Abstract
Purpose
To estimate the effectiveness of a multimodal educational intervention to increase use of shared decision-making (SDM) behaviors by inpatient pediatric and internal medicine hospitalists and trainees at teaching hospitals at Stanford University and the University of California, San Francisco.
Method
The 8-week Patient Engagement Project Study intervention, delivered at 4 services between November 2014 and January 2015, included workshops, campaign messaging, report cards, and coaching. For 12-week pre- and postintervention periods, clinician peers used the 9-point Rochester Participatory Decision-Making Scale (RPAD) to evaluate rounding teams’ SDM behaviors with patients during ward rounds. Eligible teams included a hospitalist and at least 1 trainee (resident, intern, medical student), in addition to nonphysicians. Random-effects models were used to estimate intervention effects based on RPAD scores that sum points on 9 SDM behaviors per patient encounter.
Results
In total, 527 patient encounters were scored during 175 rounds led by 49 hospitalists. Patient and team characteristics were similar across pre- and postintervention periods. Improvement was observed on all 9 SDM behaviors. Adjusted for the hierarchical study design and covariates, the mean RPAD score improvement was 1.68 points (95% CI, 1.33 to 2.03; P < .001; Cohen d = 0.82), with intervention effects ranging from 0.7 to 2.5 points per service. Improvements were associated with longer patient encounters and a higher percentage of trainees per team.
Conclusions
The intervention increased behaviors supporting SDM during ward rounds on 4 independent services. The findings recommend use of clinician-focused interventions to promote SDM adoption in the inpatient setting.
Shared decision making (SDM) is a process by which a patient and a physician make a medical decision together, incorporating patient values and the best clinical evidence.1,2 SDM has been associated with increased treatment adherence, lower health care expenditures, and decreased disease severity.3–8 Despite its value and recommendation by professional organizations, many physicians have not yet adopted SDM.9,10
Over the past decade, multiple studies have shown that interventions can improve SDM behaviors. Légaré and colleagues performed a systematic review of trials on the adoption of SDM by health care professionals; in general, their findings suggest that educational interventions aimed at physicians, patients, or both groups improve adoption of SDM.11 Significant improvement was not observed overall in secondary outcomes, although in a few studies, patient-reported outcomes such as mood (anxiety, subclinical depression), patient satisfaction, and patient knowledge showed small improvement.12–15
In a 2012 publication, Légaré and colleagues reviewed the training components of 54 individual training programs. The most common teaching methods included case-based discussions, large-group educational sessions, audit with feedback, exposure to printed educational materials, and role-play.16 A 2016 update noted an increase in the number of studies describing SDM training programs targeting health professionals, but only a small proportion assessed intervention effectiveness.17 Rusiecki and colleagues recently implemented and evaluated an SDM curriculum for internal medicine residents using standardized patients. They reported that a subset of participants (i.e., U.S. medical graduates) showed improvement in SDM skills during subsequent clinical encounters.18
Although a substantial body of literature has examined SDM in the ambulatory setting, little is known regarding SDM behaviors in the inpatient setting, where much of internal medicine and pediatric training occurs.19 The inpatient setting presents complexities that may compete with SDM, including environmental, team-based, and clinical challenges19: Hospitalized patients have higher acuity, requiring closer monitoring; medical teams vary in professional composition and experience; and clinical circumstances often require rapid decision making. Inpatient SDM interventions must incorporate the pace of inpatient practice, the acute nature of decisions, the involvement of multiple medical professionals, and the values of individual patients.20
Prior observations of team behaviors pertaining to social and behavioral sciences during inpatient medicine and pediatric ward rounds found that SDM setup and practice were among the least observed behaviors.21 A follow-up study22 using the Rochester Participatory Decision-Making Scale (RPAD)23 highlighted opportunities to improve SDM behaviors on rounds. A 2017 study of a communication skills course for inpatient oncology nurses--a course that included a few behaviors to promote SDM (checking understanding, asking open-ended questions)--demonstrated improvements in overall observable skills in simulated patient encounters but not in actual clinical encounters.24
We conducted the Patient Engagement Project (PEP) Study, a quasi-experimental intervention study, to estimate the effectiveness of a multimodal educational intervention on increasing physicians’ SDM behaviors during inpatient rounds. This study addresses how to approach changing clinicians’ behaviors in the real-life, complex inpatient team environment. We independently implemented the study on 4 services to assess its replicability.
Method
We conducted the PEP Study on the medicine and pediatric services at teaching hospitals at Stanford University and the University of California, San Francisco. All 4 services in the study provide care to diverse patient populations, with approximately half of patients enrolled in Medicare/Medicaid. IRB approval was obtained from both universities.
Study design
The intervention involved an 8-week SDM campaign targeting the 4 study services (Med-1, Med-2, Peds-1, and Peds-2) between November 2014 and January 2015. The intervention was preceded and followed by 12-week pre- and postintervention periods (August to November 2014 and December 2014 to April 2015) of structured observation of SDM behaviors during bedside rounds at each service by physicians serving as peer observers. To estimate typical SDM behaviors per period, adjusted for variations on hospitalist-led rounding teams, the study design called for recruiting 8 participating hospitalists per service and recording collective SDM behaviors during 3 rounds per hospitalist-led rounding team per study period. Hospitalists could participate in 1 or both periods, with preferential recruitment in the postintervention period of those who had participated in the preintervention period.
Study participants/intervention targets
Hospitalists were notified of the study via email in August 2014 without mention of study aims or outcomes. Those who supervised rounds at least 1 month per year and who were not study investigators were eligible for enrollment and could opt out as desired. To capture the impact of hospitalists (attendings and fellows) as trainers, eligible study rounds included at least 1 physician-trainee (resident, intern, or medical student).
Rounding teams included other disciplines (e.g., nursing, pharmacy, social work), and team composition varied among patients in each morning round to meet each patient’s needs. Although the intervention targeted physicians and trainees, any member of the team could influence the team’s SDM score.
Total patients per round included all those listed on the team census. Among these, SDM encounters were restricted to patients who were present during observed rounds (including guardian for pediatrics), did not have altered mental status, and were deemed medically stable by the hospitalist.
SDM educational intervention
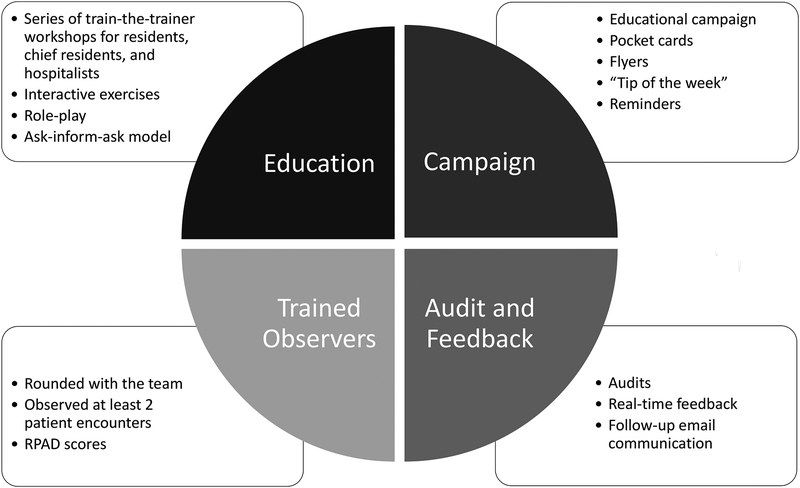
The PEP Study SDM intervention was developed through review of the literature and expert consensus by internal medicine and pediatric educators and hospitalists.19 The 4-part educational bundle of the 8-week intervention was based on medical education and quality improvement literature that demonstrates the effectiveness of interactive teaching and feedback to promote behavior change (Figure 1).25
Figure 1.
Patient Engagement Project (PEP) Study 4-part education bundle. The PEP educational intervention was delivered independently to the medicine and pediatric services at teaching hospitals at Stanford University and the University of California, San Francisco between November 2014 and January 2015.
Independently within each of the 4 services, faculty and trainees were invited to participate in all components of the intervention through emails, posters in team rooms, and announcements. PEP’s workshops and campaign messages were integrated into their everyday workflows, including attending rounds, noon conferences, and faculty meetings, to reach all hospitalists on service, not just study participants.
Interactive workshops.
The 8-week educational intervention began with two SDM workshops: a 45-minute workshop for trainees, held during lunch-hour conferences, and a 90-minute workshop for faculty and fellows, held during regularly scheduled service meetings. Workshop leaders reviewed SDM principles and introduced the RPAD23 as a specific rubric for teaching and assessing SDM communication techniques. After presentation of a video of inpatient rounds,26 attendees independently used the RPAD to evaluate the SDM behaviors depicted in the example video and then discussed their findings as a group. After watching the video, attendees engaged in role-play to practice SDM communication techniques and provided feedback to one another using the rubric. Workshop leaders facilitated group discussion on how to incorporate SDM into practice at the institutional level.
Campaign messages.
Hospitalists and trainees received ongoing exposure to SDM messaging at their service sites during the 8-week intervention period through posters, workstation screen savers, and flyers that reinforced the key principles of SDM. They also received pocket cards and “tip of the week” email messages to reinforce SDM best practices that were presented in the interactive workshops. Campaign messages were stopped after the 8-week intervention period, but posters remained on display in the hospital work rooms and hospitalists and trainees retained their pocket cards.
Team-based coaching.
During the 8-week intervention period, an expert faculty observer accompanied hospitalist-led teams during rounds and completed an RPAD for at least 2 patient encounters per team. They provided teams with real-time verbal feedback that addressed specific behaviors to promote SDM and opportunities for improvement.
Audit and feedback.
In addition to the real-time verbal feedback, hospitalists received written reports after the rounds observed during the team-based coaching. The reports included the team’s mean RPAD summary and component scores, comparisons with scores of other teams on the same service, and any qualitative feedback from patients.
Observer training
Eleven peer-observers, with 2 to 3 deployed per service, provided coaching and feedback during the intervention period and collected study outcomes during the pre- and postintervention periods. The peer-observers were trained as a group to perform RPAD ratings using videos of patient encounters from an online resource for health care communication.26 To develop consensus, observers discussed their ratings of each RPAD item and documented nuances using the PEP Study RPAD rating guide, which annotates the items defined by Shields et al23 to minimize interobserver variability (Supplemental Digital Appendix 1 at [LWW INSERT LINK]). Next, observers independently scored four videos from the online resource and discussed and vetted their scores to improve calibration. Finally, they independently viewed and scored 4 additional videos; the standard deviation of the RPAD scores was less than 1 point for all 4 videos.
Data collection
Patient and team characteristics.
During the pre- and postintervention periods, peer-observers recorded patient and team characteristics using a standardized form. Rounds-level data included date, start and end times, hospitalist name, and the round’s patient census. Patient-level data included primary language, decision topic(s) discussed, seniority of the clinician leading the discussion (hospitalist, resident, intern, medical student), team composition, and duration of the patient encounter (including time spent with the patient’s guardian or advocate). Additional patient data obtained from electronic health records included age, gender, race, ethnicity, admission date, and admitting diagnosis.
SDM measures.
Peer-observed SDM behaviors were quantified per patient encounter using the 9-item RPAD23 for the pre- and postintervention periods. Peer-observers scored each RPAD item using a 3-point scale (0 = absent, 0.5 = partial, 1 = present); then scores were summed across the 9 items to obtain RPAD scores ranging from 0 to 9 points, with higher scores indicating higher-quality SDM.
Statistical methods
We used descriptive statistics to describe nested levels of the study design--including hospitalists, rounds per hospitalist, and patients per round (total and SDM encounters)--and to examine balance in patient and round characteristics across periods. For the latter, we also used random-effects maximum likelihood regression models to estimate mean characteristics by period and statistical significance of period and period-by-service interaction effects. Random effects allowed for distinct covariance terms by hospitalist and round, grouped within service. Duration of patient encounter had a right-skew distribution and was log-transformed for analysis.
To analyze intervention effects in the sample of SDM encounters, we first used descriptive statistics to estimate mean RPAD scores per service and period and calculated mean differences between periods. We then estimated intervention effects using random-effects regression models, reporting unadjusted mean differences (95% CIs) between periods, P values, and Cohen d.27 Subsequently, we added 8 covariates expected to influence RPAD scores, intervention effects, or both, as well as their 2-way interactions with period and service: 4 patient-level covariates (log-duration of patient encounter, gender, SDM topic {Diagnosis/Not; Treatment/Not}, and status of lead discussant {Trainee/Trainer}) and 4 round/team characteristics (team size, trainee percentage, patient census, and round duration). After examining the fit of the model via goodness-of-fit statistics and residuals, we modeled log-duration of patient encounter as a nonlinear (quadratic) function.
We conducted 2 exploratory analyses in models adjusted for study design but not covariates. One allowed for distinct random effects by observers in place of attendings. The other modeled the proportion of each RPAD score represented by components discussing treatment plans (Items 4, 5) as a function of 4-level SDM topic, stratified by period. Analyses were conducted using SAS Version 9.4 (SAS Institute Inc., Cary, North Carolina).
Results
We observed 35 and 34 hospitalists (49 total) leading 87 and 88 (175 total) team rounds in the pre- and postintervention periods, respectively (2.5 and 2.6 rounds per hospitalist; P = .41), and scored 254 and 273 (571 total) SDM encounters (2.9 and 3.1 encounters per round) (Table 1). The mean patient census per round increased by 10% across periods (9.0 versus 9.9; P = .10), whereas the percentage of patients participating in SDM encounters remained stable (33% versus 34%; P = .68).
Table 1.
Comparison of Patient Engagement Project Study Design Features Across Study Periodsa
| Overall | |||
| Hospitalists, no. | 35 | 34 | 20 / 49 (41%) |
| Rounds per hospitalist, mean | 2.5 | 2.6 | |
| Patients per round, mean (% SDM) | 9.0 (33%) | 9.9 (34%) | |
| SDM encounters, no. | 254 | 273 | |
| Med-1 | |||
| Hospitalists, no. | 6 | 6 | 6 / 6 (100%) |
| Rounds per hospitalist, mean | 2.2 | 2.7 | |
| Patients per round, mean (% SDM) | 7.9 (38%) | 8.7 (38%) | |
| SDM encounters, no. | 34 | 46 | |
| Med-2 | |||
| Hospitalists, no. | 12 | 13 | 3 / 22 (14%) |
| Rounds per hospitalist, mean | 2.8 | 2.2 | |
| Patients per round, mean (% SDM) | 8.5 (31%) | 9.7 (33%) | |
| SDM encounters, no. | 83 | 83 | |
| Peds-1 | |||
| Hospitalists, no. | 9 | 9 | 7 / 11 (64%) |
| Rounds per hospitalist, mean | 2.7 | 2.9 | |
| Patients per round, mean (% SDM) | 10.4 (26%) | 10.1 (35%) | |
| SDM encounters, no. | 62 | 86 | |
| Peds-2 | |||
| Hospitalists, no. | 8 | 6 | 4 / 10 (40%) |
| Rounds per hospitalist, mean | 2.2 | 2.8 | |
| Patients per round, mean (% SDM) | 8.9 (50%) | 10.8 (34%) | |
| SDM encounters, no. | 75 | 58 |
Abbreviation: SDM indicates shared decision making.
The 8-week Patient Engagement Project Study intervention was delivered at four 4 services (Med-1, Med-2, Peds-1, and Peds-2) at teaching hospitals at Stanford University and the University of California, San Francisco between November 2014 and January 2015. Rounds were observed during 12-week pre- and postintervention periods (August to November 2014 and December 2014 to April 2015) to evaluate rounding teams’ SDM behaviors with patients. SDM encounters were restricted to patients who were present during observed rounds (including guardian for pediatrics), did not have altered mental status, and were deemed medically stable by the hospitalist.
%SDM indicates percentage of patients participating in SDM encounters.
Although rounds and patients are unique to each period, 20/49 (41%) hospitalists participated in both periods and accounted for 61% of SDM encounters. Quantities of hospitalists, rounds, and SDM encounters at Med-2 were approximately double those at Med-1.22
Patient and team characteristics
The study samples were balanced between the pre- and postintervention periods with respect to most characteristics (Table 2). The mean patient ages on pediatric and medicine services were 6.6 and 58 years, respectively; about half the patients were non-Caucasian and more than 80% were native English speakers. Physician-trainees composed about half of rounding team members, and interns led more than half of patient encounters. Individual patient encounters lasted a median of 13 minutes. Of SDM discussions, 12% to 15% focused on diagnoses, 47% to 48% on treatment plans, and 21% to 30% on both.
Table 2.
Comparison of Patient and Team Characteristics for SDM Encounters Across Patient Engagement Project Study Periodsa
| Characteristic | Preintervention period | Postintervention period | P value | |
|---|---|---|---|---|
| SDM patients assessed, no. | 254 | 273 | ||
| Patient age (years). median (Q1–Q3) | ||||
| Pediatrics | 6.0 (2.0–13) | 5.0 (1.0–12) | .55 | .48 |
| Medicine | 59 (42–69) | 62 (46–71) | .36 | .53 |
| Male patient, no. (%) | 128 (51) | 141 (52) | .92 | .02 |
| Patient race, no. (%) | .25 | .15 | ||
| White | 136 (54) | 128 (47) | ||
| Asian | 28 (11) | 33 (12) | ||
| African American | 26 (10) | 29 (11) | ||
| Pacific Islander/Native American | 10 (3.9) | 3 (1) | ||
| Other | 54 (21) | 64 (23) | ||
| Unknown | 0 (0) | 16 (6) | ||
| Patient primary language, no. (%) | .39 | .80 | ||
| English | 220 (87) | 228 (84) | ||
| Spanish | 21 (8.3) | 29 (11) | ||
| Other | 13 (5.1) | 9 (3) | ||
| Unknown | 0 (0) | 7 (3) | ||
| Duration of SDM patient encounter (minutes) | .09 | .77 | ||
| Median (Q1–Q3) | 13.0 (9–18) | 13.0 (8–18) | ||
| ≤ 6, no. (%) | 34 (13) | 40 (15) | ||
| 7–12, no. (%) | 80 (32) | 90 (33) | ||
| 13–18, no. (%) | 78 (31) | 80 (29) | ||
| 19–24, no. (%) | 27 (11) | 36 (1) | ||
| > 24, no. (%) | 35 (14) | 27 (10) | ||
| Primary decision discussed, no. (%)b | < .001 | < .001 | ||
| Treatment | 120 (47) | 130 (48) | ||
| Diagnosis | 39 (15) | 34 (12) | ||
| Diagnosis and treatment | 77 (30) | 57 (21) | ||
| Neither (other) | 18 (7.1) | 52 (19) | ||
| Presenting clinician, no. (%) | .18 | .10 | ||
| Medical student | 89 (35) | 84 (31) | ||
| Intern | 132 (52) | 160 (59) | ||
| Resident | 30 (12) | 21 (8) | ||
| Attending hospitalist | 4 (1.6) | 2 (1) | ||
| Unknown | 0 (0) | 6 (2) | ||
| Rounds observed, no. | 88 | 87 | ||
| Rounds duration in hours, median (Q1–Q3)c | 1.8 (1.5–2.4) | 2.1 (1.6–2.9) | .14 | < .001 |
| Team size, median (Q1–Q3) | 7.0 (6–10) | 7.0 (5–9) | .01 | .73 |
| Trainee percentage of team, median (Q1–Q3)d | 50 (40–55) | 50 (40–60) | .08 | 11 |
Abbreviations: SDM indicates shared decision making; Q1, 25th percentile, Q3, 75th percentile.
Continuous characteristics are summarized by median (Q1–Q3) and categorical characteristics by no. (%); P values arise from random-effects models that address variation of each characteristic between periods and differential period effects among services (interaction effect). The 8-week Patient Engagement Project Study intervention was delivered at four 4 services (Med-1, Med-2, Peds-1, and Peds-2) at teaching hospitals at Stanford University and the University of California, San Francisco between November 2014 and January 2015. Rounds were observed during 12-week pre- and postintervention periods (August to November 2014 and December 2014 to April 2015) to evaluate rounding teams’ SDM behaviors with patients. SDM encounters were restricted to patients who were present during observed rounds (including guardian for pediatrics), did not have altered mental status, and were deemed medically stable by the hospitalist.
SDM topics could address other matters, such as discharge timing.
Round-level hours per patient = round duration divided by the patient census per round.
Trainees were residents, interns, and medical students; hospitalists were attendings and fellows. Other team members could include nurses, pharmacists, social workers, interpreters, and consultants.
Patient gender, the distribution of SDM topics, and mean duration of rounds showed statistically significant service-by-period interactions (Table 2). The proportion of male SDM patients fell from 77% to 55% at Peds-1 and rose from 33% to 48% at Med-2. SDM discussions focused on treatment became more common at Med-1 (from 32% to 67%), whereas topics other than diagnosis or treatment became more common at Med-2 (from 6% to 45%). The mean duration of rounds decreased at Med-1 (by 46 minutes) and increased at Med-2 and Peds-1 (by 34 to 38 minutes). Overall mean team size decreased statistically significantly by 1.1 members.
Effect of the SDM intervention campaign
Mean RPAD scores improved overall, from 3.91 preintervention to 5.77 postintervention, representing a 1.86-point absolute difference (Table 3). Adjusted for the hierarchical study design, the mean improvement was 1.69 points (95% CI, 1.42 to 1.96; P < .001; Cohen d, 1.08), with all service-specific 95% CIs excluding 0. Adjusted for covariates, the overall intervention effect changed little, but all 95% CIs widened, such that the overall Cohen d was 0.82 and the Med-1 CI included 0.
Table 3.
Estimates of Mean RPAD Scores and Intervention Effects According to 3 Methods of Analysisa
| Analysis method | Overall RPAD score | Period
effect, P value |
RPAD score | Service-by-period, P value |
|||
|---|---|---|---|---|---|---|---|
| Descriptive statistics | |||||||
| Preintervention | 3.91 | 4.72 | 4.37 | 3.01 | 4.15 | ||
| Postintervention | 5.77 | 5.62 | 5.67 | 5.69 | 6.13 | ||
| Difference, mean | 1.86 | 0.90 | 1.30 | 2.68 | 1.98 | ||
| Model adjusted for service, period, and their interaction | |||||||
| Preintervention | 4.07 | 4.72 | 4.34 | 3.06 | 4.14 | ||
| Postintervention | 5.76 | 5.62 | 5.68 | 5.59 | 6.13 | ||
| Difference, mean (95% CI) |
1.69 (1.42–1.96) |
< .001 | 0.90 (0.35–1.45) |
1.34 (0.81–1.87) |
2.53 (1.92–3.13) |
1.95 (1.55–2.44) |
< .001 |
| Cohen d | 1.08 | 0.73 | 0.83 | 1.27 | 1.55 | ||
| Model as above, also adjusted for patient and team covariatesb | |||||||
| Preintervention | 4.13 | 4.53 | 4.31 | 3.50 | 4.19 | ||
| Postintervention | 5.81 | 5.24 | 5.65 | 6.02 | 6.34 | ||
| Difference, mean (95% CI) |
1.68 (1.33–2.03) |
< .001 | 0.71 (-0.32–1.75) |
1.35 (0.62–2.07) |
2.52 (1.59–3.45) |
2.15 (1.56–2.74) |
.03 |
| Cohen d | 0.82 | 0.30 | 0.61 | 0.83 | 1.25 | ||
Abbreviation: RPAD indicates Rochester Participatory Decision-Making Scale.23
The 8-week Patient Engagement Project Study intervention was delivered at four 4 services (Med-1, Med-2, Peds-1, and Peds-2) at teaching hospitals at Stanford University and the University of California, San Francisco between November 2014 and January 2015. Rounds were observed during 12-week pre- and postintervention periods (August to November 2014 and December 2014 to April 2015) to evaluate rounding teams’ SDM behaviors with patients. SDM encounters were restricted to patients who were present during observed rounds (including guardian for pediatrics), did not have altered mental status, and were deemed medically stable by the hospitalist. Services are ordered by increasing standardized intervention effects using Cohen d statistic.
Adjusted for 4 team-level continuous covariates (round census, round duration, team size, and 5-level trainee percentage of team) and 4 patient-level covariates (log-scale duration of patient encounter with linear and quadratic terms, gender {Male/Female}, SDM topic {Diagnosis/Not; Treatment/Not}, and status of lead discussant {Trainee/Trainer}). Random variation among rounds per hospitalist at {Med-1, Peds-1, Peds-2, Med-2} accounted for {0.0, 4.3, 2.8, 6.3} percent of overall variation, respectively.
Based on the design-adjusted model, random-effect estimates of heterogeneity among hospitalists were statistically significant at Peds-1 and Med-2 (each P < .04). A parallel exploratory analysis found no statistically significant heterogeneity among observers (P > .08 per service).
Associations of patient and team characteristics with SDM improvements
The intervention effect increased with the log-duration of patient encounter; at {4.8, 13, 35} minutes, improvements in RPAD scores were {1.2, 1.7, 2.2}, respectively (duration-by-period, P = .02). The increasing intervention effect reflects relatively steep increases in RPAD scores up to the median duration of 13 minutes; the scores plateaued in the preintervention period but continued to rise less steeply in the postintervention period.
The RPAD scores improved by 0.27 points per 10% increment in trainees on the team (trainee%-by-period P = .02), arising from RPAD scores being negatively associated with trainee percentage preintervention and positively associated postintervention.
Although the intervention effect was independent of both gender and SDM topic, RPAD scores were higher by 0.33 points (P = .003) for female patients than for male patients and higher by 0.24 points (P = .05) when diagnosis was not discussed. No other covariate effect was statistically significant at P < .05 in the multivariable model.
SDM improvements by RPAD item scores
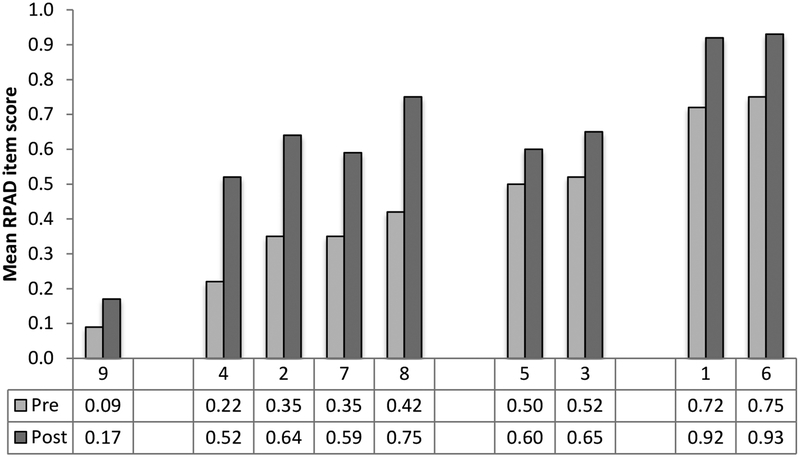
Scores improved on all 9 RPAD items in the postintervention period (Figure 2). In particular, 4 behaviors explained 63% of the unadjusted difference of 1.86 between periods: examine barriers (16%), discuss uncertainties (16%), solicit patients’ questions (13%), and ask open-ended questions (18%). All four mean item-level scores were below 0.5 preintervention and above 0.5 postintervention. However, the lowest preintervention score, for “physician checks own understanding,” showed little improvement. In an exploratory analysis stratified by period, we found no association between proportion of RPAD scores addressing treatment plans and SDM topic (both P ≥ .35).
Figure 2.
Patient Engagement Project (PEP) Study pre- and postintervention scores. The PEP educational intervention was delivered at pediatric and medicine services at Stanford University and the University of California, San Francisco between November 2014 and January 2015. The pre- and postintervention periods were August to November 2014 and December 2014 to April 2015. Overall, mean item-level scores improved on all 9 behaviors of the Rochester Participatory Decision-Making Scale (RPAD) between the pre- and postintervention periods. RPAD item definitions23: 9 = Physician checks his/her understanding of patient’s point of view; 4 = Examine barriers to follow-through with treatment plan; 2 = Discussion of the uncertainties associated with the situation; 7 = Physician asks, “Any questions?”; 8 = Physician asks open-ended questions; 5 = Physician gives patient opportunity to ask questions and checks patient’s understanding of treatment plan; 3 = Clarification of agreement; 1 = Explain the clinical issue or nature of the decision; and 6 = Physician’s medical language matches patient’s level of understanding.
Discussion
The PEP Study demonstrated that a multimodal educational intervention achieved clinically and statistically significant improvements in SDM communications and behaviors of clinical teams caring for pediatric and adult inpatients. The overall adjusted mean 1.7-point improvement occurred despite an overall higher patient census and lower team size during the postintervention period. Further, the intervention was replicable: 4 independent inpatient services with widely different practice characteristics22 each achieved adjusted mean improvements in RPAD scores of 0.7 to 2.5 points. At Med-1 (0.7-point improvement), the preintervention RPAD score was above average, and the postintervention score was comparable to other services’ scores. Covariate adjustment diminished the estimated intervention effect only at Med-1, suggesting its covariate profile was atypical.
The features most strongly associated with improved SDM behaviors were longer patient encounters and higher percentage of physician-trainees on teams. The latter finding may reflect trainees’ receptiveness to learning SDM concepts via the educational intervention and hospitalists’ support of SDM behaviors. Clinician-educators may consciously model SDM behaviors more often when more trainees are present, evoking positive patient responses to the enhanced attention. The behavior least employed and least improved by the intervention was “physician checks own understanding.” Perhaps team dynamics inhibited members’ use of this behavior, which could reveal their lack of understanding. However, the multidimensional drivers of health make each case unique. Future educational interventions should particularly emphasize the importance of this behavior.25
We chose to use the RPAD instrument in the PEP Study based on expert consensus that it highlights SDM behaviors seen in the inpatient setting better than the commonly used OPTION12 tool.28 Although the RPAD tool was not originally designed for inpatient use and has not yet been validated in a hospital setting,23 the general communication behaviors used to promote SDM are applicable across both settings. Two OPTION12 items (3, 10) pertaining to SDM setup would more likely occur before rounds rather than during study observation, and OPTION12 does not capture the elements of health literacy and self-assessment (Items 6, 9) captured by the RPAD.20 However, four RPAD items (1, 4, 5, 7) have close OPTION12 analogs (1, 7, 8, 9, respectively).
We gauge our findings relative to 3 studies of primary care outpatients: a cross-sectional study using the RPAD23 and two studies using the (Dutch) OPTION12 tool, one comparing differences in general practitioners’ SDM behaviors between 2007 and 201529 and the other comparing SDM behaviors of trained and untrained general practitioners, using intervention components similar to PEP’s.30 On the 0-to-1 scale of the OPTION12 tool, PEP’s unadjusted mean score (preintervention, 3.91/9 = 0.43) is higher than that of the other RPAD-based study (3.13/9 = 0.35)23 and baseline mean scores of the OPTION12-based studies (0.14 and 0.23).29,30 PEP’s higher score might be explained by team-based SDM, which allows multiple individuals to contribute to the score; by inpatients versus outpatients; or by methodologic differences between studies (e.g., RPAD versus OPTION12 items). Comparing intervention effects, Cohen d ranks the effect of PEP (0.82) between the passive intervention29 (0.74) and the active intervention30 (0.94). Like PEP, both comparator intervention studies reported higher improvements with longer encounters, and one reported higher improvements among female patients and when discussing treatment.30
Our finding of higher RPAD scores associated with SDM discussions of treatment evaluations led us to examine whether RPAD scores differ systematically by SDM topic, since Items 4 and 5 particularly address treatment plans. We found no evidence that this wording biases RPAD scores. Nonetheless, SDM discussions of diagnoses might be more challenging than discussions of treatments, as suggested by their 1:4 prevalence as primary SDM topics. Future research could examine underpinnings of the distribution of topics and, if needed, offer clinicians SDM training tailored to discussions of diagnostic evaluations, including role-play and live opportunities with team-based coaching and feedback.
To maximize accuracy of scores, PEP employed peer-observers. Although observers were trained to be unobtrusive, their presence might have encouraged more ideal physician behavior and higher RPAD scores (Hawthorne effect). However, both pre- and postintervention observations were subject to the same confounding factors, and biases were mitigated through use of an RPAD rating guide and intermittent recalibration through video review and ratings. Other studies blinded observers by recording patient encounters23,29,30 but recordings were not permitted by our IRBs. PEP’s limited pool of observers precluded blinding them to study period and deploying multiple observers per round; thus patient-level intra- and interrater reliability were not assessed. However, PEP observers scored multiple patients and rounds; averaged over multiple rounds, scores should differ little among observers on a given service. Indeed, statistically significant heterogeneity among observers was not found.
We developed an educational intervention that institutions could implement to improve SDM behaviors of providers. We standardized core aspects of the intervention, including workshop curricula, formative feedback after implementation on rounds by participating hospitalists, and campaign messages. Importantly, PEP’s workshops and campaign messages were designed to reach all hospitalists on service, not just study participants. By intervening on multiple generations of physicians at teaching hospitals, our multicomponent intervention approach has the potential for SDM behaviors to become normative. To quantify the study’s impact, we limited PEP Study observations to morning rounds because they reflect a routine component of clinical practice that is amenable to standardized capture of pre- and postintervention assessments of active hospitalists. We believe these rounds afford key opportunities for trainee education and attending physician role modeling that strengthen and reinforce the educational focus of our intervention.
In conclusion, we demonstrate that clinician-focused educational interventions can promote the adoption of SDM behaviors by inpatient medicine and pediatrics teams. Unlike prior studies that focused on SDM behaviors of individual providers, the PEP Study shows the feasibility of an innovative team-based approach to improving inpatient clinicians’ use of SDM behaviors, even on large, multidisciplinary teams.
Supplementary Material
Acknowledgments:
The authors are grateful to additional members of the Patient Engagement Project who collected study observations and/or delivered the intervention: Poonam Hosamani, MD, clinical assistant professor, Department of Medicine, Stanford University School of Medicine, Stanford, California; Adeena Khan, MD, assistant professor, Department of Medicine, University of California, San Francisco, San Francisco, California; Lisa Shieh, MD, clinical professor, Department of Medicine, Stanford University School of Medicine, Stanford, California; and Lijia Xie, MD, hospitalist, Highland Hospital, Oakland, California).
Funding/Support: We gratefully acknowledge financial support from grant R25 AT006573, awarded to Dr. Satterfield by the National Institutes of Health National Center for Complementary and Integrative Health (formerly National Center for Complementary and Alternative Medicine) and Office of Behavioral and Social Sciences Research.
Footnotes
Other disclosures: None reported.
Ethical approval: Institutional review board approval to conduct the PEP Study was obtained from both the University of California, San Francisco and Stanford University.
Previous presentations: This study was presented at the Pediatric Academic Societies 2016 Annual Meeting (April 2016, Baltimore, Maryland), where it received the 2016 Academic Pediatric Association Ray E. Helfer Innovation in Medical Education Award. It also was presented at the Society of General Internal Medicine 2016 Annual Meeting, May 2016, Hollywood, Florida, and Learn Serve Lead 2016: The AAMC Annual Meeting, November 2016, Seattle, Washington.
Contributor Information
Stephanie M. Harman, Department of Medicine, Stanford University School of Medicine, Stanford, California..
Rebecca Blankenburg, Department of Pediatrics, Stanford University School of Medicine, Stanford, California..
Jason M. Satterfield, Department of Medicine, University of California, San Francisco, School of Medicine, San Francisco, California..
Brad Monash, Departments of Medicine and Pediatrics, University of California, San Francisco, School of Medicine, San Francisco, California..
Stephanie Rennke, Department of Medicine, University of California, San Francisco, School of Medicine, San Francisco, California..
Patrick Yuan, Department of Epidemiology and Biostatistics, University of California, San Francisco, School of Medicine, San Francisco, California..
Debbie S. Sakai, Department of Pediatrics, Stanford University School of Medicine, Stanford, California..
Eric Huynh, University of California, Berkeley, Haas School of Business, Berkeley, California..
Ian Chua, Department of Pediatrics, Children’s National Medical Center, Washington, D.C.; Department of Pediatrics, Stanford University School of Medicine, Stanford, California.
Joan F. Hilton, Department of Epidemiology and Biostatistics, University of California, San Francisco, School of Medicine, San Francisco, California..
References
- 1.Towle A, Godolphin W. Framework for teaching and learning informed shared decision making. BMJ. 1999;319(7212):766–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Elwyn G, Edwards A, Hood K, et al. Achieving involvement: Process outcomes from a cluster randomized trial of shared decision making skill development and use of risk communication aids in general practice. Fam Pract. 2004;21(4):337–346. [DOI] [PubMed] [Google Scholar]
- 3.Weingart SN, Zhu J, Chiappetta L, et al. Hospitalized patients’ participation and its impact on quality of care and patient safety. Int J Qual Health Care. 2011;23(3):269–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shay LA, Lafata JE. Where is the evidence? A systematic review of shared decision making and patient outcomes. Med Decis Making. 2015;35(1):114–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fiks AG, Mayne S, Localio AR, Alessandrini EA, Guevara JP. Shared decision-making and health care expenditures among children with special health care needs. Pediatrics. 2012;129(1):99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fiks AG, Mayne SL, Karavite DJ, et al. Parent-reported outcomes of a shared decision-making portal in asthma: A practice-based RCT. Pediatrics. 2015;135(4):e965–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Valenzuela JM, Smith LB, Stafford JM, et al. Shared decision-making among caregivers and health care providers of youth with type 1 diabetes. J Clin Psychol Med Settings. 2014;21(3):234–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fiks AG, Hughes CC, Gafen A, Guevara JP, Barg FK. Contrasting parents’ and pediatricians’ perspectives on shared decision-making in ADHD. Pediatrics. 2011;127(1):e188–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blair L, Légaré F. Is shared decision making a utopian dream or an achievable goal? Patient. 2015;8(6):471–476. [DOI] [PubMed] [Google Scholar]
- 10.Légaré F, Witteman HO. Shared decision making: Examining key elements and barriers to adoption into routine clinical practice. Health Aff (Millwood). 2013;32(2):276–284. [DOI] [PubMed] [Google Scholar]
- 11.Légaré F, Thompson-Leduc P. Twelve myths about shared decision making. Patient Educ Couns. 2014;96(3):281–286. [DOI] [PubMed] [Google Scholar]
- 12.Elwyn G, Lloyd A, May C, et al. Collaborative deliberation: A model for patient care. Patient Educ Couns. 2014;97(2):158–164. [DOI] [PubMed] [Google Scholar]
- 13.Roter DL, Wexler R, Naragon P, et al. The impact of patient and physician computer mediated communication skill training on reported communication and patient satisfaction. Patient Educ Couns. 2012;88(3):406–413. [DOI] [PubMed] [Google Scholar]
- 14.van Peperstraten A, Nelen W, Grol R, et al. The effect of a multifaceted empowerment strategy on decision making about the number of embryos transferred in in vitro fertilisation: Randomised controlled trial. BMJ. 2010;341:c2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hess EP, Knoedler MA, Shah ND, et al. The chest pain choice decision aid: A randomized trial. Circ Cardiovasc Qual Outcomes. 2012;5(3):251–259. [DOI] [PubMed] [Google Scholar]
- 16.Légaré F, Politi MC, Drolet R, et al. Training health professionals in shared decision-making: An international environmental scan. Patient Educ Couns. 2012;88(2):159–169. [DOI] [PubMed] [Google Scholar]
- 17.Diouf NT, Menear M, Robitaille H, Painchaud Guérard G, Légaré F. Training health professionals in shared decision making: Update of an international environmental scan. Patient Educ Couns. 2016;99(11):1753–1758. [DOI] [PubMed] [Google Scholar]
- 18.Rusiecki J, Schell J, Rothenberger S, Merriam S, McNeil M, Spagnoletti C. An innovative shared decision-making curriculum for internal medicine residents: Findings from the University of Pittsburgh Medical Center. Acad Med. 2018;93(6):937–942. [DOI] [PubMed] [Google Scholar]
- 19.Rennke S, Yuan P, Monash B, et al. The SDM 3 Circle Model: A literature synthesis and adaptation for shared decision making in the hospital. J Hosp Med. 2017;12(12):1001–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elwyn G, Tilburt J, Montori V. The ethical imperative for shared decision-making. Eur J Pers Cent Healthc. 2013;1(1):129–131. [Google Scholar]
- 21.Satterfield JM, Bereknyei S, Hilton JF, et al. The prevalence of social and behavioral topics and related educational opportunities during attending rounds. Acad Med. 2014;89(11):1548–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blankenburg R, Hilton JF, Yuan P, et al. Shared decision-making during inpatient rounds: Opportunities for improvement in patient engagement and communication. J Hosp Med. 2018;13(7):453–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shields CG, Franks P, Fiscella K, Meldrum S, Epstein RM. Rochester Participatory Decision-Making Scale (RPAD): Reliability and validity. Ann Fam Med. 2005;3(5):436–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Banerjee SC, Manna R, Coyle N, et al. The implementation and evaluation of a communication skills training program for oncology nurses. Transl Behav Med. 2017;7(3):615–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Starmer AJ, Spector ND, Srivastava R, et al. Changes in medical errors after implementation of a handoff program. N Engl J Med. 2014;371(19):1803–1812. [DOI] [PubMed] [Google Scholar]
- 26.Doc.com. Online video library of communication skills. http://doc.com. Accessed March 6, 2019.
- 27.Cohen J Statistical Power Analysis for the Behavioral Sciences. New York, NY: Routledge Academic; 1988. [Google Scholar]
- 28.Stubenrouch FE, Pieterse AH, Falkenberg R, et al. OPTION5 versus OPTION12 instruments to appreciate the extent to which healthcare providers involve patients in decision-making. Patient Educ Couns. 2016;99(6):1062–1068. [DOI] [PubMed] [Google Scholar]
- 29.Meijers MC, Noordman J, Spreeuwenberga P, Olde Hartman TC, van Dulmen S. Shared decision-making in general practice: An observational study comparing 2007 with 2015 [published online ahead of print August 30, 2018]. Fam Pract. doi: 10.1093/fampra/cmy070. [DOI] [PubMed] [Google Scholar]
- 30.Sanders AR, Bensing JM, Essed MA, Magnée T, de Wit NJ, Verhaak PF. Does training general practitioners result in more shared decision making during consultations? Patient Educ Couns. 2017;100(3):563–574. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.