Abstract
Catalytic chemistry that involves the activation and transformation of main group substrates is relatively undeveloped and current examples are generally mediated by expensive transition metal species. Herein, we describe the use of inexpensive and readily available tBuOK as a catalyst for P–P and P–E (E = O, S, or N) bond formation. Catalytic quantities of tBuOK in the presence of imine, azobenzene hydrogen acceptors, or a stoichiometric amount of tBuOK with hydrazobenzene, allow efficient homodehydrocoupling of phosphines under mild conditions (e.g. 25 °C and < 5 min). Further studies demonstrate that the hydrogen acceptors play an intimate mechanistic role. We also show that our tBuOK catalysed methodology is general for the heterodehydrocoupling of phosphines with alcohols, thiols and amines to generate a range of potentially useful products containing P–O, P–S, or P–N bonds.
Subject terms: Homogeneous catalysis, Synthetic chemistry methodology, Inorganic chemistry
Catalytic transformations involving main group compounds is much less developed than carbon chemistry. Here, the authors report the use of catalytic or stoichiometric tBuOK in presence of a hydrogen acceptor for efficient P–P and P–heteroatom coupling reactions.
Introduction
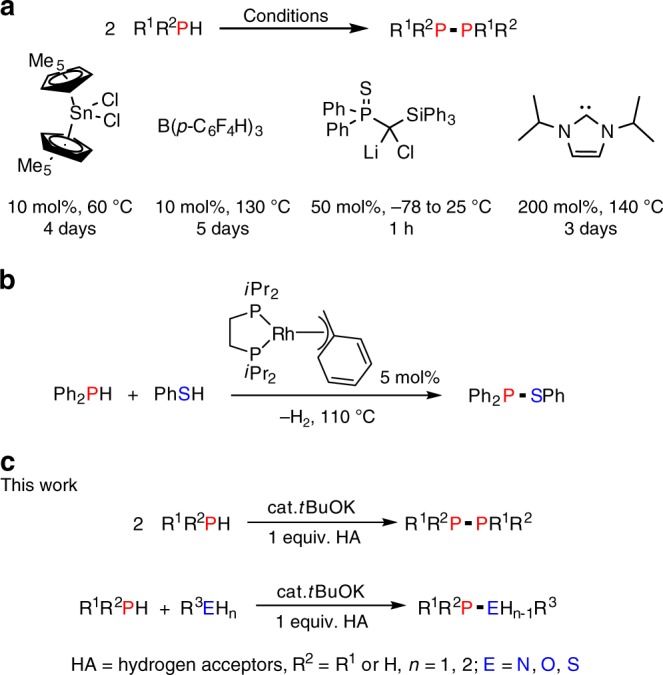
When compared to the traditional stoichiometric salt metathesis and reductive coupling reactions that still dominate the formation of element–element bonds in main group chemistry, catalytic methods represent a highly attractive alternative synthetic approach1–7. For example, substantial advances have been made in catalytic dehydrocoupling of p-block substrates to form both homonuclear (E–E) or heteronuclear (E–E′) bonds (E, E′ = p-block element). However, most of the current catalysts in use are based on precious transition metals such as Rh8–11, Ir12–15 and Ru16 raising concerns about their high price as a result of their low natural abundance and their presence as potentially toxic residues in polymer products17–19. Although significant progress has been made in terms of the use of earth abundant metals such as Zr20–22, Fe23,24 and Ni25,26, the development of transition metal-free dehydrocoupling catalysts also offers considerable potential. For example, recent reports describe advances concerning the use of main group species such as [(Dipp-nacnac)MgnBu]227, Al(NMe2)328 and B(C6F5)329, especially in the areas of N–B and P–Si bond formation. Rare examples of the use of alkali metal reagents such as KN(SiMe3)230, 1-lithium-2-tert-butyl-1,2-dihydropyridine31, or group 1 salts containing a carbazolido NNN pincer ligand32 for the dehydrocoupling of Me2NH•BH3 to form [Me2NBH2]2 have also been described.
Molecular compounds containing P–P bonds have numerous applications in coordination7,33,34 and synthetic chemistry29,35–37, and diphosphines readily react with alkenes or alkynes to form bidentate ligands38–40. Probably because of the potential catalyst deactivation due to unproductive phosphine coordination, only few transition metal catalysts (Zr, Rh) have been successfully applied for P–P bond formation via catalytic dehydrocoupling3,7. Furthermore, in most cases, relatively high temperatures (110–140 °C) and long reaction times (3–4 days) were required2,41–44. Recently, transition metal-free catalysts (C5Me5)2SnCl245, B(p-C6F4H)346 and stoichiometric reagents, namely lithium chloride carbenoids47 and N-heterocyclic carbenes48, have also been reported to mediate phosphine dehydrocoupling although either harsh reaction conditions, long reaction times, or non-commercially available reagents were still required (Fig. 1a).
Fig. 1.
Developed catalysts for P–P and P–E bond formation. a Main group catalysts and conditions for the homodehydrocoupling of phosphines. b Heterodehydrocoupling of diphenylphosphine with thiophenol by in situ generated rhodium phosphido complexes. c Our current work using tBuOK as the catalyst for the homo- and heterodehydrocoupling of phosphines
In addition to species containing P–P bonds, compounds containing P–O, P–S and P–N bonds also have widespread importance in catalysis, organic synthesis, and in biochemistry and agrochemistry49,50. Apart from an example of heterodehydrocoupling of phosphines with protic substrates (PhS-H) to form P–S bonds mediated by in situ generated rhodium phosphido complexes at 110 °C (Fig. 1b)2, catalytic heterodehydrocoupling of phosphines with other protic substrates is unexplored. In particular, the development of a cost-effective, general, and practical transition metal-free catalytic homodehydrocoupling and heterodehydrocoupling of phosphines is highly desirable. Herein, we report our findings on the use of inexpensive and commercially available reagent tBuOK as a general catalyst for dehydrocoupling reactions involving phosphines and the formation of P–P, P–O, P–N and P–S bonds (Fig. 1c).
Results
Dehydrocoupling of phosphines
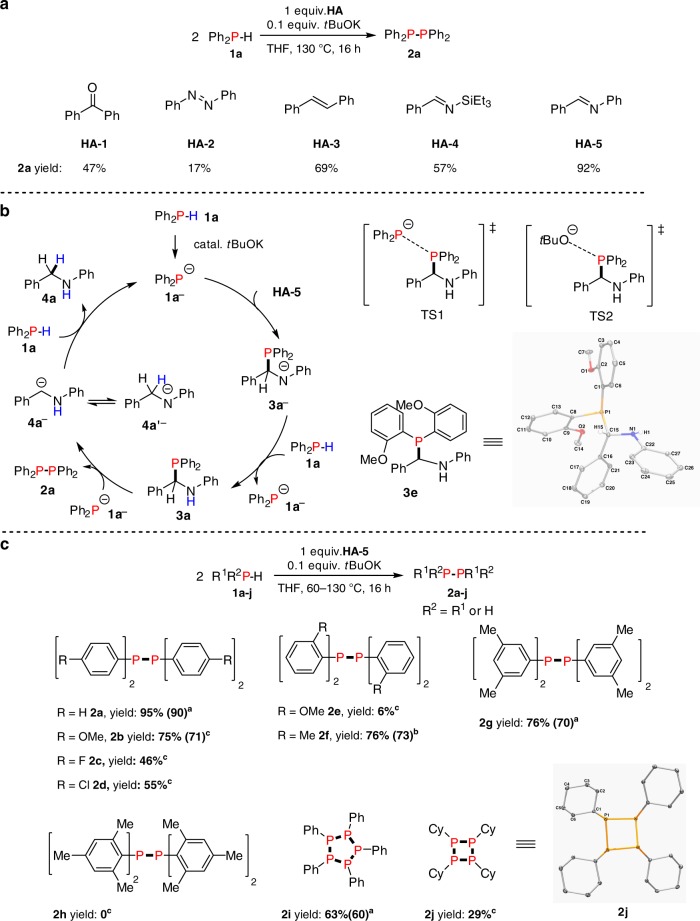
Phosphorus and hydrogen possess similar Pauling electronegativities leading to only a weak polarisation for P–H bonds. As strong Lewis acids such as B(p-C6F4H)3 have been shown to dehydrocouple phosphines (R1R2PH, R2 = R1 or H) via a mechanism that involves sequential hydride and proton removal from phosphorus46, we rationalised that a strong base might be able to mediate the same process. Based on the recently revealed, highly versatile role of tBuOK in initiating C–C51,52, C–Si,53 Si–O54 and C–N bond formation,55 we studied the use of tBuOK as a catalyst for the homodehydrocoupling of phosphines. Initially, we chose diphenylphosphine (1a) as a model substrate and the reaction was attempted with 0.1 mmol of 1a with 10 mol% tBuOK in 0.5 mL THF at 130 °C. After 16 h no reaction had taken place. Given the recent examples of the introduction of a hydrogen acceptor (HA) for the promotion of dehydrocoupling reactions29,46,56,57, we then explored the use of different types of HA for this reaction with dramatically different results (Fig. 2a and Supplementary Table 1). HA-1 to HA-5 were most effective: for example, benzophenone (HA-1) led to 86% conversion of 1a and the formation of 2a in 47% yield whereas trans-stilbene (HA-3) gave full conversion of 1a accompanied by the production of 2a in 69% yield. In the latter case residual 1a was found to be converted to the hydrophosphination product. Most impressively, with the addition of N-benzylideneaniline (HA-5) we achieved >99% conversion of 1a with the formation of 2a in 92% yield. Computationally, the parent dehydrocoupling reaction of 2 equiv. 1a to 2a and H2 in the absence of an HA was thermodynamically uphill by 2.64 kcal mol−1, and likely possesses a significant kinetic barrier. The dehydrocoupling reactions with added HA-1 to HA-5 were all calculated to be thermodynamically exergonic (see Supplementary Discussion and Supplementary Data 1, 2 in the Supplementary Information).
Fig. 2.
tBuOK-catalysed dehydrocoupling of phosphines in the presence of HA. a The effect of selected HA in the homodehydrocoupling of Ph2PH. b Proposed reaction mechanism via 3a as key intermediate and SN2-type reaction to produce 2a, K+ ions are removed from the catalytic cycles for clarity. Proposed transition state structures TS1, and TS2, for attack of 3a by incoming 1a− or tBuO−. X-ray crystal structure of hydrophosphination product 3e with non-hydrogen atoms shown as 30% probability ellipsoids. c, Reaction generality using different phosphines: reactions were performed with 0.1 mmol phosphines, 0.01 mmol tBuOK, 0.1 mmol HA-5, 0.5 mL THF in a J. Young NMR tube and yields were based on the phosphine as the limiting reagent and determined by 31P{1H} NMR spectroscopy using a capillary of PCl3 as a calibration standard, the numbers in brackets were isolated yields of products. a60 °C, b100 °C, c130 °C. X-ray crystal structure of 2j with non-hydrogen atoms shown as 30% probability ellipsoids
With HA-5, efforts to perform the reaction under milder conditions were made using different bases, temperatures, and catalyst loadings (Supplementary Tables 2–5). Notably, with 0.01 mmol of tBuOK (10 mol%) and 0.1 mmol of HA-5, 82% yield of 2a was obtained after 64 h at 25 °C (Supplementary Table 2, entry 4). Even with 2.5 mol% tBuOK at 60 °C, we could still achieve 85% yield after 24 h (Supplementary Table 2, entry 6). It is worth noting that 10 mol% of different alkali metal tert-butoxides were screened as catalysts for the dehydrocoupling reaction with added HA-5 where the activity followed the order of K>Na>Li (Supplementary Table 4). We then chose 60 °C and 10 mol% tBuOK as the reaction conditions (which gave a 95% yield of 2a after 8 h, Supplementary Table 4) for further mechanistic studies. It is known that P–H bonds can be activated by strong bases to initiate the subsequent hydrophosphination of the C=N or C=C double bond58–60. We explored the potential role of the hydrophosphination product from 1a and HA-5 and carried out several control experiments; the results are summarised in Supplementary Fig. 1. Heating 1a or a mixture of 1a and HA-5 at 60 °C in THF gave no reaction after 8 h (Supplementary Fig. 1, Eqs. 1–2). When 10 mol% of tBuOK was added to the THF solution containing 1a and HA-5, the rapid emergence of a new peak at 3.3 ppm in the 31P{1H} nuclear magnetic resonance (NMR) spectrum was observed (Supplementary Fig. 2). This was assigned to the hydrophosphination adduct 3a upon synthesis and subsequent characterisation of a sample of this species (Supplementary Fig. 1, Eq. 3). After 8 h compound 3a was quantitatively converted to a mixture of the homodehydrocoupling product 2a, and N-benzylaniline 4a, and HA-5 in a 1:1:1 molar ratio (Supplementary Fig. 1, Eq. 5 and Supplementary Figs. 3–5). Monitoring the transformation of 3a to 2a at 30 min intervals showed that no other P-containing species were involved, except for minor amounts of 5a by-product which were also observed (Supplementary Fig. 6). It is worth noting that 3a is in equilibrium with HA-5 and 1a (Supplementary Fig. 1, Eq. 4), and that the transformation of 3a to the 1:1:1 mixture of 2a, 4a, and HA-5 requires the presence of the tBuOK catalyst (Supplementary Fig. 1, Eq. 5)61. Our experiments suggest that 3a is the key intermediate for the homodehydrocoupling of 1a.
Based on the results of the stepwise experiments we proposed the reaction mechanism outlined in Fig. 2b. Firstly, 1a is deprotonated by tBuOK to form diphenylphosphide 1a−. This activation facilitates the addition of 1a to HA-5 to form the hydrophosphination adduct 3a. Based on the knowledge that SN2 reactions at phosphorus are possible62–64 and similar halophilic reactions exist, we anticipated that hydrophosphination adduct 3a is attacked by anionic 1a− at the phosphorus centre through a SN2-type of reaction (TS1) which eventually yields 2a. Similarly, 3a could be attacked by the tBuO− anion (TS2) which leads to the side product Ph2P-OtBu 5a detected by 31P{1H} NMR as a peak at 86.9 ppm (Supplementary Fig. 6). Here we propose a carbanionic leaving group (4a−) which may tautomerise to the amide anion (4a′−), which can also deprotonate 1a and regenerate 1a− and close the catalytic cycle. Similar transient carbanionic species are also proposed in Brook rearrangements of silylated amines in the presence of a catalytic amount of base65,66, and carbanionic leaving groups are also known in reactions of phosphine oxides with organometallic reagents67. Another possibility is following the nucleophilic attack of the phosphorus of 3a by an anion such as 1a− or tBuO− there may be a process in which the P–C bond cleavage process is accompanied by protonation by an incoming protic substrate at the incipiently generated and partially carbanionic site.
When HA-5 was substituted at the para-position of the benzylidene phenyl group with an electron-withdrawing group (–COOMe) the initial reaction rate was substantially increased, on the other hand with an electron-donating (–Me) group the initial reaction rate was much slower (Supplementary Fig. 7). These kinetic observations provide further support for the proposed reaction mechanism described in Fig. 2b as an electron-withdrawing group should stabilise the anionic leaving group 4a−.
The substrate generality for the reaction was then studied (Fig. 2c). With diphenyl phosphine (1a) as substrate, a 95% yield of the dehydrocoupling product 2a could be obtained. In the case of para-methoxy-substituted secondary phosphines, a 75% yield of 2b was gained. For chloro- and fluoro-substituted phosphines, more moderate yields were observed for 2c and 2d, perhaps due to the side reactions involving C–F and C–Cl bonds such as dehalogenation68,69. When bis(ortho-methoxyphenyl) phosphine was used we can only obtain 6% of the homodehydrocoupling product 2e due to the existence of a large quantity of intermediate 3e, which was crystallographically characterised (Fig. 2b, Supplementary Table 7), which suggests that the sterics of the substituents at phosphorus play a key role and is consistent with our proposed mechanism involving both P-based nucleophiles and electrophiles. Thus, for example, with less bulky meta-methylphenyl substituents on phosphorus a 76% yield of 2g was observed. On the other hand, the presence of a bulkier mesityl substituent (as in 2h) totally suppressed the hydrophosphination and the subsequent dehydrocoupling reaction. We also extended our protocol to primary phosphines and found that phenylphosphine was selectively transformed into 5-membered cyclic ring [PhP]5 2i in 63% yield. Interestingly, when the aliphatic primary phosphine cyclohexylphosphine was used, cyclic 2j, which contains a 4-membered ring, was produced in 29% yield and this species was characterised crystallographically (Fig. 2c, Supplementary Table 7).
Based on our proposed mechanism in Fig. 2b, the high conversion of 1a but lower yield of diphosphine 2a for the cases of HA-3 and HA-4 can be explained by the presence of unconverted hydrophosphination intermediate (Supplementary Figs. 10, 11). However, in the case of azobenzene (HA-2), where the yield of 2a is particularly low (17%), no hydrophosphination product was detected at 130 °C in THF over 16 h. Instead, in addition to the peak for 2a, two further peaks were present in the 31P{1H} NMR spectrum and these were assigned to Ph2P-OtBu (5a, 86.9 ppm) and Ph2P-NHPh (6a, 26.9 ppm) (Supplementary Fig. 9). We then explored whether the reaction using HA-2 could be further optimised (Supplementary Table 6). Remarkably, on lowering the reaction temperature from 130 to 25 °C while using 10 mol% of tBuOK resulted in the formation of 2a in 75% yield within 5 min (Supplementary Table 6, entry 1).
Low-temperature 31P NMR spectroscopy was performed in THF to provide some initial insight into the reaction (Supplementary Fig. 13). Gradually increasing the temperature from −60 °C to room temperature clearly showed the decay of 1a and growth of the diphosphine 2a. At 25 °C, two additional peaks at 60.8 ppm (hydrophosphination adduct Ph2P-N(Ph)-NHPh, 7a)70 and 86.9 ppm (Ph2P-OtBu, 5a) appeared. Further experiments showed that when the stoichiometry of the reaction was changed and a mixture composed of 2 equiv. of 1a and 1 equiv. of HA-2 was treated using 10 mol% of tBuOK resulted in complete conversion to a 1:1 mixture of 2a and hydrazobenzene within 5 min at 25 °C (Fig. 3a, Supplementary Figs. 14 and 15).
Fig. 3.
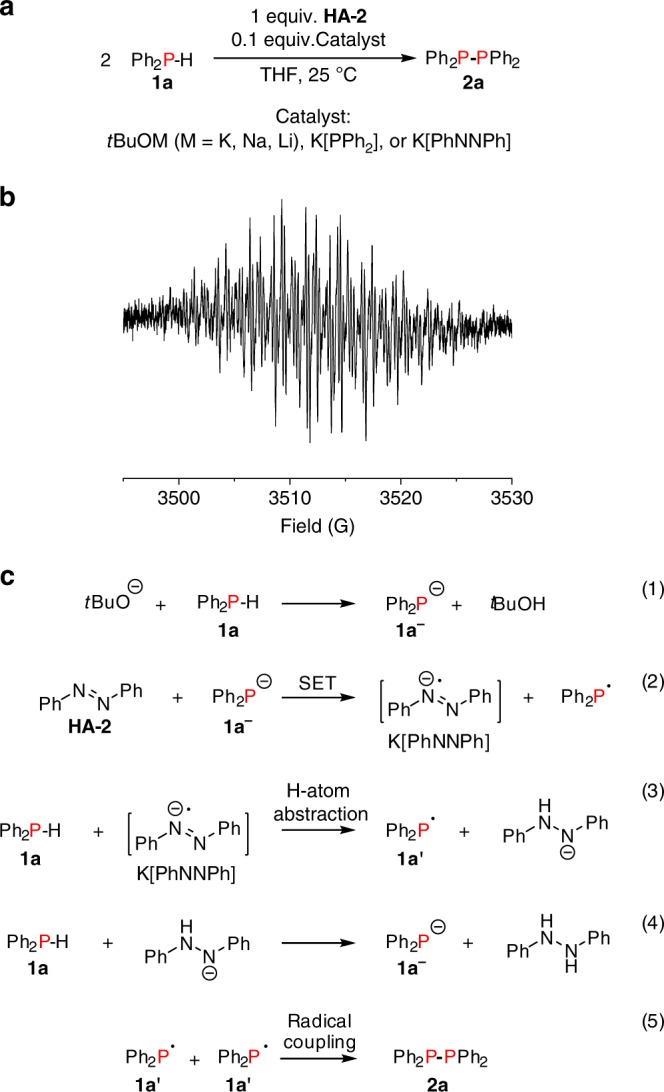
Dehydrocoupling of 1a in presence of azobenzene (HA-2). a Homodehydrocoupling of 1a catalysed by tBuOM (M = K, Na, Li), K[PPh2] (1a−), or K[PhNNPh] at 25 °C. b X-band EPR spectrum of K[PhNNPh] in THF/2-Me-THF (20:1) generated in the 1:1 stoichiometric reaction of HA-2 with tBuOK. c Proposed radical chain mechanism for dehydrocoupling of 1a to 2a mediated by in situ generated radical anion K[PhNNPh]
A series of stoichiometric and catalytic reactions were performed in an attempt to determine potential active species present over the course of catalysis. In the 1:1 stoichiometric reaction of orange azobenzene (HA-2) with tBuOK in THF-d8 a dark brown reaction mixture resulted for which analysis by 1H and 13C{1H} NMR revealed the presence of only unreacted diamagnetic HA-2 and tBuOK (Supplementary Fig. 16). However a solution-state EPR spectrum in THF/2-Me-THF (20:1) at 25 °C revealed a signal at approximately g = 2 which is characteristic of an organic radical and shows a hyperfine structure consistent with the previously reported azobenzenyl radical anion K[PhNNPh] (Fig. 3b, Supplementary Fig. 16)71,72. The 1:1 stoichiometric reaction of HA-2 with K[PPh2] (1a−) also yielded a deep brown solution however by 1H and 31P{1H} NMR the only diamagnetic species observed was 2a, as HA-2 was fully converted to its radical anion K[PhNNPh], which was also observed by EPR (Supplementary Fig. 17).
The radical anion K[PhNNPh] could also be independently prepared from potassium metal and HA-2 for use in further studies on its role as a catalyst (Supplementary Fig. 18). Upon the addition of 10 mol% K[PhNNPh] to a 1:1 stoichiometric mixture of 1a and HA-2 gave approximately a 1:1 mixture of 7a and 2a by 31P{1H} NMR spectroscopy along with hydrazobenzene (Supplementary Fig. 19). Remarkably, treatment of a mixture of 2 equiv. of 1a and 1 equiv. of HA-2 in THF with either 10 mol% 1a− or independently synthesised K[PhNNPh] resulted in complete conversion to a 1:1 mixture of 2a and hydrazobenzene within 5 min at 25 °C (Fig. 3a, Supplementary Figs. 14 and 15), analogous to the results obtained when 10 mol% tBuOK was used. The K[PhNNPh] radical anion was also observed by EPR spectroscopy in the reaction of HA-2 with tBuOK and 0.5 equiv. of the substrate 1a (Supplementary Fig. 20).
In addition, the use of 10 mol% tBuONa also resulted in complete conversion of 2 equiv. of 1a and 1 equiv. of HA-2 within 5 min at 25 °C, while tBuOLi was significantly slower requiring 6 h at 25 °C to reach full conversion, likely due to the poor solubility of tBuOLi in THF (Fig. 3a). While both 1a− and K[PhNNPh] can catalyse the dehydrocoupling of 1a to 2a, inexpensive and commercially available tBuOK provides a convenient and practical entry point into the catalysis.
We also explored the reactivity of the hydrophosphination product 7a which was observed as a P-containing product along with 2a in reactions involving 10 mol% of an alkali metal-based catalyst (tBuOK, 1a−, or K[PhNNPh]) and a 1:1 mixture of 1a and HA-2. Treatment of 7a with 1 equiv. of tBuOK or 1a− also resulted in conversion to 2a, hydrazobenzene, and K[PhNNPh] (Supplementary Fig. 21). In addition, treatment of a pure sample of independently synthesised 7a with 10 mol% tBuOK resulted in ~43% conversion to 2a and hydrazobenzene and ~7% conversion to 5a within 5 min at 25 °C (Supplementary Fig. 22). Similar product distributions were also obtained using either 10 mol% 1a− or K[PhNNPh] as catalysts (Supplementary Figs. 23 and 24). Interestingly the reaction of a 1:1 stoichiometric mixture of 1a and 7a with 10 mol% tBuOK or K[PhNNPh] resulted in complete conversion to 2a and hydrazobenzene (Supplementary Figs. 25 and 26).
Radical trapping experiments with 1,4-cyclohexadiene, and experiments with added radical initiator di(t-butyl)peroxide (DTBP) were performed which are further supportive of a radical process (Supplementary Figs. 27 and 28). On the basis of the stoichiometric reactions and our key observation of the radical anion K[PhNNPh] and the demonstrated competency of this species in catalysis we propose a radical mechanism initiated by one-electron reduction of HA-2 to produce the radical anion K[PhNNPh] (Fig. 3c).
The oxidation potential for tBuOK is at +0.10 V vs. SCE in DMF,51 meanwhile the reduction potential for azobenzene (HA-2) is at −1.36 V vs. SCE in DMF.73 Ashby and coworkers attempted to experimentally measure the oxidation potential of K[PPh2] (1a−) in THF and were unsuccessful due to adsorption onto the Pt electrode, but concluded based on their studies on single electron transfer (SET) to organic iodides that they estimate the oxidation potential of 1a− to be in the range of +0.8 to +1.3 V vs. SCE.74 It seems unlikely that tBuOK acts directly as the primary electron donor to azobenzene given that there is a significant mismatch of redox potentials and in our experiments only a small amount of radical was produced in the 1:1 reaction of HA-2 with tBuOK. It is more likely that 1a− generated under the catalytic conditions from the deprotonation reaction of 1a by tBuOK is engaged in electron transfer to HA-2 to initiate the radical chain process (Fig. 3c). In addition, the diphenylphosphide anion has been shown to participate in SET chemistry to a range of organic molecules either under photoirradiation,75,76 and without photoirradiation.74,77,78
It appears that in all reactions where an EPR signal was detectable that the only persistent radical species observed is the azobenzenyl radical anion K[PhNNPh], this assignment is on the basis of the g-value and coupling constants, which matched closely with the literature values71,72 with varying degrees of spectral line broadening likely due to concentration and other solvent effects (see Supplementary Figs. 16–21 and 29). It is noteworthy that the P-based radical •PPh2 (1a′) does not persist under our reaction conditions since previously the EPR spectrum for this unstable intermediate was collected at 77 K,79 and therefore rapidly couples to give 2a (Fig. 3c).
It is noteworthy that the hydrophosphination adduct 7a can be converted both with and without a second equivalent of 1a upon the addition of either catalytic or stoichiometric quantities of tBuOK or 1a−. This gave a mixture of 2a, hydrazobenzene, and K[PhNNPh]. However, given that in the variable temperature NMR experiment (Supplementary Fig. 13) 7a was not observed until the sample was warmed to 25 °C from −60 °C, it is likely that 7a is an intermediate on a secondary pathway en route to 2a and not an essential or central intermediate on the primary pathway to 2a. This is in contrast to what was previously demonstrated using HA-5 where the resulting hydrophosphination adduct 3a was demonstrated to be a crucial intermediate.
A comparison between the use of N-benzylideneaniline (HA-5) and azobenzene (HA-2) was performed using different substituted phosphines (Fig. 4). For diphenylphosphine, although the yield of 2a with HA-2 was lower than for the case of HA-5 (75% vs 95%), it was achieved at milder temperature (25 °C vs 60 °C) and with considerably shorter reaction time (<5 min vs. 8 h). From this point of view, HA-2 is still a practical hydrogen acceptor. Thus, additional phosphine substrates were explored: we found that in some cases HA-2 gave better conversion and yield of P–P coupled products than HA-5 (Fig. 4). For example, with fluoro- and chloro-substituted phosphines, more than 30% yield increases (78% vs. 46% and 87% vs. 55%) were observed in each case at much lower temperature (−20 °C vs 130 °C) and short reaction time (<5 min vs. 120 h). For bis(mesityl)phosphine a 59% yield of diphosphine 2 h was obtained using HA-2, while in the case of HA-5, no product was observed at all. The yield of [PhP]5 (2i) was very high (92%) for the dehydrocoupling of phenylphosphine. When cyclohexylphosphine was used as a substrate, [CyP]4 (2j) was produced in 66% yield.
Fig. 4.
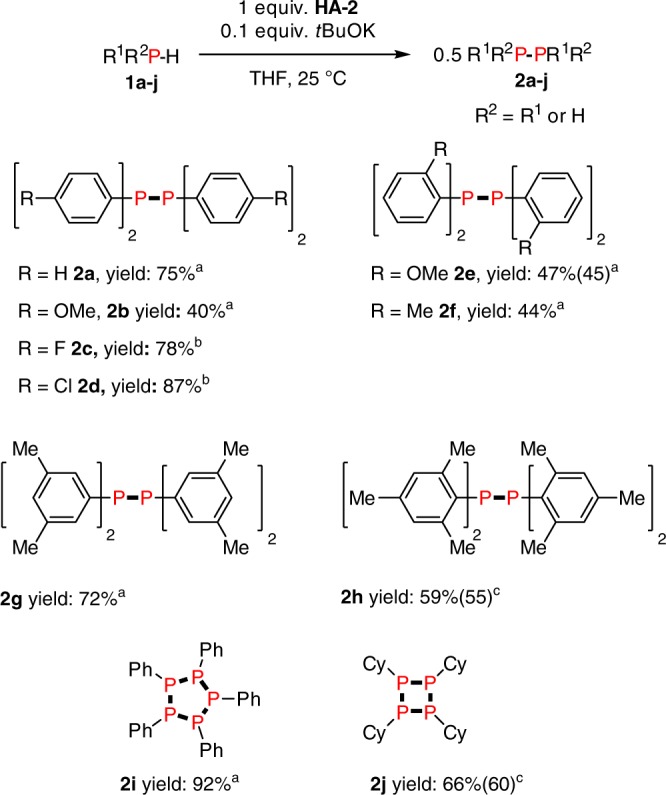
Reaction generality for the dehydrocoupling of different phosphines with HA-2: reactions were performed with 0.1 mmol phosphines, 0.01 mmol tBuOK, 0.1 mmol HA-2, 0.5 mL THF in a J. Young NMR tube and yields were based on the phosphine as the limiting reagent and determined by 31P{1H} NMR spectroscopy using a capillary of PCl3 as a calibration standard, the numbers in brackets were isolated yields. a25 °C within 5 min, b−20 °C within 5 min, c130 °C for 1 h
Heterodehydrocoupling of phosphines
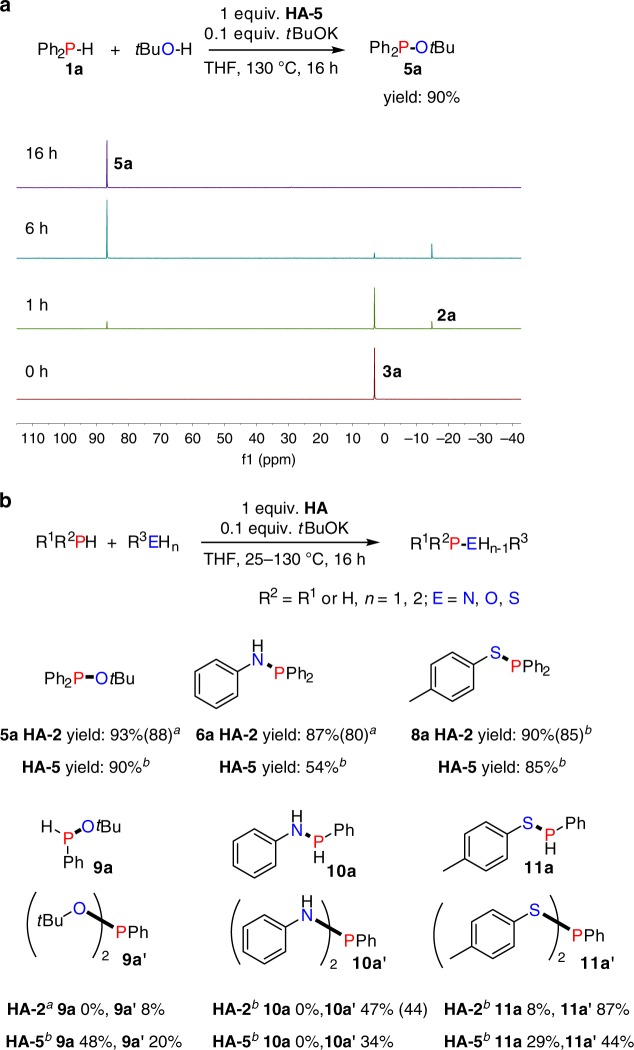
Next, given the virtual absence of previous examples2, we investigated whether our tBuOK-catalysed dehydrocoupling protocol could be extended to the heterodehydrocoupling of phosphines with different protic substrates (R3E-Hn; E = N, O, S, n = 1, 2). The initial reaction was performed using 0.1 mmol Ph2PH and 0.1 mmol tBuOH as substrates together with 0.1 mmol HA-5 and 10 mol% tBuOK in 0.5 mL THF at 130 °C. We followed the reaction profile by 31P NMR spectroscopy at different time intervals (Fig. 5a); the imine hydrophosphination adduct 3a is formed quantitatively after combining all the reagents. After heating the reaction mixture for 1 h we could observe both homodehydrocoupling product 2a and heterodehydrocoupling product 5a. Both 2a and 5a were present during the reaction and eventually all 2a was converted to give 5a in 90% yield together with some other minor side products. Computationally the heterodehydrocoupling reaction of 1a with tBuOH in the presence of HA-5 to produce 5a and 4a was exergonic by 10.62 kcal mol−1, similar to the computed value of ΔG = −10.09 kcal mol−1 for the analogous homodehydrocoupling process (see Supplementary Discussion in the Supplementary Information and Supplementary Data 1 and 2). The similarly favourable thermodynamics calculated for the P–P and P–O coupling reactions suggest that both reactions are possible, and likely competitive. The further conversion of 2a to 5a in the presence of additional tBuOH allows for high yield of 5a.
Fig. 5.
Heterodehydrocoupling of phosphines. a 31P{1H} NMR spectra of tBuOK-catalysed heterodehydrocoupling of 1a with tBuOH in THF. b Reaction generality of catalytic heterodehydrocoupling of phosphines with alcohol, amine and thiol: reactions were performed with 0.1 mmol phosphines, 0.1–0.3 mmol of R3E-Hn; E = N, O, S, n = 1, 2 and 0.01 mmol tBuOK, 0.1 mmol HA-5 or HA-2, 0.5 mL THF in a J. Young NMR tube and yields were based on the phosphine as the limiting reagent and were determined by 31P{1H} NMR spectroscopy using a capillary of PCl3 as a calibration standard, the numbers in brackets were isolated yields. a25 °C, b130 °C
We then performed the heterodehydrocoupling reactions of secondary and primary phosphines with alcohols, amines or thiols in the presence of either azobenzene (HA-2) or imine (HA-5). As shown in Fig. 5b, we observed that HA-2 was able to mediate the heterodehydrocoupling of diphenylphosphine with either tBuOH or aniline at 25 °C with very high yields (93% and 87%, respectively). At 25 °C with p-thiocresol there was no reaction; however, after heating at 130 °C for only 3 h, the heterodehydrocoupling product 8a was obtained in 90% yield. Using HA-5 on the other hand, all reactions involving diphenylphosphine required heating at 130 °C to obtain excellent yields (90% and 85%) except for the case of reaction with aniline where only a moderate yield of 6a (54%) was observed. When phenylphosphine was used as the substrate with HA-2 the heterodehydrocoupling with tBuOH produced only 8% of the doubly-dehydrocoupled product 9a′, while with HA-5 the yields were 48% for singly-dehydrocoupled product 9a and 20% for 9a′. When phenylphosphine was reacted with aniline in the presence of HA-2 we also observed the formation of only doubly dehydrocoupled 10a′ in 47% yield and with HA-5 a 34% yield of 10a′ was obtained. The reaction with p-thiocresol mediated by HA-2 produced 87% doubly-dehydrocoupled product 11a′ alongside only 8% of 11a, while the use of HA-5 led to the formation of 44% 11a′ and 29% of 11a.
Dehydrocoupling of phosphines with hydrazobenzene
During our studies of the dehydrocoupling mechanism using HA-2 we were surprised to discover that in the presence of hydrogenation product from HA-2, hydrazobenzene, with Ph2PH (1a), the homodehydrocoupling product 2a could also be observed (Fig. 6). We therefore determined whether it was possible to use hydrazobenzene as an HA directly. The reaction with 0.1 mmol 1a and 0.1 mmol hydrazobenzene and 0.5 mL THF in a J. Young NMR tube was monitored using 31P NMR spectroscopy (Fig. 6a). It is noteworthy that the formation of 2a took place readily at 25 °C. However, a stoichiometric amount of tBuOK was required for a full conversion as the gradual addition of tBuOK to 0.1 mmol increased the yield of 2a to 93%.
Fig. 6.
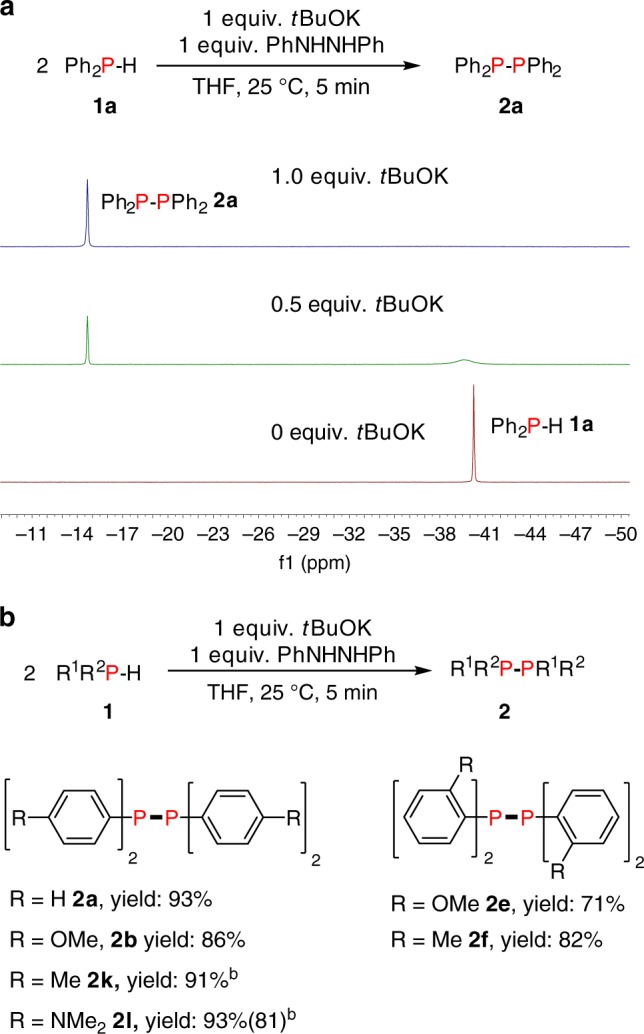
Catalytic homodehydrocoupling of phosphines using hydrazobenzene as HA. a 31P{1H} NMR spectra in THF with different amounts of added tBuOK for the dehydrocoupling of 1a in the presence of hydrazobenzene. b Catalytic results with different substituted phosphines: reactions were performed with 0.1 mmol phosphines, 0.1 mmol tBuOK and 0.1 mmol hydrazobenzene, 0.5 mL THF at 25 °C in a J. Young NMR tube and yields were based on the phosphine as the limiting reagent and determined by 31P{1H} NMR spectroscopy using a capillary of PCl3 as a calibration standard, the number in brackets shows an isolated yield
The 1:1 stoichiometric reaction of hydrazobenzene with either tBuOK or K[PPh2] in THF at 25 °C resulted in immediate formation of a brown reaction mixture which displayed the diagnostic signal in the EPR spectrum corresponding to the K[PhNNPh], analogous to reactions where HA-2 was used (Supplementary Fig. 29). Examples of the dehydrogenation reaction of hydrazobenzenes to azobenzenes mediated by tBuOK and other alkali metal compounds are known in the literature80,81. It therefore seems likely that in situ generation of HA-2 and subsequent radical species are involved in the dehydrocoupling of phosphines involving hydrazobenzene and a stoichiometric amount of tBuOK.
Subsequently, the generality of using hydrazobenzene in the homodehydrocoupling of phosphines was studied (Fig. 6b). We found that improved yields were obtained compared to the use of HA-2 or HA-5 and all reactions were completed within 5 min. Our system was tolerant to various functional groups, thus phosphines with methoxy- and methyl- and N,N-dimethyl-substituents at para and ortho- positions all reacted well and gave the homodehydrocoupling products (2a–b, 2e–f, 2k–l) in very high yields (71–93%).
After establishing the hydrazobenzene-mediated homodehydrocoupling of phosphines, we then explored the possible extension to heterodehydrocoupling processes (Fig. 7). In general, we found that the reaction was successful, albeit with moderate product yields. For example, in the presence of 1 equiv. hydrazobenzene, diphenylphosphine reacted with aniline and produced 6a in 52% yield. For primary phenylphosphine, doubly-dehydrocoupled products 9a′ and 11a′ were formed with tBuOH and p-thiocresol in moderate to good yield (45% and 59%, respectively).
Fig. 7.
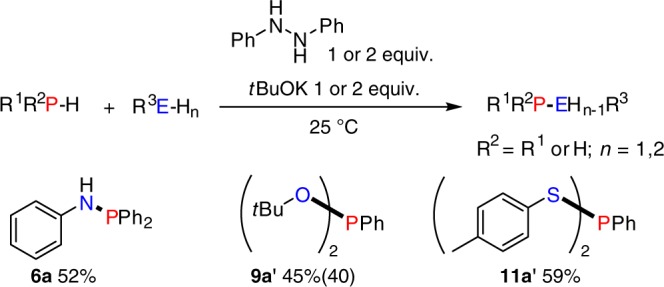
Hydrazobenzene-mediated heterodehydrocoupling of phosphines with alcohols, thiols, and amines: reactions were performed with 0.1 mmol phosphines, 0.1–0.2 mmol tBuOK and 0.1–0.2 mmol hydrazobenzene in 0.5 mL THF at 25 °C for 8 h in a J. Young NMR tube and yields were based on the phosphine as the limiting reagent and were determined by 31P{1H} NMR spectroscopy using a capillary of PCl3 as a calibration standard, the number in brackets shows an isolated yield
Discussion
We have demonstrated that cheap and commercially available tBuOK was capable of catalysing the homodehydrocoupling of phosphines (R1R2PH, R2 = R1 or H) in the presence of hydrogen acceptors such as an imine or azobenzene to produce molecules containing P–P bonds. Milder reaction conditions (25 °C) and fast reaction times (<5 min) allow this process to be more practical and general for a variety of different substituted diphosphines. This is in contrast to the previously reported processes which generally use expensive transition metal catalysts or reagents requiring multi-step syntheses, harsh reaction conditions, and substantially longer reaction times. We found that imines and azobenzene play an intimate mechanistic role in facilitating the dehydrocoupling reactions rather than simply functioning as H2-acceptors. It appears that two different reaction mechanisms operate: for imines an ionic mechanism involving a hydrophosphination adduct as a key intermediate was elucidated whereas for azobenzene, evidence for an alternative, radical coupling mechanism was apparent. In addition, saturated hydrazobenzene was also able to mediate the homodehydrocoupling reactions involving phosphines with stoichiometric amount of tBuOK and superior yields of the dehydrocoupled products are obtained compared to azobenzene. Significantly, a general procedure for the heterodehydrocoupling of phosphines with various main group substrates (R3EHn, E = O, S, N, n = 1 or 2) was also established and this provides a convenient route to compounds containing P–O, P–S, or P–N bonds. This chemistry may prove useful in the future for the preparation of phosphorus compounds with applications in biological and agrochemistry as well as in catalysis. Our current work focuses on further expansion of the substrate scope and additional detailed mechanistic studies.
Methods
tBuOK-catalysed homodehydrocoupling of phosphines
A J. Young NMR tube was charged with 0.1 mmol of the phosphines and 0.1 mmol of the corresponding hydrogen acceptors azobenzene (HA-2) or N-benzylideneaniline (HA-5), 0.5 mL of 0.02 M tBuOK THF solution was added to the J. Young NMR tube. The NMR tube was sealed and heated at various temperature as indicated in the corresponding Fig. 2 and Fig. 4 or Supplementary Tables 1–6 for the time shown and analysed by 31P NMR spectroscopy. For isolated products, the solvents/volatiles were removed under vacuum and the residue was washed with hexanes, solid products were recrystallised through vapour diffusion of hexanes into THF solutions of the product.
tBuOK-catalysed heterodehydrocoupling of phosphines
A J. Young NMR tube was charged with 0.1 mmol of the phosphines and 0.1 mmol of azobenzene (HA-2) or N-benzylideneaniline (HA-5), 0.5 mL of 0.02 M tBuOK THF solution was added to the J. Young NMR tube, then 0.1–0.3 mmol of the corresponding alcohol, thiol, or amine was added. The NMR tube was sealed and heated for the time shown in the Fig. 5 and analysed by 31P NMR spectroscopy. For isolated products, the reactions were performed in 0.5 mmol scale of phosphine and distilled under lower pressure for liquid products or purified via diffusion crystallisation (THF/hexane).
Homodehydrocoupling of phosphines using hydrazobenzene
A J. Young NMR tube was charged with 0.1 mmol of the phosphines and 0.1 mmol hydrazobenzene, 0.5 mL of 0.2 M tBuOK THF solution was added to the J. Young NMR tube. The NMR tube was sealed and analysed directly by 31P NMR spectroscopy. The isolated products were obtained via diffusion crystallisation (THF/hexane).
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
We would like to thank Dr. Saurabh S. Chitnis and Dr. Marius I. Arz for helpful discussions. We thank Paul Lawrence, Tom Leman for their help on the low temperature NMR spectroscopy experiments. We also acknowledge the EPSRC National Electron Paramagnetic Resonance Spectroscopy Research Facility and Service, and Richard Procter of Prof. Michael Ingleson’s group at University of Manchester for their assistance with EPR measurements. This work was supported by European Union for Marie Skłodowska-Curie Actions for L.W. (H2020-MSCA-IF-2015_701972) and V.T.A. (H2020-MSCA-IF-2016_748371). V.T.A. also is grateful to NSERC of Canada for a postdoctoral fellowship. I.M also thanks the government of Canada for a Canada 150 Research Chair and University of Bristol for support.
Author contributions
L.W., V.T.A., and I.M. conceived the project and designed the experiments. L.W. and V.T.A. performed the experiments and analysed the data. H.J. performed computational chemistry. V.T.A. performed X-ray crystallography. A.B. and D.C. performed EPR measurements and modelled EPR spectral data. L.W., V.T.A., H.J., and I.M. wrote the manuscript. All the authors discussed the results and commented on the manuscript. L.W. and V.T.A. share co-first authorship given their contributions indicated above.
Data availability
All data to support the conclusions in this paper are available in the main text or the supplementary materials. Crystallographic data for 2j (CCDC 1842531) and 3e•(THF) (CCDC 1842532) are available from the Cambridge Crystallographic Data Centre. Copies of the data can be obtained free of charge from [www.ccdc.cam.ac.uk/data_request/cif].
Competing interests
The authors declare no competing interests.
Footnotes
Peer review information: Nature Communications thanks R Baker and the other anonymous reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Lipeng Wu, Vincent T. Annibale.
Supplementary information
Supplementary Information accompanies this paper at 10.1038/s41467-019-09832-4.
References
- 1.Gauvin, F., Harrod, J. F. & Woo, H. G. in Adv. Organomet. Chem. Vol. 42 (eds Gordon, F., Stone A. & Robert, West) 363–405 (Academic Press, Cambridge, 1998).
- 2.Han LB, Tilley TD. Selective homo- and heterodehydrocouplings of phosphines catalyzed by rhodium phosphido complexes. J. Am. Chem. Soc. 2006;128:13698–13699. doi: 10.1021/ja065346+. [DOI] [PubMed] [Google Scholar]
- 3.Waterman R. Dehydrogenative bond-forming catalysis involving phosphines. Curr. Org. Chem. 2008;12:1322–1339. doi: 10.2174/138527208785909592. [DOI] [Google Scholar]
- 4.Less, R. J., Melen, R. L., Naseri, V. & Wright, D. S. Recent perspectives on main group-mediated dehydrocoupling of P-P bonds. Chem. Commun. 4929–4937 (2009). [DOI] [PubMed]
- 5.Leitao EM, Jurca T, Manners I. Catalysis in service of main group chemistry offers a versatile approach to p-block molecules and materials. Nat. Chem. 2013;5:817–829. doi: 10.1038/nchem.1749. [DOI] [PubMed] [Google Scholar]
- 6.Melen RL. Dehydrocoupling routes to element-element bonds catalysed by main group compounds. Chem. Soc. Rev. 2016;45:775–788. doi: 10.1039/C5CS00521C. [DOI] [PubMed] [Google Scholar]
- 7.Greenberg S, Stephan DW. Stoichiometric and catalytic activation of P-H and P-P bonds. Chem. Soc. Rev. 2008;37:1482–1489. doi: 10.1039/b612306f. [DOI] [PubMed] [Google Scholar]
- 8.Dorn H, et al. Transition metal-catalyzed formation of phosphorus−boron bonds: a new route to phosphinoborane rings, chains, and macromolecules. J. Am. Chem. Soc. 2000;122:6669–6678. doi: 10.1021/ja000732r. [DOI] [Google Scholar]
- 9.Johnson HC, et al. Mechanistic studies of the dehydrocoupling and dehydropolymerization of amine–boranes using a [Rh(Xantphos)]+catalyst. J. Am. Chem. Soc. 2014;136:9078–9093. doi: 10.1021/ja503335g. [DOI] [PubMed] [Google Scholar]
- 10.Choffat F, et al. Synthesis and characterization of linear poly(dialkylstannane)s. Macromolecules. 2007;40:7878–7889. doi: 10.1021/ma071463t. [DOI] [Google Scholar]
- 11.Sloan ME, Clark TJ, Manners I. Homogeneous catalytic dehydrogenation/dehydrocoupling of amine-borane adducts by the Rh(I) Wilkinson’s complex analogue RhCl(PHCy2)3 (Cy = cyclohexyl) Inorg. Chem. 2009;48:2429–2435. doi: 10.1021/ic801752k. [DOI] [PubMed] [Google Scholar]
- 12.Paul USD, Braunschweig H, Radius U. Iridium-catalysed dehydrocoupling of aryl phosphine-borane adducts: synthesis and characterisation of high molecular weight poly(phosphinoboranes) Chem. Commun. 2016;52:8573–8576. doi: 10.1039/C6CC04363A. [DOI] [PubMed] [Google Scholar]
- 13.Kumar A, et al. Multiple metal-bound oligomers from Ir-catalysed dehydropolymerisation of H3B.NH3 as probed by experiment and computation. Chem. Sci. 2014;5:2546–2553. doi: 10.1039/C4SC00735B. [DOI] [Google Scholar]
- 14.Staubitz A, et al. Catalytic dehydrocoupling/dehydrogenation of N-methylamine-borane and ammonia-borane: synthesis and characterization of high molecular weight polyaminoboranes. J. Am. Chem. Soc. 2010;132:13332–13345. doi: 10.1021/ja104607y. [DOI] [PubMed] [Google Scholar]
- 15.Denney MC, Pons V, Hebden TJ, Heinekey DM, Goldberg KI. Efficient catalysis of ammonia borane dehydrogenation. J. Am. Chem. Soc. 2006;128:12048–12049. doi: 10.1021/ja062419g. [DOI] [PubMed] [Google Scholar]
- 16.Marziale AN, et al. The mechanism of borane–amine dehydrocoupling with bifunctional ruthenium catalysts. J. Am. Chem. Soc. 2013;135:13342–13355. doi: 10.1021/ja311092c. [DOI] [PubMed] [Google Scholar]
- 17.Sun CL, Shi ZJ. Transition-metal-free coupling reactions. Chem. Rev. 2014;114:9219–9280. doi: 10.1021/cr400274j. [DOI] [PubMed] [Google Scholar]
- 18.Rossin A, Peruzzini M. Ammonia–borane and amine–borane dehydrogenation mediated by complex metal hydrides. Chem. Rev. 2016;116:8848–8872. doi: 10.1021/acs.chemrev.6b00043. [DOI] [PubMed] [Google Scholar]
- 19.Han D, Anke F, Trose M, Beweries T. Recent advances in transition metal catalysed dehydropolymerisation of amine boranes and phosphine boranes. Coord. Chem. Rev. 2019;380:260–286. doi: 10.1016/j.ccr.2018.09.016. [DOI] [Google Scholar]
- 20.Imori T, Lu V, Cai H, Tilley TD. Metal-catalyzed dehydropolymerization of secondary stannanes to high molecular weight polystannanes. J. Am. Chem. Soc. 1995;117:9931–9940. doi: 10.1021/ja00145a001. [DOI] [Google Scholar]
- 21.Metters OJ, et al. Catalytic dehydrocoupling of amine–boranes using cationic zirconium(IV)–phosphine frustrated lewis pairs. ACS Catal. 2016;6:6601–6611. doi: 10.1021/acscatal.6b02211. [DOI] [Google Scholar]
- 22.Roering AJ, MacMillan SN, Tanski JM, Waterman R. Zirconium-catalyzed heterodehydrocoupling of primary phosphines with silanes and germanes. Inorg. Chem. 2007;46:6855–6857. doi: 10.1021/ic7013144. [DOI] [PubMed] [Google Scholar]
- 23.Baker RT, et al. Iron complex-catalyzed ammonia–borane dehydrogenation. A potential route toward B–N-containing polymer motifs using earth-abundant metal catalysts. J. Am. Chem. Soc. 2012;134:5598–5609. doi: 10.1021/ja210542r. [DOI] [PubMed] [Google Scholar]
- 24.Vance JR, et al. Iron-catalyzed dehydrocoupling/dehydrogenation of amine–boranes. J. Am. Chem. Soc. 2014;136:3048–3064. doi: 10.1021/ja410129j. [DOI] [PubMed] [Google Scholar]
- 25.Keaton RJ, Blacquiere JM, Baker RT. Base metal catalyzed dehydrogenation of ammonia−borane for chemical hydrogen storage. J. Am. Chem. Soc. 2007;129:1844–1845. doi: 10.1021/ja066860i. [DOI] [PubMed] [Google Scholar]
- 26.Vogt M, de Bruin B, Berke H, Trincado M, Grützmacher H. Amino olefin nickel(I) and nickel(0) complexes as dehydrogenation catalysts for amine boranes. Chem. Sci. 2011;2:723–727. doi: 10.1039/c0sc00483a. [DOI] [Google Scholar]
- 27.Liptrot DJ, Hill MS, Mahon MF, MacDougall DJ. Group 2 promoted hydrogen release from NMe2H⋅BH3: intermediates and catalysis. Chem. Eur. J. 2010;16:8508–8515. doi: 10.1002/chem.201000127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cowley HJ, Holt MS, Melen RL, Rawson JM, Wright DS. Catalytic dehydrocoupling of Me2NHBH3 with Al(NMe2)3. Chem. Commun. 2011;47:2682–2684. doi: 10.1039/c0cc05294a. [DOI] [PubMed] [Google Scholar]
- 29.Wu L, Chitnis SS, Jiao H, Annibale VT, Manners I. Non-metal-catalyzed heterodehydrocoupling of phosphines and hydrosilanes: mechanistic studies of B(C6F5)3-mediated formation of P-Si bonds. J. Am. Chem. Soc. 2017;139:16780–16790. doi: 10.1021/jacs.7b09175. [DOI] [PubMed] [Google Scholar]
- 30.Bellham P, Hill MS, Kociok-Köhn G. Alkali metal-mediated dehydrocoupling of Me2NH.BH3. Dalton Trans. 2015;44:12078–12081. doi: 10.1039/C5DT00178A. [DOI] [PubMed] [Google Scholar]
- 31.McLellan R, Kennedy AR, Mulvey RE, Orr SA, Robertson SD. 1-Alkali-metal-2-alkyl-1,2-dihydropyridines: soluble hydride surrogates for catalytic dehydrogenative coupling and hydroboration applications. Chem. Eur. J. 2017;23:16853–16861. doi: 10.1002/chem.201703609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nolla-Saltiel R, Geer AM, Lewis W, Blake AJ, Kays DL. Dehydrogenation of dimethylamine-borane mediated by Group I pincer complexes. Chem. Commun. 2018;54:1825–1828. doi: 10.1039/C7CC08385H. [DOI] [PubMed] [Google Scholar]
- 33.Molitor S, Mahler C, Gessner VH. Synthesis and solid-state structures of gold(I) complexes of diphosphines. New J. Chem. 2016;40:6467–6474. doi: 10.1039/C6NJ00786D. [DOI] [Google Scholar]
- 34.Geier, S. J. & Stephan, D. W. Activation of P5R5 (R = Ph, Et) by a Rh-β-diketiminate complex. Chem. Commun. 2779–2781 (2008). [DOI] [PubMed]
- 35.Annibale VT, Ostapowicz TG, Westhues S, Wambach TC, Fryzuk MD. Synthesis of a sterically bulky diphosphine synthon and Ru(II) complexes of a cooperative tridentate enamide-diphosphine ligand platform. Dalton Trans. 2016;45:16011–16025. doi: 10.1039/C6DT02352E. [DOI] [PubMed] [Google Scholar]
- 36.Chitnis SS, et al. Addition of a cyclophosphine to nitriles: an inorganic click reaction featuring protio, organo, and main-group catalysis. Angew. Chem. Int. Ed. 2017;56:9536–9540. doi: 10.1002/anie.201704991. [DOI] [PubMed] [Google Scholar]
- 37.Feldmann KO, Weigand JJ. P–N/P–P bond metathesis for the synthesis of complex polyphosphanes. J. Am. Chem. Soc. 2012;134:15443–15456. doi: 10.1021/ja305406x. [DOI] [PubMed] [Google Scholar]
- 38.Hirano K, Miura M. Recent advances in diphosphination of alkynes and alkenes. Tetrahedron Lett. 2017;58:4317–4322. doi: 10.1016/j.tetlet.2017.10.018. [DOI] [Google Scholar]
- 39.Hajdók I, Lissner F, Nieger M, Strobel S, Gudat D. Diphosphination of electron poor alkenes. Organometallics. 2009;28:1644–1651. doi: 10.1021/om801179k. [DOI] [Google Scholar]
- 40.Sato Y, Kawaguchi Si, Nomoto A, Ogawa A. Highly selective phosphinylphosphination of alkenes with tetraphenyldiphosphine monoxide. Angew. Chem. Int. Ed. 2016;55:9700–9703. doi: 10.1002/anie.201603860. [DOI] [PubMed] [Google Scholar]
- 41.Fermin MC, Stephan DW. Catalytic oligomerization of primary phosphines by the anionic zirconocene trihydride: [Cp∗2ZrH3] J. Am. Chem. Soc. 1995;117:12645–12646. doi: 10.1021/ja00155a033. [DOI] [Google Scholar]
- 42.Böhm VPW, Brookhart M. Dehydrocoupling of phosphanes catalyzed by a rhodium(I) complex. Angew. Chem. Int. Ed. 2001;40:4694–4696. doi: 10.1002/1521-3773(20011217)40:24<4694::AID-ANIE4694>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 43.Waterman R. Selective dehydrocoupling of phosphines by triamidoamine zirconium catalysts. Organometallics. 2007;26:2492–2494. doi: 10.1021/om070189o. [DOI] [Google Scholar]
- 44.King AK, Buchard A, Mahon MF, Webster RL. Facile, catalytic dehydrocoupling of phosphines using β-diketiminate iron(II) complexes. Chem. Eur. J. 2015;21:15960–15963. doi: 10.1002/chem.201503399. [DOI] [PubMed] [Google Scholar]
- 45.Naseri V, Less RJ, Mulvey RE, McPartlin M, Wright DS. Stoichiometric and catalytic Sn-mediated dehydrocoupling of primary phosphines. Chem. Commun. 2010;46:5000–5002. doi: 10.1039/c0cc00827c. [DOI] [PubMed] [Google Scholar]
- 46.Dobrovetsky R, Takeuchi K, Stephan DW. Metal-free Lewis acid mediated dehydrocoupling of phosphines and concurrent hydrogenation. Chem. Commun. 2015;51:2396–2398. doi: 10.1039/C4CC09526J. [DOI] [PubMed] [Google Scholar]
- 47.Molitor S, Becker J, Gessner VH. Selective dehydrocoupling of phosphines by lithium chloride carbenoids. J. Am. Chem. Soc. 2014;136:15517–15520. doi: 10.1021/ja509381w. [DOI] [PubMed] [Google Scholar]
- 48.Schneider H, Schmidt D, Radius U. The reductive P-P coupling of primary and secondary phosphines mediated by N-heterocyclic carbenes. Chem. Commun. 2015;51:10138–10141. doi: 10.1039/C5CC02517F. [DOI] [PubMed] [Google Scholar]
- 49.Ashford RD. Ashford’s Dictionary of Industrial Chemicals: Properties, Production, Uses. London: Wavelength; 1994. [Google Scholar]
- 50.Murphy PJ. Organophosphorus Reagents. Oxford: Oxford University Press; 2004. [Google Scholar]
- 51.Barham JP, et al. KOtBu: a privileged reagent for electron transfer reactions? J. Am. Chem. Soc. 2016;138:7402–7410. doi: 10.1021/jacs.6b03282. [DOI] [PubMed] [Google Scholar]
- 52.Kumar Amit, Janes Trevor, Chakraborty Subrata, Daw Prosenjit, von Wolff Niklas, Carmieli Raanan, Diskin-Posner Yael, Milstein David. C−C Bond Formation of Benzyl Alcohols and Alkynes Using a Catalytic Amount of KOt Bu: Unusual Regioselectivity through a Radical Mechanism. Angewandte Chemie International Edition. 2019;58(11):3373–3377. doi: 10.1002/anie.201812687. [DOI] [PubMed] [Google Scholar]
- 53.Liu WB, et al. Potassium tert-butoxide-catalyzed dehydrogenative C-H silylation of heteroaromatics: a combined experimental and computational mechanistic study. J. Am. Chem. Soc. 2017;139:6867–6879. doi: 10.1021/jacs.6b13031. [DOI] [PubMed] [Google Scholar]
- 54.Weickgenannt A, Oestreich M. Potassium tert-butoxide-catalyzed dehydrogenative Si-O coupling: reactivity pattern and mechanism of an underappreciated alcohol protection. Chem. Asian J. 2009;4:406–410. doi: 10.1002/asia.200800426. [DOI] [PubMed] [Google Scholar]
- 55.Evoniuk CJ, et al. Coupling N–H deprotonation, C–H activation, and oxidation: metal-free C(sp3)–H aminations with unprotected anilines. J. Am. Chem. Soc. 2017;139:16210–16221. doi: 10.1021/jacs.7b07519. [DOI] [PubMed] [Google Scholar]
- 56.Yin Q, Klare HF, Oestreich M. Catalytic Friedel-Crafts C-H borylation of electron-rich arenes: dramatic rate acceleration by added alkenes. Angew. Chem. Int. Ed. 2017;56:3712–3717. doi: 10.1002/anie.201611536. [DOI] [PubMed] [Google Scholar]
- 57.Pérez M, Caputo CB, Dobrovetsky R, Stephan DW. Metal-free transfer hydrogenation of olefins via dehydrocoupling catalysis. Proc. Natl Acad. Sci. 2014;111:10917–10921. doi: 10.1073/pnas.1407484111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bunlaksananusorn T, Knochel P. t-BuOK-catalyzed addition phosphines to functionalized alkenes: a convenient synthesis of polyfunctional phosphine derivatives. Tetrahedron Lett. 2002;43:5817–5819. doi: 10.1016/S0040-4039(02)01177-2. [DOI] [Google Scholar]
- 59.King RB, Cloyd JC, Kapoor PN. Syntheses and properties of novel polyphosphines containing various combinations of primary, secondary, and tertiary phosphorus atoms. J. Chem. Soc. Perkin Trans. 1973;1:2226–2229. doi: 10.1039/p19730002226. [DOI] [Google Scholar]
- 60.Yoshimura A, et al. An efficient base-catalyzed double addition of H-phosphine oxides to alkynes. Tetrahedron Lett. 2016;57:3382–3384. doi: 10.1016/j.tetlet.2016.06.079. [DOI] [Google Scholar]
- 61.Andrieu, J., Dietz, J., Poli, R. & Richard, P. Reversible P-C bond formation for saturated α-aminophosphine ligands in solution: stabilization by coordination to Cu(I). New J. Chem.23, 581–583 (1999).
- 62.van Bochove MA, Swart M, Bickelhaupt FM. Nucleophilic substitution at phosphorus (SN2@P): disappearance and reappearance of reaction barriers. J. Am. Chem. Soc. 2006;128:10738–10744. doi: 10.1021/ja0606529. [DOI] [PubMed] [Google Scholar]
- 63.León T, Riera A, Verdaguer X. Stereoselective synthesis of P-stereogenic aminophosphines: ring opening of bulky oxazaphospholidines. J. Am. Chem. Soc. 2011;133:5740–5743. doi: 10.1021/ja200988c. [DOI] [PubMed] [Google Scholar]
- 64.Kolodiazhnyi OI, Kolodiazhna A. Nucleophilic substitution at phosphorus: stereochemistry and mechanisms. Tetrahedron: Asymmetry. 2017;28:1651–1674. doi: 10.1016/j.tetasy.2017.10.022. [DOI] [Google Scholar]
- 65.Brook AG, Duff JM. Base-catalyzed rearrangement of aminomethylsilanes to methylaminosilanes. J. Am. Chem. Soc. 1974;96:4692–4693. doi: 10.1021/ja00821a065. [DOI] [Google Scholar]
- 66.Duff JM, Brook AG. Studies of the rearrangements of aminoalkylsilanes to alkylaminosilanes. Can. J. Chem. 1977;55:2589–2600. doi: 10.1139/v77-358. [DOI] [Google Scholar]
- 67.Capozzi, M. A. M., Cardellicchio, C. & Naso, F. Enantioselective routes to sulfoxides based upon the use of carbanionic leaving groups. Eur. J. Org. Chem. 1855–1963 (2004).
- 68.Lin S, et al. An expedient synthesis of carbazoles through potassium tert-butoxide-promoted intramolecular direct C–H bond arylation. Eur. J. Org. Chem. 2017;2017:443–447. doi: 10.1002/ejoc.201601293. [DOI] [Google Scholar]
- 69.Liu W, Hou F. Transition-metal-free dehalogenation of aryl halides promoted by phenanthroline/potassium tert-butoxide. Tetrahedron. 2017;73:931–937. doi: 10.1016/j.tet.2017.01.002. [DOI] [Google Scholar]
- 70.Fedotova YV, et al. Phosphinohydrazines and phosphinohydrazides M(–N(R)–N(R)–PPh2)n of some transition and main group metals: synthesis and characterization: Rearrangement of Ph2P–NR–NR– ligands into aminoiminophosphorane, RN=PPh2–NR–, and related chemistry. J. Organomet. Chem. 2004;689:3060–3074. [Google Scholar]
- 71.Andrez J, Pécaut J, Bayle PA, Mazzanti M. Tuning lanthanide reactivity towards small molecules with electron-rich siloxide ligands. Angew. Chem. Int. Ed. 2014;53:10448–10452. doi: 10.1002/anie.201405031. [DOI] [PubMed] [Google Scholar]
- 72.Buser U, Ess CH, Gerson F. The radical anion of (E)-azobenzene revisited. Magn. Reson. Chem. 1991;29:721–725. doi: 10.1002/mrc.1260290715. [DOI] [Google Scholar]
- 73.Sadler JL, Bard AJ. Electrochemical reduction of aromatic azo compounds. J. Am. Chem. Soc. 1968;90:1979–1989. doi: 10.1021/ja01010a010. [DOI] [Google Scholar]
- 74.Ashby EC, Gurumurthy R, Ridlehuber RW. Investigation of the purity of alkali metal diphenylphosphides and their reactions with organic halides. Evidence for single electron transfer. J. Org. Chem. 1993;58:5832–5837. doi: 10.1021/jo00073a051. [DOI] [Google Scholar]
- 75.Rossi RA, Pierini AB, Peñéñory AB. Nucleophilic substitution reactions by electron transfer. Chem. Rev. 2003;103:71–168. doi: 10.1021/cr960134o. [DOI] [PubMed] [Google Scholar]
- 76.Bangerter BW, Beatty RP, Kouba JK, Wreford SS. Coupling reactions of diorganophosphides with organic halides. Evidence for a one-electron path. J. Org. Chem. 1977;42:3247–3251. doi: 10.1021/jo00440a010. [DOI] [Google Scholar]
- 77.Ashby EC, Sun X, Duff JL. Single electron transfer in nucleophilic aliphatic substitution. Evidence for single electron transfer in the reactions of 1-halonorbornanes with various nucleophiles. J. Org. Chem. 1994;59:1270–1278. doi: 10.1021/jo00085a012. [DOI] [Google Scholar]
- 78.Ashby EC, Deshpande AK. Electron transfer in the reactions of geminal dihalides with Ph2P-. Evidence for the formation of a carbene intermediate from a radical precursor. J. Org. Chem. 1995;60:7117–7124. doi: 10.1021/jo00127a014. [DOI] [Google Scholar]
- 79.Fullam, B. W., Mishra, S. P. & Symons, M. C. R. Unstable intermediates. Part CXLIX. An electron spin resonance study of various phosphinyl radicals formed by solid-state radiolysis. J. Chem. Soc. Dalton Trans. 2145–2148 (1974).
- 80.Xu L, Wang YC, Ma W, Zhang WX, Xi Z. Mechanistic insights into N—N bond cleavage in catalytic guanylation reactions between 1,2-diarylhydrazines and carbodiimides. J. Org. Chem. 2014;79:12004–12009. doi: 10.1021/jo501865b. [DOI] [PubMed] [Google Scholar]
- 81.Wang L, Ishida A, Hashidoko Y, Hashimoto M. Dehydrogenation of the NH-NH bond triggered by potassium tert-butoxide in liquid ammonia. Angew. Chem. Int. Ed. 2017;56:870–873. doi: 10.1002/anie.201610371. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
All data to support the conclusions in this paper are available in the main text or the supplementary materials. Crystallographic data for 2j (CCDC 1842531) and 3e•(THF) (CCDC 1842532) are available from the Cambridge Crystallographic Data Centre. Copies of the data can be obtained free of charge from [www.ccdc.cam.ac.uk/data_request/cif].