Abstract
Chronic lithium treatment stimulates adult hippocampal neurogenesis, but whether increased neurogenesis contributes to its therapeutic mechanism remains unclear. We use a genetic model of neural progenitor cell (NPC) ablation to test whether a lithium-sensitive behavior requires hippocampal neurogenesis. NPC-ablated mice were treated with lithium and assessed in the forced swim test (FST). Lithium reduced time immobile in the FST in NPC-ablated and control mice but had no effect on activity in the open field, a control for the locomotion-based FST. These findings show that hippocampal NPCs that proliferate in response to chronic lithium are not necessary for the behavioral response to lithium in the FST. We further show that 4–6 week old immature hippocampal neurons are not required for this response. These data suggest that increased hippocampal neurogenesis does not contribute to the response to lithium in the forced swim test and may not be an essential component of its therapeutic mechanism.
Keywords: lithium, GSK-3, bipolar disorder, forced swim test, neurogenesis, neural progenitor cells
Introduction
Lithium is a first-line treatment for bipolar disorder, which affects 1–2% of the population [17–19, 46, 52]. Lithium treatment has a narrow therapeutic index and can inhibit several widely-expressed targets [18, 29, 38, 42, 46]. Understanding the mechanisms by which lithium causes behavior phenotypes will help distinguish therapeutic mechanisms from side effects and facilitate the development of targeted therapies.
Behaviors altered by lithium in mice include the forced swim test (FST) [30, 40], tail suspension test [21, 30], elevated zero maze [40, 41], and exploratory behavior [40, 41]. Interestingly, chronic exposure to antidepressants influence these behaviors similarly [33, 51, 58].
Lithium causes these behaviors through direct inhibition of glycogen synthase kinase-3 (GSK-3) [29, 40, 53, 57]. Lithium reduces time immobile in the FST [40], as do structurally diverse GSK-3 inhibitors [20, 25, 47, 49], genetic deletion of Gsk3a [20, 25, 47, 49], or heterozygous deletion of Gsk3b [40]. The effects of lithium or Gsk3b haploinsufficiency on the FST are reversed by overexpression of Gsk3b in the central nervous system, supporting that the effects of lithium are specifically due to inhibition of GSK-3 [41]. Lithium and antidepressants that mimic lithium in multiple behavior tests enhance inhibitory phosphorylation of GSK-3 [8, 10, 32, 45], and these phosphorylation sites are required for several lithium-responsive behaviors, further supporting the central role of GSK-3 in behavioral responses to lithium [1, 4, 50].
GSK-3 inhibition and other agents that activate Wnt signaling induce proliferation of neural progenitor cells (NPCs) [9, 13, 22, 27, 28, 44, 62, 64, 67]. Antidepressants similarly induce proliferation of hippocampal NPCs [26, 35, 54, 59] and require hippocampal neurogenesis to produce behaviors that are also sensitive to lithium [2, 26, 54]. Airan et al. [2, 26, 54] reported that neurogenesis is required for response to fluoxetine in the FST. Furthermore, antidepressants of several classes induce GSK-3 phosphorylation in cultured cells and mouse brain [4, 32, 45]. Given strong parallels between antidepressants and lithium in behavior, hippocampal neurogenesis, and signaling, we hypothesized that lithium may require hippocampal neurogenesis for behavioral response.
To test whether hippocampal neurogenesis is required for lithium-induced behavior, we inducibly ablated NPCs using an established transgenic mouse model [6, 15] and assessed whether the response to lithium was mitigated by ablation of proliferating NPCs and their progeny. The FST was selected for its large effect size, facilitating analysis of two variables-lithium and neurogenesis—and because the response to lithium in the FST is highly reproducible across genetic strains and laboratories [3, 30, 40, 41, 43, 56]. We additionally assessed these mice in the Open Field to rule out confounding changes in locomotor activity.
Materials and methods
Animals
Heterozygous glial fibrillary acidic protein-thymidine kinase (GFAP-TK) mice (Jackson Laboratory, line 005698) were bred to C57BL/6J mice (Jackson Laboratory, line 000664) to produce experimental cohorts. Males and females were used as follows: Fig. 1C: n = 12, 4 per group, 8 males, 4 females; Fig. 2: n = 38, 8–10 per group, 20 males, 18 females; Fig. 3: n = 29, 7–8 per group, 16 males, 13 females. Mice were housed 2–3 per cage in standard conditions with 12-hour light/dark cycles, with controlled temperature and humidity. All mouse protocols were approved by the University of Pennsylvania Institutional Animal Care and Use Committee.
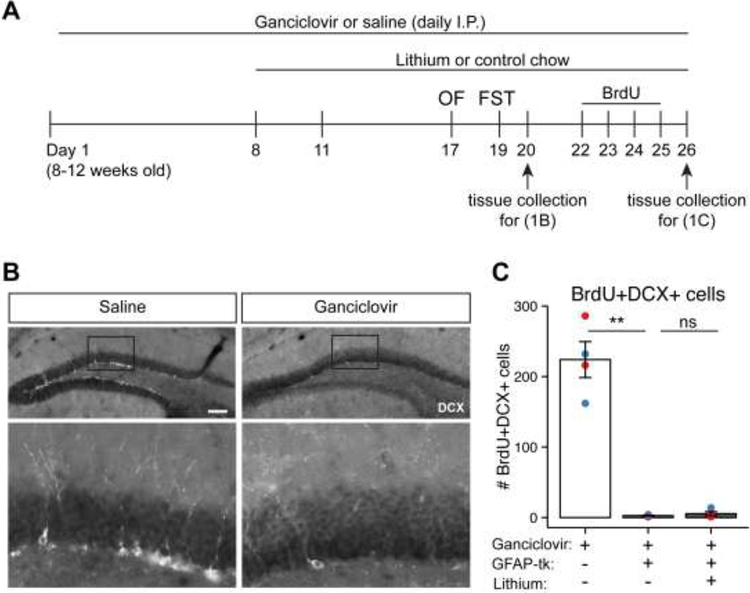
Figure 1.
Intraperitoneal ganciclovir ablates adult hippocampal neurogenesis.
A) Experiment schedule for quantification of NPC ablation.
B) Fluorescent immunohistochemistry for DCX, a marker of immature neurons that derive from GFAP-positive NPCs [15], in brains collected one day after the FST. Daily ganciclovir depletes DCX-positive NPC progeny in the dentate gyrus, indicating ablation of hippocampal neurogenesis. Scale bar: 100um.
C) Quantification of NPC ablation from mice treated with BrdU (after completing behavior testing). BrdU+ DCX+ double-positive cells in the SGZ are depleted in GFAP-TK mice compared to wild-type. Lithium treatment does not change the extent of NPC ablation. Bar graph and error bars represent mean ± SEM; n = 4 per group. Individual data points are color-coded by sex (red = male, blue = female). Significance: ** p < 0.005 by Welch’s two sample t-test. OF, Open Field; FST, Forced Swim Test; I.P., intraperitoneal; DCX, Doublecortin.
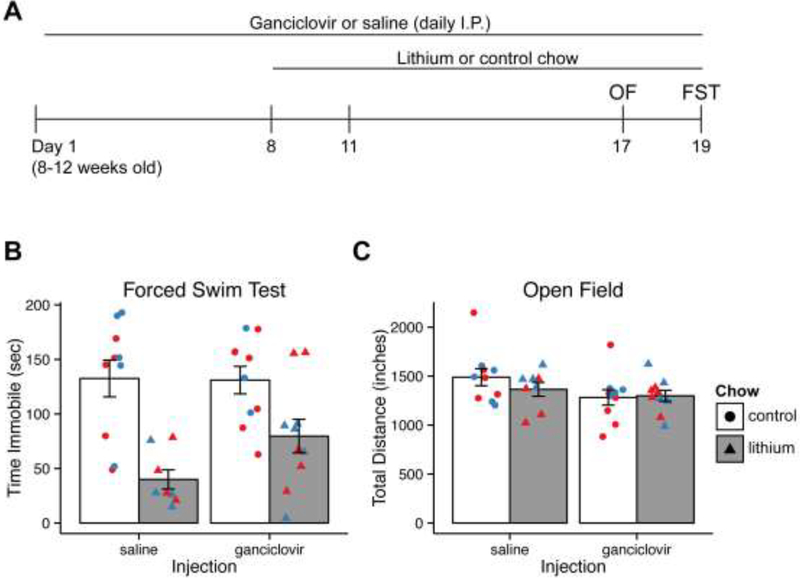
Figure 2.
Lithium response in FST is unaffected by NPC ablation.
A) Experiment schedule to ablate NPCs during lithium treatment.
B) Time immobile in the FST is reduced by lithium, and ganciclovir does not mitigate the effect of lithium.
C) Activity in the open field is unaffected by any treatment condition.
Bar graph and error bars represent mean +/− SEM; n = 8–10 per group. Individual data points are color-coded by sex (red = male, blue = female). OF, Open Field; FST, Forced Swim Test; I.P., intraperitoneal.
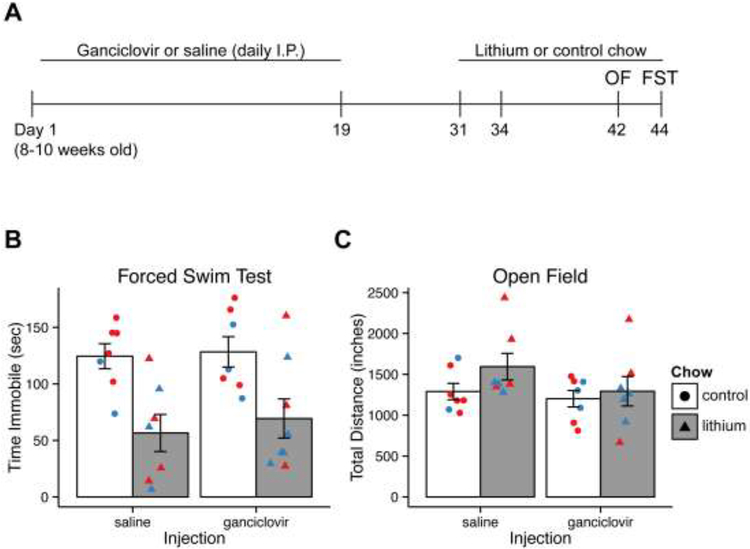
Figure 3.
Ablation of 4–6 week old immature neurons does not mitigate lithium response in the FST.
A) Experiment schedule to assess the role of 4–6 week old immature neurons.
B) Time immobile in the FST is reduced by lithium, and ganciclovir does not mitigate the effect of lithium.
C) Activity in the open field is unaffected by any treatment condition.
Bar graph and error bars represent mean +/− SEM; n = 7–8 per group. Individual data points are color-coded by sex (red = male, blue = female). OF, Open Field; FST, Forced Swim Test; I.P., intraperitoneal.
NPC ablation and lithium treatment schedule
The GFAP-TK NPC ablation model uses a transgenic Gfap promoter to drive expression of herpes simplex virus thymidine kinase (HSV-TK) [6]. HSV-TK converts ganciclovir into a cytotoxic guanosine analog that terminates DNA replication and kills proliferating cells [12, 14, 24].
To investigate the role of 0–3 week old NPCs and progeny on the FST, 8–12 week old GFAP-TK mice received 50 mg/kg ganciclovir (APP Pharmaceutical) or saline by intraperitoneal injection for 19 days. On day 8 of ganciclovir treatment, half of the mice were switched from control chow to 0.2% LiCl chow for 3 days followed by 0.4% LiCl chow (Teklad Custom Diet #5001, Envigo), (Fig. 1A and2A). Mice had ad lib access to standard drinking water and 450 mM NaCl. A separate cohort of mice was subsequently treated with intraperitoneal injection of 50 mg/kg 5-bromo-2’-deoxyuridine (BrdU) for four consecutive days and then perfused for tissue collection 24 hours after the last BrdU injection (Fig. 1A).
The role of 4–6 week old neurons on the FST was investigated using a similar experimental design. On day 31 after the start of ganciclovir treatment, half of the mice were switched from control chow to 0.2% LiCl chow for 3 days followed by 0.4% LiCl chow.
Open Field
Mice were tested in a photobeam activity system open field apparatus as described [40, 41]; distance traveled was determined with a Matlab-based (MathWorks) open source image analysis program [48].
Forced Swim Test
Mice were tested in the FST as described [40, 41] with water 25 ± 1 degree C; the last 4 minutes of 6 minute trials were manually graded for time immobile.
Tissue Collection and Immunohistochemistry
Anesthetized mice were perfused with paraformaldehyde and brains harvested and stained for Doublecortin (DCX) (Abcam ab18723 1:100) as described [36]. Double staining for BrdU (US Biologicals B2850–10G 1:1000) and DCX (Abcam ab18723 1:1000) was performed as described [37]. Double-positive (DCX+ BrdU+) cells in the subgranular zone (SGZ, 3 cell bodies or less from the hilus) were counted in 5 coronal sections per mouse sampled throughout the dentate gyrus.
Statistics
Data from both sexes were combined and analyzed together, as preliminary data with GFAP-TK mice showed no effect of sex on the response to lithium in the FST. (A two-way ANOVA on this cohort demonstrated a significant effect of lithium treatment F(1,53)=19.72, p<0.001, but no significant effect of sex F(1,53)=1.53, p=0.22 or Li treatment*sex interaction F(3,53)=1.43, p=0.24). Furthermore, prior work has demonstrated no effect of sex or estrous cycle on NPC proliferation [31] or on the FST in C57BL/6 mice [63, 66], and both sexes respond to lithium in the FST [7]. Additionally, a 3-way ANOVA comparing lithium, ablation, and sex did not reveal a main effect of sex or significant interaction in our data. Data were tested for normality and homogeneity of variance before assessment by t-test or 2-way ANOVA. Welch’s two sample t-test was performed when homogeneity of variance was not met. P-values in figure 1C were adjusted by multiplying by the number of tests performed (2). Significant p-values were defined as less than 0.05. All statistical analyses were performed using R and RStudio [60, 61]. Graphs were made using ggplot2 [65].
Results
Ablation of hippocampal neurogenesis
GFAP-TK mice express HSV-TK in NPCs in the dentate gyrus. Proliferating NPCs that express HSV-TK are ablated by treatment with ganciclovir [15, 55]. Importantly, GFAP-TK NPC ablation phenocopies hippocampal irradiation of NPCs in neurogenesis-dependent behaviors [11, 55], further validating this model to probe for neurogenesis-dependent behaviors.
We established a modified protocol for ganciclovir delivery (see Methods and Fig. 1A) that maximized ablation of hippocampal neurogenesis without inducing previously described gastrointestinal toxicity [6]. Mice appeared healthy and had normal home cage behavior. Body weights were indistinguishable between saline and ganciclovir treated groups. Thus, the ganciclovir dosing schedule was well-tolerated and resulted in robust impairment of neurogenesis. DCX-positive cells were markedly reduced after 19 days of ganciclovir (Fig. 1B), suggesting ablation of hippocampal neurogenesis at the time of the FST [5, 15]. Similarly, GFAP-TK mice that received BrdU, a synthetic thymidine analog used to detect proliferating cells, for four days after completion of behavior testing had almost complete loss of BrdU+DCX+ cells in the SGZ (2.75 ± 0.63, mean ± SEM) compared to wild-type littermates (224 ± 26, adjusted p = 0.003 by Welch’s t-test). Lithium did not prevent ganciclovir-induced reduction of double-positive cells in GFAP-TK mice (5.5 ± 3.0, adjusted p = 1 by Welch’s t-test) (Fig. 1C). The loss of BrdU+DCX+ cells indicates that proliferating NPCs were depleted.
No effect of NPC ablation on lithium response in FST
Chronic lithium treatment increases NPC proliferation [9, 23, 27, 67] and reduces time immobile in the FST [40]. We hypothesized that hippocampal NPC proliferation is necessary for lithium-induced decrease in immobility in the FST. To test this, GFAP-TK mice were treated daily with ganciclovir or saline and started on a lithium or control diet a week later while continuing ganciclovir (Fig. 2A). The lithium diet was previously established to achieve therapeutic levels of serum lithium (0.6–1.2mM) [40]. After 12 days of lithium, mice were assessed in the FST. A two way ANOVA was performed to assess ablation (ganciclovir) and lithium effects. The analysis revealed a significant effect of lithium on time immobile (F(1,34) = 23.9, p<0.001). However, there was no effect of ablation (F(1,34) = 1.6, p=0.22) or lithium × ablation interaction on time immobile (F(1,34) = 2.07, p = 0.16), indicating that NPC ablation did not affect the FST and that the effect of lithium on the FST was resilient to NPC depletion (Fig. 2B).
To assess the effects of NPC ablation and lithium treatment on their general state, mice were tested for overall activity levels in an open field arena. No differences were seen in the distance traveled between the groups [lithium: F(1,34) = 0.59, p = 0.45; ablation: F(1,34) = 3.5, p = 0.07; lithium × ablation: F(1,34) = 0.88, P = 0.36] (Fig. 2C). These results show that neither ganciclovir-mediated ablation of NPCs nor lithium treatment influence overall state or activity, supporting that time immobile in the FST is not due to hypoactivity.
Depleting 4–6 week old immature neurons does not affect lithium phenotype in FST
Immature neurons (4–6 week old neurons) have outsized effects on the hippocampal circuit due to their greater synaptic plasticity and increased excitability compared to mature granule cell neurons [16, 39, 68, 69]. Furthermore, hippocampal neurogenesis-dependent behaviors can require these 4–6 week old immature neurons rather than younger or older neurons [11]. A report of neurogenesis-dependence for response to fluoxetine in the FST did not discriminate between stages of neural differentiation [2]. We therefore hypothesized that although actively proliferating NPCs and their 0–3 week old progeny are unnecessary for the effect of lithium in the FST, this behavior phenotype could still be hippocampal neurogenesis-dependent with a requirement for 4–6 week old immature neurons.
To assess the role of 4–6 week old neurons, mice were treated with the same ganciclovir paradigm and then behavior was tested 6 weeks after starting ganciclovir (Fig. 3A). Lithium or control chow was introduced two weeks prior to behavior testing. Mice appeared healthy and had normal home cage behavior. Body weights and serum lithium concentrations were indistinguishable between saline and ganciclovir treated groups.
A two-way ANOVA to assess the effects of ablation and lithium revealed a significant effect of lithium on time immobile (F(1,25) = 17.632, p<0.001), but no effect of ablation (F(1,25) = 0.32, p = 0.58). Additionally, there was no significant interaction between ablation and lithium on time spent immobile (F(1,25) = 0.089, p = 0.778), indicating that ablation of 4–6 week old neurons did not mitigate the effect of lithium on time spent immobile in the FST (Fig. 3B).
Open field activity was measured two days before FST to assess overall activity levels. No effect on distance traveled in the open field was observed with ablation or lithium treatment [lithium: F(1,24) = 1.98, p = 0.17; ablation: F(1,24) = 1.91, p = 0.18; lithium × ablation: F(1,24) = 0.57, p = 0.46] (Fig. 3C). Once again, neither ganciclovir-mediated ablation nor lithium influenced overall state or activity.
Discussion
These experiments using GFAP-TK to ablate adult hippocampal neurogenesis reveal that hippocampal neurogenesis is not required for the behavioral response to lithium in the FST. Ablation of NPCs during the time-frame of lithium dosing or ablation of 4–6 week old immature neurons has no effect on lithium-induced reduced time immobile in the FST.
Lithium treatment of bipolar disorder patients takes weeks to stabilize mood [17, 19, 46, 52], suggesting that chronic lithium treatment requires cellular changes for its therapeutic mechanism of action. Chronic lithium induces proliferation of NPCs in rodents [9, 23, 27, 67] and evokes responses in several behaviors that are also sensitive to antidepressants, including the FST. Chronic antidepressant treatment induces hippocampal neurogenesis [34, 35], and some antidepressant-induced behaviors also require hippocampal neurogenesis, including, in some reports, the FST [2, 54]. However, our results show that ablating NPC proliferation and depleting their 0–3 week old progeny does not diminish the lithium response in the FST. Thus, NPC proliferation does not have a critical role in this behavioral response to lithium.
A requirement for hippocampal neurogenesis in the FST response to fluoxetine was demonstrated 8 weeks after ablation of neurogenesis [2, 54]. Furthermore, Denny and colleagues [11] found that 4–6 week old immature neurons but not 2 week old neurons were required for a neurogenesis-dependent behavior test. We therefore tested the hypothesis that 4–6 week old immature neurons may play a role in the response to lithium in the FST. However, depletion of 4–6 week old immature neurons did not alter the effect of lithium on time immobile in the FST. Therefore, immature hippocampal granule cell neurons do not play a measurable role in the response to lithium in the FST.
The FST has a large effect size that allows for statistical power while probing two variables-cellular ablation and lithium treatment. Furthermore, the effect of lithium on time immobile in the FST is highly reproducible among different labs and mouse genetic backgrounds [3, 30, 40, 41, 43, 56]. These strengths of the FST make it an optimal behavioral assay when using mouse genetic models to assess the importance of neuronal populations to lithium-influenced behavior. While behaviors in rodents may be difficult to extrapolate directly to human psychiatric disorders, establishing the mechanism of lithium action on neuronal function and behavior requires a robust behavior assay.
Conclusions
This study demonstrates that adult hippocampal neurogenesis is not necessary for lithium effects on the FST, the most reproducible lithium-sensitive behavior in rodents. The lithium response in the FST is unaffected by ablation of proliferating NPCs and their 0–3 week old progeny or 4–6 week old immature neurons. We conclude that lithium-induced NPC proliferation is not essential for the effect of lithium on this behavior. The cellular mediators of behavioral responses to lithium remain to be determined by future studies.
Highlights.
The effect of lithium on mouse behavior in the forced swim test does not require hippocampal neurogenesis.
Hippocampal neurogenesis is ablated in GFAP-TK mice treated with intraperitoneal ganciclovir.
Ablation of hippocampal neurogenesis does not disrupt overall activity in the open field.
Acknowledgements
The authors thank David Gootenberg for assistance with R programming, and Ivana Horceny for technical assistance. Assistance with behavior procedures was provided by the Neurobehavior Testing Core at UPenn/ITMAT and IDDRC CHOP/Penn (U54 HD086984). This work was funded by T32MH14654–40 and F32MH113334 (MES), R01MH100923 (PSK), and NIH DA023555 and NASA NNX15AE09G/80NSSC17K0060 (AJE). The authors declare no conflicts of interest.
Abbreviations:
- BrdU
5-bromo-2’-deoxyuridine
- DCX
Doublecortin
- FST
forced swim test
- GFAP-TK
glial fibrillary acidic protein-thymidine kinase
- GSK-3
glycogen synthase kinase-3
- HSV-TK
herpes simplex virus thymidine kinase
- NPC
neural progenitor cell
- SGZ
subgranular zone
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of interest: none
Contributor Information
W. Timothy O’Brien, Email: obrienw@pennmedicine.upenn.edu.
Peter S. Klein, Email: pklein@pennmedicine.upenn.edu.
References
- [1].Ackermann TF, Kempe DS, Lang F, Lang UE, Hyperactivity and enhanced curiosity of mice expressing PKB/SGK-resistant glycogen synthase kinase-3 (GSK-3), Cell Physiol Biochem 25 (2010) 775–786. [DOI] [PubMed] [Google Scholar]
- [2].Airan RD, Meltzer LA, Roy M, Gong Y, Chen H, Deisseroth K, High-speed imaging reveals neurophysiological links to behavior in an animal model of depression, Science 317 (2007) 819–823. [DOI] [PubMed] [Google Scholar]
- [3].Bersudsky Y, Shaldubina A, Belmaker RH, Lithium’s effect in forced-swim test is blood level dependent but not dependent on weight loss, Behav Pharmacol 18 (2007) 77–80. [DOI] [PubMed] [Google Scholar]
- [4].Beurel E, Song L, Jope RS, Inhibition of glycogen synthase kinase-3 is necessary for the rapid antidepressant effect of ketamine in mice, Mol Psychiatry 16 (2011) 1068–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Brown JP, Couillard-Després S, Cooper-Kuhn CM, Winkler J, Aigner L, Kuhn HG, Transient expression of doublecortin during adult neurogenesis, J Comp Neurol 467 (2003) 1–10. [DOI] [PubMed] [Google Scholar]
- [6].Bush TG, Savidge TC, Freeman TC, Cox HJ, Campbell EA, Mucke L, Johnson MH, Sofroniew MV, Fulminant jejuno-ileitis following ablation of enteric glia in adult transgenic mice, Cell 93 (1998) 189–201. [DOI] [PubMed] [Google Scholar]
- [7].Can A, Piantadosi SC, Gould TD, Differential antidepressant-like response to lithium treatment between mouse strains: effects of sex, maternal care, and mixed genetic background, Psychopharmacology (Berl) 228 (2013) 411–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Chalecka-Franaszek E, Chuang DM, Lithium activates the serine/threonine kinase Akt-1 and suppresses glutamate-induced inhibition of Akt-1 activity in neurons, Proc Natl Acad Sci U S A 96 (1999) 8745–8750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Chen G, Rajkowska G, Du F, Seraji-Bozorgzad N, Manji HK, Enhancement of hippocampal neurogenesis by lithium, J Neurochem 75 (2000) 1729–1734. [DOI] [PubMed] [Google Scholar]
- [10].De Sarno P, Li X, Jope RS, Regulation of Akt and glycogen synthase kinase-3 beta phosphorylation by sodium valproate and lithium, Neuropharmacology 43 (2002) 1158–1164. [DOI] [PubMed] [Google Scholar]
- [11].Denny CA, Burghardt NS, Schachter DM, Hen R, Drew MR, 4- to 6-week-old adult-born hippocampal neurons influence novelty-evoked exploration and contextual fear conditioning, Hippocampus 22 (2012) 1188–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Elion GB, Furman PA, Fyfe JA, de Miranda P, Beauchamp L, Schaeffer HJ, Selectivity of action of an antiherpetic agent, 9-(2-hydroxyethoxymethyl) guanine, Proc Natl Acad Sci U S A 74 (1977) 5716–5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Fiorentini A, Rosi MC, Grossi C, Luccarini I, Casamenti F, Lithium improves hippocampal neurogenesis, neuropathology and cognitive functions in APP mutant mice, PLoS One 5 (2010) e14382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Furman PA, McGuirt PV, Keller PM, Fyfe JA, Elion GB, Inhibition by acyclovir of cell growth and DNA synthesis of cells biochemically transformed with herpesvirus genetic information, Virology 102 (1980) 420–430. [DOI] [PubMed] [Google Scholar]
- [15].Garcia ADR, Doan NB, Imura T, Bush TG, Sofroniew MV, GFAP-expressing progenitors are the principal source of constitutive neurogenesis in adult mouse forebrain, Nat Neurosci 7 (2004) 1233–1241. [DOI] [PubMed] [Google Scholar]
- [16].Ge S, Yang C-H, Hsu K-S, Ming G-L, Song H, A critical period for enhanced synaptic plasticity in newly generated neurons of the adult brain, Neuron 54 (2007) 559–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Geddes JR, Miklowitz DJ, Treatment of bipolar disorder, Lancet 381 (2013) 1672–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Gitlin M, Lithium side effects and toxicity: prevalence and management strategies, Int J Bipolar Disord 4 (2016) 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Goodwin FK, & Jamison KR, Manic-Depressive Illness, Oxford University Press, New York, NY, US, 1990. [Google Scholar]
- [20].Gould TD, Einat H, Bhat R, Manji HK, AR-A014418, a selective GSK-3 inhibitor, produces antidepressant-like effects in the forced swim test, Int J Neuropsychopharmacol 7 (2004) 387–390. [DOI] [PubMed] [Google Scholar]
- [21].Gould TD, O’Donnell KC, Dow ER, Du J, Chen G, Manji HK, Involvement of AMPA receptors in the antidepressant-like effects of lithium in the mouse tail suspension test and forced swim test, Neuropharmacology 54 (2008) 577–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hanson ND, Nemeroff CB, Owens MJ, Lithium, but not fluoxetine or the corticotropin-releasing factor receptor 1 receptor antagonist R121919, increases cell proliferation in the adult dentate gyrus, J Pharmacol Exp Ther 337 (2011) 180–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Hashimoto R, Senatorov V, Kanai H, Leeds P, Chuang D-M, Lithium stimulates progenitor proliferation in cultured brain neurons, Neuroscience 117 (2003) 55–61. [DOI] [PubMed] [Google Scholar]
- [24].Heyman RA, Borrelli E, Lesley J, Anderson D, Richman DD, Baird SM, Hyman R, Evans RM, Thymidine kinase obliteration: creation of transgenic mice with controlled immune deficiency, Proc Natl Acad Sci U S A 86 (1989) 2698–2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kaidanovich-Beilin O, Lipina TV, Takao K, van Eede M, Hattori S, Laliberté C, Khan M, Okamoto K, Chambers JW, Fletcher PJ, MacAulay K, Doble BW, Henkelman M, Miyakawa T, Roder J, Woodgett JR, Abnormalities in brain structure and behavior in GSK-3alpha mutant mice, Mol Brain 2 (2009) 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kang E, Wen Z, Song H, Christian KM, Ming G-L, Adult Neurogenesis and Psychiatric Disorders, Cold Spring Harb Perspect Biol 8 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kara N, Narayanan S, Belmaker RH, Einat H, Vaidya VA, Agam G, Chronic Lithium Treatment Enhances the Number of Quiescent Neural Progenitors but Not the Number of DCX-Positive Immature Neurons, Int J Neuropsychopharmacol 18 (2015) pyv003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Kitamura Y, Doi M, Kuwatsuka K, Onoue Y, Miyazaki I, Shinomiya K, Koyama T, Sendo T, Kawasaki H, Asanuma M, Gomita Y, Chronic treatment with imipramine and lithium increases cell proliferation in the hippocampus in adrenocorticotropic hormone-treated rats, Biol Pharm Bull 34 (2011) 77–81. [DOI] [PubMed] [Google Scholar]
- [29].Klein PS, Melton DA, A molecular mechanism for the effect of lithium on development, Proc Natl Acad Sci U S A 93 (1996) 8455–8459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Koche RP, Smith ZD, Adli M, Gu H, Ku M, Gnirke A, Bernstein BE, Meissner A, Reprogramming factor expression initiates widespread targeted chromatin remodeling, Cell Stem Cell 8 (2011) 96–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Lagace DC, Fischer SJ, Eisch AJ, Gender and endogenous levels of estradiol do not influence adult hippocampal neurogenesis in mice, Hippocampus 17 (2007) 175–180. [DOI] [PubMed] [Google Scholar]
- [32].Li X, Zhu W, Roh M-S, Friedman AB, Rosborough K, Jope RS, In vivo regulation of glycogen synthase kinase-3beta (GSK3beta) by serotonergic activity in mouse brain, Neuropsychopharmacology 29 (2004) 1426–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Lucki I, Dalvi A, Mayorga AJ, Sensitivity to the effects of pharmacologically selective antidepressants in different strains of mice, Psychopharmacology (Berl) 155 (2001) 315–322. [DOI] [PubMed] [Google Scholar]
- [34].Malberg JE, Implications of adult hippocampal neurogenesis in antidepressant action, J Psychiatry Neurosci 29 (2004) 196–205. [PMC free article] [PubMed] [Google Scholar]
- [35].Malberg JE, Eisch AJ, Nestler EJ, Duman RS, Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus, J Neurosci 20 (2000) 9104–9110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Mandyam CD, Harburg GC, Eisch AJ, Determination of key aspects of precursor cell proliferation, cell cycle length and kinetics in the adult mouse subgranular zone, Neuroscience 146 (2007) 108–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Mazumdar J, O’Brien WT, Johnson RS, LaManna JC, Chavez JC, Klein PS, Simon MC, O2 regulates stem cells through Wnt/β-catenin signalling, Nat Cell Biol 12 (2010) 1007–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].McKnight RF, Adida M, Budge K, Stockton S, Goodwin GM, Geddes JR, Lithium toxicity profile: a systematic review and meta-analysis, Lancet 379 (2012) 721–728. [DOI] [PubMed] [Google Scholar]
- [39].Ming G-L, Song H, Adult neurogenesis in the mammalian brain: significant answers and significant questions, Neuron 70 (2011) 687–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].O’Brien WT, Harper AD, Jové F, Woodgett JR, Maretto S, Piccolo S, Klein PS, Glycogen synthase kinase-3beta haploinsufficiency mimics the behavioral and molecular effects of lithium, J Neurosci 24 (2004) 6791–6798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].O’Brien WT, Huang J, Buccafusca R, Garskof J, Valvezan AJ, Berry GT, Klein PS, Glycogen synthase kinase-3 is essential for β-arrestin-2 complex formation and lithium-sensitive behaviors in mice, J Clin Invest 121 (2011) 3756–3762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].O’Brien WT, Klein PS, Validating GSK3 as an in vivo target of lithium action, Biochem Soc Trans 37 (2009) 1133–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].O’Donnell KC, Gould TD, The behavioral actions of lithium in rodent models: leads to develop novel therapeutics, Neurosci Biobehav Rev 31 (2007) 932–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].O’Leary OF, O’Connor RM, Cryan JF, Lithium-induced effects on adult hippocampal neurogenesis are topographically segregated along the dorso-ventral axis of stressed mice, Neuropharmacology 62 (2012) 247–255. [DOI] [PubMed] [Google Scholar]
- [45].Okamoto H, Voleti B, Banasr M, Sarhan M, Duric V, Girgenti MJ, Dileone RJ, Newton SS, Duman RS, Wnt2 expression and signaling is increased by different classes of antidepressant treatments, Biol Psychiatry 68 (2010) 521–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Oruch R, Elderbi MA, Khattab HA, Pryme IF, Lund A, Lithium: a review of pharmacology, clinical uses, and toxicity, Eur J Pharmacol 740 (2014) 464–473. [DOI] [PubMed] [Google Scholar]
- [47].Pan JQ, Lewis MC, Ketterman JK, Clore EL, Riley M, Richards KR, Berry-Scott E, Liu X, Wagner FF, Holson EB, Neve RL, Biechele TL, Moon RT, Scolnick EM, Petryshen TL, Haggarty SJ, AKT kinase activity is required for lithium to modulate mood-related behaviors in mice, Neuropsychopharmacology 36 (2011) 1397–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Patel TP, Gullotti DM, Hernandez P, O’Brien WT, Capehart BP, Morrison, Barclay r., Bass C, Eberwine JE, Abel T, Meaney DF, An open-source toolbox for automated phenotyping of mice in behavioral tasks, Front Behav Neurosci 8 (2014) 349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Pérez-Domper P, Palomo V, Gradari S, Gil C, de Ceballos ML, Martínez A, Trejo JL, The GSK-3-inhibitor VP2.51 produces antidepressant effects associated with adult hippocampal neurogenesis, Neuropharmacology (2016). [DOI] [PubMed] [Google Scholar]
- [50].Polter A, Beurel E, Yang S, Garner R, Song L, Miller CA, Sweatt JD, McMahon L, Bartolucci AA, Li X, Jope RS, Deficiency in the inhibitory serine-phosphorylation of glycogen synthase kinase-3 increases sensitivity to mood disturbances, Neuropsychopharmacology 35 (2010) 1761–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Porsolt RD, Bertin A, Jalfre M, Behavioral despair in mice: a primary screening test for antidepressants, Arch Int Pharmacodyn Ther 229 (1977) 327–336. [PubMed] [Google Scholar]
- [52].Price LH, Heninger GR, Lithium in the treatment of mood disorders, N Engl J Med 331 (1994) 591–598. [DOI] [PubMed] [Google Scholar]
- [53].Ryves WJ, Harwood AJ, Lithium inhibits glycogen synthase kinase-3 by competition for magnesium, Biochem Biophys Res Commun 280 (2001) 720–725. [DOI] [PubMed] [Google Scholar]
- [54].Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, Belzung C, Hen R, Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants, Science 301 (2003) 805–809. [DOI] [PubMed] [Google Scholar]
- [55].Saxe MD, Battaglia F, Wang J-W, Malleret G, David DJ, Monckton JE, Garcia ADR, Sofroniew MV, Kandel ER, Santarelli L, Hen R, Drew MR, Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus, Proc Natl Acad Sci U S A 103 (2006) 17501–17506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Shaldubina A, Johanson RA, O’Brien WT, Buccafusca R, Agam G, Belmaker RH, Klein PS, Bersudsky Y, Berry GT, SMIT1 haploinsufficiency causes brain inositol deficiency without affecting lithium-sensitive behavior, Mol Genet Metab 88 (2006) 384–388. [DOI] [PubMed] [Google Scholar]
- [57].Stambolic V, Ruel L, Woodgett JR, Lithium inhibits glycogen synthase kinase-3 activity and mimics wingless signalling in intact cells, Curr Biol 6 (1996) 1664–1668. [DOI] [PubMed] [Google Scholar]
- [58].Steru L, Chermat R, Thierry B, Simon P, The tail suspension test: a new method for screening antidepressants in mice, Psychopharmacology (Berl) 85 (1985) 367–370. [DOI] [PubMed] [Google Scholar]
- [59].Surget A, Saxe M, Leman S, Ibarguen-Vargas Y, Chalon S, Griebel G, Hen R, Belzung C, Drug-dependent requirement of hippocampal neurogenesis in a model of depression and of antidepressant reversal, Biol Psychiatry 64 (2008) 293–301. [DOI] [PubMed] [Google Scholar]
- [60].Team R, RStudio: Integrated Development Environment for R Boston, MA, 2016. [Google Scholar]
- [61].Team RC, R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria, 2015. [Google Scholar]
- [62].Valvezan AJ, Klein PS, GSK-3 and Wnt Signaling in Neurogenesis and Bipolar Disorder, Front Mol Neurosci 5 (2012) 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Voikar V, Koks S, Vasar E, Rauvala H, Strain and gender differences in the behavior of mouse lines commonly used in transgenic studies, Physiol Behav 72 (2001) 271–281. [DOI] [PubMed] [Google Scholar]
- [64].Wexler EM, Geschwind DH, Palmer TD, Lithium regulates adult hippocampal progenitor development through canonical Wnt pathway activation, Mol Psychiatry 13 (2008) 285–292. [DOI] [PubMed] [Google Scholar]
- [65].Wickham H, ggplot2: Elegant Graphics for Data Analysis, Springer-Verlag; New York, 2009. [Google Scholar]
- [66].Xin F, Fischer E, Krapp C, Krizman EN, Lan Y, Mesaros C, Snyder NW, Bansal A, Robinson MB, Simmons RA, Bartolomei MS, Mice exposed to bisphenol A exhibit depressive-like behavior with neurotransmitter and neuroactive steroid dysfunction, Horm Behav 102 (2018) 93–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Zanni G, Michno W, Di Martino E, Tjärnlund-Wolf A, Pettersson J, Mason CE, Hellspong G, Blomgren K, Hanrieder J, Lithium Accumulates in Neurogenic Brain Regions as Revealed by High Resolution Ion Imaging, Sci Rep 7 (2017) 40726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Zhao C, Deng W, Gage FH, Mechanisms and functional implications of adult neurogenesis, Cell 132 (2008) 645–660. [DOI] [PubMed] [Google Scholar]
- [69].Zhao C, Teng EM, Summers RG Jr., Ming GL, Gage FH, Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus, J Neurosci 26 (2006) 3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]