Abstract
Background:
Women living with HIV (WLH) in rural communities face challenges to obtaining treatment and accurate disease-related information. Nutritional deficits exacerbate disease progression.
Setting:
WLH were recruited from primary-health centers in rural India.
Method:
A quasi-experimental trial of a comprehensive Accredited Social Health Activist (ASHA)-supported intervention compared four distinct ASHA-based programs [1) standard education alone (SE); 2) nutrition education (+NE); 3) nutrition supplements (+NS); or 4) nutrition education and nutrition supplements (+NENS)] on key disease and nutrition-related outcomes [CD4 count, body mass index (BMI), serum albumin and hemoglobin]. Assessments occurred at baseline, and months 6 (immediately post-intervention), 12, and 18. Multilevel modeling examined effects of program (group) over time.
Findings:
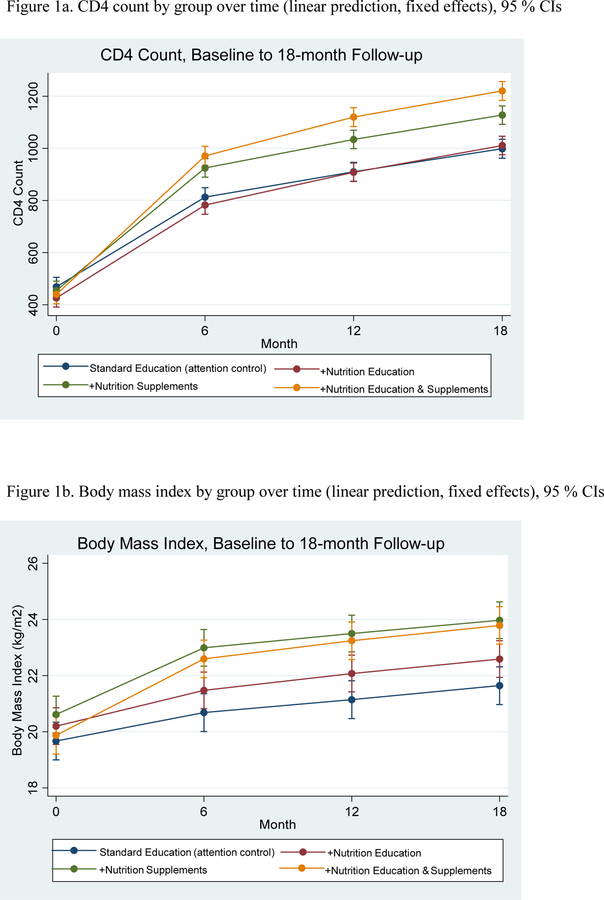
Among 600 WLH enrolled (n=150 per arm), mean age, CD4 count and BMI (kg/m2) were 34.31, 447.42, and 20.09, and 30.4 respectively, at baseline. At 18-month follow-up, Program 4 (+NENS) experienced greatest improvements in CD4 counts compared to Program 1 (+SE) (adjusted difference=223.81, 95% CI=170.29, 277.32). For BMI, Programs 3 (+NS; adjusted difference=2.33, 95% CI, 1.39, 3.26) and 4 (+NENS; adjusted difference=2.14, 95% CI, 1.17, 3.12) exhibited greater gains compared to Program 1 (+SE). Programs 3 and 4 were not significantly different from each other (adjusted difference=−0.18, 95% CI, −1.12, 0.76). Hemoglobin and serum albumin also improved over time; Program 4 (+NENS) exhibited the greatest gains.
Conclusions:
A low-cost ASHA-supported behavioral and nutritional intervention improved outcomes for WLH. Gains were sustained at 18-month follow-up. Similar approaches may help improve HIV and other infectious disease-related outcomes in vulnerable populations.
Trial Registration:
Keywords: Infectious diseases/HIV-AIDS, Clinical Trial, Nutrition, Nursing, Health Education, Community Health Workers, Behavioral Medicine, CD4 Lymphocyte Count, Body Mass Index
Introduction
There is a reciprocal relationship between adequate nutrition and effective immune functioning, a critical factor for managing HIV. Undertreated HIV increases metabolic demands among people living with HIV,1 suggesting that interventions that incorporate nutritional components may promote better outcomes, particularly in food insecure settings.2 Meta-analytic findings demonstrate that food insecure individuals with HIV have, on average lower CD4 counts,3 a critical marker of HIV disease progression and mortality.4 High Body Mass Index (BMI) tends to be protective against disease progression, highlighting the important role of overall adequate caloric intake for those with HIV.5
In resource limited-settings, such as India, poor nutrition may be related to limited access to nutritious food and low levels of nutritional knowledge, exhibited in low macronutrient and micronutrient intake.6 Micronutrient deficits, including iron deficiency, are exacerbated among people living with HIV due to malabsorption.7 In fact, hemoglobin (a marker of anemia) is an independent risk factor of AIDS and death.8 Low serum albumin has also been linked with these poor outcomes8–10 by promoting inflammatory processes that limit nutrient effectiveness including lowering protein-intake effectiveness.11 Serum albumin may also be a particularly useful marker of disease progression in resource-limited settings, since it is a moderately inexpensive metric to analyze in a laboratory.10,12
Despite the robust findings between nutrition-linked indicators and key markers of HIV disease progression, prior research has not consistently found micronutrient supplements (e.g., single or multiple formulations of vitamins and trace elements) to be effective in improving clinically-relevant outcomes (CD4 count, viral load, nutritional status, hospital admissions and mortality).13 Such findings suggest the need for a more comprehensive approach that involves improvements to diet and nutrient intake via nutritious food, targeting a wider range of nutritional deficiencies (including protein deficiency) and accounting for the interconnected and complex systems that comprise nutrition processes. Improving nutrition via diet may also help address the nutritional and overall health needs of people living with HIV as a component of integrated care.
The Comprehensive Health Seeking and Coping Paradigm (CHSCP)14 provides a guiding framework for designing and implementing community-based interventions in vulnerable populations. Originating from a Stress and Coping15 and Health Seeking perspective,16 the framework provides a biopsychosocial view of factors impacting health. For food-insecure women in India, preexisting factors include socio-demographic risk factors (e.g., sex, income), health history (HIV-status, opportunistic infections), and nutritional parameters (e.g., poor nutrition, anemia). Barriers to improved immune status include stigma, low social support, and lack of HIV/AIDS knowledge; barriers to nutritional adequacy include inadequate knowledge and nutrition/food insecurity. To address these challenges, we implemented the CHSCP and the Asha (Accredited Social Health Activist)-based model of support to design, along with community members, a comprehensive, community-based intervention that incorporated psychosocial support and life-skills training (based on the targeted needs of the community)17 with nutrition education and nutrition supplements. Asha are members of the community with little or no formal training in healthcare; and who are trained to assist doctors, nurses, and other health professionals, expanding existing structures in resource-limited settings. Importantly, the Asha-based model seeks to instill in women living with HIV (WLH) the knowledge (e.g., importance of ART regimens; healthy eating) and skills (e.g., job skills, healthy cooking) to sustain positive change in their own lives after participation in intervention procedures. We have previously shown that ASHA-supported nutritional interventions were effective in improving CD4 counts, body mass index (BMI), and other health outcomes among WLH at the end of the 6-month intervention.18
The present study investigated whether the effects of this Asha-supported nutritional intervention were sustained one year (12 months) after completion of all intervention procedures (18-months after baseline assessments). We hypothesized that food supplements and nutrition training added to an ASHA-supported intervention would each significantly improve key outcomes, and that combining supplements and nutrition education would produce greater improvements in key outcomes, with gains that would be sustained over time.
Methods
Study design
We conducted a prospective, quasi-experimental study among WLH in rural Andhra Pradesh (AP), India to evaluate the effects of an Asha-supported behavioral and nutrition intervention on critical HIV-related outcomes of disease progression and nutrition. ASHA are lay community healthcare workers trained to facilitate participant’s understanding of and adherence to healthcare regimens. The Asha in our study were hired and trained specifically for our study; the training and financial compensation was comparable to that received by government-supported Asha and thus the Asha would be qualified to work for government-based jobs following completion of the study.
We implemented a 2×2 factorial design with assessments taken at baseline, 6 months (immediately following completion of intervention procedures), and at 12 and 18 months. The intervention sought to improve physiological outcomes by improving nutrition and bolstering access to existing health care resources. Participants were allocated 1:1:1:1 into the four study arms: 1) standard education (including Asha support) alone (SE); 2) SE plus enhanced nutrition education (+NE); 3) SE plus nutrition supplements (+NS); and 4) SE plus the combination of enhanced nutrition education and nutrition supplements (+NENS). The food supplements were selected from locally consumed food (urad dal [black gram] and tur dal [pigeon peas]) consisting of high protein legumes and high polyunsaturated vegetable oils at 500K per adult and 250K per child in the entire household.
Standard education (a component of every arm) included individual 1:1 weekly Asha support, 6 group sessions covering the content in Modules 1–3 (see Table 1), and referral to life skills classes. Life skills classes were designed to provide economic resources and sustainable jobs to the women in the program (e.g., selling fruit and vegetables, sewing and embroidery, computer skills). The entire program was designed in a culturally-relevant manner with data derived from focus groups held with WLH in India.17 An important component of the intervention design was imparting skills and knowledge to WLH that would allow positive change to occur even after intervention procedures were terminated. For example, women learned healthcare skills (how to ask doctors questions, the importance of refilling and taking ART medications), job skills (to continue to generate income), and nutrition skills (e.g., where to buy healthy food, how to cook culturally-desirable healthy food).
Table 1.
Overview of the ASHA-based Behavioral and Nutrition Intervention Components
| Module 1A and 1B: Keeping Healthy | 1. Getting support; talking to my Asha and my doctor/nurse; learning about HIV/AIDS. |
| 2. Learning about and taking ART recognizing an opportunistic infection; healthy eating | |
| 3. Keeping with healthy routines. | |
| 4. Deciding with whom to share health problems. | |
| Module 2A and 2B: Care-giving | 1. Breastfeeding safely or learning to sterilize the formula. |
| 2. Promoting care-giving for family while caring for my own mental and physical well-being. | |
| 3. Engaging in life skill education. | |
| 4. Staying in sync with my children; identifying their needs. | |
| Module 3A and 3B: Staying Upbeat | 1. Finding the joys in life; creating a social network for myself |
| 2. Dealing well with problems; Keeping positive relations with family | |
| 3. Taking active steps to improve my life every day | |
| 4. Getting psychological assistance | |
| Module 4A and 4B: Healthy Eating for Self and Family (Nutrition Education) (Only Programs 2 & 4 Complete) | 1. Learning about low-cost foods that are nutritious |
| 2. Where to purchase food | |
| 3. Learning how to monitor food intake | |
| 4. Considering ways to eat healthy | |
| 5. Participating in a cooking class | |
| Nutrition Supplements (Only for Programs 3 & 4) | 1. Consists of high protein foods with minimal saturated fat, fiber, and cholesterol. |
| 2. Food choices evaluated using India national guidelines and our team’s nutrition experts. | |
| 3. Food supplements consist of high protein legumes and high polyunsaturated vegetable oils at 500K per adult and 250K per child in household. (Food given to entire household to ensure that the women would consume the supplements rather than giving it away to family members). | |
| 4. Foods provided were popular in the region (e.g., urad dal [black gram] or tur dal [pigeon peas]). | |
The study was approved by University Human Subjects Protection Committees in the United States and the All India Institute of Medical Sciences and the Ministry of Health in India.
Sample and Setting
WLH between ages of 18–50 with a verified HIV-positive diagnosis, ART prescription for the last three months, and who were currently receiving care in one of the 16 Primary Health Centers (PHCs) located in Nellore and Prakasam districts of AP were recruited for participation. The PHCs were grouped into four regional clusters; the clusters were created so that they contained villages that were close to each other and yet distant from villages contained in other clusters. This design was implemented rather than randomizing each of the individual 16 PHCs into one of the four arms in order to increase feasibility and promote intervention fidelity by reducing cross-contamination. Additional inclusion criteria included having a child aged 3 – 8 (separate analyses not relevant to the data presented herein focused on child outcomes). As part of enrollment criteria, all women were receiving HIV-treatment.
Exclusionary factors included CD4 T-cell levels < 100 cells/mm3, participation in previous ASHA-interventions (used as pilot data) by our team, or currently pregnant. (No women became pregnant over the course of the study). Baseline data collection began April 30, 2014. Enrollment was staggered to best implement the group-based intervention every six months over a two-year period; four cohorts were enrolled with 100 participants in each (25 per arm, per cohort). Cohorts were enrolled in equal proportions across sites concurrently.
Procedures
Flyers were posted in selected PHCs. Interested women met with a trained research staff who was blinded to the program hypotheses in a private location to receive study information, and complete the eligibility screener. Verified diagnosis of HIV was assessed by showing government hospital-generated ART card. CD4+ T cell count was also measured during screening. WLH received cash compensation for each study procedure, including initial screening, four blood draws, thirteen 24-hour diet recalls that included nine BMIs and urine collections, four interviews, and 6–8 group sessions depending on program assignment, totaling USD $172-$182 (9,036 – 9,562 INR) over the course of the 18-month study. Participants were also reimbursed for transportation costs and provided childcare as needed. All WLH interested in participating provided informed consent. A second informed consent was completed by all eligible participants based on the results of eligibility screening.
Asha Selection and Training
Asha were recruited from villages similar to the participants and ranged in age between 20–50; attained an education of 8th grade or higher; and were interested in caring for women and children with HIV. Some had received previous Asha training by the Indian government; training by our team was provided to those who had not.
Sixteen Asha were rigorously trained by the key investigators and Project Director (PD); all received training by our team to ensure comparability of treatment. The focus was on the study protocol, needs of WLH, including emotional and social support, basics of HIV disease progression, importance of adherence to ART, strategies to assist coping with illness, and maintaining participant confidentiality. Four Asha were assigned to each of the four programs and received training specific to that program design. Quality assurance assessments were done quarterly. Nursing guidance and research staff were led by PIs, physicians and nurses in the community. The Asha were employed full time by our team for study duration.
Primary Outcomes.
CD4+ T cells were measured through blood samples collected at screening and at 6-, 12- and 18-month follow-up. CD4+ T cell testing was performed at the Nellore District Hospital diagnostic laboratory using the Act Diff Coulter Analyzer (Beckman Coulter; Brea, CA). Laboratory personnel were blinded to the intervention group of the participants. Body Mass Index: Height and weight were measured at baseline, then monthly for six months, and then at 12- and 18-month follow up. Trained research staff used a calibrated scale to weigh WLH while barefoot, and took heights using a stadiometer with BMI calculated by dividing weight in kilograms by height in meters squared.
Secondary outcomes.
Hemoglobin was assessed in grams / liter (g/L) in the blood; anemia was defined according to WHO guidelines19 for severe (<80g/L), moderate (80–109 g/L), mild (110–119 g/L) or no anemia (≥ 120 g/L ) hemoglobin levels. Serum albumin concentration was evaluated as g/L concentration in the blood and analyzed as a continuous variable. (Note: these were added post hoc from initial ClinicalTrials.gov submission).
Key covariates.
Demographic data were collected at baseline. In addition, data were collected for the following risk factors for HIV-related outcomes. Percent past month ART adherence was represented by a self-reported measure that assessed adherence to ART via a Visual Analogue Scale ranging from 0 to 100, with the number representing percent of pills taken in past month 20,21, previously used in India and correlated with viral load 21. Physical activity was evaluated with the International Physical Activity Questionnaire was summarized into Metabolic Equivalents of Energy Expenditure (MET) minutes per week.22 Quality of Life (QOL) assessed by the internationally validated Quality of Life Enjoyment and Satisfaction Questionnaire.23 Food insecurity by The Household Food Insecurity Access Scale,24 a widely used measure previously implemented in India with a similar population.25 Depression was assessed by The Center for Epidemiological Studies Depression Scale (CES-D), short version,26 a 10-item scale with well-established reliability and validity including in India27. Internalized stigma was represented with a 10-item scale, previously used in India, that measured the extent respondents believed they deserved to be shunned (5 items, e.g., should avoid social interactions) or blamed/shamed because of HIV (5 items, e.g., shamed the family, feel guilty).28 Social support was derived from The MOS Social Support Survey 29 as both a composite scale and an assessment of number of friends one could turn to in the past month (composite scale was used in inferential statistics). Opportunistic infections (OI) were assessed by using a list of 8 common OI endorsed by our collaborating physicians that included tuberculosis, pneumocystis carinii, Herpes Simplex Virus/Varicella Zoster, candida, fungal dermatoses, diarrhea, febrile illness, or other. Participants indicated whether they had experienced any of these OI in the previous 6 months, with a summation of endorsed OI into one index measure.
Statistical analysis
Sample size was estimated based on effect sizes drawn from pilot study results of a behavioral intervention for WLH in India.30–32 Alpha was set to 0.01 to mitigate for multiple dependent variables. We estimated an intraclass correlation of 0.05 and a design effect of 2.45 (to account for potential PHC clustering effects) and a 90% attrition rate after 18 months. For each main effect of parent study, we estimated differences of about half a standard deviation (SD) in size (d=0.48, medium effect size) between the outcome means of nutrition and supplement groups compared to the SE arm. Based on these parameters, the overall sample size of 600 (150 per group) provided 80% power for detecting the differences in outcomes across intervention groups.
All analyses were conducted using STATA 15 (Stata Corp). Although an intention-to-treat analysis was planned for the primary analysis, all participants provided complete data at all time points and no attrition or mortality over time occurred; thus analyses did not account for missingness. Significance tests were 2-sided and α=0.05.
Primary analyses examined between and within group differences over time between baseline and follow-ups (6, 12, and 18-month) for the four programs. Given the random allocation was by cluster and not individually based, equivalent distributions of covariates according to group membership were not expected; between group differences at baseline were tested in bivariate models and covariates significantly different by group included in subsequent analyses. A multilevel, random-intercept mixed model was constructed, nested within participants to allow for clustering of data within each individual. This model included fixed effects for group (program), time, group (program) x time, and covariates that explained between group differences at baseline or were clinically relevant. Random slope models were also tested to allow slopes between groups to vary. Random-intercept only models exhibited best model fit based on likelihood ratio chi-squared tests. An independent covariance pattern was specified as our final models were random intercept only (participant); results are presented using restricted maximum likelihood (REML) for optimal control of Type 1 error.33 In addition to models that separately compared Programs 2–4 to the reference group (Program 1), factorial models (which use an interaction term for Program 4, defined by participation in both nutrition education and nutrition supplements, rather than creating a dummy coded variable for each of the four groups) were also constructed. Finally, recovery from anemia (defined as hemoglobin ≥ 120) at 18 months was examined using logistic regression (1=anemia recovery; 0=anemic).
Results
Beginning in April 2014, 972 women were screened, of whom, 600 (61.73%) were eligible (see Supplemental Figure 1. CONSORT Flow Diagram). Of the eligible participants, 100% agreed to participate in the study, resulting in 600 women enrolled from the four intervention clusters (150 from each cluster). Table 2 presents descriptive statistics for total sample and each of the four arms as well as between group differences between arms at baseline. As detailed in Table 2, arms differed on the following variables at baseline: age, quality of life, MET, internalized stigma, food insecurity and education. Baseline ART adherence did not differ between arms but was included as a covariate in subsequent analyses given its critical importance in understanding HIV-related outcomes over time.21
Table 2.
Description of the sample on key variables at baseline and 18-month follow-up, N=600
| Total Sample (N=600) |
Program 1: Standard Education (n=150) |
Program 2 + Nutrition Education (n=150) |
Program 3: + Nutrition Supplements (n=150) |
Program 4: + Nutrition Education & Supplements (n=150) |
Test of between group differencesa | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Demographics | |||||||||||
| M | SD | M | SD | M | SD | M | SD | M | SD | ||
| Age | 34.31 | 6.96 | 36.05 | 6.96 | 34.66 | 6.90 | 33.61 | 6.41 | 32.91 | 7.17 | F(3, 596)=23.79, p<.001 |
| Number of children | 1.86 | 0.80 | 1.83 | 0.73 | 1.78 | 0.78 | 1.91 | 0.93 | 1.92 | 0.76 | F(3, 596)=1.05, p=.369 |
| Monthly income (Rupees) | 2113.5 | 678.68 | 2110 | 758.91 | 2158.67 | 629.97 | 2137.33 | 626.97 | 2048 | 707.93 | F(3, 596)=0.75, p=0.523 |
| Marital status | n | % | n | % | n | % | n | % | n | % | |
| Married | 238 | 39.67 | 51 | 34.0 | 67 | 44.67 | 53 | 35.33 | 67 | 44.67 | (6)=8.72, p=.190 |
| Widowed | 308 | 51.50 | 87 | 58.0 | 68 | 45.33 | 85 | 56.67 | 68 | 45.33 | |
| Divorced / Separated | 54 | 9.00 | 12 | 8.0 | 15 | 10.00 | 12 | 8.00 | 15 | 10.00 | |
| Disease-related variables | |||||||||||
| M | SD | M | SD | M | SD | M | SD | M | SD | ||
| Percent past month ART adherence | 30.37 | 13.23 | 30.37 | 12.75 | 32.0 | 14.35 | 28.6 | 12.99 | 30.5 | 12.69 | F(3, 596)=1.66, p=.174 |
| Number of OIsb past 6-months | 4.58 | 1.20 | 4.61 | 1.35 | 4.52 | 1.35 | 4.58 | 0.96 | 4.63 | 1.13 | F(3, 596)=0.22, p=.883 |
| CD4+ T count | 447.42 | 273.60 | 442.57 | 272.66 | 459.61 | 284.48 | 426.19 | 275.34 | 461.29 | 262.75 | F(3, 596)=0.54, p=.653 |
| Nutrition-related variables | |||||||||||
| M | SD | M | SD | M | SD | M | SD | M | SD | ||
| Total MET min/week | 4608.57 | 1663.57 | 4957.09 | 1873.23 | 4569.73 | 1416.82 | 4691.85 | 1718.60 | 4215.60 | 1539.27 | F(3, 596)=5.25, p=.001 |
| Body mass index | 20.09 | 4.16 | 19.75 | 3.77 | 20.20 | 3.97 | 20.55 | 4.67 | 19.86 | 4.16 | F(3, 596)=1.15, p=.329 |
| Weight (kgs) | 46.22 | 10.37 | 25.17 | 9.65 | 46.42 | 9.65 | 47.43 | 11.22 | 45.84 | 10.86 | F(3, 596)=1.28, p=.283 |
| Height (cm) | 151.52 | 5.98 | 151.06 | 5.81 | 151.57 | 6.06 | 151.62 | 6.27 | 151.62 | 6.27 | F(3, 596)=0.46, p=.710 |
| Food insecurity | 21.15 | 3.44 | 20.27 | 4.29 | 21.63 | 2.58 | 20.76 | 3.45 | 21.94 | 2.98 | F(3, 596)=7.78, p<.001 |
| Hemoglobin (grams/liter) | 71.62 | 12.87 | 71.36 | 11.34 | 72.59 | 11.38 | 72.37 | 14.57 | 70.19 | 13.83 | F(3, 596)=1.08 p=.358 |
| Anemiad | n | % | n | % | n | % | n | % | n | % | |
| Severe (hemoglobin < 80) | 436 | 72.67 | 113 | 75.33 | 107 | 71.33 | 103 | 68.67 | 113 | 75.33 | (3)=2.42, p=0.491 |
| Moderate (hemoglobin 80–109) | 164 | 27.33 | 37 | 24.67 | 43 | 28.67 | 47 | 31.33 | 37 | 24.67 | |
| Mild (hemoglobin 110–119) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Not anemic (hemoglobin ≥ 120) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Psychological Variables | |||||||||||
| M | SD | M | SD | M | SD | M | SD | M | SD | ||
| Quality of life | 0.30 | 0.29 | 0.26 | 0.33 | 0.34 | 0.25 | 0.27 | 0.33 | 0.34 | 0.25 | F(3, 596)=2.92 p=.034 |
| Internalized stigma | 2.30 | 0.25 | 2.24 | 0.41 | 2.30 | 0.16 | 2.33 | 0.19 | 2.33 | 0.15 | F(3, 596)=3.54, p=.015 |
| Summary CESDe depression | 9.18 | 3.08 | 9.12 | 3.11 | 9.09 | 3.06 | 9.16 | 3.13 | 9.35 | 3.04 | F(3, 596)=0.22, p=.879 |
| Social support (scale) | 1.08 | 0.22 | 1.13 | 0.41 | 1.06 | 0.12 | 1.05 | 0.08 | 1.06 | 0.08 | F(3, 596)=4.52, p=.004 |
| Number of friends | 0.37 | 0.65 | 0.50 | 0.93 | 0.27 | 0.49 | 0.35 | 0.53 | 0.35 | 0.55 | F(3, 596)=3.24, p=.022 |
ANOVA for continuous outcomes; chi square for categorical outcomes
OI= Opportunistic infection
Could not be computed due to lack of variability between groups
Defined according to World Health Organization guidelines for anemia
CESD=Center for Epidemiologic Studies Depression Scale
Primary Outcomes
Tables 3 and 4 present results from mixed effects models examining effects of group by assessment timepoint for primary (CD4 count and BMI) and secondary (hemoglobin and serum albumin) outcomes, respectively, adjusted for key covariates and baseline ART adherence. CD4 count: Fixed effects for time indicated that overall, groups improved CD4 count over assessments (baseline and 6-, 12-, and 18-month follow-up). Group by assessment timepoint effects were significant, demonstrating that the three programs containing a nutrition component (+NE, +NS, +NENS) improved more compared to the control (SE); see Figure 1a. Analyses of the separate factorial model indicated a statistically significant interaction term for Program 4 (18-month compared to baseline: b=52.51, 95% CI, 24.19 80.82, p <.001), suggesting a synergistic effect of nutrition education and supplements on CD4 counts. BMI: Fixed effects for time indicated that overall BMI increased over assessments (baseline and 6-, 12-, and 18-month follow-up); i.e. BMI increased significantly more among participants in the three programs that contained a nutrition component (+NE, +NS, +NENS) compared to the BMI among Program 4 (SE) participants. Factorial effects signifying participation in Program 4 (SE) were not statistically significant (18 month compared to baseline: b=0.14, 95% CI, −.01, .30, p=.074); see Figure 1b. Model-estimated marginal (adjusted) means for the four programs over time are presented in Supplemental Tables 1 and 2 and Figure 1.
Table 3.
Mixed Effectsa for Primary Outcomes, N=600
| Primary Outcomes | |||||||
|---|---|---|---|---|---|---|---|
| CD4 Count | BMI | ||||||
| b | 95% CI | p-value | b | 95% CI | p-value | ||
| Fixed effects | |||||||
| Groupb | |||||||
| Standard Education (+SE)c | |||||||
| + Nutrition Education (+NE) | −39.94 | −91.79, 11.90 | .131 | 0.53 | −0.41, 1.48 | .271 | |
| + Nutrition Supplements (+NS) | −11.63 | −63.00, 39.73 | .657 | 0.94 | −0.01, 1.88 | .048 | |
| + Nutrition Education & Supplements (+ NENS) | −26.68 | −80.19, 26.84 | .329 | 0.20 | −0.77, 1.18 | .682 | |
| Time | |||||||
| Baselined | |||||||
| 6 month | 343.97 | 329.86, 358.13 | <.001 | 1.01 | 0.94, 1.09 | <.001 | |
| 12 months | 441.19 | 427.08, 455.35 | <.001 | 1.47 | 1.39, 1.55 | <.001 | |
| 18 months | 529.62 | 515.46, 543.78 | <.001 | 1.97 | 1.90, 2.05 | <.001 | |
| Group x Timee | |||||||
| 6-mo x (+NE) | 12.17 | −7.85, 32.19 | .233 | 0.26 | 0.15, 0.37 | <.001 | |
| 12-mo x (+NE ) | 40.68 | 20.66, 60.70 | <.001 | 0.40 | 0.29, 0.51 | <.001 | |
| 18-mo x (+NE) | 54.94 | 34.92, 74.96 | <.001 | 0.42 | 0.30, 0.53 | <.001 | |
| 6-mo x (+NS) | 125.69 | 105.67, 145.71 | <.001 | 1.36 | 1.25, 1.47 | <.001 | |
| 12-mo x (+NS) | 137.71 | 117.69, 157.73 | <.001 | 1.41 | 1.30, 1.52 | <.001 | |
| 18-mo x (+NS) | 143.04 | 123.02, 163.06 | <.001 | 1.38 | 1.27, 1.49 | <.001 | |
| 6-mo x (+NENS)f | 186.85 | 166.82, 206.87 | <.001 | 1.71 | 1.60, 1.82 | <.001 | |
| 12-mo x (+NENS)f | 238.43 | 218.41, 258.44 | <.001 | 1.90 | 1.79, 2.01 | <.001 | |
| 18-mo x (+NENS)f | 250.49 | 230.47, 270.51 | <.001 | 1.94 | 1.83, 2.05 | <.001 | |
| Estimate (95% CI) | Estimate (95% CI) | ||||||
| Random effects | |||||||
| Constant | 45213.71 (40221.93, 50825.00) | 16.17 (14.42, 18.14) | |||||
| Residual | 3912.42 (3664.18, 4177.47) | 0.12 (0.11, 0.13) | |||||
| Model Statistics | Wald X2(24)=38216.79, p < .001 | Wald X2(24)=26509.52 p < .001 | |||||
Multilevel model fixed effects: group (ASHA + =reference group), time (baseline=reference group), group X time, % ART adherence and covariates explaining group differences at baseline (age, quality of life, total MET, internalized stigma, social support, food insecurity, education).
SE=Program 1 (Standard Education); +NE=Program 2 (Nutrition Education); +NS=Program 3 (Nutrition Supplements); +NENS=Program 4 (Nutrition Education & Nutrition Supplements).
SE (Program 1)=reference group.
Baseline (Month 0) is the reference group.
Baseline and SE (Program 1) are the reference groups.
Addative effect are presented, rather than the interaction term.
Table 4.
Mixed Effectsa for Secondary Outcomes, N=600
| Secondary Outcomes | ||||||||
|---|---|---|---|---|---|---|---|---|
| Hemoglobin | Serum Albumin | |||||||
| b | 95% CI | p-value | b | 95% CI | p-value | |||
| Fixed effects | ||||||||
| Groupb | ||||||||
| Standard Education (+SE)c | ||||||||
| + Nutrition Education (+NE) | 1.99 | −0.02, 3.99 | .052 | −0.37 | −1.27, 0.52 | .413 | ||
| + Nutrition Supplements (+NS) | 1.39 | −0.61, 3.39 | .172 | −0.70 | −1.59, 0.19 | .125 | ||
| + Nutrition Education & Supplements (+ NENS) | −0.37 | −2.42, 1.67 | .721 | 0.38 | −0.54, 1.29 | .419 | ||
| Time | ||||||||
| Baselined | ||||||||
| 6 month | 32.13 | 30.55, 33.71 | <.001 | 7.69 | 6.95, 8.42 | <.001 | ||
| 12 months | 38.54 | 36.96, 40.12 | <.001 | 10.37 | 9.64, 11.11 | <.001 | ||
| 18 months | 41.53 | 39.95, 43.11 | <.001 | 12.19 | 11.46, 12.93 | <.001 | ||
| Group x Timee | ||||||||
| 6-mo x (+NE) | 0.50 | −1.74, 2.73 | .663 | 1.35 | 0.31, 2.39 | .011 | ||
| 12-mo x (+NE ) | 1.83 | −0.40, 4.07 | .108 | 1.11 | 0.07, 2.15 | .037 | ||
| 18-mo x (+NE) | 1.53 | −0.71, 3.76 | .181 | 1.12 | 0.08, 2.16 | .035 | ||
| 6-mo x (+NS) | 2.51 | 0.28, 4.75 | .028 | 2.96 | 1.92, 4.00 | <.001 | ||
| 12-mo x (+NS) | 1.10 | −1.13, 3.33 | .335 | 2.37 | 1.33, 3.41 | <.001 | ||
| 18-mo x (+NS) | 0.92 | −1.31, 3.15 | .420 | 2.14 | 1.10, 3.18 | <.001 | ||
| 6-mo x (+NENS)f | 6.09 | 3.86, 8.33 | <.001 | 2.61 | 1.57, 3.65 | <.001 | ||
| 12-mo x (+NENS)f | 7.44 | 5.21, 9.67 | <.001 | 2.45 | 1.41, 3.49 | <.001 | ||
| 18-mo x (+NENS)f | 6.91 | 4.68, 9.15 | <.001 | 2.29 | 1.25, 3.33 | <.001 | ||
| Estimate (95% CI) | Estimate (95% CI) | |||||||
| Random effects | ||||||||
| Constant | 27.13 (22.92, 32.11) | 4.67(3.88, 5.61) | ||||||
| Residual | 48.74 (45.65, 52.04) | 10.56 (9.91, 11.30) | ||||||
| Model Statistics | Wald X2(24)=15385.32, p < .001 | Wald X2(24)=6381.54, p < .001 | ||||||
Multilevel model fixed effects: group (ASHA + =reference group), time (baseline=reference group), group X time, % ART adherence and covariates explaining group differences at baseline (age, quality of life, total MET, internalized stigma, social support, food insecurity, education).
SE=Program 1 (Standard Education); +NE=Program 2 (Nutrition Education); +NS=Program 3 (Nutrition Supplements); +NENS=Program 4 (Nutrition Education & Nutrition Supplements).
SE (Program 1)=reference group.
Baseline (Month 0) is the reference group.
Baseline and SE (Program 1) are the reference groups.
Addative effect are presented, rather than the interaction term.
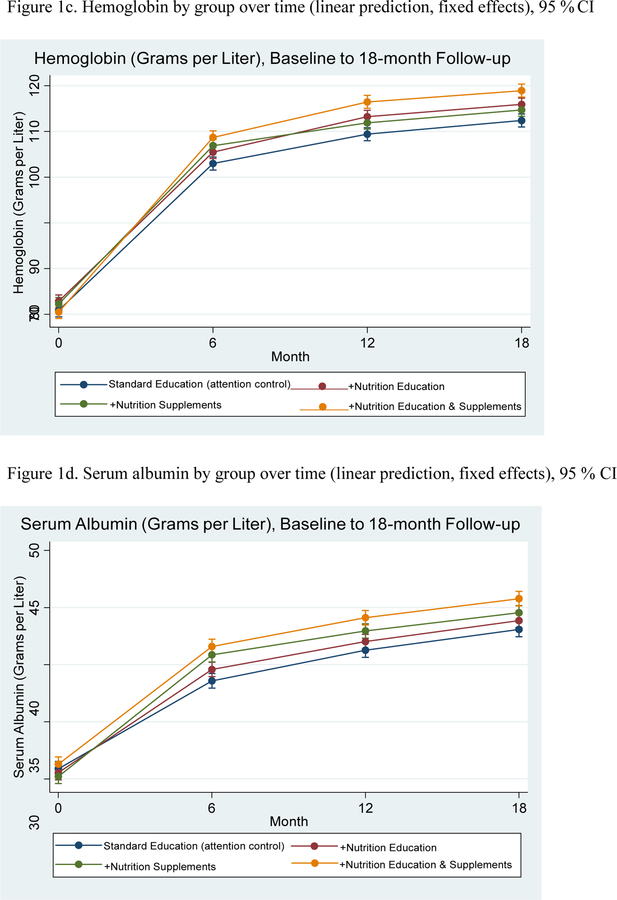
Figure 1.
Primary and Secondary Outcomes over 18-months
Secondary Outcomes
At baseline, 100% of the women in the sample were anemic, with the vast majority meeting WHO guidelines for severe anemia (n=436, 72%, see Table 2); at 18 month follow-up, none of the women in any of the four arms were severely anemic and 19.67% of the total sample had normal hemoglobin levels (n=118; n=15 in +SE, n=23 in +NE, n=26 in Asha + NS, and n=54 in +NENS). Results of logistic regression analyses found that, controlling for covariates, participants in each of the three programs with a nutrition component (+NE, +NS, +NENS) were more likely to recover from anemia compared to the control (Program 4, SE), who received standard education (+NE: OR=2.55, 95% CI, 1.2, 5.36, p<.001; + NS: OR=2.21, 95% CI, 1.08, 4.52, p=.030; +NENS: OR=9.59, 95% CI, 4.67, 19.70, p<.001). The interaction term signifying synergistic effect of the two nutrition interventions was not significant (OR=1.70, 95% CI, 0.67, 4.30, p=.261). Mixed models examining hemoglobin levels as a continuous indicator over time demonstrated a similar pattern of results, except that the interaction term at 18 month follow-up was statistically significant (b=4.47, 95% CI, 1.31, 7.63, p=.006), suggesting synergistic effects for combining nutrition supplements and education on hemoglobin as a continuous variable.
Table 4 and Figure 1d present mixed models of serum albumin levels over time. All groups improved over time; with the greatest gains exhibited in the nutrition supplements and education group (Program 4, +NENS; see Table 4). The interaction term (signifying participation in Program 4, +NENS) was not significant (b=−1.03, 95% CI, −2.51, 0.44, p=.169).
Discussion
This study is, to the best of our knowledge, the most comprehensive clinical trial of a behavioral- and nutrition-based intervention for WLH. We evaluated a number of key primary and secondary outcomes at baseline, 6-months (immediately after termination of intervention activities) and 12 and 18-month longitudinal follow-up. We found that participating in any of the Asha-enhanced programs resulted in improved physiological and nutritional outcomes during follow-up, but gains were most dramatic in the most comprehensive program (Program 4, +NENS). Gains were evident at 6 months and were retained over the year following termination of intervention procedures. This may be due to the focus on teaching skills and habits that can be maintained after intervention procedures are terminated. Psychosocial problems (e.g., depression, stigma) were also addressed as part of the care delivered in all four arms; previous analyses by our team demonstrate that key psychological variables improved over time in all groups; key psychosocial variables (e.g., depression, stigma) were not different between groups post-intervention.34
Importantly, we were able to see dramatic gains compared to previous work on nutrition supplementation in HIV population, which found relatively small effects on improving HIV-related outcomes.13 This may because of our intervention’s unique focus on food and nutrition training involving food, as opposed to vitamins or similar supplements. BMI, which is correlated with a number of salutary outcomes relevant for those with HIV (e.g., higher CD4 count, decreased risk of AIDS-defining conditions)5 also improved in all groups with highest gains in programs that included supplements in addition to education and support. This suggests that reducing food insecurity through increased caloric intake should be a key goal in treating HIV in disadvantaged communities.
A study conducted in India demonstrated that anemia is more common in women who are HIV positive with low iron levels correlated with low CD4 levels.35 Serum albumin has been linked to mortality among malnourished individuals with HIV from developing countries.36 Therefore, our findings on these secondary outcomes suggest that nutrition education and food supplements have the potential to improve critical disease-related outcomes, improving quality of life and decreasing mortality in this population.
Limitations and Future Directions.
We note several limitations. Randomization occurred at the cluster level among four clusters, which resulted in between-group differences at baseline. Although we controlled for baseline differences in subsequent analyses, we cannot rule out the possibility of residual confounding leading to the observed differences by group. In addition, as in any clinical or behavioral study, a degree of self-selection likely occurred; those who chose to participate may have been healthier or more motivated to seek care than WLH who did not. However, interest in participating in the study was very high across the PHCs and bias is likely to be very minimal. Viral load was cost-prohibited to collect on all women at the time of study implementation. Future research with an Asha-based model should test for viral load as a primary outcome.
Increased dietary diversity in addition to caloric intake may have helped improved outcomes: our team intends to present more in-depth analyses of the nutrition intake data in a follow-up to the findings presented herein. While our team has maintained close relationships with our community partners and continues to work with public health officials in India to disseminate findings, the long-term sustainability of this community-run approach outside of a research setting is unknown.
Findings demonstrate the capacity of integrated, comprehensive behavioral and nutritional interventions to improve a number of immune and nutrition-related outcomes in resource-limited settings. An Asha-supported approach may be broadly applicable to treating HIV and other public health crises around the world.
Supplementary Material
Supplemental Figure 1. CONSORT Flow Diagram
Table S1. Marginal Contrasts of Mixed Effects for Primary Outcomes
Table S2. Marginal Contrasts of Mixed Effects for Secondary Outcomes
Funding:
Research reported in this publication was supported by National Institute of Mental Health of the National Institutes of Health under award number R01MH098728
Research was funded by National Institute of Mental Health of the National Institutes of Health under award number R01MH098728 to Dr. Adeline M. Nyamathi.
Footnotes
No authors have any conflicts of interests to disclose.
Contributor Information
Adeline N. Nyamathi, University of California, Irvine, 826 Health Sciences Rd, Irvine, CA 92617, USA.
Sanghyuk S. Shin, University of California, Irvine, 826 Health Sciences Rd, Irvine, CA 92617, USA.
Sanjeev Sinha, All India Institute of Medical Sciences, Ansari Nagar (East), AIIMS campus, New Delhi-110029, India.
Catherine L. Carpenter, University of California, Los Angeles, 900 Veteran Ave, Los Angeles, CA 90095, USA.
Dana Rose Garfin, University of California, Irvine, 826 Health Sciences Rd, Irvine, CA 92617, USA.
Padma RK, Department of Medicine, All India Institute of Medical Sciences, India..
Kartik Yadav, University of California, Irvine, 826 Health Sciences Rd, Irvine, CA 92617, USA.
Maria L. Ekstrand, University of California, San Francisco, 505 Parnassus Ave, San Francisco, CA 94143, USA.
References
- 1.Kosmiski L Energy expenditure in HIV infection 1 – 3 2011;94:1677–1682. doi: 10.3945/ajcn.111.012625.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Young S, Wheeler A, McCoy S, Weiser SD. A review of the role of food insecurity in adherence to care and treatment among adult and pediatric populations living with HIV and AIDS. AIDS Behav 2014;18(0 5):505–515. doi: 10.1016/j.immuni.2010.12.017.Two-stage. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aibibula W, Cox J, Hamelin A-M, Mamiya H, Klein MB, Brassard P. Food insecurity and low CD4 count among HIV-infected people: a systematic review and meta-analysis. AIDS Care 2016;28(12):1577–1585. doi: 10.1080/09540121.2016.1191613. [DOI] [PubMed] [Google Scholar]
- 4.Lewden C, Chene G, Morlat P, et al. HIV-infected adults with a CD4 cell count greater than 500 cells/mm3 on long-term combination antiretroviral therapy reach same mortality rates as the general population. J Acquir Immune Defic Syndr 2007;46(1):72–77. doi: 10.1097/QAI.0b013e318134257a. [DOI] [PubMed] [Google Scholar]
- 5.Martinez SS, Campa A, Bussmann H, et al. Effect of BMI and fat mass on HIV disease progression in HIV- infected, antiretroviral treatment-naïve adults in Botswana. Br J Nutr 2016;115(12):2114–2121. doi: 10.1017/S0007114516001409.Effect. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hadgu TH, Worku W, Tetemke D, Berhe H. Undernutrition among HIV positive women in Humera hospital, Tigray, Ethiopia, 2013: Antiretroviral therapy alone is not enough, cross sectional study. BMC Public Health 2013;13(1):1–10. doi: 10.1186/1471-2458-13-943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Faintuch J, Soeters PB, Osmo HG. Nutritional and metabolic abnormalities in pre-AIDS HIV infection. Nutrition 2006;22:683–690. doi: 10.1016/j.nut.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 8.Shah S, Smith CJ, Lampe F, et al. Haemoglobin and albumin as markers of HIV disease progression in the highly active antiretrovial therapy era: Relationships with gender. HIV Med 2007;8(1):38–45. doi: 10.1111/j.1468-1293.2007.00434.x. [DOI] [PubMed] [Google Scholar]
- 9.Guenter P, Muurahainen N, Simons G, et al. Relationships among nutritional status, disease progression, and survival in HIV infection. J Acquir Immune Defic Syndr 1993;6(10):1130–1138. http://www.ncbi.nlm.nih.gov/pubmed/8105073. [PubMed] [Google Scholar]
- 10.Feldman JG, Gange SJ, Bacchetti P, et al. Serum albumin is a powerful predictor of survival among HIV-1-infected women. JAIDS 2003;33:66–73. [DOI] [PubMed] [Google Scholar]
- 11.Don BR, Kaysen G. Serum albumin: Relationship to inflammation and nutrition. Semin Dial 2004;17(6):432–437. doi: 10.1111/j.0894-0959.2004.17603.x. [DOI] [PubMed] [Google Scholar]
- 12.Olawumi H, Olatunjo P. The value of serum albumin in pretreatment assessment and monitoring of therapy in HIV / AIDS patients. HIV Med 2006:351–355. [DOI] [PubMed]
- 13.Visser ME, Durao S, Sinclair D, Irlam JH, Siegfried N. Micronutrient supplementation in adults with HIV infection. Cochrane Database Syst Rev 2017;(5):1–147. doi: 10.1002/14651858.CD003650.pub4.www.cochranelibrary.com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nyamathi A Comprehensive health seeking and coping paradigm. J Adv Nurs 1989;14(4):281–290. doi: 10.1111/j.1365-2648.1989.tb03415.x. [DOI] [PubMed] [Google Scholar]
- 15.Lazarus R, Folkman S Stress, Appraisal and Coping; 1984. doi: 10.1016/j.apmr.2009.07.011. [DOI] [Google Scholar]
- 16.Schlotfel. Schlotfeldt R, Nursing in the future. Nurs Outlook 1981;(29):295–301. [PubMed] [Google Scholar]
- 17.Nyamathi A, Ekstrand M, Srivastava N, et al. ASHA-Life Intervention Perspectives Voiced by Rural Indian Women Living With AIDS. Health Care Women Int 2016;37(4):412–425. doi: 10.1080/07399332.2015.1066790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nyamathi AM, Carpenter CL, Yadav K, et al. Impact of a behavioral intervention on physical and psychological health among rural women living with HIV/AIDS at six month follow-up. AIDS [Google Scholar]
- 19.Who, Chan M. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity Geneva, Switz World Heal Organ; 2011:1–6. doi:2011. [Google Scholar]
- 20.Ekstrand ML, Chandy S, Heylen E, Steward W, Singh G. Developing useful highly active antiretroviral therapy adherence measures for India: The prerana study. J Acquir Immune Defic Syndr 2010;53(3):415–417. doi: 10.1097/QAI.0b013e3181ba3e4e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ekstrand ML, Shet A, Chandye S, et al. Suboptimal adherence associated with virological failure and resistance mutations to first-line highly active antiretroviral therapy (HAART) in Bangalore, India. Int Health 2011;3(1):27–34. doi: 10.1016/j.jacc.2007.01.076.White. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Craig CL, Marshall AL, Sjöström M, et al. International physical activity questionnaire: 12-Country reliability and validity. Med Sci Sports Exerc 2003. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 23.Endicott J, Nee J, Harrison W, Blumenthal R. Quality of Life Enjoyment and Satisfaction Questionnaire: a new measure. Psychopharmacol Bull 1993;29(2):321–326. doi: 10.1111/j.1365-2850.2011.01735.x. [DOI] [PubMed] [Google Scholar]
- 24.Coates J, Swindale a, Bilinsky P. Household Food Insecurity Access Scale (HFIAS) for measurement of food access: indicator guide. Washington, DC Food Nutr Tech …. 2007 doi: 10.1007/s13398-014-0173-7.2. [DOI] [Google Scholar]
- 25.Heylen E, Panicker ST, Chandy S, Steward WT, Ekstrand ML. Food Insecurity and Its Relation to Psychological Well-Being Among South Indian People Living with HIV. AIDS Behav 2015;19(8):1548–1558. doi: 10.1007/s10461-014-0966-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Radloff LS. The CES-D Scale: A Self-Report Depression Scale for Research in the General Population. Appl Psychol Meas 1977;1(3):385–401. doi: 10.1177/014662167700100306. [DOI] [Google Scholar]
- 27.Nyamathi A, Heravian A, Zolt-Gilburne J, et al. Correlates of depression among rural women living with AIDS in Southern India. Issues Ment Health Nurs 2011;32(6):385–391. doi: 10.3109/01612840.2011.577269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Steward WT, Bharat S, Ramakrishna J, Heylen E, Ekstrand ML. Stigma is associated with delays in seeking care among HIV-infected people in India. J Int Assoc Provid AIDS Care 2013;12(2):103–109. doi: 10.1177/1545109711432315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sherbourne CD, Stewart AL. The MOS social support survey. Soc Sci Med 1991. doi: 10.1016/0277-9536(91)90150-B. [DOI] [PubMed] [Google Scholar]
- 30.Nyamathi A, Sinha S, Ganguly KK, Ramakrishna P, Suresh P, Carpenter CL. Impact of protein supplementation and care and support on body composition and CD4 count among HIV-infected women living in rural India: Results from a randomized pilot clinical trial. AIDS Behav 2013;17(6):2011–2021. doi: 10.1007/s10461-013-0420-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nyamathi A, Hanson AY, Salem BE, et al. Impact of a rural village women (Asha) intervention on adherence to antiretroviral therapy in southern India. Nurs Res 2012;61(5):353–362. doi: 10.1097/NNR.0b013e31825fe3ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nyamathi A, Salem B, Ernst EJ, et al. Correlates of Adherence Among Rural Indian Women Living With HIV/AIDS. J HIV AIDS Soc Serv 2012;11(4):327–345. doi: 10.1080/15381501.2012.735164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luke SG. Evaluating significance in linear mixed-effects models in R. Behav Res Methods 2017;49(4):1494–1502. doi: 10.3758/s13428-016-0809-y. [DOI] [PubMed] [Google Scholar]
- 34.Garfin DR, Shin SS, Ekstrand ML, et al. Depression, social support, and stigma as predictors of quality of life over time : results from an Asha-based HIV / AIDS intervention in India. AIDS Care 2019;0(0):1–9. doi: 10.1080/09540121.2018.1563281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kharb S, Kumawat M, Lallar M, Ghalaut PS, Nanda S. Serum iron, Folate, Ferritin and CD4 Count in HIV Seropositive Women. Indian J Clin Biochem 2017;32(1):95–98. doi: 10.1007/s12291-016-0571-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dao CN, Peters PJ, Kiarie JN, et al. Hyponatremia, Hypochloremia, and Hypoalbuminemia Predict an Increased Risk of Mortality During the First Year of Antiretroviral Therapy Among HIV-Infected Zambian and Kenyan Women. AIDS Res Hum Retroviruses 2011. doi: 10.1089/aid.2010.0345. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. CONSORT Flow Diagram
Table S1. Marginal Contrasts of Mixed Effects for Primary Outcomes
Table S2. Marginal Contrasts of Mixed Effects for Secondary Outcomes