Abstract
Fresh and dried Zanthoxylum bungeanum Maxim volatiles of two main cultivars including Dahongpao and Meihuajiao, were determined through GC–MS and compared. In all the tested samples, linalool, d-limonene, eucalyptol, 3-nonanone, and β-myrcene were identified as the five predominant components. The percentages of these components in fresh Dahongpao were 23.89%, 21.04%, 7.46%, 5.63% and 5.87%, respectively. Similar percentages, 27.28%, 17.62%, 6.39%, 1.66% and 7.8%, were found in dried Dahongpao. In general, the contents of linalool and β-myrcene in dried Dahongpao and Meihuajiao were slightly higher than those in fresh samples, whereas the contents of d-limonene, eucalyptol, and 3-nonanone were lower. Partial least squares discriminant analysis results showed that the two cultivars could be clearly differentiated based on volatiles, whereas, the fresh and dried Zanthoxylum bungeanum Maxim samples could not. This demonstrated that the drying process had no significant effect on the volatiles.
Keywords: Zanthoxylum bungeanum Maxim, Volatiles, Gas chromatography–mass spectrometry, Partial least squares discriminant analysis
Introduction
Zanthoxylum bungeanum Maxim (Z. bungeanum Maxim) belongs to the Rutaceae family and is generally referred to as Huajiao in China. It is widely grown in the provinces of Gansu, Sichuan, Hebei, Shanxi, and Shandong (Yang et al., 2013; Song et al., 2017). It has also been widely employed as a spice flavoring owing to its unique taste and aroma (Feng et al., 2013; Tao et al., 2017). Huajiao contains many medicinal components and is used as a traditional Chinese medicine (Lan et al., 2014; Abuajah et al., 2015). Currently, there is an increasing interest in Z. bungeanum Maxim, because its flavor can stimulate saliva production to increase appetite and it is effective for treating epigastric pain, pruritus, dysentery, and eczema (Lan et al., 2014; Zhang et al., 2017). In addition, the essential oils or specific volatiles obtained from Z. bungeanum Maxim have antimicrobial, antioxidant, and insect repellent and feeding deterrent properties (Liu et al., 2009; Wei et al., 2011; Xia et al., 2011).
Volatiles are considered as an important factors for determining fruit quality, as well as sensory cues for the nutritional makeup of plant products (Kader, 2008). The unique smell of Z. bungeanum Maxim is mainly derived from its volatile organic compounds (VOCs; Tao et al., 2017). However, Z. bungeanum Maxim deteriorates rapidly after harvesting leading to a loss of aroma. Drying is an effective approach for maintaining the flavor of Z. bungeanum Maxim and prolonging its shelf-life by slowing enzyme activity, preventing harmful microbial growth, and slowing many water-mediated reactions (Andrés-Bello et al., 2011; Tian et al., 2016). However, drying can change the volatile profile of plant samples, and lead to a characteristic aroma associated with the breakdown of proteins into amino acids (Hiraide et al., 2004). This phenomenon has been observed in rhizomes of turmeric (Curcuma longa Linn.; Kutti Gounder and Lingamallu, 2012), mango (Mangifera indica L. cv. Kent; Adeline et al. 2016), and shiitake (Lentinus edodes) mushrooms (Tian et al., 2016).
Previous studies have investigated the volatile compositions of the pericarp and essential oil of dried Z. bungeanum Maxim (Yang, 2008; Gong et al., 2009; Wei et al., 2011; Diao et al., 2013; Liu et al., 2017). Yang (2008) compared the aromatic constituents of dried red and green Huajiao (Z. bungeanum and Z. schinifolium). Whereas, Liu et al. (2017) analyzed the volatiles in essential oils derived from six dried Z. bungeanum Maxim cultivars. However, both fresh and dried Huajiao are widely used as flavorings, to the best of our knowledge, the differences between their volatiles compositions are still unknown.
In this work, gas chromatography-mass spectrometry (GC–MS) was used to investigate the volatiles in fresh and dried Z. bungeanum Maxim, specifically the Dahongpao and Meihuajiao cultivars. A partial least squares discriminant analysis (PLS-DA) approach was employed to analyze the differences in their volatiles profiles and to identify the characteristic components in the samples.
Materials and methods
Plant materials and chemical reagents
Mature fruits of the Dahongpao and Meihuajiao cultivars of Z. bungeanum Maxim were collected from Longnan (Gansu Province, China) in August 2017. The external color and morphological features, as well as average single panicle weight and hundred grain weight for each cultivar are shown in Fig. 1 and Table 1, respectively. Cyclohexanol was obtained from Sigma (St. Louis, MO, USA). All other analytical grade reagents were were purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China).
Fig. 1.
Dahongpao and Meihuajiao used in the present study
Table 1.
Average single panicle weight and hundred-grain weight of Dahongpao and Meihuajiao at mature period
| Cultivars | Average single panicle weight (g) | Hundred-grain weight (g) |
|---|---|---|
| Dahongpao | 3.92 ± 1.18 | 5.80 |
| Meihuajiao | 4.96 ± 2.15 | 5.38 |
Data are expressed as mean ± standard deviation (n = 9)
Sample treatment
For each cultivar, one portion of raw material was frozen in liquid nitrogen and ground into a fine powder using a 6750 freezer-mill apparatus (Glen Creston). The powder was subsequently stored at − 80 °C until analysis. Another portion of raw material was dried in shade for 5 days and then powdered using a mechanical grinder. Every experiment was performed in triplicate.
Determination of volatile components
The volatile contents were determined as previously reported with some modifications (Xi et al., 2014; Zheng et al., 2016). Typically, 1.5 g pulp powder was homogenized in 3 mL saturated NaCl solution, and 20 μL cyclohexanol was then added as an internal standard to quantify VOCs. The solution was incubated at 40 °C for 30 min. A solid-phase microextraction needle with a 1 cm long fiber (Supelco Co., Bellefonte PA, USA) was used for volatile extraction.
A GC–MS-QP2010 gas chromatograph-mass spectrometer system (Shimadzu Corporation, Kyoto, Japan) with a Rtx-5MS capillary column (30 m × 0.32 mm × 0.5 μm, J&W Scientific, Folsom CA, USA) was used to identify the VOCs. The injection port temperature was 240 °C and the injection volume was 1 μL. Ultrapure helium was employed as the carrier gas with a flow rate of 1.0 mL/min. The GC oven temperature was programmed to hold at 40 °C for 3 min, increase to 250 °C at a rate of 4 °C min−1, and then hold at 250 °C for 5 min. Mass spectra were obtained by electron ionization at 70 eV in the scan range of 40–500 mass units. The transfer line, ion source, and detector were held at 250, 200, and 150 °C, respectively. The chromatograms and mass spectra were analyzed using GC–MS Postrun Analysis software (Shimadzu, GC–MS-QP2010, Japan). The compounds were identified by comparing their mass spectra with those in the data system library (NIST08). Semi-quantitative determinations were obtained by using cyclohexanol as the internal standard. The contents of the volatiles were calculated from their GC peak areas relative to the GC peak area of the internal standard. Three replicates were done for each sample.
Statistical analysis
All data were expressed as the mean ± standard deviation of three replicates. A multivariate PLS-DA approach (mixOmics package; Lê Cao et al., 2011) was used to analyze the differences among the volatile compositions of fresh and dried Z. bungeanum Maxim.
Results and discussion
Volatile contents of selected samples
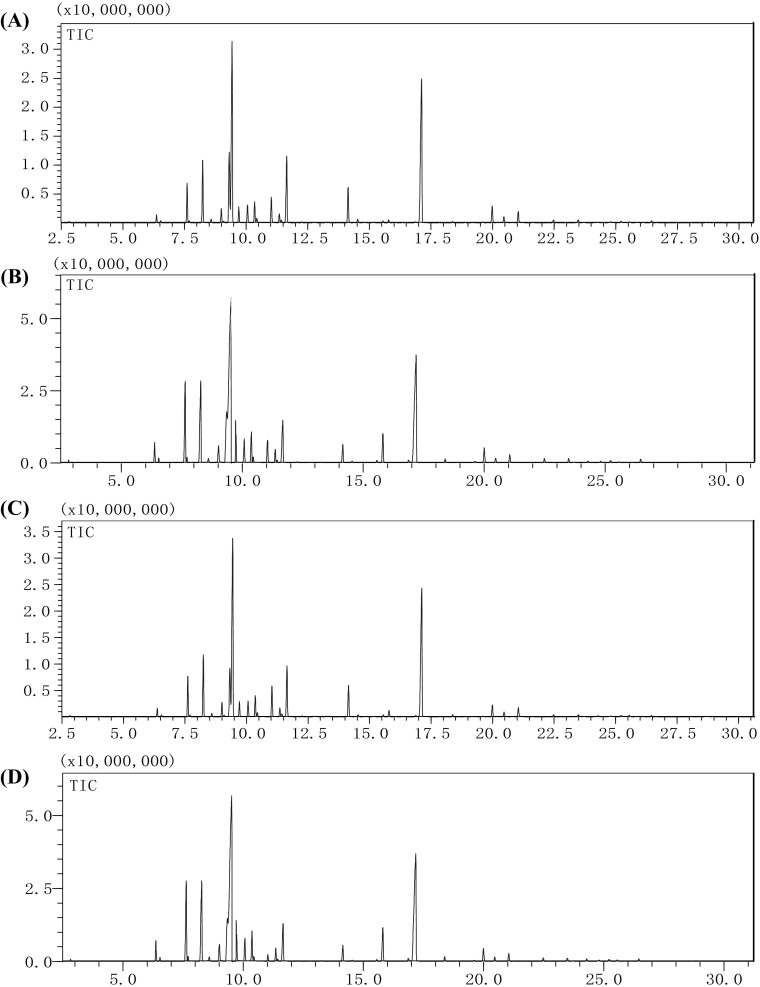
Representative total ion chromatograms of the samples are shown in Fig. 2, and results are listed in Table 2. A total of 114 major VOCs including 50 olefins, 28 alcohols, 16 esters, 8 alkanes, 9 aldehydes, and 5 ketones, were tentatively identified and semi-quantified through GC–MS. The number of VOCs identified in our study was much greater than that identified in previous reports on Z. bungeanum Maxim (Yang, 2008; Wang et al., 2010; Wei et al., 2011; Diao et al., 2013; Liu et al., 2017). Namely, 78 and 89 VOCs were found in fresh and dried Dahongpao, whereas, 79 and 80 components were identified in fresh and dried Meihuajiao. It is worth noting that, although a greater number of VOCs was observed in fresh samples than in dried samples for both Dahongpao and Meihuajiao. Drying did not remarkably change the number of VOCs observed for these Huajiao cultivars. The total VOCs percentage contents of fresh Dahongpao and Meihuajiao were 88.11% and 87.70% of the total relative area (RA) and were lower than those of dry samples (Table 2).
Fig. 2.
Stacking chart of total ion chromatograms (TICs) of volatile components in fresh and dried Dahongpao and Meihuajiao. (Dahongpao, A: fresh, B: dried; Meihuajiao, C: fresh, D: dried). X, Retention time (min); Y, intensity
Table 2.
Volatile components in fresh and dried Dahongpao and Meihuajiao
| No. | Volatile components | RA (%) | |||
|---|---|---|---|---|---|
| Dahongpao | Meihuajiao | ||||
| Fresh | Dried | Fresh | Dried | ||
| Aldehydes | |||||
| 1 | (E)-2-hexenal | 0.05 ± 0.00 | nd | 0.06 ± 0.02 | nd |
| 2 | (E,E)-2,4-hexadienal | 0.02 ± 0.00 | 0.03 ± 0.02 | 0.02 ± 0.01 | 0.01 ± 0.00 |
| 3 | Citronellal | 0.12 ± 0.00 | 0.11 ± 0.04 | 0.11 ± 0.00 | 0.10 ± 0.03 |
| 4 | (E)-2-nonenal | 0.04 ± 0.00 | 0.03 ± 0.02 | 0.03 ± 0.00 | 0.02 ± 0.02 |
| 5 | Decanal | nd | nd | nd | 0.02 ± 0.00 |
| 6 | Undecanal | 0.01 ± 0.00 | nd | 0.01 ± 0.00 | 0.01 ± 0.00 |
| 7 | 2-Undecenal | nd | nd | 0.02 ± 0.00 | nd |
| Sum | 0.24 ± 0.00 | 0.16 ± 0.08 | 0.23 ± 0.03 | 0.16 ± 0.05 | |
| Alcohols | |||||
| 8 | Eucalyptol | 7.46 ± 1.09 | 6.39 ± 0.17 | 6.79 ± 0.17 | 5.91 ± 0.31 |
| 9 | β-Terpineol | 0.52 ± 0.09 | 0.83 ± 0.25 | 0.59 ± 0.07 | 0.76 ± 0.14 |
| 10 | (Z)-nerol | 0.06 ± 0.01 | 0.09 ± 0.00 | 0.04 ± 0.00 | nd |
| 11 | Linalool oxide | 0.02 ± 0.01 | 0.10 ± 0.07 | 0.02 ± 0.01 | nd |
| 12 | Cis-p-2-menthen-1-ol | 0.21 ± 0.01 | 0.29 ± 0.11 | 0.20 ± 0.02 | 0.14 ± 0.06 |
| 13 | 8-Hydroxylinalool | nd | 0.01 ± 0.00 | nd | 0.03 ± 0.02 |
| 14 | Neoiso-isopulegol | 0.04 ± 0.02 | 0.05 ± 0.04 | 0.03 ± 0.00 | 0.02 ± 0.00 |
| 15 | 2,7-Dimethyl-1-octanol | 0.02 ± 0.00 | 0.03 ± 0.02 | 0.01 ± 0.00 | 0.02 ± 0.02 |
| 16 | 4-Terpineol | 4.10 ± 0.22 | 4.33 ± 0.16 | 4.03 ± 0.12 | 4.55 ± 0.43 |
| 17 | (s)-(−)-perillyl alcohol | nd | 0.06 ± 0.00 | nd | nd |
| 18 | 3,7-Dimethyl-2-octen-1-ol | nd | 0.10 ± 0.01 | nd | 0.02 ± 0.00 |
| 19 | Ethanol | 0.21 ± 0.00 | nd | 0.21 ± 0.02 | nd |
| 20 | Linalool | 23.89 ± 1.02 | 17.62 ± 0.40 | 21.29 ± 0.38 | 18.29 ± 0.84 |
| 21 | α-Terpineol | 1.94 ± 0.25 | 1.29 ± 0.04 | 1.50 ± 0.04 | 1.22 ± 0.09 |
| 22 | 8-p-menthene-1,2-diol | 0.05 ± 0.02 | 0.01 ± 0.00 | 0.06 ± 0.01 | 0.02 ± 0.00 |
| 23 | 1,8-cineole | 0.11 ± 0.01 | 0.13 ± 0.01 | 0.1 ± 0.00 | 0.11 ± 0.00 |
| 24 | Citronellol | 0.07 ± 0.01 | 0.10 ± 0.00 | 0.06 ± 0.00 | 0.04 ± 0.01 |
| 25 | Geraniol | 1.34 ± 0.07 | 1.11 ± 0.06 | 1.81 ± 0.03 | 1.24 ± 0.12 |
| 26 | Tetrahydrolavandulol | nd | 0.06 ± 0.01 | nd | 0.02 ± 0.00 |
| 27 | Trans-nerolidol | nd | nd | 0.02 ± 0.00 | nd |
| 28 | Nerolidol | 0.03 ± 0.00 | 0.04 ± 0.02 | 0.02 ± 0.00 | 0.02 ± 0.00 |
| 29 | τ-Cadinol | 0.07 ± 0.02 | 0.04 ± 0.01 | 0.07 ± 0.01 | 0.03 ± 0.00 |
| 30 | β-Eudesmol | 0.03 ± 0.00 | 0.03 ± 0.01 | 0.02 ± 0.00 | 0.01 ± 0.00 |
| 31 | α-Cadinol | nd | 0.05 ± 0.00 | 0.07 ± 0.02 | 0.04 ± 0.01 |
| 32 | τ-Muurolol | 0.05 ± 0.00 | 0.03 ± 0.01 | 0.07 ± 0.00 | nd |
| 33 | α-Bisabolol | nd | nd | nd | 0.02 ± 0.01 |
| 34 | Carotol | 0.03 ± 0.00 | 0.02 ± 0.01 | 0.02 ± 0.00 | nd |
| 35 | Epiglobulol | nd | 0.02 ± 0.00 | nd | nd |
| Sum | 40.25 ± 2.85 | 32.83 ± 1.41 | 37.03 ± 0.90 | 32.51 ± 2.06 | |
| Esters | |||||
| 36 | Isobutyl acetate | 0.06 ± 0.02 | 0.11 ± 0.00 | 0.08 ± 0.01 | 0.11 ± 0.00 |
| 37 | Isobutyl propionate | nd | 0.01 ± 0.00 | nd | nd |
| 38 | Methyl 3-methylpentanoate | nd | 0.02 ± 0.02 | nd | nd |
| 39 | Methyl isocaproate | 0.01 ± 0.00 | 0.01 ± 0.01 | 0.01 ± 0.00 | nd |
| 40 | Methyl 4-methyl-2-pentenoate | 0.01 ± 0.00 | 0.01 ± 0.02 | 0.01 ± 0.00 | 0.01 ± 0.00 |
| 41 | Hexyl acetate | nd | nd | 0.01 ± 0.00 | 0.02 ± 0.03 |
| 42 | Hexyl propionate | 0.02 ± 0.01 | nd | nd | nd |
| 43 | Nonyl acetate | 0.04 ± 0.01 | 0.03 ± 0.01 | 0.03 ± 0.00 | 0.04 ± 0.02 |
| 44 | Methyl phenylacetate | 0.01 ± 0.00 | 0.01 ± 0.01 | 0.01 ± 0.00 | 0.02 ± 0.02 |
| 45 | 2-Phenylethyl acetate | 0.01 ± 0.00 | 0.09 ± 0.00 | 0.02 ± 0.00 | 0.08 ± 0.00 |
| 46 | Bornyl acetate | 0.03 ± 0.00 | 0.02 ± 0.00 | 0.02 ± 0.00 | nd |
| 47 | Myrtenyl acetate | nd | 0.01 ± 0.00 | nd | nd |
| 48 | α-Terpineol acetate | 0.04 ± 0.01 | 0.04 ± 0.00 | 0.05 ± 0.01 | 0.04 ± 0.00 |
| 49 | Linalyl octanoate | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.02 ± 0.01 | 0.01 ± 0.00 |
| 50 | Trans-carveyl acetate | 0.05 ± 0.02 | nd | 0.04 ± 0.00 | nd |
| 51 | Trans-farnesol acetate | 0.02 ± 0.01 | 0.02 ± 0.00 | 0.02 ± 0.00 | 0.03 ± 0 |
| Sum | 0.27 ± 0.06 | 0.35 ± 0.08 | 0.26 ± 0.02 | 0.29 ± 0.07 | |
| Alkanes | |||||
| 52 | 1,2-Diisopropenylcyclobutane | 0.03 ± 0.00 | 0.04 ± 0.00 | 0.06 ± 0.00 | nd |
| 53 | 7-Propylidene-bicyclo[4.1.0]heptane | 0.01 ± 0.00 | nd | nd | 0.01 ± 0.00 |
| 54 | 8-Methylene-3-oxatricyclo[5.2.0.0(2,4)]nonane | nd | 0.05 ± 0.00 | nd | nd |
| 55 | Decane | 0.03 ± 0.00 | nd | 0.03 ± 0.00 | nd |
| 56 | 4-Methyl-dodecane | nd | nd | nd | 0.01 ± 0.00 |
| 57 | 1,2-Epoxy-p-menth-8-ene | nd | 0.03 ± 0.02 | 0.01 ± 0.00 | 0.02 ± 0.02 |
| 58 | 2-Methylene-4,8,8-trimethyl-4-vinyl-bicyclo[5.2.0]nonane | nd | nd | nd | 0.02 ± 0.01 |
| 59 | Hexadecane | nd | nd | nd | 0.01 ± 0.00 |
| Sum | 0.07 ± 0.00 | 0.12 ± 0.02 | 0.10 ± 0.00 | 0.07 ± 0.03 | |
| Olefins | |||||
| 60 | p-Cymene | 0.27 ± 0.01 | 0.33 ± 0.01 | 0.25 ± 0.04 | 0.23 ± 0.10 |
| 61 | α-Cubebene | 0.20 ± 0.06 | 0.25 ± 0.03 | 0.26 ± 0.03 | 0.29 ± 0.07 |
| 62 | γ-Gurjunene | 0.02 ± 0.01 | 0.05 ± 0.01 | 0.03 ± 0.01 | 0.02 ± 0.00 |
| 63 | γ-Cadinene | 0.54 ± 0.11 | 0.46 ± 0.12 | 0.38 ± 0.25 | 0.39 ± 0.07 |
| 64 | Sabinene | nd | 0.37 ± 0.01 | nd | 0.30 ± 0.02 |
| 65 | Azulene | nd | 0.03 ± 0.01 | 0.01 ± 0.00 | nd |
| 66 | Tricyclene | nd | 0.01 ± 0.01 | nd | nd |
| 67 | (−)-Camphene | 0.02 ± 0.00 | 0.04 ± 0.02 | 0.02 ± 0.00 | 0.02 ± 0.00 |
| 68 | Sabenene | 3.21 ± 0.63 | 6.53 ± 0.10 | nd | 6.60 ± 0.01 |
| 69 | β-Copaene | 0.03 ± 0.00 | nd | nd | nd |
| 70 | Styrene | 0.01 ± 0.00 | 0.01 ± 0.01 | 0.01 ± 0.00 | nd |
| 71 | 1,3,5,7-Cyclooctatetraene | 0.01 ± 0.00 | nd | 0.01 ± 0.00 | nd |
| 72 | α-Thujene | 0.60 ± 0.13 | 1.26 ± 0.02 | 0.78 ± 0.01 | 1.40 ± 0.04 |
| 73 | α-Pinene | 0.15 ± 0.03 | 0.54 ± 0.22 | 0.19 ± 0.00 | 0.34 ± 0.12 |
| 74 | β-Phellandrene | nd | nd | nd | 0.05 ± 0.00 |
| 75 | β-Pinene | 0.16 ± 0.03 | 0.55 ± 0.17 | 0.18 ± 0.00 | 0.32 ± 0.02 |
| 76 | 2-Methyl-6-methylene-1,7-octadiene | 0.01 ± 0.00 | nd | 0.01 ± 0.00 | 0.02 ± 0.00 |
| 77 | β-Myrcene | 5.63 ± 0.81 | 7.8 ± 0.29 | 6.56 ± 0.03 | 7.94 ± 0.19 |
| 78 | α-Phellandrene | 0.36 ± 0.03 | 0.48 ± 0.14 | 0.40 ± 0.01 | 0.46 ± 0.07 |
| 79 | 3-Carene | nd | nd | nd | 0.07 ± 0 |
| 80 | α-Terpilene | 1.34 ± 0.18 | 1.47 ± 0.00 | 1.55 ± 0.03 | 1.62 ± 0.04 |
| 81 | d-limonene | 21.04 ± 3.56 | 27.28 ± 2.64 | 26.11 ± 0.18 | 31.98 ± 1.19 |
| 82 | β-Ocimene | 2.48 ± 0.75 | 4.27 ± 0.07 | 3.36 ± 0.14 | 4.30 ± 0.27 |
| 83 | γ-Terpilene | 1.88 ± 0.26 | 2.22 ± 0.06 | 2.31 ± 0.04 | 2.48 ± 0.10 |
| 84 | Terpinolene | 1.51 ± 0.61 | 1.02 ± 0.00 | 1.23 ± 0.01 | 1.07 ± 0.02 |
| 85 | 1,3,8-p-menthatriene | 0.13 ± 0.01 | 0.12 ± 0.09 | 0.11 ± 0.02 | 0.12 ± 0.08 |
| 86 | 1,3,6-Heptatriene | nd | 0.06 ± 0.00 | nd | nd |
| 87 | Santolina triene | 0.01 ± 0.00 | 0.05 ± 0.01 | nd | nd |
| 88 | Cosmene | nd | nd | nd | 0.03 ± 0.02 |
| 89 | Dihydroocimen | 0.04 ± 0.00 | 0.08 ± 0.00 | nd | nd |
| 90 | Copaene | 0.05 ± 0.02 | 0.07 ± 0.01 | 0.05 ± 0.01 | 0.04 ± 0.00 |
| 91 | 7-Tetradecene | nd | 0.03 ± 0.01 | nd | nd |
| 92 | Caryophyllene | 0.35 ± 0.03 | 0.35 ± 0.04 | 0.29 ± 0.00 | nd |
| 93 | Cis-limonene oxide | nd | 0.02 ± 0.02 | nd | 0.01 ± 0.00 |
| 94 | γ-Elemene | 0.04 ± 0.00 | 0.06 ± 0.01 | 0.02 ± 0.01 | 0.03 ± 0.00 |
| 95 | Di-epi-α-cedrene | nd | 0.04 ± 0.00 | nd | 0.01 ± 0.00 |
| 96 | (+)-Epi-bicyclosesquiphellandrene | 0.03 ± 0.00 | 0.07 ± 0.04 | 0.03 ± 0.01 | 0.03 ± 0.01 |
| 97 | α-Cubebene | 0.02 ± 0.00 | 0.02 ± 0.00 | nd | nd |
| 98 | (Z,Z,Z)-1,5,9,9-tetramethyl-1,4,7-cycloundecatriene | 0.28 ± 0.17 | 0.35 ± 0.04 | 0.31 ± 0.01 | 0.33 ± 0.05 |
| 99 | (E)-β-farnesene | 0.13 ± 0.02 | 0.14 ± 0.02 | 0.12 ± 0.01 | 0.11 ± 0.02 |
| 100 | Isocaryophillene | nd | nd | 0.15 ± 0.13 | nd |
| 101 | 2-Isopropyl-5-methyl-9-methylene-bicyclo[4.4.0]dec-1-ene | 0.03 ± 0.00 | nd | nd | 0.03 ± 0.02 |
| 102 | α-Bergamotene | 0.22 ± 0.05 | 0.21 ± 0.03 | 0.21 ± 0.02 | 0.22 ± 0.04 |
| 103 | α-Muurolene | 0.04 ± 0.01 | 0.04 ± 0.01 | 0.05 ± 0.00. | 0.05 ± 0.00 |
| 104 | 8-Isopropenyl-1,5-dimethyl-cyclodeca-1,5-diene | nd | 0.03 ± 0.01 | nd | 0.02 ± 0.00 |
| 105 | β-Patchoulene | nd | 0.03 ± 0.00 | nd | 0.02 ± 0.01 |
| 106 | β-Guaiene | nd | nd | 0.01 ± 0.00 | 0.01 ± 0.00 |
| 107 | α-Farnesene | 0.31 ± 0.03 | 0.24 ± 0.03 | 0.27 ± 0.00 | 0.28 ± 0.05 |
| 108 | Germacrene B | 0.20 ± 0.13 | 0.27 ± 0.04 | 0.21 ± 0.02 | 0.24 ± 0.05 |
| 109 | 1-Hydroxy-1,7-dimethyl-4-isopropyl-2,7-cyclodecadiene | nd | 0.03 ± 0.02 | nd | 0.02 ± 0.00 |
| Sum | 41.35 ± 7.68 | 57.28 ± 4.37 | 45.48 ± 1.02 | 61.50 ± 2.68 | |
| Ketones | |||||
| 110 | 3-Nonanone | 5.87 ± 3.79 | 1.66 ± 0.3 | 4.51 ± 1.44 | 0.84 ± 0.17 |
| 111 | Trans-isopulegone | 0.01 ± 0.00 | nd | 0.02 ± 0.01 | nd |
| 112 | 1,8-Epoxy-p-menthan-2-one | nd | 0.07 ± 0.00 | nd | nd |
| 113 | Piperitone | 0.05 ± 0.00 | 0.07 ± 0.00 | 0.04 ± 0.00 | 0.02 ± 0.00 |
| 114 | 7-Geranyloxycoumarin | nd | nd | 0.03 ± 0.02 | nd |
| Sum | 5.93 ± 3.79 | 1.80 ± 0.30 | 4.60 ± 1.47 | 0.86 ± 0.17 | |
| Total | 88.11 ± 14.38 | 92.54 ± 6.26 | 87.7 ± 3.44 | 95.39 ± 5.06 | |
Data are expressed as mean ± standard deviation (n = 3)
nd not detected, RT retention time, RA relative area
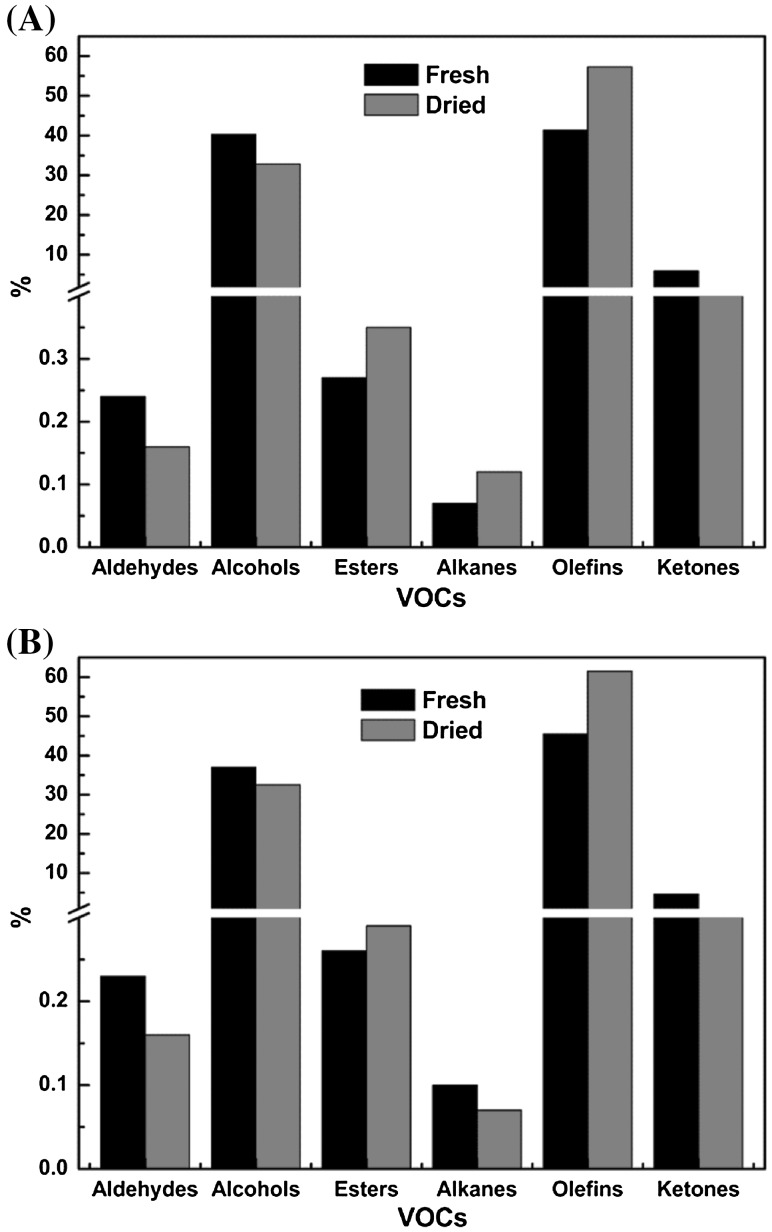
VOCs originate mainly from the enzymatic or chemical oxidation of unsaturated fatty acids, which can further interact with free amino acids, peptides, and proteins (Dashdorj et al., 2015; Ho et al., 2015). Other VOCs are derived from Maillard reactions and the Strecker degradation of free amino acids (Scalone et al., 2015). The proportion of the content of each chemical family of volatile compounds in fresh and dried Z. bungeanum Maxim samples are shown in Fig. 3. Olefins, alcohols, and ketones were identified as the principal VOCs both in fresh and dried Dahongpao samples. In fresh Dahongpao, olefins were the most abundant component, accounting for 41.35% of the RA, followed by alcohols (40.25%) and ketones (5.93%). In dried Dahongpao, the proportion of olefins, alcohols, and ketones were 57.28, 32.83, and 1.8% of the RA, respectively. The percentage of olefins in dried Dahongpao was higher than that in the fresh sample, but the percentages of alcohols, ketones and aldehydes were lower. These findings were consistent with those from previous studies (Deng et al., 2015; Zhang et al., 2015; Tian et al., 2016), and indicated that the drying process had some effect on VOC content. The reduction in the alcohol and ketone contents may have been due to the evaporation or thermal decomposition of these compounds during the drying process. In fresh Meihuajiao samples as well, the three major classes of VOCs were olefins (45.48%), alcohols (37.03%), and ketones (4.6%). The corresponding percentages of the above compounds in dried samples were 61.5%, 32.51% and 0.86%, respectively. These results showed a similar tendency as those for Dahongpao. However, fresh and dried Meihuajiao contained more olefins than Dahongpao, whereas their alcohol and ketone contents were lower. Thus, the difference in VOC composition mainly depended on the cultivar.
Fig. 3.
Percentage of different types of VOCs in Dahongpao and Meihuajiao
The major VOCs in all the samples tested were d-limonene, linalool, β-myrcene, eucalyptol, sabenene, 4-terpineol, β-ocimene, α-terpilene, γ-terpilene, geraniol, α-terpineol, terpinolene, α-thujene, and 3-nonanone. Linalool, d-limonene, eucalyptol, 3-nonanone, and β-myrcene were the most abundant VOCs in Dahongpao, accounting for 23.89%, 21.04%, 7.46%, 5.63% and 5.87% of the RA, respectively. Similarly, 27.28%, 17.62%, 6.39%, 1.66% and 7.8% of these major VOCs were found in dried Dahongpao. The linalool and β-myrcene contents of the samples increased significantly after drying, whereas the relative amount of d-limonene, eucalyptol, and 3-nonanone decreased. Similar results were also observed for fresh and dried Meihuajiao. Thus, the above results indicated that the drying process had affected the VOCs contents of both cultivars. Similar effects have been observed during the drying processes of turmeric rhizomes (Curcuma longa Linn.; Kutti Gounder and Lingamallu, 2012), mango (Mangifera indica L. cv. Kent; Adeline et al. 2016), roasted almonds (Prunus dulcis; Xiao et al., 2014), and shiitake (Lentinus edodes) mushrooms (Tian et al., 2016).
To date, a few studies have investigated the VOCs of Z. bungeanum Maxim (Yang, 2008; Gong et al., 2009; Diao et al., 2013). A previous study showed that the major VOCs of Z. bungeanum Maxim included 4-terpinenol (19.7%), 1,8-cineole (16.0%), p-cymene (7.9%), g-terpinene (7.3%), and a-terpineol (7.2%; Gong et al., 2009). However, another work reported that the major VOCs of Z. schinifolium (green huajiao) were linalool (28.2%), limonene (13.2%), sabinene (12.1%), myrcene (6.12%), linalyl acetate (3.90%), 4-terpinenol (3.72%), and β-phellandrene (3.38%; Diao et al., 2013). Moreover, yet another report indicated that the major VOCs of green huajiao were linalool (29%), limonene (14%), and sabinene (13%), but linalyl acetate (15%), linalool (13%), and limonene (12%) were the main VOCs in Dahongpao (Yang, 2008). Liu et al. (2017) also determined that the major VOCs in six Z. bungeanum Maxim cultivars including Qin’an, Danghongpao A, Danghongpao B, Danghongpao C, Meifengjiao, and Shizitou showed obvious variations based on the species, location of cultivation, and cultivar. The differences in the reported VOCs contents were attributed to the different cultivars investigated, as well as the different geographic origins of the samples and their aging (Iseli et al., 2007). In this work, we found that linalool, d-limonene, eucalyptol, 3-nonanone, and β-myrcene were the dominant volatiles in both fresh and dried Dahongpao and Meihuajiao. Moreover, we found that the contents of these VOCs were different for Dahongpao and Meihuajiao, which indicated that the major VOCs in Huajiao were mainly determined by the cultivar (Gong et al., 2009).
Characterization of VOCs in different samples by PLS-DA
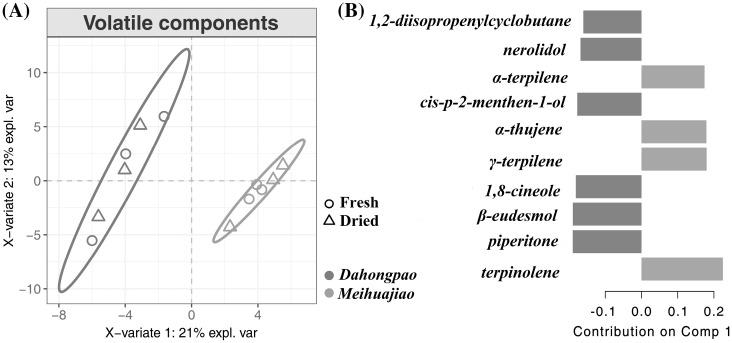
The multivariate PLS-DA method is generally used to differentiate among several groups of samples. The technique is based upon linking two data matrices, namely, the explanatory dataset X and the explicative dataset Y (Lê Cao et al., 2016; Yang et al., 2018). In this work, a PLS-DA model was developed based on the VOCs identified and two treatments (Fig. 4). In the score plot, Dahongpao and Meihuajiao formed clusters, as indicated by the blue circle and yellow circle, respectively, regardless of whether the samples were fresh or dried (Fig. 4A). This result indicated that the variety was the main factor determining the VOC composition of Z. bungeanum Maxim. However, the volatiles of the fresh and dried samples were not separated clearly, revealing that the drying process did not significantly change the VOC profile of Z. bungeanum Maxim.
Fig. 4.
Score plots (A) and loading (B) of PLS-DA for VOCs in Dahongpao and Meihuajiao
The PLS-DA loading plot was employed to display the specific VOCs to explain the differences between Dahongpao and Meihuajiao (Fig. 4B). In the loading model, α-terpilene, α-thujene, γ-terpilene, and terpinolene were the main contributors for the composition of Meihuajiao, while 1,2-diisopropenylcyclobutane, nerolidol, cis-p-2-menthen-1-ol, 1,8-cineole, β-eudesmol, and piperitone were the major contributors for Dahongpao. The results of the PLS-DA loading analysis were in agreement with the VOC contents. For example, several specific components were located at positive positions of the loading plot, indicating that these contents were higher in Meihuajiao than in Dahongpao.
The VOCs in Z. bungeanum Maxim can undergo lipid oxidation or degradation, Maillard reactions, and Strecker degradation of free amino acids during the drying process (Dashdorj et al., 2015; Deng et al., 2015; Scalone et al., 2015). In addition to the species, geographic origin, and aging, the drying conditions are an important factor affecting the composition of VOCs via the Maillard reaction and lipid oxidation (Wu and Mao, 2008; Zhang et al., 2018). In present study, the dried samples exhibited lower levels of alcohols and ketones due to the evaporation or thermal decomposition during the drying process. Therefore, results indicated that the Z. bungeanum Maxim cultivar significantly influenced the VOC composition of the resulting sample, but the drying process did not induce remarkable changes in the VOCs of Z. bungeanum Maxim.
In this work, the effect of the drying process on the VOCs in Z. bungeanum Maxim was identified and compared using two red cultivars, Dahongpao and Meihuajiao. Compared with fresh samples, the total content of VOCs increased in dried Dahongpao and Meihuajiao. Olefins were the major class of VOCs in the tested Huajiao samples, followed by alcohols and ketones. The percentages of alcohols and ketones were higher in the fresh samples than those in the dried samples, but the olefins content was lower. The d-limonene, linalool, β-myrcene, eucalyptol, and sabenene were the predominant VOCs in Dahongpao and Meihuajiao. The contents of linalool and β-myrcene in the dried Dahongpao and Meihuajiao samples were higher than those of the fresh samples, whereas the d-limonene, eucalyptol, and 3-nonanone contents were lower. However, a PLS-DA model showed that the drying process had no significant effect on the volatiles. Also, the different cultivars can be characterized by their different volatile component profiles. This study provides important information for the food processing industry and the utilization of Z. bungeanum Maxim.
Acknowledgements
This work was supported by Scientific Research Projects of Chongqing University of Arts and Sciences (2017RTZ20, P2017TZ14) and Scientific and Technological Projects of Longnan, Gansu ([2016]04).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Wenlin Zhang, Email: zhangwenlin88519@126.com.
Si Tan, Email: 764661056@qq.com.
Wanpeng Xi, Email: xwp1999@zju.edu.cn.
Jianlei Yang, Email: 936379502@qq.com.
Qinhong Liao, Email: lqhwisdom@cqwu.edu.cn.
Jianbin Lan, Email: lanjb1013@163.com.
Yukui Lv, Email: 444127784@qq.com.
Jianmin Tang, Phone: +023 49891753, Email: tangjmjy@163.com.
References
- Abuajah CI, Ogbonna AC, Osuji CM. Functional components and medicinal properties of food: a review. J. Food Sci. Technol. 2015;52:2522–2529. doi: 10.1007/s13197-014-1396-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adeline B, Renaud B, Marc L, Isabelle M, Ziya G. Aroma compounds in fresh and dried mango fruit (Mangifera indica L. cv. Kent): impact of drying on volatile composition. Int. J. Food Sci. Technol. 2016;51:789–800. doi: 10.1111/ijfs.13038. [DOI] [Google Scholar]
- Andrés-Bello A, García-Segovia P, Martínez-Monzó J. Vacuum frying: an alternative to obtain high-quality dried products. Food Eng. Rev. 2011;3:63. doi: 10.1007/s12393-011-9037-5. [DOI] [Google Scholar]
- Dashdorj D, Amna T, Hwang I. Influence of specific taste-active components on meat flavor as affected by intrinsic and extrinsic factors: an overview. Eur. Food Res. Technol. 2015;241:157–171. doi: 10.1007/s00217-015-2449-3. [DOI] [Google Scholar]
- Deng Y, Luo Y, Wang Y, Zhao Y. Effect of different drying methods on the myosin structure, amino acid composition, protein digestibility and volatile profile of squid fillets. Food Chem. 2015;171:168–176. doi: 10.1016/j.foodchem.2014.09.002. [DOI] [PubMed] [Google Scholar]
- Diao WR, Hu QP, Feng SS, Li WQ, Xu JG. Chemical composition and antibacterial activity of the essential oil from green huajiao (Zanthoxylum schinifolium) against selected foodborne pathogens. J. Agric. Food Chem. 2013;61:6044–6049. doi: 10.1021/jf4007856. [DOI] [PubMed] [Google Scholar]
- Feng ZZ, Xue ZR, Kai Z, Qiang H, Min LA, Hong G. Characterization and comparison of the pungent components in commercial Zanthoxylum bungeanum oil and Zanthoxylum schinifolium oil. J. Food Sci. 2013;78:1516–1522. doi: 10.1111/1750-3841.12236. [DOI] [PubMed] [Google Scholar]
- Gong Y, Huang Y, Zhou L, Shi X, Guo Z, Wang M, Jiang W. Chemical composition and antifungal activity of the fruit oil of Zanthoxylum bungeanum Maxim. (Rutaceae) from China. J. Essent. Oil Res. 2009;21:174–178. doi: 10.1080/10412905.2009.9700141. [DOI] [Google Scholar]
- Hiraide M, Miyazaki Y, Shibata Y. The smell and odorous components of dried shiitake mushroom, Lentinula edodes I: relationship between sensory evaluations and amounts of odorous components. J. Wood Sci. 2004;50:358–364. doi: 10.1007/s10086-003-0568-0. [DOI] [Google Scholar]
- Ho CT, Zheng X, Li S. Tea aroma formation. Food Sci. Hum. Wellness. 2015;4:9–27. doi: 10.1016/j.fshw.2015.04.001. [DOI] [Google Scholar]
- Iseli V, Potterat O, Hagmann L, Egli J, Hamburger M. Characterization of the pungent principles and the essential oil of Zanthoxylum schinifolium pericarp. Pharmazie. 2007;62:396–400. [PubMed] [Google Scholar]
- Kader AA. Flavor quality of fruits and vegetables. J. Sci. Food Agric. 2008;88:1863–1868. doi: 10.1002/jsfa.3293. [DOI] [Google Scholar]
- Kutti Gounder D, Lingamallu J. Comparison of chemical composition and antioxidant potential of volatile oil from fresh, dried and cured turmeric (Curcuma longa) rhizomes. Ind. Crop Prod. 2012;38:124–131. doi: 10.1016/j.indcrop.2012.01.014. [DOI] [Google Scholar]
- Lê Cao KA, Boitard S, Besse P. Sparse PLS discriminant analysis: biologically relevant feature selection and graphical displays for multiclass problems. BMC Bioinformatics. 2011;12:253. doi: 10.1186/1471-2105-12-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lê Cao KA, Costello ME, Lakis VA, Bartolo F, Chua XY, Brazeilles R, Rondeau P. MixMC: a multivariate statistical framework to gain insight into microbial communities. PLoS One. 2016;11:e0160169. doi: 10.1371/journal.pone.0160169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan Y, Li H, Chen Y, Zhang Y, Liu N, Zhang Q, Wu Q. Essential oil from Zanthoxylum bungeanum Maxim. and its main components used as transdermal penetration enhancers: a comparative study. J. Zhejiang Univ. Sci. B. 2014;15:940–952. doi: 10.1631/jzus.B1400158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu ZL, Chu SS, Jiang GH. Feeding deterrents from Zanthoxylum schinifolium against two stored-product insects. J. Agr. Food Chem. 2009;57:10130–10133. doi: 10.1021/jf9012983. [DOI] [PubMed] [Google Scholar]
- Liu S, Wang S, Song S, Zou Y, Wang J, Sun B. Characteristic differences in essential oil composition of six Zanthoxylum bungeanum Maxim. (Rutaceae) cultivars and their biological significance. J. Zhejiang Univ. Sci. B. 2017;18:917–920. doi: 10.1631/jzus.B1700232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scalone GLL, Cucu T, De Kimpe N, De Meulenaer B. Influence of free amino acids, oligopeptides, and polypeptides on the formation of pyrazines in maillard model systems. J. Agric. Food Chem. 2015;63:5364–5372. doi: 10.1021/acs.jafc.5b01129. [DOI] [PubMed] [Google Scholar]
- Song Y, Ke J, Li S, Shen G, Luo Q, Wu H, Liu X, Chen A, Zhang Z. Comparison and optimization of two extract methods (atmospheric pressure and pressurized pretreatment) of pectin from Zanthoxylum bungeanum Maxim. seeds by response surface methodology. Sep. Sci. Technol. 2017;52:1806–1814. doi: 10.1080/01496395.2017.1294604. [DOI] [Google Scholar]
- Tao X, Peng W, Xie D, Zhao C, Wu C. Quality evaluation of Hanyuan Zanthoxylum bungeanum Maxim. using computer vision system combined with artificial neural network: a novel method. Int. J. Food Prop. 2017;20:3056–3063. doi: 10.1080/10942912.2016.1271808. [DOI] [Google Scholar]
- Tian Y, Zhao Y, Huang J, Zeng H, Zheng B. Effects of different drying methods on the product quality and volatile compounds of whole shiitake mushrooms. Food Chem. 2016;197:714–722. doi: 10.1016/j.foodchem.2015.11.029. [DOI] [PubMed] [Google Scholar]
- Wang L, Wang Z, Li X, Zhang H, Zhou X, Zhang H. Analysis of volatile compounds in the pericarp of Zanthoxylum bungeanum Maxim. by ultrasonic nebulization extraction coupled with headspace single-drop Microextraction and GC–MS. Chromatographia. 2010;71:455–459. doi: 10.1365/s10337-010-1497-x. [DOI] [Google Scholar]
- Wei S, Zhang H, Wang Y, Wang L, Li X, Wang Y, Zhang H, Xu X, Shi Y. Ultrasonic nebulization extraction-heating gas flow transfer-headspace single drop microextraction of essential oil from pericarp of Zanthoxylum bungeanum Maxim. J. Chromatogr. A. 2011;1218:4599–4605. doi: 10.1016/j.chroma.2011.05.047. [DOI] [PubMed] [Google Scholar]
- Wu T, Mao L. Influences of hot air drying and microwave drying on nutritional and odorous properties of grass carp (Ctenopharyngodon idellus) fillets. Food Chem. 2008;110:647–653. doi: 10.1016/j.foodchem.2008.02.058. [DOI] [Google Scholar]
- Xi W, Zhang Q, Lu X, Wei C, Yu S, Zhou Z. Improvement of flavour quality and consumer acceptance during postharvest ripening in greenhouse peaches by carbon dioxide enrichment. Food Chem. 2014;164:219–227. doi: 10.1016/j.foodchem.2014.05.017. [DOI] [PubMed] [Google Scholar]
- Xia L, You J, Li G, Sun Z, Suo Y. Compositional and antioxidant activity analysis of Zanthoxylum bungeanum seed oil obtained by supercritical CO2 fluid extraction. J. Am. Oil Chem. Soc. 2011;88:23–32. doi: 10.1007/s11746-010-1644-4. [DOI] [Google Scholar]
- Xiao L, Lee J, Zhang G, Ebeler SE, Wickramasinghe N, Seiber J, Mitchell AE. HS-SPME GC/MS characterization of volatiles in raw and dry-roasted almonds (Prunus dulcis) Food Chem. 2014;151:31–39. doi: 10.1016/j.foodchem.2013.11.052. [DOI] [PubMed] [Google Scholar]
- Yang X. Aroma constituents and alkylamides of red and green huajiao (Zanthoxylum bungeanum and Zanthoxylum schinifolium) J. Agr. Food Chem. 2008;56:1689–1696. doi: 10.1021/jf0728101. [DOI] [PubMed] [Google Scholar]
- Yang LC, Li R, Tan J, Jiang ZT. Polyphenolics composition of the leaves of Zanthoxylum bungeanum Maxim. grown in Hebei, China, and their radical scavenging activities. J. Agric. Food Chem. 2013;61:1772–1778. doi: 10.1021/jf3042825. [DOI] [PubMed] [Google Scholar]
- Yang YQ, Yin HX, Yuan HB, Jiang YW, Dong CW, Deng YL. Characterization of the volatile components in green tea by IRAE-HS-SPME/GC-MS combined with multivariate analysis. PLoS One. 2018;13:e0193393. doi: 10.1371/journal.pone.0193393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Qin W, Lin D, Shen Q, Saleh ASM. The changes in the volatile aldehydes formed during the deep-fat frying process. J. Food Sci. Technol. 2015;52:7683–7696. doi: 10.1007/s13197-015-1923-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Wang J, Zhu L, Li T, Jiang W, Zhou J, Peng W, Wu C. Zanthoxylum bungeanum Maxim. (Rutaceae): a systematic review of its traditional uses, botany, phytochemistry, pharmacology, pharmacokinetics, and toxicology. Int. J. Mol. Sci. 2017;18:2172. doi: 10.3390/ijms18102172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Liu X, Yang Z, Song H, Zhang Y, Jin Y. Effect of soaking and temperature process on the volatile compounds in soymilk made by soymilk maker. J. Food Sci. Tech. 2018;55:1591–1598. doi: 10.1007/s13197-018-3072-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Zhang Q, Quan J, Zheng Q, Xi W. Determination of sugars, organic acids, aroma components, and carotenoids in grapefruit pulps. Food Chem. 2016;205:112–121. doi: 10.1016/j.foodchem.2016.03.007. [DOI] [PubMed] [Google Scholar]