Abstract
Background
Coronary endothelial dysfunction is a precursor of atherosclerosis and adverse outcomes. Whether endothelial dysfunction is a localized or generalized phenomenon in humans remains uncertain.
Objectives
We simultaneously measured femoral and coronary vascular function with the hypothesis that peripheral vascular endothelial function will be reflective of coronary endothelial function.
Approach and Results
Eighty-five subjects underwent coronary angiography for evaluation of chest pain or abnormal stress tests. Endothelium-dependent and -independent vascular function were measured using intra-coronary and intra-femoral infusions of acetylcholine and sodium nitroprusside, respectively. Coronary flow reserve was assessed using intra-coronary adenosine infusion. Flow velocity was measured in each circulation using a Doppler wire (FloWire, EndoSonics). Coronary vascular resistance (CVR) and femoral vascular resistance (FVR) were calculated as mean arterial pressure (mmHg)/coronary blood flow (ml/min) and mean arterial pressure (mmHg)/femoral average peak velocity (cm/s), respectively. Mean age was 53±11 years, 37% female, 44% hypertensive, 12% diabetic and 38% had obstructive coronary artery disease. There was a correlation between the change in FVR with acetylcholine and acetylcholine-mediated changes in both the CVR (r=0.27; p=0.014) and in the epicardial coronary artery diameter (r=−0.25; p=0.021), indicating that subjects with normal endothelial function in the femoral circulation had normal endothelial function in the coronary epicardial and microcirculation, and vice versa. The coronary vasodilator response to adenosine also correlated with the femoral vasodilatation with acetylcholine (r=0.4; p=0.0002). There was no correlation between the coronary and femoral responses to sodium nitroprusside.
Conclusions
Endothelial functional changes in the peripheral and coronary circulations were modestly correlated. Thus, peripheral microvascular endothelial function reflects endothelium-dependent coronary epicardial and microvascular function, and the coronary flow reserve.
Keywords: peripheral microvascular function, coronary vascular function, endothelial function, microvasculature, coronary flow reserve
INTRODUCTION
The endothelium modulates vascular health by releasing a variety of paracrine factors that modulate smooth muscle vasomotion, platelet activation, and over the longer term influence endothelial layer integrity and susceptibility to atherosclerosis 1–5. These endothelium-derived factors include nitric oxide (NO) that is synthesized in the endothelial cells by the constitutive action of endothelial nitric oxide synthase on L-arginine that is converted to L-citrulline and NO. NO then diffuses into the lumen and contributes to passivation of platelet aggregation and into the smooth muscle to stimulate cyclic guanosine monophosphate-dependent relaxation. Other endothelium-dependent vasodilators include endothelium-derived hyperpolarizing factors, prostaglandins, carbon monoxide and others 6–9. Release of these endothelium-dependent agonists can be stimulated by pharmacologic probes such as acetylcholine, bradykinin, and substance P, and by physical stimuli including increased flow or shear 10–14.
We and others have demonstrated that the endothelial reactivity in response to these pharmacologic probes indicative of vasomotion during physiologic stimulation as well. Coronary flow and epicardial responses to pacing, cold pressor testing and mental stress correlate with the responses to acetylcholine 7, 15–17. We also have shown that forearm microvascular dilation in response to exercise mediated by NO release is greater in healthy subjects compared to those with hypercholesterolemia 18. Importantly, endothelial dysfunction in the coronary circulation is an independent predictor of worse long term prognosis in patients with and without significant coronary artery disease (CAD) 19–21. Moreover, coronary microvacular endothelial dysfunction is commonly observed in patients with chest pain and non-obstructed coronary arteries 22. Finally, treatments associated with improvement in cardiovascular outcomes, including physical exercise, statins and angiotensin antagonists, at least partly, impart their beneficial effects by improving endothelial function 23–26.
Endothelial function in the coronary and peripheral circulations can be measured using invasive techniques using infusions of endothelium-dependent agonists 27–31. In subjects with normal coronary endothelial function, acetylcholine promotes vasodilation of both the coronary epicardial and microvascular circulations by stimulating endothelial muscarinic receptors that activates endothelial nitric oxide synthase to synthesize NO and other endothelium-dependent vasodilators. However, exposure to cardiovascular risk factors such as hypercholesterolemia, hypertension, aging and atherosclerosis leads to endothelial dysfunction and absence or reduction in NO synthesis. In these circumstances, the direct constrictor effects of acetylcholine on vascular smooth cells predominate, and the net effect is epicardial coronary vasoconstriction and reduced microvascular vasodilation 7, 32–36
Non-invasive techniques that utilize increased shear as a stimulus for nitric oxide release are commonly used to test brachial arterial endothelial function. A significant correlation between flow-mediated brachial artery dilation and coronary epicardial endothelial function, both estimates conductance vessel function, has been reported previously 37–40. However, whether endothelial function in both the coronary epicardial and microcirculation is similar to endothelial function in the peripheral circulation, assessed in detail using acetylcholine infusions, remains unknown, and was the aim of this study. Herein, we measured endothelium-dependent and -independent coronary epicardial and microvascular function and coronary flow reserve and compared these measures with endothelium-dependent and -independent function in the femoral circulation. Studies were performed simultaneously in patients with and without atherosclerotic risk factors and CAD. Our hypothesis was that endothelial function in different vascular beds is similar, and therefore endothelial dysfunction in the coronary epicardial arteries and microcirculation will be reflected in the femoral microcirculation.
MATERIAL AND METHODS
The data that support the findings of this study are available from the corresponding author upon reasonable request. We studied 85 subjects with chest pain or abnormal cardiac tests who underwent cardiac catheterization at the Cardiology branch of the National Institutes of Health. The study was approved by the Institutional Review Board, and all subjects gave written informed consent. Subjects with an acute coronary syndrome, myocardial infarction in the past 3 months, heart failure of New York Heart Association class III to IV, severe valvular disease, or those with left main or 3-vessel disease were excluded. CAD was defined as any angiographic evidence of plaque, luminal irregularity, or stenosis in any epicardial coronary artery. Non-obstructive CAD was defined as angiographic evidence of <50% stenosis in the epicardial coronary artery. Cardiac risk factors included age, body mass index (BMI), history of tobacco smoking (current or use within the past year), diabetes, hypertension, and hyperlipidemia. Diabetes was defined as a fasting blood glucose level >126 mg/dL or if the subject was receiving insulin or oral hypoglycemic therapy. Hypertension was defined as systolic blood pressure ≥140 mm Hg or diastolic pressure ≥90 mm Hg on at least 3 occasions, or the patient was being treated with medications. Hyperlipidemia was defined as a fasting serum low-density lipoprotein >130 mg/dL or if the subject was being treated with lipid-lowering medications or dietary modification.
Subjects were asked to refrain from smoking, drinking alcohol or caffeinated beverages for at least 24 hours before the studies. Additionally, all cardiac medications were withdrawn at least 48 hours before the study.
Coronary Vasomotor Function Assessment
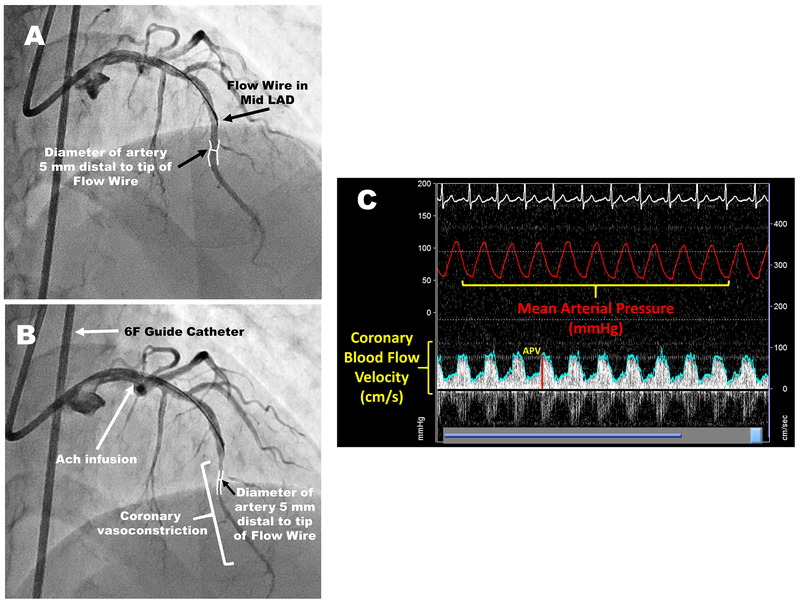
After performing diagnostic coronary angiography, a 3F infusion catheter was introduced via a 7F guide catheter into a non-obstructed coronary artery (stenosis severity ≤50%). Blood flow velocity was measured, as previously described, with a 0.014- or 0.018-in Doppler wire (FloWire, EndoSonics) 19. Coronary blood flow (CBF) was calculated as arterial cross-sectional area X average flow velocity X 0.5 41. The change in CBF was calculated as: (CBFpeak-CBFrest/CBFrest) x100. Coronary artery diameter measured in a 0.25- to 0.5-cm segment of vessel beginning 0.25 cm beyond the tip of the flow wire. Coronary vascular resistance (CVR) was calculated as: mean arterial pressure/CBF. Left coronary angiograms were obtained in the best projection to estimate epicardial coronary artery diameter at baseline and after each infusion to determine the percent change in diameter during drug administration compared to baseline and calculate the CBF in response to each vasoactive medication. Quantitative angiography was performed by one observer blinded to the identity of the study infusion with ARTREK software (Quantim 2001, StatView, ImageComm Systems, Inc) or the PIE medical CAAS system 36 (Figure 1).
Figure 1: Coronary angiograms, intracoronary Doppler tracing and hemodynamic measurements during the study protocol.
The Doppler Wire is positioned in the mid-left anterior descending coronary artery. Coronary artery diameter is measured 5 mm distal to the Doppler wire tip at rest (A) and after acetylcholine infusion (B). Acetylcholine infusion resulted in severe coronary vasoconstriction. (C) Intracoronary Doppler tracing and hemodynamic measurements of the subject during the study protocol. LAD: left anterior descending artery; Ach: acetylcholine; APV: average peak velocity.
Endothelium-dependent vasodilation was assessed using intracoronary acetylcholine infusions for 2-minutes at 15 µg/min [estimated intracoronary concentrations of 10−6 mol/L). Ten minutes after acetylcholine infusion, endothelium-independent function was assessed using a 3-minute intracoronary infusion of 20 µg/min sodium nitroprusside, followed by measurement of coronary flow reserve with a 2-minute infusion of 2.2 mg/min of adenosine. After each medication infusion, cine image was obtained for quantitative coronary angiography.
Femoral Vascular Function Assessment
A 6F multipurpose catheter (CORDIS, Inc) was introduced through a 7F femoral arterial sheath to just above the sheath in the femoral artery. A 0.018-in Doppler wire was introduced and positioned 1 cm beyond the catheter tip. Because the Doppler Wire was positioned proximal to the delivery of the vasoactive medications, measurements of femoral artery diameter changes were not made. However, to exclude any significant changes in femoral artery diameter at the level of Doppler Wire in response to different vasoactive medications, we measured the changes in femoral artery diameter at the level of the flow wire in a preliminary study of 24 patients using serial angiography. Baseline diameter was 5.1±0.9 mm, while in response to 300 µg/min acetylcholine was 5.1±0.9 mm and 40 µg/min sodium nitroprusside was 5.1±0.9 mm. There was no constriction observed in femoral artery diameter with acetylcholine in patients with atherosclerosis or its risk factors. Hence, the femoral vascular resistance (FVR) was calculated as the mean arterial pressure/femoral average peak velocity 31, 42.
After obtaining the resting femoral arterial average peak flow velocity, endothelium-dependent vasodilation in the femoral circulation was assessed using intra-femoral infusions of acetylcholine (300 µg/min) for 2-minutes. Following a 15-minute rest period and return to baseline flow velocity, intra-femoral sodium nitroprusside infusion (40 µg/min for 2-minutes) was initiated to assess endothelium-independent function and the femoral average peak velocity measurement.
Statistical analysis
Clinical variables are summarized as mean ± standard deviation for continuous variables and using count (%) for categorical variables. Data normality was assessed using the Kolmogorov-Smirnov test. Paired t-tests were used when no gross violation of normality was present and Wilcoxon signed-rank tests were used for non-normally distributed variables. All probability variables were two-tailed and a significance level of 0.05 was used for all analyses. Univariate and multivariate linear regression analyses were performed to determine the relationships between changes in CVR and FVR and clinical variables in both circulations in response to acetylcholine and sodium nitroprusside. Covariates entered in this regression model were age, BMI, sex, history of hypertension, diabetes, current smoking, hyperlipidemia and presence of CAD. Correlation analyses were performed using Spearman’s correlation coefficient. Analysis was performed using IBM SPSS version 25 (IBM, New York, NY).
RESULTS
Baseline characteristics of the enrolled subjects are summarized in Table 1. Mean age was 53±11 years and 54 (64%) were male subjects.
Table 1:
Characteristics of the study population
| All subjects (N=85) | |
|---|---|
| Age (years), mean±SD | 53±11 |
| Female, N (%) | 31 (37%) |
| BMI (kg/m2), mean±SD | 29±5 |
| White, N (%) | 68 (80%) |
| Cholesterol (mg/dL), mean±SD | 218±49 |
| LDL (mg/dL), mean±SD | 142±42 |
| HDL (mg/dL), mean±SD | 41±12 |
| CAD, N (%) - No CAD - Non-obstructive CAD - Obstructive CAD |
47 (55%) 6 (7%) 32 (38%) |
| History of hypertension, N (%) | 37 (44%) |
| History of diabetes, N (%) | 10 (12%) |
| History of smoking, N (%) | 52 (61%) |
BMI: body mass index, LDL: Low-density lipoprotein, HDL: High-density lipoprotein, CAD: coronary artery disease. Obstructive CAD: coronary artery luminal stenosis ≥50%.
Coronary Vascular Function
There was no significant change in the mean arterial pressure in response to intra-coronary acetylcholine infusions (108±15 [rest] vs 108±15 [acetylcholine] mm Hg, p=0.87). The mean coronary epicardial artery diameter change in response to acetylcholine was 1.4±10% (range −25% to 30%), with 38 (45%) subjects demonstrating constriction (defined as % coronary diameter < 0%), indicating endothelial dysfunction. CBF increased by 122±98% (range −30 to 432%) and CVR decreased by −46±23% (range −80% to 43%) with acetylcholine (Table 2). In multivariate regression analyses, increased age was an independent predictor of the decrease in CBF in response to acetylcholine (Supplemental Table I).
Table 2:
Vasomotor parameters and changes in response to vasoactive medications
| Mean±SD | %Change | |
|---|---|---|
| Coronary Study | ||
| Coronary blood flow (mL/min) - Baseline - Acetylcholine infusion - Sodium nitroprusside infusion - Adenosine infusion |
42±30 94±70 99±68 172±110 |
122±98% 152±84% 361±169% |
| Coronary vascular resistance (mmHg.mL −1.min) - Baseline - Acetylcholine infusion - Sodium nitroprusside infusion - Adenosine infusion |
3.6±2.3 2.0±1.6 2.0±1.3 0.98±0.7 |
−46±23% −59±15% −75±9% |
| Coronary artery diameter (mm) - Baseline - Acetylcholine infusion - Sodium nitroprusside infusion |
2.5±0.68 2.5±0.73 2.9±0.78 |
1.4±10% 19.4±16% |
| Femoral Study | ||
| Femoral blood flow velocity (cm/sec) - Baseline - Acetylcholine infusion - Sodium nitroprusside infusion |
19±8 46±26 42±16 |
139±108% 124±57% |
| Femoral artery resistance (mmHg.mL −1.min) - Baseline - Acetylcholine infusion - Sodium nitroprusside infusion |
5.9±2.3 2.8±1.6 2.3±0.9 |
−54±17% −59±10% |
In response to sodium nitroprusside infusion, mean arterial pressure decreased from 108±15 mmHg [rest] to 99±15 mmHg; p<0.0001. Coronary artery diameter change was 19±16%; CBF increased by 152±84% (range 0.5% to 392%) and CVR decreased by −59±15% (range −83% to −8%). Presence of CAD was an independent predictor of reduced vasodilation in response to sodium nitroprusside. There was no correlation between the demographic and risk factor variables and the % changes in CBF or CVR in response to sodium nitroprusside infusion (Supplemental Table I).
CBF increased by 360±168% (range 103% to 880%) and CVR decreased by −75±9 (range −88% to −53%) with intracoronary adenosine infusion. Presence of CAD and diabetes were independent predictors of decreased CBF and vasodilator reserve in response to adenosine (Supplemental Table I).
Femoral Vascular Function
There was a significant reduction in mean arterial pressure in response to intra-femoral acetylcholine (101±14 [rest] vs 95±16 [acetylcholine] mmHg, p<0.0001 and intra-femoral sodium nitroprusside (100±14 [rest] vs 87±15 [sodium nitroprusside] mmHg; p<0.0001) infusions. In response to acetylcholine, the femoral average peak velocity increased by 139±108 % (range −5 to 511%) and the FVR decreased by −52±17% (range −93% to −10%). With sodium nitroprusside, femoral average peak velocity increased by 124±57% (range 33 to 273%) and FVR decreased by −59±10% (range −81% to −29%). In multivariate analyses adjusted for the aforementioned covariates, increasing age and male sex were independent predictors of reduced femoral vasodilation with acetylcholine infusion. Only male sex was an independent predictor of reduced vasodilation in response to sodium nitroprusside (Table 3).
Table 3:
Univariate and multivariate determinants of femoral vascular function
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| β value | p value | β value | p value | |
| % change in femoral vascular resistance in response to acetylcholine | ||||
| Age | 0.69 | <0.001 | 0.55 | 0.002 |
| BMI | 0.4 | 0.3 | 0.17 | 0. 65 |
| Male sex | 7.8 | 0.047 | 7.5 | 0.045 |
| Hypertension | 4.2 | 0.27 | 2.1 | 0.56 |
| Diabetes | 12.2 | 0.037 | 5.6 | 0.32 |
| Smoking | −2.5 | 0.53 | −4.9 | 0.19 |
| Hyperlipidemia | 6.8 | 0.08 | 5.0 | 0.15 |
| Presence of CAD | 10.2 | 0.007 | 3.9 | 0.31 |
| % change in femoral vascular resistance in response to sodium nitroprusside | ||||
| Age | −0.15 | 0.18 | −0.14 | 0.22 |
| BMI | 0.38 | 0.11 | 0.26 | 0.3 |
| Male sex | 4.1 | 0.09 | 4.9 | 0.042 |
| Hypertension | 4.1 | 0.08 | 2.7 | 0.3 |
| Diabetes | 5.8 | 0.12 | 6.1 | 0.11 |
| Smoking | −3.0 | 0.21 | −2.8 | 0.25 |
| Hyperlipidemia | 2.0 | 0.41 | −1.8 | 0.44 |
| Presence of CAD | −2.3 | 0.34 | −2.4 | 0.37 |
| % change in femoral blood flow velocity in response to acetylcholine | ||||
| Age | −3.1 | 0.005 | −1.8 | 0.072 |
| BMI | −4.2 | 0.08 | −0.9 | 0.36 |
| Male sex | −52.5 | 0.03 | −2.1 | 0.038 |
| Hypertension | −35.2 | 0.14 | −0.9 | 0.33 |
| Diabetes | −70.3 | 0.053 | −0.7 | 0.49 |
| Smoking | 20.3 | 0.40 | 1.6 | 0.11 |
| Hyperlipidemia | −33.4 | 0.16 | −0.9 | 0.33 |
| Presence of CAD | −73.9 | 0.001 | −2.0 | 0.049 |
| % change in femoral blood flow velocity in response to sodium nitroprusside | ||||
| Age | 1.3 | 0.03 | 2.0 | 0.045 |
| BMI | −1.5 | 0.27 | −0.4 | 0.72 |
| Male sex | −21.0 | 0.12 | −2.1 | 0.037 |
| Hypertension | −20.5 | 0.12 | −1.4 | 0.18 |
| Diabetes | −16.0 | 0.43 | −0.9 | 0.37 |
| Smoking | 20.9 | 0.12 | 1.7 | 0.09 |
| Hyperlipidemia | 6.8 | 0.61 | 0.3 | 0.78 |
| Presence of CAD | 17.6 | 0.18 | 0.8 | 0.43 |
BMI: body mass index, CAD: coronary artery disease.
Relationship between the microvascular responses in the coronary and femoral circulations
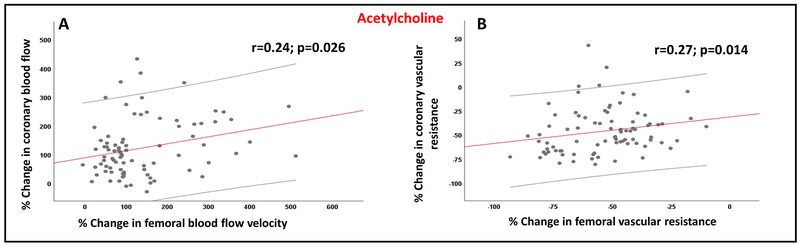
Compared to the resting average peak velocities in the coronary circulation, resting average peak velocities in the femoral circulation were significantly lower (59.9±32 versus 46.1±26; p=0.0002). There was a significant correlation between the magnitude of coronary and femoral microvascular vasodilation in response to acetylcholine (Figure 2). Thus, the % change in FVR correlated with the % change in CVR (r=0.27; p=0.014) and the % change in femoral average peak velocity correlated with the % change in CBF in response to acetylcholine infusions (r=0.24; p=0.026). In contrast, there was no correlation between the endothelium-independent coronary and femoral microvascular vasodilation in response to sodium nitroprusside infusion (Supplemental Figure 1).
Figure 2: Relationship between coronary and femoral microvascular function in response to acetylcholine.
Relationship between the percent changes in (A) femoral blood flow velocity and coronary blood flow in response to acetylcholine infusions, (B) femoral vascular resistance and coronary vascular resistance in response to acetylcholine infusions.
Relationship between the epicardial coronary arterial and femoral microvascular responses
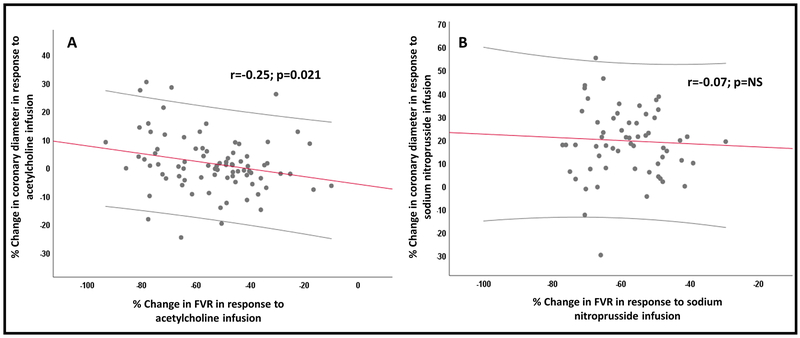
There was a significant correlation between changes in the epicardial coronary diameter and femoral microvascular dilation in response to acetylcholine (r=−0.25; p=0.021) (Figure 3). Subjects with epicardial coronary vasoconstriction in response to acetylcholine (N=38 subjects) had attenuated femoral vasodilation (FVR: −50±16.6 vs −57±17.6 mmHg.mL −1.min; p=0.044) compared to those with epicardial coronary vasodilation (N=44 subjects). Similarly, the femoral average peak velocity in response to acetylcholine was significantly lower in subjects with epicardial coronary vasoconstriction compared to those with epicardial coronary vasodilation (114±94 vs 165±117 cm/sec; p=0.033). There was no association between in femoral microvascular dilation and the coronary diameter changes in response to sodium nitroprusside given in each circulation (Figure 3).
Figure 3: Relationship between coronary epicardial and femoral microvascular functions.
Relationship between the percent changes in (A) femoral vascular resistance and change in coronary diameter in response to acetylcholine infusion, (B) femoral vascular resistance and change in coronary diameter in response to sodium nitroprusside infusion. NS: not significant.
Relationship between the coronary flow reserve with adenosine and femoral microvascular responses
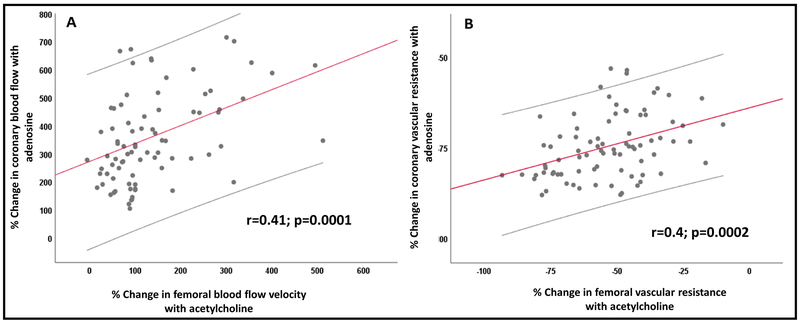
There was a significant correlation between coronary microvascular dilation in response to adenosine (coronary flow reserve) and femoral microvascular dilation in response to acetylcholine infusions (Figure 4). Thus, the change in CVR with adenosine correlated with the change in FVR with acetylcholine (r=0.4; p=0.0002). There was no association between the coronary flow reserve and femoral vasodilation with sodium nitroprusside.
Figure 4: Relationship between coronary flow reserve and femoral microvascular function.
Relationship between the percent changes in (A) femoral blood flow velocity and coronary blood flow in response to acetylcholine and adenosine infusions, respectively, (B) femoral vascular resistance and coronary vascular resistance in response to acetylcholine and adenosine infusions, respectively.
DISCUSSION
In a study that comprehensively examined the relationship between the coronary and peripheral endothelium-dependent and -independent vascular function in response to acetylcholine and sodium nitroprusside, respectively, performed simultaneously during the catheterization procedure, in a diverse population with and without coronary risk factors and CAD, we found that peripheral vascular endothelial responses to acetylcholine reflect (a) endothelial function in both the coronary epicardial arteries and microcirculation, and (b) the coronary flow reserve in response to adenosine (Graphic Abstract). There was no relationship between the peripheral and coronary endothelium-independent function assessed using sodium nitroprusside. Thus, subjects with peripheral vascular endothelial dysfunction had (a) coronary epicardial endothelial dysfunction, (b) coronary microvascular dysfunction, and c) abnormal coronary flow reserve in response to adenosine.
Previous studies have only investigated the relationship between conductance vessel endothelial function in the brachial and coronary epicardial arteries and reported similarity in endothelial function of the two circulations 37, 38. To our knowledge this is the first study to assess endothelium-dependent and -independent microvascular function using acetylcholine and sodium nitroprusside infusions, respectively, in both the coronary and peripheral circulations. Conditions such as coronary microvascular dysfunction, where patients present with chest pain despite non-obstructive epicardial coronary arteries, are associated with impaired coronary microvascular endothelial function and reduced flow reserve 22, 43. In this population and those with CAD, the magnitude of endothelial dysfunction and the magnitude of abnormality in flow reserve is predictive of adverse cardiovascular outcomes 20, 21, 44–46. However, measurement of coronary endothelial function and flow reserve requires invasive testing that is expensive, time consuming, and not free of risk. There is thus a critical need for simpler tests that can accurately estimate coronary endothelial function and flow reserve. This would aid clinicians in both the diagnosis and prognostication of patients undergoing coronary angiography. Our study shows that assessment of peripheral endothelial function at the end of the diagnostic catheterization procedure, using a single infusion of acetylcholine, can provide valuable information regarding the coronary vascular endothelial function and coronary flow reserve. Importantly, a previous study has demonstrated the importance of acetylcholine-mediated vasodilator responses in the forearm microcirculation as an independent predictor of long term outcomes in CAD patients 47.
Previous studies have suggested that coronary vascular dysfunction is associated with peripheral vascular structural changes. Also, subjects with coronary microvascular dysfunction appear to have narrower retinal arterioles 48. Another study demonstrated that vasoreactivity in peripheral microvessels was abnormal in patients with vasospastic coronary syndromes and those with microvascular angina 49. Whether the abnormal microvascular function in the coronary and peripheral circulation we observed is a functional abnormality or secondary to generalized structural changes in the microcirculation remains unknown. In a study examining the coronary microcirculation from ventricular biopsy tissue in patients with chest pain and normal coronary arteries, no structural abnormality was observed, except for mitochondrial changes 50. Thus, it is likely that the observed microvascular changes are functional in nature due to endothelial dysfunction. This is further supported by the observation that smooth muscle function was not correlated with endothelial function in either circulation. We and others have reported previously that endothelial function, but not endothelium-independent function is impaired with increasing age, in men compared to women, and in the presence of cardiovascular risk factors in both the coronary and peripheral circulations 13, 29–31, 51, 52. Finally, we have also previously shown that acetylcholine-mediated femoral microvascular responses, the technique used in this study, are abnormal in patients with cardiovascular risk factors or atherosclerosis compared to those without risk factors 31.
Interestingly, we also found that peripheral microvascular endothelial responses to acetylcholine correlated with the coronary flow reserve, measured as the vasodilator response to intracoronary adenosine. In a previous small study comparing patients with normal and abnormal coronary flow reserve, flow-mediated brachial arterial vasodilation was also noted to be lower in the latter group 53. Another study demonstrated a correlation between the coronary flow reserve and microvascular endothelial function in the subcutaneous fat 49. Our study is the first demonstration of the relationship between peripheral microvascular endothelial dysfunction and abnormalities in coronary flow reserve. The significant relationship between endothelial function in response to acetylcholine and coronary flow reserve in response to adenosine was perhaps due to the presence of adenosine receptors in human endothelial cells 54. Additionally, it is known that vasodilation in response to adenosine leads to increased shear stress which triggers endothelial nitric oxide release which causes further vasodilation. Thus, the adenosine-induced vasodilation is at least partly due to nitric oxide release 55.
The mechanisms regulating endothelium-independent smooth muscle dilatation of the coronary and peripheral microvasculature are complex and incompletely understood. Sodium nitroprusside causes vasodilation by donating nitric oxide directly to the smooth muscle. Recent study showed that vasodilation with sodium nitroprusside in the coronary and peripheral circulation were not correlated with each other, or with the acetylcholine responses, in either circulation 49. One reason for the lack of correlation is because the effect of the NO donor on the endothelium-independent vasodilation is indeed different in the coronary compared to the peripheral circulation, while the effects of acetylcholine is similar. However, we used a single dose of sodium nitroprusside. A dose-response curve with multiple escalating doses of sodium nitroprusside could have shown a better correlation between the two vascular beds. However, the use of sodium nitroprusside infusions at higher doses is limited due to the systemic vasodilation that occurs at high doses.
Strengths and Limitations
This is the largest study to date that comprehensively examines coronary epicardial and microvascular endothelium-dependent and -independent function, and compares it to endothelium-dependent and -independent vasodilation in the peripheral circulation using the same agonists. Coronary vascular function was measured in a single unobstructed coronary artery and thus may not reflect endothelial function of obstructed coronary arteries. We also performed comparisons of single doses of these agonists, based on prior experience 20. It is possible that a dose-response curve with multiple doses of each agonist would have provided better correlations than observed with a single dose. Finally, this study was not powered to examine the differences in vascular function based on racial and gender disparities. This is an area that should be further explored in future studies.
CONCLUSIONS
Herein, we demonstrate that coronary endothelium-dependent epicardial and microvascular function, and coronary flow reserve can be estimated by testing microvascular endothelial function, but not endothelium-independent function in the femoral circulation. Our findings suggest that endothelial dysfunction is a systemic disorder rather than localized one. A simple test that measures femoral vascular response to acetylcholine can provide important insights into the coronary vascular endothelial function and coronary flow reserve, tests that can assist in the diagnosis of coronary microvascular dysfunction and in prognostication. Whether strategies designed to improve peripheral endothelial dysfunction will also similarly improve coronary endothelial dysfunction need to be investigated.
Supplementary Material
Relationship between femoral endothelium-dependent microvascular function and coronary vascular function.
Femoral endothelium-dependent microvascular function reflects coronary flow reserve, and endothelium-dependent coronary epicardial and microvascular function.
Supplemental Figure I: Relationship between coronary and femoral microvascular function in response to sodium nitroprusside.
Relationship between the percent changes in (A) femoral blood flow velocity and coronary blood flow in response to sodium nitroprusside infusions, and (B) femoral vascular resistance and coronary vascular resistance in response to sodium nitroprusside infusions. NS=not significant.
HIGHLIGHTS.
-
-
In subjects with and without risk factors, coronary endothelium-dependent epicardial and microvascular function, and coronary flow reserve can be estimated by testing microvascular endothelial function in the femoral circulation.
-
-
Endothelial dysfunction is a systemic disorder rather than localized one.
-
-
Endothelial functional changes are generalized in the human circulation, and measures of peripheral endothelial function reflects coronary endothelial function.
-
-
A simple test that measures femoral vascular response to acetylcholine can provide important insights into the coronary vascular endothelial function and the flow reserve, tests that can assist in the diagnosis of coronary microvascular dysfunction and in prognostication.
ACKNOWLEDGMENTS
Acknowledgments: We are grateful to the staff and investigators of the cardiac catheterization laboratory at the Cardiology Branch of the NHLBI, Bethesda MD for their assistance with the conduct of this study.
Source of Funding: Ahmed Al-Badri is supported by American Heart Association Postdoctoral Fellowship Award Grant (18POST34080330). Arshed A. Quyyumi is currently supported by National Institutes of Health (NIH) grants 5P01HL101398-02, 1P20HL113451-01, 1R56HL126558-01, 1RF1AG051633-01, R01 NS064162-01, R01 HL89650-01, HL095479-01, 1U10HL110302-01, 1DP3DK094346-01, and 2P01HL086773-06A1.
*This work is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute or National Institutes of Health.
Abbreviations
- CAD
Coronary artery disease
- BMI
body mass index
- CBF
coronary blood flow
- CVR
coronary vascular resistance
- FVR
femoral vascular resistance
Footnotes
Disclosures: Authors have nothing to disclose.
REFERENCES
- 1.Furchgott RF and Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–376. [DOI] [PubMed] [Google Scholar]
- 2.Furchgott RF. Role of endothelium in responses of vascular smooth muscle. Circulation research. 1983;53:557–573. [DOI] [PubMed] [Google Scholar]
- 3.Vanhoutte PM. The endothelium--modulator of vascular smooth-muscle tone. The New England journal of medicine. 1988;319:512–513. [DOI] [PubMed] [Google Scholar]
- 4.Bassenge E and Busse R. Endothelial modulation of coronary tone. Progress in cardiovascular diseases. 1988;30:349–380. [DOI] [PubMed] [Google Scholar]
- 5.Vane JR, Anggard EE and Botting RM. Regulatory functions of the vascular endothelium. The New England journal of medicine. 1990;323:27–36. [DOI] [PubMed] [Google Scholar]
- 6.Ozkor MA and Quyyumi AA. Endothelium-derived hyperpolarizing factor and vascular function. Cardiology research and practice. 2011;2011:156146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Quyyumi AA, Dakak N, Andrews NP, Gilligan DM, Panza JA and Cannon RO 3rd. Contribution of nitric oxide to metabolic coronary vasodilation in the human heart. Circulation. 1995;92:320–326. [DOI] [PubMed] [Google Scholar]
- 8.Halcox JP, Narayanan S, Cramer-Joyce L, Mincemoyer R and Quyyumi AA. Characterization of endothelium-derived hyperpolarizing factor in the human forearm microcirculation. American journal of physiology Heart and circulatory physiology. 2001;280:H2470–2477. [DOI] [PubMed] [Google Scholar]
- 9.Campia U, Choucair WK, Bryant MB, Quyyumi AA, Cardillo C and Panza JA. Role of cyclooxygenase products in the regulation of vascular tone and in the endothelial vasodilator function of normal, hypertensive, and hypercholesterolemic humans. The American journal of cardiology. 2002;89:286–290. [DOI] [PubMed] [Google Scholar]
- 10.Ignarro LJ, Byrns RE, Buga GM and Wood KS. Endothelium-derived relaxing factor from pulmonary artery and vein possesses pharmacologic and chemical properties identical to those of nitric oxide radical. Circulation research. 1987;61:866–879. [DOI] [PubMed] [Google Scholar]
- 11.Gardiner SM, Compton AM, Bennett T, Palmer RM and Moncada S. Control of regional blood flow by endothelium-derived nitric oxide. Hypertension (Dallas, Tex : 1979). 1990;15:486–492. [DOI] [PubMed] [Google Scholar]
- 12.Ozkor MA, Murrow JR, Rahman AM, Kavtaradze N, Lin J, Manatunga A and Quyyumi AA. Endothelium-derived hyperpolarizing factor determines resting and stimulated forearm vasodilator tone in health and in disease. Circulation. 2011;123:2244–2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Quyyumi AA, Mulcahy D, Andrews NP, Husain S, Panza JA and Cannon RO 3rd. Coronary vascular nitric oxide activity in hypertension and hypercholesterolemia. Comparison of acetylcholine and substance P. Circulation. 1997;95:104–110. [DOI] [PubMed] [Google Scholar]
- 14.Prasad A, Husain S, Schenke W, Mincemoyer R, Epstein N and Quyyumi AA. Contribution of bradykinin receptor dysfunction to abnormal coronary vasomotion in humans. Journal of the American College of Cardiology. 2000;36:1467–1473. [DOI] [PubMed] [Google Scholar]
- 15.AlBadri A, Wei J, Mehta PK, Landes S, Petersen JW, Anderson RD, Samuels B, Azarbal B, Handberg EM, Li Q, Minissian M, Shufelt C, Pepine CJ and Bairey Merz CN. Acetylcholine versus cold pressor testing for evaluation of coronary endothelial function. PloS one. 2017;12:e0172538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hammadah M, Kim JH, Al Mheid I, et al. Coronary and Peripheral Vasomotor Responses to Mental Stress. Journal of the American Heart Association. 2018;7:e008532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yeung AC, Vekshtein VI, Krantz DS, Vita JA, Ryan TJ, Ganz P and Selwyn AP. The Effect of Atherosclerosis on the Vasomotor Response of Coronary Arteries to Mental Stress. New England Journal of Medicine. 1991;325:1551–1556. [DOI] [PubMed] [Google Scholar]
- 18.Ozkor MA, Hayek SS, Rahman AM, Murrow JR, Kavtaradze N, Lin J, Manatunga A and Quyyumi AA. Contribution of endothelium-derived hyperpolarizing factor to exercise-induced vasodilation in health and hypercholesterolemia. Vascular medicine (London, England). 2015;20:14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suwaidi JA, Hamasaki S, Higano ST, Nishimura RA, Holmes DR Jr. and Lerman A Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation. 2000;101:948–954. [DOI] [PubMed] [Google Scholar]
- 20.Halcox JP, Schenke WH, Zalos G, Mincemoyer R, Prasad A, Waclawiw MA, Nour KR and Quyyumi AA. Prognostic value of coronary vascular endothelial dysfunction. Circulation. 2002;106:653–658. [DOI] [PubMed] [Google Scholar]
- 21.AlBadri A, Bairey Merz CN, Johnson BD, Wei J, Mehta PK, Cook-Wiens G, Reis SE, Kelsey SF, Bittner V, Sopko G, Shaw LJ, Pepine CJ and Ahmed B. Impact of Abnormal Coronary Reactivity on Long-Term Clinical Outcomes in Women. Journal of the American College of Cardiology. 2019;73:684–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ong P, Athanasiadis A, Borgulya G, Mahrholdt H, Kaski JC and Sechtem U. High prevalence of a pathological response to acetylcholine testing in patients with stable angina pectoris and unobstructed coronary arteries. The ACOVA Study (Abnormal COronary VAsomotion in patients with stable angina and unobstructed coronary arteries). Journal of the American College of Cardiology. 2012;59:655–662. [DOI] [PubMed] [Google Scholar]
- 23.Prasad A, Husain S and Quyyumi AA. Abnormal flow-mediated epicardial vasomotion in human coronary arteries is improved by angiotensin-converting enzyme inhibition: a potential role of bradykinin. Journal of the American College of Cardiology. 1999;33:796–804. [DOI] [PubMed] [Google Scholar]
- 24.Prasad A, Tupas-Habib T, Schenke WH, Mincemoyer R, Panza JA, Waclawin MA, Ellahham S and Quyyumi AA. Acute and chronic angiotensin-1 receptor antagonism reverses endothelial dysfunction in atherosclerosis. Circulation. 2000;101:2349–2354. [DOI] [PubMed] [Google Scholar]
- 25.Halcox JPJ, Nour KRA, Zalos G, Mincemoyer R, Waclawiw MA, Rivera CE, Willie G, Ellahham S and Quyyumi AA. The effect of sildenafil on human vascular function, platelet activation, and myocardial ischemia. Journal of the American College of Cardiology. 2002;40:1232–1240. [DOI] [PubMed] [Google Scholar]
- 26.Murrow JR, Sher S, Ali S, Uphoff I, Patel R, Porkert M, Le NA, Jones D and Quyyumi AA. The differential effect of statins on oxidative stress and endothelial function: atorvastatin versus pravastatin. Journal of clinical lipidology. 2012;6:42–49. [DOI] [PubMed] [Google Scholar]
- 27.Cardillo C, Kilcoyne CM, Quyyumi AA, Cannon RO 3rd and Panza JA Selective defect in nitric oxide synthesis may explain the impaired endothelium-dependent vasodilation in patients with essential hypertension. Circulation. 1998;97:851–856. [DOI] [PubMed] [Google Scholar]
- 28.Gilligan DM, Panza JA, Kilcoyne CM, Waclawiw MA, Casino PR and Quyyumi AA. Contribution of endothelium-derived nitric oxide to exercise-induced vasodilation. Circulation. 1994;90:2853–3858. [DOI] [PubMed] [Google Scholar]
- 29.Gilligan DM, Guetta V, Panza JA, Garcia CE, Quyyumi AA and Cannon RO 3rd. Selective loss of microvascular endothelial function in human hypercholesterolemia. Circulation. 1994;90:35–41. [DOI] [PubMed] [Google Scholar]
- 30.Panza JA, Quyyumi AA, Brush JE Jr. and Epstein SE Abnormal endothelium-dependent vascular relaxation in patients with essential hypertension. The New England journal of medicine. 1990;323:22–27. [DOI] [PubMed] [Google Scholar]
- 31.Dakak N, Husain S, Mulcahy D, Andrews NP, Panza JA, Waclawiw M, Schenke W and Quyyumi AA. Contribution of nitric oxide to reactive hyperemia: impact of endothelial dysfunction. Hypertension (Dallas, Tex : 1979). 1998;32:9–15. [DOI] [PubMed] [Google Scholar]
- 32.Lefer AM and Ma XL. Decreased basal nitric oxide release in hypercholesterolemia increases neutrophil adherence to rabbit coronary artery endothelium. Arteriosclerosis, Thrombosis, and Vascular Biology. 1993;13:771–776. [DOI] [PubMed] [Google Scholar]
- 33.Zeiher AM, Drexler H, Wollschläger H and Just H. Modulation of coronary vasomotor tone in humans. Progressive endothelial dysfunction with different early stages of coronary atherosclerosis. Circulation. 1991;83:391–401. [DOI] [PubMed] [Google Scholar]
- 34.Hodgson JM and Marshall JJ. Direct vasoconstriction and endothelium-dependent vasodilation. Mechanisms of acetylcholine effects on coronary flow and arterial diameter in patients with nonstenotic coronary arteries. Circulation. 1989;79:1043–1051. [DOI] [PubMed] [Google Scholar]
- 35.Newman CM, Maseri A, Hackett DR, el-Tamimi HM and Davies GJ. Response of angiographically normal and atherosclerotic left anterior descending coronary arteries to acetylcholine. The American journal of cardiology. 1990;66:1070–1076. [DOI] [PubMed] [Google Scholar]
- 36.Quyyumi AA, Dakak N, Andrews NP, Husain S, Arora S, Gilligan DM, Panza JA and Cannon RO 3rd. Nitric oxide activity in the human coronary circulation. Impact of risk factors for coronary atherosclerosis. The Journal of clinical investigation. 1995;95:1747–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Teragawa H, Ueda K, Matsuda K, Kimura M, Higashi Y, Oshima T, Yoshizumi M and Chayama K. Relationship between endothelial function in the coronary and brachial arteries. Clinical cardiology. 2005;28:460–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anderson TJ, Uehata A, Gerhard MD, Meredith IT, Knab S, Delagrange D, Lieberman EH, Ganz P, Creager MA, Yeung AC and et al. Close relation of endothelial function in the human coronary and peripheral circulations. Journal of the American College of Cardiology. 1995;26:1235–1241. [DOI] [PubMed] [Google Scholar]
- 39.Parrinello R, Sestito A, Di Franco A, Russo G, Villano A, Figliozzi S, Nerla R, Tarzia P, Stazi A, Lanza GA and Crea F. Peripheral arterial function and coronary microvascular function in patients with variant angina. Cardiology. 2014;129:20–24. [DOI] [PubMed] [Google Scholar]
- 40.Rigo F, Pratali L, Palinkas A, Picano E, Cutaia V, Venneri L and Raviele A. Coronary flow reserve and brachial artery reactivity in patients with chest pain and “false positive” exercise-induced ST-segment depression. The American journal of cardiology. 2002;89:1141–1144. [DOI] [PubMed] [Google Scholar]
- 41.Doucette JW, Corl PD, Payne HM, Flynn AE, Goto M, Nassi M and Segal J. Validation of a Doppler guide wire for intravascular measurement of coronary artery flow velocity. Circulation. 1992;85:1899–1911. [DOI] [PubMed] [Google Scholar]
- 42.Husain S, Andrews NP, Mulcahy D, Panza JA and Quyyumi AA. Aspirin Improves Endothelial Dysfunction in Atherosclerosis. Circulation. 1998;97:716–720. [DOI] [PubMed] [Google Scholar]
- 43.Reis SE, Holubkov R, Conrad Smith AJ, Kelsey SF, Sharaf BL, Reichek N, Rogers WJ, Merz CN, Sopko G and Pepine CJ. Coronary microvascular dysfunction is highly prevalent in women with chest pain in the absence of coronary artery disease: results from the NHLBI WISE study. American heart journal. 2001;141:735–741. [DOI] [PubMed] [Google Scholar]
- 44.Britten MB, Zeiher AM and Schachinger V. Microvascular dysfunction in angiographically normal or mildly diseased coronary arteries predicts adverse cardiovascular long-term outcome. Coronary artery disease. 2004;15:259–64. [DOI] [PubMed] [Google Scholar]
- 45.Pepine CJ, Anderson RD, Sharaf BL, Reis SE, Smith KM, Handberg EM, Johnson BD, Sopko G and Bairey Merz CN. Coronary microvascular reactivity to adenosine predicts adverse outcome in women evaluated for suspected ischemia results from the National Heart, Lung and Blood Institute WISE (Women’s Ischemia Syndrome Evaluation) study. Journal of the American College of Cardiology. 2010;55:2825–2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schachinger V, Britten MB and Zeiher AM. Prognostic impact of coronary vasodilator dysfunction on adverse long-term outcome of coronary heart disease. Circulation. 2000;101:1899–1906. [DOI] [PubMed] [Google Scholar]
- 47.Heitzer T, Schlinzig T, Krohn K, Meinertz T and Munzel T. Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation. 2001;104:2673–2678. [DOI] [PubMed] [Google Scholar]
- 48.Wang L, Wong TY, Sharrett AR, Klein R, Folsom AR and Jerosch-Herold M. Relationship between retinal arteriolar narrowing and myocardial perfusion: multi-ethnic study of atherosclerosis. Hypertension (Dallas, Tex : 1979). 2008;51:119–126. [DOI] [PubMed] [Google Scholar]
- 49.Ford TJ, Rocchiccioli P, Good R, et al. Systemic microvascular dysfunction in microvascular and vasospastic angina. European heart journal. 2018;39:4086–4097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Opherk D, Zebe H, Weihe E, Mall G, Durr C, Gravert B, Mehmel HC, Schwarz F and Kubler W. Reduced coronary dilatory capacity and ultrastructural changes of the myocardium in patients with angina pectoris but normal coronary arteriograms. Circulation. 1981;63:817–825. [DOI] [PubMed] [Google Scholar]
- 51.Casino PR, Kilcoyne CM, Quyyumi AA, Hoeg JM and Panza JA. The role of nitric oxide in endothelium-dependent vasodilation of hypercholesterolemic patients. Circulation. 1993;88:2541–2547. [DOI] [PubMed] [Google Scholar]
- 52.Egashira K, Inou T, Hirooka Y, Yamada A, Maruoka Y, Kai H, Sugimachi M, Suzuki S and Takeshita A. Impaired coronary blood flow response to acetylcholine in patients with coronary risk factors and proximal atherosclerotic lesions. The Journal of clinical investigation. 1993;91:29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Park CS, Youn HJ, Kim JH, Cho EJ, Jung HO, Jeon HK, Lee JM, Ihm SH, Oh YS, Chung WS, Kim JH, Choi KB and Hong SJ. Relation between peripheral vascular endothelial function and coronary flow reserve in patients with chest pain and normal coronary angiogram. International journal of cardiology. 2006;113:118–120. [DOI] [PubMed] [Google Scholar]
- 54.Olanrewaju HA, Qin W, Feoktistov I, Scemama JL and Mustafa SJ. Adenosine A(2A) and A(2B) receptors in cultured human and porcine coronary artery endothelial cells. American journal of physiology Heart and circulatory physiology. 2000;279:H650–656. [DOI] [PubMed] [Google Scholar]
- 55.Smits P, Williams SB, Lipson DE, Banitt P, Rongen GA and Creager MA. Endothelial release of nitric oxide contributes to the vasodilator effect of adenosine in humans. Circulation. 1995;92:2135–2141. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Relationship between femoral endothelium-dependent microvascular function and coronary vascular function.
Femoral endothelium-dependent microvascular function reflects coronary flow reserve, and endothelium-dependent coronary epicardial and microvascular function.
Supplemental Figure I: Relationship between coronary and femoral microvascular function in response to sodium nitroprusside.
Relationship between the percent changes in (A) femoral blood flow velocity and coronary blood flow in response to sodium nitroprusside infusions, and (B) femoral vascular resistance and coronary vascular resistance in response to sodium nitroprusside infusions. NS=not significant.