STRUCTURED ABSTRACT
Purpose:
Clinical interventions in programs such as cardiac rehabilitation (CR) are guided by clinical characteristics of participating patients. This study describes changes in CR participant characteristics over 20 yr.
Methods:
To examine changes in patient characteristics over time, we analyzed data from 1996 to 2015 (n = 5396) garnered from a systematically and prospectively gathered database. Linear, logistic, multinomial logistic or negative binomial regression were used, as appropriate. Effects of sex and index diagnosis were considered both as interactions and as additive effects.
Results:
Analyses revealed that mean age increased (60.7 to 64.2 yr), enrollment of women increased (26.8% to 29.6%), and index diagnosis has shifted; coronary artery bypass surgery decreased (37.2% to 21.6%) while heart valve (HV) repair/replace increased (0 to 11.4%). Risk factors also shifted with increases in body mass index (28.7 vs 29.6 kg/m2), obesity (33.2% to 39.6%), hypertension (51% to 62.5%), type 2 diabetes mellitus (17.3% to 21.7%), and those reporting current smoking (6.6% to 8.4%). Directly measured peak aerobic capacity remained relatively stable throughout. The proportion of patients on statin therapy increased from 63.6% to 98.9% coinciding with significant improvements in lipid levels.
Conclusions:
Compared to 1996, participants entering CR in 2015 were older, more overweight, and had a higher prevalence of coronary risk factors. Lipid values improved substantially concurrent with increased statin use. While the percentage of female participants increased, they continue to be underrepresented. HV patients now constitute 11.4% of the patients enrolled. Clinical programs need to recognize changing characteristics of attendees to best tailor interventions.
Keywords: cardiac rehabilitation, patient characteristics, risk factors, time
CONDENSED ABSTRACT
Cardiac rehabilitation programming is guided by clinical characteristics. Compared to 1996, participants in 2015 were older, more overweight, and had a higher prevalence of risk factors. Lipid values improved with increased statin use. While female participation increased, women continue to be underrepresented. Heart valve patients now constitute 11.4% of enrollees.
Appropriate clinical care should reflect the characteristics of the patients who will receive it. As the population ages,1 and proportions of risk factors change (eg, significant decrease in smoking),2 clinical care must change as well. Only by being cognizant of the changing demographic and clinical profile of patients can clinical programs deliver appropriate care and interventions. In this research, we used cardiac rehabilitation (CR) as an illustration of how clinical populations are changing over time and how those changes may affect the care that is offered.
Contemporary outpatient CR began in the early 1970s, when exercise programs were extended beyond hospital discharge to highly structured, physician supervised, and electrocardiographically monitored exercise programs.3 The focus was almost entirely on exercise training to reverse physical decline resulting from extended bed rest and participation was mostly limited to middle-class men <65 yr of age with uncomplicated myocardial infarction (MI) or coronary bypass surgery (CABG). Since that time CR has proven highly effective in broader populations, decreasing cardiac mortality by 26% over 3 yr and hospitalizations by 31% in the year after an acute coronary event.4 These positive health outcomes have been shown for a variety of diagnoses and CR programs grew to include chronic stable angina pectoris and heart transplant.5 More recently, in 2006, heart valve replacement/repair and percutaneous coronary intervention (without MI) were added as eligible diagnoses, followed by chronic systolic heart failure (HF) in 2014 and peripheral artery disease (PAD) in 2017.6–9 Thus, presently, referral to CR is a class 1-A recommendation of the American Heart Association.10
Contemporary CR has evolved into a multidisciplinary disease management program guided by case-managers11–13 and treatment is individualized based upon patient characteristics. Exercise programming and counseling varies substantially from an 83-year-old frail woman participant vs a 55-year-old but overweight male participant. Similarly, programming is significantly different for an individual with early onset coronary heart disease (CHD) and multiple coronary risk factors vs an older individual with isolated aortic valvular disease in the absence of CHD or left ventricular dysfunction.
There has also been increased effort to include a greater proportion of eligible patients.6 Historically women, the elderly, minorities and patients of low-socioeconomic status have been underrepresented.14 This is despite studies demonstrating that these patients similarly benefit from participation in CR.15–19 As more patients are enrolled (eg more women), clinical characteristics may also shift.
Over time CR programs have needed to adapt as patient demographics shifted and diagnoses of participants have expanded.20 To provide the requisite care and programming, understanding the characteristics of patients entering CR is critical. To this end, we examined the demographic and clinical characteristics of patients entering a CR program over a 20-yr period from 1996 to 2015.
METHODS
We analyzed prospectively gathered data on 5396 consecutive individuals entering the University of Vermont Medical Center phase 2 CR program between January 1996 and December 2015. For patients with multiple admissions to CR, only data from the initial enrollment was included. During the initial visit, we collected data on age, sex, anthropometric variables (stadiometer obtained height in meters, digital scale measured weight kilograms, and tape measured waist circumference in centimeters (measured at the level of the umbilicus), conventional risk factors (type 2 diabetes mellitus [DM], diagnosed by medical history and use of hypoglycemic medication; hypertension (HTN), diagnosed by medical history), current smoking, and fasting lipid levels (total cholesterol [TC], low-density lipoprotein cholesterol [LDL-C], high-density lipoprotein cholesterol [HDL-C], and triglycerides [TG]). Measures of total cholesterol, TG, HDL-C, and glucose were obtained in the morning following a 12-hr fast prior to starting CR. LDL-C was calculated by using the formula of Friedewald et al.21 Index diagnosis at entry into CR was recorded in a hierarchal fashion (ie, all patients with CABG for any indication were coded as CABG, then those with MI without CABG were coded as MI, then those without CABG/MI who received percutaneous coronary intervention were coded as PCI; followed by those who has angina treated medically and other diagnosis, such as congestive heart failure or valvular heart disease). Information on treatment with evidence-based cardiovascular drugs (antiplatelet agents, β-adrenergic blockers, statins, and angiotensin-converting enzyme inhibitors or angiotensin receptor blockers) was recorded. All patients underwent a symptom-limited treadmill test with collection and analysis of expired gas for determination of peak aerobic capacity (peak ) in mL/kg/min (relative) or in L/min (absolute). Comorbidity scores were calculated with a scale used in prior studies, but which has been not formally validated.22 A comorbidities score was calculated as a combination of the presence and severity of conditions that could limit activities within the CR program including chronic obstructive pulmonary disease, peripheral vascular disease, orthopedic problems, and cerebrovascular accident/stroke. Presence of a comorbid condition was obtained via electronic health record while severity was determined by direct observation during the baseline exercise test. Absence of a comorbid condition was given a score of 0. For each diagnosis a score of 1–3 signified; (1) not symptomatic during exercise, (2) symptomatic during exercise or (3) exercise-limiting.22
Statistical Analysis
Frequencies and means are presented visually by four 5-yr time periods (1996–2000, 2001–2005, 2006–2010, 2011–2015) based on date of entry into CR. In the statistical analyses; however, time was modeled as a linear trend with year as a continuous variable. The type of regression analysis used depended on outcome type: linear regression analysis for continuous outcomes, logistic or multinomial logistic regression for categorical outcomes, and negative binomial regression for count data (number of comorbidities). The impact of patients’ sex and index diagnosis (valve replacement vs other) on time trends were considered both as interactions and as additive effects; in other words, whether trends differed significantly in slope/trajectory over time and whether there was a group difference that was consistent over time. All analyses were conducted using SAS 9.4 (SAS Institute). Across all tests, statistical significance was defined as P < .05 (2-tailed).
RESULTS
Changes Over Time in Characteristics of the Overall Sample at Entry into CR
Over the twenty years, there were significant changes in clinical characteristics of our total population (Table 1). Age increased significantly from 60.8 to 64.2 yr with a yearly increase of 0.23 yr (t = 8.5, P < .0001). Accordingly, the percent of the population 65 yr of age or greater significantly increased from 40.2% to 49.2% with a yearly increase of 0.6% (χ2 = 21.6, P < .0001) and the percent 75 yr of age or greater increased from 11.2% to 19.9% with a yearly increase of 0.4% (χ2 = 18.6, P < .0001). The percentage of women increased as well from 26.8% to 29.6% with a yearly increase of 0.3% (χ2 = 7.7, P < .05). BMI and the percent of patients considered obese (BMI > 30) increased significantly (28.7 to 29.6, yearly increase 0.07, t = 5.1, P < .0001; and 33.9% to 38.8%, yearly increase 0.5%, χ2 = 15.4, P < .0001, respectively). Mean weight and waist circumference did not change significantly over time. However, this is due to an increasing proportion of women over time. When controlling for sex, both weight and waist circumference increase significantly over time (all P values < .01).
Table 1.
Demographic and Clinical Characteristics at Entry into Cardiac Rehabilitationa
| Period 1: 1996 – 2000 (n = 1174) | Period 2: 2001 – 2005 (n = 1371) | Period 3: 2006 – 2010 (n = 1407) | Period 4: 2011 – 2015 (n = 1444) | Average Yearly Change | Effect of Time | |
|---|---|---|---|---|---|---|
| Sex Difference |
Effect of Sex |
|||||
| Age, yr | ||||||
| All | 60.7 ± 11.5 | 62.3 ± 11.8 | 63.5 ± 11.1 | 64.2 ± 11.6 | 0.23 | 8.46c |
| Men | 59.9 ± 11.4 | 61.7 ± 11.2 | 62.4 ± 10.7 | 63.4 ± 11.3 | 3 | 8.63d |
| Women | 63.1 ± 11.4 | 64.3 ± 13.4 | 66.1 ± 11.5 | 66.1 ± 12.1 | ||
| Women, % | ||||||
| All | 26.8 | 24.1 | 28 | 29.6 | 0.30% | 7.72b |
| Older, % aged ≥ 65 yr | ||||||
| All | 40.2 | 44.8 | 44.8 | 49.2 | 0.56% | 21.56d |
| Men | 37.4 | 42.2 | 40.2 | 45.4 | 13% | 90.41d |
| Women | 47.9 | 52.9 | 56.6 | 58.4 | ||
| Elderly, % aged ≥ 75 yr | ||||||
| All | 11.2 | 17.2 | 17.6 | 19.9 | 0.44% | 18.58d |
| Men | 10.4 | 14.3 | 15 | 17.1 | 8.5% | 40.12d |
| Women | 13.7 | 26.3 | 24.4 | 26.6 | ||
| Weight, kg | ||||||
| All | 84.8 ± 18.1 | 86.9 ± 18.5 | 86.8 ± 19.6 | 86.7 ± 19.1 | 1.93 | ns |
| Men | 88.4 ± 17.2 | 90.7 ± 17.2 | 91.0 ± 18.4 | 90.5 ± 17.9 | 14.5 | -29.46d |
| Women | 75.0 ± 17.0 | 74.6 ± 17.4 | 76.2 ± 18.3 | 77.5 ± 18.7 | ||
| Waist, cm | ||||||
| All | 100.9 ± 13.7 | 101.8 ± 14.4 | 102.8 ± 14.5 | 101.8 ± 14.1 | ns | ns |
| Men | 103.0 ± 12.7 | 104.4 ± 13.4 | 104.7 ± 13.6 | 103.7 ± 13.1 | 8.5 | -18.91d |
| Women | 95.2 ± 14.7 | 93.0 ± 14.0 | 97.7 ± 15.5 | 97.1 ± 15.4 | ||
| Body mass index, kg/m2 | ||||||
| All | 28.7 ± 5.4 | 29.0 ± 5.5 | 30.1 ± 6.0 | 29.6 ± 5.9 | 0.07 | 5.05d |
| Men | 28.5 ± 5.0 | 29.1 ± 5.2 | 30.1 ± 5.5 | 29.4 ± 5.3 | ns | ns |
| Women | 29.1 ± 6.3 | 28.5 ± 6.4 | 30.0 ± 7.1 | 30.0 ± 7.1 | ||
| Obesity | ||||||
| All | 33.2 | 36.6 | 42.9 | 39.6 | 0.46% | 15.41d |
| Men | 31.1 | 37.1 | 43.7 | 38.1 | ns | ns |
| Women | 39.1 | 35.1 | 40.7 | 43 | ||
| Diabetes mellitus | ||||||
| All | 17.3 | 21.5 | 24.7 | 21.7 | 0.31% | 9.38b |
| Men | 16.1 | 21.6 | 25.4 | 21.2 | ns | ns |
| Women | 20.6 | 21.1 | 23.1 | 23.1 | ||
| Hypertension | ||||||
| All | 51 | 59.4 | 70.6 | 62.5 | 0.80% | 48.06d |
| Men | 48.7 | 58 | 69.9 | 63.3 | 11% | 11.87c |
| Women | 57.5 | 63.7 | 72.3 | 60.7 | ||
| Current smoking | ||||||
| All | 6.6 | 5.3 | 7.2 | 8.4 | 0.16% | 7.06b |
| Men | 7.3 | 5.3 | 6.8 | 8.6 | ns | ns |
| Women | 4.8 | 5.4 | 8.1 | 7.9 | ||
| Comorbidity score | ||||||
| All | 0.3 ± 0.9 | 0.4 ± 1.0 | 0.5 ± 1.2 | 0.6 ± 1.2 | 0.016 | 25.8d |
| Men | 0.3 ± 0.9 | 0.3 ± 1.0 | 0.5 ± 1.2 | 0.5 ± 1.2 | 0.12 | 5.47b |
| Women | 0.4 ± 0.9 | 0.4 ± 1.1 | 0.6 ± 1.2 | 0.7 ± 1.3 | ||
| Relative peak, mL/kg/min | ||||||
| All | 18.2 ± 5.9 | 18.0 ± 6.2 | 18.3 ± 6.5 | 18.2 ± 6.0 | ns | 0.29 |
| Men | 19.4 ± 6.1 | 19.0 ± 6.4 | 19.5 ± 6.7 | 19.2 ± 6.2 | 3.8 | -23.09d |
| Women | 14.9 ± 3.9 | 14.8 ± 4.3 | 15.0 ± 4.5 | 15.5 ± 4.2 | ||
| Absolute peak, L/min | ||||||
| All | 1.5 ± 0.6 | 1.6 ± 0.6 | 1.6 ± 0.7 | 1.6 ± 0.6 | ns | 1.4 |
| Men | 1.7 ± 0.6 | 1.7 ± 0.6 | 1.8 ± 0.7 | 1.7 ± 0.6 | 0.6 | -35.53d |
| Women | 1.1 ± 0.4 | 1.1 ± 0.4 | 1.1 ± 0.4 | 1.2 ± 0.4 | ||
Abbreviations: ns, nonsignificant;, oxygen uptake.
Data analyzed for statistical significance using ANOVA with 3 degrees of freedom or by χ2.
Data reported as mean ± standard deviation or percent.
P < .05.
P < .001.
P < .0001.
There were also significant increases in the prevalence of cardiac risk factors (Table 1). Prevalence of diabetes increased significantly from 17.3% to 21.7% with a yearly increase of 0.3%, (χ2 = 9.4, P < .05). Those with a diagnosis of hypertension have increased from 51% to 62.5% with a yearly increase of 0.8%, (χ2 = 48.1, P < .0001). Those reporting current smoking have increased from 6.6% to 8.4% with a yearly increase of 0.2%, (χ2 = 7.1, P < .05). Comorbidity scores also significantly increased from 0.3 to 0.6/4 with a yearly increase of 0.02 (χ2 = 25.8, P < .0001). Baseline aerobic capacity did not change over time. This lack of change persisted even when controlling for sex, age, and diagnosis.
There have also been significant changes in the index diagnosis (χ2(df=6) = 371.71, P < .0001; Table 2). The percentage of CABG patients decreased substantially when comparing time periods 1 and 4, 37.2% to 21.6% with a yearly decrease of 1.1%. Those entering the program due to medically treated angina also decreased from 5.4 to 1.5% with a yearly decrease of 0.29%. These changes mirrored significant increases in MI (29.7% to 39.6%), PCI for angina (18.6% to 22.4%), and HV surgery which went from zero to 11.4% of the population.
Table 2.
Index Diagnosis at Entry into Cardiac Rehabilitation
| Period 1: 1996 – 2000 (n = 1174) | Period 2: 2001 – 2005 (n = 1371) | Period 3: 2006 – 2010 (n = 1407) | Period 4: 2011 – 2015 (n = 1444) | Average Yearly Change | Effect of Time | |
|---|---|---|---|---|---|---|
| Sex Difference |
Effect of Sex |
|||||
| CABG, n = 1675 (31%) | ||||||
| All | 37.2 | 38.0 | 29.2 | 21.6 | −1.07% | 98.59a |
| Men | 39.2 | 40.2 | 33.2 | 23 | 11.2 | 48.93a |
| Women | 31.8 | 30.9 | 16 | 12.2 | ||
| MI, n = 1705 (32%) | ||||||
| All | 29.7 | 29.1 | 27.8 | 39.6 | 0.60% | 32.05a |
| Men | 27.8 | 28.3 | 25.7 | 38.4 | 5.3 | 25.32a |
| Women | 34.9 | 31.8 | 33.3 | 41.4 | ||
| Angina (PCI), n = 1209 (22%) | ||||||
| All | 18.6 | 18.3 | 29.6 | 22.7 | 0.37% | 14.91a |
| Men | 19.8 | 19 | 29.1 | 21.8 | ns | |
| Women | 15.2 | 15.9 | 31 | 24.3 | ||
| Angina (Medical Treatment), n = 159 (3%) | ||||||
| All | 5.4 | 3.7 | 1.8 | 1.5 | −0.29% | 43.59a |
| Men | 4.8 | 3.1 | 1.8 | 1.2 | 1.4 | 28.46a |
| Women | 7.0 | 5.5 | 1.8 | 2.1 | ||
| Valve Replacement, n = 218 (4%) | ||||||
| All | 4.7 | 10.6 | 0.82% | 176.03a | ||
| Men | 3.6 | 10 | 4.2 | 109.57a | ||
| Women | 7.6 | 14.5 | ||||
| CHF, n = 190 (4%) | ||||||
| All | 2.4 | 4.6 | 3.6 | 3.4 | ns | |
| Men | 2.9 | 4.1 | 3.5 | 3.2 | ns | |
| Women | 1.0 | 6.1 | 3.8 | 4.0 | ||
Abbreviations: CABG, coronary artery bypass graft; CHF, congestive heart failure; MI myocardial infarction; ns, nonsignificant; PCI, percutaneous coronary intervention.
Notes:
• 4% of patients were categorized as “other” and are not represented in this table.
• Overall effect of time was significant using multinomial logistic regression.
• Average yearly change calculated by pairwise logistic regression (target diagnosis vs all others).
• Statistical significance analyzed using χ2 or t-tests, as appropriate.
P < .0001.
Substantial changes have occurred in the percentage of patients taking cardiovascular medications at entry to CR (Table 3). Use of all 4 medications tracked increased. Antiplatelet agent use increased from 91.7% to 98.3% with a yearly increase of 0.5%, (χ2 = 71.8, P < .0001), β-adrenergic blockers increased from 68.7% to 82.7% with a yearly increase of 1%, (χ2 = 95.71, P < .0001), and angiotensin-converting enzyme inhibitors or angiotensin receptor blockers increased from 29.8% to 52.9% with a yearly increase of 1.6%, (χ2 = 180.5, P < .0001). The most striking change was seen in statin use which increased from 63.6% to 98.9% with a yearly increase of 2.7%, (χ2 = 627.83, P < .0001). The effect of this increase in statin use is reflected in the lipid levels seen in the Figure. At entry into CR, the mean ± standard deviation values for total cholesterol (189 + 40 vs 161 + 44 mg/dL), triglycerides (198 + 128 vs 145 + 112 mg/dL), LDL-C (113 + 34 vs 89 + 37 mg/dL) all decreased while HDL-C increased (39 + 10 vs 43 + 13 mg/dL) (all P values < .0001) from time period 1 to time period 4.
Table 3.
Cardiovascular Medications at Entry into Cardiac Rehabilitationa
| Period 1: 1996 – 2000 (n = 1174) | Period 2: 2001 – 2005 (n = 1371) | Period 3: 2006 – 2010 (n = 1407) | Period 4: 2011 – 2015 (n = 1444) | Average Yearly Change | Effect of Time | |
|---|---|---|---|---|---|---|
| Sex Difference | Effect of Sex | |||||
| Antiplatelet Agents | ||||||
| All | 91.7 | 92.9 | 95.7 | 98.3 | 0.50% | 71.8b |
| Men | 93.2 | 94.1 | 95.8 | 98.2 | 2.8% | 15.12c |
| Women | 87.3 | 88.8 | 95.7 | 98.4 | ||
| β-adrenergic Blocker | ||||||
| All | 68.7 | 71.6 | 79.5 | 82.7 | 1% | 95.71b |
| Men | 69.3 | 73.1 | 80.6 | 82.4 | ns | ns |
| Women | 67.3 | 67.1 | 76.9 | 83.4 | ||
| ACE inhibitor or ARB | ||||||
| All | 29.8 | 35.6 | 48.4 | 52.9 | 1.60% | 180.5b |
| Men | 29.1 | 36 | 49 | 53.2 | ns | ns |
| Women | 31.7 | 34.4 | 47 | 52.1 | ||
| Statin | ||||||
| All | 63.6 | 69.1 | 94.6 | 98.9 | 2.70% | 627.83b |
| Men | 65.5 | 71.1 | 95 | 99.2 | 8% | 14.22c |
| Women | 58.6 | 62.8 | 93.4 | 98.1 | ||
Abbreviations: ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; ns, nonsignificant.
Note: Statistical significance analyzed using χ2 or t-tests, as appropriate.
All data reported as percent.
P < .0001.
P < .001.
Sex Differences
A significant main effect of sex was seen for several characteristics (Table 1). Overall, mean age was 3 yr older in the female patients (t = 8.63, P < .0001). Weight and waist circumference were significantly lower in females (14.5 kg lower, t = −29.46, P < .0001 and 8.5 cm lower, t = −18.91, P < .0001, respectively). However, BMI and percent considered obese did not differ significantly by sex.
DM and current smoking also did not differ significantly by sex. However, HTN was more common in females (11% higher, χ2 = 11.87, P < .001) and they had higher comorbidity scores (χ2 = 5.47, P = .02). Fitness level was significantly lower in females. Women had a peak that was on average 3.8 mLO2/kg/min lower than men (t = −23.09, P < .0001).
Index diagnosis differed by sex (Table 2). Patients with a diagnosis of CABG were more likely to be male (11% higher, χ2 = 48.93, P < .001) and MI and HV patients were more likely to be female (5% higher, χ2 = 25.32, P < .001 and 4% higher, χ2 = 109.57, P < .001, respectively).
Medication use differed in some respects by sex (Table 3). Women were less likely to be taking antiplatelet agents or statins (10% fewer, χ2 = 15.12, P < .001 and 8% fewer, χ2 = 14.22, P < .001, respectively). However, the significant interaction (sex and time) in antiplatelet use as well as the nonsignificant differences in use of angiotensin-converting enzyme inhibitors/angiotensin receptor blockers and β-adrenergic blockers, as well as visual examination of the data demonstrate that over time prescription of all 4 medications have increased and differences by sex are dissipating.
Effects of Inclusion of Valve Patients
Given that the percent of valve patients has changed dramatically over the 20-yr period, the inclusion of these patients has shifted the characteristics of the population. Accordingly, data were examined for main effects of inclusion of HV patients as well as potential interactions between time and inclusion of these patients. Table 4 demonstrates how characteristics differ if HV patients were separated out.
Table 4.
Sample Characteristics of CR Population with and without Valve Patientsa
| Period 3: 2006 – 2010 (n = 1407) | Period 4: 2011 – 2015 (n = 1444) | Main Effect of Valve Procedure | Average Difference Between Valve and All Others | |
|---|---|---|---|---|
| Valve Replacement/Repair | ||||
| All | 4.69 | 10.53 | ||
| Age | ||||
| All | 63.5 ± 11.1 | 64.2 ± 11.6 | ||
| Valve | 65.8 ± 12.8 | 66.4 ± 12.9 | 2.9b | 2.30 |
| All others | 63.4 ± 11.0 | 63.8 ± 11.3 | ||
| Women | ||||
| All | 28.0 | 29.6 | 15.02c | 11.00% |
| Valve | 45.5 | 38.2 | ||
| All others | 27.1 | 28.6 | ||
| Weight, kg | ||||
| All | 86.8 ± 19.6 | 86.7 ± 19.1 | −6.61d | −8.0 |
| Valve | 80.0 ± 18.1 | 78.9 ± 16.5 | ||
| All others | 87.2 ± 19.6 | 87.6 ± 19.1 | ||
| Waist, cm | ||||
| All | 102.8 ± 14.5 | 101.8 ± 14.1 | −6.6d | −7.2 |
| Valve | 97.3 ± 14.4 | 94.6 ± 12.0 | ||
| All others | 103.1 ± 14.4 | 102.6 ± 14.2 | ||
| Body Mass Index, kg/m2 | ||||
| All | 30.1 ± 6.0 | 29.6 ± 5.9 | −5.93d | −2.1 |
| Valve | 28.3 ± 5.4 | 27.4 ± 5.0 | ||
| All others | 30.1 ± 6.1 | 29.9 ± 5.9 | ||
| Obesity | ||||
| All | 42.9 | 39.6 | ||
| Valve | 33.8 | 25.0 | 16.77d | −12% |
| All others | 43.3 | 41.2 | ||
| Diabetes Mellitus | ||||
| All | 24.7 | 21.7 | ||
| Valve | 12.1 | 8.5 | 22.48d | −11.00% |
| All others | 25.4 | 23.2 | ||
| Hypertension | ||||
| All | 70.6 | 62.5 | 4.30b | |
| Valve | 62.1 | 58.6 | −1.80% | |
| All others | 70.9 | 63.0 | 0.89% | |
| Current Smoking | ||||
| All | 7.2 | 8.4 | ||
| Valve | 0 | 3.9 | 7.83b | −3% |
| All others | 7.5 | 9.0 | ||
| Comorbidity Score | ||||
| All | 0.5 ± 1.2 | 0.6 ± 1.2 | ||
| Valve | 0.5 ± 1.0 | 0.4 ± 1.0 | ns | ns |
| All others | 0.6 ± 1.2 | 0.6 ± 1.3 | ||
| Antiplatelet Agents | ||||
| All | 95.7 | 98.3 | ||
| Valve | 84.8 | 95.4 | 20.72d | −21% |
| All others | 96.3 | 98.6 | ||
| β-adrenergic Blocker | ||||
| All | 79.5 | 82.7 | ||
| Valve | 81.8 | 77.0 | ns | ns |
| All others | 79.5 | 83.5 | ||
| ACE Inhibitor or ARB | ||||
| All | 48.4 | 52.9 | ||
| Valve | 28.8 | 32.2 | 38.54d | −14% |
| All others | 49.4 | 55.4 | ||
| Statin, % | ||||
| All | 94.6 | 98.9 | ||
| Valve | 90.0 | 96.0 | ns | ns |
| All others | 94.8 | 99.1 | ||
| Relative Peak , mL/kg/min | ||||
| All | 18.3 ± 6.5 | 18.2 ± 6.0 | ||
| Valve | 16.3 ± 5.0 | 16.8 ± 5.3 | −3.74c | −1.4 |
| All Others | 18.4 ± 6.6 | 18.4 ± 6.0 | ||
| Absolute peak, L/min | ||||
| All | 1.6 ± 0.7 | 1.6 ± 0.6 | ||
| Valve | 1.3 ± 0.5 | 1.3 ± 0.5 | −6.45d | −0.25 |
| All others | 1.6 ± 0.7 | 1.6 ± 0.6 | ||
Abbreviations: ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; ns, nonsignificant; VO2, oxygen uptake.
Data reported as percent or mean ± standard deviation.
Notes:
• Statistical significance analyzed using χ2 or t-tests, as appropriate.
• For hypertension, a significant interaction was found so the differences listed are the yearly changes that differ for valve patients and all other patients.
P < .05.
P< .001.
P < .0001.
A significant main effect of diagnoses was seen on several characteristics (Table 4). Mean age was 2.3 yr higher in the HV patients as compared to other diagnoses (t = 2.9, P < .05). The percentage of women was higher as well (11% higher, χ2 = 15.02, P < .001). Weight, waist circumference, BMI, and percent considered obese all were lower in the HV population (8 kg lower, t = −6.61, P < .0001; 7.2 cm lower, t = −6.6, P < .0001; 2.1 lower, t = −5.93, P < .0001; and 12% lower, x2 = 16.77, P < .0001, respectively).
Risk factors and clinical characteristics differed by HV status. Heart valve patients are less likely to have traditional CAD risk factors. Current smoking and diabetes were significantly lower in HV patients (3% lower, χ2 = 7.83, P < .05; 11% lower, χ2 = 22.48, P < .0001). Peak aerobic capacity, at entry, differed by diagnosis. Peak for HV patients was, on average, 1.4 mLO2/kg/min lower than nonHV patients (t = −3.74, P < .001). HTN is the only characteristic that had a significant interaction between time and diagnosis with it increasing over time in other diagnoses but decreasing in HV patients (χ2 = 16.77, P < .0001). However, this is likely an artifact of HV patients only being included in the last 2 time periods.
Given these differences in risk factors, medication usage also differed between HV patients and the other diagnoses. The use of antiplatelet agents and angiotensin-converting enzyme inhibitors or angiotensin receptor blockers were significantly lower in HV patients (21% lower, χ2 = 20.72, P < .0001, and 14% lower χ2 = 38.54, P < .0001, respectively). However, neither β-adrenergic blockers nor statin use differed significantly in the HV patients compared to other diagnoses.
DISCUSSION
In the present study of over 5000 individuals entering CR between the years of 1996 to 2015, we found that patients have become older, more overweight, and more likely to have risk factors such as DM and current smoking. Patients also have more comorbidities and a greater percentage were women. Yet, fitness measures were essentially unchanged. The type of patients entering CR has shifted the overall clinical profile. While still underrepresented, more women are entering CR. The women are significantly older, and less fit, with more comorbidities than men, which shifts the overall clinical sample accordingly. Additionally, coinciding with the introduction of drug-eluting stents in 2003, there has been an increase in the percentage of patients undergoing PCI and a resulting decrease in the numbers undergoing CABG.23 Other clinical changes have likely affected clinical characteristics. For example, American Heart Association Get with the Guidelines Initiative may have increased statin prescription and use.24 Finally, the inclusion of other patients (eg, HV) also shifts the characteristics of the sample. HV diagnoses increased from 0% to 11.4% of the entrants to CR. HV patients do not necessarily have clinical CHD, and differed compared to the traditional population, being less obese, less likely to have DM, less likely to smoke, and older. As a result, HV patients alter the clinical and demographic characteristics of CR (Tables 1 and 4). For example, if valve patients were excluded, the percent of patients categorized as obese would have increased from 33.2% to 41.1% (instead of to 39.5%).
This dataset demonstrates how a change in entry diagnoses, such as the inclusion of HV patients, can significantly change the characteristics of a clinical population. Changes such as this one is likely to continue with inclusion of other populations, such as patients with a diagnosis of HF (added 2014), and patients with symptomatic PAD (added 2017). These changes will likely further increase the heterogeneity of the CR population. In addition, CR continues to be underutilized, only 35.5% of people who survived an MI attended CR.25 If efforts to increase CR participation are successful, programs will likely see patients with different sets of characteristics than are seen currently. Currently underrepresented in CR are patients with some of the highest risk profiles, such as lower-SES patients and nonEnglish speaking individuals.26
Cardiac rehabilitation programming depends upon the characteristics of patients and goals of therapy, related both to physical functioning and prevention of future cardiac events. Historically, most CR patients have had CHD and a large body of literature has demonstrated that improving fitness and cardiac risk factors yields improved clinical outcomes.4 As other diagnoses are included, patients will have different needs. As demonstrated, HV patients differ from other CHD patients, being older and less aerobically fit. Consequently, training programs for HV patients might have increased focus on improving aerobic fitness and strength rather than on lowering cholesterol or losing weight. Those who have CHF will work on improving fitness but will also need education specific to managing their unique clinical characteristics.27,28 PAD patients are more likely to smoke and have DM and, thus, will need specific interventions around those risk factors. In addition, the focus of their exercise training differs as specific training protocols have been shown to increase time until onset of claudication and overall walking time.29 It will continue to be important to adjust CR programming based upon the diverse needs of an increasingly heterogenous patient population. Staffing requirements will need to be considered as well. Given the increase in patient heterogeneity, programs could benefit from having staff with diverse skill sets, able to handle the unique needs of patients with different medical needs. The ability to individualize treatment plans will need to increase. Patient complexity will also differ, suggesting a potential need for increasing staffing ratios. The greater prevalence of obesity, diabetes, smoking and comorbidities will require behavioral programs for weight loss, close monitoring of diabetes, and increasing expertise at smoking cessation interventions. Programs need to assure that patient needs are addressed, and care is delivered in a safe and appropriate fashion with a focus on individualizing treatment plans to optimize patient outcomes.
Limitations of this study include that it was performed at a single community-based university affiliated center with a relatively homogeneous population. Medical history was used to define some variables (eg, DM and HTN) possibly underestimating the true prevalence of these conditions. Additionally, diagnostic criteria for these conditions have changed over time. Detailed information on glycosylated hemoglobin levels and blood pressure were not available although there were no clinically meaningful changes in blood pressure in the sample during the 20-year time period. Diagnostic categories were not comprehensive because, for example, in the MI category, the proportions of non-ST segment elevation MI or ST segment elevation MI were not known, and the proportion of PCI or other treatment following MI were not presented. Furthermore, we decided to classify CABG and MI as mutually exclusive categories due to the profound impact of CABG upon patient recovery. Additionally, given how diagnoses were categorized we were unable to detail how characteristics changed within a single diagnosis or within combinations of diagnoses. Detailed psychosocial characteristics were not available for the whole time-period but depression scores were stable throughout. Finally, determination of smoking status was by patient history and self-report.
Conclusions
Over a 20-yr period, cardiac patients entering CR have become older, more obese, and have a higher prevalence of coronary risk factors. Lipid values have improved remarkably associated with increasing use of statin medications. The diagnoses of patients enrolled has changed with the percentage of patients enrolling in CR after CABG decreasing (37.2% to 21.6%), HV patients now constituting over 10% of patients, and other diagnoses, such as PCI patients, having increased as well. Given the diversity of and changing demographics throughout the United States, a consistent monitoring of age, sex and diagnosis within individual programs will need to take place to tailor interventions to specific patient needs.
Figure.
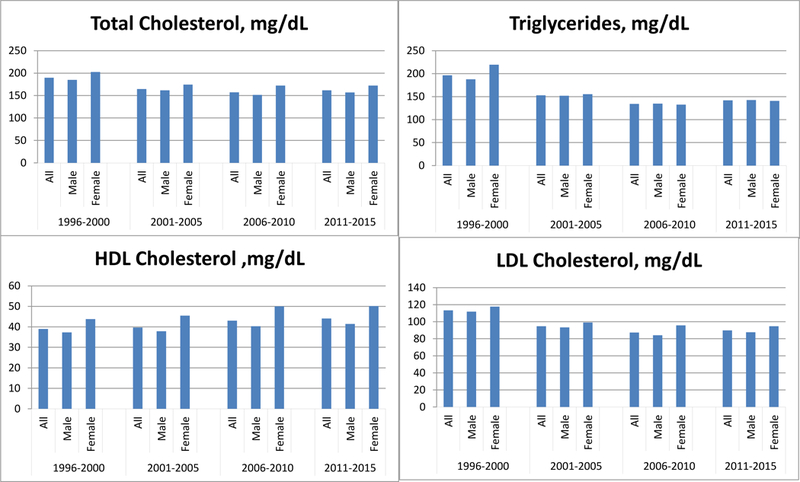
Lipid levels in patients entering cardiac rehabilitation over a 20-yr period.
Acknowledgements
This research was supported in part by National Institutes of Health Center of Biomedical Research Excellence award P20GM103644 from the National Institute of General Medical Sciences and Tobacco Centers of Regulatory Science award P50DA036114 from the National Institute on Drug Abuse and U.S. Food and Drug Administration. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Food and Drug Administration.
Footnotes
Conflict of interest: The authors declare no conflicts of interest.
REFERENCES
- 1.Colby SL, Ortman JM. Projections of the size and composition of the US population: 2014 to 2060 Current Population Reports, P25–1143, U.S. Census Bureau, Washington, DC, 2014. [Google Scholar]
- 2.U.S. Department of Health and Human Services. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2014. [Google Scholar]
- 3.Wenger NK. Current status of cardiac rehabilitation. J Am Coll Cardiol. 2008;51(17):1619–1631. [DOI] [PubMed] [Google Scholar]
- 4.Heran BS, Chen JMH, Ebrahim S, et al. Exercise-based cardiac rehabilitation for coronary heart disease. Cochrane Database of Systematic Reviews. 2011(7), CD001800–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Williams MA, Ades PA, Hamm LF, et al. Clinical evidence for a health benefit from cardiac rehabilitation: an update. Am Heart J. 2006;152(5):835–841. [DOI] [PubMed] [Google Scholar]
- 6.Ades PA, Keteyian SJ, Wright JS, et al. Increasing cardiac rehabilitation participation from 20% to 70%: a road map from the Million Hearts Cardiac Rehabilitation Collaborative. Mayo Clin Proc. 2017;92(2):234–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Forman DE, Sanderson BK, Josephson RA, Raikhelkar J, Bittner V. Heart failure as a newly approved diagnosis for cardiac rehabilitation: challenges and opportunities. J Am Coll Cardiol. 2015;65(24):2652–2659. [DOI] [PubMed] [Google Scholar]
- 8.McMahon SR, Ades PA, Thompson PD. The role of cardiac rehabilitation in patients with heart disease. Trends Cardiovasc Med. 2017;27(6):420–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Centers for Medicare and Medicaid Services. Proposed Decision Memo for Supervised Exercise Therapy (SET) for Symptomatic Peripheral Artery Disease (PAD) (CAG-00449N). 2017. [Google Scholar]
- 10.Smith SC Jr., Benjamin EJ, Bonow RO, et al. AHA/ACCF Secondary Prevention and Risk Reduction Therapy for Patients with Coronary and other Atherosclerotic Vascular Disease: 2011 update: a guideline from the American Heart Association and American College of Cardiology Foundation. Circulation. 2011;124(22):2458–2473. [DOI] [PubMed] [Google Scholar]
- 11.Haskell WL, Alderman EL, Fair JM, et al. Effects of intensive multiple risk factor reduction on coronary atherosclerosis and clinical cardiac events in men and women with coronary artery disease. The Stanford Coronary Risk Intervention Project (SCRIP). Circulation. 1994;89(3):975–990. [DOI] [PubMed] [Google Scholar]
- 12.DeBusk RF, Haskell WL, Miller NH, et al. Medically directed at-home rehabilitation soon after clinically uncomplicated acute myocardial infarction: a new model for patient care. Am J Cardiol. 1985;55(4):251–257. [DOI] [PubMed] [Google Scholar]
- 13.Ades PA. Cardiac rehabilitation and secondary prevention of coronary heart disease. New Engl J Med. 2001;345(12):892–902. [DOI] [PubMed] [Google Scholar]
- 14.Suaya JA, Shepard DS, Normand S-LT, Ades PA, Prottas J, Stason WB. Use of cardiac rehabilitation by Medicare beneficiaries after myocardial infarction or coronary bypass surgery. Circulation. 2007;116(15):1653–1662. [DOI] [PubMed] [Google Scholar]
- 15.Mayer-Berger W, Simic D, Mahmoodzad J, et al. Efficacy of a long-term secondary prevention programme following inpatient cardiovascular rehabilitation on risk and health-related quality of life in a low-education cohort: a randomized controlled study. Eur J Prev Cardiol. 2014;21(2):145–152. [DOI] [PubMed] [Google Scholar]
- 16.Nielsen KM, Meillier LK, Larsen ML. Extended cardiac rehabilitation for socially vulnerable patients improves attendance and outcome. Dan Med J. 2013;60(3):A4591. [PubMed] [Google Scholar]
- 17.Colbert JD, Martin BJ, Haykowsky MJ, et al. Cardiac rehabilitation referral, attendance and mortality in women. Eur J Prev Cardiol. 2015;22(8):979–986. [DOI] [PubMed] [Google Scholar]
- 18.Menezes AR, Lavie CJ, Forman DE, Arena R, Milani RV, Franklin BA. Cardiac rehabilitation in the elderly. Prog Cardiovasc Dis. 2014;57(2):152–159. [DOI] [PubMed] [Google Scholar]
- 19.Rodrigues P, Santos M, Sousa MJ, et al. Cardiac rehabilitation after an acute coronary syndrome: the impact in elderly patients. Cardiology. 2015;131(3):177–185. [DOI] [PubMed] [Google Scholar]
- 20.Audelin MC, Savage PD, Ades PA. Changing clinical profile of patients entering cardiac rehabilitation/secondary prevention programs: 1996 to 2006. J Cardiopulm Rehabil Prev. 2008;28(5):299–306. [DOI] [PubMed] [Google Scholar]
- 21.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. [PubMed] [Google Scholar]
- 22.Ades PA, Maloney A, Savage P, Carhart RL Jr., Determinants of physical functioning in coronary patients: response to cardiac rehabilitation. Arch Intern Med. 1999;159(19):2357–2360. [DOI] [PubMed] [Google Scholar]
- 23.Gogo PB Jr, Dauerman HL, Mulgund J, et al. ; CRUSADE Investigators. Changes in patterns of coronary revascularization strategies for patients with acute coronary syndromes (from the CRUSADE Quality Improvement Initiative). Am J Cardiol. 2007;99(9):1222–1226. [DOI] [PubMed] [Google Scholar]
- 24.Smaha LA. The American Heart Association get with the guidelines program. Am Heart J. 2004;148(5):S46–48. [DOI] [PubMed] [Google Scholar]
- 25.Fang J Use of outpatient cardiac rehabilitation among heart attack survivors—20 states and the District of Columbia, 2013 and four states, 2015. MMWR Morb Mortal Wkly Rep. 2017;66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gaalema DE, Elliott RJ, Monford ZH, Ades PA, Higgins ST. Effect of socioeconomic status on propensity to change risk behaviors following myocardial infarction: implications for healthy lifestyle medicine. Prog Cardiovasc Dis. 2017;60(1):159–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ades PA, Keteyian SJ, Balady GJ, et al. Cardiac rehabilitation exercise and self-care for chronic heart failure. JACC Heart Fail. 2013;1(6):540–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rengo JL, Savage PD, Barrett T, Ades PA. Cardiac rehabilitation participation rates and outcomes for patients with heart failure. J Cardiopulm Rehabil Prev. 2018; 38(1):38–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gerhard-Herman MD, Gornik HL, Barrett C, et al. 2016 AHA/ACC Guideline on the Management of Patients With Lower Extremity Peripheral Artery Disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;135(12):e726–e779. [DOI] [PMC free article] [PubMed] [Google Scholar]


