Abstract
Background:
Alcohol use influences HIV disease severity via multiple mechanisms. Whether HIV disease severity is sensitive to changes in alcohol use among people with HIV (PWH) is understudied.
Setting:
National Veterans Health Administration.
Methods:
Pairs of AUDIT-C screens within 9-15 months (2/1/08-9/30/14) were identified among PWH from the Veterans Aging Cohort Study (VACS). Initial and follow-up VACS Index 2.0 pairs obtained 0-270 days after initial and follow-up AUDIT-Cs, respectively, determined change in VACS Index 2.0, a composite HIV severity measure. Change in VACS Index 2.0 was regressed on AUDIT-C change scores (−12 to +12) adjusted for demographics, initial VACS Index 2.0, and days between VACS Index measures.
Results:
Among 23,297 PWH (76,202 observations), most had no (51%) or low-level (38%) alcohol use initially. Most (54%) had no subsequent change; 21% increased and 24% decreased drinking. Initial VACS Index 2.0 scores ranged from 0-134, change scores ranged from −65 to +73, with average improvement of 0.76 points (SD 9.48). AUDIT-C change was associated with VACS Index 2.0 change (p<0.001). Among those stable alcohol use (AUDIT-C change ≤│1│point), VACS Index 2.0 improvements ranged 0.36-0.60 points. For those with maximum AUDIT-C increase (change from 0 to 12), VACS Index 2.0 worsened 3.74 points (95% CI −4.71, −2.78); for those with maximum AUDIT-C decrease (change from 12 to 0), VACS Index 2.0 changed minimally [−0.60 (95% CI −1.43, 0.23)].
Conclusion:
In this national sample, improvement in HIV severity was generally greatest among those with stable alcohol use (primarily those with no use).
Keywords: HIV, alcohol, HIV disease severity, VACS Index, alcohol use
INTRODUCTION
Alcohol use has substantial adverse influences on the health of people with HIV (PWH),1,2 but is highly prevalent in this population.3 Among myriad adverse HIV-related outcomes, higher levels of alcohol use are associated with greater HIV disease severity and risk of death.2,4-7
Emerging longitudinal research has focused on understanding population-level patterns of alcohol use associated with increased HIV disease severity among PWH,8 and examining whether individual-level changes in alcohol use over time may influence HIV-relevant outcomes,9 including disease severity.10 Research of this type is nascent but has potential to identify PWH with the greatest alcohol-related risk, and to determine levels of changes in alcohol use that may facilitate clinically-relevant improvements or decrements in markers of HIV severity. Thus, further work is needed to understand whether individual-level changes in reported alcohol use correspond to changes in markers of HIV disease severity, and to examine this relationship within important clinical subgroups (e.g., persons with uncontrolled or untreated HIV).
Alcohol use influences morbidity and mortality among PWH via both behavioral (e.g., ART adherence) and biological (e.g., immune suppression, chronic inflammation) mechanisms sometimes mediated through other common comorbid conditions.1 We previously identified significant associations between individual-level changes in alcohol use and changes in traditional clinical markers of HIV severity—CD4 cells/ml and HIV RNA viral load.10 However, no study has evaluated a more-comprehensive, composite measure of HIV disease severity—an outcome that more broadly reflects the wide range of mechanisms via which alcohol use influences adverse outcomes among PWH.1 The Veterans Aging Cohort Study (VACS) Index11 is a composite measure of HIV disease severity that includes multiple clinical indicators (age, traditional markers of HIV severity—CD4 and HIV-1 RNA, hemoglobin, renal and hepatic function tests, and viral hepatitis C infection). Its original version predicts all-cause mortality, cause-specific mortality, and other clinical outcomes in PWH with proven reproducibility, accuracy, and validity.12-14 The recently-developed VACS Index 2.0 incorporates additional clinical indicators and uses continuous measures for greater precision,15 resulting in significantly improved discrimination for predicting mortality and better ability to detect change.15,16 Because it incorporates a broader range of clinical outcomes that influence health among PWH,1,17 this combined clinical measure is more sensitive to HIV disease severity than traditional markers of HIV severity alone,12,16,18 and, thus, provides improved clinical utility for understanding the dynamic and complex relationship between alcohol use and HIV disease severity.1,15,16 Finally, because all indicators included in VACS Index 2.0 are routinely monitored according to best care practices for PWH,12,19,20 this outcome can be applied across HIV care settings.15,16 Therefore, using VA clinical data from VACS, we sought to evaluate the association between individual-level changes in alcohol use and subsequent changes in the VACS Index 2.0 in a national sample of PWH.
METHODS
Data Source and Sample.
Following previously-reported methods,10 VA electronic health record (EHR) data from VACS were used to identify a national sample of PWH21 receiving VA care who had at least one documented Alcohol Use Identification Test Consumption (AUDIT-C) alcohol screen 2/1/2008 to 5/1/2014.10 Among these, we identified PWH with a follow-up AUDIT-C screen recorded 9-15 months after initial screening. For each initial screen, follow-up screens were identified as those documented closest to 12 (and within 9-15) months after initial screening to best approximate the AUDIT-C’s past-year timeframe. Follow-up screens were identified through 7/31/2015. To maximize power, patients included in the sample could contribute multiple pairs of alcohol screens during the study if there were at least 9 months between initial screens. For each pair of alcohol screens, an initial and follow-up VACS Index 2.0 was identified within 0-270 days after initial and follow-up AUDIT-C scores, respectively.
Measures
For each pair of observations, the primary exposure measure—change in alcohol use over time—was measured as the difference between each initial and follow-up AUDIT-C score. AUDIT-C scores range from 0 to 12, with increasing AUDIT-C scores reflecting increasing mean alcohol consumption levels and increased symptoms of alcohol use disorder.22,23 Possible AUDIT-C change scores ranged from −12 to 12 and were calculated as the change from follow-up to baseline so that negative values reflect increased drinking, positive values reflect decreased drinking, and 0 values reflect stable drinking over time. For descriptive purposes, AUDIT-C change scores were categorized as: −6 to −12, −3 to −5, −2, −1, 0, 1, 2, 3 to 5, and 6 to 12. Similarly, initial and follow-up AUDIT-C scores were categorized according to risk groups corresponding to increased risk of adverse outcomes: 0 non-drinking; 1-3 (1-2 for women) low-level alcohol use; 4-5 (3-5 for women), medium-level alcohol use; 6-7 high-level alcohol use; and 8-12 very high-level alcohol use.2,24,25
The primary outcome was change in HIV disease severity measured using VACS Index 2.0. VACS Index 2.0 calculates HIV disease severity using traditional clinical measures of HIV severity (CD4 count and HIV-1 RNA), as well as hemoglobin, white blood cell count, estimated liver fibrosis (FIB-4) and liver injury (hepatitis C infection and albumin), estimated kidney injury (eGFR), and body mass index (BMI), and thus captures many of the major organ systems directly affected by microbial translocation, chronic inflammation, and its sequalae, key mechanisms influenced by alcohol use.1,15 VACS Index 2.0 scores range from 0 to 164; higher scores reflect higher disease severity and increasing risk of mortality.15 Change in VACS Index 2.0 was based on the difference between the initial and follow-up score and calculated so that negative change scores indicate increased disease severity over time while positive change scores indicate improvements, and 0 indicates no change.
Other measures included demographic characteristics measured using EHR documentation at initial AUDIT-C administration: age (<50, 50-65, >65), gender (male/female), and race/ethnicity (black, Hispanic, white, and other/unknown). We also measured depressive and anxiety disorders, serious mental illness, and substance use disorders (stimulant use disorder, opioid use disorder, other drug use disorder, and alcohol use disorder) using International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes documented in the year prior to each patient’s first initial AUDIT-C. Finally, two HIV clinical measures were derived for each AUDIT-C pair: a three-category variable measuring anti-retroviral treatment (ART) over time (treated with ART within a year prior to initial screen, initiated ART between initial and follow-up screens, and no documented ART treatment) based on having filled one or more prescriptions, and a dichotomous measure of detectable viral load at the time of initial screen, defined as HIV-RNA ≥500 copies/ml at the most recent measurement within one year prior. Though changes in the sensitivity of tests for HIV-RNA have lowered commonly used cut-points,26,27 ≥500 copies/ml was a relevant target for all years of the study.
Analysis
We conducted descriptive analyses at the patient level at the time of each individual’s first documented AUDIT-C. We summarized the distribution of initial and follow-up AUDIT-C scores, as well as AUDIT-C and VACS Index 2.0 change scores. We described mean initial VACS Index 2.0 scores across AUDIT-C risk group categories and tested their association using analysis of variance tests.
At the observation level, we evaluated the association between AUDIT-C change scores and VACS Index 2.0 change scores using linear regression. AUDIT-C change scores were flexibly modeled using restricted cubic splines to allow for a smooth association across levels of AUDIT-C change without assuming linearity.28,29 Spline knots where segments connect were set at scores −3, −1, 0, 1, and 3 based on examination of the unadjusted association.29 Our model was first unadjusted and then adjusted for all measured demographic characteristics (age, gender, and race/ethnicity), initial VACS Index 2.0 score, and days between VACS Index 2.0 measures. The adjusted model was considered primary. Standard errors were estimated using the robust sandwich estimator to account for correlation due to multiple observations (i.e., screen pairs) per patient.30 Because not all PWH receiving VA care who had an initial AUDIT-C screen also had a follow-up screen documented, inverse probability weights were estimated using logistic regression (an indicator for being in the analytic sample was regressed on demographic and clinical characteristics) and used to weight analyses back to the full sample of PHW with any alcohol screening.31
The significance of the association between AUDIT-C and VACS Index 2.0 change scores was tested using an overall (omnibus) Wald Test assessing significance of the spline terms. For the primary model, we estimated and plotted the mean estimated VACS Index 2.0 change for each possible AUDIT-C change score (−12 to +12). Mean changes were obtained based on fixed covariate values: mean age, male gender, black race, mean initial VACS Index 2.0 score, mean days between VACS Index 2.0 measures.
Based on prior research,32 we hypothesized that increases in AUDIT-C scores (reflecting increased drinking) would be associated with worsening HIV severity. On the other hand, due to possible social desirability bias in reported drinking reductions and the possibility that abstinence or decreases in drinking may result from illness33 or reflect unstable drinking,34 we hypothesized that decreases in AUDIT-C score might not be reflected in changes in HIV severity. Therefore, we tested a priori-specified contrasts of estimated mean VACS Index 2.0 change scores across several AUDIT-C change score combinations. Specifically, we tested whether mean change in VACS Index 2.0 differed for PWH with a 2-point increase in AUDIT-C (AUDIT-C change score −2) compared with those remaining stable (AUDIT-C change score 0); PWH with a 5-point increase in AUDIT-C (AUDIT-C change −5) relative to those remaining stable (AUDIT-C change 0); and PWH with a 5-point increase in AUDIT-C (AUDIT-C change −5) relative to those with an 8-point increase (AUDIT-C change −8).
We undertook three sets of secondary analyses. First, because alcohol use is associated both with being treated with and adherent to ART7,9,35 and ART treatment is reflected in HIV viral load,36,37 we repeated our primary model stratified by two factors: 1) treatment with ART (ART at initial screen, ART initiation between screens, no ART treatment) and 2) detectable (500+ copies/ml) vs. non-detectable (<500 copies/ml) viral load at initial screening. Second, to minimize the likelihood that patients may have changed their drinking between an AUDIT-C and the calculation of VACS Index 2.0, we repeated primary analyses in the subgroup of patients with AUDIT-C and VACS Index 2.0 measurements ≤30 days apart at both initial and follow-up screening (n=64,944 observations; 85% of all observations). Finally, we repeated primary model stratified by initial AUDIT-C risk group based on previous research that found associations between changes in alcohol use and changes in medical outcomes depended on initial level of drinking.32
All analyses were conducted using Stata Statistical Software, release 14. 38 This study was approved by Institutional Review Boards at VA Connecticut and VA Puget Sound Healthcare Systems, including waivers of written consent and HIPAA authorization.
RESULTS
Among 23,297 eligible PWH (contributing 76,202 observations), patients were largely male (97%) and ≥50 years (64%), and over half were black (49%) or Hispanic (8%) (Table 1). Nearly a third of patients had documented depressive disorder, and nearly one-tenth had anxiety disorders and/or serious mental illness. Approximately 14% had a documented alcohol use disorder and nearly 8% had other drug use disorders. Most (74%) were on ART at the time of their first initial AUDIT-C; 16% initiated ART between their first pair of screens, and another 9% had no documented ART at initial screening (Table 1).
Table 1.
Characteristics of eligible VA Patients with HIV at first Alcohol Use Disorders Identification Test Consumption (AUDIT-C) Measure during the study period (n=23,781 individuals)
| Characteristic | N | (%) |
|---|---|---|
| Demographics | ||
| Gender (Female) | 659 | (2.8) |
| Age | ||
| <50 | 8,601 | (36.2) |
| 50 – 64 | 12,894 | (54.2) |
| ≥65 | 2,286 | (9.6) |
| Race/Ethnicity | ||
| Black | 11,577 | (48.7) |
| Hispanic | 1,979 | (8.3) |
| White | 9,545 | (40.1) |
| Other/Unknown | 680 | (2.9) |
| Mental Health and Substance Use Disorder | ||
| Depressive Disorder | 7,091 | (29.8) |
| Anxiety Disorder | 2,096 | (8.8) |
| Serious Mental Illness | 2,275 | (9.6) |
| Stimulant Use Disorder | 349 | (1.5) |
| Opioid Use Disorder | 885 | (3.7) |
| Other Drug Use Disorder | 1,822 | (7.7) |
| HIV Clinical Measure | ||
| On Antiretroviral Treatment (ART) at Initial Screen | 17,604 | (74.0) |
| Initiated ART Between Screens | 3,871 | (16.3) |
| Not on ART at Either Screen | 2,306 | (9.7) |
| Alcohol Use Severity | ||
| Alcohol Use Disorder | 3,311 | (13.9) |
| Baseline AUDIT-C Category | ||
| 0 | 11,134 | (46.8) |
| 1-3 (1-2 for women) | 9,441 | (39.7) |
| 4-5 (3-5 for women) | 1,859 | (7.8) |
| 6-7 | 580 | (2.4) |
| 8-12 | 767 | (3.2) |
The majority of PWH (87%) reported no (47% AUDIT-C=0) or lower-risk (40% AUDIT-C=1-3 men;1-2 women) alcohol use at their first AUDIT-C (Table 1). Slightly more than half (54%) had no change in AUDIT-C over time; 21% increased drinking, and 24% decreased drinking. Most with no change (97%) had no (74% AUDIT-C=0) or low-level (23% AUDIT-C 1-3/1-2) alcohol use initially. First initial VACS Index 2.0 scores ranged from 0-134 with a mean of 51.1 (16.7 standard deviation). VACS Index 2.0 scores were generally higher for patients in higher AUDIT-C risk groups (ANOVA p<0.001; Table 2). VACS Index 2.0 change scores ranged from −65 to +73, with improvement of 0.76 points (SD 9.48) average, reflecting a modest improvement in HIV severity over time. The mean time between initial AUDIT-Cs and VACS Index 2.0 measurement was 8.1 days (SD 26.9) and between follow-up AUDIT-Cs and VACS Index 2.0 was 10.4 days (SD 31.9).
Table 2.
Mean Initial VACS Index 2.0 Score Across Initial AUDIT-C Score Risk Groups at the time of first AUDIT-C screen (n = 23,781 individuals)
| N | Initial VACS Index 2.0 Score Mean (SD) |
||
|---|---|---|---|
| Total Sample | 23,781 | 51.1 (16.7) | |
| Initial AUDIT-C Risk Group | p-value* | ||
| 0 | 11134 | 52.9 (16.7) | <0.001 |
| 1-3 (1-2 for women) | 9441 | 48.7 (16.3) | |
| 4-5 (3-5 for women) | 1859 | 49.9 (16.4) | |
| 6-7 | 580 | 52.0 (18.6) | |
| 8-12 | 767 | 56.6 (17.3) |
Analysis of variance test
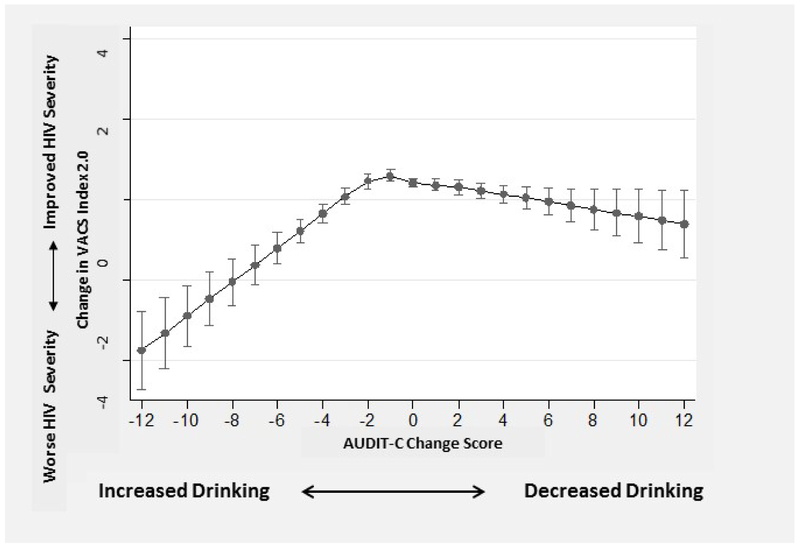
Changes in VACS Index 2.0 were associated with AUDIT-C change scores in the unadjusted and adjusted model (p-values for spline terms <0.001). In primary analyses, those with the smallest AUDIT-C change scores had the greatest improvements in HIV severity over time while changes in either direction were associated with worsening HIV disease severity (Figure 1). Those with increased alcohol use had worse estimated declines (Figure 1). Specifically, among those with relatively stable alcohol use (AUDIT-C change ≤│1│ point), VACS Index 2.0 improvements ranged from 0.36 to 0.60 points. For those with AUDIT-C change −12 indicating maximum increased alcohol use, VACS Index 2.0 worsened 3.74 points (95% CI −4.71, −2.78); for those with AUDIT-C change score +12 indicating maximum decreased alcohol use, VACS Index 2.0 changed minimally (estimated mean= −0.60 (95% CI −1.43, 0.23), Figure 1).
Figure 1.
Association* Between Change in AUDIT-C and Change in VACS Index 2.0 in a National Sample of VA Patients with HIV (n = 76,202 observations)
* adjusted for demographics (age, gender, and race),initial VACS Index 2.0 score, and time between VACS Index 2.0 measures.
Results of contrast tests comparing mean estimated change in VACS Score 2.0 across specific AUDIT-C change scores are presented in Table 3. In a priori contrast tests, no statistically significant difference in estimated mean VACS Index 2.0 change was observed between those with AUDIT-C change scores of 0 [estimated mean VACS Index 2.0 change of 0.42 (95% CI, 0.31-0.52)] and those with AUDIT-C change score of −2 [estimated mean VACS Index 2.0 change of 0.46 (95% CI 0.27-0.65)] (p=0.67). However, differences were observed between those with AUDIT-C change scores of 0 and −5 and between those with AUDIT-C change scores of −5 and −8 (Table 3). We conducted several post hoc contrast tests to assess effects of decreases in drinking. Those with no change (AUDIT-C change score = 0) did not differ in mean VACS Index 2.0 change from those with a small decrease in drinking (AUDIT-C change score = +2), but did relative to those with a larger decrease in drinking (AUDIT-C change score = +5). Differences in mean VACS Index 2.0 change were also observed for those with AUDIT-C change scores of +1 relative to −1; +5 relative to −5, +5 relative to +8, and −8 relative to +8 (Table 3).
Table 3.
Association between AUDIT-C change scores and change in VACS Index 2.0 score: Results of contrast tests* for comparison of mean changes in VACS Index 2.0 between a priori- and post hoc- specified AUDIT-C change scores among n = 76,206 observations
| Specified AUDIT-C change scores |
Predicted change in VACS Index 2.0 Score Associated with Specified AUDIT-C Change Scores |
p-value comparing predicted change in VACS Index 2.0 Score estimated for specified AUDIT-C Change Scores* |
||
|---|---|---|---|---|
| ΔA** | ΔA'** | ΔY (95% CI) | ΔY' (95% CI) | |
| A priori contrasts to test hypothesis | ||||
| 0 | −2 | 0.42 (0.31, 0.52) | 0.46 (0.27, 0.65) | 0.6704 |
| 0 | −5 | 0.42 (0.31, 0.52) | −0.77 (−1.08, 0.47) | <0.001 |
| −5 | −8 | −0.77 (−1.08, 0.47) | −2.05 (−2.62, −1.47) | <0.001 |
| Post-hoc contrast tests to assess effects of decreases in drinking | ||||
| 0 | +2 | 0.42 (0.31, 0.52) | 0.31 (0.12, 0.50) | 0.2939 |
| 0 | +5 | 0.42 (0.31, 0.52) | 0.04 (−0.23, 0.30) | 0.0076 |
| +1 | −1 | 0.36 (0.21, 0.51) | 0.60 (0.45, 0.75) | 0.0140 |
| +5 | −5 | 0.04 (−0.23, 0.30) | −0.77 (−1.08, 0.47) | 0.0001 |
| +5 | +8 | 0.04 (−0.23, 0.30) | −0.24 (−0.73, 0.26) | 0.0444 |
| +8 | −8 | −0.24 (−0.73, 0.26) | −2.05 (−2.62, −1.47) | <0.001 |
KEY: Above, mean change from initial to follow-up VACS Index 2.0 Score (ΔY) associated with one AUDIT-C change score (ΔA) are compared to mean changes in VACS Index 2.0 Score (ΔY') associated with another AUDIT-C change score (ΔA'); negative AUDIT-C change score indicates increased AUDIT-C; positive AUDIT-C change score indicates decreased AUDIT-C
p-value for contrast, tested using primary model (Block 2) adjusted for demographics, initial VACS Index Score 2.0, and days between measures.
N for AUDIT-C Δ −8 = 173; N for AUDIT-C Δ −5 = 408; N for AUDIT-D Δ −2 = 3,479; N for AUDIT-C Δ −1 = 9,157; N for AUDIT-C Δ 0 = 41,466; N for AUDIT-C Δ +1 = 9,963; N for AUDIT-C Δ +2 = 3,939; N for AUDIT-C Δ +5 = 510; N for AUDIT-C Δ +8 = 220.
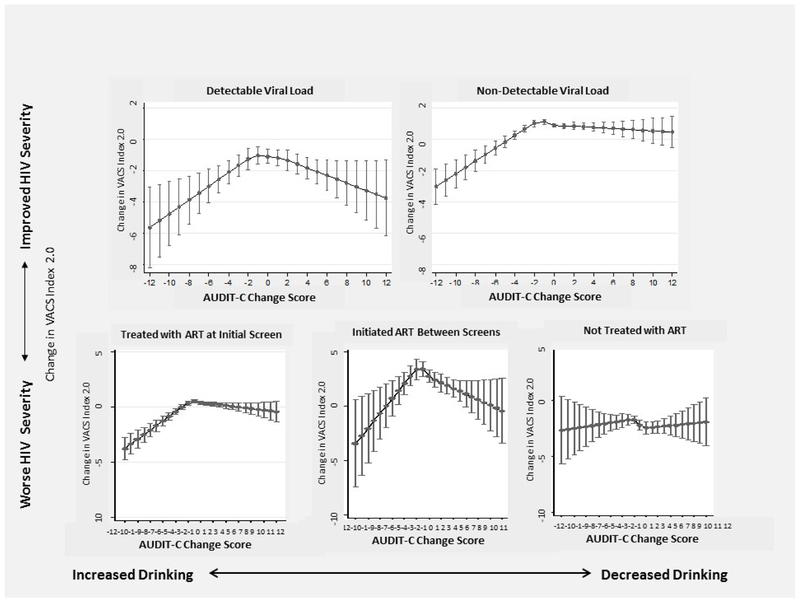
Secondary analyses stratified by ART treatment and viral load status resulted in similar findings (Figure 2) though precision was decreased for those initiating ART between screens and those without documented ART due to small sample sizes. Associations were unchanged when limited to those with AUDIT-C and VACS Index 2.0 measurements ≤ 30 days apart (data not shown) and similar when stratified by baseline AUDIT-C risk groups (Supplemental Figure).
Figure 2.
Association* between Change in AUDIT-C and Change in VACS Index 2.0 in a National Sample of Patients with HIV: Stratified by Viral Load and ART Treatment Status, (n = 76,202 observations)
* adjusted for demographics (age, gender, and race), initial VACS Index 2.0 score, and time between VACS Index 2.0 measures.
DISCUSSION
In this national cohort of PWH receiving healthcare in the VA, individual-level changes in drinking measured by AUDIT-C scores were associated with changes in HIV disease severity measured by the VACS Index 2.0, a composite risk index that includes measurement of multiple mechanisms via which alcohol use influences HIV.1 On average, HIV disease severity improved slightly among patients over time and improved the most among those who remained relatively stable in drinking, 97% of whom reported drinking at lower levels at the time of initial measurement. Those with increases in alcohol use worsened in HIV severity slightly more than those with decreases in alcohol use. Analyses stratified by ART treatment and viral load status, as well as initial alcohol use risk groups, resulted in generally similar findings.
Results of the present study extend a large literature that primarily has described cross-sectional associations between alcohol use and HIV disease severity4-6 and are consistent with prior longitudinal studies suggesting that stable or decreased alcohol use may result in the greatest health benefits.10,32 Though potentially surprising that greater improvements in HIV severity were not observed among PWH who decreased drinking, these results are possibly due to social desirability bias in reported drinking reductions, as well as the possibility that alcohol abstinence may result from illness39 or may reflect unstable drinking and not be sustained.34 Findings support and build on those of two previous longitudinal studies that assessed associations between HIV disease severity and measures of alcohol use over time.8,10 The first was conducted in a VACS survey sub-sample (3,539 PWH recruited from 8 urban HIV clinics) using group-based trajectory analyses. That study found that those with high likelihood of consistent heavy alcohol use over time were most likely to belong to an extreme HIV severity risk group identified using the original VACS Index, thus identifying population-level groups that are at greatest risk for morbidity and mortality over time.8 The second assessed associations between individual-level changes in alcohol use and subsequent changes in clinical measures of HIV (CD4 count and HIV RNA) at the individual level, and found that PWH with relatively stable drinking over time had the greatest improvements in clinical markers of HIV severity, while those whose drinking increased over time had the smallest improvements.10 That study was the first to our knowledge to assess whether change in a modifiable risk factor (alcohol use) at the individual-level was associated with change in severity of an undesirable HIV-related health outcome (e.g., detectable viral load). The present study extends these studies by replicating individual-level findings using a validated risk index, which combines multiple clinical outcomes, is more sensitive to HIV disease severity than traditional HIV biomarkers12,18 and more comprehensively measures mechanisms via which alcohol use and HIV severity are linked.1 As expected, the magnitude of associations were stronger in the present study using VACS Index 2.0 compared to the previous study assessing traditional clinical biomarkers,10 highlighting that this comprehensive risk index is more sensitive to changes in alcohol use over time than are measures used clinically to monitor HIV disease and suggesting that the VACS Index 2.0 provides improved clinical utility for understanding the dynamic relationship between alcohol use and HIV disease severity over time.
Findings from longitudinal analyses,10 including the present study, suggest that PWH with stable non-drinking or low-level alcohol use may fare best over time in relation to changes in HIV severity. While the potential benefits of stable alcohol use were fairly consistent across each level of baseline alcohol use, the vast majority (97%) of those with stable alcohol use started at low levels of alcohol use or non-drinking. Thus, we were optimally powered to identify associations among those with stable low-level alcohol use or non-use. Previous research suggests that no level of alcohol use may be safe for PWH,12,18,40 and that any and increasing levels of alcohol use may influence all stages of the HIV care continuum.1,7 Further, the present study’s results from analyses stratified by initial AUDIT-C risk group suggest that, among those with initial severe unhealthy alcohol use, those with little or no change over time may have less improvement in VACS Index 2.0 compared with those with greater reductions in alcohol use. Further work is needed to investigate the influence of stable alcohol use among those with high levels of drinking.
Among PWH whose alcohol use changed over time, those whose AUDIT-C change scores indicated increased alcohol use fared slightly worse in HIV disease severity than those whose scores indicated decreased alcohol use. These findings have several implications for clinicians treating PWH. First, alcohol use should be monitored over time among PWH—via alcohol screening scores or other monitoring instruments. Second, patients who increase alcohol use should be considered a priority group for alcohol interventions underutilized among PWH receiving healthcare.41,42 Finally, research is needed to understand how to improve outcomes of PWH with variable alcohol screening scores.
This study has several limitations. Findings may not be generalizable to PWH not receiving care in VA or to women, given the small sample. Further work should assess gender-specific associations, given associations may differ for women and men.43 Reliance on EHR data may have limited measurement of alcohol use as clinically documented screening may miss patients with any and unhealthy alcohol use, and patients may under-report alcohol use and/or report greater than experienced reductions in alcohol use over time.44-46 Additionally, findings from this study cannot be considered causal. Further work is needed to understand how individual-level changes in alcohol use influence HIV severity and whether observed associations reflect changes in alcohol use and/or changes in health that led to changes in alcohol use.33 Finally, previous research suggests that a 5-point difference in VACS Index scores is associated with a 20% increased risk of 5-year mortality.47 Investigation of the clinical relevance of changes in HIV severity observed at all levels of AUDIT-C change is needed among PWH.
Despite these limitations, this study found that changes in a practical widely-used alcohol screen are associated with changes in HIV severity over time in a large national sample of PWH receiving care in the VA, the nation’s largest provider of HIV care. Findings from this study support and extend those of previous studies by using a validated, composite outcome measure that better reflects the multiple mechanisms via which alcohol use influences HIV severity and mortality risk and provides greater specificity regarding individual-level risk associated with changes in alcohol use. Findings highlight that stable low-level alcohol use over time may result in optimal stability of or improvements in HIV-related severity and risk.
Supplementary Material
Supplemental Figure. Adjusted* Association between Change in AUDIT-C and Change in VACS Index 2.0* in a National Sample of Patients with HIV: Stratified by Baseline AUDIT-C Risk Group (n = 76,206 observations)
ACKNOWLEDGEMENTS
This research was funded by a grant from the National Institute on Alcohol Abuse and Alcoholism (R21AA022866-01; Williams/Bradley PIs) and COMpAAAS/Veterans Aging Cohort Study (U24-AA020794, U01-AA020790, U01-AA020795, U01-AA020799; U10 AA013566). Dr. Williams is supported by a Career Development Award from VA Health Services Research & Development (CDA 12-276), and Dr. Bradley is supported by a mid-career mentorship award from NIAAA (K24-AA022128). The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication. Dr. Bryant, the study’s scientific collaborator, is employed by the funder (the National Institutes of Alcohol Abuse and Alcoholism) and helped guide analysis, interpretation and presentation of data and participated in the decision to submit the manuscript for publication.
SOURCES OF FUNDING
This research was funded by a grant from the National Institute on Alcohol Abuse and Alcoholism (R21AA022866-01; Williams/Bradley PIs) and COMpAAAS/Veterans Aging Cohort Study (U24-AA020794, U01-AA020790, U01-AA020795,U01-AA020799; U10 AA013566). Dr. Williams is supported by a Career Development Award from VA Health Services Research & Development (CDA 12-276), and Dr. Bradley is supported by a mid-career mentorship award from NIAAA (K24-AA022128). The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication. An employee of the funder (K. Bryant) served as a scientific collaborator and helped guide analysis, interpretation and presentation of data and participated in the decision to submit the manuscript for publication. Views presented in the manuscript are those of the authors and do not represent the official position of the U.S. Government, the Department of Veterans Affairs, or other affiliated institutions.
Footnotes
Preliminary findings of this study were presented at the Research Society on Alcoholism (RSA) Conference in San Diego, CA in June 2018.
CONFLICTS OF INTEREST
All authors declare no potential conflicts of interest.
REFERENCES
- 1.Williams EC, Hahn JA, Saitz R, Bryant K, Lira MC, Samet JH. Alcohol use and Human Immunodeficiency Virus (HIV) Infection: current knowledge, implications, and future directions. Alcohol Clin Exp Res. 2016;4 (10):2056–2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Justice AC, McGinnis KA, Tate JP, et al. Risk of mortality and physiologic injury evident with lower alcohol exposure among HIV infected compared with uninfected men. Drug Alcohol Depend. 2016;161:95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williams EC, Joo YS, Lipira L, Glass JE. Psychosocial stressors and alcohol use, severity, and treatment receipt across human immunodeficiency virus (HIV) status in a nationally representative sample of US residents. Subst Abus. 2016;38(3):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deiss RG, Mesner O, Agan BK, et al. Characterizing the Association Between Alcohol and HIV Virologic Failure in a Military Cohort on Antiretroviral Therapy. Alcohol Clin Exp Res. 2016;40(3):529–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Samet JH, Cheng DM, Libman H, Nunes DP, Alperen JK, Saitz R. Alcohol consumption and HIV disease progression. J Acquir Immune Defic Syndr. 2007;46(2):194–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baum MK, Rafie C, Lai S, Sales S, Page JB, Campa A. Alcohol use accelerates HIV disease progression. AIDS Res Hum Retroviruses. 2010;26(5):511–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Williams EC, McGinnis KA, Edelman EJ, et al. Level of Alcohol Use Associated with HIV Care Continuum Targets in a National U.S. Sample of Persons Living with HIV Receiving Healthcare. AIDS and behavior. 2019;23(1):140–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marshall BDL, Tate JP, McGinnis KA, et al. Long-term alcohol use patterns and HIV disease severity. AIDS. 2017;31(9):1313–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barai N, Monroe A, Lesko C, et al. The Association Between Changes in Alcohol Use and Changes in Antiretroviral Therapy Adherence and Viral Suppression Among Women Living with HIV. AIDS Behav. 2017;21(7):1836–1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williams EC, McGinnis KA, Bobb JF, et al. Changes in alcohol use associated with changes in HIV disease severity over time: A national longitudinal study in the Veterans Aging Cohort. Drug Alcohol Depend. 2018;189:21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Department of Veterans Affairs. Study of Barriers for Women Veterans to VA Health Care: Final Report. https://www.womenshealth.va.gov/docs/Womens%20Health%20Services_Barriers%20to%20Care%20Final%20Report_April2015.pdf Washington, D.C.: Department of Veterans Affairs;2015. [Google Scholar]
- 12.Justice AC, Modur SP, Tate JP, et al. Predictive accuracy of the Veterans Aging Cohort Study index for mortality with HIV infection: a North American cross cohort analysis. J Acquir Immune Defic Syndr. 2013;62(2):149–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown ST, Tate JP, Kyriakides TC, et al. The VACS index accurately predicts mortality and treatment response among multi-drug resistant HIV infected patients participating in the options in management with antiretrovirals (OPTIMA) study. PloS one. 2014;9(3):e92606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bebu I, Tate J, Rimland D, et al. The VACS index predicts mortality in a young, healthy HIV population starting highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2014;65(2):226–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tate JP, Brown ST, Gibert C, et al. White Blood Count, Albumin, and BMI Enhance VACS Index Prognostic Model, But Nadir CD4 and CD8 Metrics Do Not. Poster presentation, IDWeek Conference San Diego, CA, October 2017. [Google Scholar]
- 16.Tate JP, Sterne JA, Justice AC, for the Veterans Aging Cohort Study (VACS) and the Antiretroviral Therapy Cohort Collaboration (ART-CC). Improved discrimination of mortality with Veterans Aging Cohort Study Index 2.0 in HIV-positive individuals. AIDS. 2019;[Epub ahead of print]. [Google Scholar]
- 17.Justice A, Falutz J. Aging and HIV: an evolving understanding. Curr Opin HIV AIDS. 2014;9(4):291–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Justice AC, Lasky E, McGinnis KA, et al. Medical disease and alcohol use among veterans with human immunodeficiency infection: a comparison of disease measurement strategies. Med Care. 2006;44(8 Suppl 2):S52–60. [DOI] [PubMed] [Google Scholar]
- 19.Justice AC, Freiberg MS, Tracy R, et al. Does an index composed of clinical data reflect effects of inflammation, coagulation, and monocyte activation on mortality among those aging with HIV? Clin Infect Dis. 2012;54(7):984–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tate JP, Justice AC, Hughes MD, et al. An internationally generalizable risk index for mortality after one year of antiretroviral therapy. AIDS. 2013;27(4):563–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fultz SL, Skanderson M, Mole LA, et al. Development and verification of a “virtual” cohort using the National VA Health Information System. Med Care. 2006;44(8 Suppl 2):S25–30. [DOI] [PubMed] [Google Scholar]
- 22.Rubinsky AD, Dawson DA, Williams EC, Kivlahan DR, Bradley KA. AUDIT-C scores as a scaled marker of mean daily drinking, alcohol use disorder severity, and probability of alcohol dependence in a U.S. general population sample of drinkers. Alcohol Clin Exp Res. 2013;37(8):1380–1390. [DOI] [PubMed] [Google Scholar]
- 23.Rubinsky AD, Kivlahan DR, Volk RJ, Maynard C, Bradley KA. Estimating risk of alcohol dependence using alcohol screening scores. Drug Alcohol Depend. 2010;108(1-2):29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harris AH, Bradley KA, Bowe T, Henderson P, Moos R. Associations between AUDIT-C and mortality vary by age and sex. Popul Health Manag. 2010;13(5):263–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kinder LS, Bryson CL, Sun H, Williams EC, Bradley KA. Alcohol screening scores and all-cause mortality in male Veterans Affairs patients. J Stud Alcohol Drugs. 2009;70(2):253–260. [DOI] [PubMed] [Google Scholar]
- 26.North American AIDS Cohort. North American AIDS Cohort collaboration on research and design https://statepiaps7.jhsph.edu/naaccord/ 2017.
- 27.Dombrowski JC, Kitahata MM, Van Rompaey SE, et al. High levels of antiretroviral use and viral suppression among persons in HIV care in the United States, 2010. J Acquir Immune Defic Syndr. 2013;63(3):299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khamis H, Kepler M. Multivariate cubic spline smoothing in multiple prediction. Comput Methods Programs Biomed. 2002;67(2):131–136. [DOI] [PubMed] [Google Scholar]
- 29.Royston P Choice of scale for cubic smoothing spline models in medical applications. Stat Med. 2000;19(9):1191–1205. [DOI] [PubMed] [Google Scholar]
- 30.Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 31.Little RJA, Rubin DB. Complete-Case and Available-Case Analysis, Including Weighting Methods In: Statistical Analysis with Missing Data, Second Edition. Hoboken, NJ: John Wiley & Sons, Inc.; 2002. [Google Scholar]
- 32.Bradley KA, Rubinsky AD, Lapham GT, et al. Predictive Validity of Clinical AUDIT-C Alcohol Screening Scores and Changes in Scores for Three Objective Alcohol-related Outcomes in a Veterans Affairs (VA) Population. Addiction. 2016;111(11):1975–1984. [DOI] [PubMed] [Google Scholar]
- 33.Shaper AG, Wannamethee G, Walker M. Alcohol and mortality in British men: explaining the U-shaped curve. Lancet. 1988;2(8623):1267–1273. [DOI] [PubMed] [Google Scholar]
- 34.Dawson DA, Goldstein RB, Grant BF. Rates and correlates of relapse among individuals in remission from DSM-IV alcohol dependence: a 3-year follow-up. Alcohol Clin Exp Res. 2007;31(12):2036–2045. [DOI] [PubMed] [Google Scholar]
- 35.Hendershot CS, Stoner SA, Pantalone DW, Simoni JM. Alcohol use and antiretroviral adherence: review and meta-analysis. J Acquir Immune Defic Syndr. 2009;52(2):180–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taddei TH, Lo Re V 3rd, Justice AC. HIV, aging, and viral coinfections: taking the long view. Curr HIV/AIDS Rep. 2016;13(5):269–278. [DOI] [PubMed] [Google Scholar]
- 37.Centers for Disease Control and Prevention. Understanding the HIV Care Continuum. https://www.cdc.gov/hiv/pdf/library/factsheets/cdc-hiv-care-continuum.pdf Atlanta, GA: CDC;2017. [Google Scholar]
- 38.StataCorp. Stata Statistical Software: Release 14, College Station, TX: StataCorp LP; 2015. [Google Scholar]
- 39.Shaper AG, Wannamethee G, Walker M. Alcohol and mortality in British men: explaining the U shaped curve. Lancet. 1988;8623(2):1267–1273. [DOI] [PubMed] [Google Scholar]
- 40.Justice AC, McGinnis KA, Tate JP, et al. Risk of mortality and physiologic injury evident with lower alcohol exposure among HIV infected compared with uninfected men. Drug Alcohol Depend. 2016;161:95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Williams EC, Lapham GT, Shortreed SM, et al. Among patients with unhealthy alcohol use, those with HIV are less likely than those without to receive evidence-based alcohol-related care: A national VA study. Drug Alcohol Depend. 2017;174:113–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Edelman EJ, Williams EC, Marshall BDL. Addressing unhealthy alcohol use among people living with HIV: recent advances and research directions. Current opinion in infectious diseases. 2018;31(1):1–7. [DOI] [PubMed] [Google Scholar]
- 43.Matson TE, McGinnis KA, Rubinsky AD, et al. Gender and alcohol use: influences on HIV care continuum in a national cohort of patients with HIV. AIDS. 2018;32(15):2247–2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Williams EC, Achtmeyer CE, Thomas RM, et al. Factors Underlying Quality Problems with Alcohol Screening Prompted by a Clinical Reminder in Primary Care: A Multi-site Qualitative Study. J Gen Intern Med. 2015;30(8):1125–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bradley KA, Chavez LJ, Lapham GT, et al. When Quality Indicators Undermine Quality: Bias in a Quality Indicator of Follow-Up for Alcohol Misuse. Psychiatr Serv. 2013;64(10):1018–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hawkins EJ, Kivlahan DR, Williams EC, Wright SM, Craig T, Bradley KA. Examining quality issues in alcohol misuse screening. Subst Abus. 2007;28(3):53–65. [DOI] [PubMed] [Google Scholar]
- 47.Tate JP, Brown ST, Gibert C, et al. Albumin, white blood cell count, and body mass index improve Veterans Aging Cohort Study (VACS) Index discrimination of mortality risk in HIV-positive individuals. In preparation. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure. Adjusted* Association between Change in AUDIT-C and Change in VACS Index 2.0* in a National Sample of Patients with HIV: Stratified by Baseline AUDIT-C Risk Group (n = 76,206 observations)