first in over 8 years to look at the question of ALLO and menopausal mood symptoms.
Discussion and Future Directions
The evidence reviewed above points to a definitive, if ill-defined, role for ALLO in psychiatric symptoms related to reproductive transitions. We know that ALLO rises during puberty and fluctuates across the menstrual cycle, with higher levels present in the luteal phase. We also know that it rises drastically in pregnancy and crashes abruptly at childbirth, and that it probably decreases (and certainly stops fluctuating) after the menopausal transition. We know little about the relationship between ALLO and mood around the time of menarche. We do have evidence that either the increased level or the change of ALLO in the luteal phase in cycling women induces negative mood effects in vulnerable women, and that low ALLO (possibly through an intermediary such as receptor plasticity or other interacting systems) may play a role in depressive symptoms in the peripartum. We have speculation that an increase in ALLO (mediated by receptor changes) at the time of weaning may lead to a paradoxical reaction and increased negative mood effects, and some very limited evidence that low ALLO may be connected to specific depressive symptoms in after menopause.
Taken together, the literature shows us that ALLO dysregulation is an important factor in all of these transition points – and also shows us how much we do not know. Is ALLO related to mood changes in menarche? If receptor plasticity is an important mediator of ALLO’s effects, can blocking or stimulating receptors alter ALLO’s relationship to mood? What is the relationship between ALLO and anxiety, which few studies have addressed but which is a potent area for research given the GABA-ergic nature of ALLO? Other than receptor plasticity, what are other links in the chain when ALLO levels are associated with subsequent symptoms – should we be looking at the immune system, at cortisol, or at other elements of the stress response? And how do we connect ALLO with its direct effects in the brain? Beginning to answer these questions may help us to unlock the confusing relationship between biology and the mind at moments when we know what the biological changes are – and may thus help to pave the way for similar connections at times when those changes are less clear.
Acknowledgments.
The authors wish to acknowledge Cate Kiefe, an artist with the Department of Medical Illustration at Johns Hopkins University School of Medicine, who drew the figure.
Footnotes
Declaration of Interest. The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper. Dr. Osborne has research funding from the Brain and Behavior Foundation (Young Investigator Award), the NIH (NIMH (1K23 MH110607–01A1), and the Doris Duke Foundation (Early Clinician Investigator Award).
References
- Afroz S, Shen H, & Smith SS (2017). alpha4betadelta GABAA receptors reduce dendritic spine density in CA1 hippocampus and impair relearning ability of adolescent female mice: Effects of a GABA agonist and a stress steroid. Neuroscience, 347, 22–35. doi: 10.1016/j.neuroscience.2017.01.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agis-Balboa RC, Guidotti A, & Pinna G (2014). 5alpha-reductase type I expression is downregulated in the prefrontal cortex/Brodmann’s area 9 (BA9) of depressed patients. Psychopharmacology (Berl), 231(17), 3569–3580. doi: 10.1007/s00213-014-3567-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreen L, Sundstrom-Poromaa I, Bixo M, Andersson A, Nyberg S, & Backstrom T (2005). Relationship between allopregnanolone and negative mood in postmenopausal women taking sequential hormone replacement therapy with vaginal progesterone. Psychoneuroendocrinology, 30(2), 212–224. doi: 10.1016/j.psyneuen.2004.07.003 [DOI] [PubMed] [Google Scholar]
- Backstrom T, Bixo M, Johansson M, Nyberg S, Ossewaarde L, Ragagnin G, … van Wingen G (2014). Allopregnanolone and mood disorders. Prog Neurobiol, 113, 88–94. doi: 10.1016/j.pneurobio.2013.07.005 [DOI] [PubMed] [Google Scholar]
- Backstrom T, Bixo M, & Stromberg J (2015). GABAA Receptor-Modulating Steroids in Relation to Women’s Behavioral Health. Curr Psychiatry Rep, 17(11), 92. doi: 10.1007/s11920-015-0627-4 [DOI] [PubMed] [Google Scholar]
- Backstrom T, Haage D, Lofgren M, Johansson IM, Stromberg J, Nyberg S, … Bengtsson SK (2011). Paradoxical effects of GABA-A modulators may explain sex steroid induced negative mood symptoms in some persons. Neuroscience, 191, 46–54. doi: 10.1016/j.neuroscience.2011.03.061 [DOI] [PubMed] [Google Scholar]
- Barbaccia ML, Lello S, Sidiropoulou T, Cocco T, Sorge RP, Cocchiarale A, … Romanini C (2000). Plasma 5alpha-androstane-3alpha,17betadiol, an endogenous steroid that positively modulates GABA(A) receptor function, and anxiety: a study in menopausal women. Psychoneuroendocrinology, 25(7), 659–675. [DOI] [PubMed] [Google Scholar]
- Bernardi F, Hartmann B, Casarosa E, Luisi S, Stomati M, Fadalti M, … Genazzani AR (1998). High levels of serum allopregnanolone in women with premature ovarian failure. Gynecol Endocrinol, 12(5), 339–345. [DOI] [PubMed] [Google Scholar]
- Bernardi F, Pieri M, Stomati M, Luisi S, Palumbo M, Pluchino N, … Genazzani AR (2003). Effect of different hormonal replacement therapies on circulating allopregnanolone and dehydroepiandrosterone levels in postmenopausal women. Gynecol Endocrinol, 17(1), 65–77. [PubMed] [Google Scholar]
- Bixo M, Johansson M, Timby E, Michalski L, & Backstrom T (2018). Effects of GABA active steroids in the female brain with a focus on the premenstrual dysphoric disorder. J Neuroendocrinol, 30(2). doi: 10.1111/jne.12553 [DOI] [PubMed] [Google Scholar]
- Bloch M, Schmidt PJ, Danaceau M, Murphy J, Nieman L, & Rubinow DR (2000). Effects of gonadal steroids in women with a history of postpartum depression. Am J Psychiatry, 157(6), 924–930. doi: 10.1176/appi.ajp.157.6.924 [DOI] [PubMed] [Google Scholar]
- Bristot G, Ascoli B, Gubert C, Panizzutti B, Kapczinski F, & Rosa AR (2014). Progesterone and its metabolites as therapeutic targets in psychiatric disorders. Expert Opin Ther Targets, 18(6), 679–690. doi: 10.1517/14728222.2014.897329 [DOI] [PubMed] [Google Scholar]
- Burke CS, Susser LC, & Hermann AD (2018). GABAA dysregulation as an explanatory model for late-onset postpartum depression associated with weaning and resumption of menstruation. Arch Womens Ment Health. doi: 10.1007/s00737-018-0871-9 [DOI] [PubMed] [Google Scholar]
- Carver CM, & Reddy DS (2013). Neurosteroid interactions with synaptic and extrasynaptic GABA(A) receptors: regulation of subunit plasticity, phasic and tonic inhibition, and neuronal network excitability. Psychopharmacology (Berl), 230(2), 151–188. doi: 10.1007/s00213-013-3276-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan AF, Mortola JF, Wood SH, & Yen SS (1994). Persistence of premenstrual syndrome during low-dose administration of the progesterone antagonist RU 486. Obstet Gynecol, 84(6), 1001–1005. [PubMed] [Google Scholar]
- Crowley SK, O’Buckley TK, Schiller CE, Stuebe A, Morrow AL, & Girdler SS (2016). Blunted neuroactive steroid and HPA axis responses to stress are associated with reduced sleep quality and negative affect in pregnancy: a pilot study. Psychopharmacology (Berl), 233(7), 1299–1310. doi: 10.1007/s00213-016-4217-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit H, Schmitt L, Purdy R, & Hauger R (2001). Effects of acute progesterone administration in healthy postmenopausal women and normally-cycling women. Psychoneuroendocrinology, 26(7), 697–710. [DOI] [PubMed] [Google Scholar]
- Deligiannidis KM, Kroll-Desrosiers AR, Mo S, Nguyen HP, Svenson A, Jaitly N, … Shaffer SA (2016). Peripartum neuroactive steroid and gamma-aminobutyric acid profiles in women at-risk for postpartum depression. Psychoneuroendocrinology, 70, 98–107. doi: 10.1016/j.psyneuen.2016.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deligiannidis KM, Sikoglu EM, Shaffer SA, Frederick B, Svenson AE, Kopoyan A, … Moore CM (2013). GABAergic neuroactive steroids and resting-state functional connectivity in postpartum depression: a preliminary study. J Psychiatr Res, 47(6), 816–828. doi: 10.1016/j.jpsychires.2013.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadalti M, Petraglia F, Luisi S, Bernardi F, Casarosa E, Ferrari E, … Bernasconi S (1999). Changes of serum allopregnanolone levels in the first 2 years of life and during pubertal development. Pediatr Res, 46(3), 323–327. doi: 10.1203/00006450-199909000-00013 [DOI] [PubMed] [Google Scholar]
- Genazzani AR, Petraglia F, Bernardi F, Casarosa E, Salvestroni C, Tonetti A, … Luisi M (1998). Circulating levels of allopregnanolone in humans: gender, age, and endocrine influences. J Clin Endocrinol Metab, 83(6), 2099–2103. doi: 10.1210/jcem.83.6.4905 [DOI] [PubMed] [Google Scholar]
- Gilbert Evans SE, Ross LE, Sellers EM, Purdy RH, & Romach MK (2005). 3alpha-reduced neuroactive steroids and their precursors during pregnancy and the postpartum period. Gynecol Endocrinol, 21(5), 268–279. doi: 10.1080/09513590500361747 [DOI] [PubMed] [Google Scholar]
- Girdler SS, Straneva PA, Light KC, Pedersen CA, & Morrow AL (2001). Allopregnanolone levels and reactivity to mental stress in premenstrual dysphoric disorder. Biol Psychiatry, 49(9), 788–797. [DOI] [PubMed] [Google Scholar]
- Giuliani FA, Escudero C, Casas S, Bazzocchini V, Yunes R, Laconi MR, & Cabrera R (2013). Allopregnanolone and puberty: modulatory effect on glutamate and GABA release and expression of 3alpha-hydroxysteroid oxidoreductase in the hypothalamus of female rats. Neuroscience, 243, 64–75. doi: 10.1016/j.neuroscience.2013.03.053 [DOI] [PubMed] [Google Scholar]
- Guintivano J, Sullivan PF, Stuebe AM, Penders T, Thorp J, Rubinow DR, & Meltzer-Brody S (2018). Adverse life events, psychiatric history, and biological predictors of postpartum depression in an ethnically diverse sample of postpartum women. Psychol Med, 48(7), 1190–1200. doi: 10.1017/s0033291717002641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellgren C, Akerud H, Skalkidou A, Backstrom T, & Sundstrom-Poromaa I (2014). Low serum allopregnanolone is associated with symptoms of depression in late pregnancy. Neuropsychobiology, 69(3), 147–153. doi: 10.1159/000358838 [DOI] [PubMed] [Google Scholar]
- Hellgren C, Comasco E, Skalkidou A, & Sundstrom-Poromaa I (2017). Allopregnanolone levels and depressive symptoms during pregnancy in relation to single nucleotide polymorphisms in the allopregnanolone synthesis pathway. Horm Behav, 94, 106–113. doi: 10.1016/j.yhbeh.2017.06.008 [DOI] [PubMed] [Google Scholar]
- Kanes S, Colquhoun H, Gunduz-Bruce H, Raines S, Arnold R, Schacterle A, … Meltzer-Brody S (2017). Brexanolone (SAGE-547 injection) in post-partum depression: a randomised controlled trial. Lancet, 390(10093), 480–489. doi: 10.1016/s0140-6736(17)31264-3 [DOI] [PubMed] [Google Scholar]
- Klatzkin RR, Morrow AL, Light KC, Pedersen CA, & Girdler SS (2006). Associations of histories of depression and PMDD diagnosis with allopregnanolone concentrations following the oral administration of micronized progesterone. Psychoneuroendocrinology, 31(10), 1208–1219. doi: 10.1016/j.psyneuen.2006.09.002 [DOI] [PubMed] [Google Scholar]
- Le Melledo JM, & Baker G (2004). Role of progesterone and other neuroactive steroids in anxiety disorders. Expert Rev Neurother, 4(5), 851–860. doi: 10.1586/14737175.4.5.851 [DOI] [PubMed] [Google Scholar]
- Maguire J, & Mody I (2008). GABA(A)R plasticity during pregnancy: relevance to postpartum depression. Neuron, 59(2), 207–213. doi: 10.1016/j.neuron.2008.06.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez PE, Rubinow DR, Nieman LK, Koziol DE, Morrow AL, Schiller CE, … Schmidt PJ (2016). 5alpha-Reductase Inhibition Prevents the Luteal Phase Increase in Plasma Allopregnanolone Levels and Mitigates Symptoms in Women with Premenstrual Dysphoric Disorder. Neuropsychopharmacology, 41(4), 1093–1102. doi: 10.1038/npp.2015.246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEvoy K, Payne JL, & Osborne LM (2018). Neuroactive Steroids and Perinatal Depression: a Review of Recent Literature. Curr Psychiatry Rep, 20(9), 78. doi: 10.1007/s11920-018-0937-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer-Brody S, Colquhoun H, Riesenberg R, Epperson CN, Deligiannidis KM, Rubinow DR, … Kanes S (2018). Brexanolone injection in post-partum depression: two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet, 392(10152), 1058–1070. doi: 10.1016/s0140-6736(18)31551-4 [DOI] [PubMed] [Google Scholar]
- Morgan ML, Rapkin AJ, Biggio G, Serra M, Pisu MG, & Rasgon N (2010). Neuroactive steroids after estrogen exposure in depressed postmenopausal women treated with sertraline and asymptomatic postmenopausal women. Arch Womens Ment Health, 13(1), 91–98. doi: 10.1007/s00737-009-0106-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow AL (2007). Recent developments in the significance and therapeutic relevance of neuroactive steroids--Introduction to the special issue. Pharmacol Ther, 116(1), 1–6. doi: 10.1016/j.pharmthera.2007.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TV, Reuter JM, Gaikwad NW, Rotroff DM, Kucera HR, Motsinger-Reif A, … Schmidt PJ (2017). The steroid metabolome in women with premenstrual dysphoric disorder during GnRH agonist-induced ovarian suppression: effects of estradiol and progesterone addback. Transl Psychiatry, 7(8), e1193. doi: 10.1038/tp.2017.146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg S, Backstrom T, Zingmark E, Purdy RH, & Poromaa IS (2007). Allopregnanolone decrease with symptom improvement during placebo and gonadotropin-releasing hormone agonist treatment in women with severe premenstrual syndrome. Gynecol Endocrinol, 23(5), 257–266. doi: 10.1080/09513590701253511 [DOI] [PubMed] [Google Scholar]
- Osborne LM, Gispen F, Sanyal A, Yenokyan G, Meilman S, & Payne JL (2017). Lower allopregnanolone during pregnancy predicts postpartum depression: An exploratory study. Psychoneuroendocrinology, 79, 116–121. doi: 10.1016/j.psyneuen.2017.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossewaarde L, Hermans EJ, van Wingen GA, Kooijman SC, Johansson IM, Backstrom T, & Fernandez G (2010). Neural mechanisms underlying changes in stress-sensitivity across the menstrual cycle. Psychoneuroendocrinology, 35(1), 47–55. doi: 10.1016/j.psyneuen.2009.08.011 [DOI] [PubMed] [Google Scholar]
- Predieri B, Luisi S, Casarosa E, De Simone M, Balli F, Bernasconi S, … Iughetti L (2007). High basal serum allopregnanolone levels in overweight girls. Int J Obes (Lond), 31(3), 543–549. doi: 10.1038/sj.ijo.0803406 [DOI] [PubMed] [Google Scholar]
- Rouge-Pont F, Mayo W, Marinelli M, Gingras M, Le Moal M, & Piazza PV (2002). The neurosteroid allopregnanolone increases dopamine release and dopaminergic response to morphine in the rat nucleus accumbens. Eur J Neurosci, 16(1), 169–173. [DOI] [PubMed] [Google Scholar]
- Rupprecht R (2003). Neuroactive steroids: mechanisms of action and neuropsychopharmacological properties. Psychoneuroendocrinology, 28(2), 139–168. [DOI] [PubMed] [Google Scholar]
- Schiller CE, Schmidt PJ, & Rubinow DR (2014). Allopregnanolone as a mediator of affective switching in reproductive mood disorders. Psychopharmacology (Berl), 231(17), 3557–3567. doi: 10.1007/s00213-014-3599-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt PJ, Nieman LK, Danaceau MA, Adams LF, & Rubinow DR (1998). Differential behavioral effects of gonadal steroids in women with and in those without premenstrual syndrome. N Engl J Med, 338(4), 209–216. doi: 10.1056/nejm199801223380401 [DOI] [PubMed] [Google Scholar]
- Schule C, Nothdurfter C, & Rupprecht R (2014). The role of allopregnanolone in depression and anxiety. Prog Neurobiol, 113, 79–87. doi: 10.1016/j.pneurobio.2013.09.003 [DOI] [PubMed] [Google Scholar]
- Shen H, Gong QH, Aoki C, Yuan M, Ruderman Y, Dattilo M, … Smith SS (2007). Reversal of neurosteroid effects at alpha4beta2delta GABAA receptors triggers anxiety at puberty. Nat Neurosci, 10(4), 469–477. doi: 10.1038/nn1868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slopien R, Pluchino N, Warenik-Szymankiewicz A, Sajdak S, Luisi M, Drakopoulos P, & Genazzani AR (2018). Correlation between allopregnanolone levels and depressive symptoms during late menopausal transition and early postmenopause. Gynecol Endocrinol, 34(2), 144–147. doi: 10.1080/09513590.2017.1371129 [DOI] [PubMed] [Google Scholar]
- Syan SK, Minuzzi L, Costescu D, Smith M, Allega OR, Coote M, … Frey BN (2017). Influence of endogenous estradiol, progesterone, allopregnanolone, and dehydroepiandrosterone sulfate on brain resting state functional connectivity across the menstrual cycle. Fertil Steril, 107(5), 1246–1255.e1244. doi: 10.1016/j.fertnstert.2017.03.021 [DOI] [PubMed] [Google Scholar]
- Timby E, Backstrom T, Nyberg S, Stenlund H, Wihlback AN, & Bixo M (2016). Women with premenstrual dysphoric disorder have altered sensitivity to allopregnanolone over the menstrual cycle compared to controls-a pilot study. Psychopharmacology (Berl), 233(11), 2109–2117. doi: 10.1007/s00213-016-4258-1 [DOI] [PubMed] [Google Scholar]
- Uzunova V, Ceci M, Kohler C, Uzunov DP, & Wrynn AS (2003). Region-specific dysregulation of allopregnanolone brain content in the olfactory bulbectomized rat model of depression. Brain Res, 976(1), 1–8. [DOI] [PubMed] [Google Scholar]
- Uzunova V, Wrynn AS, Kinnunen A, Ceci M, Kohler C, & Uzunov DP (2004). Chronic antidepressants reverse cerebrocortical allopregnanolone decline in the olfactory-bulbectomized rat. Eur J Pharmacol, 486(1), 31–34. [DOI] [PubMed] [Google Scholar]