Abstract
Objective:
To determine if accelerated knee osteoarthritis (KOA) is preceded and characterized by destabilizing meniscal tears or other pathologic changes.
Methods:
We selected 3 sex-matched groups from the first 48 months of the Osteoarthritis Initiative who had a knee without KOA at baseline (Kellgren-Lawrence [KL]<2): Accelerated KOA (AKOA) developed KL grade≥3, typical KOA increased radiographic scoring, and no KOA had the same KL grade over time. An index visit was the visit when the radiographic criteria for AKOA and typical KOA were met (no KOA was matched to AKOA). The observation period was up to 2 years before and after an index visit. Radiologists reported destabilizing meniscal tears (root tears, radial tears, complex tears), miscellaneous pathology (acute ligamentous or tendinous injuries, attrition, subchondral insufficiency fractures, other incidental findings), and meniscal damage in ≥2 out of 6 regions (3 regions/meniscus: anterior horn, body, posterior horn) on magnetic resonance images. We quantified bone marrow lesions (BML) and cartilage damage on magnetic resonance images. We performed linear mixed models.
Results:
At 1 year before the index visit, >75% of adults with AKOA had meniscal damage in ≥2 regions (vs typical KOA: OR=3.19, 95%CI=1.70 to 5.97). By the index visit, meniscal damage in ≥2 regions was ubiquitous in AKOA; including 42% with a destabilizing meniscal tear (typical KOA=14%). These changes corresponded to larger BMLs and greater cartilage loss.
Conclusion:
AKOA is characterized by destabilizing meniscal tears in a knee compromised by meniscal damage in ≥2 regions, large BMLs, and cartilage loss.
Knee osteoarthritis (KOA) is typically a slowly progressive disorder. However, a subset of individuals develops accelerated KOA (AKOA), which is defined by the rapid onset and progression of disease within 4 years (from pre-radiographic disease to advanced-stage disease)(1–3). Two out of 3 adults with AKOA transition from develop AKOA in less than 12 months (1–4). Individuals with AKOA have substantially greater pain and disability than peers without AKOA and this is evident prior to radiographic onset (3, 4). AKOA may be a unique subset of KOA characterized by specific pathologies (e.g., meniscal root tears(5–9)) that differentiate it from typical KOA; however, this hypothesis has not been specifically examined. If specific pathologies differentiate people at risk for AKOA it may help identify adults with early-stage or high-risk for AKOA and inspire novel prevention strategies.
The purpose of this longitudinal study was to determine if AKOA is preceded and characterized over time by destabilizing meniscal tears (i.e., root tears, radial tears, complex tears), miscellaneous pathologies (i.e., acute ligamentous or tendinous injuries, attrition, subchondral insufficiency fractures, other incidental findings), or other structural changes (i.e., meniscal damage in 2 or more regions, medial or lateral meniscal pathology or extrusion, and greater changes in bone marrow lesions [BMLs] and articular cartilage) than adults with typical or no KOA. We hypothesize that AKOA is a unique disorder characterized by destabilizing meniscal tears, miscellaneous pathologies, and greater cartilage loss and increases in BML size than in individuals with typical or no KOA.
MATERIALS AND METHODS
We identified adults using OAI data from baseline and the first 4 annual follow-up visits. The OAI is a cohort of 4,796 adults with or at risk for symptomatic KOA recruited at 4 clinical sites (Memorial Hospital of Rhode Island, The Ohio State University, University of Maryland and Johns Hopkins University, and the University of Pittsburgh) between 2004 and 2006. Institutional review boards at each clinical site and the coordinating center (University of California, San Francisco) approved the study. Participants offered informed consent prior to enrollment.
Participant selection
We identified 3 groups based on radiographic criteria. Adults with AKOA included anyone with a knee without radiographic osteoarthritis at baseline (Kellgren-Lawrence [KL] grade<2) that developed advanced-stage KOA (KL grade 3 or 4) within 48 months (n=125)(2). Individuals with typical KOA had no radiographic KOA in both knees at baseline and at least 1 knee that increased radiographic scoring within 48 months (n=187; i.e., KL change from 0 to 1, 0 to 2, or 1 to 2). Individuals with no KOA had no KOA in both knees at baseline and had no change in KL grade in either knee from baseline to 48 months (n=1,325). We matched the typical and no KOA groups to those with AKOA on sex (n=125/group).
Index knee
The index knee among adults with AKOA or typical KOA was the first knee to develop AKOA or typical KOA, respectively. For individuals with no KOA, the index knee was the same as that person’s matched member of the AKOA group.
Definition of index visit
For individuals with either AKOA or typical KOA, the index visit was the visit when a person met the definition for AKOA or typical KOA, respectively. For someone with no KOA, the index visit was the same as that person’s matched member of the AKOA group.
Knee radiographs
Bilateral weight-bearing, fixed-flexion posteroanterior knee radiographs were obtained at each visit. Blinded central readers recorded KL grades (0 to 4; intrarater agreement: weighted kappa=0.70 to 0.80) and tibiofemoral joint space narrowing (0 to 3 with within grade changes permitted (10, 11); intrarater agreement: weighted kappa=0.75 to 0.87; files: kXR_SQ_BU##_SAS [versions 0.6, 1.6, 3.5, 5.5, and 6.3]) (12). These radiographs were also used to measure baseline static knee alignment (femorotibial alignment angle; intra-reader reliability intraclass correlation coefficient [ICC]=0.98; file: KXR_FTA_Duryea00, version 0.2)(13).
Magnetic resonance imaging acquisition
Magnetic resonance (MR) images were acquired annually with 1 of 4 identical Siemens (Erlangen, Germany) Trio 3-Tesla MR systems at each clinical site. Readers performing semi-quantitative scoring were provided all the sequences acquired on each index knee at each visit (e.g., those noted below; coronal intermediate-weighted, turbo spin echo, without fat suppression). BML quantitative measurements were performed using a sagittal intermediate-weighted, turbo spin echo, fat-suppressed MR sequence. Cartilage damage was quantified using a 3-dimensional dual-echo steady-state sequence. These sequences have been described elsewhere (14).
Meniscal readings
Two musculoskeletal radiologists reviewed MR images (RW:255 cases, JM:120 cases) blinded to group but unblinded to time. They reported the presence of meniscal pathology in 3 regions (anterior, body, posterior) of each meniscus based on the International Society of Arthroscopy, Knee Surgery, and Orthopaedic Sports Medicine meniscal tear classification, which was modified for MR imaging (15, 16). Possible meniscal pathologies were normal, degeneration, horizontal, flap horizontal, vertical longitudinal, radial, morphologic deformity, maceration, complex, or vertical flap tear. The readers also reported the presence of a root tear, which was a complete radial tear at the anterior or posterior medial and lateral meniscal roots. These tears would be considered radial tears; however, due to the potential pathological significance of root tears the readers reported these separately. The readers also scored medial and lateral meniscal extrusion (0 to 3) based on the MR Imaging Osteoarthritis Knee Score (Grade 1: <2 mm, Grade 1: 2 to 2.9 mm, Grade 2: 3 to 5 mm, and Grade 3: >5mm) (17).
Since some meniscal findings were infrequently identified we created 6 dichotomous variables (defined below) to ensure statistical power. Our primary outcome for meniscal pathology was the presence of a destabilizing meniscal tear in either meniscus. Destabilizing meniscal tears compromise meniscal function and may increase the risk for accelerated progression (6, 7). Destabilizing meniscal tears consisted of a root tear, radial tear, or complex tear, which almost always featured a radial component. We also created five variables for secondary meniscal findings: 1) meniscal damage in 2 or more regions: knee with any meniscal pathology in ≥2 out of 6 regions (kappa=0.89), 2) medial meniscal pathology: any pathology in the medial meniscus (excludes extrusion; kappa=0.90), 3) medial meniscal extrusion (scores: 0 vs ≥1; kappa=0.91), 4) lateral meniscal pathology: any pathology in the lateral meniscus (excludes extrusion; kappa=0.63), and 5) lateral meniscal extrusion (scores: 0 vs ≥ 1; 100% agreement). All kappa statistics represent inter-reader agreement among 24 knees.
Miscellaneous pathology
The radiologists were asked to record the presence of any other pathologies: attrition, acute ligamentous or tendinous injuries, subchondral insufficiency fractures, and any other incidental findings. We recorded attrition from 0 (normal) to 3 (severe), based on the perceived degree of deviation from a normal contour for the medial and lateral femur and tibia. We defined the presence of attrition as a score of 1 or more. We defined acute ligamentous or tendinous injury as per routine clinical practice, using the presence/absence of focal fiber disruption and intrinsic ligamentous or subjacent soft tissue edema to detect the presence of injury. We differentiated acute from chronic injuries as per clinical judgement, using the criteria above (focal fiber disruption and edema) as the key differentiators from a chronic finding with attenuation and no edema. We defined subchondral insufficiency fracture as a linear low signal in the subchondral bone on a fat suppressed image and subadjacent edema (18). Since each pathologic finding was rare we combined them into a primary outcome variable based on the presence of any miscellaneous pathology.
Semi-automated bone marrow lesion segmentation
One reader (ACS) measured tibiofemoral BML volume with a validated semi-automated segmentation method (19, 20). After the reader identified crude boundaries of the bones, the program automatically identified the precise bone boundaries and performed a thresholding and curve evolution process twice to segment areas of high-signal intensity. We eliminated false-positive regions by defining a BML based on 2 criteria: 1) the distance between a BML to the articular surface should be ≥10 mm (21–23) and 2) a BML should span more than one MR image. We summed BML measurements to generate a whole knee BML volume (intra-reader reliability: ICC3,1=0.91). The reader was unaware of group assignment and was unblinded to the order of time. The primary investigator (JBD) reviewed all measurements.
Cartilage damage index
To quantify change in tibiofemoral cartilage damage, we used the validated cartilage damage index (CDI) (24, 25). One reader (JED) manually marked the bone-cartilage boundary on automatically selected images, and measured the cartilage thickness at predefined informative locations, which the software located. The software then computed the CDI by summing the products of cartilage thickness, cartilage length (anterior-posterior), and voxel size from each informative location. The CDI was calculated for the medial and lateral femur and the medial and lateral tibia (intra-reader reliability: ICC3,1=0.86 to 0.99). The reader was unaware of group assignment and was unblinded to the order of time. All measurements were reviewed by the primary investigator.
Clinical data
Age, body mass index, self-reported injury, frequent knee pain, days with limited activity in prior month, overall global rating, and Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain were acquired at each visit based on a standard protocol.
Statistical analysis
We calculated descriptive baseline characteristics and compared groups using chi-square tests or one-way analyses of variance with post hoc comparisons of the three groups with a Bonferroni correction.
To determine if the presence of destabilizing meniscal tears, miscellaneous pathology, or other secondary meniscal findings differed between groups across time we used generalized linear mixed models assuming compound symmetry. Independent variables included group (3 levels) and time (up to 5 levels). We also tested a group-by-time interaction. Significant interactions were followed up with 3 cross-sectional post hoc comparisons at each time (e.g., at the index visit: AKOA vs typical KOA, AKOA vs no KOA, and typical vs no KOA). For our primary analyses we adjusted for sex (matching variable)(26) and factors related to someone having no MR images at the next annual visit (i.e., age, body mass index, injury, frequent knee pain, days with limited activity in prior month, overall global rating, and WOMAC pain). When a model failed to converge because of a limited number of outcomes in a group, we performed a logistic regression for each time point separately with group (2 levels: AKOA versus no AKOA [typical and no KOA]) as an independent variable and adjusted for sex.
To explore if cartilage damage or BML volume (continuous measures) differed between groups, we performed linear mixed models assuming compound symmetry. Independent variables included group (3 levels) and time (up to 5 levels). We also tested a group-by-time interaction. Significant interactions were followed up as described above. For our primary analyses we adjusted for sex and factors related to missing MR images at the next visit (see above). We calculated the mean change in each measure during the 2 years preceding the index visit based on the least-squares means from the primary analyses.
We conducted several sensitivity analyses:
-
1)
adjusted only for sex,
-
2)
controlling for contralateral KOA: only included people who developed AKOA and had no radiographic KOA in the contralateral knee at baseline (n=54/group with matching),
-
3)
controlled for contralateral KL grade: we added baseline contralateral KL grade as a covariate to the models in our primary analyses (n=125/group),
-
4)
controlled for static knee alignment: we added baseline femorotibial angle as a covariate to the models in our primary analyses (n=125/group),
-
5)
assessed people with the fastest development of AKOA: only included individuals who developed AKOA in ≥12 months (n=71/group with matching);
-
6)
assessed people with complete data: among people with complete data at every visit (AKOA n=12, typical KOA n=29, no KOA n=25; no matching),
-
7)
assessed a commonly reported radiographic definition of incident KOA: only included those who developed typical KOA and had KL=2 (n=76/group with matching), and
-
8)
controlled for baseline disease severity: only included adults who had KL=0 in the index knee at baseline (AKOA n=42, typical KOA n=71, no KOA n=92; no matching).
Since the sensitivity analyses were always among a smaller sample size, we performed them adjusted for sex only, unless noted above. As an additional sensitivity analysis, we assessed model diagnostics on the primary analyses and reran the analyses excluding people with potential influential data (i.e., large Cook’s D or Cook’s D Covariance Parameters). We also repeated the primary BML analyses with the log of BML volume because BML volume was skewed with a few knee-visits having large BML volumes.
All analyses were performed with SAS Enterprise 7.15 (Cary, NC, USA).
RESULTS
Descriptive characteristics
Table 1 provides an overview of the descriptive characteristics of the groups. Overall, participants were predominantly female (63%), overweight, and 24 to 39% reported frequent knee pain within the prior 12 months. Compared with adults with typical or no KOA, those with AKOA were on average older, heavier, and reporting greater global impact of arthritis, and more likely to have questionable signs of KOA (KL grade=1; Table 1). Over time, 70%, 25%, 4% of adults with AKOA developed joint space narrowing in the medial, lateral, or both tibiofemoral compartments, respectively. In contrast, only 26% and 0% of adults with either typical or no KOA developed joint space narrowing, respectively.
Table 1.
Descriptive Characteristics of those with Accelerated, Typical, and No Knee Osteoarthritis (KOA) at Osteoarthritis Initiative Baseline
| Variables (means, SD; except where noted) |
Accelerated KOA (n=125) |
Typical KOA (n=125) |
No KOA (n=125) |
p-value |
|---|---|---|---|---|
| Females (n, %) | 79 (63%) | 79 (63%) | 79 (63%) | 1.00 |
| Index knee KL Grade=0 (n, %) | 42 (34%)a,b | 71 (57%)a | 92 (74%) | <0.001 |
| Frequent knee pain in past 12 months (n, %) | 44 (35%) | 49 (39%)a | 30 (24%) | 0.03 |
| Age (years)c | 63 (9)a,b | 58 (8) | 57 (8) | <0.001 |
| Body mass index (kg/m2)d | 29.7 (4.6)a,b | 28.1 (4.4) | 26.9 (4.4) | <0.001 |
| Global impact rating (0 to 10; higher score=greater impact) | 1.7 (1.9)a,b | 1.1 (1.5) | 0.8 (1.1) | <0.001 |
| How many days limited activities in past 30 days (0 to 30)? | 3.2 (7.3)a | 1.7 (4.8) | 1.4 (4.3) | 0.03 |
| WOMAC pain (0 to 20; higher score=more pain) | 2.3 (3.1) | 1.8 (2.3) | 1.6 (2.4) | 0.08 |
Notes. Index knee Kellgren-Lawrence (KL) grade could be 0 or 1. SD=standard deviation, MR = magnetic resonance
statistically significant difference with No KOA (p <0.017).
statistically significant difference with Typical KOA (p <0.017).
Age Ranges: AKOA: 45 to 79 years, typical KOA: 45 to 79 years, no KOA: 46 to 80 years.
Body Mass Index Ranges: AKOA: 17.0 to 40.6 kg/m2, typical KOA: 18.6 to 40.9 kg/m2, no KOA: 19.3 to 45.6 kg/m2.
Destabilizing meniscal tear
Destabilizing meniscal tears were rare in adults with no KOA (<5%; Table 2, Figure 1). Hence, we compared AKOA versus no AKOA. At 2 years prior to the index visit, people who developed AKOA had >4 times the odds of a destabilizing meniscal pathology than adults without AKOA (Table 2, Figure 1). By the index visit, adults with AKOA were >7 times more likely to have a destabilizing meniscal tear than those without AKOA and this persisted for the next 2 visits. Among adults with AKOA 86% of destabilizing tears occurred in the same compartment as joint space narrowing.
Table 2.
Adults with Accelerated Knee Osteoarthritis (AKOA) are Approximately Twice as Likely to have Destabilizing Meniscal Tears and Miscellaneous Pathology Starting at the Index Visit than Adults with Either Typical or No Knee Osteoarthritis (KOA).
| Variable | Visit | AKOA | Typical KOA | No KOA | AKOA vs No AKOA |
|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | OR (95%CI) | ||
| Destabilizing Meniscal Tear | −2 | 12 (13) | 3 (5) | 2 (2) | 4.7 (1.6 to 14.1) |
| −1 | 25 (21) | 13 (11) | 3 (2) | 4.0 (2.0 to 7.9) | |
| Index | 44 (42) | 17 (14) | 5 (4) | 7.3 (4.1 to 13.1) | |
| 1 | 39 (49) | 18 (16) | 4 (4) | 8.0 (4.3 to 14.9) | |
| 2 | 19 (53) | 14 (17) | 1 (2) | 9.8 (4.1 to 23.6) | |
| Miscellaneous Pathology: Overall | −2 | 6 (7) | 1 (2) | 2 (2) | 3.6 (0.9 to 14.9) |
| −1 | 6 (5) | 1 (1) | 2 (2) | 4.4 (1.1 to 17.9) | |
| Index | 16 (15) | 2 (2) | 3 (2) | 8.6 (3.1 to 24.2) | |
| 1 | 13 (16) | 3 (3) | 2 (2) | 8.1 (2.8, 23.8) | |
| 2 | 8 (22) | 3 (4) | 1 (2) | 9.7 (2.7, 34.5) | |
| Miscellaneous Pathology: Attrition | −2 | 1 (1%) | 0 (0%) | 1 (1%) | |
| −1 | 2 (2%) | 0 (0%) | 1 (1%) | ||
| Index | 11 (11%) 2 cases had fractures |
1 (1%) | 1 (1%) | ||
| 1 | 11 (14%) | 1 (1%) | 1 (1%) | ||
| 2 | 7 (19%) | 2 (2%) | 1 (2%) | ||
| Miscellaneous Pathology: subchondral insufficiency fractures, acute ligamentous injuries, acute tendinous injuries, osteochondral lesions, and tibial contusions | −2 | 5 (5%) | 1 (2%) | 1 (1%) | |
| −1 | 4 (3%) | 1 (1%) | 1 (1%) | ||
| Index | 7 (7%) | 1 (1%) | 2 (2%) | ||
| 1 | 2 (3%) | 2 (2%) | 1 (1%) | ||
| 2 | 1 (3%) | 1 (1%) | 0 (0%) | ||
OR = odds ratio, 95%CI = 95% confidence intervals. Original models with AKOA, Typical KOA, and No KOA failed to converge. Presented logistic regression models are adjusted for sex only.
Figure 1.
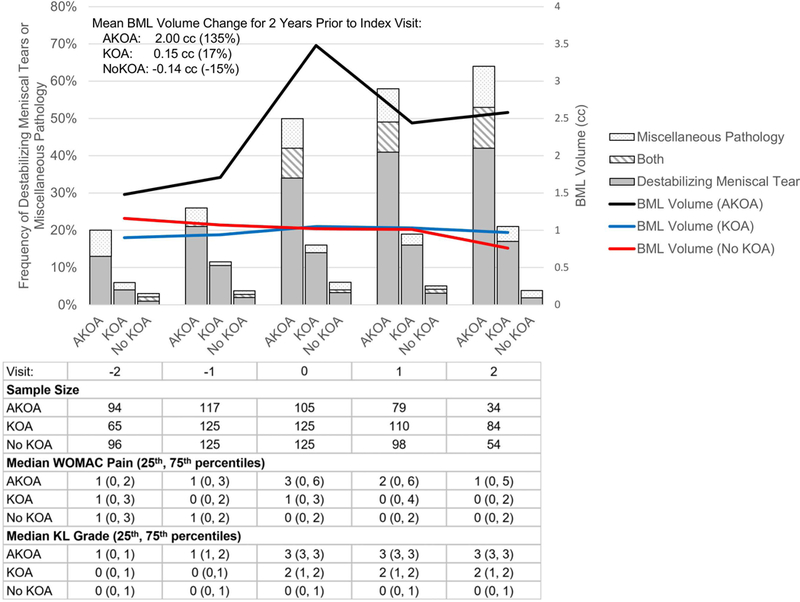
Accelerated Knee Osteoarthritis (AKOA) is Characterized by a Large Increase in Bone Marrow Lesion (BML) Volume that Corresponds to Destabilizing Meniscal Tears and Miscellaneous Pathologies
Miscellaneous pathology
Since very few cases (< 5%) of miscellaneous pathology occurred among adults with typical or no KOA we compared AKOA versus no AKOA. Starting at the year before the index visit adults with AKOA had >4 times greater odds of having miscellaneous pathology than their peers (OR = 4.4, Table 2, Figure 2). At the index visit individuals with AKOA were ~8.6 times as likely to have miscellaneous pathology compared to those without AKOA (Table 2, Figure 1). This persisted for up to 2 years after the index visit. Table 2 shows the frequency of miscellaneous pathology separately for attrition or other findings (i.e., subchondral insufficiency fractures, acute ligamentous injuries, acute tendinous injuries, osteochondral lesions, and tibial contusions).
Figure 2.
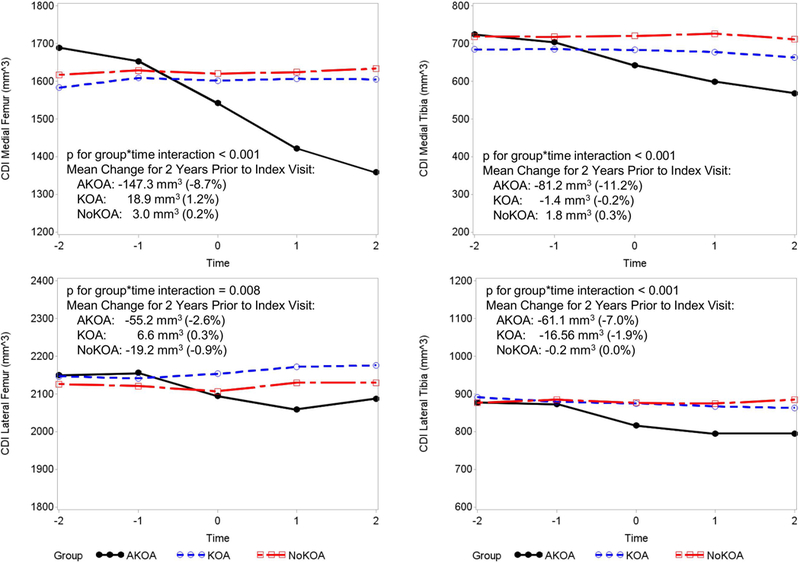
Adults with Accelerated Osteoarthritis (AKOA) have a Loss of Cartilage Compared with Adults with typical or no knee osteoarthritis (KOA, NoKOA).
During the 4-year observation period, all cases of miscellaneous pathology in a specific compartment, excluding those with no KOA, developed joint space narrowing in the same compartment. Adults with AKOA who had ligamentous or musculotendinous injuries all developed medial joint space narrowing. Two adults with typical KOA and a cruciate ligament injury experienced medial or no joint space narrowing.
Collectively, more than three times as many adults with AKOA (49%) had a destabilizing meniscal tear or miscellaneous pathology compared with typical KOA (15%; no KOA 6%) at the index visit (Figure 1).
Secondary meniscal findings
At 2 years prior to the index visit, adults with AKOA were more likely to have meniscal damage in 2 or more regions (66% vs 30%), any medial meniscal pathology (excluding extrusion; 72% vs 39%), and medial meniscal extrusion (20% vs 6%), compared with adults with no KOA (Table 3). Thereafter, individuals with AKOA were more likely to have these pathologic features as well as lateral meniscal extrusion than adults with either typical or no KOA. By the index visit, >90% of adults with AKOA had meniscal damage in 2 or more regions and 85% had medial meniscal pathology. Furthermore, 77% of individuals with AKOA had meniscal extrusion at the index visit compared to only 32% and 6% with typical KOA, or no KOA.
Table 3.
Secondary Meniscal Findings Indicate that Adults Who Develop Accelerated Knee Osteoarthritis (AKOA) Have More Meniscal Pathology Than Adults with Either Typical or No Knee Osteoarthritis as Early as 2 Years Before Radiographic Onset
| Variable | Visit | AKOA | Typical KOA |
No KOA | AKOA vs No KOA | KOA vs No KOA | AKOA vs KOA |
|---|---|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | OR (95%CI) | OR (95%CI) | OR (95%CI) | ||
| Meniscal Damage in ≥2 regions (group-by-time interactionp<0.001) | −2 | 61 (66) | 26 (40) | 29 (30) | 3.12 (1.69, 5.76) | 1.87 (1.00, 3.53) | 1.66 (0.89, 3.13) |
| −1 | 90 (77) | 63 (51) | 35 (28) | 7.60 (4.00, 14.45) | 2.38 (1.33, 4.28) | 3.19 (1.70, 5.97) | |
| Index | 99 (93) | 67 (54) | 37 (30) | 19.87 (9.08, 43.46) | 2.71 (1.56, 4.70) | 7.34 (3.42, 15.76) | |
| 1 | 73 (91) | 61 (55) | 30 (31) | 21.25 (9.24, 48.88) | 2.88 (1.64, 5.04) | 7.39 (3.27, 16.71) | |
| 2 | 33 (92) | 47 (56) | 14 (26) | 27.03 (9.55, 76.48) | 2.90 (1.60, 5.26) | 9.32 (3.36, 25.83) | |
| Medial Meniscal Pathology (group-by-time interaction p<0.001) | −2 | 66 (72) | 36 (55) | 37 (39) | 5.89 (2.80, 12.42) | 3.25 (1.62, 6.49) | 1.81 (0.85, 3.87) |
| −1 | 93 (79) | 78 (63) | 50 (40) | 9.10 (4.15, 19.94) | 3.23 (1.65, 6.31) | 2.82 (1.29, 6.16) | |
| Index | 90 (85) | 81 (65) | 49 (40) | 12.49 (5.44, 28.71) | 3.94 (2.02, 7.66) | 3.17 (1.39, 7.25) | |
| 1 | 70 (88) | 72 (65) | 39 (40) | 12.24 (5.28, 28.38) | 4.00 (2.05, 7.80) | 3.06 (1.32, 7.08) | |
| 2 | 31 (86) | 54 (64) | 23 (43) | 8.50 (3.74, 19.33) | 3.94 (1.99, 7.80) | 2.16 (0.95, 4.88) | |
| Medial Meniscal Extrusion (group-by-time interaction p<0.001) | −2 | 18 (20) | 10 (15) | 6 (6) | 4.17 (1.46, 11.88) | 3.50 (1.15, 10.63) | 1.19 (0.52, 2.71) |
| −1 | 43 (37) | 26 (21) | 7 (6) | 11.02 (4.11, 29.57) | 3.57 (1.26, 10.12) | 3.09 (1.52, 6.30) | |
| Index | 68 (64) | 36 (29) | 6 (5) | 34.09 (11.73, 99.07) | 8.13 (2.82, 23.43) | 4.19 (2.24, 7.84) | |
| 1 | 51 (64) | 36 (33) | 3 (3) | 43.28 (13.52, 138.52) | 12.29 (3.91, 38.60) | 3.52 (1.86, 6.65) | |
| 2 | 28 (78) | 29 (35) | 2 (4) | 53.97 (14.69, 198.32) | 13.90 (4.01, 48.19) | 3.88 (1.87, 8.06) | |
| Lateral Meniscal Pathology (group p=0.002; group-by-time interaction p=0.97) | −2 | 43 (47) | 19 (29) | 20 (21) | 2.96 (1.60, 5.48) | 1.74 (0.92, 3.26) | 1.71 (0.95, 3.06) |
| −1 | 52 (44) | 40 (33) | 25 (20) | 2.99 (1.63, 5.49) | 1.85 (1.00, 3.40) | 1.62 (0.92, 2.85) | |
| Index | 54 (51) | 41 (33) | 26 (21) | 3.21 (1.76, 5.86) | 1.94 (1.07, 3.51) | 1.65 (0.95, 2.87) | |
| 1 | 37 (46) | 36 (33) | 23 (24) | 2.80 (1.54, 5.08) | 1.80 (1.00, 3.22) | 1.56 (0.90, 2.71) | |
| 2 | 19 (53) | 32 (38) | 9 (17) | 2.74 (1.48, 5.07) | 1.81 (1.01 (3.24) | 1.51 (0.85, 2.69) | |
| Lateral Meniscal Extrusion | −2 | 3 (3) | 1 (2) | 0 (0) | Model Failed to Converge (see text for more details) | ||
| −1 | 9 (8) | 4 (3) | 1 (1) | ||||
| Index | 15 (14) | 5 (4) | 1 (1) | ||||
| 1 | 10 (13) | 5 (5) | 1 (1) | ||||
| 2 | 3 (8) | 4 (5) | 0 (0) | ||||
No statistically significant group-by-time interaction was detected; however, there was a significant group effect. OR = odds ratio, 95%CI = 95% confidence intervals
All models adjusted for sex (matching variable) and factors related to someone having no MR images at the next annual visit (i.e., age, body mass index, injury, frequent knee pain, days with limited activity in prior month, overall global rating, and WOMAC pain).
Regardless of time, individuals with either AKOA or typical KOA were more likely than those with no KOA to have lateral meniscal pathology (AKOA: OR=2.94, 95% CI=1.64 to 5.26; typical KOA: OR=1.82, 95% CI=1.03 to 3.25). All sensitivity analyses supported the secondary meniscal findings with a few exceptions.
Bone marrow lesion volume
At one year prior to the index visit, adults who developed AKOA had greater BML volume than comparators (versus typical KOA: p=0.024, versus no KOA: p=0.046) and these findings persisted for at least 2 years after the index visit (all p<0.001; Table 4, Figure 1). In addition, during the two years leading up to the index visit, the adults with AKOA exhibited on average 13 times greater longitudinal increase in BML volume than adults with typical KOA, (2.00 vs 0.15 cc; Figure 1). All the sensitivity analyses led to similar patterns described above.
Table 4.
Adults who develop accelerated knee osteoarthritis (AKOA) exhibit different structural findings than adults with typical knee osteoarthritis (KOA) or no KOA as early as 2 years before meeting the criteria for AKOA or typical KOA.
| Outcome | Visit | AKOA | Typical KOA | No KOA |
|---|---|---|---|---|
| n =125 | n = 125 | n = 125 | ||
| LSMeans (SE) | ||||
| Total Bone Marrow Lesion Volume (cc) | −2 | 1.48 (0.24) | 0.90 (0.32) | 1.16 (0.25) |
| (group-by-time interaction p<0.001) | −1 | 1.71 (0.23)1,2 | 0.94 (0.25) | 1.07 (0.23) |
| Index | 3.48 (0.22)1,2 | 1.05 (0.20) | 1.02 (0.20) | |
| 1 | 2.45 (0.25)1,2 | 1.03 (0.20) | 1.01 (0.21) | |
| 2 | 2.58 (0.35)1,2 | 0.97 (0.23) | 0.76 (0.27) | |
| Medial Femur Cartilage Damage Index | −2 | 1689 (28)1 | 1583 (33) | 1617 (28) |
| (group-by-time interaction p<0.001) | −1 | 1653 (27) | 1609 (28) | 1629 (26) |
| Index | 1542 (27)2 | 1602 (25) | 1620 (25) | |
| 1 | 14212 (29)1,2 | 1607 (25) | 1624 (26) | |
| 2 | 1359 (35)1,2 | 1605 (30) | 16334 (29) | |
| Medial Tibia Cartilage Damage Index | −2 | 724 (15) | 685 (16) | 719 (15) |
| (group-by-time interaction p<0.001) | −1 | 704 (14) | 685 (15) | 718 (14) |
| Index | 643 (14)1,2 | 683 (13)2 | 721 (13) | |
| 1 | 600 (15)1,2 | 678 (13)2 | 727 (14) | |
| 2 | 569 (18)1,2 | 664 (14)2 | 712 (15) | |
| Lateral Femur Cartilage Damage Index | −2 | 2150 (34) | 2147 (39) | 2126 (34) |
| (group-by-time interaction p=0.008) | −1 | 2156 (34) | 2141 (34)301 | 2121 (33) |
| Index | 2095 (34) | 2154 (31) | 2107 (31) | |
| 1 | 2060 (35)1 | 2172 (31) | 2130 (32) | |
| 2 | 2088 (42) | 2176 (33) | 2130 (35) | |
| Lateral Tibia Cartilage Damage Index | −2 | 877 (19) | 892 (21) | 877 (19) |
| (group-by-time interaction p<0.001) | −1 | 873 (19) | 879 (19) | 885 (18) |
| Index | 816 (19)1,2 | 875 (17) | 877 (18) | |
| 1 | 795 (20)1,2 | 867 (18) | 875 (18) | |
| 2 | 795 (22)1,2 | 863 (18) | 885 (19) | |
Significant against KOA;
Significant against No KOA
All models adjusted for sex (matching variable) and factors related to someone having no MR data at the next annual visit (i.e., age, body mass index, injury, frequent knee pain, days with limited activity in prior month, overall global rating, and WOMAC pain).
Articular cartilage loss
Articular cartilage loss prior to the index visit
At 2 years prior to the index visit, adults with AKOA had greater medial femoral cartilage compared with typical KOA (p=0.016; Table 4, Figure 2). The mean changes in CDI during the 2 years prior to the index visit was greater among people with AKOA compared with typical or no KOA (Figure 2).
Articular cartilage loss at the index visit
At the index visit, the AKOA group had less cartilage in the medial and lateral tibia than adults with typical KOA or no KOA (all p<0.04; Table 4, Figure 2). Furthermore, adults with AKOA had less medial femoral cartilage than those with no KOA (p=0.041).
Articular cartilage loss after the index visit
After the index visit, adults with AKOA had less cartilage in the medial and lateral tibia and medial femur compared with individuals with either typical or no KOA. During this time frame the typical KOA group had less cartilage in the medial tibia than the no KOA group (all p<0.023; Table 4, Figure 2).
All the CDI sensitivity analyses led to similar results; however, when we omitted influential observations (outliers), the group-by-time interaction for lateral femur cartilage damage was no longer statistically significant (p=0.48; 5 observations removed).
DISCUSSION
Adults with AKOA are differentiated from adults with typical KOA by early meniscal damage in 2 or more regions, subsequent larger BMLs, a substantially greater rate of cartilage loss, and eventually a destabilizing meniscal tear. These observations provide strong support for the hypothesis that AKOA is a unique subset of KOA likely precipitated by a destabilizing meniscal tear in a joint compromised by the presence of multiple structural abnormalities (e.g., large BMLs, cartilage loss, meniscal damage in 2 or more regions, large effusion-synovitis volumes(27)). Since adults with AKOA experience a shortened window to advance-stage disease it is critical that we recognize and are proactive when a patient without radiographic KOA presents with knee pain and signs of meniscal damage in 2 or more regions and large BMLs. For these individuals, early (pre-radiographic) recognition is urgently needed as is research to develop primary prevention strategies (e.g., injury prevention programs) to reduce their risk of a destabilizing meniscal tear or miscellaneous pathology.
These findings add to the evidence that AKOA (4, 28–30) is different to the typically perceived archetype of slow-progressing osteoarthritis. AKOA has a unique risk profile (e.g., coronal tibial slope with malalignment) (1, 3, 29–31), greater prodromal symptoms (4, 28), and greater preradiographic disease burden(3). These new findings complement evidence from a prior publication using this case-control sample that AKOA is preceded and characterized by large effusion-synovitis volumes and altered infrapatellar fat pad signal intensity(27), which may reflect local inflammation. The meniscal extrusion and meniscal damage in 2 or more regions likely contributed to effusion-synovitis(32), altered signal intensity in the infrapatellar fat pad, large BMLs(33–35), and cartilage loss(36, 37). Furthermore, the early evidence of large BMLs, effusion-synovitis, and meniscal damage in 2 or more regions may explain the greater knee pain and dysfunction (4, 20, 37–41) reported by people up to 3 years prior to developing radiographic evidence of AKOA (4, 28). Knee pain is a risk factor for a new injury (42) that may lead to destabilizing tears or other pathologies, which could be a catalyst for an accelerated onset and progression to joint failure. Hence, it is critical to recognize people with knee pain early and be proactive when they report knee pain or a new injury.
Our findings that adults who go onto develop AKOA had greater baseline prevalence of KL=1 and pre-radiographic evidence of meniscal damage in 2 or more regions, dramatic increases in BML volume, and articular cartilage loss is consistent with evidence that questionable signs of radiographic KOA (KL=1) predict incident KOA (43) and AKOA (3). Our MR-based findings of greater pre-radiographic disease burden were even found among adults who had definitively no radiographic KOA (KL=0) at baseline. This highlights that radiographs are nonspecific and offer an inconclusive interpretation of early disease status. Early (pre-radiographic) evidence of meniscal damage, BMLs, and cartilage damage are important as they associate with prevalent and incident knee symptoms (44) as well as incident radiographic KOA (45–47). Our findings confirm that pre-radiographic disease burden is a risk factor for KOA, especially AKOA.
Our findings that AKOA is characterized by destabilizing meniscal tear or miscellaneous pathology, especially subchondral bone damage, supports prior evidence that certain types of meniscal pathology (e.g., root tears) may increase the risk for accelerated progression (6, 7) and that other more aggressive forms of rapidly progressive osteoarthritis progression may be defined by significant subchondral bone damage (48, 49). It is important to acknowledge that it remains unclear if AKOA has any relation to type 2 rapidly progressive osteoarthritis, which was characterized by a more dramatic joint space narrowing (≥2mm within 1 year) and greater abnormal bone loss/destruction (48, 50). However, the role of bone in both forms of rapid disease onset/progression warrants further study.
Since almost 50% of people who develop AKOA have a destabilizing meniscal tear or miscellaneous pathology (versus typical KOA: 15% or no KOA = 6%) it is critical to question the current standard of grouping people with AKOA with peers who develop typical KOA. Within the OAI, AKOA accounts for more than 1 in 5 cases of incident KOA during the first 4 years of follow-up (1). Hence, adults with AKOA may have a strong influence on the results from epidemiological studies and clinical trials. Many findings that are attributed to KOA may be attributable to AKOA rather than typical KOA. For example, we recently demonstrated that excluding cases of incident AKOA from analyses that defined incident KOA as a ≥2 point increase in KL grade (i.e., KL=0 to 2, 3, or 4; or KL=1 to 3 or 4) led to smaller effect estimates (Cohen’s d (51)) when comparing cases to controls for average WOMAC knee pain over time (all incident KOA d=0.41, excluding AKOA d=0.14) or recent knee injury (all incident KOA OR=5.4, excluding AKOA OR=4.0) (2). Adults with AKOA may be distinct from those with typical KOA regarding symptomatic and structural changes. This raises critical concerns about suggestions that clinical trials for KOA would benefit from recruiting people with faster progression. Instead, these findings may indicate that separate trials may be needed for AKOA and other forms of KOA because AKOA has a unique natural history defined by the onset of a destabilizing meniscal tear or miscellaneous pathology in a joint compromised by the presence of multiple pathologies. It will be beneficial to explore if adults with AKOA respond differently to therapeutic interventions than adults with typical KOA.
While this study characterized AKOA it is important to acknowledge some limitations. First, the timing of disease onset was hard to discern, which made it challenging to define the moment of incident disease. Despite this limitation the OAI offered an unprecedented opportunity to study annual images to observe how AKOA develops. Secondly, we were unable to definitively demonstrate that AKOA is a distinct disease versus an accelerated progression of typical features. However, the occurrence of unique structural changes (e.g., destabilizing tears and miscellaneous pathologies) support the hypothesis that AKOA is a different KOA subset. Thirdly, we classified a meniscal tear as destabilizing if it predisposed a meniscus to extrusion. Hence, we may have neglected other types of tears (e.g., bucket handle tears) that other investigators may consider unstable. We also included small radial tears as destabilizing tears despite some of these possibly being stable. However, despite the potential for misclassification we still observed a significant association between destabilizing meniscal tears and AKOA. Fourth, we found no universal pathology that was associated with AKOA. Hence, AKOA may be a collection of subsets of KOA that rapidly develops. Fifth, we had missing MR data that could influence the results. However, we conducted robust analyses that adjusted for factors related to missing MR data at the next visit. We also completed numerous sensitivity analyses to explore the robustness of these results. Our study was also limited by our sample size and the number of structural features we explored. Future research with a larger sample size of adults at risk for AKOA may help further refine our understanding of AKOA and help develop a clinically useful predictive model. Despite these limitations, the OAI offered an unprecedented opportunity to address this study question and we pursued the analyses with well-defined a priori hypotheses.
In conclusion, we found that in an early phase, individuals who develop AKOA are differentiated from individuals with typical KOA by the presence of meniscal damage in 2 or more regions, subsequent development of large BMLs, substantially greater rate of cartilage loss, and ultimately a destabilizing meniscal tear.
Acknowledgments
These analyses were financially supported by a grant from the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number R01-AR065977. The OAI is a public-private partnership comprised of five contracts (N01-AR-2-2258; N01-AR-2-2259; N01-AR-2-2260; N01-AR-2-2261; N01-AR-2-2262) funded by the National Institutes of Health, a branch of the Department of Health and Human Services, and conducted by the OAI Study Investigators. Private funding partners include Merck Research Laboratories; Novartis Pharmaceuticals Corporation, GlaxoSmithKline; and Pfizer, Inc. Private sector funding for the OAI is managed by the Foundation for the National Institutes of Health. This manuscript was prepared using an OAI public use data set and does not necessarily reflect the opinions or views of the OAI investigators, the NIH, or the private funding partners. Dr. Lo is supported by K23 AR062127, an NIH/NIAMS funded mentored award, providing support for design and conduct of the study, analysis, and interpretation of the data. This work is supported in part with resources at the VA HSR&D Center for Innovations in Quality, Effectiveness and Safety (#CIN 13–413), at the Michael E. DeBakey VA Medical Center, Houston, TX. The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs. The funding sources had no role in study design; in the collection, analysis and interpretation of data; in the writing of the report; nor in the decision to submit the article for publication.
Footnotes
The authors have no other conflicts of interest regarding this work.
REFERENCES
- 1.Driban JB, Eaton CB, Lo GH, Ward RJ, Lu B, McAlindon TE. Association of knee injuries with accelerated knee osteoarthritis progression: data from the Osteoarthritis Initiative. Arthritis Care Res (Hoboken) 2014;66:1673–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Driban JB, Stout AC, Lo GH, Eaton CB, Price LL, Lu B, et al. Best performing definition of accelerated knee osteoarthritis: data from the Osteoarthritis Initiative. Ther Adv Musculoskelet Dis 2016;8:165–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Riddle DL, Stratford PW, Perera RA. The incident tibiofemoral osteoarthritis with rapid progression phenotype: development and validation of a prognostic prediction rule. Osteoarthritis Cartilage 2016;24:2100–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Driban JB, Price LL, Eaton CB, Lu B, Lo GH, Lapane KL, et al. Individuals with incident accelerated knee osteoarthritis have greater pain than those with common knee osteoarthritis progression: data from the Osteoarthritis Initiative. Clin Rheumatol 2016;35:1565–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhatia S, LaPrade CM, Ellman MB, LaPrade RF. Meniscal root tears: significance, diagnosis, and treatment. Am J Sports Med 2014;42:3016–30. [DOI] [PubMed] [Google Scholar]
- 6.Guermazi A, Hayashi D, Jarraya M, Roemer FW, Zhang Y, Niu J, et al. Medial posterior meniscal root tears are associated with development or worsening of medial tibiofemoral cartilage damage: the multicenter osteoarthritis study. Radiology 2013;268:814–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krych AJ, Reardon PJ, Johnson NR, Mohan R, Peter L, Levy BA, et al. Non-operative management of medial meniscus posterior horn root tears is associated with worsening arthritis and poor clinical outcome at 5-year follow-up. Knee Surg Sports Traumatol Arthrosc 2017;25:383–9. [DOI] [PubMed] [Google Scholar]
- 8.Ozkoc G, Circi E, Gonc U, Irgit K, Pourbagher A, Tandogan RN. Radial tears in the root of the posterior horn of the medial meniscus. Knee Surg Sports Traumatol Arthrosc 2008;16:849–54. [DOI] [PubMed] [Google Scholar]
- 9.Robertson DD, Armfield DR, Towers JD, Irrgang JJ, Maloney WJ, Harner CD. Meniscal root injury and spontaneous osteonecrosis of the knee: an observation. J Bone Joint Surg Br 2009;91:190–5. [DOI] [PubMed] [Google Scholar]
- 10.Felson DT, Nevitt MC, Yang M, Clancy M, Niu J, Torner JC, et al. A new approach yields high rates of radiographic progression in knee osteoarthritis. J Rheumatol 2008;35:2047–54. [PMC free article] [PubMed] [Google Scholar]
- 11.Felson DT, Niu J, Guermazi A, Sack B, Aliabadi P. Defining radiographic incidence and progression of knee osteoarthritis: suggested modifications of the Kellgren and Lawrence scale. Ann Rheum Dis 2011;70:1884–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eckstein F, Wirth W, Nevitt MC. Recent advances in osteoarthritis imaging--the osteoarthritis initiative. Nat Rev Rheumatol 2012;8:622–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iranpour-Boroujeni T, Li J, Lynch JA, Nevitt M, Duryea J, Investigators OAI. A new method to measure anatomic knee alignment for large studies of OA: data from the Osteoarthritis Initiative. Osteoarthritis Cartilage 2014;22:1668–74. [DOI] [PubMed] [Google Scholar]
- 14.Peterfy CG, Schneider E, Nevitt M. The osteoarthritis initiative: report on the design rationale for the magnetic resonance imaging protocol for the knee. Osteoarthritis Cartilage 2008;16:1433–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anderson AF, Irrgang JJ, Dunn W, Beaufils P, Cohen M, Cole BJ, et al. Interobserver reliability of the International Society of Arthroscopy, Knee Surgery and Orthopaedic Sports Medicine (ISAKOS) classification of meniscal tears. Am J Sports Med 2011;39:926–32. [DOI] [PubMed] [Google Scholar]
- 16.Antony B, Driban JB, Price LL, Lo GH, Ward RJ, Nevitt M, et al. The relationship between meniscal pathology and osteoarthritis depends on the type of meniscal damage visible on magnetic resonance images: data from the Osteoarthritis Initiative. Osteoarthritis Cartilage 2017;25:76–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hunter DJ, Guermazi A, Lo GH, Grainger AJ, Conaghan PG, Boudreau RM, et al. Evolution of semi-quantitative whole joint assessment of knee OA: MOAKS (MRI Osteoarthritis Knee Score). Osteoarthritis Cartilage 2011;19:990–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramnath RR, Kattapuram SV. MR appearance of SONK-like subchondral abnormalities in the adult knee: SONK redefined. Skeletal Radiol 2004;33:575–81. [DOI] [PubMed] [Google Scholar]
- 19.Pang JC, Driban JB, Destenaves G, Miller E, Lo GH, Ward RJ, et al. Quantification of bone marrow lesion volume and volume change using semi-automated segmentation: data from the osteoarthritis initiative. BMC Musculoskelet Disord 2013;14:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Driban JB, Price L, Lo GH, Pang J, Hunter DJ, Miller E, et al. Evaluation of bone marrow lesion volume as a knee osteoarthritis biomarker--longitudinal relationships with pain and structural changes: data from the Osteoarthritis Initiative. Arthritis Res Ther 2013;15:R112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Driban JB, Lo GH, Lee JY, Ward RJ, Miller E, Pang J, et al. Quantitative bone marrow lesion size in osteoarthritic knees correlates with cartilage damage and predicts longitudinal cartilage loss. BMC Musculoskelet Disord 2011;12:217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roemer FW, Frobell R, Hunter DJ, Crema MD, Fischer W, Bohndorf K, et al. MRI-detected subchondral bone marrow signal alterations of the knee joint: terminology, imaging appearance, relevance and radiological differential diagnosis. Osteoarthritis Cartilage 2009;17:1115–31. [DOI] [PubMed] [Google Scholar]
- 23.Roemer FW, Khrad H, Hayashi D, Jara H, Ozonoff A, Fotinos-Hoyer AK, et al. Volumetric and semiquantitative assessment of MRI-detected subchondral bone marrow lesions in knee osteoarthritis: a comparison of contrast-enhanced and non-enhanced imaging. Osteoarthritis Cartilage 2010;18:1062–6. [DOI] [PubMed] [Google Scholar]
- 24.Zhang M, Driban JB, Price LL, Lo GH, Miller E, McAlindon TE. Development of a Rapid Cartilage Damage Quantification Method for the Lateral Tibiofemoral Compartment Using Magnetic Resonance Images: Data from the Osteoarthritis Initiative. BioMed research international 2015;2015:634275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang M, Driban JB, Price LL, Harper D, Lo GH, Miller E, et al. Development of a rapid knee cartilage damage quantification method using magnetic resonance images. BMC Musculoskelet Disord 2014;15:264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pearce N Analysis of matched case-control studies. BMJ : British Medical Journal 2016;352:i969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davis JE, Ward RJ, Mackay JW, Lu B, Price LL, McAlindon TE, et al. Effusion-Synovitis and Infrapatellar Fat Pad Signal Intensity Alteration Differentiate Accelerated Knee Osteoarthritis. Rheumatology (Oxford) 2018;[In Press]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davis J, Eaton CB, Lo GH, Lu B, Price LL, McAlindon TE, et al. Knee symptoms among adults at risk for accelerated knee osteoarthritis: data from the Osteoarthritis Initiative. Clin Rheumatol 2017;36:1083–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Driban JB, McAlindon TE, Amin M, Price LL, Eaton CB, Davis JE, et al. Risk factors can classify individuals who develop accelerated knee osteoarthritis: Data from the osteoarthritis initiative. J Orthop Res 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Driban JB, Stout AC, Duryea J, Lo GH, Harvey WF, Price LL, et al. Coronal tibial slope is associated with accelerated knee osteoarthritis: data from the Osteoarthritis Initiative. BMC Musculoskelet Disord 2016;17:299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Driban JB, Eaton CB, Amin M, Stout AC, Price LL, Lu B, et al. Glucose homeostasis influences the risk of incident knee osteoarthritis: Data from the osteoarthritis initiative. J Orthop Res 2017;35:2282–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roemer FW, Guermazi A, Hunter DJ, Niu J, Zhang Y, Englund M, et al. The association of meniscal damage with joint effusion in persons without radiographic osteoarthritis: the Framingham and MOST osteoarthritis studies. Osteoarthritis Cartilage 2009;17:748–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lo GH, Hunter DJ, Nevitt M, Lynch J, McAlindon TE, Group OAII. Strong association of MRI meniscal derangement and bone marrow lesions in knee osteoarthritis: data from the osteoarthritis initiative. Osteoarthritis Cartilage 2009;17:743–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Englund M, Guermazi A, Roemer FW, Yang M, Zhang Y, Nevitt MC, et al. Meniscal pathology on MRI increases the risk for both incident and enlarging subchondral bone marrow lesions of the knee: the MOST Study. Ann Rheum Dis 2010;69:1796–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Y, Wluka AE, Pelletier JP, Martel-Pelletier J, Abram F, Ding C, et al. Meniscal extrusion predicts increases in subchondral bone marrow lesions and bone cysts and expansion of subchondral bone in osteoarthritic knees. Rheumatology (Oxford) 2010;49:997–1004. [DOI] [PubMed] [Google Scholar]
- 36.Hunter DJ, Zhang YQ, Niu JB, Tu X, Amin S, Clancy M, et al. The association of meniscal pathologic changes with cartilage loss in symptomatic knee osteoarthritis. Arthritis Rheum 2006;54:795–801. [DOI] [PubMed] [Google Scholar]
- 37.Hunter DJ, Zhang W, Conaghan PG, Hirko K, Menashe L, Li L, et al. Systematic review of the concurrent and predictive validity of MRI biomarkers in OA. Osteoarthritis Cartilage 2011;19:557–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lo GH, McAlindon TE, Niu J, Zhang Y, Beals C, Dabrowski C, et al. Bone marrow lesions and joint effusion are strongly and independently associated with weight-bearing pain in knee osteoarthritis: data from the osteoarthritis initiative. Osteoarthritis Cartilage 2009;17:1562–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Felson DT, Niu J, Guermazi A, Roemer F, Aliabadi P, Clancy M, et al. Correlation of the development of knee pain with enlarging bone marrow lesions on magnetic resonance imaging. Arthritis Rheum 2007;56:2986–92. [DOI] [PubMed] [Google Scholar]
- 40.Han W, Aitken D, Zhu Z, Halliday A, Wang X, Antony B, et al. Signal intensity alteration in the infrapatellar fat pad at baseline for the prediction of knee symptoms and structure in older adults: a cohort study. Ann Rheum Dis 2016;75:1783–8. [DOI] [PubMed] [Google Scholar]
- 41.Wang X, Jin X, Han W, Cao Y, Halliday A, Blizzard L, et al. Cross-sectional and Longitudinal Associations between Knee Joint Effusion Synovitis and Knee Pain in Older Adults. J Rheumatol 2016;43:121–30. [DOI] [PubMed] [Google Scholar]
- 42.Driban JB, Lo GH, Eaton CB, Price LL, Lu B, McAlindon TE. Knee Pain and a Prior Injury Are Associated with Increased Risk of a New Knee Injury: Data from the Osteoarthritis Initiative. J Rheumatol 2015;42:1463–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kerkhof HJ, Bierma-Zeinstra SM, Arden NK, Metrustry S, Castano-Betancourt M, Hart DJ, et al. Prediction model for knee osteoarthritis incidence, including clinical, genetic and biochemical risk factors. Ann Rheum Dis 2014;73:2116–21. [DOI] [PubMed] [Google Scholar]
- 44.Sharma L, Chmiel JS, Almagor O, Dunlop D, Guermazi A, Bathon JM, et al. Significance of preradiographic magnetic resonance imaging lesions in persons at increased risk of knee osteoarthritis. Arthritis & rheumatology (Hoboken, NJ) 2014;66:1811–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roemer FW, Kwoh CK, Hannon MJ, Hunter DJ, Eckstein F, Fujii T, et al. What comes first? Multitissue involvement leading to radiographic osteoarthritis: magnetic resonance imaging-based trajectory analysis over four years in the osteoarthritis initiative. Arthritis & rheumatology (Hoboken, NJ) 2015;67:2085–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Englund M, Guermazi A, Roemer FW, Aliabadi P, Yang M, Lewis CE, et al. Meniscal tear in knees without surgery and the development of radiographic osteoarthritis among middle-aged and elderly persons: The Multicenter Osteoarthritis Study. Arthritis Rheum 2009;60:831–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sharma L, Hochberg M, Nevitt M, Guermazi A, Roemer F, Crema MD, et al. Knee tissue lesions and prediction of incident knee osteoarthritis over 7 years in a cohort of persons at higher risk. Osteoarthritis Cartilage 2017;25:1068–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roemer FW, Hayes CW, Miller CG, Hoover K, Guermazi A. Imaging atlas for eligibility and on-study safety of potential knee adverse events in anti-NGF studies (Part 1). Osteoarthritis Cartilage 2015;23 Suppl 1:S22–42. [DOI] [PubMed] [Google Scholar]
- 49.Flemming DJ, Gustas-French CN. Rapidly Progressive Osteoarthritis: a Review of the Clinical and Radiologic Presentation. Curr Rheumatol Rep 2017;19:42. [DOI] [PubMed] [Google Scholar]
- 50.Hochberg MC. Serious joint-related adverse events in randomized controlled trials of anti-nerve growth factor monoclonal antibodies. Osteoarthritis Cartilage 2015;23 Suppl 1:S18–21. [DOI] [PubMed] [Google Scholar]
- 51.Cohen J A power primer. Psychol Bull 1992;112:155–9. [DOI] [PubMed] [Google Scholar]


