Abstract
The roots of Rehmannia glutinosa (RG) have been widely used for medicinal purposes in Asia. The traditional processing of RG involves repetitive steaming and drying, and 9-time-steamed RG (NSRG) is the most commonly consumed form. For a development of a convenient processing method, RG was puffed at various pressures resulting in significantly increased solid extraction yield by up to 14%. The amount of the Maillard reaction product 5-hydroxymethylfurfural and the antioxidant capacities determined by the ABTS and DPPH radical scavenging assays were enhanced at increasing puffing pressure. Treatment of lipopolysaccharide-stimulated RAW 264.7 macrophages with RG extracts revealed that puffing of RG enhanced its suppression of the pro-inflammatory cytokine IL-6 by up to 37%. The 5-hydroxymethylfurfural contents, ABTS/DPPH radical scavenging capacities, and IL-6 regulatory effects of puffed RG samples were greater than those of the NSRG control, indicating that puffing is a desirable processing technique for development of nutraceuticals using RG.
Keywords: Puffing, Cytokine, Antioxidant, Extraction yield, Rehmannia glutinosa
Introduction
The roots of Rehmannia glutinosa (RG), generally known as Jihwang in Korea, are widely used as an herb in traditional oriental medicine. Previous studies have shown that RG has anti-cancer (Xu et al., 2017a, 2017b), blood-glucose regulatory (Zhang et al., 2004), anti-inflammatory (Wang et al., 2015), antioxidant (Zhang et al., 2004), and immunoregulatory (Kim et al., 1998) effects. Among the three forms in which RG is consumed, i.e., raw, dried, and steamed, the most commonly consumed form is 9-time-steamed RG (NSRG), which is prepared by repetition of alcohol soaking, steaming, and drying (Hong et al., 1993). The repetition of these processes is thought to soften the physical matrices of the roots, increasing the extraction of bioactive compounds. With respect to chemical changes, the heating process degrades complex polymers into smaller molecules, increasing the bioavailability and functionality of the effective ingredients. Despite the health benefits of NSRG, this manufacturing process is complex, costly, and time-consuming. In addition, the use of soaking solution and incomplete drying may cause microbial contamination. Therefore, there is demand for alternative processing methods for RG.
Gun puffing is a well-accepted food processing method that alters the physicochemical properties of food matrices and ingredients (Mariotti et al., 2006). Briefly, food material in a chamber of a semi-closed system is heated to increase chamber pressure. Once the proper pressure is reached, the chamber is opened to instantly lower the chamber pressure. Due to internal expansion involving evaporation of moisture from the food, the matrix becomes fragmented, and the food volume increases. Application of heat and pressure also triggers chemical changes, including the Maillard reaction. Indeed, it was previously reported that puffing yielded Maillard reaction products (MRPs) including 5-hydroxymethylfurfural (5-HMF) in wheat kernels (Cattaneo et al., 2015).
In an effort to apply puffing to replace the traditional 9-time steaming of RG, the current study examined the effects of puffing pressure on extraction yield and bio-functionality of RG. In detail, the antioxidant and anti-inflammatory properties of extracts of puffed RG were investigated and compared with those of a non-puffed control.
Materials and Methods
Reagents
Dried RG and control NSRG were purchased from a local market (Gyeongdong Market, Seoul, Korea), and genetic identification was performed by the Plant DNA Bank in Korea. Dulbecco’s modified Eagle’s medium (DMEM), fetal bovine serum (FBS), and antibiotic/antimycotic solution were purchased from Welgene (Gyeongsan, Korea). Lipopolysaccharide (LPS), phosphate-buffered saline (PBS), ABTS, 2,2′-azobis(2-methylpropionamidine) dihydrochloride (AAPH), 5-HMF, and ascorbic acid were purchased from Sigma-Aldrich (St. Louis, MO, USA), while DPPH was purchased from Aladdin (Shanghai, China). An enzyme-linked immunosorbent assay (ELISA) kit for interleukin-6 (IL-6) was purchased from eBioscience (San Diego, CA, USA). Ethanol and methanol were purchased from Daejung (Siheung, Korea).
Puffing of RG
RG was puffed by a gun puffing method as previously reported, in which puffing condition for plant roots was optimized for prevention of carbonization yet yielding the highest extraction (Chang et al., 2014; Kim et al., 2008). Briefly, dried RG was mixed with rice at a ratio of 1:5 (w/w) in a preheated chamber to optimize the puffing conditions by preventing carbonization of RG at high temperature. This mixture was further heated to increase the chamber pressure to 490.4 kPa, after which the valve was opened to release the pressure to 294.3 kPa. The chamber was reheated until the chamber pressure was elevated to 685.5, 784.5, 882.6, or 980.7 kPa, and the chamber was instantly opened to inflate the puffed materials. Puffed RG was stored at − 20 °C in dark conditions until use.
Ambient ethanol extraction
Dried RG, puffed RG, and NSRG were ground with a commercial blender (NB600A, Prische, Seoul, Korea), and 5 g of each powder was extracted in 100 mL of 70% ethanol while stirring at room temperature for 30 min. The extracts were passed through a funnel in a filtering flask with no. 53 filter paper (Hyundai Micro, Seoul, Korea). An aliquot of the filtrate was dried on an aluminum dish in a hot-air dryer (HB-502M, Han Beak Scientific Co., Bucheon, Korea) at 105 °C for 24 h, and the extraction yield was calculated by the equation below. Extracts were concentrated using a rotary vacuum evaporator (N-11, Eyela, Tokyo, Japan) and stored at 4 °C.
where W, weight of the sample (g); E, total volume of extract (mL); E′, used volume of extract (mL); W1, weight of the aluminum dish (g); W2, weight of the aluminum dish and solids (g).
Assessment of 5-HMF content and antioxidant capacity
An end-product of the Maillard reaction, 5-HMF, was quantified by the Biocenter of Gyeonggido Business & Science Accelerator through a previously reported HPLC method (Castellari et al., 2001). The ABTS radical scavenging activity of each extract was measured by a modified version of a previously reported method (Kim and Lee, 2004). In brief, a mixture of 1.0 mM AAPH, 2.5 mM ABTS, and PBS was stirred for 30 min at 70 °C. After filtration through a 0.22-μm syringe filter, 980 μL of the ABTS solution was added to 20 μL of each extract. The mixtures were reacted for 10 min at 37 °C, the absorbance was measured at 734 nm, and blank subtraction was performed. Ascorbic acid served as a standard, and the radical scavenging activity of the extract was expressed as μg vitamin C equivalents (VCE)/g dry RG.
In parallel, the DPPH free radical scavenging activity of each extract was measured in a 96-well plate using a microplate reader (Bio-Rad, Hercules, CA, USA). Briefly, 0.2 mM DPPH free radical solution was prepared in 80% methanol. Then, 100 μL of the DPPH solution was added to 100 μL of each extract, and the mixture was shaken vigorously and left in the dark for 30 min. Reduction of the DPPH radical was determined based on the absorbance at 515 nm. The percentage of DPPH radical scavenging was calculated by the following equation.
where Abs sample or Abs control is the absorbance of DPPH• solution with or without extracts, respectively.
In vitro assessment of inflammatory responses
RAW 264.7 murine macrophages were obtained from the Korea Cell Line Bank (Seoul, Korea). Cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% antibiotic/antimycotic solution (10,000 U/mL penicillin G, 10,000 μg/mL streptomycin, 25 μg/mL amphotericin B) at 37 °C in a 5% CO2 incubator. Cells (1 × 105 cells/mL) were pretreated with each extract at 100 μg/mL for 24 h and then stimulated by incubation with 500 ng/mL of LPS for 12 h.
Following supernatant harvest by centrifugation at 300×g, the cellular production of the pro-inflammatory cytokine IL-6 in the culture medium was quantified by a sandwich ELISA kit according to the manufacturer’s instructions. Briefly, a diluted capture antibody was added to a 96-well plate and incubated overnight at 4 °C. The excess antibody was removed from the plate by 3 washes with sterile PBS. A 200 μL aliquot of the assay diluent was added to each well and incubated for 1 h at room temperature to prevent non-specific binding. Excess assay diluent was removed by 3 washes with sterile PBS. Then, 100 μL of the diluted standards and culture supernatants were added to each well and incubated for 2 h, followed by the addition of a 100 μL mixture of detection antibody and a streptavidin–horseradish peroxidase conjugate (SAv-HRP). After washing, 100 μL of substrate solution was added and incubated for 30 min in the dark, and the reaction was terminated by addition of 50 μL of stop solution. Lastly, IL-6 was quantified based on absorbance at 450 nm, for which standards were provided in the kit.
Statistical analysis
Data are representative of repeated experiments and are expressed as the mean ± standard error of the mean (SEM, n = 4–5). Different letters in a set of data indicate significant differences at p < 0.05, as determined by one-way ANOVA and Tukey’s post hoc test in Prism 5.0 (GraphPad Software, La Jolla, CA, USA).
Results and discussion
Increase in extraction yield
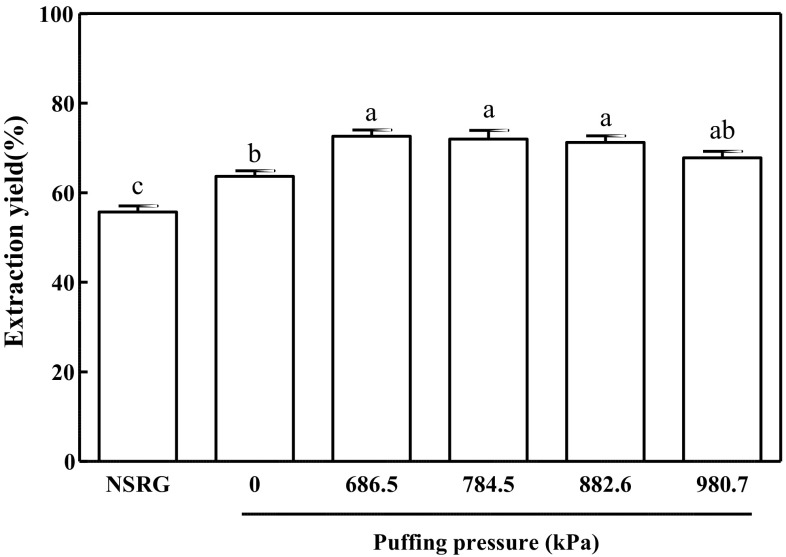
There have been multiple reports that puffing enhanced the extraction yields of plant-derived foods (An et al., 2011; Kim et al., 2008; Yoon et al., 2010). Given that RG is generally consumed as an extract for both nutritional and medicinal purposes, the solid extraction yield of RG is critical for its industrial application. Therefore, the current study investigated whether puffing would also enhance the extract yields of RG. For this purpose, non-puffed RG served as a negative control, whereas traditionally prepared NSRG served as a positive control. As shown in Fig. 1, the extraction yield of RG puffed at 686.5 kPa (72.60 ± 1.44%) was 14% greater than that of non-puffed RG (63.67 ± 1.27%). Of interest, the extraction yield of NSRG (55.67 ± 1.38%) was less than that of non-puffed RG. Similarly, it was previously reported that the extraction yield and crude saponin content of ginseng extract were increased by puffing at 784.5 kPa (Kim et al., 2008), indicating that puffing is a favorable method of processing plant roots as foods.
Fig. 1.
Extraction yields of puffed Rehmannia glutinosa
Enhancement of 5-HMF content
The Maillard reaction is a well-characterized chemical reaction of sugars and amino acids that mainly result from heating. The end-products of this reaction are known as Maillard reaction products (MRPs), including 5-HMF, which is used as an indicator of NSRG quality (Baek et al., 2012; Lee et al., 2002). In the present study, unprocessed RG was found to contain a trace amount of 5-HMF (0.01 ± 0.01 μg/mg, Fig. 2), while traditional processing with repeated steaming and drying induced the Maillard reaction, resulting in increased concentration of 5-HMF in NSRG (3.36 ± 0.21 μg/mg 5-HMF in NSRG). Of interest, the 5-HMF content of puffed RG increased significantly in proportion to puffing pressure. The quantities of 5-HMF in puffed RG were 0.84 ± 0.05, 4.60 ± 0.20, 8.31 ± 0.14, and 16.27 ± 0.71 μg/mg at puffing pressures of 686.5, 784.5, 882.6, and 980.7 kPa, respectively.
Fig. 2.
5-HMF content of puffed Rehmannia glutinosa
Augmentation of antioxidant capacity
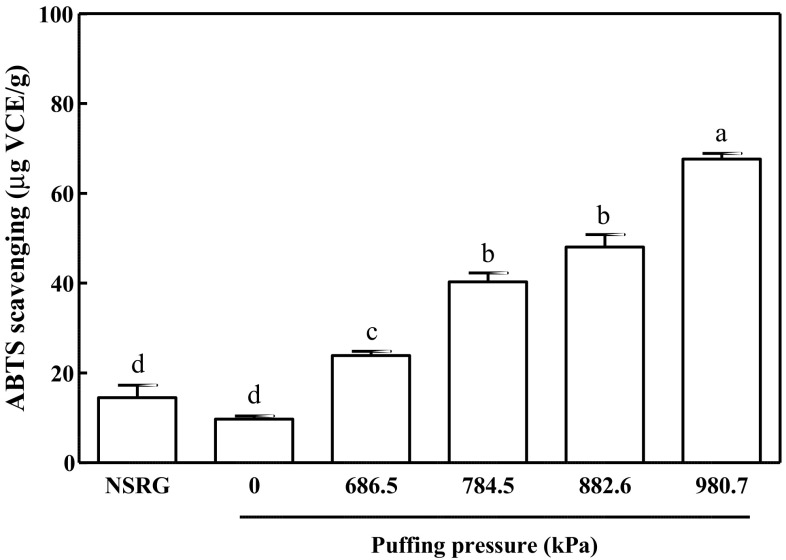
Previous studies have shown that MRPs including 5-HMF have antioxidant properties (Li et al., 2009; Patrignani et al., 2016; Yilmaz and Toledo, 2005). The ABTS and DPPH radical scavenging assays are commonly used for colorimetric assessment of antioxidant capacities of food ingredients. Upon interacting with an antioxidant, the blue-green ABTS anion radical solution loses its reactivity and turns light blue. In the ABTS assay, the antioxidant capacity of NSRG (14.50 ± 2.80 μg VCE/g) exhibited no significant difference from non-puffed RG (9.701 ± 0.72 μg VCE/g) (Fig. 3). In contrast, puffing of RG at pressures of 686.5, 784.5, 882.6, and 980.7 kPa significantly increased the antioxidant capacities of the extracts to 23.88 ± 0.92, 40.26 ± 2.00, 48.05 ± 2.78, and 67.62 ± 1.25 μg VCE/g, respectively.
Fig. 3.
ABTS radical scavenging activity of puffed Rehmannia glutinosa
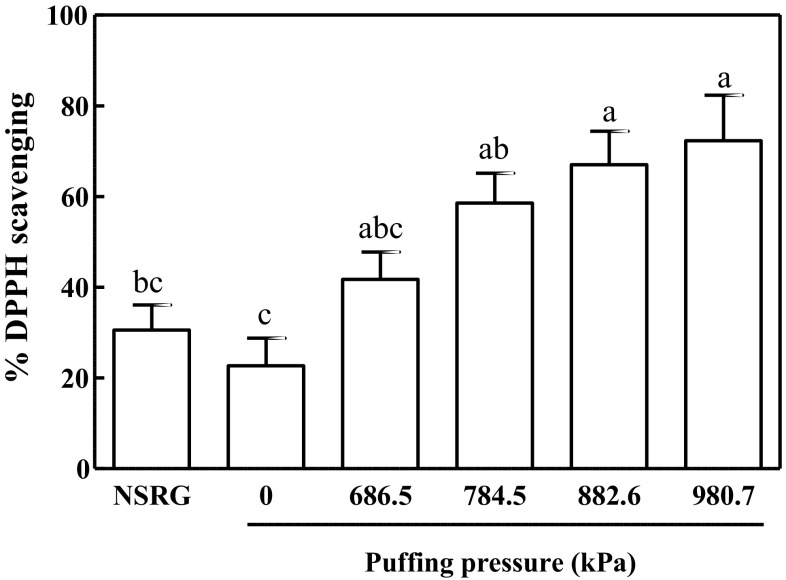
The DPPH radical scavenging assay, in which a purple radical solution becomes pale yellow upon interacting with an antioxidant, revealed that the % DPPH radical scavenging capacity increased in proportion to puffing pressure. In detail, puffing of RG at 686.5, 784.5, 882.6, and 980.7 kPa resulted in 41.73 ± 6.07, 58.59 ± 6.57, 67.04 ± 7.36, and 72.27 ± 10.10% DPPH scavenging, respectively, compared to 22.68 ± 6.08% DPPH scavenging by non-puffed RG (Fig. 4). Of interest, NSRG had no greater DPPH scavenging capacity (30.57 ± 5.51%) than non-puffed RG, indicating that puffing is a desirable process for enhancement of antioxidant capacity of RG.
Fig. 4.
DPPH radical scavenging activity of puffed Rehmannia glutinosa
It should be noted that the two antioxidant capacity assays, the ABTS and DPPH scavenging assays, utilize distinct solutions. The ABTS assay is carried out in an aqueous environment, whereas the DPPH method is performed with an 80% organic medium. Given that the extracts were prepared in 70% ethanol, the effective antioxidant molecules in NSRG may have been alcohol-soluble, contributing to the improvement of DPPH scavenging assay results. The water-soluble molecules, however, also seemed to be effective for antioxidant properties as observed by ABTS radical scavenging. In this regard, Serpen et al. (2007) reported that DPPH is more sensitive to assessment of anti-oxidant capacity of MRPs, including 5-HMF, whereas ABTS is suitable for phenolic-containing coumpounds. Nevertheless, it is remarkable that the 5-HMF contents, ABTS and DPPH radical scavenging capacities of the puffed RG extracts correlated strongly with puffing pressure.
Regulation of IL-6 production
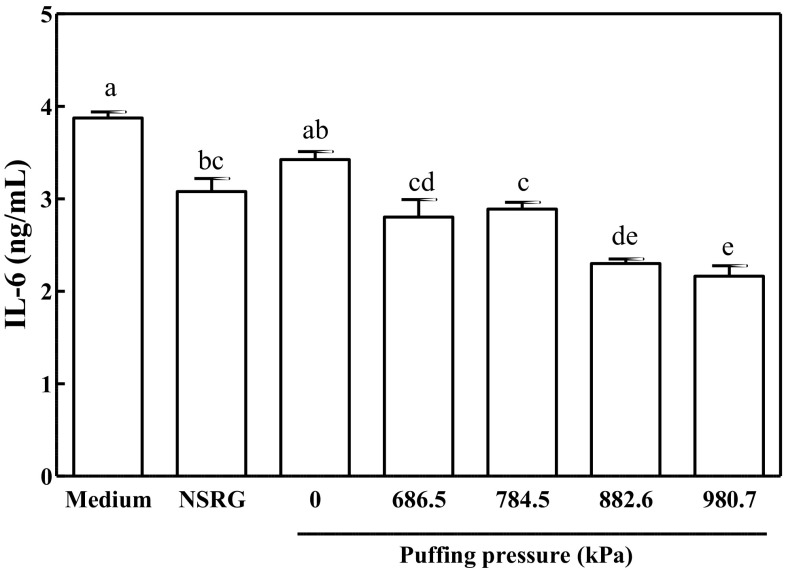
Recently, nutraceutical use of phytochemicals to regulate inflammatory responses has been a focus of research (Koh et al., 2018; Saqub et al., 2018). RG has also been investigated for its anti-inflammatory properties, both in vitro (Baek et al., 2012; Han et al., 2012) and in vivo (Zhou et al., 2015). The current study examined whether puffing would influence the secretion of the pro-inflammatory cytokine IL-6 in LPS-stimulated RAW 264.7 macrophages. Since extraction yield was affected by puffing pressure (Fig. 1), the final solid content of the culture medium was diluted to 100 μg/mL prior to cell treatment. As shown in Fig. 5, production of IL-6 was significantly lower in the NSRG-treated positive control (3.08 ± 0.14 ng/mL) than in the medium-treated negative control (3.88 ± 0.06 ng/mL). Incubation of cells with the non-puffed RG extract had no effect on IL-6 production (3.42 ± 0.09 ng/mL), but puffing of RG suppressed the release of this cytokine to 2.80 ± 0.19, 2.89 ± 0.07, 2.30 ± 0.05, and 2.16 ± 0.11 ng/mL at 686.6, 784.5, 882.6, and 980.7 kPa, respectively). As for suppression of cytokine production by 5-HMF, it was previously reported that 5-HMF effectively attenuated the nuclear translocation of nuclear factor (NF)-κB by increased expression of inhibitor of NF-κB (IκB)-α in the cytoplasm (Kim et al., 2011). Considering that NF-κB has long been known for a key cellular signaling for IL-6 production (Libermann and Baltimore, 1990), it is supposed that increased 5-HMF in puffed RG augments IκB, resulting in the suppressed NF-κB nuclear translocation and downstream cytokine productions.
Fig. 5.
IL-6 secretion of macrophages cultivated with puffed Rehmannia glutinosa
Baek et al. (2012) previously reported that extracts of raw RG (up to a concentration of 1 mg/mL) suppressed reactive oxygen species and inflammatory cytokine production in macrophages. It should also be highlighted that the model cell line was THP-1 human cells, and cytokines were quantified in terms of mRNA levels by RT-PCR. The current observation in murine cells indicates that 100 μg/mL of non-puffed RG extract is not effective for regulating inflammatory responses. In this regard, it was previously reported that the bioavailability of dietary polyphenols is extremely low; in fact, only 0.1% of (−)-epigallocatechin-3-gallate (EGCG) in tea was judged to be bioavailable in vivo (Chen et al., 1997). With regard to the active compounds for anti-inflammatory effects of RG, previous studies indicated 2,5-dihydroxyacetophenone (Han et al., 2012) and catalpol (Baek et al., 2012) as putative contributors, even though most studies utilized the crude extracts of RG.
Overall, the current study investigated the beneficial effects of puffing RG, a simple process replacing the traditional repetitions of steaming and drying. The results clearly demonstrated that puffing increased the extraction yield, the content of 5-HMF, and the antioxidant capacities of the RG. Puffed RG extracts also exhibited suppressed IL-6 production indicating enhanced anti-inflammatory properties at equivalent solid concentrations. These observations imply that puffing may be a promising process for the nutraceutical application of RG.
Acknowledgements
This research was supported by a Grant (D171701) from Gyeonggi Technology Development Program funded by Gyeonggi Province. This study was also supported by Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, and Forestry (Project No. 116021-3).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yeji Kwon, Email: dcgirl111@naver.com.
Seungmin Yu, Email: dbtmdals1004@hanmail.net.
Gwang Su Choi, Email: cgs91@naver.com.
Jang Hwan Kim, Email: zongy5000@naver.com.
Mooyeol Baik, Email: mooyeol@khu.ac.kr.
Seung Tae Su, Email: bibongherb@bibongherb.com.
Wooki Kim, Phone: +82-31-201-3482, Email: kimw@khu.ac.kr.
References
- An YE, Ahn SC, Yang DC, Park SJ, Kim BY, Baik MY. Chemical conversion of ginsenosides in puffed red ginseng. LWT-Food Sci. Technol. 2011;44:370–374. doi: 10.1016/j.lwt.2010.09.013. [DOI] [Google Scholar]
- Baek G-H, Jang Y-S, Jeong S-I, Cha J, Joo M, Shin S-W, Ha K-T, Jeong H-S. Rehmannia glutinosa suppresses inflammatory responses elicited by advanced glycation end products. Inflammation. 2012;35:1232–1241. doi: 10.1007/s10753-012-9433-x. [DOI] [PubMed] [Google Scholar]
- Castellari M, Sartini E, Spinabelli U, Riponi C, Galassi S. Determination of carboxylic acids, carbohydrates, glycerol, ethanol, and 5-HMF in beer by high-performance liquid chromatography and UV-refractive index double detection. J. Chromatogr. Sci. 2001;39:235–238. doi: 10.1093/chromsci/39.6.235. [DOI] [PubMed] [Google Scholar]
- Cattaneo S, Hidalgo A, Masotti F, Stuknytė M, Brandolini A, DeNoni I. Heat damage and in vitro starch digestibility of puffed wheat kernels. Food Chem. 2015;188:286–293. doi: 10.1016/j.foodchem.2015.05.019. [DOI] [PubMed] [Google Scholar]
- Chang YJ, Choi H-W, Kim H-S, Lee H, Kim W, Kim DO, Kim B-Y, Baik M-Y. Physicochemical properties of granular and non-granular cationic starches prepared under ultra high pressure. Carbohydr. Polym. 2014;99:385–393. doi: 10.1016/j.carbpol.2013.09.010. [DOI] [PubMed] [Google Scholar]
- Chen L, Lee MJ, Li H, Yang CS. Absorption, distribution, elimination of tea polyphenols in rats. Drug Metab. Dispos. 1997;25:1045–1050. [PubMed] [Google Scholar]
- Han Y, Jung HW, Lee JY, Kim JS, Kang SS, Kim YS, Park Y-K. 2,5-dihydroxyacetophenone isolated from Rehmanniae Radix Preparata inhibits inflammatory responses in lipopolysaccharide-stimulated RAW264.7 macrophages. J. Med. Food. 2012;15:505–510. doi: 10.1089/jmf.2011.1940. [DOI] [PubMed] [Google Scholar]
- Hong SP, Kim YC, Kim KH, Park JH, Park MK. Characteristic component of Rehmanniae Radix Preparata compared to Rehmanniae Radix and Rehmanniae Radix Crudus. Anal. Sci. Technol. 1993;6:401–404. [Google Scholar]
- Kim J, Ahn S, Choi S, Hur N, Kim B, Baik M. Changes in effective components of ginseng by puffing. Appl. Biol. Chem. 2008;51:188–193. [Google Scholar]
- Kim HK, Choi Y-W, Lee EN, Park JK, Kim S-G, Park D-J, Kim B-S, Lim Y-T, Yoon S. 5-Hydroxymethylfurfural from black garlic extract rrevents TNFα-induced monocytic cell adhesion to HUVECs by suppression of vascular cell adhesion molecule-1 expression, reactive oxygen species generation and NF-κB activation. Phytother. Res. 2011;25:965–974. doi: 10.1002/ptr.3351. [DOI] [PubMed] [Google Scholar]
- Kim D, Lee C. Comprehensive study on vitamin C equivalent antioxidant capacity (VCEAC) of various polyphenolics in scavenging a free radical and its structural relationship. Crit. Rev. Food Sci. Nutr. 2004;44:253–273. doi: 10.1080/10408690490464960. [DOI] [PubMed] [Google Scholar]
- Kim H, Lee E, Lee S, Shin T, Kim Y, Kim J. Effect of Rehmannia glutinosa on immediate type allergic reaction. Int. J. Immunopharmacol. 1998;20:231–240. doi: 10.1016/S0192-0561(98)00037-X. [DOI] [PubMed] [Google Scholar]
- Koh YC, Yang G, Lai CS, Weerawatanakorn M, Pan MH. Chemopreventive effects of phytochemicals and medicines on M1/M2 polarized macrophage role in inflammation-related diseases. Int. J. Mol. Sci. 2018;19:E2208. doi: 10.3390/ijms19082208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J-H, Go J-A, Hwang E-Y, Hong S-P. Quantitative determination of 5-hydroxymethyl-2-furaldehyde from Rehmanniae Radix Preparata according to various processings. Korean J. Herbol. 2002;17:145–149. [Google Scholar]
- Li Y-X, Li Y, Qian ZJ, Kim M-M, Kim S-K. In vitro antioxidant activity of 5-HMF isolated from marine red alga Laurencia undulata in free-radical-mediated oxidative systems. J. Microbiol. Biotechnol. 2009;19:1319–1327. doi: 10.4014/jmb.0901.00004. [DOI] [PubMed] [Google Scholar]
- Libermann TA, Baltimore D. Activation of interleukin-6 gene expression through the NF-kappa B transcription factor. Mol. Cell. Biol. 1990;10:2327–2334. doi: 10.1128/MCB.10.5.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariotti M, Alamprese C, Pagani MA, Lucisano M. Effect of puffing on ultrastructure and physical characteristics of cereal grains and flours. J. Cereal Sci. 2006;43:47–56. doi: 10.1016/j.jcs.2005.06.007. [DOI] [Google Scholar]
- Patrignani M, Rinaldi GJ, Lupano CE. In vivo effects of Maillard reaction products derived from biscuits. Food Chem. 2016;196:204–210. doi: 10.1016/j.foodchem.2015.09.038. [DOI] [PubMed] [Google Scholar]
- Saqub U, Sarkar S, Suk K, Mohammad O, Baig MS, Savai R. Phytochemicals as modulators of M1-M2 macrophages in inflammation. Oncotarget. 2018;9:17937–17950. doi: 10.18632/oncotarget.24788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serpen A, Capuano E, Fogliano V, Gökmen V. A ne prodecure to measure the antioxidant activity of insoluble food components. J. Agric. Food Chem. 2007;55:7676–7681. doi: 10.1021/jf071291z. [DOI] [PubMed] [Google Scholar]
- Wang J-L, Meng X, Lu R, Wu C, Luo Y-T, Yan X, Li X-J, Kong X-H, Nie G-X. Effects of Rehmannia glutinosa on growth performance, immunological parameters and disease resistance to Aeromonas hydrophila in common carp (Cyprinus carpio L.) Aquaculture. 2015;435:293–300. doi: 10.1016/j.aquaculture.2014.10.004. [DOI] [Google Scholar]
- Xu L, Kwak M, Zhang W, Zeng L, Lee PC-W, Jin J-O. Rehmannia glutinosa polysaccharide induces toll-like receptor 4 dependent spleen dendritic cell maturation and anti-cancer immunity. Oncoimmunology. 2017;6:1325981. doi: 10.1080/2162402X.2017.1325981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Zhang W, Zeng L, Jin J-O. Rehmannia glutinosa polysaccharide induced an anti-cancer effect by activating natural killer cells. Int. J. Biol. Macromol. 2017;105:680–685. doi: 10.1016/j.ijbiomac.2017.07.090. [DOI] [PubMed] [Google Scholar]
- Yilmaz Y, Toledo R. Antioxidant activity of water-soluble Maillard reaction products. Food Chem. 2005;93:273–278. doi: 10.1016/j.foodchem.2004.09.043. [DOI] [Google Scholar]
- Yoon S-R, Lee G-D, Park J-H, Lee I-S, Kwon J-H. Ginsenoside composition and antiproliferative activities of explosively puffed ginseng (Panax ginseng C.A. Meyer) J. Food Sci. 2010;75:C378–C382. doi: 10.1111/j.1750-3841.2010.01592.x. [DOI] [PubMed] [Google Scholar]
- Zhang RX, Jia ZP, Kong LY, Ma HP, Ren J, Li MX, Ge X. Stachyose extract from Rehmannia glutinosa Libosch. to lower plasma glucose in normal and diabetic rats by oral administration. Pharmazie. 2004;59:552–556. [PubMed] [Google Scholar]
- Zhou J, Xu G, Yan J, Li K, Bai Z, Cheng W, Huang K. Rehmannia glutinosa (Gaertn.) DC. polysaccharide ameliorates hyperglycemia, hyperlipemia and vascular inflammation in streptozotocin-induced diabetic mice. J. Ethnopharmacol. 2015;164:229–238. doi: 10.1016/j.jep.2015.02.026. [DOI] [PubMed] [Google Scholar]